Abstract
Stem cells offer unprecedented opportunities for the development of strategies geared toward the treatment of retinal degenerative diseases. A variety of cellular sources have been investigated for various potential clinical applications, including tissue regeneration, disease modeling, and screening for non–cell-based therapeutic agents. As the field transitions from more than a decade of preclinical research to the first phase I/II clinical trials, we provide a concise overview of the stem cell sources most commonly used, weighing their therapeutic potential on the basis of their technical strengths/limitations, their ethical implications, and the extent of the progress achieved to date. This article serves as a framework for further in-depth analyses presented in the following chapters of this Special Issue.
Keywords: retinal degeneration, stem cells, therapies
Over the last decade, stem cells have arisen as a highly promising resource for potential therapeutic strategies to treat a wide array of eye diseases. Within the context of the Ocular Research Symposium, we have been tasked with presenting a concise overview of the different stem cell sources that are more relevant for the development of potential treatments for retinal degenerative diseases affecting photoreceptors and RPE cells, to serve as a framework for more in-depth discussion in other chapters of the present Special Issue. We will therefore focus on the most significant results obtained with human stem cells, with emphasis on strategies for cell replacement, making reference to findings from animal models only when they aid in clarifying the potential of a specific cell source for human therapy. We will then succinctly discuss the prospects for broader therapeutic applications of the different stem cell sources described, taking into account the advantages and limitations of each type. Finally, we will present what we consider some of the most immediate needs and opportunities to successfully bring stem cell–based photoreceptor/RPE replacement into the clinical arena.
Stem Cell Sources
Stem cells are characterized by three general properties: their capacity for unlimited self-renewal, their unspecialized status, and their ability to differentiate into multiple cell types.
Within this general definition, stem cells can be classified according to different criteria. A broad classification concerns their potency; thus, multipotent stem cells have the ability to differentiate into a limited number of cell types, whereas pluripotent stem cells have the ability to differentiate into any of the cell types found in the adult body. Multipotent stem cells can in turn be subclassified according to their origin, as, for example, fetal and adult stem cells (i.e., derived from a variety of developing fetal tissues or from adult functional tissues, respectively), or neural and non-neural (i.e., derived from neuroectodermal versus non-neuroectodermal lineages). Among the different multipotent stem cell types identified to date, those most commonly evaluated for potential therapies for retinal degenerative diseases include (1) fetal stem cells from neural lineages, including fetal retinal progenitor cells (fRPCs) and fetal cortical progenitor cells (fCPCs); (2) adult stem cells from neural lineages including ciliary epithelium–derived stem cells (CESCs), retinal pigmented epithelium stem cells (RPESCs), and Müller glial cells (MCs); and (3) adult stem cells from non-neural lineages, including umbilical tissue–derived stem cells (UTSCs), and bone marrow–derived stem cells (BMDSCs). Pluripotent stem cells, on the other hand, encompass embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).
Multipotent Fetal Stem Cells From Neural Lineages
Fetal Retinal Progenitor Cells
Seminal studies in mouse models using retinal cells from donor animals for transplantation have indicated that successful integration into the host tissue is highly dependent on the developmental stage of the donor cells.1–4 Specifically, postmitotic photoreceptor precursors seem to provide the highest efficiency for successful transplantation. Moreover, some of these studies also showed a certain degree of functional restoration. These and other results have prompted some groups to undertake a similar approach for the treatment of human patients. Such an approach depends on the use of fetal-derived retinal progenitor cells as a donor source. The fRPCs are obtained from the retina of human fetuses between 14 and 20 weeks of gestation, a time at which photoreceptor progenitors in the developing retinal neuroepithelium are exiting the cell cycle and starting their differentiation process.5 Thus, there have been several studies in which fRPCs have been transplanted into patients affected by retinitis pigmentosa (RP) and AMD, using various approaches including the transplantation of microaggregate suspensions of fRPCs, fetal neural retinal sheets consisting of an isolated photoreceptor cell layer or a full retinal component, RPE sheets, or neural retina with associated RPE.6–8 A critical outcome from these studies is the apparent lack of adverse effects.9,10 In addition, as reported by Radtke et al.8 regarding a phase II clinical trial in which fetal retina/RPE sheets were transplanted on 10 RP and AMD patients, this treatment led to a certain level of short-term visual improvement, as assessed by ETDRS (Early Treatment Diabetic Retinopathy Study) visual acuity scores up to 12 months after surgery. However, no long-term benefits were observed, except for the case of one patient that maintained visual improvement at a 6-year follow-up. It is uncertain whether the improvement was due to a trophic effect of the implant or to functional integration of the transplanted cells.
Currently, a new phase I/II clinical trial has been approved by the Food and Drug Administration (FDA) that aims at testing the safety and tolerability of a single subretinal injection of dissociated fRPCs in patients with RP (clinicaltrials.gov, #NCT02464436). This will be a dose escalation study using cells derived from a 20-week-old human fetus and expanded in vitro, which will follow patients up to 1 year after injection. Despite being a safety study, visual function, transplant, and host retina integrity will also be assessed as secondary outcomes.
Fetal Cortical Progenitor Cells
Preclinical studies using the RCS rat (a well-characterized model of photoreceptor degeneration) showed that subretinal transplantation of human neural progenitor cells derived from human prenatal cortex results in long-term rescue of visual function associated with extensive survival and migration of the transplanted cells and morphologic and functional preservation of host photoreceptors.11–13 Even though the mechanism underlying this neuroprotective effect is not completely understood, it might be explained at least in part by the apparent ability of fCPCs to phagocytose photoreceptor outer segments.14 A phase I clinical trial has recently been completed in patients with dry AMD that were subjected to unilateral subretinal transplantation of a suspension of these cells (clinicaltrials.gov, #NCT01632527). Safety reports have been favorable at this point, and a phase II clinical trial for proof-of-concept of the safety and efficacy of fCPCs subretinal transplantation in subjects with geographic atrophy is currently underway (clinicaltrials.gov, #NCT02467634).
Multipotent Adult Stem Cells From Neural Lineages
Ciliary Epithelium–Derived Stem Cells
Initially characterized in the mouse,15,16 these cells were subsequently identified in the adult human retina from cadaveric eyes.17–22 They constitute a small subpopulation of cells residing within the ciliary body epithelium: approximately 10,000 cells per human eye.17 During adult life, these cells remain in a quiescent state, but when isolated from the eye, they are able to clonally proliferate under both adherent and neurosphere-forming conditions.17 Some studies have also reported that CESCs show a relative ability to differentiate into cells expressing markers characteristic of retinal progenitors and of more mature retinal cell types including photoreceptors and RPE.17,22,23 Furthermore, studies from Coles et al.17 show that, on transplantation into early postnatal immune deficient NOD/SCID mice and embryonic chick eyes, these cells migrated into the host retina and expressed markers normally seen in photoreceptor, ganglion and horizontal cells (reviewed in Ref. 10). Despite exhibiting characteristics of neural stem cells in vitro, there is no evidence to date of their ability to trigger a similar behavior within their normal in vivo environment. It is also important to note that several more recent reports have generated significant controversy regarding the true stem cell nature of these cells, suggesting that CESCs are not bona fide multipotent stem cells as originally proposed, but rather represent a subpopulation of neuroepithelial progenitors with robust self-renewal capabilities but very limited ability to differentiate into multiple lineages (reviewed and discussed in Refs. 24–27).
RPE Stem Cells
Although RPE cells are not neuronal in nature, they differentiate from the optic neuroepithelium, sharing this common origin with the cells from the neural retina. Studies in animal models have explored the possibility of retinal repair from RPE cells for decades, shedding valuable insights into the differences in the cellular and molecular mechanisms that are recruited in models with high versus low regenerative abilities. In this context, amphibians such as frogs and salamanders stand out for their unparalleled capacity to regenerate their neural retina from the RPE upon injury or complete loss28,29 (reviewed by Ref. 30). In this process known as transdifferentiation, the RPE cells lose their specialized characteristics (including their pigment), regaining their “stemness,” to subsequently proliferate and differentiate into all the different retinal cell types, restoring an anatomically (and in the case of salamanders also functionally) normal retina and adjacent RPE.30,31 Chickens also possess this capacity, albeit to a much more limited extent: it only occurs during a specific window of embryonic development (embryonic days 3.5–4.5), and the RPE layer is not restored in the process but rather converts into a neural retinal phenotype, yielding a retina with reverse polarity (reviewed by Refs. 30 and 32). Unfortunately the potential of mammalian RPE to regenerate retinal neurons is significantly more restricted. Studies of dissociated adult rat RPE cultured in vitro have demonstrated the ability of these cells to dedifferentiate and proliferate, although with very low efficiency, and to express some markers of early neuronal lineage.33 In addition, work in rats and humans has indicated the existence of a very small population of proliferative cells in the peripheral RPE.34
Promisingly, a more recent study using dissociated adult RPE cells from human cadaveric donors showed that a small subpopulation (10%) of harvested RPE cells were able to dedifferentiate in vitro, proliferate with an acceptable level of efficiency (being able to undergo several rounds of division), and robustly redifferentiate into stable cobblestone RPE monolayers, suggesting that these cells could act as a potential cellular source for RPE replacement therapy.35,36 Furthermore, under defined culture conditions, they were also able to differentiate into both neural (particularly early retinal progenitors) and mesenchymal lineages, indicating their multipotent nature36 (reviewed by Refs. 10 and 35).
Müller Glial Cells
Müller cells are not considered typical stem cells due to their differentiated status, but they are able to acquire stem cell-like characteristics under specific conditions to different extents depending on the animal species. For example, zebrafish are able to spontaneously regenerate their neural retina from MCs in response to injury inflicted by different types of insults and constitute the archetype of this kind of regeneration (reviewed in Refs. 37–39). Chicks and rodents, on the other hand, display a much more limited capacity for MC proliferation and neuron generation after pharmacologic damage or growth factor stimulation (reviewed in Ref. 39). Interestingly, the mammalian MC transcriptome overlaps significantly with that of retinal progenitor cells.40,41 Humans have not been shown to display endogenous regeneration from this cell source. However, studies done in vitro have identified a population of human MCs that display stem cell characteristics (hMSCs) and a relative capacity to generate retinal neurons, including rod photoreceptor cells.42–44 Furthermore, on transplantation into a rodent model of photoreceptor degeneration, hMSC-derived photoreceptor-like cells were able to migrate and integrate into the host outer nuclear layer, leading to an improvement in photoreceptor function as assessed by electroretinography (ERG)45 (reviewed in Ref. 10).
Multipotent Adult Stem Cells From Non-Neural Lineages
Stem cells of non-neuroectodermal lineage are currently being tested in a number of clinical trials for various retinal diseases, including AMD, RP, and Stargardt's disease, although they are mostly aimed at a potential trophic paracrine effect that could preserve photoreceptor function and not necessarily at cell replacement (reviewed in Refs. 46–49). Because this topic is specifically addressed in another article, here we present a very succinct description and refer the reader to the appropriate chapter for further information.
Umbilical Tissue–Derived Stem Cells
The UTSCs have been shown to slow vision loss in RCS rats,50 and a phase I clinical trial was conducted using subretinal injection of these cells to evaluate safety and efficacy outcomes in patients with RP (clinicaltrials.gov, #NCT00458575). This study was terminated in 2010, but a new phase I/II clinical trial using subretinal transplantation of UTSCs in AMD patients began that same year and is still ongoing (clinicaltrials.gov, #NCT01226628).
Bone Marrow–Derived Stem Cells
Intravitreal transplantation of autologous BMDSCs in RP patients has been conducted by a group in Brazil, with a phase I clinical trial meeting safety criteria (clinicaltrials.gov, #NCT01068561),51 and a phase II clinical trial still under evaluation but showing transient improvement in patients' quality of life (clinicaltrials.gov, #NCT01560715).52 Similar clinical trials are being carried out in Egypt (clinicaltrials.gov, #NCT02016508) and Spain (clinicaltrials.gov, #NCT02280135).
Autologous bone marrow–derived CD34+ hematopoietic stem cells are also being pursued as a therapeutic agent in AMD, Stargardt's disease, RP, and retinal vascular occlusion through intravitreal injection. Reports from the phase I clinical trial show good tolerability, with no worsening of the best-corrected visual acuity and full-field ERG after 6 months (clinicaltrials.gov, #NCT01736059).53
Additional clinical trials aimed at evaluating safety and efficacy of bone marrow-derived mesenchymal stem cells for the treatment of RP and AMD are currently underway in Thailand (ClinicalTrials.gov, NCT01531348), India (ClinicalTrials.gov, NCT01914913) and the United States (ClinicalTrials.gov, NCT01920867).
Pluripotent Stem Cells
Embryonic Stem Cells
Human embryonic stem cells are harvested from the inner cell mass of 5-day-old preimplantation embryos; they are pluripotent and therefore have the potential to differentiate into all the cell types found in the adult body. Within the last decade, significant progress has been made in identifying appropriate culture conditions to induce hESCs to follow a retinal differentiation pathway. Initially, differentiation protocols designed to mimic the sequential induction steps that take place in the embryo during the development of the retina demonstrated that these cells were capable of differentiating into several of the retinal cell types in adherent conditions.54–56 Due to their potential value for clinical applications, special emphasis was given to the differentiation of photoreceptor and RPE cells, resulting in well-defined conditions to obtain relatively advanced differentiated rods (displaying robust rhodopsin expression among other photoreceptor-specific proteins) and RPE cells (exhibiting morphologic, molecular, and physiological characteristics of mature RPE) with varying efficiencies57–61 (reviewed in Refs. 47 and 62).
Subsequently, it was shown that under appropriate three-dimensional (3D) culture conditions, hESCs were capable of differentiating into self-organizing 3D structures resembling the histologic composition of the early optic vesicle and more advanced, stratified neural retina.63–65
To date, studies reporting subretinal transplantation of hESC-derived neuroretinal cells are still scarce.54,57,66 In these studies, the transplanted hESC-derived RPCs showed a very limited ability to migrate into the outer nuclear layer (ONL) of the host retina. Despite this, and even though the hESC-derived photoreceptor-like cells failed to form outer segments, a light response with b-wave restoration was observed on transplantation into Crx–/– mice.57 On the other hand, several studies have evaluated the feasibility of transplanting hESC-derived RPE cells, as both single cell suspension and monolayer sheets, in animal models of retinal degeneration, showing acceptable survival of the transplanted cells, retardation of photoreceptor loss, and rescue of visual parameters67–71 (reviewed in Refs. 10 and 72).
Furthermore, two prospective phase I/II clinical trials to test the safety and tolerability of subretinal transplantation of hESC-derived RPE cells have been recently carried out (clinicaltrials.gov, #NCT01345006 and #NCT01344993). These studies involved subretinal injection of a suspension of dissociated hESC-derived RPE cells in patients affected by Stargadt's disease and dry AMD and showed no evidence of adverse proliferation, rejection, or serious ocular or systemic safety issues related to the transplanted tissue, although there were some adverse events associated with vitreoretinal surgery and immunosuppression.73 Although a trend toward improved vision was noted, it still requires further investigation. In addition, a phase I, open label, safety and feasibility study involving the implantation of the hESC-derived monolayer of RPE immobilized on a polyester membrane in subjects with wet AMD and rapid vision loss has just been initiated (clinicaltrials.gov, #NCT01691261).
Induced Pluripotent Stem Cells
Induced pluripotent stem cells are generated from differentiated somatic cells through a process of reprogramming. Pioneering this technology, Takahashi and Yamanaka demonstrated in 2006 that the forced expression of four specific transcription factors was sufficient to convert adult murine fibroblasts into ESC-like cells.74 In 2007, an important milestone was achieved with the generation of iPSCs from adult human cells.75,76 Since then, human iPSCs have been generated from a variety of somatic cell types through different reprograming methods and various combinations of transcription factors.77–79 After reprograming, these cells acquire a pluripotent state equivalent to that of ESCs, with an indefinite capacity to self-renew and the ability to differentiate into all the different cell types found in the adult body.
Efforts to induce human iPSCs to differentiate along a retinal lineage have rendered similar results to those obtained with hESCs. Two-dimensional stepwise differentiation protocols analogous to those used with hESCs showed that human iPSCs are capable of differentiating into several of the major retinal cell types, including photoreceptors and RPE cells.80–85 Likewise, when cultured in 3D conditions, they gave rise to self-organizing 3D retinal tissue with the different major retinal cell types arranged in their proper layers.86,87 What is more, a recent report has shown that in these human iPSC–derived 3D retinal tissues, photoreceptors can achieve an advanced degree of maturation including the formation of outer segments, expression of phototransduction proteins, and response to light, a first for the field.87
Transplantation of dissociated and purified human iPSC–derived photoreceptors into the subretinal space of normal adult mice showed that a small proportion of the transplanted cells was able to migrate into the ONL of the host retina and express photoreceptor markers, although no inner processes projecting toward the inner nuclear layer or inner and outer segments were present; functional evaluation was not performed.82
Human iPSC-derived RPE cells display many structural and functional features of fairly mature native RPE58,60,88–90 (reviewed in Refs. 47 and 62). Transplantation studies involving subretinal injection of dissociated human iPSC–derived RPE in rodents led to restoration of some aspects of RPE function and improvement in visual function.91,92
More recently, Kamao et al.93 showed that autologous transplantation of iPSC-derived RPE sheets in nonhuman primates, as well as transplantation of human iPSC–derived RPE sheets into rats, did not lead to undesired effects (including immune reaction and tumor growth) and induced preservation of photoreceptor cells and restored ERG responses. These preclinical studies paved the way for the recently started clinical trial in Japan, in which a female patient with exudative AMD became the world's first recipient of an experimental transplant of iPSC-derived autologous cells.94,95 This clinical trial is currently being revised with the purpose of transitioning to a human leukocyte antigen (HLA)-matched allogeneic iPSC strategy.96
The ability to generate patient-derived iPSCs opens the way for novel and much needed applications including elucidation of disease-causing mechanisms, drug and gene therapy testing, toxicology studies, and even personalized treatment-efficacy evaluation.97–100 Importantly, the now feasible ability to generate 3D retinal organoids allows for the more accurate modeling of retinal diseases, as exemplified in recent studies.101,102
Potential of the Different Stem Cell Sources for Therapeutic Applications
Stem cell–based strategies most commonly explored for the development of therapeutic treatments include (1) endogenous cell replacement through the activation of stem cell–like populations residing within the diseased retina; (2) exogenous cell replacement by transplantation of stem cell–derived retinal cells to replace the diseased cell population(s); (3) neuroprotection through a paracrine effect from transplanted cells; (4) in vitro stem cell–based disease models to aid in better understanding the mechanisms underlying the different pathologies; and (5) the use of the aforementioned stem cell–based disease models to identify and validate non–cell-based therapeutic approaches such as neuroprotective agents, drug delivery methods, and gene therapy strategies, among others (Fig.).
Figure.
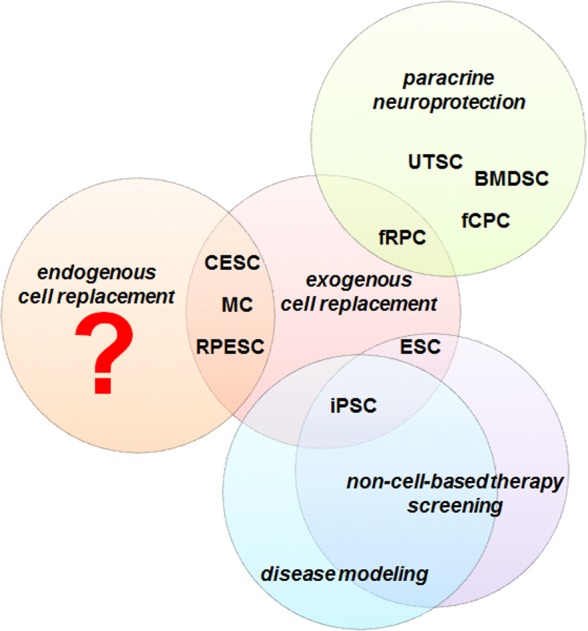
Potential of different stem cell sources for therapeutic applications. Colored circles represent the stem cell–based strategies most commonly explored for the development of therapeutic treatments for retinal degenerative diseases.
The ideal stem cell source for feasible, wide-range therapeutic applications that could be standardized for use in a global scale would have the following characteristics: (1) unlimited/renewable source; (2) high efficiency of differentiation into the cells of interest; (3) no immunogenic or tumorigenic risk; (4) across-the-board range of application; and (5) no ethical corollaries. Therefore, even though we focused primarily on strategies for cell replacement, the potential of the different stem cell sources for their use in therapeutic procedures will be discussed within this broader context.
Ciliary Epithelium–Derived Stem Cells, RPESCs, and MCs
The ability to induce the diseased retina to repair itself through endogenous mechanisms like those observed in some animal models as described above, would undoubtedly be the best scenario and is therefore one of the major goals of regenerative medicine for vision repair. Although the ability of the human retina to undergo endogenous regeneration is yet to be demonstrated, the progress made in elucidating the mechanisms capable of regulating endogenous retinal repair through the activation of CESCs, RPESCs, or MCs in animal models is shedding significant light. Furthermore, the availability of human stem cell–based models that would allow investigating such mechanisms in greater detail could prove instrumental in accelerating the process of discovery. Ciliary epithelium–derived stem cells, RPESCs, and MCs have also been proposed as possible sources for exogenous cell replacement. In the particular case of CESCs and MCs, the feasibility of such an approach, however, is significantly hampered by their limited ability to self-renew and differentiate in vitro and their yet undetermined capacity to generate mature retinal neurons. RPESCs, on the other hand, could potentially be applied to exogenous RPE replacement as discussed above, whereas their applicability to other retina cell types present limitations similar to those for CESCs and MCs. The immunogenicity risk and the availability of sufficient cadaveric donor tissue for treating large clinical populations pose additional challenges for the broad applicability of these cells sources.
Fetal Retinal Progenitor Cells, fCPCs, and ESCs
Compared with CESCs, RPESCs, and MCs, fetal neural progenitor cells and ESCs have a much higher capacity to self-renew and differentiate. They still share, however, the issues of immunogenicity (because they are derived from nonautologous tissues), tumorigenic potential in the case of ESCs,103 and limited accessibility to donor tissue in the case of fetal neural stem cells because they depend on the availability of human fetuses at the appropriate developmental stages. In turn, the origin of the donor tissue has been the subject of heated ethical debate in all social and political arenas. At the center of the debate is the issue of the moral status of the embryo, because the isolation of ESCs requires the destruction of human embryos, and isolation of fNPCs relies on the availability of healthy human fetuses aborted at late gestational periods (within the fourth–fifth month). This lack of societal consensus has to be carefully considered when attempting to establish a therapeutic strategy for standard of care that will be applied to a diverse patient population. These aspects acquire particular relevance when considering that stem cell sources such as iPSCs already offer a suitable and feasible alternative for therapeutic approaches with none of the abovementioned ethical corollaries, together with important advantages from a clinical, scientific, and regulatory standpoint.104
Induced Pluripotent Stem Cells
This stem cell type provides a highly renewable system derived from an unlimited source of donor tissue; an autologous source with potentially negligible (if any) immunogenic risk; and none of the ethical repercussions associated with fRPCs, fCPCs, and ESCs. They are not free of limitations, however, including among others, the risk of teratoma or tumor formation from potential residual undifferentiated cells,103 their relative susceptibility to acquire genetic and epigenetic abnormalities (although recent studies suggest that this is more likely associated to the genomic background in parental cells than to the reprogramming process per se),105 and harboring the disease's genetic defect(s) for the purpose of autologous transplantation. Importantly, emerging genome editing techniques, including zinc finger nucleases, transcription activator–like effector nucleases (TALENs), and particularly clustered regularly interspaced short palindromic repeat/CAS9 RNA-guided nucleases (CRISPR/Cas) technologies, now allow for the generation of gene-corrected isogenic iPSCs better suited for transplantation therapies.106 Furthermore, their renewability makes them exceptional candidates for the establishment of worldwide banks of HLA-matched universal donors, which would make genetic correction unnecessary while also significantly alleviating the cost and workload associated with patient-specific cell line generation for autologous treatment.107
Conversely, the fact that patient-derived iPSCs carry the corresponding genetic mutation(s) offers in turn an unprecedented opportunity for the development of disease models for both understanding the pathologic mechanisms underlying specific diseases and screening for other potential therapeutic treatments besides cell transplantation.
Key Needs and Opportunities
Based on the analysis of the pluses and minuses of the different stem cell sources discussed above, it seems advisable to consider some specific goals to be pursued at both the short and long term.
In the short term, it appears reasonable to prioritize further progress in human iPSC technology based on their advantages over the other stem cell sources, most notably their broader range of applications and significant promise for cell replacement therapies. To achieve an effective transition from research and early-phase clinical trials to generalized treatment using human iPSCs, areas deserving special attention include the following:
The development of improved methods for cell line generation ensuring optimum differentiation efficiency and reproducibility across cell lines: despite significant effort to develop methods ensuring higher efficiency and systematic reproducibility of retinal cell differentiation, the results so far achieved still pose important limitations for large scale, global applications.97 Besides improved differentiation protocols, this goal may require the identification of the most appropriate combination of primary cell source and reprogramming method to achieve the best possible balance between ease of primary cell isolation, efficiency of reprograming, and efficiency/reproducibility of retinal lineage differentiation.79
Creation of banks for HLA-matched human iPSC universal donors: although autologous cell therapies would be ideal, at least from the immunologic standpoint, making this available to a large and diverse population worldwide appears hardly feasible at this time due to several practical challenges, including long generation time, individualized gene editing when necessary, and significantly high monetary cost. An alternative already being actively pursued is the establishment of haplobanks of iPSC lines homozygous for a range of HLA types representative of different geographic populations and ethnic groups.108–110 Current estimates suggest that a relatively small number of lines carefully selected based on allelic frequencies would be sufficient to cover a significant proportion of the population.108–110 Beyond technical aspects, the creation of such human iPSC haplobanks will require extensive international collaboration to address issues such as determination of the optimal HLA panel to ensure that all ethnic groups are equitably represented, donor selection criteria, procedures for donor screening and consent, and regulatory legislation.109
Establishment of efficient pipelines for production of clinical grade cell lines: it is important to continue efforts to define optimal manufacturing platforms compliant with current good manufacturing practices (GMPs) to ensure quality, stability, reproducibility, and necessary scalability of therapeutic-grade cell production while ensuring maximal cost-effectiveness, as it would be required for worldwide clinical application. In addition, it would be important to reach agreement across national and international regulatory bodies regarding at least a minimum of acceptable GMP standards to ensure that the generated cell lines reach as large a patient population as possible.
Establishment of suitable platforms for evaluating non–cell-based therapeutic strategies: the ability of stem cell–based 3D retinal organoids to recreate a more physiological in vivo–like system offers an unprecedented opportunity for clinical applications other than cell replacement, as, for example, the identification of novel therapeutic agents, validation of drug delivery methods, testing of gene therapy strategies, and preclinical efficacy/toxicity studies. The realization of this potential would require the establishment of appropriate disease models and screening platforms. In regard to the disease models per se, besides the need for faithful recapitulation of the biology of the disease, these 3D systems would need to meet relatively high levels of scalability, standardization, and reproducibility to be amenable to high-content screening. As for the screening platforms, considering that current high-throughput strategies mostly rely on 2D systems, there is also the need for designing novel screening platforms allowing analysis of relevant pathophysiologic readouts in 3D samples.
In the long term, it would be desirable to continue the efforts to unlock the mechanisms of endogenous regeneration in the adult human retina, which if successfully achieved, would provide an unparalleled opportunity for therapeutic treatment with minimally invasive intervention.
Acknowledgments
Supported in part by National Eye Institute Grant R01EY022631, the William & Mary Greve Special Scholar Award from Research to Prevent Blindness, and an honorarium from the Ocular Research Symposium Foundation.
Disclosure: V. Canto-Soler, None; M. Flores-Bellver, None; M.N. Vergara, None
References
- 1.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch U, Oriyakhel W, Kenna PF, et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008;86:691–700. doi: 10.1016/j.exer.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Pearson RA, Barber AC, Rizzi M, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakowski J, Baron M, Bainbridge J, et al. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum Mol Genet. 2010;19:4545–4559. doi: 10.1093/hmg/ddq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrickson A, Bumsted-O'Brien K, Natoli R, Ramamurthy V, Possin D, Provis J. Rod photoreceptor differentiation in fetal and infant human retina. Exp Eye Res. 2008;87:415–426. doi: 10.1016/j.exer.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das T, del Cerro M, Jalali S, et al. The transplantation of human fetal neuroretinal cells in advanced retinitis pigmentosa patients: Results of a long-term safety study. Exper Neurol. 1999;157:58–68. doi: 10.1006/exnr.1998.6992. [DOI] [PubMed] [Google Scholar]
- 7.Humayun MS, de Juan E, Jr, del Cerro M, et al. Human neural retinal transplantation. Invest Ophthalmol Vis Sci. 2000;41:3100–3106. [PubMed] [Google Scholar]
- 8.Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008;146:172–182. doi: 10.1016/j.ajo.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Seiler MJ, Aramant RB. Cell replacement and visual restoration by retinal sheet transplants. Prog Retin Eye Res. 2012;31:661–687. doi: 10.1016/j.preteyeres.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayakody SA, Gonzalez-Cordero A, Ali RR, Pearson RA. Cellular strategies for retinal repair by photoreceptor replacement. Prog Retin Eye Res. 2015;46:31–66. doi: 10.1016/j.preteyeres.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Gamm DM, Wang S, Lu B, et al. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS One. 2007;2:e338. doi: 10.1371/journal.pone.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Girman S, Lu B, et al. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2008;49:3201–3206. doi: 10.1167/iovs.08-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGill TJ, Cottam B, Lu B, et al. Transplantation of human central nervous system stem cells - neuroprotection in retinal degeneration. Eur J Neurosci. 2012;35:468–477. doi: 10.1111/j.1460-9568.2011.07970.x. [DOI] [PubMed] [Google Scholar]
- 14.Cuenca N, Fernandez-Sanchez L, McGill TJ, et al. Phagocytosis of photoreceptor outer segments by transplanted human neural stem cells as a neuroprotective mechanism in retinal degeneration. Invest Ophthalmol Vis Sci. 2013;54:6745–6756. doi: 10.1167/iovs.13-12860. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517–521. doi: 10.1006/bbrc.2000.2473. [DOI] [PubMed] [Google Scholar]
- 16.Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 17.Coles BL, Angenieux B, Inoue T, et al. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci U S A. 2004;101:15772–15777. doi: 10.1073/pnas.0401596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer EJ, Carter DA, Ren Y, et al. Neural progenitor cells from postmortem adult human retina. Br J Ophthalmol. 2005;89:102–106. doi: 10.1136/bjo.2004.057687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Sta Iglesia DD, Kielczewski JL, et al. Characteristics of progenitor cells derived from adult ciliary body in mouse, rat, and human eyes. Invest Ophthalmol Vis Sci. 2007;48:1674–1682. doi: 10.1167/iovs.06-1034. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Navarrete GC, Angulo A, Martin-Nieto J, Cuenca N. Gradual morphogenesis of retinal neurons in the peripheral retinal margin of adult monkeys and humans. J Comp Neurol. 2008;511:557–580. doi: 10.1002/cne.21860. [DOI] [PubMed] [Google Scholar]
- 21.Moe MC, Kolberg RS, Sandberg C, et al. A comparison of epithelial and neural properties in progenitor cells derived from the adult human ciliary body and brain. Exp Eye Res. 2009;88:30–38. doi: 10.1016/j.exer.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Jasty S, Srinivasan P, Pasricha G, Chatterjee N, Subramanian K. Gene expression profiles and retinal potential of stem/progenitor cells derived from human iris and ciliary pigment epithelium. Stem Cell Rev. 2012;8:1163–1177. doi: 10.1007/s12015-012-9394-3. [DOI] [PubMed] [Google Scholar]
- 23.Ballios BG, Clarke L, Coles BL, Shoichet MS, Van Der Kooy D. The adult retinal stem cell is a rare cell in the ciliary epithelium whose progeny can differentiate into photoreceptors. Biol Open. 2012;1:237–246. doi: 10.1242/bio.2012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Froen R, Johnsen EO, Nicolaissen B, Facsko A, Petrovski G, Moe MC. Does the adult human ciliary body epithelium contain “true” retinal stem cells? BioMed Res Int. 2013;2013:531579. doi: 10.1155/2013/531579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gualdoni S, Baron M, Lakowski J, et al. Adult ciliary epithelial cells, previously identified as retinal stem cells with potential for retinal repair, fail to differentiate into new rod photoreceptors. Stem Cells. 2010;28:1048–1059. doi: 10.1002/stem.423. [DOI] [PubMed] [Google Scholar]
- 26.Froen RC, Johnsen EO, Petrovski G, et al. Pigment epithelial cells isolated from human peripheral iridectomies have limited properties of retinal stem cells. Acta Ophthalmol. 2011;89:e635. doi: 10.1111/j.1755-3768.2011.02198.x. –e. [DOI] [PubMed] [Google Scholar]
- 27.Cicero SA, Johnson D, Reyntjens S, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci U S A. 2009;106:6685–6690. doi: 10.1073/pnas.0901596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergara MN, Del Rio-Tsonis K. Retinal regeneration in the Xenopus laevis tadpole: A new model system. Mol Vis. 2009;15:1000–1013. [PMC free article] [PubMed] [Google Scholar]
- 29.Islam MR, Nakamura K, Casco-Robles MM, et al. The newt reprograms mature RPE cells into a unique multipotent state for retinal regeneration. Scientific Rep. 2014;4:6043. doi: 10.1038/srep06043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbosa-Sabanero K, Hoffmann A, Judge C, Lightcap N, Tsonis PA, Del Rio-Tsonis K. Lens and retina regeneration: New perspectives from model organisms. Biochem J. 2012;447:321–334. doi: 10.1042/BJ20120813. [DOI] [PubMed] [Google Scholar]
- 31.Okada TS. Cellular metaplasia or transdifferentiation as a model for retinal cell differentiation. Curr Topics Dev Biol. 1980;16:349–380. [PubMed] [Google Scholar]
- 32.Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: A view from the eye. Cell Stem Cell. 2008;2:538–549. doi: 10.1016/j.stem.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelhardt M, Bogdahn U, Aigner L. Adult retinal pigment epithelium cells express neural progenitor properties and the neuronal precursor protein doublecortin. Brain Res. 2005;1040:98–111. doi: 10.1016/j.brainres.2005.01.075. [DOI] [PubMed] [Google Scholar]
- 34.Al-Hussaini H, Kam JH, Vugler A, Semo M, Jeffery G. Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Mol Vis. 2008;14:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 35.Saini JS, Temple S, Stern JH. Human Retinal Pigment Epithelium Stem Cell (RPESC) Adv Exp Med Biol. 2016;854:557–562. doi: 10.1007/978-3-319-17121-0_74. [DOI] [PubMed] [Google Scholar]
- 36.Salero E, Blenkinsop TA, Corneo B, et al. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell. 2012;10:88–95. doi: 10.1016/j.stem.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res. 2004;23:183–194. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackshaw S, Harpavat S, Trimarchi J, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roesch K, Jadhav AP, Trimarchi JM, et al. The transcriptome of retinal Müller glial cells. J Comp Neurol. 2008;509:225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannelli SG, Demontis GC, Pertile G, Rama P, Broccoli V. Adult human Müller glia cells are a highly efficient source of rod photoreceptors. Stem Cells. 2011;29:344–356. doi: 10.1002/stem.579. [DOI] [PubMed] [Google Scholar]
- 43.Bhatia B, Singhal S, Lawrence JM, Khaw PT, Limb GA. Distribution of Müller stem cells within the neural retina: evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp Eye Res. 2009;89:373–382. doi: 10.1016/j.exer.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 45.Jayaram H, Jones MF, Eastlake K, et al. Transplantation of photoreceptors derived from human Müller glia restore rod function in the P23H rat. Stem Cells Transl Med. 2014;3:323–333. doi: 10.5966/sctm.2013-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blenkinsop TA, Corneo B, Temple S, Stern JH. Ophthalmologic stem cell transplantation therapies. Regen Med. 2012;7:32–39. doi: 10.2217/rme.12.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: past, present and future. Development. 2013;140:2576–2585. doi: 10.1242/dev.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng TK, Fortino VR, Pelaez D, Cheung HS. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells. 2014;6:111–119. doi: 10.4252/wjsc.v6.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klassen H. Stem cells in clinical trials for treatment of retinal degeneration. Expert Opin Biol Ther. 2015. pp. 1–8. [DOI] [PubMed]
- 50.Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25:602–611. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- 51.Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial. Retina. 2011;31:1207–1214. doi: 10.1097/IAE.0b013e3181f9c242. [DOI] [PubMed] [Google Scholar]
- 52.Siqueira RC, Messias A, Messias K, et al. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell clinical trial) Stem Cell Res Ther. 2015;6:29. doi: 10.1186/s13287-015-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park SS, Bauer G, Abedi M, et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci. 2015;56:81–89. doi: 10.1167/iovs.14-15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banin E, Obolensky A, Idelson M, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006;24:246–257. doi: 10.1634/stemcells.2005-0009. [DOI] [PubMed] [Google Scholar]
- 55.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 57.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buchholz DE, Pennington BO, Croze RH, Hinman CR, Coffey PJ, Clegg DO. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med. 2013;2:384–393. doi: 10.5966/sctm.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Maruotti J, Wahlin K, Gorrell D, Bhutto I, Lutty G, Zack DJ. A simple and scalable process for the differentiation of retinal pigment epithelium from human pluripotent stem cells. Stem Cells Transl Med. 2013;2:341–354. doi: 10.5966/sctm.2012-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4:811–824. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- 62.Croze RH, Clegg DO. Differentiation of pluripotent stem cells into retinal pigmented epithelium. Develop Ophthalmol. 2014;53:81–96. doi: 10.1159/000357361. [DOI] [PubMed] [Google Scholar]
- 63.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meyer JS, Howden SE, Wallace KA, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–1218. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis. 2012;18:920–936. [PMC free article] [PubMed] [Google Scholar]
- 67.Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 68.Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 69.Vugler A, Carr AJ, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exper Neurol. 2008;214:347–361. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Hu Y, Liu L, Lu B, et al. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Res. 2012;48:186–191. doi: 10.1159/000338749. [DOI] [PubMed] [Google Scholar]
- 71.Diniz B, Thomas P, Thomas B, et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci. 2013;54:5087–5096. doi: 10.1167/iovs.12-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forest DL, Johnson LV, Clegg DO. Cellular models and therapies for age-related macular degeneration. Dis Models Mechanisms. 2015;8:421–427. doi: 10.1242/dmm.017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 74.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 76.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 77.Tanabe K, Takahashi K, Yamanaka S. Induction of pluripotency by defined factors. Proc Jap Acad Ser B Phys Biol Sci. 2014;90:83–96. doi: 10.2183/pjab.90.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rony IK, Baten A, Bloomfield JA, Islam ME, Billah MM, Islam KD. Inducing pluripotency in vitro: recent advances and highlights in induced pluripotent stem cells generation and pluripotency reprogramming. Cell Prolif. 2015;48:140–156. doi: 10.1111/cpr.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silva M, Daheron L, Hurley H, et al. Generating iPSCs: translating cell reprogramming science into scalable and robust biomanufacturing strategies. Cell Stem Cell. 2015;16:13–17. doi: 10.1016/j.stem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 80.Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 81.Osakada F, Jin ZB, Hirami Y, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–3179. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]
- 82.Lamba DA, McUsic A, Hirata RK, Wang P-R, Russell D, Reh TA. Generation purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DHW, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells. 2012;30:673–686. doi: 10.1002/stem.1037. [DOI] [PubMed] [Google Scholar]
- 84.Boucherie C, Mukherjee S, Henckaerts E, Thrasher AJ, Sowden JC, Ali RR. Brief report: self-organizing neuroepithelium from human pluripotent stem cells facilitates derivation of photoreceptors. Stem Cells. 2013;31:408–414. doi: 10.1002/stem.1268. [DOI] [PubMed] [Google Scholar]
- 85.Reichman S, Terray A, Slembrouck A, et al. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci U S A. 2014;111:8518–8523. doi: 10.1073/pnas.1324212111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Phillips MJ, Wallace KA, Dickerson SJ, et al. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012;53:2007–2019. doi: 10.1167/iovs.11-9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhong X, Gutierrez C, Xue T, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–2434. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- 89.Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011;29:825–835. doi: 10.1002/stem.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh R, Shen W, Kuai D, et al. iPS cell modeling of Best disease: Insights into the pathophysiology of an inherited macular degeneration. Hum Mol Genet. 2013;22:593–607. doi: 10.1093/hmg/dds469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y, Tsai YT, Hsu CW, et al. Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa. Molec Med. 2012;18:1312–1319. doi: 10.2119/molmed.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamao H, Mandai M, Okamoto S, et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014;2:205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cyranoski D. Stem cells cruise to clinic. Nature. 2013;494:413. doi: 10.1038/494413a. [DOI] [PubMed] [Google Scholar]
- 95.Cyranoski D. Next-generation stem cells cleared for human trial. Nature. doi: 10.1038/nature.2014.15897. [DOI]
- 96.Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33:890–891. doi: 10.1038/nbt0915-890. [DOI] [PubMed] [Google Scholar]
- 97.Borooah S, Phillips MJ, Bilican B, et al. Using human induced pluripotent stem cells to treat retinal disease. Prog Retin Eye Res. 2013;37:163–181. doi: 10.1016/j.preteyeres.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Wu WH, Hsu CW, et al. Gene therapy in patient-specific stem cell lines and a preclinical model of retinitis pigmentosa with membrane frizzled-related protein defects. Mol Ther. 2014;22:1688–1697. doi: 10.1038/mt.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng A, Li Y, Tsang SH. Personalized therapeutic strategies for patients with retinitis pigmentosa. Expert Opin Biol Ther. 2015;15:391–402. doi: 10.1517/14712598.2015.1006192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Capowski EE, Simonett JM, Clark EM, et al. Loss of MITF expression during human embryonic stem cell differentiation disrupts retinal pigment epithelium development and optic vesicle cell proliferation. Hum Mol Genet. 2014;23:6332–6344. doi: 10.1093/hmg/ddu351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Phillips MJ, Perez ET, Martin JM, et al. Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells. 2014;32:1480–1492. doi: 10.1002/stem.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 104.Cherry AB, Daley GQ. Reprogrammed cells for disease modeling and regenerative medicine. Annu Rev Med. 2013;64:277–290. doi: 10.1146/annurev-med-050311-163324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Young MA, Larson DE, Sun CW, et al. Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells. Cell Stem Cell. 2012;10:570–582. doi: 10.1016/j.stem.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li M, Suzuki K, Kim NY, Liu GH. Izpisua Belmonte JC. A cut above the rest: targeted genome editing technologies in human pluripotent stem cells. J Biol Chem. 2014;289:4594–4599. doi: 10.1074/jbc.R113.488247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gourraud PA, Gilson L, Girard M, Peschanski M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells. 2012;30:180–186. doi: 10.1002/stem.772. [DOI] [PubMed] [Google Scholar]
- 108.Pitossi FJ, Podhajcer OL. Current status of stem cells and regenerative medicine research in Argentina. Stem Cells Develop. 2014;23((suppl 1):17–19. doi: 10.1089/scd.2014.0403. [DOI] [PubMed] [Google Scholar]
- 109.Barry J, Hyllner J, Stacey G, Taylor CJ, Turner M. Setting up a haplobank: issues and solutions. Curr Stem Cell Rep. 2015;1:110–117. doi: 10.1007/s40778-015-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilmut I, Leslie S, Martin NG, et al. Development of a global network of induced pluripotent stem cell haplobanks. Regen Med. 2015;10:235–238. doi: 10.2217/rme.15.1. [DOI] [PubMed] [Google Scholar]


