Abstract
Background.
Severe stress in social situations is a core symptom of social anxiety disorder (SAD). Connectivity between the amygdala and cortical regions is thought to be important for emotion regulation, a function that is compromised in SAD. However, it has never been tested if and how this connectivity pattern changes under conditions of stress-inducing social evaluative threat. Here we investigate changes in cortical-amygdala coupling in SAD during the anticipation of giving a public speech.
Method.
Twenty individuals with SAD and age-, gender- and education-matched controls (n = 20) participated in this study. During the functional magnetic resonance imaging (fMRI) session, participants underwent three ‘resting-state’ fMRI scans: one before, one during, and one after the anticipation of giving a public speech. Functional connectivity between cortical emotion regulation regions and the amygdala was investigated.
Results.
Compared to controls, SAD participants showed reduced functional integration between cortical emotion regulation regions and the amygdala during the public speech anticipation. Moreover, in SAD participants cortical-amygdala connectivity changes correlated with social anxiety symptom severity.
Conclusions.
The distinctive pattern of cortical-amygdala connectivity suggests less effective cortical-subcortical communication during social stress-provoking situations in SAD.
Keywords: Amygdala, cortex, emotion regulation, fMRI, social anxiety, speech anticipation
Introduction
Social anxiety disorder (SAD) is characterized by persistent fear of social interactions (DSM-IV; American Psychiatric Association, 1994). Dysfunctional emotion regulation may be at the heart of its etiology and might involve ineffective cortical-subcortical coupling (Goldin et al. 2009b). However, such coupling has not been investigated in relation to social evaluative threat, a key component in social stress, which is difficult to study naturalistically in a functional magnetic resonance imaging (fMRI) context on top of that. Insight into the cortical-subcortical mechanisms is critical to advance knowledge on the neurocognitive background of SAD. Here, we test whether and how cortical-subcortical (amygdala) connectivity in SAD alters during the anticipation of speaking in public. In addition, we test whether this pattern of connectivity relates to social anxiety symptoms.
The amygdala is extensively connected to both cortical and subcortical regions, e.g. hypothalamus and brainstem nuclei, such as the periaqueductal gray and locus coeruleus (Arnsten, 2009; Freese & Amaral, 2009; Ulrich-Lai & Herman, 2009). The subcortical connections are particularly important for both the autonomic nervous system (ANS) and the hypothalamic–pituitary–adrenal (HPA) axis reactions to stressors, as research on rodents has repeatedly shown (Arnsten, 2009; Joëls & Baram, 2009; Rodrigues et al. 2009; Ulrich-Lai & Herman, 2009). As such, the amygdala may play a coordinating role in the stress response (Arnsten, 2009; Joëls & Baram, 2009; Ulrich-Lai & Herman, 2009; Shackman et al. 2013). The cortical-amygdala connections, on the other hand, appear to be important for regulatory processes aimed at altering (initial) stress or emotional responses (Arnsten, 2009; Feder et al. 2009; Buhle et al. 2014). Previous PET and fMRI studies in SAD have demonstrated increased amygdala activity during speech anticipation (Tillfors et al. 2002; Lorberbaum et al. 2004; Etkin & Wager, 2007). However, cortical-amygdala connectivity in SAD during speech anticipation has not been addressed, and, more generally, the role of amygdala activity in prolonged stress states is unclear (Pruessner et al. 2008; Wager et al. 2009b; Choi et al. 2012). It is possible that social stress alters the connectivity (van Marle et al. 2010; Veer et al. 2011) rather than the activity pattern of the amygdala.
Recent fMRI meta-analyses identified a broad set of cortical regions involved in cognitive emotion regulation, including medial and lateral prefrontal and parietal regions (Diekhof et al. 2011; Buhle et al. 2014). A limited capacity to adequately regulate emotion responses is thought to underlie several anxiety disorders (Amstadter, 2008). Some studies have started to investigate SAD in paradigms with an explicit instruction to the participants to regulate their emotional responses (Goldin et al. 2009a, b). However, these emotion regulation processes are clearly also important when situational demands are high, without following explicit emotion regulation instructions (Gross, 2010). In SAD, reduced regulatory processes could be particularly pronounced during public speech anticipation and may relate to an increased stress or anxiety response (Moscovitch et al. 2013).
Here we investigate cortical connectivity with the amygdala in social anxiety during a realistic and common stressor by applying task-free (resting state; RS) fMRI scans before, during, and after the anticipation of giving a public speech. This procedure is based on earlier work in healthy participants that showed that cortical and subcortical regions mediated the relationship between speech anticipation and both physiological responses and self-reported anxiety (Wager et al. 2009a, b). We hypothesize that compared to a control group, SAD participants are characterized by less effective emotion regulation, reflected by diminished cortical-amygdala connectivity under social stress. In addition, we investigate whether cortical-amygdala connectivity correlated with social anxiety symptoms severity.
Method
Participants
This study included 20 participants with SAD and 20 healthy control participants (selected from a pool of 24 subjects matched on age, gender, and years of education) (Table 1). SAD participants were recruited through an advertisement (n = 7), local participating treatment centers (n = 8) and, social anxiety web forums (n = 5). SAD participants had to meet criteria for general SAD according to DSM-IV as a primary diagnosis (1994) based on the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al. 1997). Two SAD participants had a secondary co-morbid current depressive episode, while four others had a history of depressive episodes. Two of these SAD participants were on stable selective serotonin reuptake inhibitor (SSRI) use. Exclusion criteria were other co-morbid anxiety, psychotic or substance abuse disorders. Healthy control participants had no history of psychiatric diseases or psychotropic medication use. Participants completed the Liebowitz Social Anxiety Scale (LSAS; Fresco et al. 2001) and Beck Depression Inventory (BDI; Beck et al. 1988) for initial screening, and the Social Phobia Anxiety Inventory (SPAI; Turner et al. 1989) to assess social anxiety symptom severity after inclusion. Several other questionnaires were also collected: Brief Fear of Negative Evaluation (BFNE; Weeks et al. 2005), the five-factor model of personality (NEO-FFI; Costa & McCrea, 1992), and the Behavioral Inhibition and Activation scale (BIS/BAS; Carver & White, 1994). The study was approved by the Medical Ethical Committee of Leiden University Medical Center and written informed consent was given by all participants.
Table 1.
Participants’ characteristics
| Social anxiety (n = 20) |
Control subjects (n = 20) |
F value | p value | |
|---|---|---|---|---|
| Age, years | 29.1 (7.5) | 27.7 (7.7) | 0.33 | 0.57 |
| Gender, male/female | 11/9 | 11/9 | ||
| Years of education | 16 (2.4) | 16.4 (2.2) | 0.26 | 0.61 |
| LSAS | 85.9 (13.9) | 21.6 (13.1) | 225.23 | <0.001 |
| BDI | 20.5 (11.6) | 5.2 (4.4) | 40.52 | <0.001 |
| SPAI-SP | 136.3 (21.3) 49.8 (24.9) | 132.9 | <0.001 | |
| BFNE | 54.3 (5.6) | 36.0 (9.2) | 44.59 | <0.001 |
| NEO-N | 43.6 (9.8) | 29.5 (6.7) | 24.54 | <0.001 |
| NEO-E | 30.8 (6.3) | 42.7 (4.8) | 39.51 | <0.001 |
| BIS | 24.7 (3.4) | 18.5 (4.2) | 25.7 | <0.001 |
| BAS-Reward | 14.9 (2.3) | 16.6 (2.2) | 5.8 | 0.021 |
Values represent the mean (standard deviation)
LSAS, Liebowitz Social Anxiety Scale; BDI, Beck
Depression Inventory; SPAI-SP, Social Phobia Anxiety
Inventory – Social Phobia subscale; BFNE, Brief Fear of Negative Evaluation; NEO-FFI, NEO Five-Factor Inventory; BIS/BAS, Behavioral Inhibition and Activation Scale.
Materials and procedures
Procedure
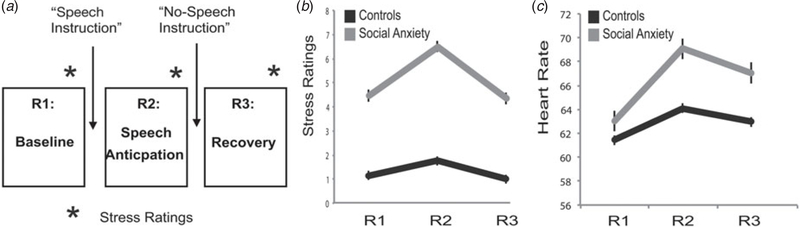
Participants were scanned during three 7.5-min RS fMRI runs (in which they were instructed to just lie still, eyes closed, without falling asleep), applying a social evaluative stress procedure comparable to Wager et al. (2009a, b). Participants were instructed beforehand that a task would follow the scanning procedure, but no details were revealed. After a first baseline run (R1, baseline), participants were instructed that this task would consist of giving a public speech, that the researchers would form the committee that would judge them on their performance, and that their speech would be video-taped for later analysis. Importantly, a topic of the speech was not yet given, and participants were told they would not have to do anything yet, in order to reduce the possibility that the observed effects are merely due to the effort of speech preparation. This instruction was immediately followed by a second RS run (R2, speech anticipation). After the second RS run, the instruction was given that participants did not have to give the public speech after all, that it was just meant to measure their initial reaction to having to give a public speech, and that after a last scan, the experiment would be finished. This instruction was followed by a third and last run (R3, recovery). Before each instruction, participants rated their stress levels on an 11-point Likert scale (See Fig. 1 for an outline of the procedure). This three-scan stress procedure was preceded by a social incentive delay task (Spreckelmeyer et al. 2009) and structural scans. After the study protocol, each subject was debriefed and asked whether they believed they would have to give the speech at the time of the instruction; all subjects (both controls and SAD) answered ‘yes’.
Fig. 1.
Experimental design and self-reported stress and heart-rate results. (a) The procedure consisted of three subsequent resting-state functional magnetic resonance imaging (fMRI) scans. After the first scan (R1, baseline), an instruction was given that a public speech would have to be performed after the scanning sequence was finished. The instruction was followed by another scan (R2, speech anticipation) after which the instruction followed that no public speech had to be given, again followed by an fMRI run (R3, recovery). After each scan, and before each instruction, a self-reported level of stress was obtained on an 11-point Likert-scale. Heart rate was measured continuously during each scan. (b) Self-reported stress levels per scan and group. (c) Average heart rate per scan and group. All error bars represent within-subjects standard error of the mean (Loftus & Masson, 1994).
Analysis
Behavioral and physiological analysis
The stress ratings at the end of each scan, before each instruction, were analyzed in a repeated-measures ANOVA with group as between and run as within-subjects factor, focusing on the quadratic contrast (speech anticipation compared to baseline and recovery). During the three scans, heart rate (HR) was continuously measured using four MRI-compatible ECG electrodes sampling at 500 Hz. Automatic peak detection was performed (using customized Matlab code) on the resulting electrocardiogram (ECG) data. Two control participants were excluded from HR analysis due to excessive noise in the ECG signal. The remaining ECG data were inspected for artifacts in peak detection, and 0.24% of the peaks had to be manually corrected. The peak detections were used to calculate the inter-beat intervals (IBI), which were transformed (60/IBI) to beats per minute. The resulting HR values were averaged per run, and analyzed in a repeated-measures ANOVA with group as between- and run as within-subjects factor.
fMRI data:
Acquisition
Imaging data were acquired on a Philips 3.0-T Achieva MRI scanner using an eight-channel SENSE head coil for radiofrequency reception (Philips Medical Systems, Best, The Netherlands). Whole-brain fMRI data were acquired using T2*-weighted gradient echo echo-planar imaging (EPI) with the following scan parameters: 200 volumes; 38 axial slices scanned in ascending order; repetition time (TR) = 2200 ms; echo time (TE) = 30 ms; flip angle = 80°; FOV = 220 × 220 mm; 2.75 mm isotropic voxels with a 0.25 mm slice gap. A high-resolution anatomical image (T1-weighted ultra-fast gradient-echo acquisition; TR = 9.75 ms; TE = 4.59 ms; flip angle = 8°; 140 axial slices; FOV = 224 × 224 mm; in-plane resolution 0.875 × 0.875 mm; slice thickness = 1.2 mm), and a high-resolution T2*- weighted gradient echo EPI scan (TR = 2.2 s; TE = 30 ms; flip angle = 80°; 84 axial slices; FOV = 220 × 220 mm; in-plane resolution 1.96 × 1.96 mm, slice thickness = 2 mm) were acquired for registration to standard space.
Preprocessing
Data were analyzed using FSL version 4.1.3 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The following preprocessing steps were applied to the EPI datasets: motion correction, removal of non-brain tissue, spatial smoothing using a Gaussian kernel of 6 mm full-width at half maximum (FWHM), grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, and a high pass temporal filter of 100 s (i.e. ≥0.01 Hz). The RS datasets were linearly registered to the high-resolution EPI image, the high-resolution EPI image to the T1-weighted image, and the T1-weighted image to the 2 mm isotropic MNI-152 standard space image (T1-weighted standard brain averaged over 152 subjects; Montreal Neurological Institute, Canada).
Connectivity analysis
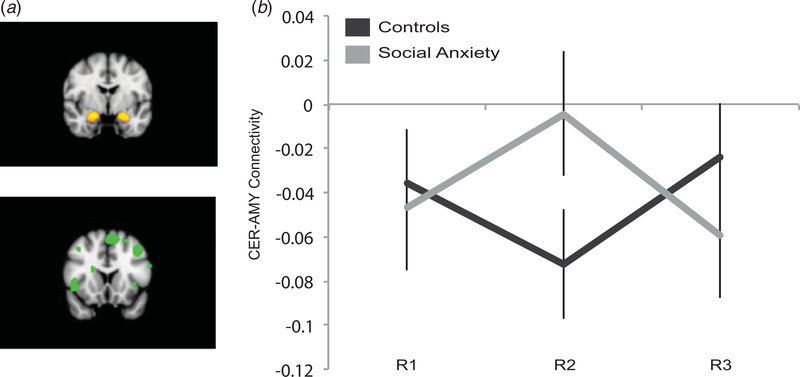
For the connectivity analysis, white matter, cerebral spinal fluid (CSF), and global (whole brain) signal were extracted and entered in a regression analysis together with the six rigid-body motion parameters. The resulting residual time-series data were used for further analysis. To investigate amygdala connectivity with cortical regions involved in emotion regulation, a representative time-series (first eigenvariate) was extracted from the residual data from the left and right amygdala (based on a 50% probability mask from the Harvard-Oxford subcortical probability atlas, provided in FSL), and the combined set of cortical regions involved in cognitive emotion regulation [CER; based on a meta-analysis on emotion regulation (Diekhof et al. 2011), see Table 2]. To quantify connectivity, the time-series for each participant and RS run were correlated (both for the left amygdala and CER, and right amygdala and CER), and the correlation coefficient transformed to Fisher’s Z score. These Z values were entered in a repeated-measures mixed-effects ANOVA with group as between-subjects and run (focusing on the quadratic contrast, i.e. comparing speech anticipation to the baseline and recovery period) and side (left and right amygdala) as within-subjects factors. By applying this approach we thus obtain a summary statistic of cortical emotion regulation – amygdala connectivity, and hence greatly reduce the number of statistical comparisons, compared to standard mass-univariate statistical test, which can suffer from low statistical power (Yarkoni, 2009; Yarkoni et al. 2010).
Table 2.
List of clusters in the meta-analysis on cognitive emotion regulation (Diekhof et al. 2011)
| Regions | Coordinates (x, y, z) |
No. of voxels |
||
|---|---|---|---|---|
| L middle temporal gyrus | −62 | −4 | −20 | 45 |
| Ventromedial PFC | 4 | 40 | −20 | 78 |
| L IFG/anterior insula | −50 | 28 | −8 | 289 |
| R IFG | 50 | 30 | −8 | 156 |
| L inferior temporal gyrus | −60 | −36 | −2 | 225 |
| R anterior insula/frontal operculum | 46 | 16 | −2 | 151 |
| L anterior insula | −38 | 18 | −4 | 34 |
| R IFG | 60 | 26 | 6 | 42 |
| R frontomarginal gyrus | 34 | 60 | 8 | 44 |
| L ACC | −8 | 28 | 28 | 36 |
| L intraparietal cortex | −44 | −64 | 36 | 226 |
| L middle frontal gyrus | −40 | 16 | 44 | 351 |
| Dorsomedial PFC | −6 | 22 | 52 | 706 |
| R Intrapariatal cortex | 50 | −60 | 42 | 44 |
| R middle frontal gyrus | 40 | 22 | 44 | 123 |
| R superior frontal gyrus | 18 | 24 | 60 | 29 |
IFG, Inferior frontal gyrus; ACC, anterior cingulate cortex; PFC, prefrontal cortex.
Additionally, whole brain voxel-wise regression analyses were also performed to support the initial approach. The representative CER time-series were used as regressors in a general linear model (GLM) voxel-wise analysis using FEAT version 5.98 (part of FSL; Smith et al. 2004). At the subject level, contrasts that tested the overall effect (across scans), the differences between the second and first scans, the second and third scans, and the quadratic effect were generated. The resulting individual parameter estimate (PE) maps were fed into a higher-level between-groups random-effects analysis (two-sample t-test). Subsequently, correction for multiple comparisons was performed for only those voxels present in the region of interest (ROI) masks (left or right amygdala) using family-wise error (FWE) correction. In a similar fashion, voxel-wise analyses were performed with the left and right amygdala as regressor and with the CER regions as targets for small volume corrections. For any effects outside our ROIs, a whole brain FWE-corrected p < 0.05 threshold was applied. Furthermore, a novel meta-analytic ‘decoding’ analysis using Neurosynth (Yarkoni et al. 2011) on the voxel-wise statistical images was performed. This analysis assesses the ‘involvement’ of the amygdala and cognitive emotion regulation voxel-wise statistical images to certain topics (e.g. ‘emotion’) based on meta-analytic data. See the online Supplementary Material, section 1 for details.
Link between behavioral variables and changes in connectivity due to speech anticipation
Correlational analyses were performed to test the relationship between the social anxiety symptoms (SPAI-SP), speech preparation related changes in brain connectivity, and self-reported stress. Furthermore, a mediation analysis was performed to test whether speech anticipatory related changes in brain connectivity mediated the relationship between social anxiety symptoms and self-reported stress (see online Supplementary Material, section 2).
Results
Stress ratings and physiological responsiveness
The stress manipulation showed a significant run (quadratic contrast)×group interaction on the reported stress levels (F1,38 = 10.87, p = 0.002). SAD participants reported higher stress after R2 (speech anticipation) than R1 (baseline) compared to controls (t38 = 2.9, p = 0.006), see Fig. 1. The average heart rate data showed a trend for a similar run (quadratic contrast)×group interaction (F1,36 = 3.04, p = 0.09), including a trend for a higher score for the differences between R2 and R1 (t36 = 1.9, p = 0.06) in the SAD compared to the control group.
fMRI: cortical-amygdala connectivity
There was a significant run×group interaction in CER-amygdala connectivity (quadratic contrast; F1,38 = 4.68, p = 0.037, see Fig. 2). This interaction can be explained by the following pattern of effects: when anticipating the public speech, SAD participants showed a transient decrease in negative functional connectivity between the amygdala and cortical regions involved in emotion regulation, whereas controls showed the opposite effect (i.e. a transient increase in negative functional connectivity; see Fig. 2). No interaction of this effect with side (left or right amygdala) was observed (p = 0.88). The main effects were non-significant (all p > 0.25), as was the omnibus run×group ANOVA (F2,76 = 2.15, p = 0.123). The same ANOVA analyses with the factors run and laterality of the amygdala was performed for each group separately; however, no significant main or interaction effect was found (all p > 0.1). Additionally, we performed a whole-brain regression analysis to confirm the above-mentioned quadratic effect findings in a voxel-wise approach. Using the CER time-series as a regressor, we found an effect in the right amygdala (x = 30/y = 0/z=−20, z = 3.14, k = 6, small volume FWE-corrected p < 0.05) for the quadratic contrast (comparing the baseline and recovery measurement to the speech anticipation). The analysis with the amygdala as source region did not yield any significant effect in our ROI for any of the group run×condition interaction effects. For both analyses, no whole-brain corrected interaction effects outside of our ROIs were found.
Fig. 2.
Cortical-amygdala connectivity. (a) Regions used for connectivity analysis, amygdala (top) and cortical emotion regulation regions (bottom). (b) Run×group interaction on cortical-amygdala connectivity. All error bars represent within-subjects standard error of the mean (Loftus & Masson, 1994).
The topic mapping approach using the Neurosynth database broadly revealed that the control group showed relatively less involvement of the amygdala connectivity with topics such as ‘emotion’, ‘social cognition’, and ‘memory’, and stronger with topics as, for instance, ‘perception’. The cognitive emotion regulation results are largely in opposing directions (see online Supplementary Material, section 1). Additionally, we explored and visualized the cortical-amygdala connectivity dynamics (see Supplementary Methods and Results, Section 4).
Link between anxiety symptoms, stress ratings, and connectivity changes due to speech anticipation
Within the social anxiety group, results showed a significant correlation between social anxiety symptoms (measured with the SPAI-SP) and increases in reported stress in the social anxiety group (r = 0.48, p = 0.048) as well as a significant correlation between SPAI-SP and stress-related changes in CER-amygdala connectivity, r = 0.53, p = 0.016). In the online Supplementary Material, section 2, a mediation model on the relationship between SPAI-SP, brain connectivity, and self-reported stress is tested.
Discussion
The present investigation revealed a distinct pattern of cortical-amygdala connectivity in SAD compared to controls when anticipating giving a public speech. The control group displayed an increase in negative connectivity during speech anticipation. The social anxiety group, however, showed reduced functional integration (moving from negative connectivity to no, or positive connectivity) during speech anticipation. This pattern in connectivity change may reflect failure to recruit adaptive control processes in the face of social stress in SAD. This finding shows similarities with studies that found a link between cortical-amygdala coupling and subjective or physiological responses during the instructed reappraisal of negative emotions (Urry et al. 2006; Wager et al. 2008; Lee et al. 2012) and indications of less cortical-amygdala connectivity during cognitive reappraisal in SAD patients (Goldin et al. 2009b).
The results of self-reported stress and HR suggest that the applied speech anticipation procedure can indeed be considered stress-inducing and is potent in differentiating the controls from the social anxiety group. This is broadly in line with various studies that have shown increases in physiological and self-reported responses to (the anticipation of) public speech in social anxiety (Davidson et al. 2000; Gramer & Saria, 2007; Blöte et al. 2009; Roelofs et al. 2009). It is of great interest that social anxiety symptoms correlated positively with both stress-related changes in cortical-amygdala connectivity and self-reported stress levels, underscoring the relevance of cortical-amygdala connectivity for social anxiety. Also, using Neurosynth (Yarkoni et al. 2011), we performed a complementary meta-analytic decoding analysis to estimate the involvement of the connectivity patterns of the amygdala and cognitive emotion regulation regions to several cognitive and emotion topics (see online Supplementary Material). The results, for instance, showed that the topic associated with social cognition loaded relatively less to amygdala connectivity but more to cortical emotion regulation connectivity in the control compared to the social anxiety group (see online Supplementary Material for the complete list of results). Hence, the decoding analysis provides interesting information, pointing at a broad differentiation between the groups in the involvement of the two regions during social threat in ‘perception and cognition’ and ‘social cognition and emotion’ topics.
It will be of great interest to further investigate whether the cortical-amygdala connectivity patterns are a state or a trait marker for SAD. For instance, one might hypothesize that after successful treatment of SAD, cortical-amygdala coupling would ‘normalize’. That is, functional connectivity under social stress could strengthen, perhaps reflective of treatment-induced increases in successful communication between cortical emotion regulation regions and the amygdala. There is an increase in studies investigating the pharmacological (Phan et al. 2013; Giménez et al. 2014) and cognitive-behavioral therapy (Goldin et al. 2013) treatment effects on neural processing in SAD. Moreover, previous studies have already shown that the amygdala activity during speech anticipation (Furmark et al. 2005) and performance (Faria et al. 2012) decreases after successful treatment, which is thought to indicate less anxiety sensitivity. It would be of great interest to test whether cortical-amygdala connectivity, as measured in our current approach, indeed normalizes after treatment, and at which rate this might occur.
Several researchers have pointed at the importance of state-related changes in RS connectivity in understanding the link between (RS) connectivity networks and cognition (Bressler & Menon, 2010; Cole et al. 2010). Our data-analytic approach is comparable to RS studies that extract representative time-series from spatial maps (based on either independent component analysis in a previous step, or on predefined network masks), and use the time-series in a regression analysis to estimate the individual representation of these networks, including their connections to other brain regions (Cole et al. 2010; Margulies et al. 2010). However, in the current study we departed from a set of regions not grouped by their temporal profile, but by their involvement in a certain function (cognitive emotion regulation) as identified in a meta-analysis. This approach assumes that no cortical region in particular drives our findings. Complex functions like emotion regulation are also most likely not sub-served by a single region, or a single connection, and the large set of regions identified by the meta-analysis on emotion regulation adds to this notion (Diekhof et al. 2011). Nonetheless, it should be noted that our approach potentially overlooks more fine-grained connectivity patterns. For instance, specific cortical-subcortical pathways have been found to be involved in the regulation or initiation of several stress responses. For example, it has been shown that medial prefrontal cortex-periaqueductal grey (mPFC-PAG) connectivity mediates HR increase during speech anticipation (Wager et al. 2009a), and other work has linked endogenous cortisol levels (Veer et al. 2012) and corticosteroid administration (Henckens et al. 2011) to amygdala-mPFC connectivity.
Limitations
Most fMRI studies suffer from low statistical power, due the large amount of dependent variables (i.e. voxels) and often relatively small number of participants (Yarkoni, 2009; Button et al. 2013). Our current analytical approach partially addresses this concern by reducing the number of outcome variables to a single measure of functional integration between a large set of cortical regions and the amygdala. However, we acknowledge that the sample size, effect size, and significance level of our main finding are moderate, which underscores the importance of independent replication of the current findings.
One preprocessing step in our analysis, which is important to point out, is the removal (by regression) of global signal fluctuations. This procedure increases the range of correlations that can be observed between regions or networks (Cole et al. 2010), but it is argued that this procedure can ‘induce’ anti-correlations, or at least make the sign of the correlations uninterpretable (Cole et al. 2010). At the very least, we agree that our findings should be interpreted in light of the global signal regression step, and ‘negative connectivity’ is therefore necessarily a relative value with respect to global signal fluctuations (see online Supplementary Material for further analyses on this and other potential confounding effects in RS analyses).
Conclusion
In this study, we have shown that SAD participants, compared to controls, display reduced functional integration between cortical emotion regulation regions and the amygdala when anticipating speaking in public. The reduced functional integration in social anxiety was, moreover, related to symptom severity. The findings suggest that SAD is characterized by less effective cortical-amygdala communication during social evaluative threat. More research is needed to test whether this potentially maladaptive change in cortical-amygdala connectivity normalizes after treatment.
Supplementary Material
Acknowledgements
K.R. and H.C. are supported by the Netherlands Organization of Scientific Research (NWO), VIDI grant no. 452-07-008. S.A.R.B.R. is supported by NWO VIDI grant no. 917-86-368.
Footnotes
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291714002657.
References
- Amstadter A (2008). Emotion regulation and anxiety disorders. Journal of Anxiety Disorders 22, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders (DSM), 4th edn American Psychiatric Press: Washington. [Google Scholar]
- Arnsten AFT (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience 10, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG (1988). Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review 8, 77–100. [Google Scholar]
- Blöte AW, Kint MJW, Miers AC, Westenberg PM (2009). The relation between public speaking anxiety and social anxiety: a review. Journal of Anxiety Disorders 23, 305–313. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V (2010). Large-scale brain networks in cognition: emerging methods and principles. Elsevier Ltd Trends in Cognitive Sciences 14, 277–290. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience 14, 365–376. [DOI] [PubMed] [Google Scholar]
- Carver C, White T (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology 67, 319–333. [Google Scholar]
- Choi JM, Padmala S, Pessoa L (2012). Impact of state anxiety on the interaction between threat monitoring and cognition. NeuroImage 59, 1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF (2010). Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in Systems Neuroscience 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrea RR (1992). Revised Neo Personality Inventory (Neo PI-R) and Neo Five-Factor Inventory (NEO-FFI). Psychological Assessment Resources: Odessa, FL. [Google Scholar]
- Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB (2000). While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry 47, 85–95. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O (2011). Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage 58, 275–285. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager T (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in ptsd, social anxiety disorder, and specific phobia. American Journal of Psychiatry 167, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria V, Appel L, Åhs F, Linnman C, Pissiota A, Frans O, Bani M, Bettica P, Pich EM, Jacobsson E, Wahlstedt K, Fredrikson M, Furmark T (2012). Amygdala subregions tied to SSRI and placebo response in patients with social anxiety disorder. Neuropsychopharmacology 37, 2222–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS (2009). Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience 10, 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, Amaral DG (2009). Anatomy of the primate amygdala In The Human Amygdala (ed. Whalen PJ & Phelps EA), pp. 3–42. Guilford: New York. [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, Goetz D (2001). The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine 31, 1025–1035. [DOI] [PubMed] [Google Scholar]
- Furmark T, Appel L, Michelgård Å, Wahlstedt K, Åhs F, Zancan S, Jacobsson E, Flyckt K, Grohp M, Bergström M, Pich EM, Nilsson L-G, Bani M, Långström B, Fredrikson M (2005). Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biological Psychiatry 58, 132–142. [DOI] [PubMed] [Google Scholar]
- Giménez M, Ortiz H, Soriano-Mas C, López-Solà M, Farré M, Deus J, Martín-Santos R, Fernandes S, Fina P, Bani M, Zancan S, Pujol J, Merlo-Pich E (2014). Functional effects of chronic paroxetine versus placebo on the fear, stress and anxiety brain circuit in Social Anxiety Disorder: initial validation of an imaging protocol for drug discovery. European Neuropsychopharmacology 24, 105–116. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ (2009a). Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry 66, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ (2009b). Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological Psychiatry 66, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ (2013). Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry 70, 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramer M, Saria K (2007). Effects of social anxiety and evaluative threat on cardiovascular responses to active performance situations. Biological Psychology 74, 67–74. [DOI] [PubMed] [Google Scholar]
- Gross JJ (2010). The future’s so bright, I Gotta wear shades. Emotion Review 2, 212–216. [Google Scholar]
- Henckens MJAG, van Wingen GA, Joëls M, Fernández G (2011). Corticosteroid induced decoupling of the amygdala in men. Cerebral Cortex 22, 2336–2345. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ (2009). The neuro-symphony of stress. Nature Publishing Group Nature Reviews Neuroscience 10, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ (2012). Amygdala-prefrontal coupling underlies individual differences in emotion regulation. NeuroImage 62, 1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ (1994). Using confidence intervals in within-subject designs. Springer Psychonomic Bulletin & Review 1, 476–490. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, Ballenger JC, Lydiard RB, Brodrick PS, Bohning DE, George MS (2004). Neural correlates of speech anticipatory anxiety in generalized social phobia. NeuroReport 15, 2701–2705. [PubMed] [Google Scholar]
- Margulies DS, Böttger J, Long X, Lv Y, Kelly C, Schäfer A, Goldhahn D, Abbushi A, Milham MP, Lohmann G, Villringer A (2010). Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. Magnetic Resonance Materials in Physics, Biology and Medicine 23, 289–307. [DOI] [PubMed] [Google Scholar]
- Moscovitch DA, Chiupka CA, Gavric DL (2013). Within the mind’s eye: negative mental imagery activates different emotion regulation strategies in high versus low socially anxious individuals. Journal of Behavior Therapy and Experimental Psychiatry 44, 426–432. [DOI] [PubMed] [Google Scholar]
- Phan KL, Coccaro EF, Angstadt M, Kreger KJ, Mayberg HS, Liberzon I, Stein MB (2013). Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biological Psychiatry 73, 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S (2008). Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry 63, 234–240. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM (2009). The influence of stress hormones on fear circuitry. Annual Review of Neuroscience 32, 289–313. [DOI] [PubMed] [Google Scholar]
- Roelofs K, van Peer J, Berretty E, Jong P, Spinhoven P, Elzinga BM (2009). Hypothalamus–pituitary–adrenal axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. Society of Biological Psychiatry Biological Psychiatry 65, 336–343. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH (2013). Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences USA 110, 6145–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Harnett Sheehan K, Janavs J, Weiller E, Keskiner A, Schinka J, Knapp E, Sheehan M, Dunbar G (1997). The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry 12, 232–241. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Kircher T, Gründer G (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience 4, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fredrikson M (2002). Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biological Psychiatry 52, 1113–1119. [DOI] [PubMed] [Google Scholar]
- Turner SM, Beidel DC, Dancu CV, Stanley MA (1989). An empirically derived inventory to measure social fears and anxiety: The Social Phobia and Anxiety Inventory. Psychological Assessment 1, 35–40. [Google Scholar]
- Ulrich-Lai YM, Herman JP (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience 26, 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJF, Hermans EJ, Qin S, Fernández G (2010). Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. NeuroImage 53, 348–354. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SARB (2011). Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. NeuroImage 57, 1534–1541. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SARB (2012). Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuro-endocrinology 37, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN (2009a). Brain mediators of cardiovascular responses to social threat, part II: prefrontal-subcortical pathways and relationship with anxiety. NeuroImage 47, 836–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF (2009b). Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage 47, 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JW, Heimberg RG, Fresco DM, Hart TA, Turk CL, Schneier FR, Liebowitz MR (2005). Empirical validation and psychometric evaluation of the brief fear of negative evaluation scale in patients with social anxiety disorder. Psychological Assessment 17, 179–190. [DOI] [PubMed] [Google Scholar]
- Yarkoni T (2009). Big correlations in little studies: inflated fMRI correlations reflect low statistical power-commentary on Vul et al. (2009). Perspectives on Psychological Science 4, 294–298. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods 8, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Van Essen DC, Wager TD (2010). Cognitive neuroscience 2.0: building a cumulative science of human brain function. Trends in Cognitive Sciences 14, 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.