Abstract
Development of resistance to chemotherapy drugs is a significant problem in treating human malignancies in the clinic. Overexpression of ABC transporter proteins, including P-170 glycoprotein (P-gp), and breast cancer resistance protein (BCRP, ABCG2) have been implicated in this multi-drug resistance (MDR). These ABC transporters are ATP-dependent efflux proteins. We have recently shown that nitric oxide (●NO) inhibits the ATPase activities of P-gp, resulting in a significant enhancement of drug accumulation and the reversal of multi-drug resistance in NCI/ADR-RES cells, a P-gp-overexpressing human MDR cell line. In this study, we used [O2-(2,4-dinitrophenyl)-1-[(4-ethoxycarbonyl)-piperazin-1yl]-diazene-1-ium-1–2-diolate] (JS-K), a tumor-specific NO-donor to study the reversal of drug resistance in both P-gp- and BCRP-overexpressing human tumor cells. We report here that while JS-K was extremely effective in reversing adriamycin resistance in the P-gp-overexpressing tumor cells (NCI/ADR-RES); it was significantly resistant to BCRP-overexpressing (MCF-7/MX) tumor cells, suggesting that JS-K may be a substrate for BCRP. Using another NO-donor (DETNO), we show that ●NO directly inhibits the ATP activities of BCRP, inducing significant increases in the accumulations of both Hoechst 33342 dye and topotecan, substrates for BCRP. Furthermore, ●NO treatment significantly reversed topotecan and mitoxantrone resistance to MCF-7/MX tumor cells. Molecular docking studies indicated that while DETNO and JS-K bind to ATP binding site in both ABC proteins, binding score was significantly reduced, compared to the ATP binding. Our results indicate that appropriately designed NO donors may find success in reversing multidrug resistance in the clinic.
Keywords: Nitric Oxide, P-gp protein, Breast Cancer Resistance Protein, Adriamycin, Mitoxantrone, Topotecan, JS-K
INTRODUCTION
Acquired resistance to chemotherapeutic drugs is the leading cause of treatment failure in the clinic today [1–5]. Multiple factors are responsible for the emergence of drug resistance, including individual variations in patients and somatic cell genetic differences in tumors. Mechanisms of drug resistance and multi-drug resistance include enhanced expression of ABC transporters that increases efflux of anticancer drugs, alterations in drug metabolism, mutations of drug targets and the activation of survival or inactivation of downstream death signaling pathways [1, 3, 5–8]. Overexpression of ABC transporters e.g., P-170 glycoprotein (P-gp, MDR1, ABCB1), breast cancer resistant protein (BCRP, ABCG2) and multi-resistance proteins (MRP’s) in tumor cells following drug treatment are commonly observed [2, 3]. ATP-dependent transporter proteins remove various anticancer drugs from the cancer cells, including drugs that are structurally different from one another. While considerable success has been achieved in restoring the activity of anticancer drugs in vitro, currently there are no compounds available that successfully inhibit/block P-gp-mediated resistance in the clinic [9–11].
Nitric Oxide (•NO), formed from L-arginine by nitric oxide synthase (NOS) in vivo, is known to induce killing of various tumor cells and therefore, it has been suggested to play an important role in the management of cancers [12]. •NO is a short-lived free radical molecule which acts as a cellular messenger and plays a key role in many important physiological functions, including vasodilatation, apoptosis, and the innate immune response [13]. Formation of reactive metabolites e.g., NO+, N2O3, and -OONO has been implicated in many of the actions of •NO. These intermediates react with sulfhydryl groups of proteins, leading to the formation of an S-nitrosothiol group, a process known as S-nitrosylation [14]. Nitrosylation of proteins has been shown to modulate the activity and stability of proteins [15]. Many human tumors show high expression of iNOS and produce significant amounts of •NO [16–19]. The effects of •NO is biphasic: at high concentrations it causes DNA damage, apoptosis, and cell death while at low concentrations it induces cell survival and tumor progression [20].
We have shown that •NO-derived species react with VP-16, a topoisomerase (topo) II poison, and the resulting products were found to be significantly less cytotoxic to tumor cells than the parent drug [21]. Our studies also indicate that •NO-derived reactive species cause resistance to topo I and II poisons in human tumor cells, possibly resulting from the nitrosylation of topoisomerase [22, 23]. Nitrosylation of topo II results in decreased activity in vitro, causing a significant decrease in DNA damage, and resistance to various topo II poisons in MCF-7 breast tumor cells [24]. Further studies showed that the decreased topo II activity resulted from the inhibition of the ATPase activity of topo II by•NO or •NO-derived species [25]. The activity of ABC transporter proteins is dependent upon their ATPase activity to efflux anticancer drugs from tumor cells [3]. We recently found that ●NO inhibits P-170 glycoprotein (P-gp) activity by inhibiting its ATPase activity in a P-gp-overexpressing NCI/ADR-RES ovarian tumor cell line, resulting in increased drug accumulations and significant reversal of adriamycin and taxol resistance in NCI/ADR-RES tumor cells [26].
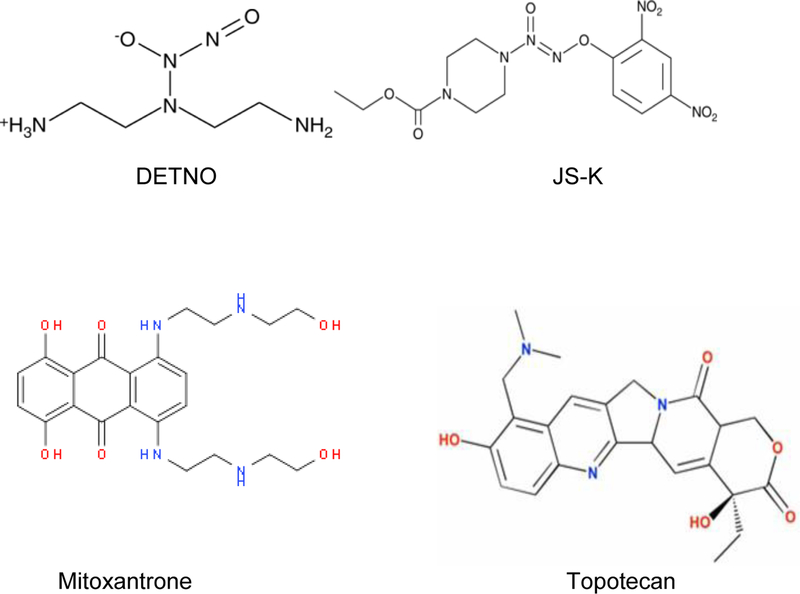
Because nitric oxide donors are toxic and are not specific to tumor cells in vivo, it is important to find very specific nitric oxide donors. In this report, we have further extended our observations in NCI/ADR-RES cells using JS-K (Figure-1), a specific nitric oxide donor which is specifically activated in tumor cells by GSH/Glutathione-S-transferase (GST) systems [27]. In addition, we used another cell line, MCF-7/MX, which overexpresses ABC transporter breast cancer resistance protein (BCRP, ABCG2), to show that •NO inhibits its ATPase activity, resulting in partial reversal of both topotecan (TPT) and mitoxantrone (MX, Figure-1) resistance, known substrates for BCRP [28].
Figure 1:
Structures of DETNO, JS-K, mitoxantrone (MX) and topotecan (TPT).
Materials and Methods
Adriamycin (ADR) was the gift of the Drug Synthesis and Chemistry Branch, Developmental Therapeutic Program of NCI, NIH. ADR was dissolved in double distilled water and stored at −80oC. Topotecan HCl (TPT) and mitoxantrone HCl (MX) were purchased from Cayman Chemicals (Ann Arbor, MI). Stock solutions of TPT and MX were prepared in double distilled water; in some cases, TPT was dissolved in DMSO, and stored at −800C The nitric oxide donors propylamine propylamine nonoate (PPNO), diethylenetriamine nonoate (DETNO) and [O2-(2,4-dinitrophenyl)-1-[(4-ethoxycarbonyl)-piperazin-1yl]-diazene-1-ium-1–2-diolate] (JS-K) were obtained from Cayman Chemicals (Ann Arbor, MI). Stock solutions of PPNO or DETNO were prepared in 0.2 N NaOH and stored at −800C. Stock solutions of JS-K were prepared in DMSO and stored at −80oC. Fresh drug solutions prepared from stock solutions were used in all experiments. The primary antibody for the detection of BCRP was obtained from Abcam (Cambridge, MA). For the inhibition of the ATPase activity of BCRP, Solvo’s BCRP-M PE ATPase Kit was obtained from Sigma-Aldrich (ST. Louis, MO). The Hoechst 33342 dye was purchased from Thermo Fisher Scientific (Waltham, MA) and was dissolved in water.
Cell Culture
Human ovarian OVCAR-8 and NCI/ADR-RES cell lines were obtained from the NCI-Frederick Cancer Center (Frederick, MD), and human MCF-7 breast tumor cells were obtained from ATCC (Manassas, VA). MCF-7/MX breast tumor cells, selected for resistance by exposure to MX as described before [29], was a gift of Dr. Erasmus Schneider. Cells were cultured in Phenol Red-free RPMI media supplemented with 10% fetal bovine serum and antibiotics. Cells were routinely used for 20–25 passages, after which the cells were discarded, and a new cell culture was started from fresh, frozen stock.
Cytotoxicity and Reversal of Resistance Studies
The cytotoxicity studies were carried out with a cell growth inhibition assay as described before [26]. Briefly, 100,000–150,000 cells/well were plated in 2 mL of complete medium onto a 6-well plate (in duplicate) and allowed to attach for 18 h. Cells were treated with various concentrations of MX or TPT for 72 h in complete medium (2 mL). DMSO, when used (0.01–0.1%), was included as the vehicle control. Cells were trypsinized, and the numbers of surviving cells were determined by counting the cells in a cell counter (Beckman, Brea, CA). For the reversal of resistance, cells were first treated with JSK or DETNO for 2 h in medium containing 1% FBS without antibiotics and 200 μL of PBS (pH 7.0) followed by the addition of various concentrations of drugs and were further incubated for 72 h in the complete medium. We used both JS-K and DETNO as our •NO donors for cytotoxicity studies.
Western Blot Assay for BCRP
The Western Blot analyses for BCRP were carried out with standard methods, and samples (5–20 μg of total protein) were electrophoresed under reducing conditions on 3–8% Tris-acetate gels (Novex, Life Technologies, Carlsbad, CA) for 50 minutes at 200 volts. After electrophoresis, proteins were transferred onto nitrocellulose membranes and probed with anti-BCRP and anti-beta actin antibodies. An Odssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE) was used to acquire images.
Confocal microscopy studies for BCRP protein in MCF-7/MX tumor cells
About 1 X 105 parental WT MCF-7 and resistant MCF-7/MX breast tumor cells were plated for 18 h at 37°C in culture plates on glass coverslips in the complete medium. The medium was removed, and cells were washed with ice-cold PBS. To investigate the presence or absence of BCRP protein, the cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature, washed twice for 5 minutes, permeabilized for 5 minutes with 0.5% Triton X-100, and washed twice for 5 minutes. After blocking with 4% fish gelatin in PBS (pH 7.4) for 2 h at room temperature, the cells were incubated with rabbit anti-BCRP antibody (diluted 1:2000) for 2h, followed by secondary anti-mouse Alexa Fluor 668 antisera (diluted 1:1000) for 1h. Coverslips containing cells were washed four times and mounted on glass slides using Prolong Gold anti-fade reagent. For the visualization of BCRP in cells, fluorescence images were captured on a Zeiss LSM710 (Carl Zeiss Inc, Oberkochen, Germany) using a C-Apochromat 40X/1.20 Water Korr M27 objective. The 633 nm laser line from a HeNe laser at 13% power was used for excitation. Subsequently, a 639–698 nm band pass emission filter was used to collect the images with a pinhole set to yield an optical Z-thickness of 2.4 μm.
Inhibition of ATPase Activity
The inhibition of the ATPase activity was carried out using Solvo’s SB-BCRP Predeasy-ATP assay kit (Sigma-Aldrich, MO, catalog # SBPE01) according to the manufacturer’s instructions. PPNO was preincubated with isolated membranes for 30 min before adding ATP to initiate the reaction.
Effects of Nitric Oxide on Accumulation of Hoechst 33342 and TPT
Accumulations of both Hoechst 33342 in MCF-7 and MCF-7/MX cells carried out as described before by Galetti et al. [30] with some modifications. Briefly, about 1 ×105 cells were seeded in six-well cover slips for 18 h in complete medium. After 18 h, the medium was removed and 2 mL of media containing 200 μl of PBS (pH 7.0) was added and cells were preincubated with different concentrations of PPNO for 30 min. Then Hoechst 33342 (5 × 10−7M) was added, and the cells were incubated for 2 h. Cells were washed with ice-cold (x 2) PBS, kept on ice in 1 ml of PBS, and examined using confocal microscopy. The accumulation of TPT was similarly carried out in MCF-7/MX using 2.0 μM TPT and 50 μM PPNO.
Molecular Modeling
ABCB1 (pdb ID: 6C0V) and ABCG2 (pdb ID: 6HBU) structures were considered as receptor configurations for molecular docking. Both protein structures were in their ATP-bound conformations. The ligand binding site of proteins were prepared for docking using the Make_Receptor module (Release 3.2.0.2) with the help of bound ATP as the ligand while docking studies were carried out using the HYBRID module (Release 3.2.0.2) of the OpenEye software package (OpenEye Scientific Software, Inc., Santa Fe, NM, U.S.A.; www.eyesopen.com). All possible DETNO and JS-K conformations were constructed with Omega2 module and the scorings were done using the default ChemGauss4 scoring function of the HYBRID module. The scoring function is based on the shape of the ligand, hydrogen bonding between ligand and receptor, hydrogen bonding interactions with implicit solvent, and metal-chelator interactions.
To study the effect of modification of cysteine by the adduct formation with •NO on the ATP binding, Cys431 of ABCB1 (pdb ID 6C0V) that was found to be in the ATP binding site was converted to NO-bound Cys. Protons were added, followed by the addition of counter ions, and the resulting structure was then solvated in a box of water with 50515 molecules. Prior to equilibration, the system consisted with 169842 atoms was subjected to the following steps: a) over a nanosecond of constrained molecular dynamics (MD) with the protein heavy atoms constrained to the original positions with a force constant of 10 kcal/mol/nm, b) minimization, c) low-temperature, constant-pressure MD simulation to assure a reasonable starting density, d) minimization, e) stepwise heating MD at constant volume, and f) a constant-volume MD run for another 15 nanoseconds with incrementally decreasing the force constants. An equilibrated solution structure was then evaluated from a subsequent unconstrained 400-ns MD trajectory calculated at 300 K under constant volume (with 1 fs time step) using the PMEMD module of the Amber 18 program [31] to accommodate long-range interactions. All standard amino acid parameters were taken from the Amber SB14 force field. Atomic charges on NO-modified Cys were derived using the ChelpG scheme with the 6–31g(d) basis, set at the HF level using the program Gaussian 09.D01[32]. An optimized structure of ABCG2 dimer was constructed (using pdb ID: 6HBU) by modeling the missing loop segments and carrying out the minimization under the Amber SB14 force field with two cysteine residues in the vicinity of the ATP binding site muted to NO-Cys.
Statistical Analysis
All experiments were carried out at least 3 times (n = 3) in duplicate. One-way analysis of variance (ANOVA) was used for statistical analysis. The Tukey Multiple comparison’s test was used for multiple comparisons. The results are expressed as mean ± SEM. The differences were considered statistically significant when p values were less than 0.05.
RESULTS
Cytotoxicity and Reversion of Drug Resistance in NCI/ADR-RES Cells by JS-K
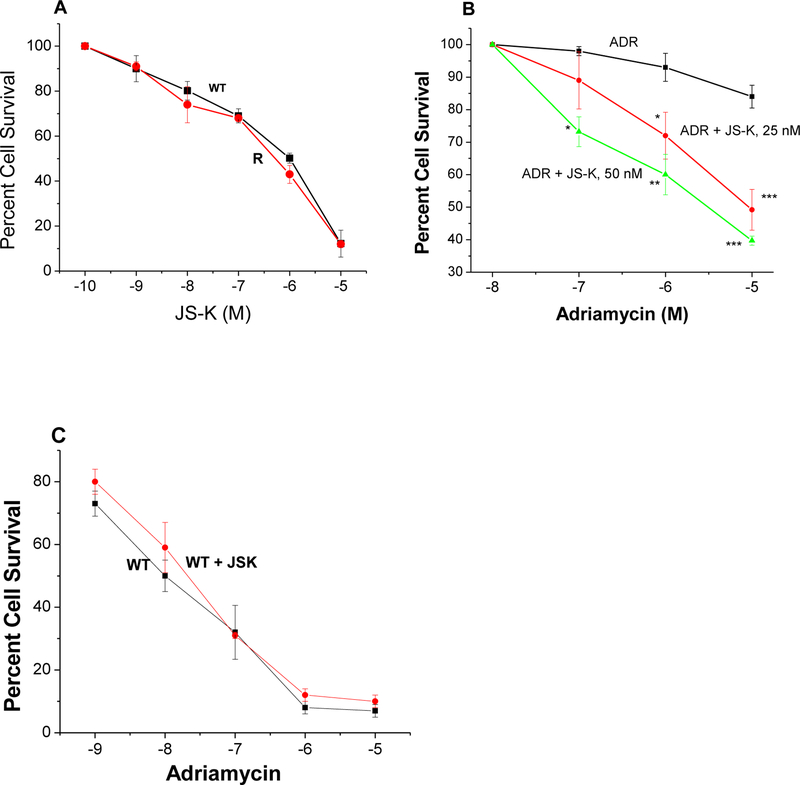
Recently, we have shown that •NO/•NO-derived species inhibit the ATPase activity and cause reversal of ADR resistance in the P-gp overexpressing NCI/ADR-RES tumor cells [26]. JS-K is activated intracellularly by GSH-GST system to generate •NO [27, 33]. In this report we examined the effects of JS-K on the reversal of ADR resistance in NCI/ADR-RES cells since the NCI/ADR-RES cells contain significantly higher amounts of GSH than the parent cell line and overexpresses GST [6]. Despite differences in GSH content and GST expressions, the data presented in Figure-2A show that JS-K is equally cytotoxic to both parental OVCAR-8 and the resistant NCI/ADR-RES tumor cells. JS-K, however, at very low doses e.g., 50nM was effective in reversing ADR resistance in NCI/ADR-RES cells (Figure-2B) without significantly modulating the cytotoxicity of ADR in the parental WT OVACAR-8 cells (Figure-2C).
Figure 2:
Cytotoxicity of JS-K in WT OVCAR-8 (■-■) and in NCI/ADR-RES (R, •-•) tumor cells (Panel A). Effects of JS-K (25 nM, •-•) and (50 nM, Δ-Δ) on ADR cytotoxicity (■-■) in NCI/ADR-RES tumor cells (Panel B), and effects of JS-K on ADR cytotoxicity in WT OVCAR-8 tumor cells (Panel C) following 72 h of drug treatment. Cells were counted as described in the methods section. Values represent three separate experiments carried out in duplicates. ***, ** and * p values ≤ 0.001, 0.005, and ≤ 0.05, respectively, compared with concentration-matched samples.
Western Blot and Confocal Microscopy for BCRP Protein in MCF-7/MX Cells
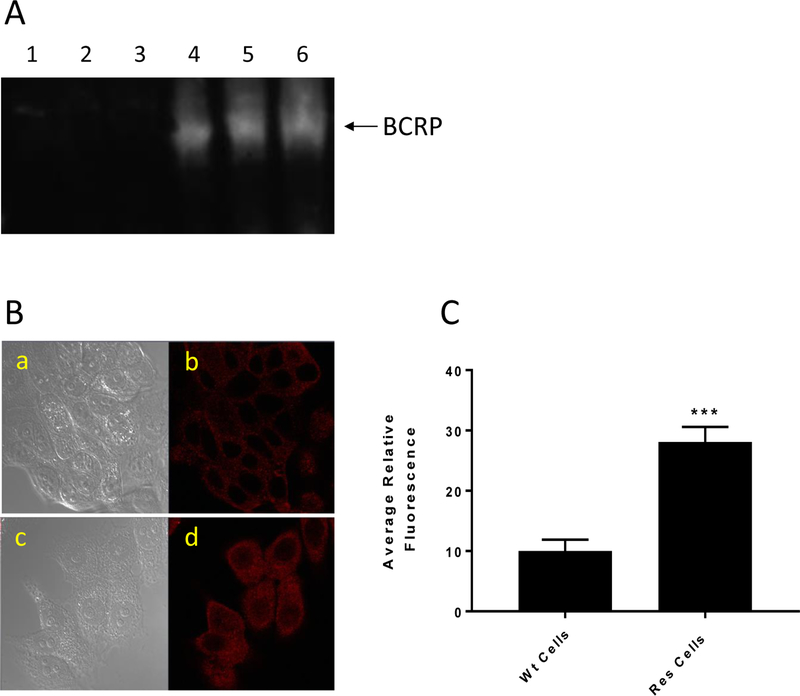
MCF-7/MX cells do not overexpress P-gp protein; however, they do express other ATP-dependent efflux proteins. The Western blot analysis (Figure-3A) shows that MCF-7/MX cells overexpress BCRP protein while this protein was not detected in the parental MCF-7 cells. We further confirmed the presence and the absence of BCRP using confocal microscopy (Figure-3B) and we found that MCF-7/MX cells overexpress BCRP protein while the parental WT MCF-7 cells express significantly less of this protein (Figure-3 B and C).
Figure 3:
The Western blot analysis for BCRP in MCF-7 and MCF-7/MX cells (A). Lanes 1, 2, 3 and 4, 5, 6 represent 5,10 and 20 μg proteins from MCF-7 and MCF-7/MX tumor cells, respectively. (B) Confocal microscopy studies for BCRP in MCF-7 and MCF-7/MX tumor cells. a, WT MCF-7 cells without BCRP antibody; b, in the presence of the antibody; c, MCF-7/MX cells without the antibody; and d, MCF-7/MX cells in the presence of the antibody. (C) quantifications of cellular fluorescence of BCRP.*** p values ≤ 0.001.
Cytotoxicity of JS-K in MCF-7 and MCF-7/MX Breast Cancer Cells
Since JS-K was effective in modulating ADR toxicity in the P-gp-overexpressing tumor cells, we also examined its effect in the BCRP-overexpressing cells. Surprisingly, MCF-7/MX tumor cells were found to be significantly resistant to JS-K (Figure–4), suggesting that JS-K may be a substrate for BCRP.
Figure-4:
Cytotoxicity of JS-K in WT MCF-7 (■-■) and in MCF-7/MX (●-●) breast tumor cells. Values represent three separate experiments carried out in duplicates. ***, p values ≤ 0.001, compared with concentration-matched samples.
Effects of DETNO in MCF-7 and MCF-7/MX Breast Tumor Cells
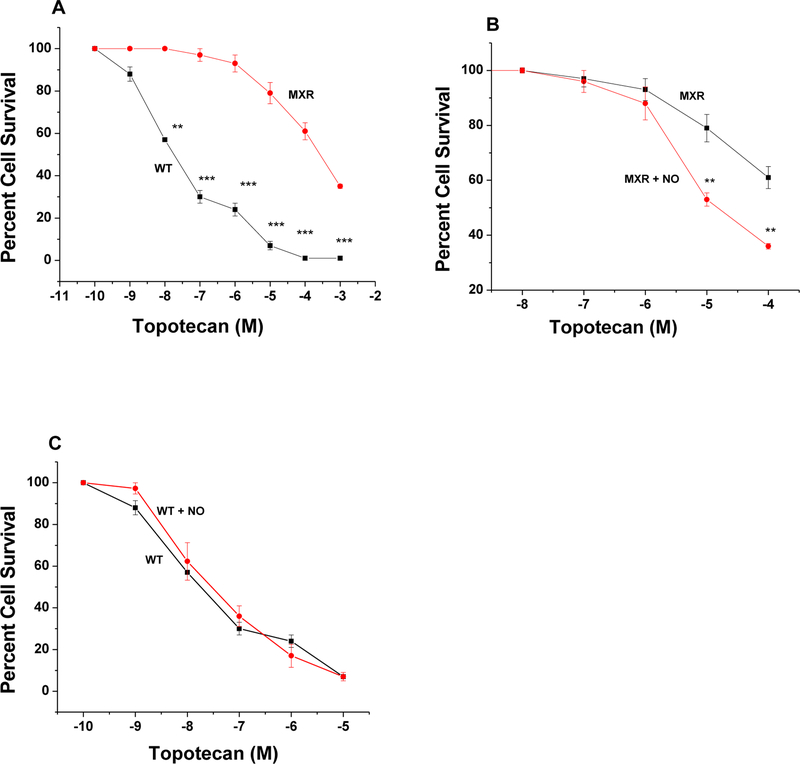
Because MCF-7/MX cells were significantly resistant to JS-K, we used DETNO to examine effects of •NO on the reversal of drug resistance as we previously found that DETNO is effective in reversing ADR and taxol resistance in NCI/ADR-RES cells [26]. As previously described and shown in Figure-5, MCF-7/MX tumor cells were also extremely resistant to TPT, a known substrate of BCRP. While DETNO had no significant effects on TPT cytotoxicity in parental WT MCF-7 tumor cells (Figure-5C) it significantly reversed TPT resistance in MCF-7/MX tumor cells (Figure-5B).
Figure-5:
Cytotoxicity of TPT in WT MCF-7 (■-■) and in MCF-7/MX (●-●) tumor cells (Panel A) and effects of DETNO (50 μM ●-●) on TPT (■-■) cytotoxicity, (Panel B) in MCF-7/MX tumor cells. Effects of DETNO (50 μM, ●-●) on TPT (■-■) cytotoxicity in WT MCF-7 tumor cells (Panel C) following 72 h of drug treatment. DETNO was preincubated for 2h before adding TPT in the complete media containing 100 μL (PBS, pH 7.0)/1mL media. Values represent three separate experiments carried out in duplicates. ***, ** and * p values ≤ 0.001, 0.005, and ≤ 0.05, respectively, compared with concentration-matched samples.
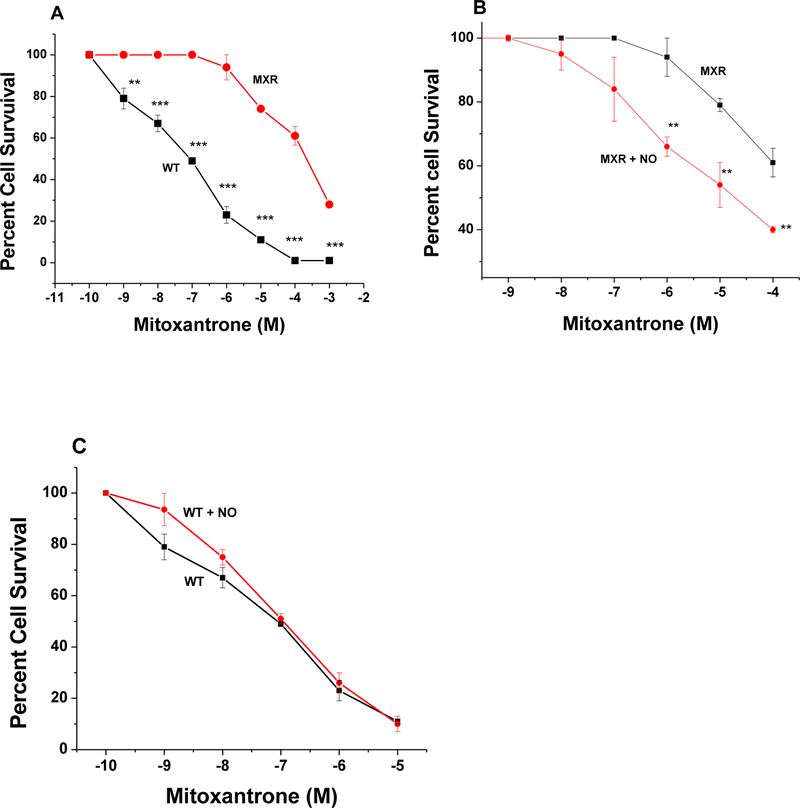
Similarly, data presented in Figure-6 show that MCF-7/MX tumor cells are also highly resistant to MX and that DETNO significantly reversed MX resistance in MCF-7/MX tumor cells without modulating MX cytotoxicity in the parental MCF-7 tumor cells.
Figure-6:
Cytotoxicity of MX in WT MCF-7 (■-■) and in MCF-7/MX (●-●) tumor cells (Panel A) and effects of DETNO (50 μM ●-●) on MX (■-■) cytotoxicity, (Panel B) in MCF-7/MX tumor cells and effects of DETNO (50 μM, ●-●) on MX (■-■) cytotoxicity in WT MCF-7 tumor cells (Panel C) following a 72h drug exposure. Cells were counted as described in the methods section. Values represent three separate experiments carried out in duplicates. ***, ** and * p values ≤ 0.001, 0.005, and ≤ 0.05, respectively, compared with concentration-matched samples.
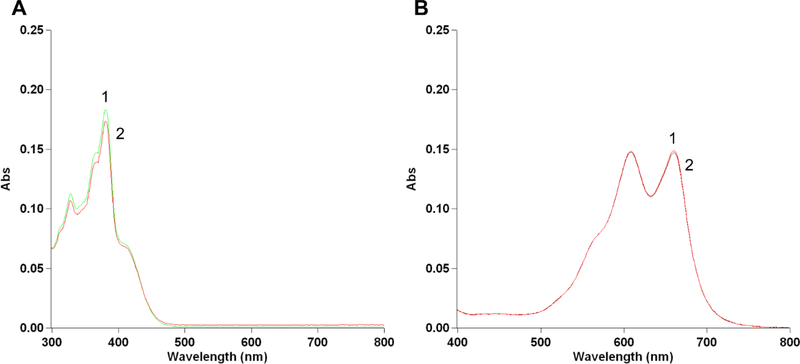
These results taken together suggest that •NO, delivered via DETNO, inhibited BCRP functions, causing the reversal of both TPT and MX. These results are consistent with our previous observations using DETNO in NCI/ADR-RES tumor cells overexpressing P-gp. It is also possible, however, that •NO may have reacted with TPT and MX as these drugs contain phenoxy-OH groups (Figure-1), resulting in altered/modified cytotoxicity [34–36]. •NO has been shown to react with some phenoxy-OH containing compounds and in case of VP-16 the resulting compound(s) were found to be inactive to tumor cells [21]. We examined the reaction of •NO with both TPT and MX and found that there were no significant reactions under our experimental conditions (Figure-7). These observations further suggest that reversal of drug resistance by •NO resulted from the inhibition of BCRP functions.
Figure-7:
Reaction of PPNO (100 μM) with TPT (1μM, Panel A) and MX (1μM, Panel B) in 100 mM phosphate buffered saline (pH 7.4). 1, no treatment; 2, with PPNO and spectra were recorded after 1h in the presence of PPNO.
Effects of •NO on Hoechst 33342 and TPT Accumulations
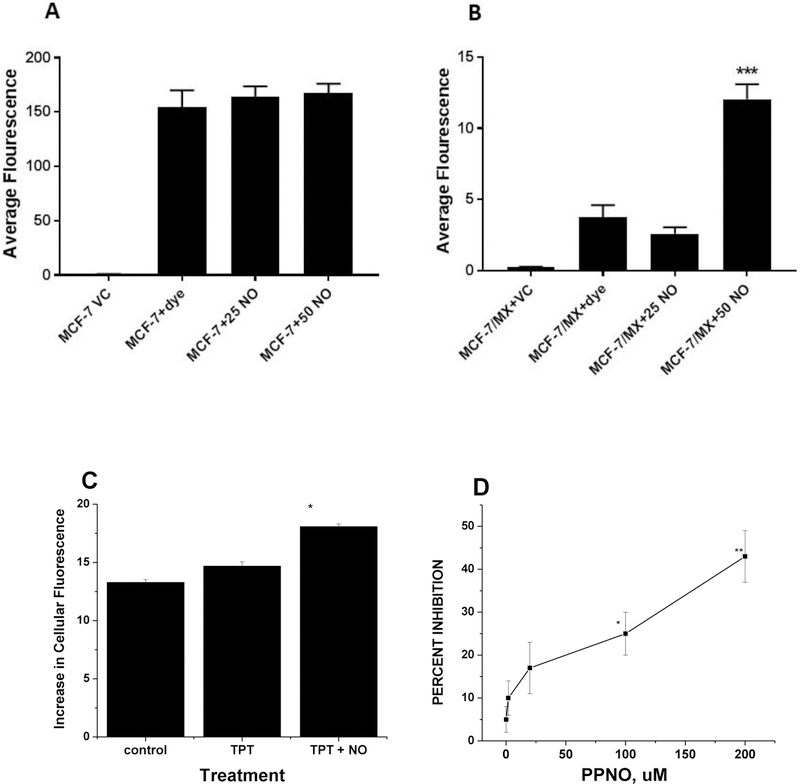
BCRP overexpressing MCF-7/MX cells have been shown to accumulate less MX than the parental MCF-7 cells [29]. Hoechst 33342 dye accumulation has been used to study the functions of BCRP protein [30, 37]. We examined the effects •NO on the accumulation of Hoechst 33342 in MCF-7/MX tumor cells. Our studies confirm that •NO significantly inhibited BCRP-dependent exclusion of the dye from MCF-7/MX tumor cells (Figure 8B) without affecting the dye accumulation in parental MCF-7 tumor cells (Figure-8A). We also examined TPT accumulation in MCF-7/MX cells using TPT autofluorescence (absorption at 405 and emission at 525 nm) as described by Hubbard et al. [38] and the results presented in Figure-8C show a significant enhancement in TPT accumulation in the presence PPNO (50 μM) in MCF-7/MX tumor cells.
Figure 8:
The relative accumulation of Hoechst 33342 dye and effects of PPNO (25 and 50 μM in WT MCF-7 (Panel A) and MCF-7/MX tumor cells (Panel B). *** p values ≤ 0.0001compared with the dye alone. Accumulation of TPT (2.0 μM) in MCF-7/MX cells for 2 h in the presence of 50 μM PPNO (Panel C). * p values ≤ 0.05 compared with TPT alone. Inhibition of the ATPase activity of BCRP in isolated BCRP membranes by PPNO. PPNO and inhibitors were incubated with membranes for 30 min prior to the addition of ATP (Panel D). ** p values ≤ 0.005 compared to controls, no treatment.
Effects of •NO on ATPase Activity of BCRP in Isolated Membranes
Since ●NO inhibited ATPase activity of both topo II and P-gp in tumor cells, we evaluated the possibility that ●NO will also inhibit the ATPase activity of BCRP in MCF-7/MX tumor cells, causing an increase drug accumulation and reversal of TPT and MX resistance. Data presented in Figure-8D show that ●NO and ●NO-related species, delivered by PPNO inhibited the ATPase activity of BCRP. This inhibition was dose-dependent and 20 μM PPNO caused a significant inhibition (20%) and at 200 μM PPNO resulted in about 40–50% inhibition of the ATPase activity in isolated membranes. These observations suggest that increases in drug accumulation and resulting reversal of MX and TPT resistance in MCF-7/MX tumor cells is due to the inhibition of the ATPase activity by ●NO.
Scoring the Binding of NO-Donors at the ATP-binding Site
We estimated the docking scores of DETNO and JS-K at the ATP binding sites of ABCB1 and ABCG2 transporters. ATP was docked at these binding sites to obtain the reference scores. Results summarized in Table 1 show high scores for ATP binding in both ABC proteins. It is clear that the shape along with the hydrogen bonding interactions of ATP contributes largely to the high total score while the ligand desolvation term impacted negatively in the ATP binding. This scoring was reduced by half for DETNO binding in both ABC proteins, compared to the ATP binding. JS-K showed a further reduction in scoring, mainly due to lack of hydrogen bonding interactions. Similar sizes make the “Shape score” of JS-K is comparable to ATP, as opposed to the diminished “Shape score” contributions of DETNO due to its smaller volume. However, even with the weaker interaction strengths both DETNO and JS-K may be located at the ATP binding site with reduced affinities.
Table 1.
Ligand interaction scores of ATP, DETNO, and JS-K bound to ABCB1 and ABCG2. Contributions from shape: H-bond, protein desolvation, and ligand desolvation are added to yield the total binding score.
| receptor | ligand | Total | Shape | H-Bond | Protein Desolvation |
Ligand Desolvation |
|---|---|---|---|---|---|---|
| ABCB1 | ATP | −15.2 | −13.4 | −20.4 | 6.9 | 11.7 |
| DETNO | −7.1 | −5.2 | −7.5 | 2.2 | 3.5 | |
| JS−K | −5.1 | −10.8 | −3.5 | 6.0 | 3.2 | |
| ABCB1 (Cys431−NO) |
ATP | −7.2 | −2.1 | −10.1 | 7.5 | 12.0 |
| ABCG2 | ATP | −13.2 | −15.1 | −19.5 | 7.7 | 13.5 |
| DETNO | −8.0 | −5.1 | −7.9 | 1.2 | 3.7 | |
| JS−K | −5.8 | −10.5 | −3.4 | 5.2 | 2.8 |
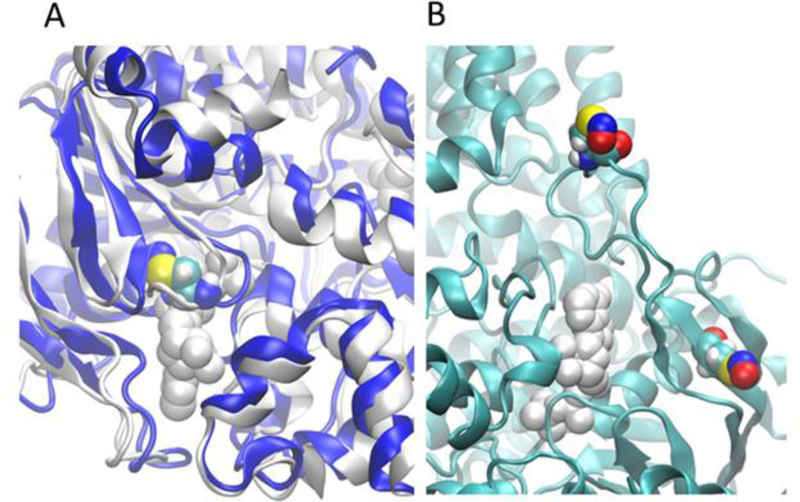
Using the NO-bound Cys431 solution structure of ABCB1 constructed from the molecular dynamic’s simulation, we analyzed the ATP binding scores under the same scheme, and the total binding score of ATP was found to be less than half of the wild type protein. Both “Shape” and hydron bonding interactions were severely compromised in the modified system (−2.1 vs −13.4 for “Shape” and −10.1 vs −20.4 for hydrogen bonding). This is clearly illustrated in Figure-9A in which the final solution structure of modified ABCB1 was superimposed with the ATP-bound cryo-EM structure. As shown in Figiure-9A, NO-Cys431 can be observed in the area otherwise occupied by ATP. Although the ATP binding site does not contain a cysteine in ABCG2, a cysteine can be found in the loop region (that is missing in the Cryo-EM structure) slightly above the entrance to ATP binding cavity and another cysteine is located in a beta-sheet next to the ATP binding site. The optimized model in Figure 9B shows that this cysteine is about 5 Å away from the binding cavity and because of this proximity, it is plausible that there could be adulterations to the ATP binding sites due to •NO binding to cysteines. Lower binding score for both DETNO and JS-K with both ABC transporter proteins indicate that these NO-donors may not bind directly to the ATP binding site but could modify cysteine residues and alter the ATP binding site, thereby reducing the ability of ATP to bind to the site. Since P-gp proteins has a cysteine directly occupying at the ATP binding site, there is a greater tendency for modification of the ATP binding due to the alteration of the binding site by NO-Cys, as shown in Figure-9A. However, this possibility is slightly reduced for BCRP due to a cysteine not present in the ATP binding site, but in the immediate vicinity of the ATP site.
Figure 9:
●NO modified cysteine (NO-Cys) may disrupt binding of ATP. (A)The ATP-bound structure (in white) is superimposed with the final solution structure (in blue) of ABCB1 (NO-Cys431) estimated using 400ns of molecular dynamics simulations. ATP is represented by white spheres and the ●NO modified cysteine (NO-Cys) is by colored spheres. (B) An optimized BCRP structure showing the ●NO modified cysteines (NO-Cys) in the vicinity of the ATP binding site.
DISCUSSION
The emergence of drug- and multi-drug resistance (MDR) to antitumor drugs is the leading cause for the failure in the management of human malignancies in the clinic [3, 5, 39]. Several mechanisms, including overexpression of ABC transporters are implicated in the development of the clinical drug resistance [3, 4, 40]. The two ABC transporter-encoding genes that have been studied extensively in MDR are ABCB1, which encodes P-glycoprotein, and ABCG2 which encodes BCRP. Along with ABCC1 (MRP), they represent the three principal MDR genes that are overexpressed in tumor cells. They are members of the ABC-transporter superfamily, and transport both hydrophobic and hydrophilic compounds. These transporters also play important roles in the normal physiology in the transport of endogenous metabolites and drugs across the placenta and the intestine and are important components of the blood–brain and blood–testis barriers [28, 41]. These transporters are ATP-dependent efflux proteins that function to remove various chemotherapeutic drugs (ADR, TPT, MX, etc.) from tumor cells, and thus decrease their effectiveness. Overexpression of P-gp and BCRP are detected in many human cancers and are correlated with decreased survival [5, 42–44].
Recent findings have shown that both P-gp and BCRP protein are important modulators of drug resistance as they have also been reported to be overexpressed in cancer stem cells (CSC), a subgroup of cancer cells, that survive cancer therapy, and promote cancer relapse due to their ability to establish higher invasiveness and resistance [45–47]. CSC’s can self-renew and differentiate into the heterogeneous lineages of cancer cells in response to chemotherapeutic agents. Thus, development of inhibitors of ABC transporters would be beneficial in the clinic in overcoming drug resistance associated with the overexpression of ABC transporters.
Our studies suggest that development of appropriate NO-donors may offer a promising future direction. In this regard, we have shown that ●NO or ●NO-related species inhibited the ATPase activity of P-gp, resulting in the reversal of ADR and taxol resistance in a P-gp overexpressing human NIH/ADR-RES tumor cells [26]. We believe the most successful course of action in developing cancer therapies with NO-donors is to develop newer ones that are target-specific to resistant tumor cells overexpressing ATP-dependent efflux proteins and release •NO following intracellular activation. Studies have shown that JS-K, is an extremely active anticancer agent against various human cancer cells both in vitro and in vivo [27, 33]. In tumor cells and in vivo, JS-K is activated to generate •NO following its reactions with GSH, catalyzed by GST [27].
In this study, we used JS-K as a tumor specific NO-donor to examine its effectiveness in overcoming drug resistance in P-gp overexpressing NCI/ADR-RES ovarian tumor cell line. We chose this cell line because it contains significantly higher amounts of GSH and overexpresses GST [6]. Our study shows that JS-K was equally cytotoxic to both the parental WT OVCAR-8 and NCI/ADR-RES cells, even though the resistant NCI/ADR-RES tumor cells contained more GSH and overexpressed GST. More importantly, JS-K was extremely effective in reversing ADR resistance in NCI/ADR-RES cells in a dose-dependent manner at very low JS-K concentrations without affecting ADR cytotoxicity in the parental WT OVCAR-8 tumor cells. Encouraged by these results, we examined effects of JS-K in MCF-7/MX tumor cells, a BCRP (ABCG2) overexpressing tumor cell line. However, we found that JS-K was extremely resistant to MCF-7/MX tumor cells, suggesting that JS-K may be a substrate for BCRP. Findlay et al. [33] also have reported that PABA/NO, a compound structurally similar to JS-K, is a substrate for multi-drug resistance protein 1 (MRP1).
Because DETNO is extremely effective in reversing P-gp-dependent drug resistance [26] we used DETNO to evaluate the reversal of drug resistance in BCRP-expressing MCF-7/MX tumor cells. We found that DETNO significantly reversed resistance to both MX and TPT in MCF-7/MX cells, known substrates of BCRP without modulating the cytotoxicity of MX or TPT in the parental WT MCF-7 tumor cells. In addition, we found that ●NO or ●NO-related species did not react with either TPT or MX under our experimental conditions. These observations suggest that ●NO or ●NO-related species was specific for BCRP. This was further shown by PPNO significantly enhancing the accumulation of Hoechst 33342 in MCF-7/MX tumor cells without affecting its accumulation in the parental WT MCF-7 tumor cells. Hoechst 33342 is a substrate of BCRP and has been used to study the role of BCRP in the drug extrusion assay [30, 37]. PPNO also significantly enhanced TPT accumulation in MCF-7/MX cells.
Our studies in isolated membranes containing BCRP confirmed that ●NO and ●NO-related species significantly inhibited the ATPase activity of the protein. These results, taken together, suggest that ●NO or ●NO-related species inhibit BCRP functions by directly inhibiting its ATPase activity, causing a significantly enhanced accumulation of MX and TPT in the BCRP-expressing MCF-7/MX tumor cells, resulting in the reversal of drug resistance. Riganti et al. [48] have shown that ●NO-dependent nitration of tyrosine in P-gp protein induces reversal of doxorubicin resistance in P-gp-expressing cells by enhancing drug accumulation. Nitration of tyrosine residue in BCRP was not studied here and therefore, it cannot be completely excluded at this time. The functional inhibition of the ATPase by ●NO or ●NO-related species would cause a depletion of cellular ATP, resulting in increased rate of oxidative phosphorylation in MDR tumor cells, promoting increased ROS generation and oxidative stress. Karwatsky et al. [49] have reported that increased ROS formation and concomitant loss of GSH are associated with cell death in resistant CHO tumor cells. JS-K is known to deplete cellular GSH and induce arylation of critical proteins, causing tumor cell death [50].
While JS-K was efficient in reversing P-gp-mediated multi-drug resistance in human ovarian NCI/ADR-RES tumor cells, it appeared to be a substrate for BCRP. In contrast, DETNO was not a substrate for BCRP. Because of the selectivity of JS-K in delivering ●NO and ●NO-related species to tumor cells, and its favorable cytotoxic profile in tumor cells, it should be possible to design analogs of JS-K that are not substrates of ABC transporters but still retain selective activation and induce killing of only tumor cells. Alternatively, it should also be possible to deliver JS-K or related compounds using novel nanotechnologies to provide high-dose targeted delivery to resistant tumor cells. Our study reported here strongly suggests that it is possible to utilize appropriately designed NO-donors to cause the reversal of both ABCB1 (P-gp)- and ABCG2 (BCRP)-mediated drug resistance by inhibiting the functional activity of ATPase in the ABC transporter overexpressing human tumor cells. These results are significant regarding the possibility of inducing the reversal in ABCB1 and ABCG2 overexpressing cancer stem cells as recent evidences would suggest that cancer stem cells are responsible for the MDR and cancer relapse [45–47].
It should be noted that while DETNO was active in reversing BCRP-mediated resistance of TPT and MX in MCF-7/MX cell line, the degree of the reversal was significantly small compared to NO-induced reversal of P-gp-mediated resistance as observed in our previous study [26]. Furthermore, we observed that much higher concentrations of ●NO (and ●NO-related species) were required for the 50% inhibition of the ATPase activity of BCRP (200 μM) than for the inhibition of the ATPase activity of P-gp (20 μM), a ten-fold difference. It would appear from our docking and binding studies (Table 1 and Figure-9) that BCRP does not directly contain a modifiable cysteine in the ATP binding site. However, it does contain cysteine residues in the periphery which can come in close proximity to the ATP binding site (5 A•). It is therefore, possible that the modification of this sterically hindered cysteine residue requires higher amounts of ●NO for the inhibition of the ATPase activity as observed in our studies.
Conclusions
Studies reported here show that ●NO and ●NO-related species inhibit ABCB1 and ABCG2 functions by inhibiting their ATPase activity, resulting in the reversal of adriamycin, topotecan and mitoxantrone resistance in human tumor cells. This reversal of the resistance results from ●NO-dependent enhancement of drug accumulation in the ABC transporter-expressing cells. Our studies further suggest that appropriately designed nitric oxide donors may be useful in the clinic for treating human tumors that overexpress P-gp and BRCP proteins (including cancer stem cells) or in other diseases where drug resistance may result from the presence of ATP-dependent efflux proteins.
Acknowledgements:
We thank Dr. Erasmus Schneider (Wadsworth Center, New York State Department of Health, Albany, NY) for providing MCF-7/MX cells. Authors thank Dr. O. Lardinois in helping with UV-Vis spectra. We also thank Maria Kadiiska, and Erik Tokar for their critical evaluation of the manuscript.
Funding: This research was supported by the intramural research program (grant number ZIA E505013922, and Z01 ES043010) of the National Institute of Environmental Health Sciences, NIH. Statements contained herein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the US Government.
ABBREVIATIONS:
- MDR
multi-drug resistance
- P-gp
P-170 glycoprotein
- BCRP
Breast Cancer Resistance Protein
- CSC
Cancer Stem Cells
- topo
topoisomerase
- ADR
Adriamycin
- MX
Mitoxantrone
- TPT
Topotecan
- ●NO
nitric oxide
- JS-K
[O2-(2,4-dinitrophenyl)-1-[(4-ethoxycarbonyl)-piperazin-1yl]-diazene-1-ium-1–2-diolate]
- DETNO
diethylenetriamine nonoate
- PPNO
propylamine propylamine nonoate
Footnotes
Conflict of Interest: The authors declare no actual or potential conflicts of interest.
REFERENCES
- [1].Endicott JA, Ling V, The biochemistry of P-glycoprotein-mediated multidrug resistance, Annu Rev Biochem, 58 (1989) 137–171. [DOI] [PubMed] [Google Scholar]
- [2].Gillet JP, Gottesman MM, Mechanisms of multidrug resistance in cancer, Methods Mol Biol, 596 (2010) 47–76. [DOI] [PubMed] [Google Scholar]
- [3].Gottesman MM, Fojo T, Bates SE, Multidrug resistance in cancer: role of ATP-dependent transporters, Nat Rev Cancer, 2 (2002) 48–58. [DOI] [PubMed] [Google Scholar]
- [4].Gottesman MM, Mechanisms of cancer drug resistance, Annu Rev Med, 53 (2002) 615–627. [DOI] [PubMed] [Google Scholar]
- [5].Gottesman MM, Pastan IH, The Role of Multidrug Resistance Efflux Pumps in Cancer: Revisiting a JNCI Publication Exploring Expression of the MDR1 (P-glycoprotein) Gene, J Natl Cancer Inst, 107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cowan KH, Batist G, Tulpule A, Sinha BK, Myers CE, Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats, Proc Natl Acad Sci U S A, 83 (1986) 9328–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sinha BK, Mimnaugh EG, Free radicals and anticancer drug resistance: oxygen free radicals in the mechanisms of drug cytotoxicity and resistance by certain tumors, Free Radic Biol Med, 8 (1990) 567–581. [DOI] [PubMed] [Google Scholar]
- [8].Beck WT, Danks MK, Wolverton JS, Kim R, Chen M, Drug resistance associated with altered DNA topoisomerase II, Adv Enzyme Regul, 33 (1993) 113–127. [DOI] [PubMed] [Google Scholar]
- [9].Krishna R, Mayer LD, Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs, Eur J Pharm Sci, 11 (2000) 265–283. [DOI] [PubMed] [Google Scholar]
- [10].Thomas H, Coley HM, Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein, Cancer Control, 10 (2003) 159–165. [DOI] [PubMed] [Google Scholar]
- [11].Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F, The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR), Curr Drug Targets, 7 (2006) 893–909. [DOI] [PubMed] [Google Scholar]
- [12].Muscara MN, Wallace JL, Nitric Oxide. V. therapeutic potential of nitric oxide donors and inhibitors, Am J Physiol, 276 (1999) G1313–1316. [DOI] [PubMed] [Google Scholar]
- [13].Murad F, Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone?, Recent Prog Horm Res, 53 (1998) 43–59; discussion 59–60. [PubMed] [Google Scholar]
- [14].Gaston B, Nitric oxide and thiol groups, Biochim Biophys Acta, 1411 (1999) 323–333. [DOI] [PubMed] [Google Scholar]
- [15].Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS, Protein S-nitrosylation: purview and parameters, Nat Rev Mol Cell Biol, 6 (2005) 150–166. [DOI] [PubMed] [Google Scholar]
- [16].Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, Martin DN, Switzer CH, Hudson RS, Wink DA, Lee DH, Stephens RM, Ambs S, Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients, J Clin Invest, 120 (2010) 3843–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Loibl S, Buck A, Strank C, von Minckwitz G, Roller M, Sinn HP, Schini-Kerth V, Solbach C, Strebhardt K, Kaufmann M, The role of early expression of inducible nitric oxide synthase in human breast cancer, Eur J Cancer, 41 (2005) 265–271. [DOI] [PubMed] [Google Scholar]
- [18].Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K, Selective expression of inducible nitric oxide synthase in human prostate carcinoma, Cancer, 82 (1998) 1897–1903. [PubMed] [Google Scholar]
- [19].Cianchi F, Cortesini C, Fantappie O, Messerini L, Schiavone N, Vannacci A, Nistri S, Sardi I, Baroni G, Marzocca C, Perna F, Mazzanti R, Bechi P, Masini E, Inducible nitric oxide synthase expression in human colorectal cancer: correlation with tumor angiogenesis, Am J Pathol, 162 (2003) 793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ridnour LA, Thomas DD, Donzelli S, Espey MG, Roberts DD, Wink DA, Isenberg JS, The biphasic nature of nitric oxide responses in tumor biology, Antioxid Redox Signal, 8 (2006) 1329–1337. [DOI] [PubMed] [Google Scholar]
- [21].Sinha BK, Bhattacharjee S, Chatterjee S, Jiang J, Motten AG, Kumar A, Espey MG, Mason RP, Role of nitric oxide in the chemistry and anticancer activity of etoposide (VP-16,213), Chem Res Toxicol, 26 (2013) 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sharma NK, Kumar A, Kumari A, Tokar EJ, Waalkes MP, Bortner CD, Williams J, Ehrenshaft M, Mason RP, Sinha BK, Nitric Oxide Down-Regulates Topoisomerase I and Induces Camptothecin Resistance in Human Breast MCF-7 Tumor Cells, PLoS One, 10 (2015) e0141897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sinha BK, Kumar A, Bhattacharjee S, Espey MG, Mason RP, Effect of nitric oxide on the anticancer activity of the topoisomerase-active drugs etoposide and adriamycin in human melanoma cells, J Pharmacol Exp Ther, 347 (2013) 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kumar A, Ehrenshaft M, Tokar EJ, Mason RP, Sinha BK, Nitric oxide inhibits topoisomerase II activity and induces resistance to topoisomerase II-poisons in human tumor cells, Biochim Biophys Acta, 1860 (2016) 1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sinha BK, Kumar A, Mason RP, Nitric oxide inhibits ATPase activity and induces resistance to topoisomerase II-poisons in human MCF-7 breast tumor cells, Biochem Biophys Rep, 10 (2017) 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sinha BK, Bortner CD, Mason RP, Cannon RE, Nitric oxide reverses drug resistance by inhibiting ATPase activity of p-glycoprotein in human multi-drug resistant cancer cells, Biochim Biophys Acta Gen Subj, 1862 (2018) 2806–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, Gu Y, Fox SD, Buzard GS, Citro ML, Waterhouse DJ, Davies KM, Ji X, Keefer LK, JS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity, Mol Cancer Ther, 2 (2003) 409–417. [PubMed] [Google Scholar]
- [28].Allen JD, Schinkel AH, Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2), Mol Cancer Ther, 1 (2002) 427–434. [PubMed] [Google Scholar]
- [29].Nakagawa M, Schneider E, Dixon KH, Horton J, Kelley K, Morrow C, Cowan KH, Reduced intracellular drug accumulation in the absence of P-glycoprotein (mdr1) overexpression in mitoxantrone-resistant human MCF-7 breast cancer cells, Cancer Res, 52 (1992) 6175–6181. [PubMed] [Google Scholar]
- [30].Galetti M, Petronini PG, Fumarola C, Cretella D, La Monica S, Bonelli M, Cavazzoni A, Saccani F, Caffarra C, Andreoli R, Mutti A, Tiseo M, Ardizzoni A, Alfieri RR, Effect of ABCG2/BCRP Expression on Efflux and Uptake of Gefitinib in NSCLC Cell Lines, PLoS One, 10 (2015) e0141795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Case DA, Ben-Shalom IY, Brozell SR, Cerutti DS, Cheatham ITE, Cruzeiro VWD, Darden TA, Duke RE, Ghoreishi D, Gilson MK, Gohlke H, Goetz AW, Greene D, Harris R, Homeyer N, Izadi S, Kovalenko A, Kurtzman T, Lee TS, LeGrand S, Li P, Lin C, Liu J, Luchko T, Luo R, Mermelstein DJ, Merz KM, Miao Y, Monard G, Nguyen C, Nguyen H, Omelyan I, Onufriev A, Pan F, Qi R, Roe DR, Roitberg A, Sagui C, Schott-Verdugo S, Shen J, Simmerling CL, Smith J, Salomon-Ferre R, Swails J, Walker RC, Wang J, Wei H, Wolf RM, Wu X, Xiao L, York DM, Kollman PA, AMBER 2018, in, University of California, San Francisco, 2018. [Google Scholar]
- [32].Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ, Gaussian 09.D01, in, Gaussian, Inc, Wallingford, CT, USA, 2009. [Google Scholar]
- [33].Findlay VJ, Townsend DM, Saavedra JE, Buzard GS, Citro ML, Keefer LK, Ji X, Tew KD, Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug, Mol Pharmacol, 65 (2004) 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reszka K, Lown JW, Photosensitization of anticancer agents−−8. One-electron reduction of mitoxantrone: an EPR and spectrophotometric study, Photochem Photobiol, 50 (1989) 297–304. [DOI] [PubMed] [Google Scholar]
- [35].Duthie SJ, Grant MH, The role of reductive and oxidative metabolism in the toxicity of mitoxantrone, adriamycin and menadione in human liver derived Hep G2 hepatoma cells, Br J Cancer, 60 (1989) 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sinha BK, van ‘t Erve TJ, Kumar A, Bortner CD, Motten AG, Mason RP, Synergistic enhancement of topotecan-induced cell death by ascorbic acid in human breast MCF-7 tumor cells, Free Radic Biol Med, 113 (2017) 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Scharenberg CW, Harkey MA, Torok-Storb B, The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors, Blood, 99 (2002) 507–512. [DOI] [PubMed] [Google Scholar]
- [38].Hubbard KE, Schaiquevich P, Bai F, Fraga CH, Miller L, Panetta JC, Stewart CF, Application of a highly specific and sensitive fluorescent HPLC method for topotecan lactone in whole blood, Biomed Chromatogr, 23 (2009) 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ozben T, Mechanisms and strategies to overcome multiple drug resistance in cancer, FEBS Lett, 580 (2006) 2903–2909. [DOI] [PubMed] [Google Scholar]
- [40].Callaghan R, Providing a molecular mechanism for P-glycoprotein; why would I bother?, Biochem Soc Trans, 43 (2015) 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mao Q, Unadkat JD, Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update, AAPS J, 17 (2015) 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Maciejczyk A, Szelachowska J, Ekiert M, Matkowski R, Halon A, Surowiak P, [Analysis of BCRP expression in breast cancer patients], Ginekol Pol, 83 (2012) 681–687. [PubMed] [Google Scholar]
- [43].Burger H, Foekens JA, Look MP, Meijer-van Gelder ME, Klijn JG, Wiemer EA, Stoter G, Nooter K, RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response, Clin Cancer Res, 9 (2003) 827–836. [PubMed] [Google Scholar]
- [44].Shaffer BC, Gillet JP, Patel C, Baer MR, Bates SE, Gottesman MM, Drug resistance: still a daunting challenge to the successful treatment of AML, Drug Resist Updat, 15 (2012) 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dean M, Fojo T, Bates S, Tumour stem cells and drug resistance, Nat Rev Cancer, 5 (2005) 275–284. [DOI] [PubMed] [Google Scholar]
- [46].Moitra K, Lou H, Dean M, Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development, Clin Pharmacol Ther, 89 (2011) 491–502. [DOI] [PubMed] [Google Scholar]
- [47].Prieto-Vila M, Takahashi RU, Usuba W, Kohama I, Ochiya T, Drug Resistance Driven by Cancer Stem Cells and Their Niche, Int J Mol Sci, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Riganti C, Miraglia E, Viarisio D, Costamagna C, Pescarmona G, Ghigo D, Bosia A, Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux, Cancer Res, 65 (2005) 516–525. [PubMed] [Google Scholar]
- [49].Karwatsky J, Lincoln MC, Georges E, A mechanism for P-glycoprotein-mediated apoptosis as revealed by verapamil hypersensitivity, Biochemistry, 42 (2003) 12163–12173. [DOI] [PubMed] [Google Scholar]
- [50].Maciag AE, Chakrapani H, Saavedra JE, Morris NL, Holland RJ, Kosak KM, Shami PJ, Anderson LM, Keefer LK, The nitric oxide prodrug JS-K is effective against non-small-cell lung cancer cells in vitro and in vivo: involvement of reactive oxygen species, J Pharmacol Exp Ther, 336 (2011) 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]