Abstract
BACKGROUND:
The comparative efficacy of cisplatin (CDDP), carboplatin, and cetuximab (CTX) delivered concurrently with radiation for locally advanced oropharyngeal squamous cell carcinoma continues to be evaluated.
METHODS:
The linked Surveillance, Epidemiology, and End Results–Medicare database was used to identify and compare patient and disease profiles, mortality, toxicity, and overall cost for patients with oropharynx cancer undergoing definitive concurrent chemoradiation with CDDP, carboplatin, or CTX between 2006 and 2011. The human papillomavirus status was unknown. The primary outcome was 2-year overall survival (OS).
RESULTS:
Four hundred nine patients receiving concurrent CDDP (n = 167), carboplatin (n = 69), or CTX (n = 173) were included. Those who were older, those who were nonwhite, and those with a Charlson Comorbidity Index ≥ 2 were less likely to receive CDDP. Two-year OS was inferior with CTX (hazard ratio [HR], 1.68; 95% confidence interval [CI], 1.08–2.60; P = .020) and no different with carboplatin (HR, 1.31; 95% CI, 0.732.35; P = .362) in a Cox proportional hazards model (reference CDDP). There was no statistically significant difference between carboplatin and CTX (HR, 1.28; 95% CI, 0.77–2.14; P = .891). Rates of antiemetic use and hospital visits for nausea/emesis/diarrhea or dehydration were statistically higher with CDDP. Pneumonia rates were higher with carboplatin. In the multivariate model, the corrected mean per-patient spending was significantly higher for CTX and carboplatin than CDDP ($61,133 and $65,721 vs $48,709).
CONCLUSIONS:
Patients who received CDDP had improved OS. CDDP was also associated with slightly lower overall costs and higher antiemetic usage and hospital visit rates, although a strong selection bias was observed because those receiving CTX and carboplatin were older and had higher comorbidity scores.
Keywords: carboplatin, cetuximab, cisplatin, concurrent chemoradiotherapy, elderly, oropharynx, Surveillance, Epidemiology, End Results (SEER)–Medicare
INTRODUCTION
In the United States, approximately 60,000 new head and neck squamous cell carcinoma (HNSCC) cases will be diagnosed in 2017, with at least 40% occurring in patients aged 65 years or older.1,2 In particular, rates of oropharyngeal squamous cell carcinoma (OPSCC) appear to be increasing, with much of the increase due to the rise in the prevalence of human papillomavirus (HPV)–related HNSCC.3 Definitive therapy for patients with OPSCC consists of either resection possibly followed by radiation with or without chemotherapy or organ preservation with definitive radiation therapy (RT). The addition of concurrent chemotherapy with cisplatin (CDDP) to RT (chemoradiation [CRT]) is the standard of care for patients with OPSCC opting for an organ-preservation approach on the basis of improved overall survival (OS), but increased potential for short- and long-term morbidity has been observed in multiple prospective randomized trials.4–6
Additional options suggested by the National Comprehensive Cancer Network (NCCN) guidelines for those who are unable to tolerate high-dose CDDP include cetuximab (CTX) and carboplatin.7 Carboplatin has a mechanism of directed cancer cell death similar to that of CDDP because it acts as an alkylating agent by binding to DNA and creating crosslinks.8 Because of the similar mechanism of action, carboplatin is considered a reasonable second-line option for patients who may not tolerate CDDP-based CRT. Another alternative to platinum-based chemotherapy emerged in 2006. Bonner et al9,10 demonstrated that the addition of CTX to RT improved both locoregional control and OS in comparison with RT alone.
Currently, there are only limited and retrospective studies comparing standard-of-care CDDP with CTX or carboplatin. Additional randomized controlled trials are ongoing.11–13 In the interim, secondary population-based data sources such as the linked Surveillance, Epidemiology, and End Results (SEER)–Medicare database provide an excellent opportunity for comparing survival, toxicity, and overall costs of treatment between concurrent CTX, carboplatin, and CDDP in patients with OPSCC undergoing RT.
MATERIALS AND METHODS
Data Source
We used data from the linked SEER-Medicare database.14 The SEER program collects information from population-based cancer registries that currently cover approximately 28% of the US population.15 When linked to Medicare claims, the database provides information on patient demographics, tumor characteristics at diagnosis, treatment, and overall and cancer-specific mortality. Medicare claims include details such as dates of service, Medicare payments, patient deductibles and copayments, diagnoses, procedures, and all-cause mortality. Diagnoses and procedures are reported with International Classification of Diseases, Ninth Revision, Clinical Modification codes, Current Procedural Terminology codes, the Healthcare Common Procedure Coding System, or the National Drug Code number. The database also contains census tract–level socioeconomic measures obtained via the linkage of the patient’s address to census data.
Sample Selection
The study protocol received a priori approval by the University of Colorado Cancer Center institutional review board. The target study population was patients with oropharyngeal tumors whose initial treatment included definitive concurrent CRT with CDDP, carboplatin, or CTX and who did not undergo surgical resection. We selected patients whose first primary tumor was a nonmetastatic squamous cell (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] morphology codes 8050–8089: squamous cell neoplasms) oropharyngeal tumor (ICD-O-3 topography codes C01.9, C05.1, C05.2, C09, and C10 [excluding C10.4]) diagnosed from 2006 (the first year in which CTX was approved for the treatment of head and neck cancer) through 2011 (n = 6312). Because we aimed to ensure that all patients had up to 24 months of follow-up, those diagnosed after 2011 were excluded. We limited the analysis to locally advanced tumors with American Joint Committee on Cancer TNM categories (6th edition). We included cases with a primary tumor size (T) category of T3 or T4 or a regional lymph node (N) category indicating any nodal involvement (N1, N2, or N3). Patients missing the month of diagnosis or with diagnoses identified by autopsy or death certificate were excluded. To capture complete Medicare claims data, patients had to be at least 65 years old at diagnosis and continuously enrolled in fee-for-service Medicare Parts A and B for 12 months before and 12 months after the month of diagnosis (or until death if it occurred within 12 months). In addition, patients with no paid claims during the 12-month observation period were excluded; this left 1552 patients for whom we had complete claims data to examine their prior health status, treatment, and outcomes of interest.
We used Current Procedural Terminology, Healthcare Common Procedure Coding System, International Classification of Diseases, Ninth Revision, and National Drug Code codes reported in the Medicare Provider Analysis and Review, Outpatient, National Claims History Physician/Supplier, and Durable Medical Equipment claims to identify patients undergoing the treatment regimens of interest (Supporting Table 1). We limited the study to patients who received initial therapy consisting of concurrent CRT, and we required the first dates of service for chemotherapy and RT to occur within 6 months of the diagnosis and within 21 days of each other to be considered concurrent. We excluded patients whose claims reported RT lasting more than 6 months (n = 16) because we believed that this indicated breaks in treatment or secondary rounds of RT. We also excluded patients identified with surgical resection in either the Medicare claims or SEER variables. Using Medicare claims, we defined surgical treatment as any head or neck surgical procedure codes billed at any time between diagnosis and 3 months after the conclusion of RT. Using SEER variables, we defined surgical treatment as surgery of the primary tumor site (other than excisional biopsies) reported as part of the first course of treatment. We further limited the sample to patients who received CDDP, carboplatin, or CTX, with no evidence of any additional chemotherapy agents, for a final analytic sample of 409 (Fig. 1).
Figure 1.
Consolidated standards of reporting trials diagram.
Outcomes
An initial analysis was conducted to identify patient characteristics (demographics and comorbidities) associated with the receipt of RT with CDDP, carboplatin, or CTX. Receipt of the agent was defined as having at least 1 claim reporting the specific agent, with no other chemotherapy drugs reported during the observation period.
Once these 3 treatment groups were defined, the primary outcome was 2-year OS, which was measured as the number of months from diagnosis until death due to any cause. We used Medicare-reported dates of death, which extended through December 2013. Surviving patients were censored 2 years after their diagnosis. SEER dates of death included the cause of death, so we used this information in a sensitivity analysis examining cancer-specific survival (CSS). CSS and OS analyses were similar between CDDP, carboplatin, and CTX; therefore, OS was selected as the primary reported endpoint of this study. SEER dates of death were available through 2011. Therefore, patients diagnosed in 2011 were excluded from CSS analyses.
We examined toxicity associated with the 3 treatment options. Using claim procedure and diagnosis codes, we identified toxicity events within the 6 months after the initiation of RT. Events included the following: surgery performed more than 3 months after the last date of radiation, gastrostomy or feeding tube placement, tracheostomy or airway obstruction, weight loss, use of antiemetics, dysphagia, esophageal stricture, acute renal failure, acute hepatic failure, aspiration pneumonia, pneumonia, speech pathology, and any emergency department (ED) visit. We also included hospital or ED visits with symptoms of dehydration, malnutrition, neutropenia/thrombocytopenia, or nausea/vomiting/emesis as toxicity events. The supporting information reports all codes used to identify these events (Supporting Tables 2 and 3).
We estimated the total spending in the 12 months after the month of diagnosis. We defined total spending as the sum of Medicare payments, patient deductibles and copays, and payments made by any other primary payers as reported on the Medicare claims. We included claims from the Medicare Provider Analysis and Review, Outpatient, National Claims History Physician/Supplier, and Durable Medical Equipment files.
Control Variables
In all multivariate analyses, we adjusted for patient sex, age at diagnosis, race, marital status, SEER reporting registry, population density (metropolitan, urban, or rural), median census tract income, primary tumor site, and American Joint Committee on Cancer T and N categories. Using Medicare claims, we also identified and controlled for whether the primary treatment facility was a teaching hospital and whether the patient received intensity-modulated RT versus any other form of RT. To address potential differences in overall health, Medicare claims from the year before the diagnosis were used to calculate Charlson Comorbidity Index values according to the National Cancer Institute’s adaptation of the algorithm described by Klabunde et al.16
Statistical Analysis
Treatment modality
Pearson chi-square tests were used to assess univariate associations between categorical variables and chemotherapy modalities. Multivariate logistic regression models were used to assess the association between patient characteristics and the receipt of concurrent CDDP, carboplatin, or CTX.
Toxicity
We used separate multivariate logistic regression analyses to estimate probabilities of experiencing toxicity events. We report results as predicted marginals, which are calculated by the averaging of the estimated probabilities of toxicity for a standardized set of patient covariates.17 Predicted marginals standardize outcomes to the entire study sample for covariance imbalance17,18 and can be interpreted as percentages for logistic models and as means for linear models. Statistically significant trends were determined with the Wald test at P < .05.
Spending
We used a 2-part model to first estimate the probability of positive spending. Using multivariate linear regression analyses, we then estimated the mean spending per patient for those who had positive spending.
Survival
OS was first examined with the Kaplan-Meier method. A univariate survival analysis was performed with the log-rank test and unadjusted Cox proportional hazards models to estimate hazard ratios (HRs). Cox proportional hazards regression analysis was used to estimate OS, which was evaluated at a significance level of P < .05. The proportional hazards assumption was assessed with a test of Schoenfeld residuals for covariates in all final models, and it returned no significant results.19 Two-year OS was selected as the endpoint to ensure complete follow-up for all patients included in this analysis.
All statistical analyses were performed with SPSS v24.0 (SPSS, Inc, Chicago, Illinois).
RESULTS
Population Characteristics
Table 1 reports the sample characteristics. The median follow-up was 24 months. Of the 409 patients included, 167 (41%) received concurrent CDDP, 173 (42%) received concurrent CTX, and 69 (17%) received concurrent carboplatin. Oropharynx sites included the base of tongue (55%), tonsil (32%), and oropharynx not otherwise specified (13%).
TABLE 1.
Patient and Treatment Characteristics
| Characteristic | All Patients, No. (%) | Cisplatin, No. (%) | Cetuximab, No. (%) | Carboplatin, No. (%) |
|---|---|---|---|---|
| Age | ||||
| 65–69 y | 144 (35) | 85 (51) | 37 (21) | 22 (32) |
| 70–74 y | 120 (29) | 50 (30) | 48 (28) | 22 (32) |
| >74 y | 145 (36) | 32 (19) | 88 (51) | 25 (36) |
| Sex | ||||
| Male | 334 (82) | 143 (86) | 134 (78) | 57 (83) |
| Female | 75 (18) | 24 (14) | 39 (23) | 12 (17) |
| Race | ||||
| White NH | 358 (88) | 155 (93) | 143 (83) | >58 (>84) |
| Nonwhite/other | 51 (12) | 12 (7) | 30 (17) | <11 (<16) |
| Residence | ||||
| Metropolitan | 335 (82) | 135 (81) | 147 (85) | 53 (77) |
| Nonmetropolitan | 74 (18) | 32 (19) | 26 (15) | 16 (23) |
| Region | ||||
| West | 163 (40) | 67 (40) | 82 (47) | 14 (20) |
| East | 74 (18) | 32 (19) | 30 (17) | 12 (17) |
| Midwest | 48 (12) | 21 (13) | 16 (9) | 11 (16) |
| South | 124 (30) | 47 (28) | 45 (26) | 32 (46) |
| Married | ||||
| Yes | 237 (58) | 99 (59) | 93 (54) | 45 (65) |
| No | 172 (42) | 68 (41) | 80 (46) | 24 (35) |
| Charlson-Deyo comorbidity score | ||||
| 0 | 234 (57) | 108 (65) | 88 (51) | 38 (55) |
| 1 | 104 (25) | 46 (28) | 38 (22) | 20 (29) |
| ≥2 | 71 (17) | 13 (8) | 47 (27) | 11 (16) |
| Facility type | ||||
| Nonteaching | 164 (40) | 62 (37) | 70 (40) | 32 (46) |
| Teaching | 245 (60) | 105 (63) | 103 (60) | 37 (54) |
| Median income | ||||
| Other | 307 (75) | 135 (81) | 128 (74) | 44 (64) |
| Lowest | 102 (25) | 32 (19) | 45 (26) | 25 (36) |
| Tumor site | ||||
| Base of tongue | 224 (55) | 97 (58) | 95 (55) | 32 (46) |
| Tonsil | 130 (32) | 52 (31) | 53 (31) | 25 (36) |
| Oropharynx NOS | 55 (13) | 18 (11) | 25 (15) | 12 (17) |
| Tumor stage | ||||
| T0–2 | 196 (48) | 85 (51) | 73 (42) | >34 (>49) |
| T3–4 | 147 (36) | 54 (32) | 69 (40) | 24 (35) |
| Missing | 66 (16) | 28 (17) | 31 (18) | <11 (<16) |
| Nodal stage | ||||
| N0–1 | 151 (37) | 47 (28) | 70 (40) | 34 (49) |
| N2–3 | 258 (63) | 120 (72) | 103 (60) | 35 (51) |
| IMRT | ||||
| No | 38 (9) | 11 (7) | 15 (9) | 12 (17) |
| Yes | 371 (91) | 156 (93) | 158 (91) | 57 (83) |
Abbreviations: IMRT, intensity-modulated radiation therapy; NH, non-Hispanic; NOS, not otherwise specified.
Treatment
In comparison with concurrent CDDP, CTX was more often used in older patients (odds ratio [OR], 6.34; 95% confidence interval [CI], 3.42–11.77; P < .001), nonwhite/other race patients (OR, 3.05; 95% CI, 1.33–7.00; P = .009), and patients with a Charlson Comorbidity Index ≥ 2 (OR, 4.64; 95% CI, 2.16–9.95; P = .001). When CDDP was compared with carboplatin, patients receiving carboplatin were also more likely to be older (OR, 3.88; 95% CI, 1.62–9.31; P = .002) and to live in the Midwest (OR, 3.53; 95% CI, 1.00–12.49; P = .051) or South (OR, 4.07; 95% CI, 1.55–10.72; P = .004; reference West); those with N2–3 disease were less likely to receive carboplatin over CDDP (OR, 0.44; 95% CI, 0.22–0.88; P = .020). There was a trend for higher utilization of CTX (OR, 1.86; 95% CI, 0.93–3.73; P = .079) and carboplatin (OR, 2.34; 95% CI, 0.91–6.06; P = .079) in patients from lower income counties (Supporting Table 4).
Survival Outcomes
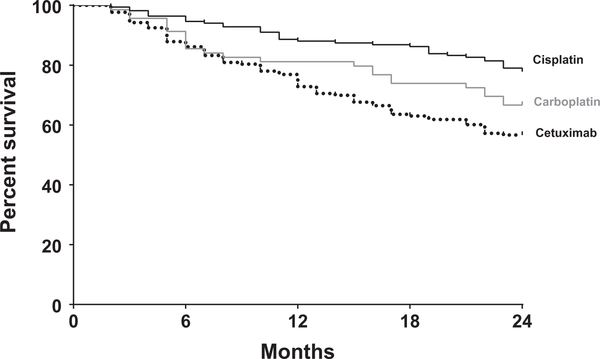
Unadjusted 2-year OS was better with CDDP (77.8%), which was followed by carboplatin (66.7%) and CTX (56.7%; log-rank P < .001; Fig. 2). In addition, unadjusted 2-year CSS was better with CDDP (83.8%), which was followed by carboplatin (72.2%) and CTX (65.7%; log-rank P = .004; Supporting Fig. 1). Further analysis with unadjusted 3-year OS and CSS demonstrated similar results (Supporting Fig. 2). In the Cox proportional hazards model, CTX corresponded to lower OS (HR, 1.68; 95% CI, 1.08–2.60; P = .020) in comparison with CDDP. There was no statistically significant difference in OS between CDDP and carboplatin (HR, 1.31; 95% CI, 0.73–2.35; P = .362). Additional predictors for longer OS for patients with oropharynx cancer were treatment at a teaching facility (HR, 0.68; 95% CI, 0.46–0.99; P = .043) and receipt of intensity-modulated RT (HR, 0.52; 95% CI, 0.30–0.89; P = .018). Variables associated with shorter OS included older age (>74 years; HR, 2.38; 95% CI, 1.50–3.77; P < .001), female sex (HR, 1.61; 95% CI, 1.07–2.42; P = .023), and T3–4 disease (HR, 1.99; 95% CI, 1.34–2.97; P < .001; Table 2). In the Cox proportional hazards model for CSS, CTX trended toward lower OS (HR, 1.66; 95% CI, 0.922.99; P = .094; Supporting Table 5).
Figure 2.
Kaplan-Meier curves of the 2-year overall survival of patients receiving concurrent cisplatin-, carboplatin-, or cetuximab-based chemoradiation. The number of patients at risk is not represented because of the Surveillance, Epidemiology, and End Results–Medicare Data Use Agreement; at least 10 patients per group are present at the time points shown.
TABLE 2.
Multivariate Analysis of Predictors of Overall Survival
| Variable | HR (95% CI) | P |
|---|---|---|
| Chemotherapy | ||
| Cisplatin | 1 | |
| Cetuximab | 1.68 (1.08–2.60) | .020 |
| Carboplatin | 1.31 (0.73–2.35) | .362 |
| Age | ||
| 65–69 y | 1 | |
| 70–74 y | 1.56 (0.94–2.57) | .084 |
| >74 y | 2.38 (1.50–3.77) | <.001 |
| Year (continuous) | 0.96 (0.86–1.07) | .470 |
| Sex | ||
| Male | 1 | |
| Female | 1.61 (1.07–2.42) | .023 |
| Race | ||
| White NH | 1 | |
| Nonwhite/other | 1.03 (0.61–1.75) | .915 |
| Married | ||
| No | 1 | |
| Yes | 1.33 (0.91–1.92) | .137 |
| Residence | ||
| Metropolitan | 1 | |
| Nonmetropolitan | 1.17 (0.69–2.00) | .558 |
| Region | ||
| West | 1 | |
| East | 1.35 (0.82–2.20) | .240 |
| Midwest | 1.20 (0.65–2.22) | .571 |
| South | 0.80 (0.50–1.28) | .351 |
| Charlson-Deyo comorbidity score | ||
| 0 | 1 | |
| 1 | 1.36 (0.91–2.02) | .134 |
| ≥2 | 0.98 (0.60–1.60) | .945 |
| Facility type | ||
| Nonteaching | 1 | |
| Teaching | 0.68 (0.46–0.99) | .043 |
| Median income | ||
| Other | 1 | |
| Lowest | 0.85 (0.53–1.36) | .499 |
| Tumor site | ||
| Base of tongue | 1 | |
| Tonsil | 0.97 (0.64–1.46) | .876 |
| Oropharynx NOS | 0.97 (0.57–1.67) | .923 |
| Tumor stage | ||
| T0–2 | 1 | |
| T3–4 | 1.99 (1.34–2.97) | <.001 |
| Missing | 0.78 (0.43–1.39) | .397 |
| Nodal stage | ||
| N0–1 | 1 | |
| N2–3 | 1.22 (0.84–1.77) | .298 |
| IMRT | ||
| No | 1 | |
| Yes | 0.52 (0.30–0.89) | .018 |
Abbreviations: CI, confidence interval; IMRT, intensity-modulated radiation therapy; HR, hazard ratio; NH, non-Hispanic; NOS, not otherwise specified.
In a subgroup analysis comparing carboplatin with CTX in the Cox proportional hazards model, there was no statistically significant difference between carboplatin and CTX (HR, 1.28; 95% CI, 0.77–2.14; P = .891).
Toxicity
Toxicity outcomes based on 1 year of claims data after the diagnosis were evaluated (Table 3). Compared with patients receiving CDDP, patients receiving concurrent CTX had lower antiemetic use (P < .001) and lower rates of hospital or ED visits for nausea/emesis/dehydration (P = .002) or dehydration (P = .002). Patients receiving concurrent carboplatin appeared to have a lower rate of hospital or ED visits for dehydration (P = .064) but a higher rate of hospital or ED visits for malnutrition (P = .064). Pneumonia rates (not otherwise specified) were also higher in the carboplatin arm (P = .012). In a multivariate analysis, patients receiving CTX (OR, 0.43; 95% CI, 0.25–0.73; P = .002) or carboplatin (OR, 0.52; 95% CI, 0.26–1.03; P = .061) had lower rates of hospital visits due to dehydration (reference CDDP). Patients receiving CTX had a lower rate of antiemetic use than patients receiving CDDP (OR, 0.05; 95% CI, 0.02–0.10; P < .001); hospital visits for nausea/vomiting were also less frequent with CTX versus CDDP (OR, 0.46; 95% CI, 0.25–0.84; P = .012). In a multivariate analysis, there was a trend for higher rates of pneumonia in the carboplatin group (OR, 1.99; 95% CI, 0.89–4.42; P = .093). Acute renal failure rates (not reported because of the lower number of events) were not significant between groups (P = .274 for CTX vs CDDP; P = .925 for carboplatin vs CDDP).
TABLE 3.
Cisplatin and Cetuximab Toxicity Outcomes: Cases With 1 Year of Prior Claims Data
| Characteristic | Overall | CDDP | CTX | Carboplatin | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | CTX vs CDDP | Carboplatin vs CDDP |
|
| Weight loss | 182 | 44.50 | 70 | 41.92 | 79 | 45.66 | 33 | 47.83 | .486 | .405 |
| Antiemetics | 289 | 70.66 | 154 | 92.22 | 74 | 42.77 | 61 | 88.41 | <.001 | .350 |
| ED visits | 161 | 39.36 | 68 | 40.72 | 66 | 38.15 | 27 | 39.13 | .628 | .821 |
| Hospital/ED visits with nausea/emesis/diarrhea | 108 | 26.41 | 56 | 33.53 | 32 | 18.50 | 20 | 28.99 | .002 | .497 |
| Hospital/ED visits with dehydration | 147 | 35.94 | 75 | 44.91 | 50 | 28.90 | 22 | 31.88 | .002 | .064 |
| Hospital/ED visits with malnutrition | 131 | 32.03 | 45 | 26.95 | 59 | 34.10 | 27 | 39.13 | .152 | .064 |
| Gastrostomy or feeding tube | 255 | 62.35 | 107 | 64.07 | 104 | 60.12 | 44 | 63.77 | .452 | .965 |
| Dysphagia | 283 | 69.19 | 117 | 70.06 | 117 | 67.63 | 49 | 71.01 | .629 | .884 |
| Aspiration pneumonia | 47 | 11.49 | 14 | 8.38 | 22 | 12.72 | 11 | 15.94 | .194 | .086 |
| Pneumonia NOS | 79 | 19.32 | 23 | 13.77 | 37 | 21.39 | 19 | 27.54 | .066 | .012 |
Abbreviations: CDDP, cisplatin; CTX, cetuximab; ED, emergency department; NOS, not otherwise specified.
Acute renal failure rates are not reported because of the low number of events, but they were not significant between groups (P =.274 for CTX vs CDDP; P =.925 for carboplatin vs CDDP).
Cost
The mean total Medicare spending during the first 12 months after the diagnosis for patients receiving concurrent CDDP, carboplatin, and CTX was $52,133, $67,560, and $62,683, respectively. In the multivariate model, the corrected mean total per-patient spending was significantly higher for CTX (P = .007) and carboplatin (P = .005) than CDDP ($61,133 and $65,721 vs $48,709).
DISCUSSION
Our study is one of the most comprehensive population-based studies directly evaluating treatment, toxicity, spending, and survival outcomes with concurrent CDDP, carboplatin, and CTX for locally advanced oropharynx cancer. Our findings demonstrate that patients receiving CDDP had longer OS than those receiving CTX or carboplatin-based CRT, although there appears to be a clear selection bias because those receiving CTX or carboplatin were older, had more comorbidities, and were of nonwhite race. Overall, the data presented in this study support the recommendations for CDDP-based CRT in elderly patients if it is tolerated, with carboplatin and CTX used as second-line options. Although toxicity rates were also similar between the treatment arms, CDDP was associated with higher antiemetic use and more frequent hospital/ED visits due to dehydration. Carboplatin use was associated with a slightly higher rate of pneumonia. Lastly, the total mean Medicare cost per patient was higher for those receiving CTX, which was followed by carboplatin and CDDP.
Current guidelines from the NCCN suggest that CDDP is the preferred treatment for patients with OPSCC, with CTX or carboplatin reserved for those who medically cannot receive CDDP.20 Other than the published phase 2 randomized trial,21 the data comparing the effectiveness of CDDP and CTX given concurrently with RT are limited to retrospective, nonrandomized studies.22–25 These studies have limited generalizability because they included diverse study populations that aggregated patients from different head and neck cancer subsites in both definitive and adjuvant settings and reported a variety of endpoints from equivalent outcomes22,26–29 rather than the superiority of CDDP to CTX.30–32
Randomized evidence comparing CDDP and RT with CTX and RT consists of a phase 2 trial from Italy.21 The study closed early because of poor accrual: it enrolled 70 of its intended 130 participants. Its findings demonstrated higher rates of hematologic, renal, and gastrointestinal toxicities with CDDP and increased rates of cutaneous toxicity and nutritional demands in patients receiving CTX; serious adverse events, including death, were more frequent with CTX. Disease control and OS were equivalent in the 2 arms, although separations on Kaplan-Meier curves were observed for local control, OS, and CSS, with each favoring the CDDP arm; however, the study was underpowered to make conclusions regarding survival differences. Overall, our findings are consistent with published prospective and retrospective studies that have demonstrated comparable-to-worse outcomes with CTX-based CRT.
Data comparing CDDP with carboplatin are more limited. A recent meta-analysis including 12 studies, 3 of which were randomized, compared the outcomes of concurrent CDDP and carboplatin for locally advanced HNSCC.33 Overall, CDDP was associated with improved 5-year OS, with no difference observed in locoregional control rates. With respect to toxicity, CDDP was associated with lower rates of hematologic side effects, whereas carboplatin use correlated with decreased gastrointestinal toxicities (grade 3 or higher nausea/vomiting); this correlated with findings in our study as well. Of the 3 randomized trials included, the most recently published evaluated patients with nasopharynx cancer and found no difference in 3-year OS rates.34 The 2 earlier published randomized trials comparing carboplatin and CDDP for HNSCC excluded cancers of the nasopharynx and demonstrated better OS and locoregional control with CDDP.35,36 Homma et al36 compared concurrent CRT with CDDP and concurrent CRT with carboplatin and reported 3-year OS rates of 80.2% and 68.5%, respectively. Notably, De Andres et al35 compared CDDP and carboplatin in the neoadjuvant setting, with complete responders to induction chemotherapy then proceeding to RT. Five-year OS rates were 49% and 25%, with CDDP favored. The results from these 2 prior randomized trials appear to support the results demonstrated in our study because we have demonstrated a relative difference in OS between carboplatin and CDDP, although this only trended in statistical significance. Additional analyses comparing carboplatin with CDDP for solid tumors, including HNSCC, have concluded that CDDP overall appears to be superior to carboplatin.8,37
To our knowledge, this is the largest analysis comparing, in a subgroup analysis, the outcomes with CTX and carboplatin. An important question for patients who cannot tolerate concurrent CDDP is what the second-line concurrent chemotherapy agent ought to be. Likely because of low patient numbers, our study is not equipped to definitively answer this question. Therefore, on the basis of the limited data at this time, the selection of carboplatin versus CTX for patients who cannot tolerate CDDP continues to be an important question, and future studies are needed to determine the optimal concurrent CRT agent for patients who are CDDP-ineligible.
Our study has limitations. The study group is limited to the Medicare fee-for-service population and those 65 years old or older; therefore, results may not be applicable for the younger head and neck cancer population. The data set also does not contain prognostic factors such as HPV/p16 positivity and smoking status. A lack of HPV/p16 positivity data in this study limits the interpretation of the results because of the prognostic importance of HPV in oropharynx cancer.38 HPV-positive oropharynx cancers continue to be on the rise, and there are numerous prospective studies evaluating treatment de-escalation because of the exceptional response rates and improved survival outcomes seen in comparison with their HPV-negative counterparts.39,40 Data from these studies as they mature will guide practice and provide more personalized medicine based on the HPV status. However, at this time, the current standard of care remains concurrent CDDP for those who can tolerate it.
Further limitations include the potential for a selection bias that occurs when treatment selection is based on unobserved patient characteristics. In our study, CDDP was given more often to younger and healthier patients. We attempted to account for potential confounding factors between those receiving CDDP, those receiving carboplatin, and those receiving CTX in the Cox proportional hazards regression model. The Charlson Comorbidity Index does not define the severity of each comorbidity. The length of radiation treatments and radiation treatment breaks also cannot be easily examined in SEER-Medicare because it is confounded by the survival time. Delays in the completion of RT for HNSCC play a critical role in local control and survival outcomes.41 Furthermore, the number of chemotherapy cycles (weekly vs CDDP every 3 weeks) could not accurately be determined. Treatment noncompliance may also play an important role in the comparatively older population included in this SEER-Medicare analysis. Finally, outcome measurements are limited to survival because SEER-Medicare does not record data on locoregional control or distant disease.
Findings from this SEER-Medicare analysis demonstrate that those receiving CDDP-based therapy had longer OS than those receiving CTX- or carboplatin-based therapy. This analysis also highlights differences in patterns of receipt of CTX, carboplatin, and CDDP. Our data overall support the current NCCN guidelines favoring CDDP for those who can tolerate it. CTX and carboplatin continue to play a major role in head and neck cancer and offer patients who cannot tolerate CDDP a good option with better OS in comparison with RT alone.10
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Dr. Sana Karam is supported by the Paul Calabresi Career Development Award for Clinical Oncology (K12, CA086913), Radiological Society of North America (RSNA) Grant (#RSD1713), Golfer’s Against Cancer, Marsico Fund, and Cancer League of Colorado.
Footnotes
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 3.Zumsteg ZS, Cook-Wiens G, Yoshida E, et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol. 2016;2:1617–1623. [DOI] [PubMed] [Google Scholar]
- 4.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. [DOI] [PubMed] [Google Scholar]
- 5.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798–1804. [DOI] [PubMed] [Google Scholar]
- 6.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-analysis of chemotherapy on head and neck cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Head and Neck Cancers, Version 2.2017. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed October 9, 2017. [DOI] [PubMed]
- 8.Lokich J, Anderson N. Carboplatin versus cisplatin in solid tumors: an analysis of the literature. Ann Oncol. 1998;9:13–21. [DOI] [PubMed] [Google Scholar]
- 9.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. [DOI] [PubMed] [Google Scholar]
- 10.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Research UK. De-ESCALaTE HPV: determination of epidermal growth factor receptor-inhibitor (cetuximab) versus standard chemotherapy (cisplatin) early and late toxicity events in human papillomavirus–positive oropharyngeal squamous cell carcinoma https://clinicaltrials.gov/ct2/show/NCT01874171. Accessed October 9, 2017.
- 12.Radiation Therapy Oncology Group. Radiation therapy with cisplatin or cetuximab in treating patients with oropharyngeal cancer. https://clinicaltrials.gov/ct2/show/NCT01302834.
- 13.Trans-Tasman Radiation Oncology Group. Weekly cetuximab/RT versus weekly cisplatin/RT in HPV-associated oropharyngeal squamous cell carcinoma (HPVOropharynx). https://clinicaltrials.gov/ct2/show/NCT01855451. Accessed October 9, 2017.
- 14.Warren JL, Klabunde CN, Schrag D et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. [DOI] [PubMed] [Google Scholar]
- 15.Mouraviev V, Villers A, Bostwick DG, et al. Understanding the pathological features of focality, grade and tumour volume of early-stage prostate cancer as a foundation for parenchyma-sparing prostate cancer therapies: active surveillance and focal targeted therapy. BJU Int. 2011;108:1074–1085. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 17.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. [DOI] [PubMed] [Google Scholar]
- 18.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleinbaum DG, Klein M. Evaluating the proportional hazards assumption In: Kleinbaum DG, Klein M, eds. Survival Analysis. New York, NY: Springer; 2012:161–200. [Google Scholar]
- 20.Ozyigit G, Cengiz M, Yazici G, et al. A retrospective comparison of robotic stereotactic body radiotherapy and three-dimensional conformal radiotherapy for the reirradiation of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e263–e268. [DOI] [PubMed] [Google Scholar]
- 21.Magrini SM, Buglione M, Corvo R, et al. Cetuximab and radiotherapy versus cisplatin and radiotherapy for locally advanced head and neck cancer: a randomized phase II trial. J Clin Oncol. 2016;34:427–435. [DOI] [PubMed] [Google Scholar]
- 22.Caudell JJ, Sawrie SM, Spencer SA, et al. Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys. 2008;71:676–681. [DOI] [PubMed] [Google Scholar]
- 23.Koutcher L, Sherman E, Fury M, et al. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced headand-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:915–922. [DOI] [PubMed] [Google Scholar]
- 24.Hu MH, Wang LW, Lu HJ, et al. Cisplatin-based chemotherapy versus cetuximab in concurrent chemoradiotherapy for locally advanced head and neck cancer treatment. Biomed Res Int. 2014;2014:904341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy A, Blanchard P, Bellefqih S, et al. Concurrent use of cisplatin or cetuximab with definitive radiotherapy for locally advanced head and neck squamous cell carcinomas. Strahlenther Onkol. 2014;190:823–831. [DOI] [PubMed] [Google Scholar]
- 26.Strom TJ, Trotti AM, Kish J, et al. Comparison of every 3 week cisplatin or weekly cetuximab with concurrent radiotherapy for locally advanced head and neck cancer. Oral Oncol. 2015;51:704–708. [DOI] [PubMed] [Google Scholar]
- 27.Smith ML, Arain AN, Herman TS, et al. Cisplatin versus cetuximab combined with radiation therapy for definitive management of locally advanced squamous cell carcinoma of the head and neck: a matched cohort retrospective analysis. Int J Radiat Oncol Biol Phys. 2016;93:E344. [Google Scholar]
- 28.Nien HH, Sturgis EM, Kies MS, et al. Comparison of systemic therapies used concurrently with radiation for the treatment of human papillomavirus–associated oropharyngeal cancer. Head Neck. 2016;38(suppl 1):E1554–E1561. [DOI] [PubMed] [Google Scholar]
- 29.Leeman JE, Li JG, Pei X, et al. Patterns of treatment failure and postrecurrence outcomes among patients with locally advanced head and neck squamous cell carcinoma after chemoradiotherapy using modern radiation techniques. JAMA Oncol. 2017;3:1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley J, Mehan P, Wildes TM, et al. Cisplatin versus cetuximab given concurrently with definitive radiation therapy for locally advanced head and neck squamous cell carcinoma. Oncology. 2013;85:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye AY, Hay JH, Laskin JJ, et al. Toxicity and outcomes in combined modality treatment of head and neck squamous cell carcinoma: cisplatin versus cetuximab. J Cancer Res Ther. 2013;9:607–612. [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Baschnagel AM, Chen P, et al. A matched-pair comparison of intensity-modulated radiation therapy with cetuximab versus intensity-modulated radiation therapy with platinum-based chemotherapy for locally advanced head neck cancer. Int J Clin Oncol. 2014;19:240–246. [DOI] [PubMed] [Google Scholar]
- 33.Guan J, Li Q, Zhang Y, et al. A meta-analysis comparing cisplatin-based to carboplatin-based chemotherapy in moderate to advanced squamous cell carcinoma of head and neck (SCCHN). Oncotarget. 2016;7:7110–7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer. 2007;43:1399–1406. [DOI] [PubMed] [Google Scholar]
- 35.De Andres L, Brunet J, Lopez-Pousa A, et al. Randomized trial of neoadjuvant cisplatin and fluorouracil versus carboplatin and fluorouracil in patients with stage IV-M0 head and neck cancer. J Clin Oncol. 1995;13:1493–1500. [DOI] [PubMed] [Google Scholar]
- 36.Homma A, Shirato H, Furuta Y, et al. Randomized phase II trial of concomitant chemoradiotherapy using weekly carboplatin or daily low-dose cisplatin for squamous cell carcinoma of the head and neck. Cancer J. 2004;10:326–332. [DOI] [PubMed] [Google Scholar]
- 37.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–422. [DOI] [PubMed] [Google Scholar]
- 38.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.RTOG Foundation. Radiation Therapy Oncology Group 1016. Phase III trial of radiotherapy plus cetuximab versus chemoradiotherapy in HPV-associated oropharynx cancer. https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1016. Accessed December 2015. [Google Scholar]
- 40.NRG Oncology. NRG-HN002. A randomized phase II trial for patients with p16 positive, non–smoking associated, locoregionally advanced oropharyngeal cancer, NCT02254278. https://www.nrgoncology.org/Clinical-Trials/NRG-HN002. Accessed December 2015. [Google Scholar]
- 41.Ohri N, Rapkin BD, Guha C, et al. Radiation therapy noncompliance and clinical outcomes in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2016;95:563–570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.