Abstract
Purpose
To understand the role and further dissect pathways downstream of tissue plasminogen activator (tPA) and the fibrinolytic pathway in modulating outflow facility.
Methods
Outflow facility of tissue plasminogen activator (Plat) knockout (KO) mice was determined and compared to that of wild-type (WT) littermates. Gene expression of urokinase plasminogen activator (Plau), plasminogen activator inhibitor (Pai-1), plasminogen (Plg), and matrix metalloproteinases (Mmp-2, -9, and -13) was measured in angle tissues. Expression of the same genes and outflow facility were measured in KO and WT mice treated with triamcinolone acetonide (TA). Amiloride was used to inhibit urokinase plasminogen activator (uPA) in Plat KO mice, and outflow facility was measured.
Results
Plat deletion resulted in outflow facility reduction and decreased Mmp-9 expression in angle tissues. Plasminogen expression was undetectable in both KO and WT mice. TA led to further reduction in outflow facility and decreases in expression of Plau and Mmp-13 in plat KO mice. Amiloride inhibition of uPA activity prevented the TA-induced outflow facility reduction in Plat KO mice.
Conclusions
tPA deficiency reduced outflow facility in mice and was associated with reduced MMP expression. The mechanism of action of tPA is unlikely to involve plasminogen activation. tPA is not the only mediator of TA-induced outflow facility change, as TA caused reduction in outflow facility of Plat KO mice. uPA did not substitute for tPA in outflow facility regulation but abrogated the effect of TA in the absence of tPA, suggesting a complex role of components of the fibrinolytic system in outflow regulation.
Keywords: tissue plasminogen activator, urokinase plasminogen activator, outflow facility, triamcinolone, IOP elevation
Intraocular pressure (IOP) elevation related to corticosteroid administration was initially reported almost 70 years ago.1 In recent years, the frequent use of potent steroids for treatment of eye and systemic diseases has made steroid-induced intraocular hypertension and glaucoma more prevalent.2 Despite extensive work to understand the mechanisms of steroid-induced IOP elevation, the pathogenesis of this condition remains unclear.
Extracellular matrix (ECM) remodeling in the trabecular meshwork (TM), which may result from enhanced production or reduced degradation of ECM, has been proposed as the mechanism that leads to reduction in outflow facility of the aqueous humor3 and subsequent IOP elevation.4 ECM turnover is to a large degree dependent on matrix metalloproteinases (MMPs), a family of zinc-containing proteases.5 Several MMPs, including MMP-1, MMP-2, MMP-9, and MMP-13, show basal expression in the TM and/or are present in the aqueous humor.6 Expression and activity of several MMPs, such as MMP-2, MMP-9, and MMP-13, have been reported to be decreased in primary open angle glaucoma patients or animal models of IOP elevation.7–9 More recently, an MMP-9 gene polymorphism has been found to be protective for glaucoma.10 MMP regulation occurs at multiple stages: they are transcriptionally controlled and synthesized as pro-MMPs (inactive) and then activated by enzymatic cleavage.11,12 One of the regulators of both transcription and activation of MMPs is tissue plasminogen activator (tPA).
tPA is a serine protease better known for its function in the conversion of plasminogen to plasmin in the fibrinolytic pathway.13 Besides its role in degrading fibrin, plasmin has been shown to serve as a trigger to proteolytically activate several pro-MMPs to their active forms, which leads to degradation of several downstream ECM proteins.14 The amount of plasmin is tightly controlled by the balance between tPA, urokinase plasminogen activator (uPA), and plasminogen activator inhibitor (PAI-1). tPA can also regulate MMP gene expression through the low-density-lipoprotein receptor-related protein 1 (LRP-1), epidermal growth factor receptor, and N-methyl-D-aspartate receptor.15–17 Thus, tPA is an important regulator of ECM turnover.4
We have previously shown that intravitreal injection of recombinant human tPA can prevent and reverse glucocorticosteroid-induced IOP elevation in sheep,18 and that overexpression of tPA is effective in both preventing and reversing steroid-induced outflow facility reduction in mice.8 In both species, an increase in tPA is associated with upregulation of MMPs. The present work was undertaken to dissect the molecular mechanisms that implicate tPA in outflow facility regulation after exposure to steroids. To better understand the role of tPA and the fibrinolytic pathway in this process, we used Plat knockout (KO) mice. These mice are phenotypically normal and have normal life span even though they display abnormalities in thrombolysis and thrombosis.19 Surprisingly, using these mice we uncovered that tPA has a much larger role in regulating outflow facility even in the absence of steroid exposure. Below we report this important finding, experiments performed to better understand some of downstream mechanisms of tPA-mediated outflow regulation, and experiments to understand the potential roles of other fibrinolytic pathway molecules.
Materials and Methods
Animals
Eight- to 12-week-old mice were used for this study. The animals were housed and bred at the State University of New York (SUNY) Downstate Medical Center, Brooklyn, New York, United States, under a 12-hour light/12-hour dark cycle and food ad libitum. The plat KO mice colony was established from animals (strain No. 002508, strain name: B6.129S2-Plattm1Mlg/J) obtained from The Jackson Laboratories (Bar Harbor, ME, USA).19 The mice had been backcrossed to C57BL/6 for eight generations before been accepted into Jackson Laboratories for distribution. The protocol was approved by the SUNY Downstate Institutional Animal Care and Use Committee, and experiments were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Mouse Tissue Collection
For TM tissue collection, all mice were perfused with RNase-free phosphate-buffered saline (PBS) containing 1% Heparin (Hospira, Lake Forest, IL, USA). For tissue used for quantitative polymerase chain reaction (qPCR), eyes were immersed in RNAlater solution (Invitrogen, Waltham, MA, USA) at 4°C and then dissected on ice to obtain the angle ring containing the TM tissues as previously described.8 TM tissues were snap-frozen in RNAlater and stored at −80°C until RNA extraction. For tissues used for zymography, TM tissues were dissected in PBS, snap-frozen in Eppendorf tubes, and stored at −80°C until use.
For hippocampus tissue collection, the brain was carefully removed, rinsed with RNase-free PBS, separated into two hemispheres, and placed on a prechilled metal surface under a dissecting microscope. The cerebellum and olfactory bulb were removed, and tissue was carefully separated to expose the medial surface of the hippocampus. A spatula was used to separate the hippocampus.20 The tissue was snap-frozen and stored at −80°C until use.
Triamcinolone Acetonide (TA) Injection
Subconjunctival PBS (vehicle) or TA (Kenalog-40; Bristol-Myers Squibb, New York, NY, USA) injections were performed as described previously.8 Briefly, 20 μL Kenalog-40 suspension was injected subconjunctivally in both eyes by using a 50-μL Hamilton syringe (Hamilton, Reno, NV, USA) with a 26-gauge needle. Control animals received bilateral injection of PBS. For experiments involving TA (or PBS) administration all mice were euthanized 1 week after subconjunctival injections.
Amiloride Treatment
Amiloride hydrochloride hydrate (Sigma-Aldrich Corp., St. Louis, MO, USA) 10 mg/kg in PBS was administered by gavage (n = 29) once daily for periods of 2 weeks. Fourteen of the 29 animals received subconjunctival injection of TA, while the rest (n = 15) received vehicle. Subconjunctival injections were performed 7 days after initiation of amiloride treatment. Eleven additional mice received PBS by gavage for 2 weeks while also receiving subconjunctival injection of PBS 1 week later and served as additional controls.
RNA Isolation and qPCR
TM tissue was isolated from eyes that were enucleated immediately after animal euthanasia. No eyes that were subjected to outflow facility determination were used for gene expression studies to avoid potential RNA degradation between the time of death and the time of tissue collection. Tissue was homogenized in TRIzol reagent (Life Technologies, Carlsbad, CA, USA). RNA was isolated per the manufacturer's instructions and resuspended in nuclease-free water. RNA concentration was determined with a Nanodrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
cDNA was synthesized by using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA) according to manufacturer's protocol. Quantitative real-time PCR (qRT-PCR) was performed by using Green-2-Go qPCR Mastermix-ROX (Bio Basic, Amherst, NY, USA) on a QuantStudio 6 Flex thermal cycler (Applied Biosystems, Carlsbad, CA, USA).
mRNA expression of mouse Plat, Plau, Pai-1, Plg, Mmp-2, Mmp-9, and Mmp-13 in the TM was determined. Primers were designed by using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome; in the public domain) and their specificity was confirmed by the presence of single band of the expected size on agarose gel electrophoresis. Primer sequences are listed in the Table. Specificity was further verified during each experiment by inspection of melting curves to ensure the absence of multiple-sized amplification products. Annealing temperature was 60°C. Values of target mRNA expression were normalized to the expression levels of Rps11 (ribosomal protein S11). The relative fold change was calculated by using the ΔΔCt method.21 The presence of outliers was tested by using the Thompson Tau test and any outliers (a total of three animals for the whole study) were excluded from analysis.
Table.
Primers for Mouse TM Tissues
| Number |
Name of Genes |
Sequences |
| 1 | Plat | FP: CGAAAGCTGACGTGGGAATA |
| RP: GTGTGAGGTGATGTCTGTGTAG | ||
| 2 | Plau | FP: GCGCCTTGGTGGTGAAAAAC |
| RP: GACACGCATACACCTCCGTT | ||
| 3 | Pai-1 | FP: ATGATGGCTCAGAGCAACAAG |
| RP: CATTGTCTGATGAGTTCAGCATC | ||
| 4 | Mmp-2 | FP: ACAGTGACACCACGTGACAA |
| RP: GGTCAGTGGCTTGGGGTATC | ||
| 5 | Mmp-9 | FP: GCGTCGTGATCCCCACTTAC |
| RP: CAGGCCGAATAGGAGCGTC | ||
| 6 | Mmp-13 | FP: TACCATCCTGCGACTCTTGC |
| RP: TTCACCCACATCAGGCACTC | ||
| 7 | Plg | FP: GACCAGTCAGATTCCTCAGTTC |
| RP: CTTCTTCCCTGTGATGGTAGTG | ||
| 8 | Rps11 | FP: AAGACGCCTAAAGAGGCTATTG |
| RP: GGTCCTCTGCATCTTCATCTTC |
FP, forward primer; RP, reverse primer.
Measurement of Outflow Facility
Mouse eyes were enucleated immediately after euthanasia and used for outflow facility measurement under either constant flow or under constant pressure. Both methods are sensitive enough to detect outflow changes across groups. For both methods, outflow facility was determined by the slope of the linear regression curve that links pressure with flow. Any eyes that developed leaks during outflow facility determination or that had pressure-flow correlations with R2 of less than 0.8 were not included in any analysis.
When experiments were initiated, we did not possess equipment necessary to measure outflow facility in mice by the constant pressure method. We thus initially measured outflow facility by using the constant flow method and using equipment described in our earlier publication.22 Because of worries that our recorder may fail at any time with no possibility of our being able to repair it, we purchased the flow sensors and instrumentation described below and started measuring outflow by the constant pressure method. This method also has the advantage of requiring much shorter equilibration times and thus provides higher throughput. Experiments shown in Figures 2 and 4 were completed with the constant flow method, while experiments shown in Figure 7 were performed with the constant pressure method. To verify that the two methods provide similar results, we repeated measurements in a number of eyes with the other method (constant pressure for Figs. 2, 4; and constant flow for some groups in Fig. 7). Both methods are sensitive enough to detect outflow difference across groups, although actual outflow values determined by the two methods were not identical. Only data obtained by using either the constant flow or constant pressure methods for each individual experiment are presented below and the method used is indicated.
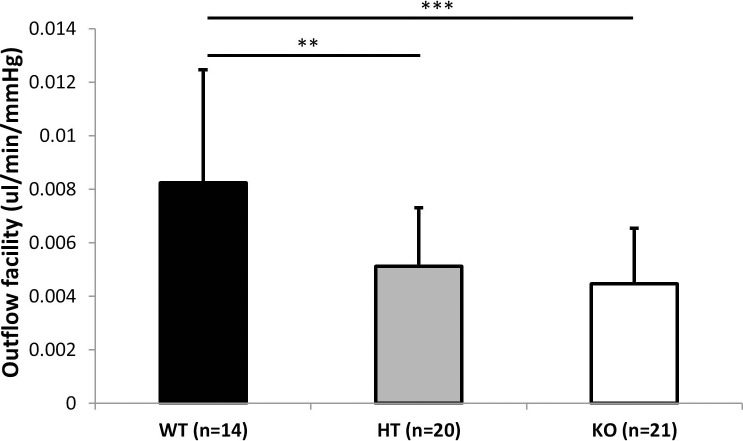
Figure 2.
Outflow facility (mean ± SD) determined by the constant flow method in Plat WT (n = 14), HT (n = 20), and KO (n = 21) mice. Asterisks indicate statistically significant differences (**P < 0.01, ***P < 0.001, ANOVA, Tukey-Kramer post hoc test).
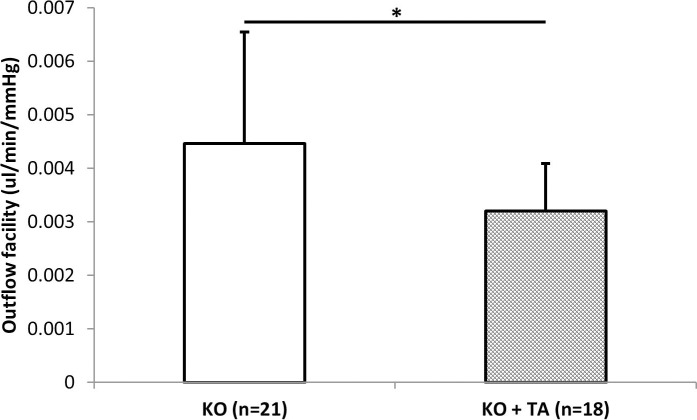
Figure 4.
Outflow facility in Plat KO mice treated with triamcinolone acetonide (KO+TA) (n = 18) or PBS (KO) (n = 21) for 7 days determined by the constant flow method. Asterisks indicate statistically significant differences (*P = 0.021, t-test).
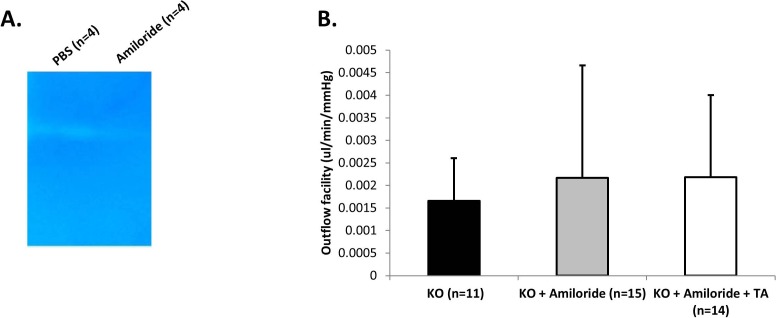
Figure 7.
(A) Representative zymograms of uPA enzymatic activity in the trabecular meshwork of KO mice treated with amiloride (n = 4) or PBS (n = 4). No activity is detected in amiloride-treated animals. (B) Outflow facility in Plat KO mice treated with vehicle both per os and subconjunctivally (KO, n = 11), amiloride per os and vehicle subconjunctivally (KO+amiloride, n = 15), or amiloride per os plus TA subconjunctivally (KO+amiloride+TA, n = 14) determined by the constant pressure method. No significant differences were found between groups (P > 0.05, ANOVA).
Constant Flow Method
Measurements were performed as previously described22 with the following modifications. After the initial stabilization, flow rate was increased sequentially from 0.09 μL/min to 0.19 μL/min in steps of 0.02 μL/min. Pressure was recorded and the pressure values at steady state for each flow rate were plotted. The slope of the regression line was used to calculate outflow facility as described previously.22
Constant Pressure Method
Measurements were performed with the initial pressure set at 8 cm H2O (5.88 mm Hg) for 10 minutes to stabilize readings. Pressure was then raised in steps of 4 cm H2O at a time until it reached 32 cm H2O (23.54 mm Hg). Flow was allowed to stabilize for 5 minutes at each pressure level before it was recorded on a microfluidic flow system (ELVEFLOW, Paris, France). The flow rates at each pressure level were plotted and the slope of the regression line was used to calculate the outflow facility value for each eye.
IOP Measurements
IOP was measured in mice after application of 0.5% proparacaine. Mice were held in a custom-made restraint that does not compress the chest or neck while IOP is measured.8 A rebound tonometer was used to measure IOP in all mice.23 Five measurements were obtained per eye and averaged. IOP measurements were performed between 10 AM and 12 PM to minimize the effect of diurnal IOP variation. All IOP measurements were performed preterminally and mice were euthanized immediately after.
Zymography
tPA/uPA activity was determined by fibrinogen/plasminogen-zymography assays. The assays were performed with minor modifications to protocols previously described.24,25 Briefly, 40 μL extraction buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM Na3VO4, pH 7.4, 1% Nonidet p40; Sigma-Aldrich Corp.) were added to TMs from four pooled eyes or from hippocampus tissue of one mouse, and the tissues were homogenized. Tissue homogenates were centrifuged at 11,180g for 5 minutes at 4°C and supernatants were collected. Protein concentration was determined by using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Aliquots containing equal amounts of tissue protein (25 μg) were mixed with gel-loading buffer and then loaded, without heating, onto 10% SDS polyacrylamide gels containing fibrinogen (5.5 mg/mL)/plasminogen (50 μg/mL) as the tPA/uPA substrate. The same amounts of murine tPA and uPA were added as positive control and run on each individual gel. A molecular weight size standard was also included on all gels (Life Technologies, Gaithersburg, MD, USA). After electrophoresis, gels were washed three times with 2.5% Triton X-100 (15 minutes each time), placed in activation buffer containing 0.1M glycine, and incubated overnight at 37°C to allow proteolysis of the substrates. Gels were stained with 0.1% Coomassie Brilliant Blue-R250 and destained in a 25% methanol and 10% acetic acid solution. The intensity of the bands was quantified by using ImageJ (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) as previously described.26 As exposure time for each gel was different, normalization to the positive control of each experiment was used to derive the relative intensity values that were plotted.
Results
tPA Expression and tPA Enzymatic Activity in Plat KO Mice
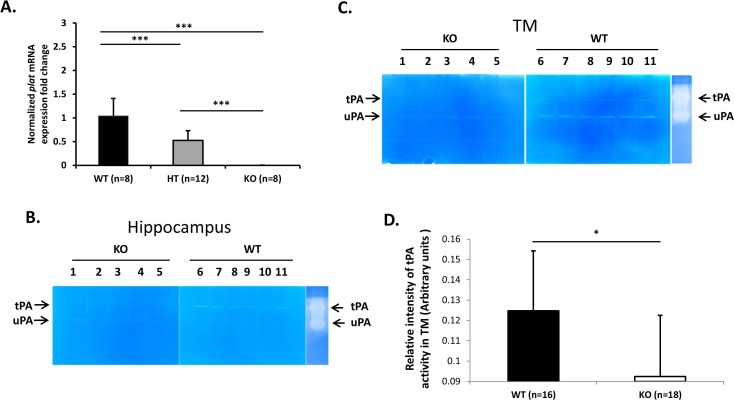
To confirm that Plat KO mice have no tPA expression, we measured Plat mRNA expression in TM tissues by RT-qPCR. As shown in Figure 1A, KO mice (n = 8) had no detectable Plat mRNA expression, while heterozygous (HT) mice (n = 12) had approximately half of the expression of WT mice (n = 8).
Figure 1.
(A) Normalized fold change (mean ± SD) for Plat mRNA expression level in angle ring tissues of Plat WT (n = 8), HT (n = 12), and KO (n = 8) mice. Fold changes were normalized to Plat mRNA expression level in WT mice. Asterisks indicate statistically significant differences (***P < 0.001, ANOVA, Tukey-Kramer post hoc test). (B) tPA and uPA enzymatic activity in hippocampus of KO (n = 5) and WT (n = 6) mice determined by in-gel zymography. Each lane represents one mouse. (C, D) tPA enzymatic activity in trabecular meshwork of WT (n = 16) and KO (n = 18) mice determined by in-gel zymography. Representative image (C) and band density quantification (D). Four eyes were pooled per lane in (C). Signal intensity is significantly different (*P < 0.05, t-test) between WT and KO mice.
In addition, enzymatic activity of tPA and uPA was determined by in-gel zymography. In the hippocampus of WT (n = 6) and KO mice (n = 5) (Fig. 1B) and in the TM of WT (n = 16) and KO mice (n = 18) (Figs. 1C, 1D), tPA activity was almost undetectable in Plat KO mice, while uPA activity can be readily detected in the same animals. Quantification of tPA activity in all TM samples analyzed is presented in Figure 1D.
Decreased Outflow Facility in Plat KO Mice
Eyes of Plat KO mice appeared normal with no obvious differences from those of WT littermates on clinical examination under an operating microscope. IOP was not significantly different between WT and KO mice (P > 0.05, t-test). Outflow facility (mean ± SD) was significantly reduced in Plat KO (0.0045 ± 0.0021 μL/min/mm Hg) (n = 21) and Plat HT mice (0.0051 ± 0.0022 μL/min/mm Hg) (n = 20) compared with WT littermates (0.0083 ± 0.0042 μL/min/mm Hg) (n = 14) (P = 0.00076, ANOVA) (Fig. 2) as determined by the constant flow method. Outflow facility of the WT group was significantly higher than that of the HT and WT groups (P < 0.01, Tukey-Kramer post hoc test for differences between WT and HT and WT and KO).
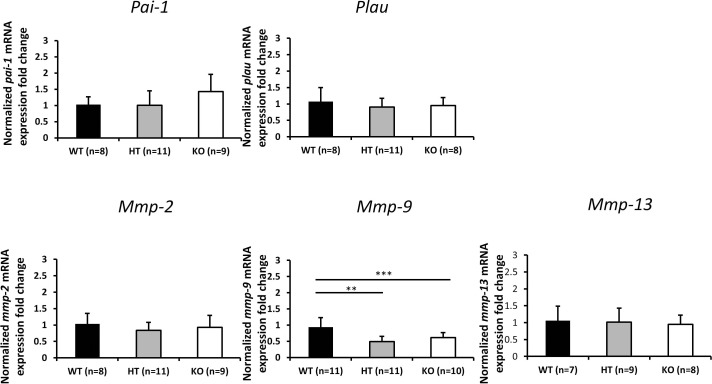
Reduced Mmp-9 mRNA Expression in Plat KO Mice
To determine potential genes that may mediate tPA-induced outflow effects, expression of various fibrinolytic and related genes (Plau, Pai-1, Plg, Mmp-2, Mmp-9, and Mmp-13) were determined by RT-qPCR. The expression of Plg in TM tissues was below the detection limit in all animals (WT, n = 8; HT, n = 11; KO, n = 9). Only Mmp-9 expression was significantly reduced in Plat HT (n = 11) and KO (n = 10) mice compared with their WT controls (n = 11) (P < 0.01, Tukey-Kramer post hoc test) (Fig. 3). Differences in the numbers of animals described above result from the amount of RNA that could be extracted from individual animals and used for amplification of specific genes and not because of exclusion of any animals except for statistical outliers (one in the KO group of Plau amplifications and one in the KO group of the Mmp-9 amplifications).
Figure 3.
Normalized fold changes (mean ± SD) for the Pai-1, Plau, Mmp-2, Mmp-9, and Mmp-13 mRNA expression levels in angle ring tissue of Plat WT, HT, and KO mice. Fold changes were normalized to mRNA expression level in WT mice. Asterisks indicate statistically significant differences (**P < 0.01, ***P < 0.001, ANOVA, Tukey-Kramer post hoc test). Differences in the numbers of animals in figure subpanels result from the amount of RNA that could be extracted from individual animals and used for amplification of specific genes and not because of exclusion of any animals except for statistical outliers (one in the KO group of Plau amplifications and one in the KO group of the Mmp-9 amplifications).
Further Reduction in Outflow Facility in Plat KO Mice Induced by TA
To determine whether tPA is the sole mediator of steroid-induced outflow facility reduction, outflow facility was measured in eyes of Plat KO mice exposed to TA for 1 week. Outflow facility (mean ± SD) determined by the constant flow method in TA-treated eyes (0.0032 ± 0.0009 μL/min/mm Hg) (n = 18) was significantly lower (P = 0.022, t-test) than that in eyes that were not exposed to TA (0.0045 ± 0.0021 μL/min/mm Hg) (n = 21) (Fig. 4).
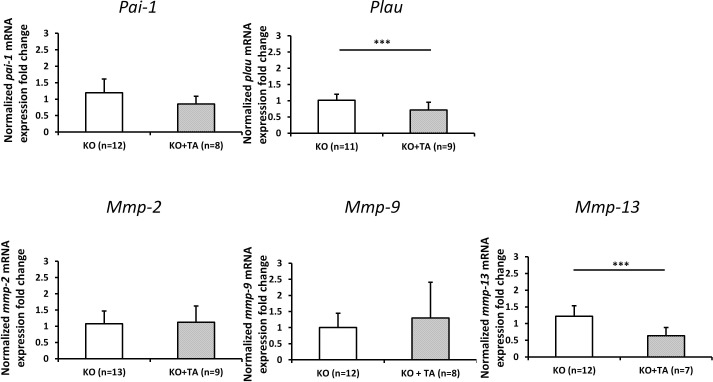
Change in Gene Expression Induced by TA in Plat KO Mice
To determine the potential mechanism that may account for the TA-induced outflow facility change in Plat KO mice, the mRNA expression of Plau, Pai-1, Mmp-2, Mmp-9, and Mmp-13 was determined by RT-qPCR. The expression of Plau was significantly downregulated in eyes from KO animals treated with TA (n = 9), compared to the expression in eyes of KO animals treated with PBS (n = 11). In addition, a significant reduction of Mmp-13 expression was detected in KO animals treated with TA (n = 7), compared with KO animals receiving vehicle (n = 12) (Fig. 5). Differences in the numbers of animals described above result from the amount of RNA that could be extracted from individual animals and used for amplification of specific genes and not because of exclusion of any animals except for statistical outliers (one in the KO+TA group of Mmp-13 amplifications).
Figure 5.
Normalized fold change (mean ± SD) of Pai-1, Plau, Mmp-2, Mmp-9, and Mmp-13 mRNA expression level in angle ring tissue from Plat KO mice treated with TA (KO+TA) or PBS (KO) for 7 days. Fold changes were normalized to mRNA expression level in KO mice without TA treatment. Asterisks indicate statistically significant differences (***P < 0.001, t-test). Differences in the numbers of animals in figure subpanels result from the amount of RNA that could be extracted from individual animals and used for amplification of specific genes and not because of exclusion of any animals except for statistical outliers (one in the KO+TA group of Mmp-13 amplifications).
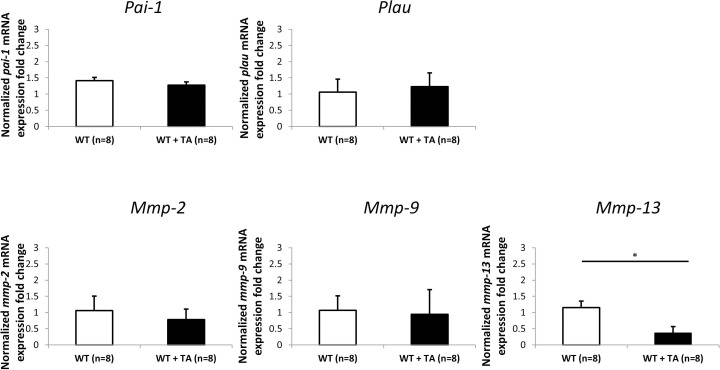
No Change in Expression of Plau, Pai-1, Mmp-2, and Mmp-9 in WT Mice Treated With TA
To compare the effects of TA treatment on the genes of the fibrinolytic and MMP pathways in Plat KO with those in tPA-sufficient animals, gene expression of Plau, Pai-1, Mmp-2, Mmp-9, and Mmp-13 was determined by qPCR. Significant reduction of only Mmp-13 expression was detected in tPA-sufficient animals treated with TA subconjunctivally (n = 8) compared with animals receiving vehicle subconjunctivally (n = 8) (Fig. 6).
Figure 6.
Normalized fold change (mean ± SD) of Plau, Pai-1, Mmp-2, Mmp-9, and Mmp-13 mRNA expression level in angle ring tissue from WT mice treated with TA (WT+TA) or PBS (WT) for 7 days (n = 8 in each group). Fold changes were normalized to mRNA expression level in WT mice without TA treatment (*P < 0.05, t-test).
uPA Inhibition in Plat KO Mice Prevents Steroid-Induced Outflow Facility Reduction
To understand whether uPA can substitute for tPA in outflow facility regulation, amiloride (given by gavage) was used to inhibit uPA enzymatic activity in Plat KO mice. Despite total inhibition of uPA enzymatic activity by amiloride in Plat KO mice (Fig. 7A) (PBS, n = 4; amiloride, n = 4), outflow facility was not significantly different between amiloride-treated mice (n = 15) and mice receiving vehicle per os (n = 11) (columns KO & KO+amiloride in Fig. 7B). However, KO mice treated with subconjunctival TA in addition to per os amiloride (n = 14) did not experience any TA-induced outflow facility reduction (column KO+amiloride+TA in Fig. 7B).
Discussion
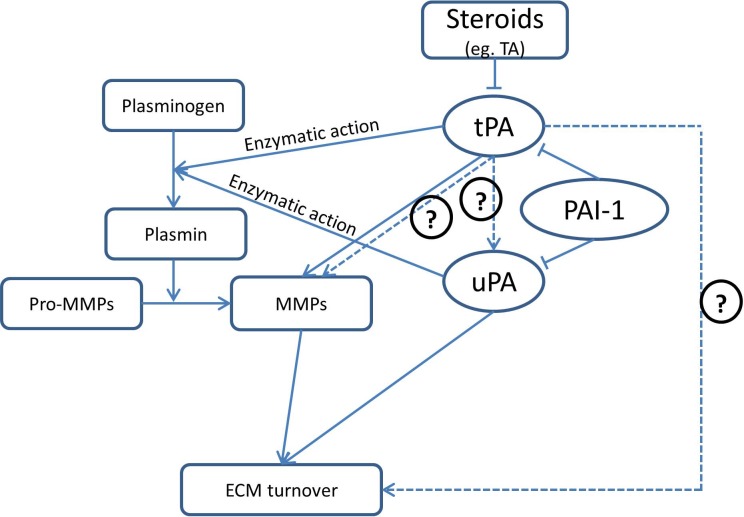
Steroid-induced IOP elevation shares many clinical and pathologic features with IOP elevation seen in primary open angle glaucoma (POAG).2 One of the major mechanisms that have been found to contribute to steroid-induced IOP elevation is ECM accumulation in outflow pathways.27 ECM turnover is regulated by a large number of enzymes, many of which degrade ECM components; tPA (Fig. 8) is one of these enzymes and has been shown to increase outflow facility in animal models of steroid-induced IOP elevation.4 Our previous studies have shown that prednisolone instillation in sheep eyes suppresses tPA expression,18 that intravitreal injection of recombinant human tPA can prevent and reverse prednisolone-induced IOP elevation in sheep,18 and that overexpression of tPA in the TM is effective in both preventing and reversing TA-induced outflow facility reduction in mice.8 In both species, an increase in tPA is associated with upregulation of MMPs.8,18 However, the exact mechanism for these effects has not been determined. In the current study, we attempted to investigate whether tPA is the sole mediator of steroid-induced outflow facility reduction. As tPA is best known for its actions in regulating the fibrinolytic pathway (which in turn can regulate ECM), we focused on the potential contributions of tPA as part of the fibrinolytic pathway in outflow regulation after exposure to steroids. For these studies we used tPA-deficient (Plat KO) mice. Our hypothesis was that if tPA was the sole mediator of steroid-induced outflow facility reduction, Plat KO mice should not experience any reductions of outflow facility when exposed to steroids. Surprisingly, work with these animals revealed that tPA plays a much larger role in outflow facility regulation.
Figure 8.
Diagrammatic map of the fibrinolytic pathway, its potential interactions with the ECM, and its modulation by steroids. Solid lines indicate relationships that have been established, while dashed lines indicate relationships supported by the current data. Arrows indicate activation or positive effects. Inhibition or negative effects are indicated by “T” endings. Question marks indicate tentative or speculative relationships.
Plat KO mice are viable and fertile. Despite abnormalities in thrombolysis and thrombosis,19 they are generally indistinguishable from WT littermates. In the current study, we also did not detect any obvious (clinical) ocular morphologic defects in Plat KO mice or any large difference in IOP between Plat KO mice and their WT littermates. Previous studies in Plat KO animals, however, have shown that tPA deficiency leads to more severe fibrosis after injury in the skin28 and liver.29 The disrupted balance between MMP activation and inhibition may contribute to ECM dysregulation in both models28,29 and can potentially explain some of the more startling results reported here.
Despite having similar IOPs, Plat KO mice had significantly reduced (∼45% lower) outflow facility as compared with that of their WT littermates. This discrepancy is not unusual because outflow facility is much more sensitive in detecting differences in aqueous humor dynamics in mice as we have previously extensively discussed.22 In addition it is also possible that Plat KO mice have increased outflow through the unconventional pathway, and/or a decreased aqueous humor production. These possible mechanisms are beyond the scope of this work and were not pursued. The reduction of outflow facility in Plat KO mice implies that tPA is a regulator of outflow facility independent of exposure to steroids (at least in mice). This is a novel finding that if confirmed for humans can potentially be used in the development of new treatments for glaucoma. It is of course true that aqueous humor physiology in mice is not identical with that in humans, but they nonetheless share many characteristics.
Interestingly, HT mice, which carry a single Plat allele, did not show outflow facilities at an intermediate level between WT and KO mice, despite the fact that Plat mRNA levels in the TM of HT mice are approximately 50% of those of WT mice (see Fig. 1). Instead, HT mouse outflow facility was closer to that of KO mice, suggesting haploinsufficiency of the Plat gene in its effects on outflow facility. It has been previously reported that HT mice have indeed significantly reduced tPA enzymatic activity in other tissues as compared with their WT littermate controls.19
To determine whether other genes in the fibrinolytic pathway may play a role in outflow facility regulation, we examined the expression of several key genes of the fibrinolytic system in anterior chamber angle rings that contain the TM. We were unable to detect any expression of plasminogen (the molecule cleaved by tPA to generate plasmin, which then cleaves fibrin to dissolve clots). This suggests that the enzymatic action of the whole fibrinolytic pathway is probably not involved in tPA-related ECM modulation in the TM. It is of course possible that trace amounts of plasminogen (below the assay detection limit) are produced locally. In addition, blood-borne plasminogen would be expected to become readily available if the blood-aqueous barrier was compromised such as during inflammatory episodes or in the presence of hyphema. However, a review of the literature suggests that plasminogen expression has not been conclusively detected in the TM, although it has been assumed to be present.30
Despite plasminogen expression being undetectable in angle ring tissue, Plau and Pai-1 expression were readily detected in both tPA-sufficient and tPA-deficient mice (see Fig. 3), as has been previously reported for mice and other species.8,31–33 The presence of locally produced tPA and uPA in the absence of plasminogen suggests that these proteases may act directly (or via MMPs) on ECM components of the TM (such as laminin and fibronectin) to modulate outflow facility as has been previously proposed.4,16,34
To determine whether tPA absence modulates outflow facility through changes in expression of MMPs, we also measured the levels of expression of a number of MMPs that have been implicated in outflow facility regulation in mice.8 MMPs participate in a number of physiological and pathologic processes.35–37 Several MMPs have been reported to be expressed in TM and/or aqueous humor at different levels in patients with and without glaucoma.6 tPA can modulate MMP activity in a fast, direct, plasmin-dependent manner by activating pro-MMPs (although a plasmin-independent activation of MMPs has also been proposed38). In addition, tPA has been shown to modulate MMP activity through the slower route of receptor-mediated alteration in MMP expression (Fig. 8). tPA-dependent activation of MMP-9 gene transcription39 through the LRP-1 receptor has been reported, but it is not the only potential mechanism for such an action. tPA has also been shown to bind to and activate the EGF receptor,17 which has been shown to have the capacity to affect MMP-9 transcription and translocation40 (Fig. 8). Interestingly, from the MMPs measured, only MMP-9 expression was decreased in the absence of tPA and in animals with one allele of Plat (Fig. 3). MMP-9 expression has also been shown to increase when tPA is administered exogenously in animal models of steroid-induced IOP elevation.8
To explore the original premise that led to this work—that is, whether tPA was the sole mediator of steroid-induced outflow facility changes—we measured outflow facility of Plat KO mice with and without steroid exposure. When Plat KO mice were treated with TA, outflow facility was further reduced by approximately 50% from the already reduced outflow facility of these KO mice, suggesting that steroid-induced facility changes can occur even in the absence of tPA (Fig. 4). This indicates that TA (and most probably other corticosteroids) potentially also affects outflow facility via pathways that do not necessarily involve tPA. Although somewhat unexpected (especially given the potentially much larger role of tPA in modulating outflow facility), this finding may reflect the fact that steroids can directly affect the physiology of TM cells41 in addition to the ECM turnover in the juxtacanalicular area. It is also possible that steroids directly modulate other proteins downstream of tPA in mice.
Interestingly, TA treatment, which is known to decrease tPA expression and activity in mice42,43 and other species18 including humans,44 did not affect Plau or Pai-1 expression in tPA-sufficient mice but decreased Plau expression in Plat KOs; this suggests that tPA somehow exerts regulatory control over the transcription of Plau. A similar cross talk between tPA and Plau has not been reported but is feasible, since tPA is known to modulate expression of a number of genes including some of its downstream targets.45
To further discern the role of tPA in steroid-induced outflow facility reduction, we determined whether patterns of Mmp expression differ in tPA-sufficient versus tPA-deficient (KO) animals. As can be readily seen by inspection of Figures 5 and 6, expression of Mmps-2, -9, and -13 follow an identical pattern after exposure to steroids in both the presence and absence of tPA. Mmp-13 is consistently the only Mmp tested for which TA caused a significant downregulation of expression. MMP-13 is a collagenase that degrades collagen type I, III, and IV. Collagen IV has been found to be more highly expressed in TM of patients with steroid-induced glaucoma than that from patients with POAG and nonglaucomatous specimens.46 In a transgenic mouse model of POAG (Tg-MYOCY437H), an increase in fibronectin, elastin, and collagen type IV and I in TM has also been reported.47 Thus, it is conceivable that MMP-13 is one of the effector molecules that play a role in TA-induced outflow facility reduction. It is actually interesting that overexpression of tPA in the TM leads to Mmp-13 upregulation and causes an increase in outflow facility.8 Although further studies are needed to dissect the exact mechanisms and roles of MMPs in steroid-induced outflow facility reduction, our results suggest that MMP-13 should be one of the potential targets.
Finally, to answer the question of whether urokinase substitutes for tPA when the latter is absent, we inhibited urokinase by systemic administration of amiloride in Plat KO animals. Amiloride is a diuretic that inhibits sodium uptake in the collecting tubule. It also competitively and selectively inhibits uPA enzymatic activity48 but does not affect the activity of tPA and other fibrinolytic pathway components.48 The effect of amiloride on outflow facility is largely unknown. There are reports that topical use of dimethylamiloride in mice could lead to IOP reduction,49 while there have been other reports that amiloride hydrochloride increases human intraocular pressure in rare cases.50 Drug administration route, molecule variant, and species differences possibly account for these differences. In the present study, we found that at the dose of amiloride used, urokinase appeared (as determined by zymography) to be completely inhibited in the angle tissues (Fig. 7). Interestingly, not only did inhibition of urokinase not affect outflow facility, but also it prevented the outflow facility reduction caused by TA in Plat KO mice (compare Figs. 4 and 7). Thus, it appears that uPA does not substitute for tPA in outflow facility regulation as has been reported for a number of other tissues,51–53 potentially owing to distinct enzymatic cofactors, receptors, and binding capacities. Furthermore, the present study suggests that uPA may use a distinct mechanism in affecting TA-induced outflow facility changes. Additional studies in this area are warranted.
In summary, we showed that tPA plays an important role in outflow facility regulation under baseline conditions independent of the presence of steroids. The effects of tPA on outflow facility do not appear to be mediated through a plasmin-dependent mechanism, at least under normal conditions in which the blood-aqueous barrier is intact. Under baseline conditions, the effect of tPA is possibly mediated via MMP-9, while underreduced levels (following TA exposure) may be through transcriptional control of MMP-13. Urokinase does not appear to substitute for tPA in its absence. However, it may have a distinct and interesting role in steroid-induced outflow facility changes. Further studies will be needed to fully understand how various components of the fibrinolytic system modulate outflow facility under both baseline conditions and after steroid exposure.
Acknowledgments
The authors thank Ilya Ribkin for his assistance in some of the outflow studies.
Supported by National Institutes of Health Grant R01 EY025543.
Disclosure: Y. Hu, None; A.O. Barron, None; S. Gindina, None; S. Kumar, None; S. Chintala, None; A. Nayyar, None; J. Danias, None
References
- 1.Gordon DM, Mc LJ, Koteen H, et al. The use of ACTH and cortisone in ophthalmology. Am J Ophthalmol. 1951;34:1675–1686. doi: 10.1016/0002-9394(51)90032-3. [DOI] [PubMed] [Google Scholar]
- 2.Phulke S, Kaushik S, Kaur S, Pandav SS. Steroid-induced glaucoma: an avoidable irreversible blindness. J Curr Glaucoma Pract. 2017;11:67–72. doi: 10.5005/jp-journals-l0028-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vranka JA, Kelley MJ, Acott TS, Keller KE. Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma. Exp Eye Res. 2015;133:112–125. doi: 10.1016/j.exer.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009;88:676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Groef L, Van Hove I, Dekeyster E, Stalmans I, Moons L. MMPs in the trabecular meshwork: promising targets for future glaucoma therapies? Invest Ophthalmol Vis Sci. 2013;54:7756–7763. doi: 10.1167/iovs.13-13088. [DOI] [PubMed] [Google Scholar]
- 7.Maatta M, Tervahartiala T, Vesti E, Airaksinen J, Sorsa T. Levels and activation of matrix metalloproteinases in aqueous humor are elevated in uveitis-related secondary glaucoma. J Glaucoma. 2006;15:229–237. doi: 10.1097/01.ijg.0000212229.57922.72. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Shah S, Tang HM, Smith M, Borras T, Danias J. Tissue plasminogen activator in trabecular meshwork attenuates steroid induced outflow resistance in mice. PLoS One. 2013;8:e72447. doi: 10.1371/journal.pone.0072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rybkin I, Gerometta R, Fridman G, Candia O, Danias J. Model systems for the study of steroid-induced IOP elevation. Exp Eye Res. 2017;158:51–58. doi: 10.1016/j.exer.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu MY, Wu Y, Zhang Y, et al. Associations between matrix metalloproteinase gene polymorphisms and glaucoma susceptibility: a meta-analysis. BMC Ophthalmol. 2017;17:48. doi: 10.1186/s12886-017-0442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaluk P, Kaczmarek L. Matrix metalloproteinase-9 in glutamate-dependent adult brain function and dysfunction. Cell Death Differ. 2007;14:1255–1258. doi: 10.1038/sj.cdd.4402141. [DOI] [PubMed] [Google Scholar]
- 12.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Loscalzo J, Braunwald E. Tissue plasminogen activator. N Engl J Med. 1988;319:925–931. doi: 10.1056/NEJM198810063191407. [DOI] [PubMed] [Google Scholar]
- 14.Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7:243–253. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Lee SR, Arai K, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, Nagai N, Yamakawa K, Kawakami J, Lijnen HR, Umemura K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009;114:3352–3358. doi: 10.1182/blood-2009-02-203919. [DOI] [PubMed] [Google Scholar]
- 17.Bertrand T, Lesept F, Chevilley A, et al. Conformations of tissue plasminogen activator (tPA) orchestrate neuronal survival by a crosstalk between EGFR and NMDAR. Cell Death Dis. 2015;6:e1924. doi: 10.1038/cddis.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerometta R, Kumar S, Shah S, Alvarez L, Candia O, Danias J. Reduction of steroid-induced intraocular pressure elevation in sheep by tissue plasminogen activator. Invest Ophthalmol Vis Sci. 2013;54:7903–7909. doi: 10.1167/iovs.13-12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmeliet P, Schoonjans L, Kieckens L, et al. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 20.Hagihara H, Toyama K, Yamasaki N, Miyakawa T. Dissection of hippocampal dentate gyrus from adult mouse. J Vis Exp. 2009;33:1543. doi: 10.3791/1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Wang X, Wu Y, et al. Role of EFNB1 and EFNB2 in mouse collagen-induced arthritis and human rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1778–1788. doi: 10.1002/art.39116. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Shah S, Deutsch ER, Tang HM, Danias J. Triamcinolone acetonide decreases outflow facility in C57BL/6 mouse eyes. Invest Ophthalmol Vis Sci. 2013;54:1280–1287. doi: 10.1167/iovs.12-11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danias J, Kontiola AI, Filippopoulos T, Mittag T. Method for the noninvasive measurement of intraocular pressure in mice. Invest Ophthalmol Vis Sci. 2003;44:1138–1141. doi: 10.1167/iovs.02-0553. [DOI] [PubMed] [Google Scholar]
- 24.Mali RS, Cheng M, Chintala SK. Intravitreous injection of a membrane depolarization agent causes retinal degeneration via matrix metalloproteinase-9. Invest Ophthalmol Vis Sci. 2005;46:2125–2132. doi: 10.1167/iovs.04-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey R, Chintala SK. Inhibition of plasminogen activators attenuates the death of differentiated retinal ganglion cells and stabilizes their neurite network in vitro. Invest Ophthalmol Vis Sci. 2007;48:1884–1891. doi: 10.1167/iovs.06-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu X, Beeton C. Detection of functional matrix metalloproteinases by zymography. J Vis Exp. 2010;45:e2445. doi: 10.3791/2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danias J, Gerometta R, Ge Y, et al. Gene expression changes in steroid-induced IOP elevation in bovine trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:8636–8645. doi: 10.1167/iovs.11-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Giorgio-Miller A, Bottoms S, Laurent G, Carmeliet P, Herrick S. Fibrin-induced skin fibrosis in mice deficient in tissue plasminogen activator. Am J Pathol. 2005;167:721–732. doi: 10.1016/S0002-9440(10)62046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao Y, Zou T, Ling CC, Hu H, Tao XM, Song HY. Disruption of tissue-type plasminogen activator gene in mice aggravated liver fibrosis. J Gastroenterol Hepatol. 2008;23(7 pt 2):e258–e264. doi: 10.1111/j.1440-1746.2007.05100.x. [DOI] [PubMed] [Google Scholar]
- 30.Tripathi BJ, Geanon JD, Tripathi RC. Distribution of tissue plasminogen activator in human and monkey eyes: an immunohistochemical study. Ophthalmology. 1987;94:1434–1438. doi: 10.1016/s0161-6420(87)33278-6. [DOI] [PubMed] [Google Scholar]
- 31.Bernatchez SF, Tabatabay C, Belin D. Urokinase-type plasminogen activator in human aqueous humor. Invest Ophthalmol Vis Sci. 1992;33:2687–2692. [PubMed] [Google Scholar]
- 32.Saiduzzafar H, Kelly R, Perkins ES. The effect of intracameral and intravenous urokinase on the resistance to aqueous outflow in cynomologus monkeys. Exp Eye Res. 1971;11:178–183. doi: 10.1016/s0014-4835(71)80021-0. [DOI] [PubMed] [Google Scholar]
- 33.Dan J, Belyea D, Gertner G, Leshem I, Lusky M, Miskin R. Plasminogen activator inhibitor-1 in the aqueous humor of patients with and without glaucoma. Arch Ophthalmol. 2005;123:220–224. doi: 10.1001/archopht.123.2.220. [DOI] [PubMed] [Google Scholar]
- 34.Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:32. doi: 10.3389/fneur.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naim A, Pan Q, Baig MS. Matrix metalloproteinases (MMPs) in liver diseases. J Clin Exp Hepatol. 2017;7:367–372. doi: 10.1016/j.jceh.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juncker-Jensen A, Lund LR. Phenotypic overlap between MMP-13 and the plasminogen activation system during wound healing in mice. PLoS One. 2011;6:e16954. doi: 10.1371/journal.pone.0016954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lijnen HR. Role of the fibrinolytic and matrix metalloproteinase systems in arterial neointima formation after vascular injury. Verh K Acad Geneeskd Belg. 2001;63:605–622. [PubMed] [Google Scholar]
- 39.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 40.Hudson LG, Moss NM, Stack MS. EGF-receptor regulation of matrix metalloproteinases in epithelial ovarian carcinoma. Future Oncol. 2009;5:323–338. doi: 10.2217/FON.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark AF, Brotchie D, Read AT, et al. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- 42.Coleman PL, Patel PD, Cwikel BJ, Rafferty UM, Sznycer-Laszuk R, Gelehrter TD. Characterization of the dexamethasone-induced inhibitor of plasminogen activator in HTC hepatoma cells. J Biol Chem. 1986;261:4352–4357. [PubMed] [Google Scholar]
- 43.Gelehrter TD, Barouski-Miller PA, Coleman PL, Cwikel BJ. Hormonal regulation of plasminogen activator in rat hepatoma cells. Mol Cell Biochem. 1983;53-54:11–21. doi: 10.1007/BF00225243. [DOI] [PubMed] [Google Scholar]
- 44.Seftor RE, Stamer WD, Seftor EA, Snyder RW. Dexamethasone decreases tissue plasminogen activator activity in trabecular meshwork organ and cell cultures. J Glaucoma. 1994;3:323–328. [PubMed] [Google Scholar]
- 45.Lee YR, Noh EM, Han JH, et al. Sulforaphane controls TPA-induced MMP-9 expression through the NF-kappaB signaling pathway, but not AP-1, in MCF-7 breast cancer cells. BMB Rep. 2013;46:201–206. doi: 10.5483/BMBRep.2013.46.4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tawara A, Tou N, Kubota T, Harada Y, Yokota K. Immunohistochemical evaluation of the extracellular matrix in trabecular meshwork in steroid-induced glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246:1021–1028. doi: 10.1007/s00417-008-0800-0. [DOI] [PubMed] [Google Scholar]
- 47.Kasetti RB, Phan TN, Millar JC, Zode GS. Expression of mutant myocilin induces abnormal intracellular accumulation of selected extracellular matrix proteins in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2016;57:6058–6069. doi: 10.1167/iovs.16-19610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vassalli JD, Belin D. Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett. 1987;214:187–191. doi: 10.1016/0014-5793(87)80039-x. [DOI] [PubMed] [Google Scholar]
- 49.Avila MY, Seidler RW, Stone RA, Civan MM. Inhibitors of NHE-1 Na+/H+ exchange reduce mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2002;43:1897–1902. [PubMed] [Google Scholar]
- 50.Midamor (Amiloride HCL). US Food and Drug Administration. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/18-200S024_Midamor_Prntlbl.pdf Accessed April 4, 2019.
- 51.Wang H, Zhang Y, Heuckeroth RO. Tissue-type plasminogen activator deficiency exacerbates cholestatic liver injury in mice. Hepatology. 2007;45:1527–1537. doi: 10.1002/hep.21613. [DOI] [PubMed] [Google Scholar]
- 52.Barazzone C, Belin D, Piguet PF, Vassalli JD, Sappino AP. Plasminogen activator inhibitor-1 in acute hyperoxic mouse lung injury. J Clin Invest. 1996;98:2666–2673. doi: 10.1172/JCI119089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitching AR, Holdsworth SR, Ploplis VA, et al. Plasminogen and plasminogen activators protect against renal injury in crescentic glomerulonephritis. J Exp Med. 1997;185:963–968. doi: 10.1084/jem.185.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]