Abstract
Cardiac electrophysiology has progressed in great strides since the electrical activity of the heart was first discovered in 1842 and documented using electrocardiography. Optical imaging of cardiac electrophysiology, or optocardiography, has seen many advances in recent years including panoramic imaging of the heart, alternating transillumination to image transmural electrical activity, optogenetic models and customizable 3D printed optical mapping systems. Most of these techniques were adopted from other fields of study and refined for cardiac electrophysiology purposes. The future of this field could see similar adaptations of photoacoustic tomography, structured light technology and optical coherence tomography contributing to optocardiography.
Past Advances: Origins of Cardiac Electrophysiology
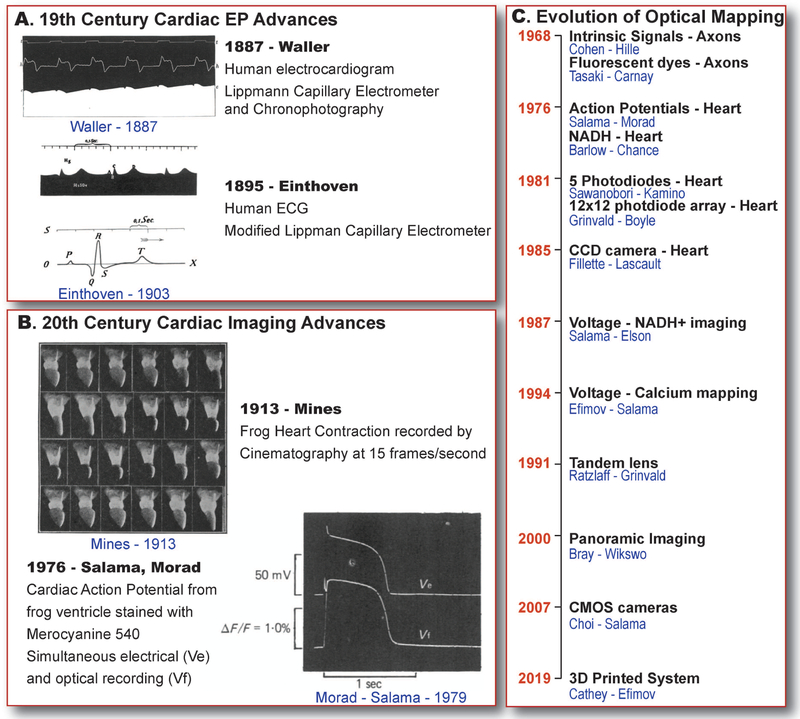
Each heart beat is initiated by an electrical excitation, followed by an increase in intracellular calcium concentration, triggering mechanical contraction that pumps blood from the heart to the rest of the body. The electrical aspect of cardiac function was first identified in 1842 by Italian Physicist, Carlo Matteucci, who discovered that each frog heart beat was accompanied by an electrical current [1]. Then, in 1856, Rudolf Albert von Koelliker along with Johannes Muller, recorded the cardiac “action potentials”, which were current fluctuations in a spontaneously beating heart [2]. Two decades later, in 1876, French physiologist, Étienne-Jules Marey recorded the first electrocardiogram (ECG) from a frog heart using a Lippmann capillary electrometer [2]. Interestingly, Marey is also credited to be the inventor of cinematography and he developed a technique called chronophotography, to capture motion. He combined his work on cardiac electrophysiology and cinematography which could have been the initiation of the field of optocardiography. Later, in 1887, Augustus Desiré Waller recorded the first human ECG from the body surface using the Lippmann capillary electrometer (Figure 1A, Top) [3]. In 1895, Willem Einthoven refined the string galvanometer technique and recorded ECGs with the five distinct and now familiar deflections that he designated as P, Q, R, S and T waves (Figure 1A, Bottom); these notations are standard today [4]. Moving on to the 20th century, George Ralph Mines recorded the contraction of frog hearts using a custom-built cinematography system in 1913. Recordings were acquired at 15 images per second (Figure 1B, Top) [5]. Up to this point, cinematography was primarily implemented only to study cardiac contraction. In the second half of the 20th century, the use of imaging techniques to record cardiac electrophysiology was developed.
Figure 1. Cardiac Electrophysiology Imaging – Past.
History of cardiac electrophysiology discoveries and inventions. A) First human electrogram recorded by Waller in 1887 [3] and Einthoven in 1895 [4]. B) Frog heart contraction recorded by Mines in 1913 [5] using cinematography and first recorded cardiac action potential from frog hearts by Salama and Morad [7]. C) Timeline of developments in optical mapping adapted from Cathey et al [8].
Present Advances
Optical imaging of electrophysiology was first reported by Cohen et al. in 1968, where axon electrophysiology was studied by imaging of intrinsic signals that correlated with the cellular electrical activity [6]. Cardiac action potential was first optically recorded in 1976 by Salama and Morad [7] (Figure 1B, Bottom). The next few decades saw significant advances in optical mapping techniques which included development of new fluorescent dyes, camera technology, multiple parameter imaging and hardware advances as detailed in our recent publication [8] and adapted in Figure 1C. A few of the more recent advances in this field are discussed below.
Cardiac experimental models:
Langendorff-perfused intact hearts, isolated atria and ventricles have been in use in optical mapping studies for decades. Wedge preparations from various regions of dog, swine, sheep and human hearts, such as the left and right atria and ventricles, sinoatrial (SA) node, atrioventricular (AV) node and right ventricular outflow tract (RVOT) have allowed the detailed study of the electrophysiology specific to these anatomical regions. This has given rise to various mechanistic discoveries such as dual pathway electrophysiology of AV node [9–11] and exit pathways of SA nodes [12]. However, one disadvantage of these acute tissue models is the inability to perform electrophysiology studies over extended periods of time. This is where long-term culturing of cardiac myocytes came into use. Not only do 2-dimensional (2D) cell cultures allow long term studies, but they also allow investigation of specific cell types and furthermore allows patient-specific electrophysiological testing using human induced pluripotent stem cell (hiPSC) derived cardiomyocytes (CM). However, 2D hiPSC-CM cultures failed so far, to reproduce key structural and functional properties of adult human CM, which are important in excitation-contraction coupling, such as the ability to form intracellular and intercellular structures: intercalated discs, cellular ion channels localization and transverse tubules. The validation of the human organotypic cardiac slice preparations overcame some of these limitations. Slices collected from various regions of the human heart can be cultured over several days [13] and current protocols in our laboratory allows for reproducible recordings for up to seven days. These 3D cardiac tissue models with native extracellular space composition and intercellular connections, could potentially be a new pre-clinical platform to test the effects of drugs on cardiac electrophysiology, over extended periods of time, in healthy and pathological human tissue.
3D printing in optical mapping:
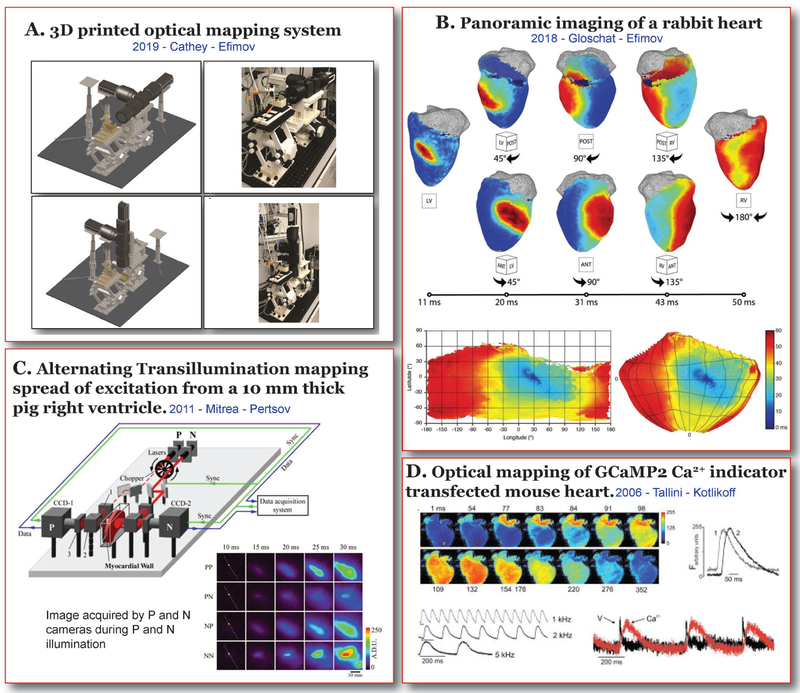
The wide range of applications within the realm of optocardiography requires specialized and expensive hardware for implementation. Recent studies [8] have identified that 3D printing of some of the hardware can reduce the overall cost of expensive equipment such as microscopes and macroscopes used in optical mapping, excluding cameras and optics. Our recent study demonstrates that a fully 3D printed system, including perfusion, and optomechanical components, can be used to build as a customized, low-cost, multiparametric, tandem lens optical mapping system that functions in both horizontal imaging mode for Langendorff-perfused hanging whole heart preparations, as well as in the upright imaging mode for various isolated cardiac preparations such as organotypic slice preparations or cell cultures as illustrated in Figure 2A. These components supported the cameras, filters and lenses and was validated to be just as functionally precise as commercially available systems. The greatest advantage of this approach lies in the ability to design an optical mapping system around an application rather than the application around a commercially available system. The customizability also allows the user with minimum engineering skills to expand the system to keep up with recent and future advances in this technique.
Figure 2. Cardiac Electrophysiology Imaging – Present.
Recent advances in Cardiac Electrophysiology Imaging. A) 3D printed dual parameter, tandem lens optical mapping system in horizontal and upright imaging modes (Cathey et al. [8]). B) Activation maps obtained by panoramic imaging of a rabbit heart (Gloschat et al. [16]). C) Alternating transillumination set up and excitation wave propagation recorded from a 10 mm thick human right ventricular preparation (Mitrea et al. [17]). D) Activation maps and calcium transient traces from a GCaMP2 transfected optogenetic mouse. (Tallini et al. [26]).
Panoramic Imaging:
Traditional optical mapping allows the study of one surface of the heart. While this can provide valuable information about cardiac electrophysiology and arrhythmias, it lacks the spatial bandwidth to study complex arrhythmias that may occur away from the field of view. The concept of panoramic imaging was first introduced in 2000 [14], where two mirrors and a single camera were used to capture multiple surfaces of the heart. Later in 2004, Kay et al, described a more refined method of panoramic optical mapping using four CCD cameras [15]. Recent studies [16] from our group has further modified this technique (Figure 2A) to image cardiac electrophysiology of mouse, rat and rabbit hearts (Figure 2B). Another key impediment in the advancement of this field was the unavailability of analysis software to handle large amounts of data from four cameras. We recently published RHYTHM2.0, an open source analysis platform for panoramic mapping data [16].
Alternating Transillumination:
While panoramic or epi/endo imaging allows for the mapping of multiple surfaces of the heart, it does not fully capture the complex three-dimensional (3D), transmural patterns of arrhythmias. The 3D structure of the heart and its transmural contribution to electrophysiology is another important aspect of arrhythmia study. Transmural mapping was previously accomplished by imaging of cut transmural surfaces of the heart. However, the mechanical damage and electrical uncoupling induced by this process has to be considered.
Transillumination is a technique that is commonly implemented in the clinics where a light source is shined through an organ or body cavity to study the structure of the tissue. A recent advancement in this field proposed a novel optical imaging technique called alternating transillumination [17,18]. This method involves the use of two cameras that are placed opposite to each other and image opposing surfaces of cardiac wedge preparations. Two rapidly alternating light sources illuminated the heart from either side, while the reflection and transillumination images were simultaneously obtained from the two surfaces. This allows for the detection of earliest points of excitation wavefronts and focal sources within the depth of the ventricular walls (Figure 2C) before it reaches the epicardial or endocardial surfaces. This technique could prove very useful in the study of arrhythmic foci and 3D reentry pathways, including scroll waves.
Optrodes:
Optrodes (derived from optical electrode) was developed as another technology that allows transmural imaging of cardiac tissue. Initial application of optrodes in cardiac imaging involved surface imaging without the need for electromechanical uncoupling of the tissue [19]. This was accomplished by positioning the optrode over the cardiac surface and applying suction to attach the optrode to the heart surface such that the optrode moved with area being imaged. Later, this technique was further modified to accommodate transmural imaging, whereby a bundle of optical fibers were arranged in a single optrode such that when inserted into the cardiac wall, it excited and collected light at different transmural depths [20,21]. Based on optrode design and number of optical fibers, this technology could allow detailed transmural imaging without the need for cut surfaces.
Optogenetics:
Optogenetics involves the use of optically active proteins, either as actuators or reporters, genetically engineered and directed to cells of interest, to study cellular processes. Actuator or sensor photoreceptor proteins undergo a conformational or energy transition when excited by light at specific wavelengths, which could then produce a functional response, such as opening an ion channel inducing either depolarization or hyperpolarization depending on the associated channel. Reporter photoreceptor proteins, when excited by light at a specific wavelength, emits light that corresponds to cellular processes such as changes in transmembrane voltage, intracellular calcium, pH etc.
Optogenetics was first introduced in 1988 to depolarize oocytes [22], while the field of cardiac electrophysiology adopted this technique more recently, in 2010 [23]. The first cardiac optogenetic model was a mouse with a channelrhodopsin-2 (ChR2) protein, which, when excited by a blue light depolarized the myocytes, producing an action potential. ChR2 channels associated with chloride currents have also been implemented to hyperpolarize and arrest the heart [24]. On the reporter side, several generations of GCaMP protein mice, from GCaMP2 (Figure 2D) [25,26] to currently available GCaMP8, and mice with voltage-sensitive fluorescent proteins (VSFPs) have been developed to record cellular calcium waves and transmembrane potential changes [27], respectively. These optogenetic mice not only allow for optical mapping without the use of dyes, but also allows cell type-specific electrophysiological studies and interactions between different cardiac cell types. For example, myocyte – non-myocyte interactions at the infarct border zone has been studied by expression of VSFP2.3 in non-myocyte cardiac cells [28].
Future Directions:
Optocardiography (cardiac electrophysiology imaging) has come a long way since the first action potential was recorded from frog hearts. In many small steps, it has advanced to accommodate the growing needs of cardiac electrophysiology studies. To conclude this review, we present here, three further optical techniques for potential cardiac imaging applications.
Photoacoustic Tomography:
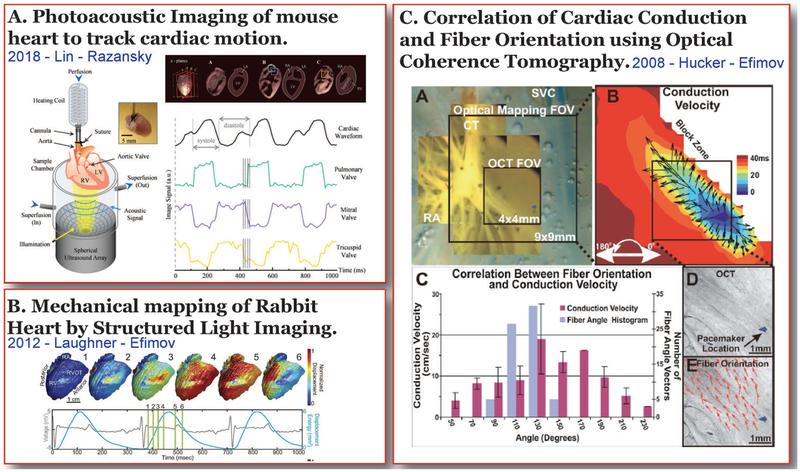
Photoacoustic tomography implements rapid optical stimulation of thermoelastic expansion and acoustic detection of a resulting sound to record structural and functional dynamics in tissues [29]. Photoacoustic tomography is a suitable imaging modality for applications that require high spatial and temporal resolutions as well as greater depth of imaging. Photoacoustic imaging was primarily developed as a non-invasive technique to detect tissue structures such as blood vessels or tumors, it has also been used in hemodynamic studies [30] and more recently to map seizure foci and propagation in rat brains [31,32]. In the field of cardiac physiology, photoacoustic tomography has been recently implemented in the study of four dimensional cardiac physiology, which includes volumes of structures across the entire mouse heart as well as cardiac motion (Figure 3A) [33]. Cardiac motion tracking using photoacoustic tomography could greatly improve the scope of conventional electrophysiology methods by allowing the study of cardiac electrical and mechanical function without isolating one from the other. This study also reported greatly improved spatial resolution of up to 150 mM and temporal resolution of about 100 Hz. Although this technology in its current form may not have the temporal resolution [31,33] necessary for cardiac electrophysiology imaging, it has the advantage of greater depth penetration in tissue.
Figure 3. Cardiac Electrophysiology Imaging – Future.
Optical imaging techniques with potential cardiac electrophysiology applications. A) Photoacoustic tomography set up that was implemented to obtain 4D cardiac volume and structural motion data from a whole mouse heart [33]. B) High resolution mechanical mapping of a rabbit heart during sinus rhythm using structured light imaging [35]. C) Optical coherence tomography used to detect cardiac fiber orientation in conjunction with optical mapping to determine cardiac conduction [37].
Structured Light Imaging:
The electrical and mechanical aspects of cardiac function are interdependent parts of excitation-contraction coupling, yet separately studied. This is because the optically recorded voltage signals from the heart are distorted by motion, and chemical electromechanical uncoupling or immobilization of the heart is necessary during optical mapping. Another technique implemented to remove motion artifacts during optical mapping involves the use of fiducial markers that are placed on the surface of the heart and its motion over a cardiac cycle is tracked to identify cardiac deformation [34]. Another approach to address this need involves a combination of structured light imaging and optical mapping by using heart surface textures to track deformation instead of fiducial markers [35].
Structured light imaging is used to dynamically image 3D shapes with a high spatial and temporal resolution. It is commonly used in industrial shape detection, security biometrics, rehabilitation medicine, entertainment purposes, and in cardiac physiology, for high resolution mechanical mapping of the heart. It utilizes a digital light processing (DLP) projector and a camera. The DLP projector projects a known texture pattern onto a 3D surface, which deforms this pattern. This deformed pattern is then detected by the second camera and the 3D surface is then digitally reconstructed with high spatial and temporal resolution[35]. However, the reconstruction of the deformation patterns is based on a set of assumptions similar to motion tracking using fiducial markers. But the current advancements in this technology has allowed for high resolution mapping of tissue deformation (unpublished) and structured light imaging could have potential use in cardiac deformation tracking during optical mapping to remove motion-induced artifacts in the optical signals.
Optical Coherence Tomography:
Another imaging tool that is implemented for structural analysis in the clinical setting is optical coherence tomography (OCT) [36]. In cardiac research, the high spatial resolution of OCT allows the detailed study of cardiac structures such as vasculature, conduction system and fiber orientation with 2–3 mm depths of penetration [37–41]. This technique in conjunction with other electrophysiological mapping methods can give crucial information regarding cardiac conduction properties and arrhythmias. For example, cardiac tissue structure near a region of infarction or 3D rotational anisotropy of cardiac fibers can be detected using OCT which can then aid in determining conduction properties and arrhythmia propensities due to these structures [37]. However, one major drawback of this technique is its relatively low depth of penetration as compared to competing imaging modalities: ultrasound and MRI.
Conclusion:
The field of optocardiography is rapidly evolving to meet the demands of newly gained knowledge in this field. Most techniques used in studies of cardiac electrophysiology were adopted and refined from other fields of study. As the saying goes, “necessity is the mother of inventions”; improvements in current technology and novel techniques will guide the future direction of this field.
HIGHLIGHTS:
The scope of this review covers almost 2 centuries of advancements in the field of cardiac electrophysiology and its imaging.
A brief history of the origins of cardiac electrophysiology and latest advancements in this field are included.
The potential of some recent advances in imaging technology, that could be adapted to improve cardiac electrophysiology imaging is detailed here.
FUNDING:
This project was supported by the Leducq Foundation [Project RHYTHM], National Institutes of Health [R21 EB023106, R01 HL126802, and R01 HL114395] to IRE and American Heart Association Postdoctoral Fellowship to SAG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.AlGhatrif M, Lindsay J: A brief review: history to understand fundamentals of electrocardiography. J Community Hosp Intern Med Perspect 2012, 2:14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins D: ECG timeline - History of the electrocardiogram. 2009. https://ecglibrary.com/ecghist.html. Last acessed: 02/12/2019.
- 3.Waller AD: A Demonstration on Man of Electromotive Changes accompanying the Heart’s Beat. J Physiol 1887, 8:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kligfield P: Derivation of the correct waveform of the human electrocardiogram by Willem Einthoven, 1890–1895. Cardiol J 2010, 17:109–113. [PubMed] [Google Scholar]
- 5.Mines GR: On Functional Analysis by Action of Electrolytes. J Physiol 1913, 19:188–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen LB, Keynes RD, Hille B: Light scattering and birefringence changes during nerve activity. Nature 1968, 218:438–441. [DOI] [PubMed] [Google Scholar]
- 7.Salama G, Morad M: Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science 1976, 191:485–487. [DOI] [PubMed] [Google Scholar]
- 8.Cathey B, Obaid S, Zolotarev AM, Pryamonosov RA, Syunyaev RA, George SA, Efimov IR: Open-Source Multiparametric Optocardiography. Sci Rep 2019, 9:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George SA, Faye NR, Murillo-Berlioz A, Lee KB, Trachiotis GD, Efimov IR: At the Atrioventricular Crossroads: Dual Pathway Electrophysiology in the Atrioventricular Node and its underlying Heterogeneities. Arrhythmia Electrophysiol Rev 2017, 6:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedorov VV, Ambrosi CM, Kostecki G, Hucker WJ, Glukhov AV, Wuskell JP, Loew LM, Moazami N, Efimov IR: Anatomic localization and autonomic modulation of atrioventricular junctional rhythm in failing human hearts. Circ Arrhythmia Electrophysiol 2011, 4:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hucker WJ, Nikolski VP, Efimov IR: Optical mapping of the atrioventricular junction. In Journal of Electrocardiology 2005, 38:121–125. [DOI] [PubMed] [Google Scholar]
- 12.Fedorov VV, Glukhov AV, Chang R, Kostecki G, Aferol H, Hucker WJ, Wuskell JP, Loew LM, Schuessler RB, Moazami N, et al. : Optical mapping of the isolated coronary-perfused human sinus node. J Am Coll Cardiol 2010, 56:1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang C, Qiao Y, Li G, Baechle K, Camelliti P, Rentschler S, Efimov IR: Human Organotypic Cultured Cardiac Slices: New Platform For High Throughput Preclinical Human Trials. Sci Rep 2016, 6:28798.* This is the first study to report the use of a novel human heart organotypic slice preparation to study cardiac electrophysiology. The slices were optically mapped soon after slicing protocol or after culturing for 24 hours. This could be a potential preclinical platform for testing the effects of drugs on human cardiac tissues.
- 14.Bray MA, Lin SF, Wikswo JP Jr: Three-dimensional surface reconstruction and fluorescent visualization of cardiac activation. IEEE Trans Biomed Eng 2000, 47:1382–1391. [DOI] [PubMed] [Google Scholar]
- 15.Kay MW, Amison PM, Rogers JM: Three-dimensional surface reconstruction and panoramic optical mapping of large hearts. IEEE Trans Biomed Eng 2004, 51:1219–1229. [DOI] [PubMed] [Google Scholar]
- 16.Gloschat C, Aras K, Gupta S, Faye NR, Zhang H, Syunyaev RA, Pryamonosov RA, Rogers J, Kay MW, Efimov IR: RHYTHM: An Open Source Imaging Toolkit for Cardiac Panoramic Optical Mapping. Sci Rep 2018, 8:2921.* This study describes the panoramic imaging of mouse, rat and rabbit hearts using four cameras. An open source software for analysis of large data that is obtained form panoramic imaging was also published along with this study.
- 17.Mitrea BG, Caldwell BJ, Pertsov AM: Imaging electrical excitation inside the myocardial wall. Biomed Opt Express 2011, 2:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walton RD, Xavier CDL, Tachtsidis I, Bernus O: Experimental validation of alternating transillumination for imaging intramural wave propagation. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 2011 2011:1676–1679. [DOI] [PubMed] [Google Scholar]
- 19.Neunlist M, Zou S zhou, Tung L: Design and use of an “optrode” for optical recordings of cardiac action potentials. Pflügers Arch Eur J Physiol 1992, 420:611–617. [DOI] [PubMed] [Google Scholar]
- 20.Byars JL, Smith WM, Ideker RE, Fast VG: Development of an Optrode for Intramural Multisite Optical Recordings of Vm in the Heart. J Cardiovasc Electrophysiol 2003, 14: 1196–1202. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell BJ, Legrice IJ, Hooks DA, Tai DCS, Pullan AJ, Smaill BH: Intramural measurement of transmembrane potential in the isolated pig heart: Validation of a novel technique. J Cardiovasc Electrophysiol 2005, 16:1001–1010. [DOI] [PubMed] [Google Scholar]
- 22.Khorana HG, Knox BE, Nasi E, Swanson R, Thompson DA: Expression of a bovine rhodopsin gene in Xenopus oocytes: demonstration of light-dependent ionic currents. Proc Natl Acad Sci U S A 1988, 85:7917–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, Fleischmann BK, Sasse P: Optogenetic control of heart muscle in vitro and in vivo. Nat Methods 2010, 7:897–900. [DOI] [PubMed] [Google Scholar]
- 24.Arrenberg AB, Stainier DYR, Baier H, Huisken J: Optogenetic control of cardiac function. Science 2010, 330:971–4. [DOI] [PubMed] [Google Scholar]
- 25.Shang W, Lu F, Sun T, Xu J, Li LL, Wang Y, Wang G, Chen L, Wang X, Cannell MB, et al. : Imaging Ca2+ nanosparks in heart with a new targeted biosensor. Circ Res 2014, 114:412–420. [DOI] [PubMed] [Google Scholar]
- 26.Tallini YN, Ohkura M, Choi B-R, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin H-B, et al. : Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci 2006, 103:4753–4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao MLC, De Boer TP, Mutoh H, Raad N, Richter C, Wagner E, Downie BR, Unsöld B, Arooj I, Streckfuss-Bömeke K, et al. : Sensing Cardiac Electrical Activity with a Cardiac Myocyte-Targeted Optogenetic Voltage Indicator. Circ Res 2015, 117:401–412. [DOI] [PubMed] [Google Scholar]
- 28.Quinn TA, Camelliti P, Rog-Zielinska EA, Siedlecka U, Poggioli T, O’Toole ET, Knöpfel T, Kohl P: Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc Natl Acad Sci 2016, 113:14852–14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia J, Yao J, Wang LHV.: Photoacoustic Tomography: Priniples and Advances. Prog Electromagn Res 2014, 147:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luke GP, Yeager D, Emelianov SY: Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann Biomed Eng 2012, 40:422–437. [DOI] [PubMed] [Google Scholar]
- 31.Xiang L, Ji L, Zhang T, Wang B, Yang J, Zhang Q, Jiang MS, Zhou J, Carney PR, Jiang H: Noninvasive real time tomographic imaging of epileptic foci and networks. Neuroimage 2013, 66:240–248. [DOI] [PubMed] [Google Scholar]
- 32.Wang B, Xiao J, Jiang H: Simultaneous real-time 3D photoacoustic tomography and EEG for neurovascular coupling study in an animal model of epilepsy. J Neural Eng 2014, 11:046013. [DOI] [PubMed] [Google Scholar]
- 33.Lin HA, Dean-Ben XL, Reiss M, Schottle V, Wahl-Schott CA, Efimov IR, Razansky D: Ultrafast Volumetric Optoacoustic Imaging of Whole Isolated Beating Mouse Heart. Sci Rep 2018, 20:14132.* This technique for the first time provides 4D imaging of an isolated mouse heart which includes fine cardiac strucutral and motion imaging. This technique allowed for hish spatial resolution at great depths (in centimenters) allowing the images of structres such as the interventricular septum.
- 34.Zhang H, Iijima K, Huang J, Walcott GP, Rogers JM: Optical Mapping of Membrane Potential and Epicardial Deformation in Beating Hearts. Biophys J 2016, 111:438–451.* This study uses ratiometric optical mapping techniques and 3D motion tracking simultaneously to map isolated pig hearts. While the combination of these two techniques have previously been implemented to record electrical signals from the heart without the use of a chemical electromechanical uncoupler, this study takes the approach one step further to analyze excitation-contraction coupling - transmembrane potential changes and cardiac deformation.
- 35.Laughner JI, Zhang S, Li H, Shao CC, Efimov IR: Mapping cardiac surface mechanics with structured light imaging. AJP Hear Circ Physiol 2012, 303:H712–H720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. : Optical coherence tomography. Science 1991, 254:1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hucker WJ, Ripplinger CM, Fleming CP, Fedorov VV., Rollins AM, Efimov IR: Bimodal biophotonic imaging of the structure-function relationship in cardiac tissue. J Biomed Opt 2008, 13:054012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ambrosi CM, Fedorov VV., Schuessler RB, Rollins AM, Efimov IR: Quantification of fiber orientation in the canine atrial pacemaker complex using optical coherence tomography. J Biomed Opt 2012, 17:071309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tearney GJ, Brezinski ME, Boppart SA, Bouma BE, Weissman N, Southern JF, Swanson EA, Fujimoto JG: Catheter-based optical imaging of a human coronary artery. Circulation 1996, 94:3013. [DOI] [PubMed] [Google Scholar]
- 40.Gupta M, Rollins AM, Izatt JA, Efimov IR: Imaging of the atrioventricular node using optical coherence tomography. J Cardiovasc Electrophysiol 2002, 13:95. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins M, Wade RS, Cheng Y, Rollins AM, Efimov IR: Optical coherence tomography imaging of the purkinje network. J Cardiovasc Electrophysiol 2005, 16:559–560. [DOI] [PubMed] [Google Scholar]