Abstract
Compared to normal tissues, the tumor microenvironment (TME) has a number of aberrant characteristics including hypoxia, acidosis, and vascular abnormalities. Many researchers have sought to exploit these anomalous features of the TME to develop anticancer therapies, and several nanoparticle-based cancer therapeutics have resulted. In this Review, we discuss the composition and pathophysiology of the TME, introduce nanoparticles (NPs) used in cancer therapy, and address the interaction between the TME and NPs. Finally, we outline both the potential problems that affect TME-based nanotherapy and potential strategies to overcome these challenges.
Graphical Abstract
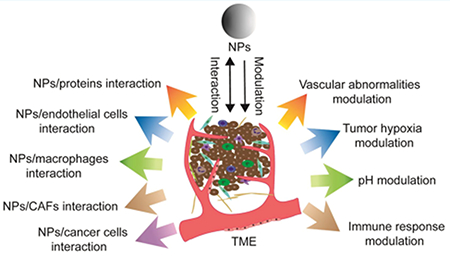
Cancer remains a leading cause of death worldwide, despite significant ongoing efforts to develop effective treatments. Nanotechnology is increasingly a focus of investigations in the biomedical arena, with the intent of improving diagnostic and therapeutic interventions for many disease states, including cancer. Many nanomaterials, with differing compositions, shapes, sizes, and functions, have been developed1–5 and have demonstrated value in drug delivery,6,7 imaging,8,9 vaccine development,10,11 and diagnostics,9 and as therapeutic agents.12 To date, several nanomaterials, including liposomes and albumin-based polymers, have been approved by the relevant regulatory authorities in numerous countries, including the US and European nations, for the treatment of cancer;13 and many other nanotechnology-based therapeutic agents are currently under clinical investigation.14
The unique physical and chemical properties of nanomaterials account for the interest in them as potential components of anticancer therapies. One such property of some nanomaterials is strong near-infrared absorbance, which has been exploited to develop photothermal agents for the treatment of cancer. Nanomaterials with potential as photothermal agents include gold-based nanostructures (nanorods and nanorings),15,16 rhodium NPs,17 polymers,18,19 carbon-based nanomaterials (carbon nanotube and graphene nanocomposites),20,21 CuS particles,22 and even some organic NPs.23–25 Some up-conversion nanomaterials are capable of converting near-infrared excitation into visible or ultraviolet light, for example, lanthanide-doped up-conversion NPs,26,27 a characteristic that can be exploited in deep-tissue bioimaging and nanomedicine. It is not only the inherent function of nanomaterials themselves that can be employed in cancer treatment, but due to their high surface-to-volume ratio, nanomaterials can also serve as excellent drug-delivery vehicles.7 Moreover, modified nanomaterials and conjugation of nanomaterials with multiple other reagents makes them invaluable candidates in biomedicine.28,29
A major barrier to successful tumor reduction with chemotherapy is insufficient drug delivery to the tumor.30 Compared to conventional drug delivery, nanomedicines preferably accumulate at the tumor area due to the enhanced permeability and retention (EPR) effect of the tumor.31 Besides passive nanomaterial-based drug delivery, nanomaterials can also function as targeting agents in either of two ways.32 The first type of targeted delivery is by loading the NP with targeting agents, e.g., siRNA,33,34 or vascular endothelial growth factor (VEGF) and human epidermal growth factor receptor 2 (HER2) antibodies,35,36 which allow nanomaterials to function as directed drug carriers. This approach has yielded promising results for cancer therapy and diagnosis in both in vitro and in vivo experiments.37 The second approach takes advantage of the unique characteristics of the TME, i.e., hypoxia, acidosis, and vascular abnormalities,38–40 and entails utilizing nanomaterials to modulate the pathophysiology of the TME.
However, as is the case with the systemic delivery of traditional drugs, nanomaterials experience several biological barriers before they reach the tumor, all of which could potentially affect drug delivery. These interactions include NP–protein interaction, blood circulation induced shear forces, and interactions with the perivascular TME.13 In order to ameliorate the potential loss of effective compound during the process of delivery, several modification strategies exist to enhance the penetration and stability of nanomedicines. Such modifications may include NP surface coating with one of the following chemistries: poly(ethylene glycol) (PEG), poly(2-alkyl-2-oxazolines), polysarcosine, poly(vinyl alcohol), other hydroxyl-containing nonionic water-soluble polymers, zwitterionic polymers (polybetaines), and mucolytic enzymes.28,41,42 This Review aims to illustrate the interactions between nanomaterials and the TME and the impact of these interactions on cancer nanomedicine.
THE TUMOR MICROENVIRONMENT
The TME, also called the stroma, is a complex tissue comprising several cell types, including vascular endothelial cells, cancer-associated fibroblasts, immune cells, cancer stem cells (CSCs), and pericytes, as well as noncell components, such as the extracellular matrix (ECM) and secreted extracellular molecules (Figure 1).43 Tumors preferably metastasize to a second location in the body with a similar environment to the original tumor site. This is the “seed and soil” theory proposed by Stephen Paget a century ago,44 which emphasizes the importance of the TME in determining the development and progression of the tumor. In the past 100 years, there have been significant research efforts dedicated to understanding the interactions between cancers and their surrounding TME. Here, we discuss the various components of the TME.
Figure 1.
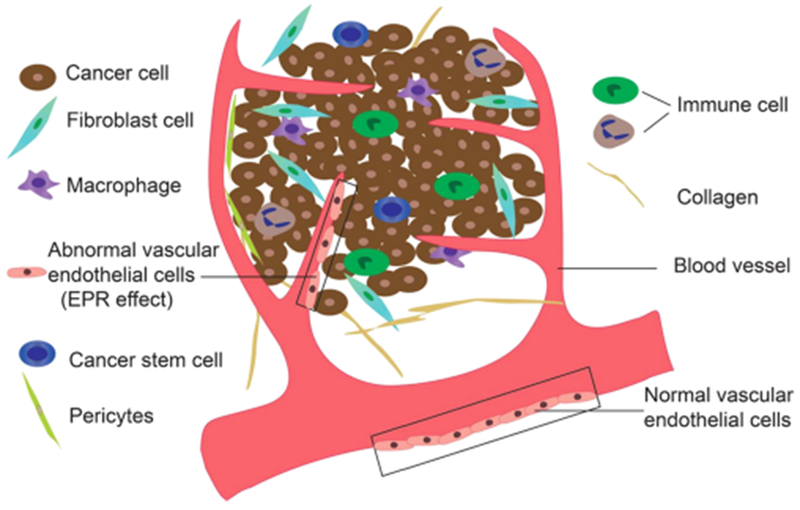
Schematic of the TME. Several components comprise the TME; these include cancer cells, fibroblast cells, macrophages, cancer stem cells, endothelial cells, immune cells, pericytes, and noncell components, such as the extracellular matrix and secreted extracellular molecules.
Tumor Endothelial Cells.
Blood vessels play important roles in the metabolism; they transport nutrients to distant organs and remove waste products. The circulatory system of tumors differs markedly from that of healthy tissues. Unlike the hierarchical branching pattern of arteries, arterioles, and capillaries or veins, venuoles, and capillaries, blood vessels in the tumor area are unorganized.45 In addition, tumor endothelial cells (TECs) do not form regular monolayers and have irregular shapes and sizes not seen in normal blood vessels.46 Moreover, there are often gaps between adjacent TECs, which result in hemorrhage and plasma leakage. Because of the rapid growth of many tumors, most tumor cells are a significant distance from any blood vessels. This distance from blood vessels restricts the oxygen supply, leading to hypoxia in the tumor. Hypoxia in turn induces overexpression of VEGF (also known as vascular permeability factor), which leads to vessel hyper-permeability and high interstitial fluid pressure. This phenomenon is well studied and is known as the “enhanced permeability and retention” (EPR) effect47 (Figure 2). The abnormal morphology of the tumor vasculature enables tumor cells to easily penetrate the blood vessels, leading to metastasis. This property of tumor blood vessels does however have the beneficial characteristic of allowing NPs to accumulate in the tumor tissue. For example, NPs 10–100 nm in size and with hydrophilic surfaces benefit in particular from the EPR effect, which may be attributed to their prolonged circulation time and decreased clearance by the kidney/liver.48,49 In addition, most tumors secrete higher levels of vascular permeability factors, such as Cyclooxygenase-2 (Cox-2),50 bradykinin,51 nitric oxide (NO),52 and peroxynitrite (ONOO−),53 which can be exploited for use in active-targeting nanotherapy. Based on the advantageous EPR effect and the overexpressed molecular targets, both passive- and active-targeting nanomaterials have been developed.54–56
Figure 2.
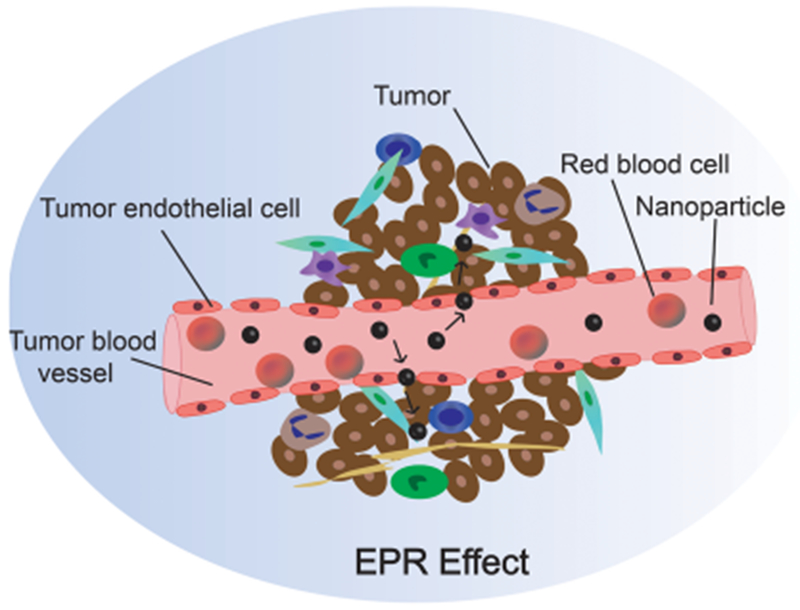
Schematic of the EPR effect. The abnormal morphology of the vasculature and enhanced permeability and retention (EPR) effect enable NPs to easily permeate tumor blood vessels and reach the tumor site.
Cancer Associated Fibroblasts.
Cancer associated fibroblasts (CAFs) are the major cellular component of the TME and the main source of collagen-producing cells.57 In contrast to the quiescent fibroblasts in normal tissues, CAFs are activated and proliferate robustly.58 CAFs express, and can be identified by, several markers, including fibroblast-specific protein 1 (FSP1), vimentin, α smooth muscle actin, fibroblast activation protein (FAP), platelet derived growth factor receptor-α (PDGFRα), PDGFRβ, desmin, and discoidin-domain-containing receptor 2.59 CAFs may enhance tumorigenesis, metastasis, and invasion of cancer cells by releasing growth factors and cytokines into the circulation.60,61 Moreover, CAFs reportedly promote the immunosuppressive environment of the TME and confer resistance to anticancer drugs on cancer cells.62–64 Li et al. encapsulated the photosensitizer ZnF16Pc into a ferritin nanocage conjugated with a sequence specific to the FAP. The complex targeted CAFs, mediated efficient and selective photodynamic therapy (PDT), and resulted in the elimination of the tumor.65
Cancer Stem Cells.
Whether cancer stem cells (CSCs) exist remains an area of active debate in the cancer biology field. However, an increasing number of publications demonstrate the existence of CSCs by identifying their cell surface markers, i.e., CD133, CD44, and aldehyde dehydrogenase (ALDH), among others, in different cancer stroma.66,67 The CSC theory declares that they represent a class of cancer cells with unlimited potential for cell division and an ability to repopulate the whole tumor. These characteristics of CSCs would, thus, explain the recurrence of tumors at either the original site or a distant area, even after successful chemotherapy and/or radiation therapy (RT).68 As the “root” of cancer, the potential of CSC targeting therapy has attracted great attention.67,69
Tumor Associated Immune Cells.
The immune cells that infiltrate the tumor area are termed tumor-infiltrating lymphocytes (TIL), and these include T cells, B cells, and Natural Killer (NK) cells. The roles that TIL play in cancer are diverse and can be both beneficial and deleterious. On one hand, some CD4+ derived T cells play a prominent antitumor role by inhibiting new blood vessel formation (Th1),70 promoting eosinophil recruitment in a manner dependent on IL-4 and IL-13 (Th2),71 or recruiting CD8+ T and NK cells into tumors (Th1 and Th17).72–74 CD8+ T cells have also been associated with tumor diminishment.75 On the other hand, forkhead box p3 (Foxp3) expressing CD4+ T cells (Treg cells) suppress the immune response and contribute to tumor cells’ immune escape.73 Developing molecular inhibitors of Treg cells, either chemical inhibitors such as cyclophosphamide or targeting reagents, like anti-GITC (glucocorticoid-induced TNF receptor) antibodies,76 represents a promising approach in immunotherapy. Macrophages and B cells also have a dual supportive and inhibitory influence on cancer, depending on the stage of the disease and the tissue involved.77 Dendritic cells (DCs) have the greatest potential to present antigens for activating antitumor T-cell responses, and as such, they offer a unique opportunity for specific targeting of tumors.78
Extracellular Matrix.
The extracellular matrix (ECM) provides structural and biochemical support to cells; it is a collection of extracellular molecules secreted by support cells. The cancer-associated ECM often displays an altered organization and enhanced post-translational modifications of ECM proteins and characteristically expresses matrix-remodeling genes such as matrix metallopeptidases (MMPs) and collagen cross-linkers.79,80 The cancer-associated ECM enhances tumor cell progression by evading growth suppressors, resisting cell death, inducing angiogenesis, and activating invasion and metastasis.81,82
Pericytes.
The currently accepted definition of mature pericytes is cells embedded within the vascular basement membrane. Low pericyte coverage may trigger metastasis and correlates with poor prognosis,83 while high pericyte coverage is associated with cancers that are the most aggressive and refractory to therapy.84 Pericyte recruitment into tumor blood vessels is mediated by PDGFRβ signaling during angiogenesis, stromal cell derived factor-1, and matrix-metalloproteinase-mediated ECM degradation.85–87
NANOPARTICLE-BASED MODULATION OF THE TME
As mentioned above, the TME is quite different from the microenvironment of normal tissues. The differences include vascular abnormalities, hypoxia, pH, and the immune response. Based on the specific characteristics of the TME, many NPs have been developed in order to diminish the tumor by adjusting the TME.
Vascular Abnormalities.
The abnormalities of the tumor vasculature make it possible for both passive targeting based NPs and active targeting NPs that engage overexpressed molecules, such as VEGF, integrin αvβ3, and vascular cell adhesion molecule-1 to enter the TME.
EPR Effect Based Passive Targeting.
Profiting from the EPR effect, NPs between 20 and 200 nm tend to accumulate at the tumor site; this accumulation occurs due to both the larger vascular endothelial pores (10–1000 nm in diameter) and the high density and permeability of the vasculature of the tumor. Moreover, due to the poor lymphatic drainage in the tumor tissue and the NPs having a large enough size to avoid renal clearance, such nanomaterials have a longer circulation time and enhanced retention in the tumor area.88,89 This was first demonstrated in 1986, when the styrene maleic acid copolymer coated anticancer protein neocarzinostatin (NCS) was found to accumulate at tumor sites more readily than NCS itself; since that time, the EPR effect theory has been extensively studied and become better understood.90
It is clear that the size and shape of, as well as modifications to, nanomaterials all affect the efficiency of the EPR effect. With respect to size, Tong et al. utilized positron emission tomography and kinetic modeling to study the tumor accumulation and elimination of different-sized 64-Cu labeled gold NPs (AuNPs), and suggested that AuNPs with relatively small volume and high aspect ratio are ideal candidates for EPR mediated tumor delivery.91 The circulation and biodistribution of NPs also depends on their shape and geometry. Geng et al. found that, compared with spherical particles, filomicelles circulated up to 1 week longer following intravenous injection in rodents.92 Others demonstrated that nanomaterials of different shapes (i.e., quantum dots and single-walled carbon nanotubes) showed different extravasational behavior based on the EPR effect, despite having similar surface coating, area, and charge. Thus, the geometry of NPs plays a complex and potentially major role in extravasation from the vasculature to tumors.93 Physical and biological barriers in the body can affect the accumulation of NPs in the tumor. The reticuloendothelial system (RES) can sequester many NPs before they reach the tumor, which not only causes a decrease in tumor accumulation, but may also damage RES-rich organs. Engineering the physicochemical properties of NPs may help minimize their RES-sequestration. NP delivery can also be impaired by interaction of the NP with the protective mucus layer on mucosal surfaces. Xu et al. showed that coating PEG onto biodegradable poly(lactic-co-glycolic acid) (PLGA) NPs leads to enhanced NP distribution throughout the mouse vagina.94 Using different ratio PEG coatings, it was shown that at least 5% PEG was required to effectively shield the NP core from interacting with mucus components both in vitro and ex vivo.
While several nanomaterials have been assessed in preclinical studies, they have almost uniformly failed in the clinic.95,96 This problem requires development of specific targeting approaches as discussed below.
Active Targeting Based on Vascular Abnormalities.
Both the abnormalities of the tumor vasculature and the heterogeneity of the TME cause difficulties in cancer therapy. However, these characteristics of the tumor also mean that several receptors are overexpressed by the tumor, and these offer the opportunity to actively target the tumor. Biomarkers with upregulated expression include galectin-1, integrins, tumor endothelial markers, cell adhesion molecules, VEGF, selectins, cell surface nucleolin, and fibrin–fibronectin complexes among others. In combination with the size-based EPR effect, several NPs have been explored in cancer treatment using active targeting.
Galectin-1 regulates the proliferation, migration, and apoptosis of endothelial cells, and its ligand, the peptide anginex, has been successfully used as a targeting agent for cancer therapy.97 Integrin αvβ3 is another endothelial cell receptor that is highly expressed in tumor-associated endothelial cells compared to normal endothelial cells: the peptide arginine-glycine-aspartate (RGD) is the specific binding motif of integrin αvβ3.98 Kluza et al. conjugated liposomes with both anginex and RGD to develop a potential tool for imaging and antiangiogenic treatment. The dual-conjugated agent greatly enhanced the number of microbubbles per cell for ultrasound imaging of tumor angiogenesis compared to either single conjugated agent.99 Due to the rapid growth of tumors, angiogenesis is essential for both tumor growth and metastasis. VEGF is an important mediator of angiogenesis; targeting of the VEGF receptor (VEGFR) on endothelial cells is a viable therapeutic approach to decrease blood vessel density and thus delay tumor growth. Anti-VEGFR antibodies were conjugated to propranolol-loaded NPs which inhibited VEGF expression and were cytotoxic in infantile hemangiomas.100
Tumor Hypoxia Modulation in the Tumor Microenvironment.
Because of the abnormally rapid growth of tumor cells, the distance from the core of the solid tumor to blood vessels is frequently beyond the diffusion range of oxygen (which is up to ~200 μm, depending on the local oxygen concentration in blood).101 This leads to hypoxia in tumor tissue, with oxygen tensions around most tumor cells varying from anoxia to 7.5 mmHg, whereas the oxygen tension in normal tissues is about 30–70 mmHg.102,103 Tumor hypoxia suppresses apoptosis and immune reactivity, supports autophagy, and increases epithelial-to-mesenchymal transition, thus increasing invasiveness and metastasis.102 Due to their distance from blood vessels, penetration of the hypoxic tumor cells by traditional anticancer drugs is limited, leading to less effective treatment. Significant efforts have been made in recent decades to improve tumor therapy efficiency.39,104 These efforts have included two major nanomedicine-based approaches, namely, generating oxygen in the tumor and deoxygenation of tumors (Figure 3).
Figure 3.
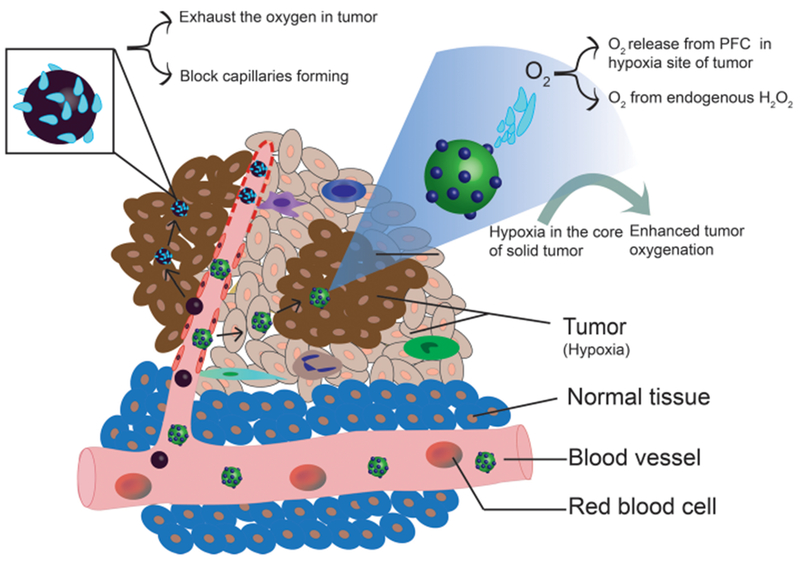
Modulation of hypoxia. There are two approaches to modulation of hypoxia in the TME. One approach is to exhaust the oxygen in the tumor thereby preventing the development of the tumor. Magnesium silicide (Mg2Si) NPs are used to exhaust the oxygen in tumor and block tumor capillaries (left); tumor growth is restricted due to lack of nutrients. The second approach involves increasing the oxygen level within the hypoxic sites of the tumor, thereby enhancing the radiation therapy (RT) efficiency. Oxygen dissolved in perfluorocarbon (PFC) was delivered to the hypoxic site to increase the oxygen level in tumor and enhance the RT efficiency (right).
Generation of Oxygen to Modulate the Tumor Microenvironment.
Radiation therapy (RT) is a widely applied cancer therapy, which employs ionizing radiation (X-ray or γ-ray) to induce DNA damage and thus inhibit tumor growth.105 Oxygen molecules form stable organic peroxides with the broken ends of DNA and thus enhance radiation-induced DNA damage during RT. However, the hypoxic nature of most solid tumors leads to hypoxia-associated resistance during RT.106,107 In order to overcome this effect of low oxygen levels on RT effectiveness, Song et al. developed TaOx@PFC–PEG nanodroplets consisting of TaOx and perfluorocarbon (PFC). The TaOx nanoparticle is an excellent X-ray absorber while PFC has high biocompatibility and readily dissolves oxygen.108,109 Following injection of the TaOx@PFC–PEG nanodroplets into mice, the oxygenation level in the tumor was increased 27%, and the RT treatment efficacy was remarkably enhanced.110 Other oxygen carriers that can be employed to overcome hypoxia within tumors include heme hybrids and hemoglobin-based oxygen carriers (HBOCs).111,112
Beside the exogenous supply of oxygen, another way to increase oxygen levels in the tumor is to generate oxygen in the TME. Compared with normal tissues, malignant cancer cells produce excessive amounts of H2O2 and, thus, significantly increase H2O2 levels in the TME.113 MnO2 NPs can act as a catalyst to generate oxygen from the H2O2, thereby overcoming the hypoxia-associated RT resistance.114
Deoxygenation in Tumors to Modulate the Tumor Microenvironment.
Starvation therapy for cancer is a concept that has been studied for several years. Blood vessels constitute a complex system for delivery of nutrition and oxygen to tissues and cells, while simultaneously removing waste products. Without sufficient vasculature, tumor cells would die for lack of adequate nutrition and oxygen. Several strategies have been used to target the blood vessels, including the FDA approved drug bevacizumab which is a humanized monoclonal antibody against VEGF.115 In addition to antibody targeting, other methods have been developed to deoxygenate the TME. Zhang et al. developed a system using polyvinylpyrrolidone (PVP)-modified magnesium silicide (Mg2Si) NP to block tumor capillaries and prevent tumors from receiving new supplies of oxygen and nutrients. In the acidic TME, Mg2Si releases silane, which in turn reacts with oxygen in tissue or blood to form silicon oxide (SiO2) aggregates. The aggregates, generated in situ, block tumor capillaries and choke off the blood supply.116 Liu et al. developed a nanostructure, TPZ-UC/PS, which included double silica-shelled upconversion nanoparticles (UCNPs), a photosensitizer (PS) molecule, and a bioreductive pro-drug (tirapazamine, TPZ). When treated with a 980 nm laser, the structure enhanced hypoxia, and in turn increased the bioreductive therapeutic effect of TPZ.117 Based on the unique hypoxic character of solid tumors, imaging approaches have also been explored. D-Fe3O4@PMn NP complexes were designed and applied in diagnostic imaging to produce significant contrast enhancement in T1- and T2-weighted magnetic resonance imaging (MRI).118
pH Modulation of the TME.
It has become increasingly apparent that energy production in cancer cells differs significantly from that in healthy cells. Normal cells generate energy through mitochondrial oxidative phosphorylation, while most cancer cells produce their energy via glycolysis, which causes significant lactic acid production even in the presence of abundant oxygen. This phenomenon is called aerobic glycolysis or the Warburg effect.119 Because of this increased glycolysis, the plasma membrane proton-pump activity, and the insufficient blood supply in most solid tumors, the TME is acidic. The extracellular pH of most tumors is in the range of 6.5–7.2, and intracellular endolysosomes exhibit even lower pH values of 5.0–5.5.120 In contrast, the extracellular pH of normal tissue and blood is constant at 7.4.121,122 Moreover, acidosis may contribute to metastatic progression by degrading the ECM, as well as increasing drug resistance.123–125 Based on the acidic character of the TME, several NPs have been developed to enhance the efficacy of tumor therapy.
pH-Sensitive Inorganic Nanosystems.
Inorganic nano-systems can be divided into two basic types. The first, such as ferromagnetic NPs, have intrinsic anticancer activity,126 and ZnO nanoparticles function as the photosensitizer.127 The second act as carriers which can release drugs under acidic conditions, and include CaCO3,128,129 calcium phosphate (CaP),130,131 and ZnO quantum dots (ZnO QDs).132,133
Under neutral pH, ferromagnetic NPs (γ-Fe2O3 or Fe3O4 NPs) can catalytically break down H2O2 into nontoxic H2O and O2. In contrast, under acidic conditions, they disproportionately generate highly toxic reactive oxygen species (ROS)—hydroxyl radicals (·OH) from H2O2.134,135 This peroxidase-like activity under acidic conditions makes ferromagnetic NPs a good candidate for use in cancer therapy. Huo et al. developed large pore-sized biodegradable dendritic silica NPs into which both glucose oxidase (GOX) and Fe3O4 NPs were loaded. GOD served to generate abundant H2O2 from the breakdown of glucose. Then, in the acidic TME, the elevated H2O2 is acted on by the Fe3O4 NPs to liberate highly toxic hydroxyl radicals, which induce apoptosis and tumor death.126 Oleylamine-capped FeS2 nanocubes were also explored for the catalytic breakdown of overproduced H2O2 into (·OH). The valence change of the ferrous ions during the self-oxidation can be leveraged to report the H2O2 levels in the tumor area, via self-enhanced MRI. Photothermal treatment also accelerates the Fenton reactivity for a synergistic photothermal therapy/chemodynamic therapy (PTT/CDT).136,137 Zhang et al. produced a core–shell CeIII-doped LiYF4@SiO2@ZnO (SCNP@SiO2@ZnO-PEG) structure, which enabled simultaneous radiotherapy and depth-insensitive PDT. SCNP seeds were excited by the radiation and emitted low energy photons that match the bandgap of ZnO nanoparticles. The subsequent excitons formed the electron hole (e− – h+), and interact with H2O and O2 to form free radicals (·OH) and (·O2). The therapy efficiency of this radiation-induced type I PDT was greatly enhanced, due to the diminished oxygen dependence.127
CaCO3 is an excellent inorganic drug carrier, which, in the acidic TME, breaks down to Ca2+ and CO2, and simultaneously releases its payload, which can include anticancer drugs such as doxorubicin (DOX),138 photosensitizers like chlorin e6 (Ce6),139 or small interfering RNAs (siRNA).140 CaP is nontoxic, biocompatible, and degradable, all of which make it attractive as a drug carrier.130 ZnO QDs are another good drug carrier for cancer therapy due to their low toxicity and their rapid dissolution to Zn2+ in an acidic environment.141 Cai et al. demonstrated that pH-sensitive ZnO QDs dissolved to Zn2+ in acidic endosomes or lysosomes after uptake by cancer cells, triggering the release of the carried DOX.132
pH-Sensitive Polymers.
Polymers have been developed and designed as “smart” drug carriers for targeted cancer therapy, since their properties change in different environments. Several polymer NPs have been developed using anionic or cationic polymers, such as poly(acrylic acid) (PAA), poly(methacrylic acid) (PMAA), poly(ethylacrylic acid) (PEAA), poly(propylacrylic acid) (PPAA), poly(butylacrylic acid) (PBAA), N-isopropylacrylamide (NIPAM), poly(glutamic acid) (PGA), and poly(N,N′-dimethylaminoethyl methacrylate) (PDEAEM), poly(β-amino ester) (PbAE), and poly(4-vinyl-pyridine) (PVP).142 For applications in cancer treatment, pH-sensitive polymers are usually covalently linked to the antitumor drug via pH-labile bonds such as imines, hydrazones, boronate monoesters, and coordination amine–cation complexes.143 Under physiological conditions, such linkages are stable, while in acidic environments (pH « 4.5–6.5), they tend to hydrolyze to release the drug. Yang et al. utilized amphiphilic triblock copolymers to self-assemble into stable vesicles in aqueous solution; long PEG segments formed the outer hydrophilic PEG layers of the vesicles, while the short PEG segments constituted the inner hydrophilic PEG layer of the complex. The antitumor drug DOX was conjugated to the hydrophobic membrane via a pH-sensitive hydrazone bond to achieve pH-responsive drug release.144 PMAA is an established pH-responsive polymer which exhibits distinct volume enlargement when the pH value is higher than the acid dissociation constant of the ionizable groups (~4.25).145 As the PMAA content increases, so the equilibrium swelling ratios increase.146 PMAA has been used to coat fluorescent YVO4:Eu cores for cell imaging based on the evaluation of pH,147 enabling the complex to have little toxicity along with enhanced cellular uptake.
Immune Response Modulation in the Tumor Microenvironment.
Tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and Tregs are the three main immune cells in the TME. They each play a critical role in enhancing tumor cell invasion and metastasis, promoting angiogenesis and ECM remodeling, and inhibiting antitumor immune surveillance. Elimination or reprogramming of the immune suppressive TME is a major challenge in the immunotherapy of cancer.148
Targeting Macrophages.
Ideally, macrophages would diminish tumors through their normal function; however, with the immune editing of the tumor, increasing evidence demonstrates that TAMs promote cancer progression and enhance tumor growth, and that TAMs are a poor prognostic factor in several tumor types.149,150 For this reason, TAMs are an attractive target for anticancer therapy. There are two major TAM subgroups: M1 and M2 TAMs. The tumor-promoting effects of TAMs are mediated primarily by M2, while M1 TAMs retain their antitumorigenic properties.150 Thus, there are two strategies for improved therapy using therapeutic NPs: the first is to convert M2 to M1 TAMs to stimulate antitumor immunity, and the second is to eradicate M2 TAMs. TAMs residing in hypoxic regions of tumors were demonstrated to promote proliferation and increase chemoresistance. Song et al. utilized hyaluronic acid (HA) to modify MnO2 NPs, thereby reprogramming the anti-inflammatory, pro-tumoral M2 TAMs to pro-inflammatory, antitumor M1 TAMs. This further enhanced the ability of MnO2 NPs to both reduce tumor hypoxia and modulate chemoresistance.151 Since M2 TAMs highly express the mannose receptor, Zhu et al. developed a PEG-sheddable, mannose-modified nanoparticle platform to target M2 TAMs. Sheddable PEG was conjugated to mannose-modified PLGA NPs via an acid-sensitive linker. In the acidic TME, acid-sensitive PEG was shed to expose PEG and mannose conjugated PLGA, allowing the particles to be internalized by the M2 TAMs, while uptake by normal macrophages in the mononuclear phagocyte system (MPS) organs was prevented because of their neutral pH.152
Ferumoxytol, a supplement approved by the FDA to treat iron deficiency disease, was recently found to promote macrophages to pro-inflammatory Th1-type responses, and in this way to significantly inhibit the growth of subcutaneous adenocarcinomas in mice.153 The liver is an organ in which most antitumor drugs accumulate. Liver-derived M2 macrophages preferentially take up NPs compared to M1 macrophages. At the same time, primary Kupffer cells, which express higher levels of M2 markers (CD163), take up more NPs than cells expressing lower levels of surface CD163. These findings suggest that targeting macrophages or Kupffer cells might be a novel approach to enhance tumor therapy by modifying the hepatic microenvironment.154
Targeting Myeloid-Derived Suppressor Cells.
Myeloid cells derive from hematopoietic stem cells in bone marrow and are destined to differentiate into macrophages and other cells. However, in many pathological conditions, including cancer, differentiation is partially blocked resulting in the accumulation of immature myeloid cells in the TME; these accumulating cells are called MDSCs. MDSCs upregulate immunosuppressive factors and thus suppress T cell functions.16,155 Thus, either enhancing differentiation of myeloid cells into mature immune cells or diminishing the accumulation of MDSCs provide two potentially significant approaches to treat cancer. All-trans retinoic acid (ATRA) is able to differentiate MDSCs into mature DCs, macrophages, or granulocytes, thus improving the tumor-specific immune response.156 Kong et al. constructed lipid-coated biodegradable hollow mesoporous silica NPs (dHMLB) with coencapsulation of ATRA, DOX, and interleukin-2 (IL-2) for chemo-immunotherapy.157 In the tumors of mice treated with the complex, the MDSCs decreased 2.4-fold, while mature DCs increased 14.3-fold compared with the controls. Another research group developed lipid nanocapsules (LNCs) which were loaded with a lauroyl-modified form of gemcitabine (GemC12), to target the monocytic (M-) MDSC subset in melanoma-bearing mice. The LNCs were preferentially taken up by monocytic cells rather than by other immune cells. Moreover, tumor-associated immunosuppression was reduced in tumor-bearing mice administered a very low dose of GemC12-loaded LNCs.158
Targeting Tregs.
Regulatory T cells (Tregs) are a subpopulation of T cells that maintain tolerance to self-antigens, thus preventing autoimmune disease. They are immunosuppressive and down-regulate both T cell proliferation and cytokine production. A large number of studies in both humans and animal models have shown that high numbers of Tregs in the TME correlate with poor prognosis and enhanced tumor malignancy.72,159 It is thought that Tregs suppress the immune response in tumors; thus, a reduction of Tregs in the TME should reverse their immunosuppressive effects and improve outcomes in cancer treatment. To date, there are four known Treg enriched markers, namely, glucocorticoid-induced TNFR-related receptor (GITR), folate receptor 4 (FR4), CD39, and CD103.160 GITR is highly expressed in tumor Tregs compared to peripheral Tregs. GITR antibodies were coated layer-by-layer on hybrid NPs in order to target Tregs within tumors; in combination with IR-780 dye, this targeting photothermal therapy successfully reduced the suppressive function of Treg cells and eradicated tumor growth in vivo.161 Neuropilin-1 (Nrp1) is expressed on the majority of Tregs, but on relatively few T effector cells; the expression of Nrp1 is highly associated with Treg cell-specific Foxp3+ expression.162 Moreover, the peptide tLyp1 peptide was identified as a substrate with high affinity and specificity for Nrp1.163 Ou et al. constructed tLyp1 peptide-conjugated hybrid NPs for targeting Treg cells in the TME. The complex down-regulated Treg cell suppression through inhibiting signal transducer and activator of transcription 3 (STAT3) and signal transducer and activator of transcription 5 (STAT5) phosphorylation. Furthermore, reduced intratumoral Treg cells, enhanced tumor inhibition, and elevated intratumoral CD8+T cells active against the tumor were observed in in vivo assays.164 Thus, the proposed Treg targeting therapy provides an effective approach to cancer therapy.
Blocking the Adaptation of Tumor Cells to the TME.
In order to adapt to the hypoxic, acidic, and low-nutrient TME, tumor cells reprogram their main metabolic pattern from mitochondrial oxidative phosphorylation (OXPHOS) to aerobic glycolysis. Thus, one strategy to diminish the tumor is to block aerobic glycolysis. Several studies have used a specific glycolysis inhibitor to block this adaptation; inhibitors used include 3-bromopyruvate (3-BP)165 and dichloroacetate (DCA).166 Zhang et al. developed tumor vascular endothelium-targeted liposomal NPs (T-Lipo-3-BP) as a controlled release system and successfully suppressed tumor growth in mice.165 Similarly, mito-DCA was developed to target the mitochondria, leading to a metabolic switch from glycolysis to glucose oxidation and resulting in cell death via apoptosis.166
In the TME, macrophages tend to be M2 polarized and exhibit up-regulated mitochondrial OXPHOS, fatty acid synthesis, and β-oxidation. In contrast, M1 macrophages predominantly use glycolysis to generate ATP.167 Exposure of macrophages to silk, PLGA, and silica NPs caused greater glucose consumption and lactate production, suggesting increased glycolytic activity; this metabolic profile is consistent with the proinflammatory M1-like phenotype, and demonstrates the potential of NPs to block adaptation of the macrophages to the TME.168
Combined Strategies to Modulate the Tumor Microenvironment.
Cancer is a complicated and persistent disease, and it is very difficult to eradicate. Thus, it may be advantageous to utilize multiple characteristics of the tumor to develop combined therapies in order to attack the cancer on multiple fronts. Yang et al. developed a biodegradable hollow manganese dioxide (H-MnO2) nanoplatform to do just this. They incorporated H-MnO2 nanoshells as the drug carrier and oxygen generator. The nanoshells were coated with PEG to stabilize the complex, and the photosensitizer chlorine e6 (Ce6) and anticancer drug DOX were incorporated.169 MnO2 degrades in the acidic pH of the TME, releasing the Mn2+, and the Ce6 and DOX loaded in the nanoshell. In addition, MnO2 can function as a catalyst or to degrade the endogenous H2O2 and produce oxygen in the tumor. The oxygen not only relieves the hypoxia of the tumor, but also contributes to the PDT induced by Ce6 under specific wavelength light activation; ROS produced by the PDT also leads to the death of the cancer cells.170 The nanoshell complex also triggered a series of antitumor immune responses. The combined therapy greatly inhibited tumor growth compared with the control group. Thus, this composition utilized the characteristic hypoxia and acidity of the TME combined with chemotherapy and PDT to achieve an enhanced therapeutic response.
Bi et al. also constructed a multifunctional nanoplatform designed to target cancer via PDT, delivery of a platinum drug, and the Fenton reaction. Their upconversion NPs (UCNPs-Pt(IV)-ZnFe2O4) enhanced therapeutic response in both in vitro and in vivo models, and by virtue of the inherent upconversion luminescence, served as a viable imaging contrast agent for multiple imaging modalities.171
THE INTERACTIONS BETWEEN THE TUMOR MICROENVIRONMENT AND NANOPARTICLES
Alongside the increasing application of nanomaterials in cancer treatment, there has been a focus on understanding the biological effects of functionalized NPs on a subcellular level, including the NP/protein interaction, the NP/TME interaction, and the consequent effects on cellular pathways.
The Interactions of Nanoparticles with Proteins.
The first change after nanomaterials enter a biological system is the formation of the so-called “corona”. The surface of the nanoparticle is quickly covered with multiple biomolecules, mostly proteins, immediately after the nanoparticle enters into the biological systems (cells, tissues, biofluids), resulting in the formation of the “protein corona”.172 One theory of the “corona” is that it exists in two distinct parts. The first is called the “hard corona” in which have proteins adsorb with high affinity to NPs and interact directly with the nanomaterial surface. The second part is termed the “soft corona” and in which proteins interact with the hard corona via weak protein–protein interactions and can be readily replaced by other high affinity proteins.173,174 This protein corona greatly changes the physicochemical properties of NPs, including the size, zeta potential, and stability. As a consequence, the biological properties and technical identities of the original nanoparticle are critically affected; changes occur in terms of cellular uptake, biological targeting, biodistribution, and toxicity.175 Studies show greater cellular uptake by immune cells of pure NPs compared to protein corona coated NPs.176 Moreover, ligand-based targeted delivery systems can lose their targeting ability due to the corona formation. The Dawson group found that even though transferrin conjugated SiO2 NPs continued to enter cells, their targeting specificity was lost for both binding to targeted receptors on cells or soluble transferrin receptors.177
However, the corona also offers potential benefits. A direct benefit is in blocking the function of proteins that enhance tumor progression, and then modulating the TME. One example is the research from the Wu group. Transforming growth factor-beta 1 (TGF-β1) is known to be an immunosuppressive agent that attenuates immune responses and results in tumor growth. Thirteen nanometer AuNPs predominantly bound to TGF-β1 through S–Au bonds and thereby destroyed the structure of the growth factor. In vivo experiments showed that in the TGF-β1-secreting murine bladder tumor 2 cells bearing syngeneic C3H/HeN mice, the addition of AuNPs blunt the growth of tumor; however, this was not the case in immunocompromised NOD-SCID mice. The discovery suggests that AuNPs may modulate tumor immunity through inhibiting immunosuppressive TGF-β1 signaling.178 Another example comes from our own work. We have previously proved that by incubating pure 20 nm AuNPs with the conditioned medium (CM) from the pancreatic cancer cell line Aspc1, the levels of Dipeptidyl Peptidase IV (DPPIV/CD26), Thrombospondin 1 (THBS1), CXCL16, Coagulation Factor III (F3), and Serpin Family E Member 1 (SerpinE1) were notably decreased (>50% decrease, p ≤ 0.05) compared with the original secretion.12 In addition, following treatment of the AuNPs, Inositol-requiring enzyme-1a (IRE1a) was greatly increased in the CM of Aspc1. These data suggest that AuNPs dramatically affect the secretory profile of pancreatic cancer cells (PCCs) and then consequently affect the expression of a large number of other secreted factors, which leads to the endoplasmic reticulum (ER) stress signaling. Moreover, AuNPs rendered ovarian cancer cell lines more sensitive to cisplatin by blunting drug resistance, reversing the epithelial–mesenchymal transition (EMT) effect and stemness induced by cisplatin, and inhibiting cisplatin-induced Akt/NF-κB. signaling.179
An elegant way to exploit corona formation is to design the NP surface to interact with specific plasma proteins that enhance NP delivery to certain organs or initiate targeted receptor-mediated cellular binding. For example, apolipoprotein was recently shown to be essential for siRNA lipoplexes to target hepatocytes in vivo.180 Retinol-conjugated polyetherimine (RcP) NPs specifically accumulated retinol binding protein 4 (RBP) in the corona and directed NPs to hepatic stellate cells (HSC), thereby alleviating hepatic fibrosis.181 Moreover, utilizing the corona to mitigate NP toxicity, to identify therapeutic targets, to manipulate NP pharmacokinetics, and to increase the drug payload capacity has recently theoretically proposed.182 Though some aspects of the corona are understood, some basic mechanisms such as the reversibility and displacement of the corona remain obscure. Chen et al. found that the adsorbed proteins in the corona formed on superparamagnetic iron oxide (SPIO) nanoworms in plasma were rapidly lost in vivo.183 This demonstrates that many of the mechanisms of corona development require further study, especially as they pertain to the in vivo system.
The Interactions between Nanoparticles and Endothelial Cells.
Nanoparticles Decrease Endothelial Barriers.
Endothelial cells are the first barrier before NPs reach the tumor. There are two main approaches to overcoming the endothelial barrier. The first strategy to conquer the endothelial barrier is to utilize the transcellular transportation systems. In order to increase the transportation efficiency, NPs can be coated with moieties that recognize specific surface receptors on endothelial cells, such as lung endothelial cell adhesion-1 molecule, glucose transporters, and the transferrin receptor.184 This strategy allows internalization and transcellular transport of the nanoparticle.
The second strategy to decrease the endothelial barrier is to use the paracellular route, i.e., through the gaps between endothelial cells. As mentioned above, the EPR effect enables NPs of specific sizes to go through the wider gaps between the endothelial cells within the TME. Aside from that specific case, in order for nanomedicine to exploit the paracellular route in broader locations than the TME, there are studies showing that NPs with specific characteristics are able to broaden the gaps between endothelial cells to micrometers. This effect is called “nanoparticle induced endothelial leakiness” (NanoEL) (Figure 4).185 The Leong group has elucidated how unmodified 22.5 nm TiO2 nanomaterials (TiO2–NM) cause NanoEL; TiO2–NM bind VE-cadherin that functions in adherent junctions in endothelial cells. The binding disrupts the VE–cadherin homophilic interaction, and then phosphorylates VE–cadherin causing the loss of interaction of VE–cadherin with both β-catenin and p120. The VE–cadherin–β-catenin–p120 complex destabilizes actin and leads to actin remodeling, which results in the leakiness between endothelial cells.186 The group further shows that the NanoEL effect differs according to both the particle size and the endothelial cell origin. AuNPs between 10 and 30 nm cause a greater NanoEL effect; human mammary- and skin-derived endothelial cells are more sensitive to AuNPs than those from the umbilical vein.185 As well as TiO2 and AuNPs, nanodiamond (ND) also increases leakiness in vascular endothelial cells. ND-induced leakiness is mediated by an increase in intracellular reactive ROS and Ca2+, which in turn triggers cytoskeletal remodeling of vascular endothelial cells187. These leakiness phenomena are unrelated to the EPR effect in tumors. They function on vascular endothelial cells and provide a new approach for antitumor nanomedicine delivery.
Figure 4.
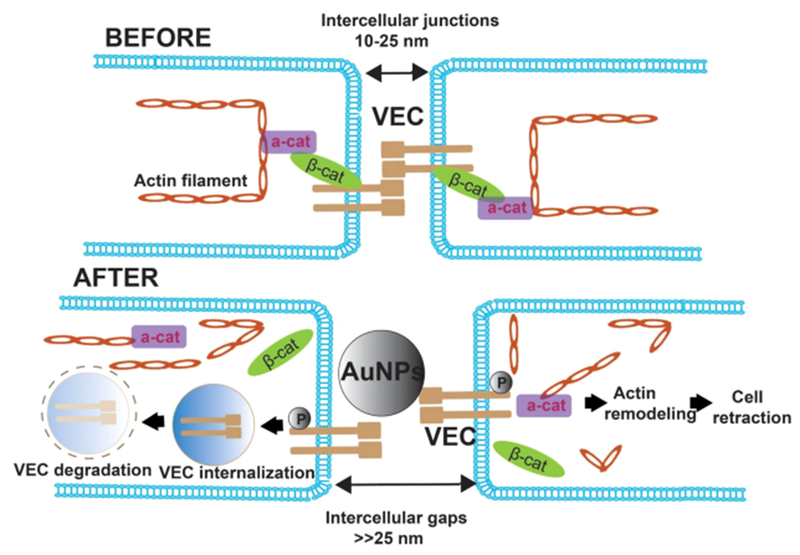
NPs induce endothelial leakiness. BEFORE: the paracellular route on the microvascular barrier is mediated by VE–cadherin, which associated with the cadherin–catenin–actin complex. AFTER: Gold NPs bind VE–cadherin, causing VE–cadherin internalization and degradation. As a result, the cadherin–catenin–actin complex disintegrates, and the actin framework is remolded, which leads to an enlarged gap between endothelial cells. In turn, leakiness between endothelial cells is enhanced. VEC: VE–cadherin, β-cat: β-catenin, α-cat: α-catenin. This figure is conceptually adapted from Figure 6 in ref 185.
Nanoparticles Increase the Anti-Angiogenesis Effect.
In addition to inducing leakiness of endothelial cells, NPs can function as anti-tumoral-angiogenesis reagents by inhibiting endothelial cell growth. Mesoporous silica NPs (MSNs) inhibit the proliferation, migration, invasion, and tube formation of human mammary microvascular endothelial cells (HMMEC). In addition, MSNs can be taken up by HMMEC and activated HMMEC to produce intracellular ROS that directly interfere with the p53 tumor suppressor pathway sequentially leading to the anti-angiogenesis effect.188 Duan et al. also found that silica NPs could decrease expression of VEGFR and cellular adhesion molecules ICAM-1 and VCAM-1 in primary human umbilical vein endothelial cell lines (HUVECs). Moreover, down-regulation of the VEGFR2/MEK1/2/Erk1/2 and VEGFR2/PI3K/Akt signaling pathways was also noted, all of which impair angiogenesis.189
Nanoparticles Increase the Autophagic Effect.
Following treatment with these 62 nm silica NPs, the accumulation of autophagic vacuoles in endothelial cells was seen both in vitro and in vivo. Furthermore, cytoskeleton reorganization including actin polymerization as well as mitochondrial damage were also found in HUVECs treated with silica NPs, indicating the toxicity of silica NPs.189 The Shen group also found that AuNPs can inhibit the proliferation, migration, and tube formation of HUVECs. In addition, AuNPs increase the expression of the autophagosome markers ATG5 and Beclin1 as well as the lysosome marker p62 in HUVECs, and convert the LC3-I to LC3-II, all of which suggest induction of autophagy.190
The Interactions between Nanoparticles and Macrophages.
Once NPs have traversed the endothelial barrier and arrived at the tumor site, a large number of them are internalized by TAMs.32,191 There has been considerable investigation regarding the fate of these internalized NPs.
Nanoparticles Induce M2 Macrophage Polarization to the M1 Phenotype.
As discussed above, monocytes are recruited to malignant tumors by expressing chemotactic cytokines192 and are polarized to anti-inflammatory M2 phenotypes,193 while in contrast, M1 macrophages retain their pro-inflammatory effect. Significant effort has been applied to either target and eliminate M2 macrophages from the TME or force M2 macrophages to polarize to the M1 phenotype.194,195 Super-paramagnetic iron oxide NPs (SPION) induce a phenotypic shift in M2 macrophages toward M1, which is characterized by up-regulated CD86, TNF α, ferritin, and cathepsin L.196 The interaction of macrophages with the FDA-approved iron oxide nanoparticle compound ferumoxytol has also been explored.153 Ferumoxytol significantly enhanced ROS and cancer cell apoptosis in a macrophage–cancer coculture system. Moreover, ferumoxytol clearly inhibits tumor growth and metastases. Other NPs also induce M2 polarization to M1, including carboxyl- and amino-functionalized polystyrene NPs,197 silica NPs,198 Temoporfin NPs,199 and glycocalyx-mimicking NPs,200 among others.201 Different surface modifications can affect this polarization of macrophages. Polyurethane NPs (PU NPs) can inhibit macrophage polarization toward the M1 phenotype; carboxyl modification on the surface caused greater inhibition than did amine modification. The inflammasome inhibition was mediated by PU NP-induced autophagy and NF-κB inactivation.202
Nanoparticles Induce Autophagy of Macrophages.
The toxicity effects of NPs are highly complex. There are reports that at high concentrations, silica NPs induce double-membrane vacuole production in macrophages, leading to their death, while at low concentrations the same NPs induce the M2 to M1 phenotype transition. Interestingly, the autophagy induced by the NPs at low concentration protects the macrophage from death.203
The majority of the synthetic NPs circulating in the body clear readily; however, the corona formed on cationic AuNPs by the intracellular proteins in macrophages make the NPs more difficult to remove, thus resulting in NP-mediated chronic toxicity. Coating the NPs with PEG, or other surface chemistry modifications, interferes with the interaction of the AuNPs with the intracellular proteins, reduces the intracellular agglomeration, and promotes macrophage exocytosis, thus decreasing toxicity.204
The Interactions between Nanoparticles and Cancer Associated Fibroblasts.
Cancer associated fibroblasts (CAFs) attract increasing research interest, due to their essential role in the TME. CAFs enhance the proliferation, migration, and invasion of cancer cells.205,206 As nanomedicine is applied in the treatment of cancer, it is essential to understand the interaction between NPs and CAFs. We have shown that pure 20 nm gold AuNPs dose-dependently inhibit the growth of CAFs, and blunt the enhancing effect of CAFs on proliferation, migration, and invasion of PCCs, thus slowing tumor growth. These data suggest that the AuNPs not only inhibit the proliferation of CAFs, but also prevent cross-talk between cancer cells and the CAFs (Figure 5).12 Another study demonstrated that metal NPs, both silver NPs (AgNPs) and AuNPs, may influence fibroblast function by inhibiting cell migration, reorganizing the cytoskeleton, negatively modulating the deposition of molecules constituting the ECM, and altering the expression of ECM receptors.207 As important components in the TME, CAFs are potential targets for cancer therapy; however, further research is necessary to fully develop this strategy.
Figure 5.
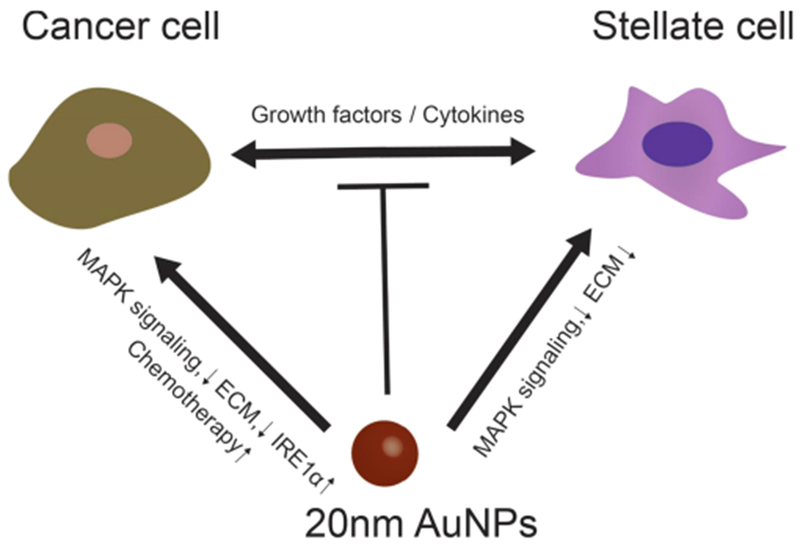
NPs inhibit the cross-talk between cancer cells and CAF cells. Gold NPs (AuNPs) inhibit the proliferation of cancer cells and stellate cells individually through several signal pathways such as preventing MAPK signaling and ECM construction. Moreover, AuNPs also decrease the expression of key modulators, such as growth factors and/or cytokines from cancer cells and fibroblasts, and thereby stop cross-talk between cells. MAPK: Mitogen-activated protein kinases. ECM: extracellular matrix. IRE1a: Inositol-requiring enzyme-1a. This figure is conceptually adapted from the TOC in ref 12.
The Interactions between Nanoparticles and Cancer Cells.
NPs have been extensively studied both as a vector for antitumor drug delivery and for their inherent ability to enhance tumor elimination.
Nanoparticles Induce Autophagy.
Many studies establish that NPs can directly induce autophagy. Harhaji et al. show that Nano-C60 induces autophagy in glioma cell lines, thus contributing to the cytostatic effect on cancer cells.208 The Wen group further explored the chemosensitization effect of Nano-C60 in cancer cells. When cells were treated with low doses of Nano-C60, DOX, or cisplatin, there was no significant effect on the cells. However, when the three agents were combined, cell death was greatly enhanced. The authors established that this chemosensitization effect was driven by the autophagy induced by Nano-C60.209 We now know that a large number of NPs induce autophagy, including rare earth oxide nanocrystals, titanium dioxide NPs,210,211 quantum dots, and neodymium oxides.212,213
The physical character of the NPs is an important factor in the induction of autophagy. Size, concentration, and dispersal conditions all impact autophagy. Compared to well-dispersed NPs, aggregated NPs induce significantly greater autophagy.214 Palladium NPs (PdNPs) affect autophagosome accumulation in two ways; at low concentrations, autophagy activation is through the mTOR signaling pathway, while at high concentrations, the autophagosome accumulation is dominated by the autophagic flux blockade resulting from lysosome impairment.215 Similarly, silica NPs induce autophagy at noncytotoxic levels, while they block autophagic flux at high doses.216 The size and charge of NPs is another important factor in modulating the induction of autophagy. Liang et al. found that AuNPs are taken up in a size-dependent manner by normal rat kidney cells; smaller particles must aggregate for efficient uptake to occur. Moreover, positively charged 50 nm AuNPs caused more autophagosome accumulation in the cell and more significant enlargement of lysosomes than negatively charged 50 nm AuNPs did.217 AgNPs showed the opposite size-dependent autophagic effect; 10 nm AgNPs enhanced autophagy induction and lysosomal activity more than either 50 or 100 nm AgNPs at noncytotoxic concentrations in human liver-derived hepatoma (HepG2) cells.218 Greater research efforts are necessary to fully elucidate the interactions between NPs and cancer cells.
Nanoparticles Inhibit Angiogenesis and Tumor Growth.
The inherent functions of unadorned NPs attract increasing research efforts. Our previous data show that 20 nm AuNPs inhibit the function of pro-angiogenic heparin-binding growth factors (HB-GFs), such as vascular endothelial growth factor 165 (VEGF165) and basic fibroblast growth factor (bFGF). However, surface modification of the AuNPs with various charged ligands prevents their ability to inhibit the HB-GFs function.219 The pure AuNPs also inhibited ovarian tumor growth in a mouse model by abrogating mitogen-activated protein kinase (MAPK) signaling and preventing EMT.220 Furthermore, AuNPs sensitize ovarian cancer cells to the anticancer drug cisplatin by reversing EMT, down-regulating stem cell markers, and preventing Akt/NF-κB signaling induction by cisplatin.179 In addition, AuNPs inhibit pancreatic tumor growth by impairing secretions of major hub node proteins and altering the cellular secretome through the ER-stress-regulated IRE1-dependent decay pathway.12
Nanoparticles Contribute to Oxidative Stress and Cell Death.
Oxidative stress is an imbalance between the production of reactive oxygen species (ROS, free radicals) and the antioxidant defense mechanisms of organisms. Oxidative stress contributes to cellular toxicity by inducing DNA damage, lipid peroxidation, and apoptosis, as well as activating signaling networks associated with decreased cell proliferation, fibrosis, and carcinogenesis.221,222 NPs can induce ROS in three distinct ways. First, NP surface-bound radicals, for example, SiO· and SiO2· on quartz particles, can lead to the production of ROS such as OH· and O2·−.223,224 Second, transition metals, including iron (Fe), copper (Cu), chromium (Cr), vanadium (V), and silica (Si), can react with endogenous or exogenous H2O2 to yield OH· and an oxidized metal ion.224 Finally, internalization of NPs by cells can directly induce signal pathways associated with ROS production. These signal pathways involve nuclear factor (erythroid-derived 2)-like 2 (Nrf2) induction and the activation of the mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) cascades. ROS may activate MAPK pathways via inhibition and/or degradation of MAPK phosphatases (MKP).225,226 CuS nanoparticles produce elevated ROS levels in tumor cells following laser radiation, block the gefitinib resistance induced by insulin growth factor-1 receptor (IGF1R) bypass activation, and down-regulate AKT/ERK/NF-κB signaling cascades.227 This is consistent with our previously published reports regarding AuNPs; we showed that 20 nm AuNPs inhibit cisplatin/gemcitabine-induced EMT, Akt, and NF-κB activation, thereby sensitizing cancer cells to chemotherapy.12,179
Nanoparticles Inhibit the Cross-Talk between Cancer Cells and Cancer Associated Fibroblasts/Endothelial Cells.
The cross-talk between TME and cancer cells is essential for tumor progression. By inhibiting the secretion of growth factors, cytokines, and chemokines, or the expression of new ligands which promote cancer cell survival, growth, or metastasis, stromal cells can regulate anticancer resistance and cancer recurrence.228 It has been shown that NPs function as an excellent reagent to induce autophagy in both cancer cells and CAFs, leading to decreased angiogenesis and tumor growth. AuNPs were found to prevent the cross-talk between cancer cells and CAFs. In an indirect coculture system, AuNPs decreased the enhancement effect of CAFs on PCCs’ proliferation, migration, and invasion, and vice versa.12 AuNP treatment also inhibits the stimulation of the fibrogenic response in pancreatic stellate cells (PSCs) by PCCs and signaling in PCCs by PSCs (Figure 5). All these data suggest that AuNPs function to prevent cross-talk between cancer cells and CAFs. We recently demonstrated that 20 nm AuNPs disrupt signal transduction from TME cells (CCs, CAFs, and ECs) to ECs and inhibit angiogenic phenotypes in vitro. When cultured with conditioned media (CM) from cells treated with AuNPs or cocultured with cells pretreated with AuNPs, both tube formation and migration of ECs were down-regulated. AuNPs removed ~95% of the VEGF165 from a VEGF single-protein solution and removed up to ~45% of VEGF165 from AuNP-treated CM.229 We have also demonstrated that 20 nm AuNPs reprogram activated pancreatic cancer associated fibroblasts to quiescence. This reprogramming to quiescence was mediated via regulation of expression of lipogenic genes such as fatty acid synthase (FASN), fatty acid binding protein 3 (FABP3), sterol regulatory element binding protein 2 (SREBP2), and lipid utilization.230
CONCLUSION AND CHALLENGES
Herein we have outlined how NPs interact with the TME, and how these interactions may be harnessed for their anticancer properties. Compared with the previous Review,122 here we emphasize the interaction between NPs having different physicochemical properties with the components of TME and reprogramming of TME by NPs. While nanomedicine is a promising tool for treatment of cancer, multiple issues need to be addressed in order to ensure a successful transition to the clinic. These issues include biocompatibility, pharmacokinetics, and efficient in vivo targeting. A major factor impacting these issues is the formation of the protein corona, and significant ongoing research efforts are required to address methods of either overcoming the deleterious impacts of the corona or harnessing the corona to generate positive outcomes. A further major issue is the potential toxicity of nanomaterials and associated health risks. This is of particular concern when considering inorganic nanomaterials, since they are likely to persist in the patient;231 efforts to ameliorate potential toxicity should be an additional focus of nanomaterial related research. Biodegradable nanomaterials25 are less likely to have serious toxicity issues, at least in the long term. Strategies to limit toxicity may include specific surface modification of the NP or utilization of the protein corona.
Although not within the purview of this Review article, identifying new molecular targets that exploit the protein corona formation around NPs is another emerging area of research. Characterizing the proteins present in the corona may provide information about the local proteome. Thus, analysis of the corona formed from disease samples such as cancer cell lysates or conditioned media, tumor tissue lysates, or patient plasma can provide information about the disease proteome. Comparison of the protein corona derived from normal samples with those from disease samples has the potential to identify targets characteristic of the disease. Importantly, formation of the protein corona could be tailored by careful decoration of the nanoparticle surface. This notion has recently been tested in a number of studies. We have demonstrated that the corona around 10 nm AuNP differs based on the surface properties of the nanosystem.232 Using this approach, we demonstrated that the hepatoma derived growth factor (HDGF) is a potential therapeutic target in ovarian cancer. Similarly, investigating the evolution of the protein corona around 20 nm AuNP, we also demonstrated that mostly basic proteins are enriched on unmodified AuNPs and no correlation was found with the molecular weights of the proteins. Using bioinformatics analysis, we identified SMNDC1, PPA1, and PI15 as potential therapeutic targets in ovarian cancer.233
In summary, modulation of the protein corona around unmodified and surface modified nanoparticles may provide unique opportunities to probe disease proteomes and identify new molecular targets responsible for disease outcome.
The past few years have witnessed a plethora of investigations to reprogram the TME in several cancers including pancreatic, ovarian, and breast cancers. These studies target components of the TME such as tumor cells, cancer associated fibroblasts, tumor endothelial cells, tumor-associated macrophages, and extracellular matrix components. However, an emerging and underrepresented area is alteration of the characteristics of the critical players in the TME via the action of NPs. This approach has recently been shown to be effective, e.g., by transforming activated fibroblasts to quiescence,230 by reversing the epithelial–mesenchymal transition in tumor cells to sensitize them to chemotherapy,179 and by disrupting triangular cross-talk to inhibit angiogenesis by gold nanoparticles.12,229 This line of investigation will not only convert “bad” cells to “good” but also help to identify critical molecules involved in their conversion and thus unravel new molecular machineries that otherwise would remain unknown.
In conclusion, despite the various problems that need to be resolved,234 nanomaterials represent a significant and extremely hopeful addition to the array of treatment options for cancer patients. Within the next few years, we anticipate that several NP-based treatments aimed at modifying the TME will have advanced to clinical trials to assess their benefit to cancer patients.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant 1R01 CA220237-01A1, 2CA136494, CA213278 (to P.M.).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Chen X, and Zhang W (2017) Diamond Nanostructures for Drug Delivery, Bioimaging, and Biosensing. Chem. Soc. Rev 46, 734–760. [DOI] [PubMed] [Google Scholar]
- (2).Teixeira MC, Carbone C, and Souto EB (2017) Beyond Liposomes: Recent Advances on Lipid Based Nanostructures for Poorly Soluble/Poorly Permeable Drug Delivery. Prog. Lipid Res 68, 1–11. [DOI] [PubMed] [Google Scholar]
- (3).Okholm AH, and Kjems J (2016) DNA Nanovehicles and the Biological Barriers. Adv. Drug Delivery Rev 106, 183–191. [DOI] [PubMed] [Google Scholar]
- (4).Cai C, Lin J, Lu Y, Zhang Q, and Wang L (2016) Polypeptide Self-Assemblies: Nanostructures and Bioapplications. Chem. Soc. Rev 45, 5985–6012. [DOI] [PubMed] [Google Scholar]
- (5).Zhang S, Geryak R, Geldmeier J, Kim S, and Tsukruk VV (2017) Synthesis, Assembly, and Applications of Hybrid Nanostructures for Biosensing. Chem. Rev 117, 12942–13038. [DOI] [PubMed] [Google Scholar]
- (6).Maeki M, Kimura N, Sato Y, Harashima H, and Tokeshi M (2018) Advances in Microfluidics for Lipid Nanoparticles and Extracellular Vesicles and Applications in Drug Delivery Systems. Adv. Drug Delivery Rev 128, 84–100. [DOI] [PubMed] [Google Scholar]
- (7).Ulbrich K, Hola K, Subr V, Bakandritsos A, Tucek J, and Zboril R (2016) Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev 116, 5338–5431. [DOI] [PubMed] [Google Scholar]
- (8).Thakor AS, Jokerst JV, Ghanouni P, Campbell JL, Mittra E, and Gambhir SS (2016) Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med 57, 1833–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Savla R, and Minko T (2017) Nanoparticle Design Considerations for Molecular Imaging of Apoptosis: Diagnostic, Prognostic, and Therapeutic Value. Adv. Drug Delivery Rev 113, 122–140. [DOI] [PubMed] [Google Scholar]
- (10).Fonjungo PN, Alemnji GA, Kebede Y, Opio A, Mwangi C, Spira TJ, Beard RS, and Nkengasong JN (2017) Combatting Global Infectious Diseases: A Network Effect of Specimen Referral Systems. Clin. Infect. Dis 64, 796. [DOI] [PubMed] [Google Scholar]
- (11).Irvine DJ, Hanson MC, Rakhra K, and Tokatlian T (2015) Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev 115, 11109–11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Saha S, Xiong X, Chakraborty PK, Shameer K, Arvizo RR, Kudgus RA, Dwivedi SK, Hossen MN, Gillies EM, Robertson JD, et al. (2016) Gold Nanoparticle Reprograms Pancreatic Tumor Microenvironment and Inhibits Tumor Growth. ACS Nano 10, 10636–10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shi J, Kantoff PW, Wooster R, and Farokhzad OC (2017) Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 17, 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wilhelm S, Tavares AJ, and Chan WCW (2016) Reply to “Evaluation of Nanomedicines: Stick to the Basics”. Nat. Rev. Mater 1, 1. [Google Scholar]
- (15).Chen X, Xia Q, Cao Y, Min Q, Zhang J, Chen Z, Chen HY, and Zhu JJ (2017) Imaging the Transient Heat Generation of Individual Nanostructures with a Mechanoresponsive Polymer. Nat. Commun 8, 1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Liu Y, Wei G, Cheng WA, Dong Z, Sun H, Lee VY, Cha SC, Smith DL, Kwak LW, and Qin H (2018) Targeting Myeloid-Derived Suppressor Cells for Cancer Immunotherapy. Cancer Immunol. Immunother 67, 1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kang S, Shin W, Choi MH, Ahn M, Kim YK, Kim S, Min DH, and Jang H (2018) Morphology-Controlled Synthesis of Rhodium Nanoparticles for Cancer Phototherapy. ACS Nano 12, 6997–7008. [DOI] [PubMed] [Google Scholar]
- (18).Lyu Y, Xie C, Chechetka SA, Miyako E, and Pu K (2016) Semiconducting Polymer Nanobioconjugates for Targeted Photothermal Activation of Neurons. J. Am. Chem. Soc 138, 9049–9052. [DOI] [PubMed] [Google Scholar]
- (19).Yue X, Zhang Q, and Dai Z (2017) Near-Infrared Light-Activatable Polymeric Nanoformulations for Combined Therapy and Imaging of Cancer. Adv. Drug Delivery Rev 115, 155–170. [DOI] [PubMed] [Google Scholar]
- (20).Chiu CF, Saidi WA, Kagan VE, and Star A (2017) Defect-Induced near-Infrared Photoluminescence of Single-Walled Carbon Nanotubes Treated with Polyunsaturated Fatty Acids. J. Am. Chem. Soc 139, 4859–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Chen YW, Su YL, Hu SH, and Chen SY (2016) Functionalized Graphene Nanocomposites for Enhancing Photothermal Therapy in Tumor Treatment. Adv. Drug Delivery Rev 105, 190–204. [DOI] [PubMed] [Google Scholar]
- (22).Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu Y, Yao Y, and Li D (2017) The Epithelial to Mesenchymal Transition (Emt) and Cancer Stem Cells: Implication for Treatment Resistance in Pancreatic Cancer. Mol. Cancer 16, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Guo B, Sheng Z, Hu D, Li A, Xu S, Manghnani PN, Liu C , Guo L, Zheng H, and Liu B (2017) Molecular Engineering of Conjugated Polymers for Biocompatible Organic Nanoparticles with Highly Efficient Photoacoustic and Photothermal Performance in Cancer Theranostics. ACS Nano 11, 10124–10134. [DOI] [PubMed] [Google Scholar]
- (24).Qi J, Fang Y, Kwok RTK, Zhang X, Hu X, Lam JWY, Ding D, and Tang BZ (2017) Highly Stable Organic Small Molecular Nanoparticles as an Advanced and Biocompatible Phototheranostic Agent of Tumor in Living Mice. ACS Nano 11 , 7177–7188. [DOI] [PubMed] [Google Scholar]
- (25).Lazarovits J, Chen YY, Song F, Ngo W, Tavares AJ, Zhang YN, Audet J, Tang B, Lin Q, Tleugabulova MC, et al. (2019) Synthesis of Patient-Specific Nanomaterials. Nano Lett. 19, 116–123. [DOI] [PubMed] [Google Scholar]
- (26).Wen S, Zhou J, Zheng K, Bednarkiewicz A, Liu X, and Jin D, (2018) Advances in Highly Doped Upconversion Nanoparticles. Nat. Commun 9, 2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wilhelm S (2017) Perspectives for Upconverting Nanoparticles. ACS Nano 11, 10644–10653. [DOI] [PubMed] [Google Scholar]
- (28).Khutoryanskiy VV (2018) Beyond Pegylation: Alternative Surface-Modification of Nanoparticles with Mucus-Inert Biomaterials. Adv. Drug Delivery Rev 124, 140–149. [DOI] [PubMed] [Google Scholar]
- (29).Sedlmeier A, and Gorris HH (2015) Surface Modification and Characterization of Photon-Upconverting Nanoparticles for Bioanalytical Applications. Chem. Soc. Rev 44, 1526–1560. [DOI] [PubMed] [Google Scholar]
- (30).Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak H,F, and Chan WCW (2016) Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater 1, 1. [Google Scholar]
- (31).Maeda H, Nakamura H, and Fang J (2013) The Epr Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in Vivo. Adv. Drug Delivery Rev 65, 71–79. [DOI] [PubMed] [Google Scholar]
- (32).Dai Q, Wilhelm S, Ding D, Syed AM, Sindhwani S, Zhang Y, Chen YY, MacMillan P, and Chan WCW (2018) Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 12, 8423–8435. [DOI] [PubMed] [Google Scholar]
- (33).Lange S, Saur D, and Rad R (2016) Sirna-Coupled Nanoparticles for Improved Therapeutic Targeting of Pancreatic Cancer. Gut 65, 1780–1781. [DOI] [PubMed] [Google Scholar]
- (34).Mahajan UM, Teller S, Sendler M, Palankar R, van den Brandt C, Schwaiger T, Kuhn JP, Ribback S, Glockl G, Evert M, et al. (2016) Tumour-Specific Delivery of Sirna-Coupled Superparamagnetic Iron Oxide Nanoparticles, Targeted against Plk1, Stops Progression of Pancreatic Cancer. Gut 65, 1838–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Salahandish R, Ghaffarinejad A, Naghib SM, Majidzadeh AK, Zargartalebi H, and Sanati-Nezhad A (2018) Nano-Biosensor for Highly Sensitive Detection of Her2 Positive Breast Cancer. Biosens. Bioelectron 117, 104–111. [DOI] [PubMed] [Google Scholar]
- (36).Shi Y, Zhou M, Zhang J, and Lu W (2015) Preparation and Cellular Targeting Study of Vegf-Conjugated Plga Nanoparticles. J. Microencapsulation 32, 699–704. [DOI] [PubMed] [Google Scholar]
- (37).Donahue ND, Acar H, and Wilhelm S (2019) Concepts of Nanoparticle Cellular Uptake, Intracellular Trafficking, and Kinetics in Nanomedicine. Adv. Drug Delivery Rev, DOI: 10.1016/j.addr.2019.04.008. [DOI] [PubMed] [Google Scholar]
- (38).De Palma M, Biziato D, and Petrova TV (2017) Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 17, 457–474. [DOI] [PubMed] [Google Scholar]
- (39).Wilson WR, and Hay MP (2011) Targeting Hypoxia in Cancer Therapy. Nat. Rev. Cancer 11, 393–410. [DOI] [PubMed] [Google Scholar]
- (40).Corbet C, and Feron O (2017) Tumour Acidosis: From the Passenger to the Driver’s Seat. Nat. Rev. Cancer 17, 577–593. [DOI] [PubMed] [Google Scholar]
- (41).Huckaby JT, and Lai SK (2018) Pegylation for Enhancing Nanoparticle Diffusion in Mucus. Adv. Drug Delivery Rev 124, 125–139. [DOI] [PubMed] [Google Scholar]
- (42).Ku SH, Jo SD, Lee YK, Kim K, and Kim SH (2016) Chemical and Structural Modifications of Rnai Therapeutics. Adv. Drug Delivery Rev 104, 16–28. [DOI] [PubMed] [Google Scholar]
- (43).Roy A, and Li SD (2016) Modifying the Tumor Microenvironment Using Nanoparticle Therapeutics. Wiley Interdiscip Rev. Nanomed Nanobiotechnol 8, 891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Fidler IJ (2003) The Pathogenesis of Cancer Metastasis: The ‘Seed and Soil’ Hypothesis Revisited. Nat. Rev. Cancer 3, 453–458. [DOI] [PubMed] [Google Scholar]
- (45).McDonald DM, and Choyke PL (2003) Imaging of Angiogenesis: From Microscope to Clinic. Nat. Med 9, 713–725. [DOI] [PubMed] [Google Scholar]
- (46).Hida K, Maishi N, Sakurai Y, Hida Y, and Harashima H (2016) Heterogeneity of Tumor Endothelial Cells and Drug Delivery. Adv. Drug Delivery Rev 99, 140–147. [DOI] [PubMed] [Google Scholar]
- (47).Maishi N, and Hida K (2017) Tumor Endothelial Cells Accelerate Tumor Metastasis. Cancer Sci 108, 1921–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Acharya S, and Sahoo SK (2011) Plga Nanoparticles Containing Various Anticancer Agents and Tumour Delivery by Epr Effect. Adv. Drug Delivery Rev 63, 170–183. [DOI] [PubMed] [Google Scholar]
- (49).Poon W, Zhang YN, Ouyang B, Kingston BR, Wu JLY, Wilhelm S, and Chan WCW (2019) Elimination Pathways of Nanoparticles. ACS Nano 13, 5785–5798. [DOI] [PubMed] [Google Scholar]
- (50).Muraki C, Ohga N, Hida Y, Nishihara H, Kato Y, Tsuchiya K, Matsuda K, Totsuka Y, Shindoh M, and Hida K (2012) Cyclooxygenase-2 Inhibition Causes Antiangiogenic Effects on Tumor Endothelial and Vascular Progenitor Cells. Int. J. Cancer 130, 59–70. [DOI] [PubMed] [Google Scholar]
- (51).Helske S, Laine M, Kupari M, Lommi J, Turto H, Nurmi L, Tikkanen I, Werkkala K, Lindstedt KA, and Kovanen PT (2007) Increased Expression of Profibrotic Neutral Endopeptidase and Bradykinin Type 1 Receptors in Stenotic Aortic Valves. Eur. Heart J 28, 1894–1903. [DOI] [PubMed] [Google Scholar]
- (52).Forstermann U, and Sessa WC (2012) Nitric Oxide Synthases: Regulation and Function. Eur. Heart J 33, 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Ferdinandy P, Danial H, Ambrus I, Rothery RA, and Schulz R (2000) Peroxynitrite Is a Major Contributor to Cytokine-Induced Myocardial Contractile Failure. Circ. Res 87, 241–247. [DOI] [PubMed] [Google Scholar]
- (54).Su B, Wang R, Xie Z, Ruan H, Li J, Xie C, Lu W, Wang J, Wang D, and Liu M (2018) Effect of Retro-Inverso Isomer of Bradykinin on Size-Dependent Penetration of Blood-Brain Tumor Barrier. Small 14, 1702331. [DOI] [PubMed] [Google Scholar]
- (55).Wang X, Yang C, Zhang Y, Zhen X, Wu W, and Jiang X (2014) Delivery of Platinum(Iv) Drug to Subcutaneous Tumor and Lung Metastasis Using Bradykinin-Potentiating Peptide-Decorated Chitosan Nanoparticles. Biomaterials 35, 6439–6453. [DOI] [PubMed] [Google Scholar]
- (56).Dai Q, Wilhelm S, Ding D, Syed AM, Sindhwani S, Zhang YW, Chen YY, MacMillan P, and Chan WCW (2018) Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 12, 8423–8435. [DOI] [PubMed] [Google Scholar]
- (57).Ishii G, Ochiai A, and Neri S (2016) Phenotypic and Functional Heterogeneity of Cancer-Associated Fibroblast within the Tumor Microenvironment. Adv. Drug Delivery Rev 99, 186–196. [DOI] [PubMed] [Google Scholar]
- (58).Kalluri R (2016) The Biology and Function of Fibroblasts in Cancer. Nat. Rev. Cancer 16, 582–598. [DOI] [PubMed] [Google Scholar]
- (59).Quail DF, and Joyce JA (2013) Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med 19, 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Yu S, Jiang Y, Wan F, Wu J, Gao Z, and Liu D (2017) Immortalized Cancer-Associated Fibroblasts Promote Prostate Cancer Carcinogenesis, Proliferation and Invasion. Anticancer Res. 37, 4311–4318. [DOI] [PubMed] [Google Scholar]
- (61).Mahale J, Smagurauskaite G, Brown K, Thomas A, and Howells LM (2016) The Role of Stromal Fibroblasts in Lung Carcinogenesis: A Target for Chemoprevention? Int. J. Cancer 138, 30–44. [DOI] [PubMed] [Google Scholar]
- (62).Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, et al. (2018) Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 33, 463–479. [DOI] [PubMed] [Google Scholar]
- (63).Zhang Q, Yang J, Bai J, and Ren J (2018) Reverse of Non-Small Cell Lung Cancer Drug Resistance Induced by Cancer-Associated Fibroblasts Via a Paracrine Pathway. Cancer Sci. 109, 944–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Wang L, Liu X, Zhou Q, Sui M, Lu Z, Zhou Z, Tang J, Miao Y, Zheng M, Wang W, et al. (2017) Terminating the Criminal Collaboration in Pancreatic Cancer: Nanoparticle-Based Synergistic Therapy for Overcoming Fibroblast-Induced Drug Resistance. Biomaterials 144, 105–118. [DOI] [PubMed] [Google Scholar]
- (65).Li L, Zhou S, Lv N, Zhen Z, Liu T, Gao S, Xie J, and Ma Q (2018) Photosensitizer-Encapsulated Ferritins Mediate Photodynamic Therapy against Cancer-Associated Fibroblasts and Improve Tumor Accumulation of Nanoparticles. Mol. Pharmaceutics 15, 3595–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Kise K, Kinugasa-Katayama Y, and Takakura N (2016) Tumor Microenvironment for Cancer Stem Cells. Adv. Drug Delivery Rev 99, 197–205. [DOI] [PubMed] [Google Scholar]
- (67).Krause M, Dubrovska A, Linge A, and Baumann M (2017) Cancer Stem Cells: Radioresistance, Prediction of Radiotherapy Outcome and Specific Targets for Combined Treatments. Adv. Drug Delivery Rev 109, 63–73. [DOI] [PubMed] [Google Scholar]
- (68).Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, , Jones DL, Visvader J, Weissman IL, and Wahl GM (2006) Cancer Stem Cells-Perspectives on Current Status and Future Directions: Aacr Workshop on Cancer Stem Cells. Cancer Res. 66, 9339–9344. [DOI] [PubMed] [Google Scholar]
- (69).Putzer BM, Solanki M, and Herchenroder O (2017) Advances in Cancer Stem Cell Targeting: How to Strike the Evil at Its Root. Adv. Drug Delivery Rev 120, 89–107. [DOI] [PubMed] [Google Scholar]
- (70).Haabeth OA, Lorvik KB, Hammarstrom C, Donaldson IM, Haraldsen G, Bogen B, and Corthay A (2011) Inflammation Driven by Tumour-Specific Th1 Cells Protects against B-Cell Cancer. Nat. Commun 2, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, and Protti MP (2011) Intratumor T Helper Type 2 Cell Infiltrate Correlates with Cancer-Associated Fibroblast Thymic Stromal Lymphopoietin Production and Reduced Survival in Pancreatic Cancer. J. Exp. Med 208, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Adeegbe DO, and Nishikawa H (2013) Natural and Induced T Regulatory Cells in Cancer. Front. Immunol 4, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Whiteside TL, Schuler P, and Schilling B (2012) Induced and Natural Regulatory T Cells in Human Cancer. Expert Opin. Biol. Ther 12, 1383–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Bronte V (2008) Th17 and Cancer: Friends or Foes? Blood 112, 214. [DOI] [PubMed] [Google Scholar]
- (75).Reading JL, Galvez-Cancino F, Swanton C, Lladser A, Peggs KS, and Quezada SA (2018) The Function and Dysfunction of Memory Cd8(+) T Cells in Tumor Immunity. Immunol Rev. 283, 194–212. [DOI] [PubMed] [Google Scholar]
- (76).Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, and Shevach EM (2004) Engagement of Glucocorticoid-Induced Tnfr Family-Related Receptor on Effector T Cells by Its Ligand Mediates Resistance to Suppression by Cd4+Cd25+ T Cells. J. Immunol 173, 5008–5020. [DOI] [PubMed] [Google Scholar]
- (77).Mantovani A, Marchesi F, Malesci A, Laghi L, and Allavena P (2017) Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol 14, 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Chiang CL, Balint K, Coukos G, and Kandalaft LE (2015) Potential Approaches for More Successful Dendritic Cell-Based Immunotherapy. Expert Opin. Biol. Ther 15, 569–582. [DOI] [PubMed] [Google Scholar]
- (79).Beaty BT, and Condeelis J (2014) Digging a Little Deeper: The Stages of Invadopodium Formation and Maturation. Eur. J. Cell Biol 93, 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. (2009) Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 139, 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Lu P, Weaver VM, and Werb Z (2012) The Extracellular Matrix: A Dynamic Niche in Cancer Progression. J. Cell Biol 196, 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Pickup MW, Mouw JK, and Weaver VM (2014) The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep. 15, 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Ramaswamy S, Ross KN, Lander ES, and Golub TR (2003) A Molecular Signature of Metastasis in Primary Solid Tumors. Nat. Genet 33, 49–54. [DOI] [PubMed] [Google Scholar]
- (84).Yonenaga Y, Mori A, Onodera H, Yasuda S, Oe H, Fujimoto A, Tachibana T, and Imamura M (2005) Absence of Smooth Muscle Actin-Positive Pericyte Coverage of Tumor Vessels Correlates with Hematogenous Metastasis and Prognosis of Colorectal Cancer Patients. Oncology 69, 159–166. [DOI] [PubMed] [Google Scholar]
- (85).Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, and Betsholtz C (2002) Analysis of Mural Cell Recruitment to Tumor Vessels. Circulation 105, 112–117. [DOI] [PubMed] [Google Scholar]
- (86).Chantrain CF, Henriet P, Jodele S, Emonard H, Feron O, Courtoy PJ, DeClerck YA, and Marbaix E (2006) Mechanisms of Pericyte Recruitment in Tumour Angiogenesis: A New Role for Metalloproteinases. Eur. J. Cancer 42, 310–318. [DOI] [PubMed] [Google Scholar]
- (87).Chen G, Chen SM, Wang X, Ding XF, Ding J, and Meng LH (2012) Inhibition of Chemokine (Cxc Motif) Ligand 12/Chemokine (Cxc Motif) Receptor 4 Axis (Cxcl12/Cxcr4)-Mediated Cell Migration by Targeting Mammalian Target of Rapamycin (Mtor) Pathway in Human Gastric Carcinoma Cells. J. Biol. Chem 287, 12132–12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Yu M, and Zheng J (2015) Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 9, 6655–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Fang J, Nakamura H, and Maeda H (2011) The Epr Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Delivery Rev 63, 136–151. [DOI] [PubMed] [Google Scholar]
- (90).Matsumura Y, and Maeda H (1986) A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 46, 6387–6392. [PubMed] [Google Scholar]
- (91).Tong X, Wang Z, Sun X, Song J, Jacobson O, Niu G, Kiesewetter DO, and Chen X (2016) Size Dependent Kinetics of Gold Nanorods in Epr Mediated Tumor Delivery. Theranostics 6, 2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, and Discher DE (2007) Shape Effects of Filaments Versus Spherical Particles in Flow and Drug Delivery. Nat. Nanotechnol 2, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Smith BR, Kempen P, Bouley D, Xu A, Liu Z, Melosh N, Dai H, Sinclair R, and Gambhir SS (2012) Shape Matters: Intravital Microscopy Reveals Surprising Geometrical Dependence for Nanoparticles in Tumor Models of Extravasation. Nano Lett. 12, 3369–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Xu Q, Ensign LM, Boylan NJ, Schon A, Gong X, Yang JC, Lamb NW, Cai S, Yu T, Freire E, et al. (2015) Impact of Surface Polyethylene Glycol (Peg) Density on Biodegradable Nanoparticle Transport in Mucus Ex Vivo and Distribution in Vivo. ACS Nano 9, 9217–9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Nichols JW, and Bae YH (2014) Epr: Evidence and Fallacy. J. Controlled Release 190, 451–464. [DOI] [PubMed] [Google Scholar]
- (96).Danhier F (2016) To Exploit the Tumor Microenvironment: Since the Epr Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Controlled Release 244, 108–121. [DOI] [PubMed] [Google Scholar]
- (97).Dings RP, van der Schaft DW, Hargittai B, Haseman J, Griffioen AW, and Mayo KH (2003) Anti-Tumor Activity of the Novel Angiogenesis Inhibitor Anginex. Cancer Lett. 194, 55–66. [DOI] [PubMed] [Google Scholar]
- (98).Danhier F, Pourcelle V, Marchand-Brynaert J, Jerome C, Feron O, and Preat V (2012) Targeting of Tumor Endothelium by Rgd-Grafted Plga-Nanoparticles. Methods Enzymol 508, 157–175. [DOI] [PubMed] [Google Scholar]
- (99).Kluza E, van der Schaft DW, Hautvast PA, Mulder WJ, Mayo KH, Griffioen AW, Strijkers GJ, and Nicolay K (2010) Synergistic Targeting of Alphavbeta3 Integrin and Galectin-1 with Heteromultivalent Paramagnetic Liposomes for Combined Mr Imaging and Treatment of Angiogenesis. Nano Lett. 10, 52–58. [DOI] [PubMed] [Google Scholar]
- (100).Zhu X, Guo X, Liu D, Gong Y, Sun J, and Dong C (2017) Promotion of Propranolol Delivery to Hemangiomas by Using Anti-Vegfr Antibody-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles. J. Biomed. Nanotechnol 13, 1694–1705. [DOI] [PubMed] [Google Scholar]
- (101).Dewhirst MW, Cao Y, and Moeller B (2008) Cycling Hypoxia and Free Radicals Regulate Angiogenesis and Radiotherapy Response. Nat. Rev. Cancer 8, 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, and Mazzone M (2014) Tumor Stroma: A Complexity Dictated by the Hypoxic Tumor Microenvironment. Oncogene 33, 1743–1754. [DOI] [PubMed] [Google Scholar]
- (103).Sharma S, and Rawat D (2019) Partial Pressure of Oxygen (Po2). StatPearls [Internet], StatPearls Publishing, Treasure Island, FL. [Google Scholar]
- (104).Fleming IN, Manavaki R, Blower PJ, West C, Williams KJ, Harris AL, Domarkas J, Lord S, Baldry C, and Gilbert FJ (2015) Imaging Tumour Hypoxia with Positron Emission Tomography. Br. J. Cancer 112, 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Kwatra D, Venugopal S, and Anant S (2013) Nanoparticles in Radiation Therapy: A Summary of Various Approaches to Enhance Radiosensitization in Cancer. Transl. Cancer Res 2, 330–342. [Google Scholar]
- (106).Yoshimura M, Itasaka S, Harada H, and Hiraoka M (2013) Microenvironment and Radiation Therapy. BioMed Res. Int 2013, 685308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Barcellos-Hoff MH, and Cordes N (2007) Radiation Therapy and the Microenvironment. Int. J. Radiat. Biol 83, 723–725. [DOI] [PubMed] [Google Scholar]
- (108).Lee HY, Kim HW, Lee JH, and Oh SH (2015) Controlling Oxygen Release from Hollow Microparticles for Prolonged Cell Survival under Hypoxic Environment. Biomaterials 53, 583–591. [DOI] [PubMed] [Google Scholar]
- (109).Teicher BA, and Rose CM (1984) Perfluorochemical Emulsions Can Increase Tumor Radiosensitivity. Science 223, 934–936. [DOI] [PubMed] [Google Scholar]
- (110).Song G, Ji C, Liang C, Song X, Yi X, Dong Z, Yang K, and Liu Z (2017) Taox Decorated Perfluorocarbon Nanodroplets as Oxygen Reservoirs to Overcome Tumor Hypoxia and Enhance Cancer Radiotherapy. Biomaterials 112, 257–263. [DOI] [PubMed] [Google Scholar]
- (111).Piras AM, Dessy A, Chiellini F, Chiellini E, Farina C, Ramelli M, and Della Valle E (2008) Polymeric Nanoparticles for Hemoglobin-Based Oxygen Carriers. Biochim. Biophys. Acta, Proteins Proteomics 1784, 1454–1461. [DOI] [PubMed] [Google Scholar]
- (112).Jia Y, Duan L, and Li J (2016) Hemoglobin-Based Nanoarchitectonic Assemblies as Oxygen Carriers. Adv. Mater 28, 1312–1318. [DOI] [PubMed] [Google Scholar]
- (113).Silva I, Rausch V, Peccerella T, Millonig G, Seitz HK, and Mueller S (2018) Hypoxia Enhances H2o2-Mediated Upregulation of Hepcidin: Evidence for Nox4-Mediated Iron Regulation. Redox Biol. 16, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Song G, Chen Y, Liang C, Yi X, Liu J, Sun X, Shen S, Yang K, and Liu Z (2016) Catalase-Loaded Taox Nanoshells as Bio-Nanoreactors Combining High-Z Element and Enzyme Delivery for Enhancing Radiotherapy. Adv. Mater 28, 7143–7148. [DOI] [PubMed] [Google Scholar]
- (115).Ellis LM, and Hicklin DJ (2008) Vegf-Targeted Therapy: Mechanisms of Anti-Tumour Activity. Nat. Rev. Cancer 8, 579–591. [DOI] [PubMed] [Google Scholar]
- (116).Zhang C, Ni D, Liu Y, Yao H, Bu W, and Shi J (2017) Magnesium Silicide Nanoparticles as a Deoxygenation Agent for Cancer Starvation Therapy. Nat. Nanotechnol 12, 378–386. [DOI] [PubMed] [Google Scholar]
- (117).Liu Y, Liu Y, Bu W, Cheng C, Zuo C, Xiao Q, Sun Y, Ni D, Zhang C, Liu J, et al. (2015) Hypoxia Induced by Upconversion-Based Photodynamic Therapy: Towards Highly Effective Synergistic Bioreductive Therapy in Tumors. Angew. Chem. Int. Ed 54, 8105–8109. [DOI] [PubMed] [Google Scholar]
- (118).Zhu H, Zhang L, Liu Y, Zhou Y, Wang K, Xie X, Song L, Wang D, Han C, and Chen Q (2016) Aptamer-Peg-Modified Fe3o4@Mn as a Novel T1- and T2-Dual-Model Mri Contrast Agent Targeting Hypoxia-Induced Cancer Stem Cells. Sci. Rep 6, 39245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Vander Heiden MG, Cantley LC, and Thompson CB (2009) Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 324, 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, Nagano T, Watanabe T, Hasegawa A, Choyke PL, et al. (2009) Selective Molecular Imaging of Viable Cancer Cells with Ph-Activatable Fluorescence Probes. Nat. Med 15, 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Cardone RA, Casavola V, and Reshkin SJ (2005) The Role of Disturbed Ph Dynamics and the Na+/H+ Exchanger in Metastasis. Nat. Rev. Cancer 5, 786–795. [DOI] [PubMed] [Google Scholar]
- (122).Dai Y, Xu C, Sun X, and Chen X (2017) Nanoparticle Design Strategies for Enhanced Anticancer Therapy by Exploiting the Tumour Microenvironment. Chem. Soc. Rev 46, 3830–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Brisson L, Reshkin SJ, Gore J, and Roger S (2012) Ph Regulators in Invadosomal Functioning: Proton Delivery for Matrix Tasting. Eur. J. Cell Biol 91, 847–860. [DOI] [PubMed] [Google Scholar]
- (124).Justus CR, Dong L, and Yang LV (2013) Acidic Tumor Microenvironment and Ph-Sensing G Protein-Coupled Receptors. Front. Physiol 4, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Cairns R, Papandreou I, and Denko N (2006) Overcoming Physiologic Barriers to Cancer Treatment by Molecularly Targeting the Tumor Microenvironment. Mol. Cancer Res 4, 61–70. [DOI] [PubMed] [Google Scholar]
- (126).Huo M, Wang L, Chen Y, and Shi J (2017) Tumor-Selective Catalytic Nanomedicine by Nanocatalyst Delivery. Nat. Commun 8, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Zhang C, Zhao K, Bu W, Ni D, Liu Y, Feng J, and Shi J (2015) Marriage of Scintillator and Semiconductor for Synchronous Radiotherapy and Deep Photodynamic Therapy with Diminished Oxygen Dependence. Angew. Chem., Int. Ed 54, 1770–1774. [DOI] [PubMed] [Google Scholar]
- (128).Liu Y, Zhi X, Yang M, Zhang J, Lin L, Zhao X, Hou W, Zhang C, Zhang Q, Pan F, et al. (2017) Tumor-Triggered Drug Release from Calcium Carbonate-Encapsulated Gold Nanostars for near-Infrared Photodynamic/Photothermal Combination Antitumor Therapy. Theranostics 7, 1650–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Wei J, Cheang T, Tang B, Xia H, Xing Z, Chen Z, Fang Y, Chen W, Xu A, Wang S, et al. (2013) The Inhibition of Human Bladder Cancer Growth by Calcium Carbonate/Caip6 Nanocomposite Particles Delivering Aib1 Sirna. Biomaterials 34, 1246–1254. [DOI] [PubMed] [Google Scholar]
- (130).Ma J, Wu H, Li Y, Liu Z, Liu G, Guo Y, Hou Z, Zhao Q, Chen D, and Zhu X (2018) Novel Core-Interlayer-Shell Dox/Znpc Co-Loaded Msns@ Ph-Sensitive Cap@Pegylated Liposome for Enhanced Synergetic Chemo-Photodynamic Therapy. Pharm. Res 35, 57. [DOI] [PubMed] [Google Scholar]
- (131).Shah SA, Sohail M, Minhas MU, Nisar Ur R, Khan S, Hussain Z, Mudassir, Mahmood A, Kousar M, and Mahmood A (2019) Ph-Responsive Cap-Co-Poly(Methacrylic Acid)-Based Hydrogel as an Efficient Platform for Controlled Gastrointestinal Delivery: Fabrication, Characterization, in Vitro and in Vivo Toxicity Evaluation. Drug Delivery Transl Res. 9, 555–577. [DOI] [PubMed] [Google Scholar]
- (132).Cai X, Luo Y, Zhang W, Du D, and Lin Y (2016) Ph-Sensitive Zno Quantum Dots-Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl Mater. Interfaces 8, 22442–22450. [DOI] [PubMed] [Google Scholar]
- (133).Ye DX, Ma YY, Zhao W, Cao HM, Kong JL, Xiong HM, and Mohwald H (2016) Zno-Based Nanoplatforms for Labeling and Treatment of Mouse Tumors without Detectable Toxic Side Effects. ACS Nano 10, 4294–4300. [DOI] [PubMed] [Google Scholar]
- (134).Chen Z, Yin JJ, Zhou YT, Zhang Y, Song L, Song M, Hu S, and Gu N (2012) Dual Enzyme-Like Activities of Iron Oxide Nanoparticles and Their Implication for Diminishing Cytotoxicity. ACS Nano 6, 4001–4012. [DOI] [PubMed] [Google Scholar]
- (135).Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, et al. (2007) Intrinsic Peroxidase-Like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol 2, 577–583. [DOI] [PubMed] [Google Scholar]
- (136).Tang Z, Zhang H, Liu Y, Ni D, Zhang H, Zhang J, Yao Z, He M, Shi J, and Bu W (2017) Antiferromagnetic Pyrite as the Tumor Microenvironment-Mediated Nanoplatform for Self-Enhanced Tumor Imaging and Therapy. Adv. Mater 29, 1701683. [DOI] [PubMed] [Google Scholar]
- (137).Tang Z, Liu Y, He M, and Bu W (2019) Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-Like Reactions. Angew. Chem. Int. Ed 58, 946–956. [DOI] [PubMed] [Google Scholar]
- (138).Min KH, Min HS, Lee HJ, Park DJ, Yhee JY, Kim K, Kwon IC, Jeong SY, Silvestre OF, Chen X, et al. (2015) Ph-Controlled Gas-Generating Mineralized Nanoparticles: A Theranostic Agent for Ultrasound Imaging and Therapy of Cancers. ACS Nano 9, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Dong Z, Feng L, Hao Y, Chen M, Gao M, Chao Y, Zhao H, Zhu W, Liu J, Liang C, et al. (2018) Synthesis of Hollow Biomineralized Caco3-Polydopamine Nanoparticles for Multimodal Imaging-Guided Cancer Photodynamic Therapy with Reduced Skin Photosensitivity. J. Am. Chem. Soc 140, 2165–2178. [DOI] [PubMed] [Google Scholar]
- (140).Zhou H, Wei J, Dai Q, Wang L, Luo J, Cheang T, and Wang S (2015) Caco(3)/Caip(6) Composite Nanoparticles Effectively Deliver Akt1 Small Interfering Rna to Inhibit Human Breast Cancer Growth. Int. J. Nanomed 10, 4255–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Zhang J, Wu D, Li MF, and Feng J (2015) Multifunctional Mesoporous Silica Nanoparticles Based on Charge-Reversal Plug-Gate Nanovalves and Acid-Decomposable Zno Quantum Dots for Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 7, 26666–26673. [DOI] [PubMed] [Google Scholar]
- (142).Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, and Liang XJ (2014) Ph-Sensitive Nano-Systems for Drug Delivery in Cancer Therapy. Biotechnol. Adv 32, 693–710. [DOI] [PubMed] [Google Scholar]
- (143).Mai BT, Fernandes S, Balakrishnan PB, and Pellegrino T (2018) Nanosystems Based on Magnetic Nanoparticles and Thermo- or Ph-Responsive Polymers: An Update and Future Perspectives. Acc. Chem. Res 51, 999–1013. [DOI] [PubMed] [Google Scholar]
- (144).Yang X, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Matson VZ, Steeber DA, and Gong S (2010) Multifunctional Stable and Ph-Responsive Polymer Vesicles Formed by Heterofunctional Triblock Copolymer for Targeted Anticancer Drug Delivery and Ultrasensitive Mr Imaging. ACS Nano 4, 6805–6817. [DOI] [PubMed] [Google Scholar]
- (145).Han J, Sun J, Bai S, Panezai H, Jin X, and Wu X (2015) “Graft to” Synthesis and Ibuprofen-Loading Performance of Ph-Sensitive Pmaa-Silica Hybrid Nanoparticles with Controlled Bimodal Mesopores. J. Pharm. Sci 104, 4299–4306. [DOI] [PubMed] [Google Scholar]
- (146).Xu Q, Huang W, Jiang L, Lei Z, Li X, and Deng H (2013) Kgm and Pmaa Based Ph-Sensitive Interpenetrating Polymer Network Hydrogel for Controlled Drug Release. Carbohydr. Polym 97, 565–570. [DOI] [PubMed] [Google Scholar]
- (147).Liu Y, Li XS, Hu J, Guo M, Liu WJ, Feng YM, Xie JR, and Du GX (2015) Fabrication of Mpeg-B-Pmaa Capped Yvo4:Eu Nanoparticles with Biocompatibility for Cell Imaging. Colloids Surf., B 136, 721–728. [DOI] [PubMed] [Google Scholar]
- (148).Lindau D, Gielen P, Kroesen M, Wesseling P, and Adema GJ (2013) The Immunosuppressive Tumour Network: Myeloid-Derived Suppressor Cells, Regulatory T Cells and Natural Killer T Cells. Immunology 138, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, and Di W (2014) A High M1/M2 Ratio of Tumor-Associated Macrophages Is Associated with Extended Survival in Ovarian Cancer Patients. J. Ovarian Res 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. (2014) Targeting Tumor-Associated Macrophages with Anti-Csf-1r Antibody Reveals a Strategy for Cancer Therapy. Cancer Cell 25, 846–859. [DOI] [PubMed] [Google Scholar]
- (151).Song M, Liu T, Shi C, Zhang X, and Chen X (2016) Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages toward M1-Like Phenotype and Attenuating Tumor Hypoxia. ACS Nano 10, 633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Zhu S, Niu M, O’Mary H, and Cui Z (2013) Targeting of Tumor-Associated Macrophages Made Possible by Peg-Sheddable, Mannose-Modified Nanoparticles. Mol. Pharmaceutics 10, 3525–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, et al. (2016) Iron Oxide Nanoparticles Inhibit Tumour Growth by Inducing Pro-Inflammatory Macrophage Polarization in Tumour Tissues. Nat. Nanotechnol 11, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).MacParland SA, Tsoi KM, Ouyang B, Ma XZ, Manuel J, Fawaz A, Ostrowski MA, Alman BA, Zilman A, Chan WC, et al. (2017) Phenotype Determines Nanoparticle Uptake by Human Macrophages from Liver and Blood. ACS Nano 11, 2428–2443. [DOI] [PubMed] [Google Scholar]
- (155).Kullberg M, Martinson H, Mann K, and Anchordoquy TJ (2015) Complement C3Mediated Targeting of Liposomes to Granulocytic Myeloid Derived Suppressor Cells. Nanomedicine 11, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, and Gabrilovich DI (2006) All-Trans-Retinoic Acid Improves Differentiation of Myeloid Cells and Immune Response in Cancer Patients. Cancer Res. 66, 9299–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Kong M, Tang J, Qiao Q, Wu T, Qi Y, Tan S, Gao X, and Zhang Z (2017) Biodegradable Hollow Mesoporous Silica Nanoparticles for Regulating Tumor Microenvironment and Enhancing Antitumor Efficiency. Theranostics 7, 3276–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Sasso MS, Lollo G, Pitorre M, Solito S, Pinton L, Valpione S, Bastiat G, Mandruzzato S, Bronte V, Marigo I, et al. (2016) Low Dose Gemcitabine-Loaded Lipid Nanocapsules Target Monocytic Myeloid-Derived Suppressor Cells and Potentiate Cancer Immunotherapy. Biomaterials 96, 47–62. [DOI] [PubMed] [Google Scholar]
- (159).Betts GJ, Clarke SL, Richards HE, Godkin AJ, and Gallimore AM (2006) Regulating the Immune Response to Tumours. Adv. Drug Delivery Rev 58, 948–961. [DOI] [PubMed] [Google Scholar]
- (160).Sacchetti C, Rapini N, Magrini A, Cirelli E, Bellucci S, Mattei M, Rosato N, Bottini N, and Bottini M (2013) In Vivo Targeting of Intratumor Regulatory T Cells Using Peg-Modified Single-Walled Carbon Nanotubes. Bioconjugate Chem. 24, 852–858. [DOI] [PubMed] [Google Scholar]
- (161).Ou W, Jiang L, Thapa RK, Soe ZC, Poudel K, Chang JH, Ku SK, Choi HG, Yong CS, and Kim JO (2018) Combination of Nir Therapy and Regulatory T Cell Modulation Using Layer-by-Layer Hybrid Nanoparticles for Effective Cancer Photoimmunotherapy. Theranostics 8, 4574–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, and Hansen W (2004) Neuropilin-1: A Surface Marker of Regulatory T Cells. Eur. J. Immunol 34, 623–630. [DOI] [PubMed] [Google Scholar]
- (163).Roth L, Agemy L, Kotamraju VR, Braun G, Teesalu T, Sugahara KN, Hamzah J, and Ruoslahti E (2012) Transtumoral Targeting Enabled by a Novel Neuropilin-Binding Peptide. Oncogene 31, 3754–3763. [DOI] [PubMed] [Google Scholar]
- (164).Ou W, Thapa RK, Jiang L, Soe ZC, Gautam M, Chang JH, Jeong JH, Ku SK, Choi HG, Yong CS, et al. (2018) Regulatory T Cell-Targeted Hybrid Nanoparticles Combined with Immuno-Checkpoint Blockage for Cancer Immunotherapy. J. Controlled Release 281, 84–96. [DOI] [PubMed] [Google Scholar]
- (165).Zhang Y, Wei J, Xu J, Leong WS, Liu G, Ji T, Cheng Z, Wang J, Lang J, Zhao Y, et al. (2018) Suppression of Tumor Energy Supply by Liposomal Nanoparticle-Mediated Inhibition of Aerobic Glycolysis. ACS Appl Mater. Interfaces 10, 2347–2353. [DOI] [PubMed] [Google Scholar]
- (166).Pathak RK, Marrache S, Harn DA, and Dhar S (2014) Mito-Dca: A Mitochondria Targeted Molecular Scaffold for Efficacious Delivery of Metabolic Modulator Dichloroacetate. ACS Chem. Biol 9, 1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Van den Bossche J, Baardman J, and de Winther MP (2015) Metabolic Characterization of Polarized M1 and M2 Bone Marrow-Derived Macrophages Using Real-Time Extracellular Flux Analysis. J. Visualized Exp, DOI: 10.3791/53424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Saborano R, Wongpinyochit T, Totten JD, Johnston BF, Seib FP, and Duarte IF (2017) Metabolic Reprogramming of Macrophages Exposed to Silk, Poly(Lactic-Co-Glycolic Acid), and Silica Nanoparticles. Adv. Healthcare Mater 6, 1601240. [DOI] [PubMed] [Google Scholar]
- (169).Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y, Peng R, and Liu Z (2017) Hollow Mno2 as a Tumor-Microenvironment-Responsive Biodegradable Nano-Platform for Combination Therapy Favoring Antitumor Immune Responses. Nat. Commun 8, 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Mokwena MG, Kruger CA, Ivan MT, and Heidi A (2018) A Review of Nanoparticle Photosensitizer Drug Delivery Uptake Systems for Photodynamic Treatment of Lung Cancer. Photodiagn. Photodyn. Ther 22, 147–154. [DOI] [PubMed] [Google Scholar]
- (171).Bi H, Dai Y, Yang P, Xu J, Yang D, Gai S, He F, Liu B, Zhong C, An G, et al. (2018) Glutathione Mediated Size-Tunable Ucnps-Pt(Iv)-Znfe2 O4 Nanocomposite for Multiple Bioimaging Guided Synergetic Therapy. Small 14, e1703809. [DOI] [PubMed] [Google Scholar]
- (172).Treuel L, Brandholt S, Maffre P, Wiegele S, Shang L, and Nienhaus GU (2014) Impact of Protein Modification on the Protein Corona on Nanoparticles and Nanoparticle-Cell Interactions. ACS Nano 8, 503–513. [DOI] [PubMed] [Google Scholar]
- (173).Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, and Dawson KA (2011) Physical-Chemical Aspects of Protein Corona: Relevance to in Vitro and in Vivo Biological Impacts of Nanoparticles. J. Am. Chem. Soc 133, 2525–2534. [DOI] [PubMed] [Google Scholar]
- (174).Walkey CD, and Chan WC (2012) Understanding and Controlling the Interaction of Nanomaterials with Proteins in a Physiological Environment. Chem. Soc. Rev 41 , 2780–2799. [DOI] [PubMed] [Google Scholar]
- (175).Monopoli MP, Aberg C, Salvati A, and Dawson KA (2012) Biomolecular Coronas Provide the Biological Identity of Nanosized Materials. Nat. Nanotechnol 7, 779–786. [DOI] [PubMed] [Google Scholar]
- (176).Simon J, Muller LK, Kokkinopoulou M, Lieberwirth I, Morsbach S, Landfester K, and Mailander V (2018) Exploiting the Biomolecular Corona: Pre-Coating of Nanoparticles Enables Controlled Cellular Interactions. Nanoscale 10, 10731–10739. [DOI] [PubMed] [Google Scholar]
- (177).Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, and Dawson KA (2013) Transferrin-Functionalized Nanoparticles Lose Their Targeting Capabilities When a Biomolecule Corona Adsorbs on the Surface. Nat. Nanotechnol 8, 137–143. [DOI] [PubMed] [Google Scholar]
- (178).Tsai YS, Chen YH, Cheng PC, Tsai HT, Shiau AL, Tzai TS, and Wu CL (2013) Tgf-Beta1 Conjugated to Gold Nanoparticles Results in Protein Conformational Changes and Attenuates the Biological Function. Small 9, 2119–2128. [DOI] [PubMed] [Google Scholar]
- (179).Xiong X, Arvizo RR, Saha S, Robertson DJ, McMeekin S, Bhattacharya R, and Mukherjee P (2014) Sensitization of Ovarian Cancer Cells to Cisplatin by Gold Nanoparticles. Oncotarget 5, 6453–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Dong Y, Love KT, Dorkin JR, Sirirungruang S, Zhang Y, Chen D, Bogorad RL, Yin H, Chen Y, Vegas AJ, et al. (2014) Lipopeptide Nanoparticles for Potent and Selective Sirna Delivery in Rodents and Nonhuman Primates. Proc. Natl. Acad. Sci. U. S.A 111, 3955–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Zhang Z, Wang C, Zha Y, Hu W, Gao Z, Zang Y, Chen J, Zhang J, and Dong L (2015) Corona-Directed Nucleic Acid Delivery into Hepatic Stellate Cells for Liver Fibrosis Therapy. ACS Nano 9, 2405–2419. [DOI] [PubMed] [Google Scholar]
- (182).Hamad-Schifferli K (2015) Exploiting the Novel Properties of Protein Coronas: Emerging Applications in Nanomedicine. Nanomedicine (London, U. K.) 10, 1663–1674. [DOI] [PubMed] [Google Scholar]
- (183).Chen F, Wang G, Griffin JI, Brenneman B, Banda NK, Holers VM, Backos DS, Wu L, Moghimi SM, and Simberg D (2017) Complement Proteins Bind to Nanoparticle Protein Corona and Undergo Dynamic Exchange in Vivo. Nat. Nanotechnol 12, 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Setyawati MI, Tay CY, Docter D, Stauber RH, and Leong DT (2015) Understanding and Exploiting Nanoparticles’ Intimacy with the Blood Vessel and Blood. Chem. Soc. Rev 44, 8174–8199. [DOI] [PubMed] [Google Scholar]
- (185).Setyawati MI, Tay CY, Bay BH, and Leong DT (2017) Gold Nanoparticles Induced Endothelial Leakiness Depends on Particle Size and Endothelial Cell Origin. ACS Nano 11 , 5020–5030. [DOI] [PubMed] [Google Scholar]
- (186).Setyawati MI, Tay CY, Chia SL, Goh SL, Fang W, Neo MJ, Chong HC, Tan SM, Loo SC, Ng KW, et al. (2013) Titanium Dioxide Nanomaterials Cause Endothelial Cell Leakiness by Disrupting the Homophilic Interaction of Ve-Cadherin. Nat. Commun 4, 1673. [DOI] [PubMed] [Google Scholar]
- (187).Setyawati MI, Mochalin VN, and Leong DT (2016) Tuning Endothelial Permeability with Functionalized Nanodiamonds. ACS Nano 10, 1170–1181. [DOI] [PubMed] [Google Scholar]
- (188).Setyawati MI, and Leong DT (2017) Mesoporous Silica Nanoparticles as an Antitumoral-Angiogenesis Strategy. ACS Appl. Mater. Interfaces 9, 6690–6703. [DOI] [PubMed] [Google Scholar]
- (189).Duan J, Yu Y, Yu Y, Li Y, Huang P, Zhou X, Peng S, and Sun Z (2014) Silica Nanoparticles Enhance Autophagic Activity, Disturb Endothelial Cell Homeostasis and Impair Angiogenesis. Part. Fibre Toxicol 11 , 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Shen N, Zhang R, Zhang HR, Luo HY, Shen W, Gao X, Guo DZ, and Shen J (2018) Inhibition of Retinal Angiogenesis by Gold Nanoparticles Via Inducing Autophagy. Int. J. Ophthalmol 11, 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C, Rao J, Tikhomirov GA, Wendland MF, Corot C, et al. (2011) Mri of Tumor-Associated Macrophages with Clinically Applicable Iron Oxide Nanoparticles. Clin. Cancer Res 17, 5695–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Mou W, Xu Y, Ye Y, Chen S, Li X, Gong K, Liu Y, Chen Y, Li X, Tian Y, et al. (2015) Expression of Sox2 in Breast Cancer Cells Promotes the Recruitment of M2Macrophages to Tumor Microenvironment. Cancer Lett. 358, 115–123. [DOI] [PubMed] [Google Scholar]
- (193).Becker M, Muller CB, De Bastiani MA, and Klamt F (2014) The Prognostic Impact of Tumor-Associated Macrophages and Intra-Tumoral Apoptosis in Non-Small Cell Lung Cancer. Histol. Histopathol 29, 21–31. [DOI] [PubMed] [Google Scholar]
- (194).Mantovani A, Sozzani S, Locati M, Allavena P, and Sica A (2002) Macrophage Polarization: Tumor-Associated Macrophages as a Paradigm for Polarized M2Mononuclear Phagocytes. Trends Immunol. 23, 549–555. [DOI] [PubMed] [Google Scholar]
- (195).Singh Y, Pawar VK, Meher JG, Raval K, Kumar A, Shrivastava R, Bhadauria S, and Chourasia MK (2017) Targeting Tumor Associated Macrophages (Tams) Via Nanocarriers. J. Controlled Release 254, 92–106. [DOI] [PubMed] [Google Scholar]
- (196).Laskar A, Eilertsen J, Li W, and Yuan XM (2013) Spion Primes Thp1 Derived M2Macrophages Towards M1-Like Macrophages. Biochem. Biophys. Res. Commun 441, 737–742. [DOI] [PubMed] [Google Scholar]
- (197).Fuchs AK, Syrovets T, Haas KA, Loos C, Musyanovych A, Mailander V, Landfester K, and Simmet T (2016) Carboxyl- and Amino-Functionalized Polystyrene Nanoparticles Differentially Affect the Polarization Profile of M1 and M2Macrophage Subsets. Biomaterials 85, 78–87. [DOI] [PubMed] [Google Scholar]
- (198).Hoppstadter J, Seif M, Dembek A, Cavelius C, Huwer H, Kraegeloh A, and Kiemer AK (2015) M2 Polarization Enhances Silica Nanoparticle Uptake by Macrophages. Front. Pharmacol 6, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Zhu Z, Scalfi-Happ C, Ryabova A, Grafe S, Wiehe A, Peter RU, Loschenov V, Steiner R, and Wittig R (2018) Photodynamic Activity of Temoporfin Nanoparticles Induces a Shift to the M1-Like Phenotype in M2-Polarized Macrophages. J. Photochem. Photobiol., B 185, 215–222. [DOI] [PubMed] [Google Scholar]
- (200).Su L, Zhang W, Wu X, Zhang Y, Chen X, Liu G, Chen G, and Jiang M (2015) Glycocalyx-Mimicking Nanoparticles for Stimulation and Polarization of Macrophages Via Specific Interactions. Small 11, 4191–4200. [DOI] [PubMed] [Google Scholar]
- (201).Miao X, Leng X, and Zhang Q (2017) The Current State of Nanoparticle-Induced Macrophage Polarization and Reprogramming Research. Int. J. Mol. Sci 18, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Huang YJ, Hung KC, Hung HS, and Hsu SH (2018) Modulation of Macrophage Phenotype by Biodegradable Polyurethane Nanoparticles: Possible Relation between Macrophage Polarization and Immune Response of Nanoparticles. ACS Appl. Mater. Interfaces 10, 19436–19448. [DOI] [PubMed] [Google Scholar]
- (203).Marquardt C, Fritsch-Decker S, Al-Rawi M, Diabate S, and Weiss C (2017) Autophagy Induced by Silica Nanoparticles Protects Raw264.7 Macrophages from Cell Death. Toxicology 379, 40–47. [DOI] [PubMed] [Google Scholar]
- (204).Oh N, and Park JH (2014) Surface Chemistry of Gold Nanoparticles Mediates Their Exocytosis in Macrophages. ACS Nano 8, 6232–6241. [DOI] [PubMed] [Google Scholar]
- (205).Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, and Takeyama H (2015) Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 7, 2443–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Tao L, Huang G, Song H, Chen Y, and Chen L (2017) Cancer Associated Fibroblasts: An Essential Role in the Tumor Microenvironment. Oncol. Lett 14, 2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Vieira LFA, Lins MP, Viana I, Dos Santos JE, Smaniotto S, and Reis M (2017) Metallic Nanoparticles Reduce the Migration of Human Fibroblasts in Vitro. Nanoscale Res. Lett 12, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Harhaji L, Isakovic A, Raicevic N, Markovic Z, Todorovic-Markovic B, Nikolic N, Vranjes-Djuric S, Markovic I,and Trajkovic V (2007) Multiple Mechanisms Underlying the Anticancer Action of Nanocrystalline Fullerene. Eur. J. Pharmacol 568, 89–98. [DOI] [PubMed] [Google Scholar]
- (209).Zhang Q, Yang W, Man N, Zheng F, Shen Y, Sun K, Li Y, and Wen LP (2009) Autophagy-Mediated Chemosensitization in Cancer Cells by Fullerene C60 Nanocrystal. Autophagy 5, 1107–1117. [DOI] [PubMed] [Google Scholar]
- (210).Man N, Yu L, Yu SH, and Wen LP (2010) Rare Earth Oxide Nanocrystals as a New Class of Autophagy Inducers. Autophagy 6, 310. [DOI] [PubMed] [Google Scholar]
- (211).Zhao Y, Howe JL, Yu Z, Leong DT, Chu JJ, Loo JS, and Ng KW (2013) Exposure to Titanium Dioxide Nanoparticles Induces Autophagy in Primary Human Keratinocytes. Small 9, 387392. [DOI] [PubMed] [Google Scholar]
- (212).Stern ST, Zolnik BS, McLeland CB, Clogston J, Zheng J, and McNeil SE (2008) Induction of Autophagy in Porcine Kidney Cells by Quantum Dots: A Common Cellular Response to Nanomaterials? Toxicol. Sci 106, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Chen Y, Yang L, Feng C, and Wen LP (2005) Nano Neodymium Oxide Induces Massive Vacuolization and Autophagic Cell Death in Non-Small Cell Lung Cancer Nci-H460 Cells. Biochem. Biophys. Res. Commun 337, 52–60. [DOI] [PubMed] [Google Scholar]
- (214).Huang D, Zhou H, and Gao J (2015) Nanoparticles Modulate Autophagic Effect in a Dispersity-Dependent Manner. Sci. Rep 5, 14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Zhang L, Chen X, Wu J, Ding S, Wang X, Lei Q, and Fang W (2018) Palladium Nanoparticles Induce Autophagy and Autophagic Flux Blockade in Hela Cells. RSC Adv. 8, 4130–4141. [Google Scholar]
- (216).Wang J, Yu Y, Lu K, Yang M, Li Y, Zhou X, and Sun Z (2017) Silica Nanoparticles Induce Autophagy Dysfunction Via Lysosomal Impairment and Inhibition of Autophagosome Degradation in Hepatocytes. Int. J. Nanomed 12, 809–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (217).Ma X, Wu Y, Jin S, Tian Y, Zhang X, Zhao Y, Yu L, and Liang XJ (2011) Gold Nanoparticles Induce Autophagosome Accumulation through Size-Dependent Nanoparticle Uptake and Lysosome Impairment. ACS Nano 5, 8629–8639. [DOI] [PubMed] [Google Scholar]
- (218).Mishra AR, Zheng J, Tang X, and Goering PL (2016) Silver Nanoparticle-Induced Autophagic-Lysosomal Disruption and Nlrp3-Inflammasome Activation in Hepg2 Cells Is Size-Dependent. Toxicol. Sci 150, 473–487. [DOI] [PubMed] [Google Scholar]
- (219).Arvizo RR, Rana S, Miranda OR, Bhattacharya R, Rotello VM, and Mukherjee P (2011) Mechanism of Anti-Angiogenic Property of Gold Nanoparticles: Role of Nanoparticle Size and Surface Charge. Nanomedicine 7, 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Arvizo RR, Saha S, Wang E, Robertson JD, Bhattacharya R, and Mukherjee P (2013) Inhibition of Tumor Growth and Metastasis by a Self-Therapeutic Nanoparticle. Proc. Natl. Acad. Sci. U. S. A 110, 6700–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Khanna P, Ong C, Bay BH, and Baeg GH (2015) Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 5, 1163–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Manke A, Wang L, and Rojanasakul Y (2013) Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int 2013, 942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (223).Fubini B, and Hubbard A (2003) Reactive Oxygen Species (Ros) and Reactive Nitrogen Species (Rns) Generation by Silica in Inflammation and Fibrosis. Free Radical Biol. Med 34, 1507–1516. [DOI] [PubMed] [Google Scholar]
- (224).Knaapen AM, Borm PJ, Albrecht C, and Schins RP (2004) Inhaled Particles and Lung Cancer. Part A: Mechanisms. Int. J. Cancer 109, 799–809. [DOI] [PubMed] [Google Scholar]
- (225).Tournier C, Thomas G, Pierre J, Jacquemin C, Pierre M, and Saunier B (1997) Mediation by Arachidonic Acid Metabolites of the H2o2-Induced Stimulation of Mitogen-Activated Protein Kinases (Extracellular-Signal-Regulated Kinase and C-Jun Nh2-Terminal Kinase). Eur. J. Biochem 244, 587–595. [DOI] [PubMed] [Google Scholar]
- (226).Guyton KZ, Liu Y, Gorospe M, Xu Q, and Holbrook NJ(1996) Activation of Mitogen-Activated Protein Kinase by H2o2. Role in Cell Survival Following Oxidant Injury. J. Biol. Chem 271, 4138–4142. [DOI] [PubMed] [Google Scholar]
- (227).Gu X, Qiu Y, Lin M, Cui K, Chen G, Chen Y, Fan C, Zhang Y, Xu L, Chen H, et al. (2019) Cus Nanoparticles as a Photodynamic Nanoswitch for Abrogating Bypass Signaling to Overcome Gefitinib Resistance. Nano Lett. 19, 3344–3352. [DOI] [PubMed] [Google Scholar]
- (228).Huai Y, Zhang Y, Xiong X, Das S, Bhattacharya R, and Mukherjee P (2019) Gold Nanoparticles Sensitize Pancreatic Cancer Cells to Gemcitabine. Cell Stress. 3, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (229).Zhang Y, Xiong X, Huai Y, Dey A, Hossen MN, Roy RV, Elechalawar CK, Rao G, Bhattacharya R, and Mukherjee P (2019) Gold Nanoparticles Disrupt Tumor Microenvironment - Endothelial Cell Cross Talk to Inhibit Angiogenic Phenotypes in Vitro. Bioconjugate Chem. 30, 1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Hossen MN, Rao G, Dey A, Robertson JD, Bhattacharya R, and Mukherjee P (2019) Gold Nanoparticle Transforms Activated Cancer-Associated Fibroblasts to Quiescence. ACS Appl Mater. Interfaces 11, 26060–26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (231).Sharma VK, Filip J, Zboril R, and Varma R S. (2015) Natural Inorganic Nanoparticles-Formation, Fate, and Toxicity in the Environment. Chem. Soc. Rev 44, 8410–8423. [DOI] [PubMed] [Google Scholar]
- (232).Arvizo RR, Giri K, Moyano D, Miranda OR, Madden B, McCormick DJ, Bhattacharya R, Rotello VM, Kocher JP, and Mukherjee P (2012) Identifying New Therapeutic Targets Via Modulation of Protein Corona Formation by Engineered Nanoparticles. PLoS One 7, e33650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Giri K, Shameer K, Zimmermann MT, Saha S, Chakraborty PK, Sharma A, Arvizo RR, Madden BJ, McCormick DJ, Kocher JP, et al. (2014) Understanding Protein-Nanoparticle Interaction: A New Gateway to Disease Therapeutics. Bioconjugate Chem. 25, 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (234).Leong HS, Butler KS, Brinker CJ, Azzawi M, Conlan S, Dufes C, Owen A, Rannard S, Scott C, Chen C, et al. (2019) On the issue of transparency and reproducibility in nanomedicine. Nat. Nanotechnol 14, 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]


