Lay summary:
A review that compares changes in body mass, glucocorticoid and sympathetic responses, and reproductive and immune function, in wild animals recently introduced into captivity to their wild counterparts. Conclusion is that captivity can be a powerful chronic stressor that may be possible to mitigate, but the impact is highly species-specific.
Keywords: stress, captivity, glucocorticoids, reproduction, immune
Abstract
Wild animals are brought into captivity for many reasons—conservation, research, agriculture and the exotic pet trade. While the physical needs of animals are met in captivity, the conditions of confinement and exposure to humans can result in physiological stress. The stress response consists of the suite of hormonal and physiological reactions to help an animal survive potentially harmful stimuli. The adrenomedullary response results in increased heart rate and muscle tone (among other effects); elevated glucocorticoid (GC) hormones help to direct resources towards immediate survival. While these responses are adaptive, overexposure to stress can cause physiological problems, such as weight loss, changes to the immune system and decreased reproductive capacity. Many people who work with wild animals in captivity assume that they will eventually adjust to their new circumstances. However, captivity may have long-term or permanent impacts on physiology if the stress response is chronically activated. We reviewed the literature on the effects of introduction to captivity in wild-caught individuals on the physiological systems impacted by stress, particularly weight changes, GC regulation, adrenomedullary regulation and the immune and reproductive systems. This paper did not review studies on captive-born animals. Adjustment to captivity has been reported for some physiological systems in some species. However, for many species, permanent alterations to physiology may occur with captivity. For example, captive animals may have elevated GCs and/or reduced reproductive capacity compared to free-living animals even after months in captivity. Full adjustment to captivity may occur only in some species, and may be dependent on time of year or other variables. We discuss some of the methods that can be used to reduce chronic captivity stress.
Introduction
The tens of thousands of vertebrate species on this planet are adapted to every condition from the Arctic to the tropics and from the mountain tops to the ocean depths. For all species, the environment contains both predictable changes (e.g. day–night transitions or seasonal variation) and unpredictable, uncontrollable threats to homeostasis and survival (Romero and Wingfield, 2016). Vertebrates have evolved a suite of defenses against the myriad unpredictable ‘shocks that flesh is heir to’ (Shakespeare, Hamlet, 3.1)—a set of conserved physiological responses known as the ‘stress response’. While the stress response can help an animal survive a threatening event, if noxious conditions are repeating or unrelenting two physiological changes take place. First, the reactive scope of the animal shrinks thereby decreasing the animal’s ability to cope (Romero et al., 2009). Second, the stress response itself can begin to cause physiological problems, a condition known as ‘chronic stress’. Even though there is no generally agreed upon definition of chronic stress or the time-frame of its onset, long-term stressor exposure or chronic stress, can lead to weight loss, immunosuppression, reproductive failure and psychological distress (Sapolsky et al., 2000). Because the stress response occurs when situations are perceived as threatening, regardless of whether the animal is experiencing physical damage, a drastic change of conditions can lead to symptoms of chronic stress even when the animal is unharmed. Consequently, when a wild animal is brought into captivity for the first time, symptoms of chronic stress can occur even though the physical needs of the animal are attended to.
In captivity, animals are provided with shelter and ample food. Nevertheless, captivity can often result in negative physiological outcomes, particularly for newly-captured animals. The conditions of captivity can be perceived as threatening, and if the perceived threat does not decrease, symptoms associated with chronic stress may result. The sources of stress in captivity are many, including cage restraint, human presence, an unfamiliar environment, and other, more subtle stressors, such as artificial light conditions (reviewed in Morgan and Tromborg, 2007). When wild animals are newly brought into captivity, it is frequently for research, conservation, agriculture (e.g. fisheries) or the exotic animal trade. To keep these animals healthy, symptoms of chronic stress should be minimized or eliminated. It is often assumed that with time, animals will adjust to captivity conditions and stress will disappear. Indeed, many animals seem to thrive in captivity. Unfortunately, many other species do not (Mason, 2010). In this review, we surveyed the literature to answer the following two questions: do wild animals eventually adjust to captivity conditions? And if so, how long does the period of adjustment typically take? This literature survey exclusively addressed wild animals introduced to captivity and not animals born in captivity.
We focused on several aspects of physiology that may be particularly affected by long-term stressor exposure. The acute stress response involves many behavioral and physiological changes, including activation of two hormonal pathways. The adrenomedullary response occurs within seconds of the onset of a stressor (Romero and Wingfield, 2016). The catecholamine hormones epinephrine and norepinephrine are rapidly released from the adrenal medulla. These cause an increase in heart rate, as well as an increase in muscle tone, an increase in blood pressure and other physiological and behavioral changes that enable an animal to survive a sudden stressor, such as a predator attack. The second hormonal response is initiated within minutes of the onset of a stressor, when a hormonal cascade triggers the synthesis and release of glucocorticoids (GCs)—steroid hormones that have wide-ranging effects on the body (Romero and Wingfield, 2016). While baseline levels of GCs help regulate metabolism, increased levels trigger an ‘emergency life history stage’, (Wingfield et al., 1998), where resources and behaviors are directed towards survival of the crisis and away from long term investments. GCs have a strong impact on the immune and reproductive systems (Sapolsky et al., 2000). In this review, we focus on captivity’s effects on mass (one of the best-documented outcomes of chronic stress), GC concentrations and the immune, reproductive and adrenomedullary systems. We also document how the adjustment to captivity is impacted by time of year and how captivity effects persist after release. Finally, we discuss some of the ways that captivity stress may be mitigated.
Methods
We surveyed the literature and gathered studies that compared wild-caught animals as they adjusted to captivity. We conducted a literature search through Web of Science using the search terms ‘captivity’ and ‘stress’ and ‘physiology’ or ‘endocrinology’ and related words. Because many papers reported on aspects of the stress response on animals that were in captivity but did not examine the effects of captivity itself, we were unable to devise search terms that included the studies we were interested in but excluded other research on stress in wild animals. We therefore devised the following criteria to determine whether papers should be included: (i) wild species were brought into captivity and physiological variables measured over the days to months of adjustment to captive conditions OR (ii) wild-caught captive animals were compared to free-living conspecifics AND (iii) the total captivity duration was at least 3 days (we did not include the many studies that measure only the acute stress effects of capture in the first 30 min to 48 hours). We further excluded two broad types of studies. One, we excluded studies where we could determine that all captive animals were captive-bred, as we were specifically interested in how well wild animals can adjust to captive conditions when taken from the wild (though we included some studies where the origin of captive animals was unclear). Second, we excluded studies of wild animals undergoing rehabilitation because it is not possible to distinguish between responses to captivity and responses to clinical interventions in animals that were injured or sick at capture. Once we had created a list of papers, we also checked the cited references of these studies for any important works our search terms missed.
There are many studies that focused on behavioral changes in captivity. However, the variables measured can be quite species-specific and difficult to interpret in a context of stress. Although we recognize the importance of behavior for the welfare of wild animals (reviewed in McPhee and Carlstead, 2010), we limited our focus to studies that included some physiological measurements (e.g. weight changes, hormone concentrations or immune measurements).
We found little standardization in experimental design in the papers examining the effect of captivity on physiology. We visually summarize the four most common experimental designs in Fig. 1. Many researchers compared animals that had been exposed to captivity (duration: 3 days to several years) to those that had not (Fig. 1A). In some cases, the free-living population was sampled when the captive population was initially captured. This was often the case in species where only a single blood sample could be drawn from an individual. In other studies, the free-living population was sampled entirely separately from the captive group. This was often the case for long-term captives, such as zoo-housed animals. Another common technique was to take a single pre-captivity sample and a single post-captivity sample on the same animal (duration of captivity 5 days to 3 months) (Fig. 1B). Other researchers used repeated sampling techniques—either sampling the same individual multiple times, or keeping different individuals in captivity for different durations before sampling. Some focused narrowly on the first few days of captivity (Fig. 1C), while others did not take a second sample until several weeks had passed (Fig. 1D). Furthermore, captive conditions varied between studies, with some studies bringing animals into closed indoor situations, whereas others placed captive animals into open outdoor pens. We considered each situation to represent captivity, but we were not able to contrast any differences in responses.
Figure 1.
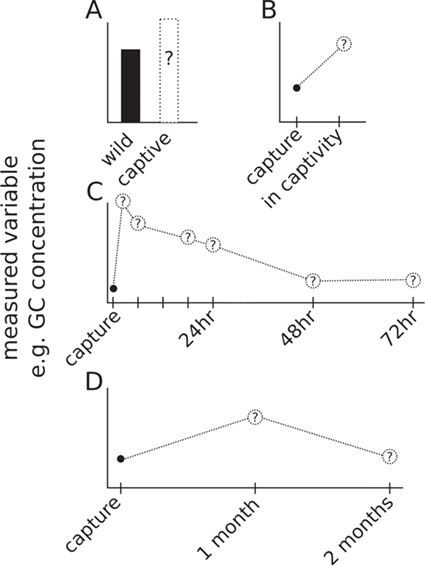
Examples of experimental designs to assess the effects of captivity on a physiological variable (e.g. GC concentration) (A) Comparison of captive individuals to free-living populations. In some cases, the free-living samples were acquired at the same time that the study population was brought into captivity. In other designs, the free-living samples were taken from entirely different populations than the origin of the captive animals (e.g. comparing zoo-housed animals to wild conspecifics). (B) Each individual measured immediately at capture and again after a period of captivity (days to months). (C and D) Each individual measured immediately at capture and resampled at multiple timepoints. Some studies focused on the first few days, with sampling points relatively close together (C). Other studies may not have taken another sample until several weeks after capture (D).
We created summary figures for the trends we observed in weight, GC hormones and the immune system with respect to captivity duration (Figures 2–4). To construct these, we tallied the total number of studies that reported on the variable for a particular time window and determined whether the variable was above, below or equal to what it was in a free-living population. If a single report showed two different patterns (e.g. males and females had different patterns or two species were reported in the same paper), each pattern of was included separately. Therefore, one ‘study’ might be included multiple times in the figure. This also holds true for reporting patterns in the literature in the text and in the tables—if one paper reported multiple patterns in different groups of individuals, it was included more than once in calculating percentages of studies and was given more than one line on the tables. We did not include studies in the figures if there were marked seasonal differences in one species (see Section 9 for seasonal differences).
Figure 2.
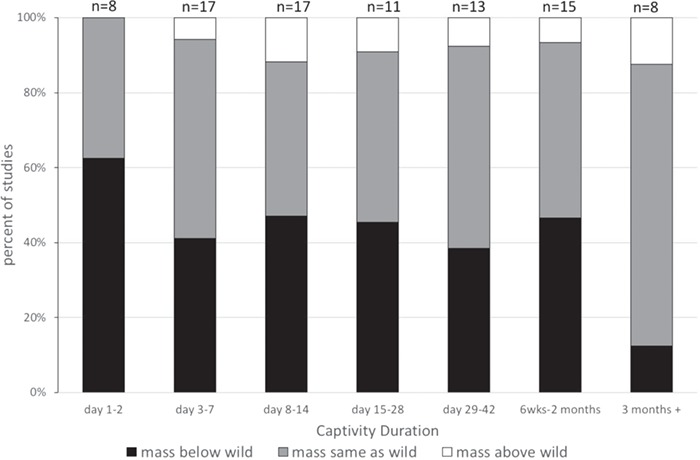
Weight change as a function of captivity duration. Data were collected from 35 studies listed in Table 1, with studies counted multiple times if they measured multiple time points after introduction to captivity. The number of species that lost weight in captivity (relative to wild, free-living animals) decreased with captivity duration.
Figure 4.
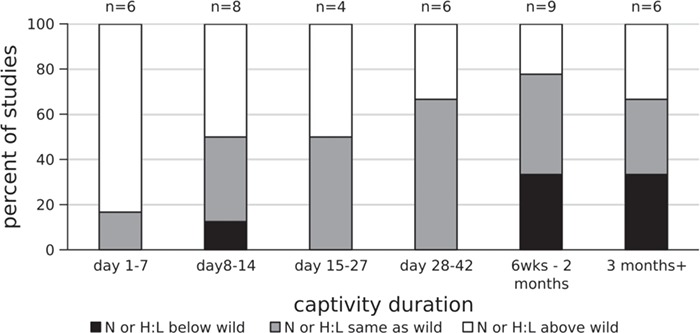
Changes in neutrophil or heterophil (N or H:L) to lymphocyte ratio in captivity as a function of time. Data were collected from 19 studies listed in Table 4, with studies counted multiple times if they measured multiple time points after introduction to captivity. The percent of studies that recorded elevated N or H:L ratio in captivity decreased with the amount of time spent in captivity.
Because most of the papers we collected did not report effect sizes, a formal meta-analysis was not possible. Consequently, we focused on qualitative differences.
Mass and body condition in captivity
After being brought into captivity from the wild, animals frequently experience a period of weight loss (Table 1). In 64% of studies (23 of 36), there was a documented decrease in mass associated with captivity during at least the initial capture period. Weight loss in captivity is likely to be attributable to chronic stress. Captive animals are not calorically restricted (as long as they choose to eat), which is not always the case in the wild, and they are not likely to use as many calories because cage restraint limits the amount of exercise that an animal can get in a day. Experimentally induced chronic stress has been demonstrated to lead to weight loss in mammals (e.g. Flugge, 1996), birds (e.g. Rich and Romero, 2005) and fish (e.g. Peters et al., 1980). In fact, weight loss is the most consistently seen effect of chronic stress (Dickens and Romero, 2013).
Table 1.
Mass changes with captivity in wild animals
| Changes in mass during adjustment to captivity | Species | Study design | Timeframe | Citation | |
|---|---|---|---|---|---|
| Weight gain in captivity | Mammals | Steller sea lions (Eumetopias jubatus) | Repeated measures; pre- vs post-captivity | Average 2 months | (Mellish et al., 2006)* |
| Richardson’s ground squirrel (Urocitellus richardsonii) | Repeated measures; multiple timepoints | 14 days | (Hare et al., 2014)* | ||
| Columbian ground squirrel (Spermophilus columbianus) | Repeated measures; multiple timepoints | 13 days | (Bosson et al., 2009)* | ||
| Brushtail possums (Trichosurus volpecula) | Repeated measures; multiple timepoints | 37 days | (Day and O'Connor, 2000)* | ||
| Birds | Ruff (Philomachus pugnax) | Repeated measures; multiple timepoints | Up to 1 year (mass increase in first few weeks, then seasonal fluctuations) | (Piersma et al., 2000)* | |
| North Island saddlebacks (Philesturnus rufusater) | Repeated measures; multiple timepoints | 3 days | (Adams et al., 2010)* | ||
| No change in mass with captivity | Mammals | Vervet monkeys (Chlorocebus aethiops)1 | Repeated measures; multiple timepoints | 8 months | (Kagira et al., 2007) |
| Brushtail possums (Trichosurus volpecula) (♂ only) | Repeated measures; multiple timepoints | 20 weeks | (Baker et al., 1998)* | ||
| Brushtail possums (Trichosurus volpecula) (♂) | Repeated measures; multiple timepoints | 8 weeks | (Begg et al., 2004)* | ||
| Birds | Curve-billed thrasher (Toxostoma curverostre) | Repeated measures;multiple timepoints | 80 days | (Fokidis et al., 2011)* | |
| Reptiles | Duvaucel’s gecoks (Hoplodactylus duvaucelli) | Captive vs free-living population | >1 year | (Barry et al., 2010)* | |
| Amphibians | Cururu toad (Rhinella icterica) | Repeated measures; pre- vs post-captivity | 13 days | (de Assis et al., 2015)* | |
| Toad (Rhinella icterica) | Captive vs free-living population, multiple timepoints2 | up to 90 days | (Titon et al., 2018)* | ||
| Weight loss in captivity | Mammals | Beluga whale (Delphinapterus leucas) | Repeated measures; pre- vs post-captivity | 10 weeks | (St Aubin and Geraci, 1988)* |
| Harbor seal (Phoca vitulina) (juveniles) | Captive vs free-living populations3 | >4 weeks | (Trumble et al., 2013)* | ||
| African green monkey (Cercopithecus aethiops) | Multiple timepoints; different individuals | 45 days | (Suleman et al., 2004)* | ||
| Bighorn sheep (Ovis canadensis canadensis) | Repeated measures;multiple timepoints | 14 days | (Franzman and Thorne. 1970)* | ||
| Birds | Zebra finches (Taeniopygia guttata) | Captive vs free-living population | 60 days4 | (Ewenson et al., 2001)* | |
| Rufous-collared sparrows (Zonotrichia capensis) | Captive vs free-living population | 2 weeks | (Ruiz et al., 2002)* | ||
| Great tit (Parus major) | Repeated measures; pre- vs post-captivity | 1 week | (Krams et al., 2013)* | ||
| House sparrow (Passer domesticus) | Repeated measures; pre- vs post-captivity | 1 week | (Fischer and Romero, 2016)* | ||
| House sparrow (Passer domesticus) | Repeated measures; pre- vs post-captivity | 1 week | (Fischer et al., 2018)* | ||
| House sparrow (Passer domesticus) | Repeated measures; pre- vs post-captivity | 5 days | (Lattin et al., 2012)* | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 27 days | (Gormally et al., 2019)* | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 66 days | (Love et al., 2017)* | ||
| Amphibians | Toad (Rhinella schneideri) | Repeated measures; pre- vs post-captivity | 60 days | (Titon et al., 2017)* | |
| Fish | Electric fish (Gnathonemus petersii) | Repeated measures; multiple timepoints | 37 days | (Landsman, 1993)* | |
| Weight lost then regained in captivity | Mammals | Rhesus macaques (Macaca mulatta) | Repeated measures; multiple timepoints | Weight decreased by Week 5, increased through 1 year | (Lilly et al., 1999)* |
| European wild rabbits (Oryctolagus cuniculus) | Repeated measures; pre- vs post-captivity (different durations) |
Weight decreased by Week 2, increased and stabilized by Week 45 | (Calvete et al., 2005)* | ||
| Brushtail possums (Trichosurus volpecula) (♀ only) | Repeated measures; multiple timepoints | Weight decreased over 5 weeks, increased through Week 20 | (Baker et al., 1998)* | ||
| Tuco-tuco (Ctenomys talarum) | Repeated measures; multiple timepoints | Weight loss on Days 10 and 20, regained by Day 30 | (Vera et al., 2011)* | ||
| Birds | White-crowned sparrow (Zonotrichia leucophrys) | Repeated measures; multiple timepoints | Weight loss at Day 1, increased through day 14 | (Wingfield et al., 1982)* | |
| Greenfinch (Chloris chloris) | Captive vs free-living population | Birds lighter at 1 month, heavier than wild at 2 months | (Sepp et al., 2010)* | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | Weight loss on Days 11–25, regained by day 35 | (Fischer et al., 2018)* | ||
| Chukar partridge (Alectoris chukar) | Repeated measures; multiple timepoints | Weight loss at Day 1, partially regained at 5 and 9 days | (Dickens et al., 2009b)* | ||
| Fish | Skipjack tuna (Katsuwonus pelamus) | Repeated measures; multiple timepoints | Weight loss at Day 2, regained at 20 days | (Bourke et al., 1987) | |
| Chukar partridge (Alectoris chukar) | Repeated measures; multiple timepoints | Weight loss at Day 1, partially regained at 5 and 9 days | (Dickens et al., 2009b)* | ||
| Fish | Skipjack tuna (Katsuwonus pelamus) | Repeated measures; multiple timepoints | Weight loss at Day 2, regained at 20 days | (Bourke et al., 1987) | |
1No at-capture values—first measured at 2 months.
2Low sample sizes at each time point.
3Captive pups were rehabilitated after rescue.
4Slight weight loss from Day 10 to Day 60.
5Females did not reach at capture weight, but all spontaneously aborted or gave birth.
*Data from this paper were used to generate Fig. 2.
In 39% of studies where animals lost weight (9 of 23), the animals eventually regained the weight they had lost. In some cases, weight loss may be very transitory and last only a couple of days. For example, North Island saddlebacks (a bird native to New Zealand) lost weight on the first day of captivity, but by Day 3, they had not only regained weight, they were heavier than they were at capture (Adams et al., 2011). Transitory weight loss may be related to adjustment to the captive diet and not to major physiological problems. In other species, it may take weeks or months to regain the lost mass. House sparrows lose weight by Day 5–7 of captivity (Lattin et al., 2012; Fischer and Romero, 2016). In a long-term study of the species, they did not regain the weight they had lost for nearly 5 weeks (Fischer et al., 2018). Similarly, female possums lost weight for 5 weeks before beginning to gain again, and although they were kept for 20 weeks, they never fully recovered their lost weight (Baker et al., 1998). In 61% of studies (14 of 23), weight that was lost was never regained, though the studies may not have been long enough for weight to stabilize.
In some cases, weight loss depended on the characteristics of the animal at capture. For example, female possums lost weight over the first 5 weeks of captivity but some males gained weight during that period (Baker et al., 1998). When curve-billed thrashers were captured, birds from urban environments had higher body condition than desert birds, but after 80 days in captivity, their body conditions had converged to an intermediate value (Fokidis et al., 2011). Captivity may impact individuals differently depending on sex, population of origin or other individual characteristics, including transitory physiological states. (See Section 9 for the effects of time of year on the ability to adjust to captivity.)
Weight loss was not the only pattern seen in captivity. In 17% of studies (6 of 36), animals gained mass above their starting condition. Some animals may benefit from the increased calories available in captivity and be able to maintain their weight. In other animals, however, ad libitum access to food and limits to exercise may cause them to become obese and face the myriad negative consequences of a high body mass or body fat content (West and York, 1998). In a study of domesticated budgerigars, birds were given ad libitum food and confined to cages that limited exercise. High body mass at the end of 28 days correlated with more DNA damage (Larcombe et al., 2015).
We visually summarized the patterns of weight changes in Fig. 2. We graphed the total percent of studies that showed weight gain, weight loss or no change in weight at different time points after introduction to captivity. There were no studies that recorded weight gain in the first day. Most weight gain seems to be reported at 15–28 days of captivity (38% of studies showed weight gain in that window). The percent of studies reporting weight loss decreased with increasing captivity duration, reflecting the fact that many studies show eventual regain of lost weight. This suggests that for many species where weight was lost, it would eventually be regained.
It is possible that seasonal fluctuations in weight may interfere with the assumptions that weight gain or loss is due to captivity. Captive ruffs and red knots have strong seasonal weight fluctuations in captivity associated with weight gain for migration and breeding (Piersma et al., 2000). If semi-naturalistic conditions are maintained in captivity (for example, if the animals are exposed to natural day length or are housed outdoors), then they may continue to experience seasonal weight changes that are not due to overfeeding or to long-term stressor exposure.
Changes in GCs during the adjustment to captivity
One of the most common variables to measure when assessing the stress of captivity was GC concentrations. GC hormones (primarily cortisol in fish and most mammals; primarily corticosterone in reptiles, birds, amphibians, and rodents) are produced in the adrenal cortex, have multiple roles throughout the body, and can influence many other physiological systems. Acute stressors cause a transitory increase in GCs, which is eventually brought down by negative feedback. Long-term stressor exposure frequently results in changes in GC regulation, although the part of the GC response affected (baseline concentrations, stress-induced concentrations, or negative feedback) and the direction of the change are different in different species and circumstances (Dickens and Romero, 2013).
GCs can be assessed in several ways (Sheriff et al., 2011). The most common method is to measure circulating plasma GCs by taking a blood sample. The sampling procedure itself can cause an increase in GCs, so researchers usually try to acquire the first sample as quickly as possible—within 3 minutes of capture or disturbance is generally considered a good guideline (Romero and Reed, 2005). In many studies, it was not possible for the researchers to meet this standard because of the difficulty of capturing and bleeding the animals. In addition, some papers were written before the 3-minute standard had been established. It is also possible to assess GCs through other means. Fecal samples can be collected to measure metabolized GCs. Fecal samples provide an integrated profile of GC secretion over several hours to several days, depending upon the species, and reflect both baseline GCs and acute stress events (Wasser et al., 2000). Fecal sampling is convenient for many species when rapid capture and blood sampling is impractical. If the first fecal sample is collected soon after capture, it will not reflect the stress of captivity and may be considered a good free-living reference. Some researchers also used urinary GC metabolites, particularly in amphibian species, where animals could be left alone in a container of water from which excreted steroids were measured.
The initial capture and handling of wild animals is expected to cause an increase in circulating GC levels (an acute stress response). While some researchers investigated captivity-induced changes in the acute stress response itself (e.g. taking a plasma sample after a standardized 30-minute restraint stress at capture and again after a period in captivity), others incorporated the acute response to capture in the same analysis as longer-term captivity effects (e.g. taking a sample at 0, 2, 6, 18, 24, 48 and 72 hours post capture). Because of the variety of different measures used, we focused particularly on the captivity effects on baseline and integrated GCs (Table 2). However, we will also discuss the effects of captivity on the acute stress response and negative feedback of GC production (Table 3). Some researchers looked for the effects of captivity at different times of year—we do not include those studies in our calculations or in Tables 2 and 3 (see Section 9).
Table 2.
Patterns of change in baseline and integrated GCs when wild animals are brought into captivity (this table does not include studies where the pattern was different in different seasons—those studies may be found in Table 6)
| GC Pattern during adjustment to captivity | Species | Study design | Timeframe | How were free-living GCs established? | Sample type | Citation | |
|---|---|---|---|---|---|---|---|
| No effect on GCs over captivity period | Mammals | Degu (Octodon degus) | Captive vs free-living populations | >1 year | Free-living population | Plasma (<2 min) | (Quispe et al., 2014)* |
| Brushtail possums (Trichosurus volpecula) (♂ only) | Repeated measures; multiple timepoints | 20 weeks | None—first sample at week 1 of captivity | Plasma (<5 min) | (Baker et al., 1998) | ||
| Brushtail possums (Trichosurus volpecula) (♂) | Repeated measures; multiple timepoints | Up to 8 weeks | None—unclear when first sample was obtained | Plasma (time not given) | (Begg et al., 2004) | ||
| Harbor seal (Phoca vitulina) (juvenile) | Captive vs free-living populations | >4 weeks | Free-living animals | Plasma (wild: 60 min captive: < 10 min) | (Trumble et al., 2013)* | ||
| Tuco-tuco (Ctenomys talarum)1 | Repeated measures; multiple timepoints | 30 days | At-capture measure | Plasma (<3 min) | (Vera et al., 2011)* | ||
| Birds | European starling (Sturnus vulgaris) | Repeated measures; pre- vs post- captivity | 4 weeks + | Feather grown in the wild | Feathers | (Fischer et al., 2017) | |
| Western screech owl (Otus kennicottii) | Captive vs free-living populations | >1 month | Free-living animals | Plasma (<5 min) | (Dufty and Belthoff, 1997)* | ||
| House sparrow (Passer domesticus) | Multiple timepoints; different individuals | Up to 4 weeks | Free-living animals | Plasma (<3 min) | (Martin et al., 2011)* | ||
| Reptiles | Tuatara (Sphenodon punctatus) (♂ only) | Captive vs free-living populations | Unknown | Free-living population | Plasma (<20 min) | (Tyrrell and Cree, 1994) | |
| Kutum (Rutilus frisii kutum) | Captive vs free-living populations | 3 days | Free-living population | Plasma (<3 min) | (Nikoo et al., 2010)* | ||
| GCs elevated in captivity | Mammals | Canada lynx (Lynx canadensis)2 | Captive vs free-living populations | Long term (unknown)3 | Free-living population | FGMs | (Fanson et al., 2012)* |
| Spider monkey (Ateles geoffroyi yucatanensis) | Captive vs free-living populations | Long term (unknown)2 | Free-living population | FGMs | (Rangel-Negrin et al., 2009)* | ||
| African wild dog (Lycaon pictus) | Captive vs free-living populations | Long term (unknown)2 | Free-living population | FGMs | (Van der Weyde et al., 2016)* | ||
| Grevy’s zebra (Equus grevyi) | Repeated measures; multiple timepoints | 6 weeks | At-capture sample; free-living population | FGMs | (Franceschini et al., 2008)* | ||
| White rhinos (Ceratotherium simum) | Repeated measures; multiple timepoints | 75 days | At-capture sample | FGMs | (Linklater et al., 2010)* | ||
| Birds | Curve-billed thrasher (Toxostoma curverostre) | Repeated measures; multiple timepoints | 80 days | At-capture samples | Plasma (<3 min) | (Fokidis et al., 2011)* | |
| White-crowned sparrow (Zonotrichia leucophrys) | Captive vs free-living populations | 35 days | Free-living population | Plasma (<1 min) | (Marra et al., 1995)* | ||
| White-throated sparrow (Zonotrichia albicollis) | Captive vs free-living populations | 35 days | Free-living population | Plasma (<1 min) | (Marra et al., 1995)* | ||
| Blackbirds (Turdus merula) | Repeated measures; pre- vs post-captivity | 22 days | At-capture sample | Plasma (<3 min) | (Adams et al., 2011)* | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 7 days | At-capture sample | Plasma (<3 min) | (Fischer and Romero, 2016)* | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 7 days | At-capture sample | Plasma (<3 min) | (Fischer et al., 2018)* | ||
| House sparrow (Passer domesticus) | Repeated measures; pre- vs post-captivity (multiple seasons) | 5 days | At-capture sample | Plasma (<3 min) | (Lattin et al., 2012)* | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 66 days | At-capture sample | Plasma (<3 min) | (Love et al., 2017)* | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 24 days | At-capture sample | Plasma (<3 min) | (Gormally et al., 2019)* | ||
| Southern pied babblers (Turdoides bicolor) | Captive vs free-living populations | 5 days | Free-living population | FGMs | (Jepsen et al., 2019)* | ||
| Reptiles | Tuatara (Sphenodon punctatus) (♀ only) | Captive vs free-living populations | Unknown | Free-living population | Plasma (<20 min) | (Tyrrell and Cree, 1994)* | |
| Garter snake (Thamnophis elegans) | Repeated measures; multiple timepoints | 4 months | At-capture sample; free-living population | Plasma (<10 min)4 | (Sparkman et al., 2014)* | ||
| Tree lizard (Urosaurus ornatus) | Multiple timepoints; different individuals | Up to 3 weeks | At-capture samples | Plasma (<1 min) | (Moore et al., 1991)* | ||
| Water snake (Nerodia sipedon) |
Repeated measures; pre- vs post-captivity | 5–8 days | At-capture samples | Plasma (<5 min) | (Sykes and Klukowski, 2009)* | ||
| Brown treesnake (Boiga irregularis) | Multiple timepoints; different individuals | 3 days | Free-living population | Plasma (<8 min) | (Mathies et al., 2001)* | ||
| Amphibians | Cururu toad (Rhinella icterica) | Repeated measures; pre- vs post-captivity | 3 months | At-capture sample | Plasma (<3 min) | (de Assis et al., 2015)* | |
| Toad (Rhinella schneideri) | Repeated measures; pre- vs post-captivity | 60 days | At-capture sample | Plasma (<3 min) | (Titon et al., 2017)* | ||
| Green frog (Rana esculenta) | Repeated measures; multiple timepoints (multiple seasons) | 3 days | At-capture sample | Plasma (<5 min) | (Zerani et al., 1991)* | ||
| Fish | Coral reef fish (Hemigymnus melapterus) | Captive vs free-living populations | 2.5 months | Free-living population | Plasma (<6 min) | (Grutter and Pankhurst, 2000)* | |
| Wedge sole (Dicologoglossa cuneate) (juvenile) | Multiple timepoints; different individuals | 45 days | At-hatching samples | Whole-body (time not given) | (Herrera et al., 2016)* | ||
| GCs increase at-capture, then decrease to approach wild baseline | Mammals | Beluga whale (Delphinapterus leucas) | Repeated measures; multiple timepoints | Peak:1 day approach free-living by 4 days | At-capture sample and free-living population | Plasma (time not given) | (St Aubin and Geraci, 1989)* |
| Chacma baboon (Papio ursinus) | Repeated measures; multiple timepoints | Peak: 4 weeks approach long-term captives by 7 weeks | None—used long term captives as baseline. | Plasma (time not given) | (Steyn, 1975) | ||
| African green monkey (Cercopithecus aethiops) | Multiple timepoints; different individuals | Peak: 1 day approach free-living by 2 days | Free-living population | Plasma (time not given) | (Suleman et al., 2004)* | ||
| Mouse lemur (Microcebus murinus) | Repeated measures; multiple timepoints | Peak: 2 days approach free-living by 4 days | At-capture sample | FGMs | (Hamalainen et al., 2014)* | ||
| Richardson’s ground squirrel (Urocitellus richardsonii) | Repeated measures; multiple timepoints | Peak: 3–5 days approach free-living by 6 days | At-capture sample | FGMs | (Hare et al., 2014)* | ||
| Bottlenose dolphin (Tursiops truncatus) | Repeated measures; multiple timepoints | Peak: 1 day approach long-term captive by 2 weeks | Long-term captives | Plasma (time not given) | (Orlov et al., 1991) | ||
| Birds | House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | Peak: Day 7 approach free-living by Day 11 | At-capture sample | Plasma (<3 min) | (Fischer et al., 2018)* | |
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | Peak: Days 1–2 approach free-living by 1 month | At-capture sample | Plasma (<3 min) | (Kuhlman and Martin, 2010)* | ||
| White-crowned sparrow (Zonotrichia leucophrys) | Repeated measures; multiple timepoints | Peak: Days 1–2 approach free-living by Day 14 | At-capture sample | Plasma (time not given) | (Wingfield et al., 1982)* | ||
| Reptiles | Skink (Egernia whitii) | Repeated measures; multiple timepoints | Peak: 1 day–1 week approach free-living by 4 weeks | At-capture sample | Plasma (<1 min) | (Jones and Bell, 2004)* | |
| Amphibians | Water frog (Rana esculenta) | Multiple timepoints; different individuals | Peak: Day 1 approach free-living by Day 7 | Free-living populations | Plasma (<3 min) | (Gobbetti and Zerani, 1996)* | |
| Cane toad (Rhinella marina) | Repeated measures; multiple timepoints | Peak: Day 5 approach free-living by Day 12 | At-capture sample | Urine | (Narayan et al., 2011)* | ||
| Cane toad (Rhinella marina) | Repeated measures; multiple timepoints | Peak: Day 4 approach free-living by Day 14 | At-capture sample | Urine | (Narayan et al., 2012)* | ||
| Fijian ground frog (Platymantis vitiana) | Repeated measures; multiple timepoints | Peak: Day 5 approach free-living by Day 25 | At-capture sample | Urine | (Narayan and Hero, 2011)* | ||
| Fish | Flounder (Paralichthys orbignyanus) | Multiple timepoints; different individuals | Peak: 1 hour approach free-living by Day 1 | Free-living animals | Plasma (<7 min) | (Bolasina, 2011)* | |
| Kahawai (Arripis trutta) | Multiple timepoints; different individuals | Peak: 2–3 hours approach free-living by Day 3 | Free-living animals | Plasma (<4 min) | (Davidson et al., 1997)* | ||
| GCs lower in captivity | Mammals | Harbor porpoise (Phocoena phocoena) | Captive vs free-living populations | Long term (unknown) | Free-living population | Plasma (time not given) | (Siebert et al., 2011)* |
| Gilbert’s potoroo (Potorous gilbertii) | Captive vs free-living populations | Long term (unknown) | Free-living population | FGMs | (Stead-Richardson et al., 2010)* | ||
| Harbor seal (Phoca vitulina) | Repeated measures; multiple timepoints | Long term (unknown) | Free-living population | Plasma (time not given) | (Gardiner and Hall, 1997)* | ||
| Black rhino (Diceros bicornis)5 | Repeated measures; multiple timepoints | 60 days | At-capture sample | FGMs | (Linklater et al., 2010)* | ||
| White whale (Delphinapterus leucas) | Repeated measures; multiple timepoints | 11 days | Long-term captives | Plasma (time not given) | (Orlov et al., 1991) | ||
| Birds | European starling (Sturnus vulgaris) | Captive vs free-living population | Unknown | Free-living population | FGMs | (Cyr and Romero, 2008) | |
| Chukar partridge (Alectoris chukar) | Repeated measures; multiple timepoints | 9 days | At-capture sample | Plasma (<3 min) | (Dickens et al., 2009a)* | ||
| Amphibians | Toad (Rhinella icterica) | Captive vs free-living population, multiple timepoints6 | Decreased from Days 30 to 60 | Free-living population | Plasma (<3 min) | (Titon et al., 2018)* | |
| High initial GCs decrease over capture period | Mammals | Rhesus macaques (Macaca mulatta) | Repeated measures; multiple timepoints | Decreased from Day 1 to 1 year | None—first sample after unknown time in trap. | Plasma (<50 min) | (Lilly et al., 1999) |
| Brushtail possums (Trichosurus volpecula) (♀ only) | Repeated measures; multiple timepoints | Decreased from Week 1 to 20 | None—first sample at Week 1 of captivity | Plasma (<5 min) | (Baker et al., 1998) | ||
| Meadow vole (Microtus pennsylvanicus) | Multiple timepoints; different individuals | Decreased from Day 1 to Day 70 | None—first sample at Day 1 | Plasma (<1 min) | (Olsen and Seabloom, 1973) | ||
| Vicuñas (Vicugna vicugna) | Repeated measures; multiple timepoints | Decreased from at-capture to Day 12 | None—first sample after stressful capture (time not given) | Plasma (time not given) | (Bonacic and Macdonald, 2003) | ||
| Eurasian otter (Lutra lutra)7 | Repeated measures; multiple timepoints | Decreased from Days 2–5 to Days 5–10 | None—first sample at Days 2–5 | Plasma (time not given) | (Fernandez-Moran et al., 2004) | ||
| Birds | Red knot (Calidris canutus) | Repeated measures; multiple timepoints | Decreased from first sample to 2 years | None—first sample at day 70 | Plasma (3–38 min) | (Piersma and Ramenofsky, 1998) | |
| Fish | Red gurnard (Chelidonichthys kumu) | Repeated measures; pre- vs post-captivity (different durations) | Decreased from first sample to 1 day | None—first sample after long line capture | Plasma (<2 min) | (Clearwater, 1997) | |
| Snapper (Pagrus auratus) | Multiple timepoints; different individuals | Decreased form at-capture to Day 2 | None—first sample after long line capture | Plasma (<10 min) | (Pankhurst and Sharples, 1992) | ||
| Sardine (Sardina pilchardus) | Multiple timepoints; different individuals | Decreased from at-capture to Day 2 | None—first sample after seine capture | Plasma (~3 min) | (Marcalo et al., 2008) | ||
1Cortisol results only.
2No difference in GCs in females pre-breeding—GCs elevated in both sexes during breeding season.
3Captive population may include some captive-raised individuals.
4Blood sampling took longer in some samples.
5GC spike in many animals during first 2 weeks, but then drops well below at capture levels.
6GCs increased in non-calling toads, but sample sizes low.
7Some animals treated with long-acting neuroleptic, which had no effect on GC levels, so values were pooled.
*Data from this paper are incorporated into Fig. 3.
Table 3.
Patterns of change in stress-induced GCs and negative feedback with captivity in wild animals
| GC pattern during adjustment to captivity | Species | Study design | Timeframe | How was free-living GCs established? | Sample type | Citation | |
|---|---|---|---|---|---|---|---|
| No change in acute stress-induced GCs over captivity period | Mammals | Tuco-tuco (Ctenomys talarum) | Captive vs free-living populations | 20 days | Free-living population | Plasma (30 and 60 min) | (Vera et al., 2011) |
| Birds | Curve-billed thrasher (Toxostoma curverostre) | Repeated measures; multiple timepoints | 80 days | At-capture samples | Plasma (30 min) | (Fokidis et al., 2011) | |
| Blackbirds (Turdus merula) | Repeated measures; pre- vs post-captivity | 22 days | At-capture sample | Plasma (30 and 60 min) | (Adams et al., 2011) | ||
| Western screech owl (Otus kennicottii) | Captive vs free-living populations | >1 month | Free-living animals | Plasma (6–10 min) | (Dufty Jr and Belthoff, 1997) | ||
| House sparrow (Passer domesticus)1 | Repeated measures; pre- vs post-captivity | 5 days | At-capture sample | Plasma (30 min) | (Lattin et al., 2012) | ||
| House sparrow (Passer domesticus) | Multiple timepoints; different individuals | Up to 1 month | At-capture sample | Plasma (60 min) | (Kuhlman and Martin, 2010) | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 7 days | At-capture sample | Plasma (30 min) | (Fischer and Romero, 2016)* | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 7 days | At-capture sample | Plasma (30 min) | (Fischer et al., 2018)* | ||
| House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 66 days | At-capture sample | Plasma (30 min) | (Love et al., 2017) | ||
| White-crowned sparrow (Zonotrichia leucophrys)2 | Repeated measures; multiple timepoints | Up to 1 year | Free-living population | Plasma (<30 min) | (Romero and Wingfield, 1999) | ||
| Fish | Winter flounder (Pseudopleuronectes americanus) | Repeated measures; multiple timepoints | Up to 1 year | Free-living population | Plasma (60 min) | (Plante et al., 2003) | |
| Acute stress-induced GCs reduced in captivity | Birds | Chukar partridge (Alectoris chukar) | Repeated measures; multiple timepoints | 9 days | At-capture sample | Plasma (30 min) | (Dickens et al., 2009a) |
| White-crowned sparrow (Zonotrichia leucophrys)3 | Repeated measures; multiple timepoints | Up to 1 year | Free-living population | Plasma (30 min) | (Romero and Wingfield, 1999) | ||
| Acute stress-induced GCs increased in captivity | Mammals | Degu (Octodon degus) | Captive vs free-living populations | >1 year | Free-living population | Plasma (30 and 60 min) | (Quispe et al., 2014) |
| Birds | White-crowned sparrow (Zonotrichia leucophrys)4 | Repeated measures; multiple timepoints | Up to 1 year | Free-living population | Plasma (30 min) | (Romero and Wingfield, 1999) | |
| Reptiles | Water snake (Nerodia sipedon) | Repeated measures; pre- vs post-captivity | 5–8 days | At-capture sample | Plasma (60 min) | (Sykes and Klukowski, 2009) | |
| Amphibians | Eastern red-spotted newt (Notophthalmus viridescens)5 | Repeated measures; multiple timepoints | >1 year | Free-living population | Plasma (30 min) | (Berner et al., 2013) | |
| Negative feedback strength decreased with captivity, then increased | Birds | Chukar partridge (Alectoris chukar) | Repeated measures; multiple timepoints | Neg. feedback reduced at Day 5; recovered at Day 9 | At-capture sample | Plasma (90 min after DEX) | (Dickens et al., 2009b) |
| Negative feedback strength increased with captivity | Birds | House sparrow (Passer domesticus) | Repeated measures; pre- vs post-captivity | 5 days | At-capture sample | Plasma 90 min after DEX) | (Lattin et al., 2012) |
| Negative feedback strength did not change with captivity | Birds | House sparrow (Passer domesticus) | Repeated measures; multiple timepoints | 66 days | At-capture sample | Plasma 90 min after DEX) | (Love et al., 2017) |
1SI GCs lower post captivity in early winter, but no change during any other time of year.
2Outside of breeding season and molt.
3During the breeding season.
4During the post-breeding/molting season.
5SI GCs higher post captivity in pre-breeding and breeding season, not in winter.
Captivity does not influence GCs in all species. In 17% (10 of 59) studies, there was no recorded difference in GCs during or after the captivity period compared to free-living levels. In most studies, however, captivity caused a change in baseline or integrated GCs. In 42% of studies (25 of 59), wild animals had increased GCs at the end of the capture period compared to concentrations in free-living animals (periods of 3 days to several years). Elevated GCs are traditionally interpreted as an indication that animals are chronically stressed. Experimentally induced chronic stress can often lead to elevated baseline GCs, although this is by no means a universal response (Dickens and Romero, 2013). Adrenal hypertrophy may be an underlying mechanism explaining the long-term elevation of GCs. For example, long-term captivity led to increased adrenal mass in African green monkeys (Suleman et al., 2004) and mouse lemurs (Perret, 1982). In nine-banded armadillos, 6 months of captivity (but not 3 months) caused adrenal changes similar to those after a harsh winter (Rideout et al., 1985) and in herring gulls 28 days of captivity led to adrenal lesions (Hoffman and Leighton, 1985).
However, many studies that reported elevated GC concentrations at the end of the captivity period may eventually have shown decreased GCs had the study been carried out for longer. For example, house sparrows had elevated baseline GCs after 1–7 days in captivity (Kuhlman and Martin, 2010; Lattin et al., 2012; Fischer and Romero, 2016). But when captive house sparrows were sampled repeatedly over 6 weeks of captivity, the high baseline GCs seen at Day 7 were dramatically reduced over Days 11–42 and approached at-capture concentrations in one study (Fischer et al., 2018), but did not decrease in another study (Love et al., 2017).
The duration of captivity in the studies we collected was quite variable, ranging from 3 days to several years. To consolidate the patterns from multiple studies with different sampling times, we graphed the percent of studies with elevated GCs (relative to free-living levels) against captivity duration (Fig. 3). We expected the percent of studies with elevated GCs to decrease as captivity duration increased (as shown in Fig. 1C and D). This pattern would indicate an adjustment to captivity conditions and is a typical a priori prediction in the literature. However, we found that 45% (5 of 11) of species continued to have elevated GCs after 3 months or more of captivity. This suggests that for many species, there is never a complete adjustment to captivity. It is also possible that a publication bias exists in the papers we collected. When researchers did not see a difference between long-term captives and free-living animals, they may have been less likely to publish, or perhaps included those results in other studies that did not appear in our literature searches. It is interesting to note that the fewest studies reported elevated GCs at around two weeks post captivity, the amount of time that many researchers allow for their study species to become acclimated to laboratory conditions (e.g. Davies et al., 2013; Lattin and Romero, 2014; McCormick et al., 2015).
Figure 3.
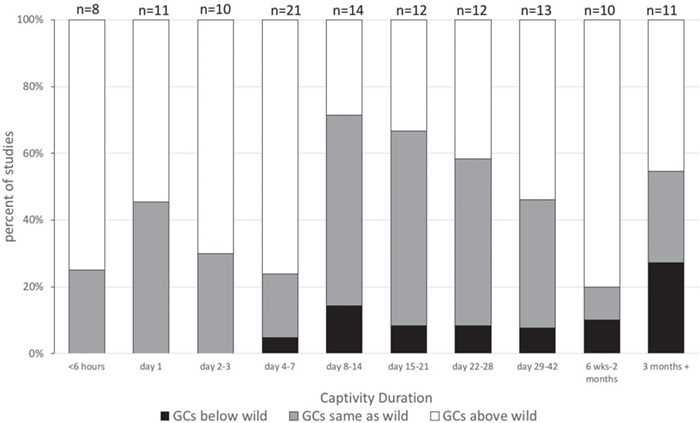
Change in baseline or integrated GCs as a function of captivity duration. Data were collected from the 47 studies listed in Table 3 that had a well-defined wild baseline value (i.e. plasma samples were collected within minutes of capture; fecal or urine samples were collected shortly after capture), with studies counted multiple times if they measured multiple time points after introduction to captivity. This figure does not include studies with seasonal effects on the GC response to capture.
The analysis in Fig. 3 contains data collected from many different taxa, study designs, etc. A more informative methodology to investigate how GCs change over time in captivity is to compare multiple timepoints within the same experiment. We found 38 studies that used repeated sampling. Researchers either repeatedly sampled individuals or captured many subjects at once and sampled them after different captivity durations. In study designs with repeated sampling, 42% of studies (16 of 38) showed an early increase in GCs followed by a decrease back to free-living levels (e.g. Fig. 1C and D, the a priori prediction for GC adjustment to captivity). Of the remaining studies, 32% (12 of 38) matched the pattern in Fig. 3 with no decrease in GC concentrations over time, 13% (5 of 38) showed decreased GC concentrations in captivity and 11% (4 of 38) reported no change in GCs whatsoever. When the expected peak and fall of GCs was observed, the timescale of adjustment to captivity varied. Baseline GCs in mouse lemurs returned to at-capture levels by Day 5 (Hamalainen et al., 2014) while the Fijian ground frog had elevated urinary GCs until Day 25 post capture (Narayan and Hero, 2011).
In some studies with repeated measures designs, the researchers did not or could not obtain a sample that represented free-living animals. In these cases, the first sample could not be acquired until minutes, hours or even days after capture. In all nine studies where this was the case (see Table 2), initially high concentrations of GCs decreased over the study period in at least some animals. This is consistent with the pattern we expect for animals successfully adjusting to captivity: capture, handling and the initial transfer to captivity result in high GCs that decrease as the animal adjusts. For example, female brushtail possums were not sampled until days after their capture and transfer to captivity, but showed decreasing plasma GCs from week 1 to week 20 of captivity (Baker et al., 1998).
These studies on baseline GCs together demonstrate a pattern wherein approximately half of species appear to adjust to captivity. Although some species seem to take longer to acclimate to captive conditions than others, it appears that many species will eventually show a reduction in GCs after an initial peak. We see this pattern across taxonomic groups, in birds, fish, reptiles, amphibians and mammals. However, we should be careful to not interpret a reduction in circulating baseline GCs, fecal GC metabolites or urinary GCs as a complete adjustment to captivity or an elimination of chronic stress. Even when baseline GCs have returned to free-living levels, other aspects of the animals’ physiologies may be negatively impacted. For example, even though circulating GCs were only elevated for 1 day in African green monkeys, adrenal mass was almost doubled after 45 days in captivity (Suleman et al., 2004). Similarly, while it is tempting to conclude that elevated GCs are diagnostic of chronic stress, it should be kept in mind that baseline GCs have many functions in metabolism and energy use. A change of baseline GCs in captivity could merely reflect a change in energy requirements and not the physiological damage we associate with chronic stress. Furthermore, a reduction in GCs in captivity, as seen in 14% of studies (8 of 59), could be interpreted as a reduction in allostatic load or as the exhaustion of adrenal capacity.
Impact of captivity on acute stress response and negative feedback of GC production
Relatively few researchers have explicitly investigated the effects of captivity on the acute GC stress response (see Table 3). Of those that have, 65% (11 of 17) found no effect of captivity (captivity duration 5–80 days). The six studies that reported changes in stress-induced GCs showed changes in opposite directions. In two studies, stress-induced GCs were decreased in captivity, even though the captive periods of 9 days (Dickens et al., 2009a) and 1 year (Romero and Wingfield, 1999) were quite different. In contrast, stress-induced GCs were increased in captivity in four studies over similar time frames. Three studies had animals in captivity for about a year (Romero and Wingfield, 1999; Berner et al., 2013; Quispe et al., 2014), with 5–8 days in the fourth study (Sykes and Klukowski, 2009).
The negative feedback of the GC response to stress, where high GC levels lead to the inhibition of GC production, is very important for the control of physiological stress (Vitousek et al., 2019). Although chronic stress has frequently been found to affect the negative feedback of GC production (Dickens and Romero, 2013), we found only three studies that explicitly measured negative feedback strength in animals immediately at capture and after a period of captivity. In each case, animals were injected with a synthetic GC (dexamethasone) after mounting a stress response to stimulate maximum negative feedback. The strength of negative feedback increased slightly in house sparrows after 5 days of captivity (Lattin et al., 2012), but in the same species showed no change after 21, 42 or 66 days (Love et al., 2017). In contrast, negative feedback strength decreased after 5 days of captivity in chukar partridges but returned to its at-capture strength by 9 days (Dickens et al., 2009b). This is an important aspect of stress physiology, one that is critical for the total amount of GC exposure, and warrants further study to determine whether it is impacted by the stress of captivity in many species.
Immune consequences of captivity
Stress has well-documented, but sometimes complex, effects on the immune system. In large part, these changes are due to the acute or long-term effects of elevated GCs on leukocyte populations. GCs can cause immune redistribution, moving lymphocytes out of the bloodstream and into the skin, spleen and lymph nodes, where they will be available in case of a wound (Dhabhar and McEwen, 1997; Johnstone et al., 2012). GCs can also cause proliferation or mobilization of neutrophils (most vertebrates) or heterophils (birds and some reptiles) (Dale et al., 1975; Gross and Siegel, 1983; Johnstone et al., 2012). Together, these effects on leukocyte populations result in a change in the neutrophil or heterophil to lymphocyte ratio (N or H:L ratio) (Dhabhar and McEwen, 1997; Johnstone et al., 2012). A change in the N or H:L ratio does not necessarily mean that an animal’s immune system is hypo- or hyperactive. Instead, this acts as another metric similar to GC secretion. A long-term increase in N or H:L ratio, like a long-term increase in circulating GCs, can be an indication that an animal is suffering from chronic stress (Davis et al., 2008).
We summarized the 23 studies that reported leukocyte counts in Table 4. Although the N or H:L is a useful metric, in some studies the researchers chose to report total number or percent of different leukocyte types without calculating or performing statistics on the relative abundances of neutrophils/heterophils and lymphocytes. In these cases, we inferred the direction (or presence) of change after captivity of the N or H:L ratio based on the changes in leukocyte counts or percentages that were reported. In two studies, only the total number of leukocytes was reported without further subdivision of leukocyte types. In 48% of studies (10 of 21), N or H:L ratio was elevated at the end of the measured captivity duration relative to its free-living value. 29% of studies (6 of 21) documented no change in N or H:L ratio over the study period. N or H:L ratio was decreased in 24% of studies (5 of 21). In one study (in the Fijian ground frog), the N:L ratio was elevated for 15 days in captivity, but then returned to wild levels by Day 25, resulting in no overall change (Narayan and Hero, 2011). Kuhlman and Martin (2010) further investigated leukocyte redistribution to the skin in house sparrows, comparing Day 1of captivity to Day 30. They concluded that the changes in H:L ratio were not due to redistribution of leukocytes, at least in this instance. We summarized the overall patterns of N or H:L ratio compared to captivity duration in Fig. 4. The number of studies reporting an increase in N or H:L ratio decreases with captivity duration. This suggests that many or most species do adjust to captivity, and an initially high N or H:L ratio may decrease given sufficient time.
Table 4.
Changes in leukocytes during captivity
| Species | Study design | How was free-living value established? | Timeframe | WBCs | H or N | L | H or N:L ratio | Citation | |
|---|---|---|---|---|---|---|---|---|---|
| Mammals | Spanish ibex (Capra pyrenaica hispanica) | Repeated measures; multiple timepoints | At-capture sample | 14 months | ↓ | – | ↓ | ↑ (n.c.) | (Peinado et al., 1995) |
| Rhesus macaques (Macaca mulatta) | Repeated measures; multiple timepoints | At-capture sample | 1 year | ↓ | ↓ | ↑ | ↓ (n.c.) | (Lilly et al., 1999) | |
| Brushtailed possums (Trichosurus vulpecula) | Repeated measures; multiple timepoints | None—first sample at Week 1 of captivity | 20 weeks | – | – | (Baker et al., 1998) | |||
| Beluga whale (Delphinapterus leucas) | Repeated measures; multiple timepoints |
At capture sample and free-living population | 2.5 months | ↑ | ↑ | ↓ | ↑ (n.c.) | (St Aubin and Geraci, 1989) | |
| brushtailed possums (Trichosurus vulpecula) | Repeated measures; multiple timepoints (different housing conditions) | None—unclear when first sample was obtained | 2 months | – | – | – | – (n.c.) | (Begg et al., 2004) | |
| Howler monkey (Alouatta caraya) | Repeated measures; pre- vs post-captivity | At-capture sample | 2 months | – | – | – | – (n.c.) | (Sanchez-Sarmiento et al., 2015) | |
| Steller sea lions (Eumetiopias jubatus) | Repeated measures; pre- vs post-captivity | At-capture sample and free-living population | 2 months | ↓ | (Mellish et al., 2006) | ||||
| Black rhinoceros (Diceros bicornis michaeli)1 | Repeated measures; pre- vs post-captivity | None – first sample after stressful capture (up to 1 hour) | 3–4 weeks | ↑ | ↓ | ↑ (n.c.) | (Kock et al., 1999) | ||
| Vicuñas (Vicugna vicugna)2 | Repeated measures; multiple timepoints | At-capture sample3 | 12 days | – | – | – | – | (Bonacic and Macdonald, 2003) | |
| Birds | Red knots (Calidris canutus) | Captive vs free-living population | Free-living population | ~1 year | – | ↓ | – | ↓ (n.c.) | (Buehler et al., 2008) |
| Ruff (Philomachus pugnax) | Repeated measures; multiple timepoints | None—does not say when first sample taken relative to capture | 1 year | – | (Piersma et al., 2000) | ||||
| Greenfinches (Chloris chloris) | Captive vs free-living population | Free-living population | 2 months | – | – | ↑ | ↓ | (Sepp et al., 2010) | |
| Zebra finches (Taeniopygia guttata) | Captive vs free-living population | Free-living population | 10 days | ↓ | ↓ | (Ewenson et al., 2001) | |||
| 2 months | – | ↓ | |||||||
| House sparrow (Passer domesticus)4 | Repeated measures; early- vs late-captivity | None—first sample 1–2 days in captivity | 1 month | – | – | ↓ | ↑ (n.c.) | (Kuhlman and Martin, 2010) | |
| Herring gull (Larus argentatus) | Repeated measures; multiple timepoints | At-capture sample | 4 weeks | ↑ | ↑ | – | ↑ (n.c.) | (Hoffman and Leighton, 1985) | |
| Rufous-collared sparrows (Zonotrichia capensis) | Captive vs free-living population | Free-living population | 2 weeks | ↑ | ↓ | ↑ | (Ruiz et al., 2002) | ||
| Reptiles | Garter snakes (Thamnophis elegans) | Repeated measures; pre- vs post-captivity | At-capture sample and free-living population | 4 months | ↑ | (Sparkman et al., 2014) | |||
| Amphibians | Cururu toad (Rhinella icterica) | Repeated measures; pre- vs post-captivity | At-capture sample | 3 months | ↑ | – | (de Assis et al., 2015) | ||
| Fijian ground frog (Platymantis vitiana) | Repeated measures; multiple timepoints | At-capture sample | 15 days | ↑ | ↓ | ↑ | (Narayan and Hero, 2011) | ||
| 25 days | – | – | – | ||||||
| Mole salamanders (Ambystoma talpoideum) | Repeated measures; pre- vs post-captivity | At-capture sample | 10 days | – | ↓ | ↑ | (Davis and Maerz, 2008) | ||
| Fish | Kutum (Rutilus frisii kutum) | Captive vs free-living population | Free-living population | 3 days | ↑ | ↑ | ↓ | ↑ (n.c.) | (Nikoo et al., 2010) |
Timeframe refers to the longest duration of captivity measured. WBC = total white blood cells; H = heterophils; N = neutrophils; L = lymphocytes; n.c. = not calculated (in this case, a count or percentage of heterophils or neutrophils and lymphocytes was measured in the paper, but H or N:L ratio was not directly compared. Presence/direction of change in the rctypes); ↑ or ↓ = higher or lower than free-living; – = no change from free-living.
1Pattern only seen in rhinos translocated from high to low (not high to high) elevation.
2Total WBCs and N:L ratio also compared to free-living wild populations of a similar species—there was no difference.
3Comparison to values collected in another study and species (llamas and alpacas).
4Circulating leukocytes and skin-infiltrating leukocytes were measured. See text for skin leukocyte patterns.
Some studies also reported the total leukocyte counts, sometimes without further subdividing them into classes. While decreased circulating leukocytes has been associated with stress (generally because of redistribution rather than destruction of cells) (Dhabhar, 2002), there was no clear pattern with the number of leukocytes in captivity. 53% of studies (9 of 17) showed no change in total white blood cells compared to free-living animals by the end of the captivity period; 23.5% (4 of 17) showed a decrease in circulating leukocytes; and 23.5% (4 of 17) showed an increase (captivity duration 3 days to 1 year, see Table 4).
Importantly, neither total leukocyte numbers nor the N or H:L ratio provide a very strong indication of immune capacity. Some researchers have used more direct measurements of immune functionality. The bacterial killing assay is a way to determine how effectively fresh whole blood can eliminate bacteria. This assay has the advantage of determining the real effectiveness of the immune system against pathogens (Millet et al., 2007). In the cururu toad, whole blood was less effective at killing bacteria after 13 days of captivity (de Assis et al., 2015) and in two other toad species, killing capacity decreased by 60 but not 30 days (Titon et al., 2017, 2018). Similarly, in red knots held in captivity for 1 year, whole blood was less effective at eliminating two Staphylococcus species than in wild living birds (though there was no difference in Escherichia coli elimination) (Buehler et al., 2008). In contrast, there was an increased proportion of E. coli killed after 3 weeks of captivity in house sparrows (Love et al., 2017).
Another way to measure immune responsiveness is by measuring a proliferative response against non-specific antigens. In some studies, this is done by culturing a sample of blood along with an antigen and quantifying cell division. In male brushtail possums, the proliferative response to the plant toxin phytohemagglutinin decreased over 20 weeks but increased by 1 year (Baker et al., 1998). In female possums, the proliferative response increased from 11 to 15 weeks in captivity, and then remained at that high level for at least a year (Baker et al., 1998). In another study in male brushtail possums, leukocyte proliferation to a Mycobacterium protein derivative increased after 4 and 6 weeks of captivity, but only when the animals were housed in high-density pens to create crowding (Begg et al., 2004). The proliferative response to phytohemagglutinin can also be measured in-vivo if PHA is injected into the skin and the degree of swelling is quantified. In zebra finches, there was no difference in the in vivo PHA response between newly captured birds and those held for 10 or 16 days (Ewenson et al., 2001).
Two studies have attempted to quantify the strength of the adaptive immune system in captivity. In red knots, plasma was plated with rabbit red blood cells. The degree of hemolysis and hemagglutination provided a measure of complement and natural antibody action. Hemolysis and hemagglutination were similar in wild and captive birds when they were measured at the same time of year, which suggests that the strength of the adaptive immune response is unaffected by captivity (Buehler et al., 2008). Conversely, newly captured killifish had a stronger response to antigen after immunization than 4–6-week captives, suggesting that the adaptive immune system was less effective after captivity (Miller and Tripp, 1982).
Overall, there does not seem to be a single pattern for immune regulation with captivity. While captivity has been shown to repress immune function in some species (e.g. reduced bacterial killing in red knots and toads), in other species, the immune system may be hyperactivated. For example, in house sparrows, gene expression for pro-inflammatory cytokines was elevated in captive birds (2- and 4-week captives) compared to newly caught animals, which was interpreted as hyperinflammation in captive birds (Martin et al., 2011). Changes in the immune response with chronic stress are thought to be most strongly tied to GC release. However, the impacts of GCs on the immune system can be complex. In the short term, GCs typically induce an immune response, while they can be immunosuppressive over the long term, although these interactions tend to be context-dependent (Dhabhar and McEwen, 1997; Martin, 2009). As the interaction between GCs and immunity is complex and context specific, and as the interaction of GCs to captivity can be complex as well (see Changes in GCs during the adjustment to captivity), it is not currently possible to predict whether captivity conditions will result in appropriate or inappropriate immune activity. However, there has been limited work in this area.
Effects of captivity on the reproductive system
Captivity has well-documented negative impacts on reproductive biology. In many species, captive breeding for research or conservation purposes can be a challenge. Even the house sparrow, so commonly used as a model species, does not readily breed in captivity (Lombardo and Thorpe, 2009). In 74% of studies (17 of 23), the transition to captivity resulted in reduced reproductive capacity in wild species (Table 5). Note, however, that these papers do not cover an extensive literature on captive breeding, including in individuals who have spent decades in captivity or were born in captivity, which is beyond the scope of this review. Here, we focus only on those papers that studied reproductive capacity of recent captives (only within the first year) and that examined a mechanism for reduced reproduction. There was no obvious taxonomic pattern for species that had reduced reproductive ability in captivity compared to those that had no documented reproductive problems. Duration of captivity did not appear to be a factor either. In one study of water frogs, reproduction in both males and females were negatively impacted by only 3 days of captivity (Zerani et al., 1991), while in jack mackerel, reproduction was inhibited after a full year of adjusting to captivity (Imanaga et al., 2014).
Table 5.
Reproductive effects of captivity in wild animals (if multiple times of year were examined, only breeding season is included in this table)
| Hormonal changes during adjustment to captivity | Species | Study design | Timeframe | Variable measured | How were free-living state established? | Citation | |
|---|---|---|---|---|---|---|---|
| Reproductive capacity decreased in captivity | Mammals | White rhino (Ceratotherium simum) | Repeated measures; multiple timepoints | 75 days | Fecal T (males) and Progestin (females) | At-capture samples | (Linklater et al., 2010) |
| Black rhino (Diceros bicornis) | Repeated measures; multiple timepoints | 60 days | Fecal T (males) and progestin (females) | At-capture samples | (Linklater et al., 2010) | ||
| Mouse lemur (Microcebus murinus) (♀ only) | Pathology of dead captive animals | Variable—usually years in captivity | Histological examination of reproductive organs (follicular growth) | Reproductive pathology increased with captivity length | (Perret, 1982) | ||
| Birds | Brown-headed cowbird (Molothrus ater) (♂ only)1 | Captive vs free-living population | 6 months +3 months photostimulation | Gonad size and plasma T | Free-living population | (Dufty Jr and Wingfield, 1986) | |
| House sparrow (Passer domesticus) (♂) | Repeated measures; multiple timepoints | 3 months | Sperm production, beak color, testes size | At-capture samples and free-living population | (Lombardo and Thorpe, 2009) | ||
| Reptiles | Brown treesnakes (Boiga irregularis) (♂) | Captive vs free-living population | 4–8 weeks | Sexual maturity (testes development) | Free-living population | (Aldridge and Arackal, 2005) | |
| Anole lizard (Anolis pulchellus) (♀) | Multiple timepoints; different individuals | 4 weeks | Plasma vitellogenin; ovary state2 | Free-living population | (Morales and Sanchez, 1996) | ||
| Tree lizard (Urosaurus ornatus) (♂) | Repeated measures; multiple timepoints | 3 weeks | Plasma T | At-capture samples | (Moore et al., 1991) | ||
| Snapping turtle (Chelydra serpentina) | Repeated measures; multiple timepoints | 1 week | Plasma T3 | At-capture sample | (Mahmoud et al., 1989) | ||
| Amphibians | Water frog (Rana esculenta) (♂) | Repeated measures; multiple timepoints | 2 weeks | Plasma T and E2 | At capture samples | (Gobbetti and Zerani, 1996) | |
| Water frog (Rana esculenta) | Repeated measures; multiple timepoints | 3 days | Plasma T and E24 | At capture sample | (Zerani et al., 1991) | ||
| Toad (Rhinella icterica) | Captive vs free-living population, multiple timepoints | 1 week | Plasma T | Free-living population | (Titon et al., 2018) | ||
| Toad (Rhinella schneideri) | Repeated measures; multiple timepoints | 60 days | Plasma T | At-capture sample | (Titon et al., 2017) | ||
| Fish | Jack mackerel (Trachurnus jabonicus) (♀) | Captive vs free-living population | 1 year | Egg maturity, reproductive stage, gnrh gene expression5 | Free-living population | (Imanaga et al., 2014) | |
| Electric fish (Gnathonemus petersii) | Repeated measures; multiple timepoints | 37 days | Sex-specific behaviors, plasma T and 11KT (males) | At capture samples | (Landsman, 1993) | ||
| Sardine (Sardina pilcardus) | Captive vs free-living population | 4 weeks | Gonadosomatic index | Free-living population | (Marcalo et al., 2008) | ||
| Red gurnard (Chelidonichthys kumu) (♀) | Multiple timepoints; different individuals AND repeated measures; multiple timepoints | 4 days | Plasma T, E2, egg development | Free-living population | (Clearwater, 1997) | ||
| No difference in reproductive capacity in captivity | Mammals | Armadillos (Dasypus novemcinctus) (♂) | Repeated measures; multiple timepoints | Up to 3 years | Plasma T | Free-living population | (Czekala et al., 1980) |
| Birds | White-crowned sparrows (Zonotrichia leucophrys)6 | Repeated measures; multiple timepoints | Up to day 20 or 33 | Plasma LH, plasma T (males only) | At-capture sample | (Wingfield et al., 1982) | |
| Reptiles | Striped plateau lizard (Sceloporus virgaltus) (♀) | Repeated measures; multiple timepoints | Up to 3 months | Plasma P, T and E27 | Free living population | (Weiss et al., 2002) | |
| Skink (Egernia whitii) (♂) | Repeated measures; multiple timepoints | 4 weeks | Plasma T | At-capture samples | (Jones and Bell, 2004) | ||
| Brown treesnake (Boiga irregularis) | Multiple timepoints; different individuals | 3 days | Plasma T and ovarian follicle development | Free-living population | (Mathies et al., 2001) | ||
| Amphibians | Water frog (Rana esculenta) (♀) | Repeated measures; multiple timepoints | 2 weeks | Plasma T and E28 | At capture samples | (Gobbetti and Zerani, 1996) | |
1Different photosimulation and social stimulation tested—maximal testicular regrowth (long days + females) still below wild, though in that group, T was the same as wild.
2Vitellogenin levels recovered by E2 use.
3T spikes during the first 24–48 hours of captivity, but decreases below at-capture levels.
4E2 spikes during first hours of captivity, but quickly decreases below at-capture levels.
5E2 higher in captive than wild.
6There was a transitory increase in LH at around Weeks 1–3 that came back to at-capture levels in multiple experiments.
7T lower in captivity, but only after egg-laying.
8E2 spike in first 6 hours of captivity but then returns to at-capture levels.
Different researchers measured different variables for reproductive capacity. Many studies analyzed reproductive steroid hormones (primarily testosterone in males and estrogen and/or progesterone in females). However, other variables were also measured, including gonad size and development, behavior and gamete development. In house sparrows, Lombardo and Thorpe (2009) found decreased sperm production, reduced testes size and a change in beak color from breeding-season black to wintering brown after 3 months of captivity. Female anole lizards experienced a rapid decrease in plasma vitellogenin (a protein necessary for yolk production) followed by regression of developing follicles (Morales and Sanchez, 1996). In electric fish, behavioral differences between males and females were reduced in captivity until they disappeared or even reversed. This occurred concurrently with decreases in testosterone and 11-ketotestosterone (a potent fish androgen) in males (Landsman, 1993).
The reduction of reproductive capacity might be tied to GC levels. GCs can be powerful suppressors of reproductive steroids (Sapolsky et al., 2000). Prolonged GC exposure can lead to decreased production of testosterone or estradiol, which can then have downstream effects on gonad development, egg maturation, sperm production and behavior. In green treefrogs, a decrease in sex steroids was concurrent with an increase in GCs (Zerani et al., 1991). However, in black rhinos, males had suppressed fecal testosterone and females had suppressed fecal progestins even though GC levels were below free-living levels for most of the captivity period (Linklater et al., 2010).
Captivity did not always result in suppression of reproduction but in most studies that did not show an effect of captivity, reproductive hormones were the only variables measured. The only exception was in the brown treesnake, where 3 days of captivity did not affect either testosterone or ovarian development (both were very low in free-living and captive animals) (Mathies et al., 2001). However, another study in brown treesnakes found underdeveloped testes in males after 4–8 weeks of captivity (Aldridge and Arackal, 2005). Captivity may affect sexual variables differently in males and females. For example, in water frogs held in captivity for 2 weeks, only males appeared to be negatively affected by captivity (Gobbetti and Zerani, 1996), which is opposite what is typically expected.
Overall, it appears that captivity tends to have a negative impact on reproduction in most species. However, there are relatively few studies that specifically examine the reproductive physiology of newly-captured animals. Furthermore, given that many animals eventually do breed in captivity while others do not, it is not clear how long-lasting these impacts may be or why they impact some species more than others.
Adrenomedullary effects of captivity
The adrenomedullary arm of the stress response can be difficult to measure. Measuring epinephrine or norepinephrine in the blood is relatively straightforward, but these hormones increase within seconds of disturbance, meaning that acquiring a free-living baseline in a wild animal is difficult without substantial acclimation to human presence. We excluded most studies that measured epinephrine or norepinephrine, as sampling techniques between wild and captive animals differed in ways that would obscure the meaning of their results. For example, plasma norepinephrine under anesthesia (collected within 50 minutes) decreased over 19 months of captivity in rhesus macaques, though a free-living sample could not be obtained under the same conditions (Lilly et al., 1999), and captive-raised bighorn sheep had a higher epinephrine response to a drop-net capture technique than did free-living sheep, though they had similar norepinephrine responses (Coburn et al., 2010).
Recording heart rate is another way to infer activity of the adrenomedullary system (Romero and Wingfield, 2016). Heart rate recordings typically involve the use of specialized and expensive equipment, but can give instantaneous updates on heart rate. In addition, scientists can measure heart rate variability, which gives a metric of how much relative control the sympathetic and parasympathetic nervous systems have over heart rate (Romero and Wingfield, 2016). However, depending on the type of heart rate recording device, it may be impossible to obtain baseline free-living heart rates. Although several researchers have had success measuring heart rate in free-living animals (e.g. white-eyed vireos; Bisson et al., 2009), to our knowledge, there has not yet been a study that directly compares heart rate in free-living and captive animals of the same species.
Heart rate has been measured in only a few species during the transition to captivity. In newly-captured bighorn sheep, heart rate during restraint and blood sampling decreased from Days 1–2 (when the animals were handled extensively and transported) until Day 14 (Franzman, 1970). The heart rate of newly-captured European starlings was high compared to birds held for more than a year in captivity but decreased to the level of long-term captives within 24 hours (Dickens and Romero, 2009). The adrenomedullary response to captivity was slightly different in house sparrows. Daytime heart rate was elevated above 1 month captive levels for at least 7 days post-capture (Fischer and Romero, 2016). These data led to a long-term repeated-measures investigation during the first 6 weeks of captivity (Fischer et al., 2018). Heart rate tended to decrease until Day 18, then plateaued. Furthermore, there was a more profound effect on the heart rate response to a sudden noise in the starling study. While long-term captives showed a robust increase in heart rate after a loud noise, a typical adrenomedullary response, newly-captured birds had a virtually eliminated heart rate response for at least 10 days (Dickens and Romero, 2009). A reduction in the startle response (as demonstrated in European starlings) could have negative consequences for animals that are released from captivity into the wild (Dickens et al., 2009a). The adrenomedullary response to sudden noises or other startling events is an adaptation that allows animals to survive sudden traumatic events, such as predator attacks or conspecific aggression. An impaired startle response could result in death if it persists after the animals are released from captivity.
Overall, there are few studies examining the effects of captivity on the adrenomedullary response. The patterns we see in European starlings and house sparrows are different—it does not appear that there is a consistent heart rate response to captivity in passerine birds, much less in all vertebrates. We believe this is an area ripe for future studies. As telemetry equipment becomes cheaper and more available, we hope to see more investigations into the adrenomedullary response to captivity and other stressors.
Effects of captivity on seasonality of hormone regulation
Some studies examined seasonal differences in the response to captivity. Table 6 shows that the time of year when animals are introduced to captivity can have a profound effect on hormonal changes. For example, baseline GCs might increase when free-living birds are in molt, decrease when free-living birds are breeding, and not change when free-living birds are captured during the winter or spring (Romero and Wingfield, 1999). Furthermore, Table 6 indicates that there is no consistent pattern across seasons or taxonomic groups. The implications of these differences are currently unknown, but the season of capture might partly explain the large variation across studies summarized in Figs 2–4. Understanding why there are seasonal differences in the acclimation to captivity would be an important contribution to this field.
Table 6.
Seasonal effects of captivity
| Hormone | Species | How was free-living GCs established? | Captivity duration | Pre-breeding | Breeding | Post-breeding/molt | Winter | Citation | |
|---|---|---|---|---|---|---|---|---|---|
| Baseline GCs | Mammals | Canada lynx (Lynx canadensis) females | Free-living population | Long term (unknown) | − | ↑ | (Fanson et al., 2012) | ||
| Canada lynx (Lynx canadensis) males | Free-living population | Long term (unknown) | ↑ | ↑ | (Fanson et al., 2012) | ||||
| Harbor seal (Phoca vitulina) | Free-living population | Long term (unknown) | ↓ | ↓ | ↓ | (Gardiner and Hall, 1997) | |||
| Birds | White-crowned sparrow (Zonotrichia leucophrys gambelii) | Free-living population | 4 months at start | − | ↓ | ↑ | − | (Romero and Wingfield, 1999) | |
| House sparrow (Passer domesticus) | Same individual pre-capture | 5 days | ↑ | − | − | − | (Lattin et al., 2012) | ||
| Reptiles | Duvaucel’s gecoks (Hoplodactylus duvaucelli) | Free-living population | >1 year at the start | − | ↑ | − | (Barry et al., 2010) | ||
| Tuatara (Sphenodon punctatus) (♀) | Free-living population | Unknown | ↑ | − | (Tyrrell and Cree, 1994) | ||||
| Tuatara (Sphenodon punctatus) (♂) | Free-living population | Unknown | − | − | (Tyrrell and Cree, 1994) | ||||
| Amphibians | Eastern red-spotted newt (Notophthalmus viridescens) | Free-living population | 2 months to > 1 year | ↑ | ↑ | − | (Berner et al., 2013) | ||
| Green frog (Rana esculenta) | Same individual pre-capture | 3 days | ↑ | ↑ | ↑ | (Zerani et al., 1991) | |||
| Fish | Winter flounder (Pseudopleuronectes americanus) (juvenile) | Free-living population | 2 months at start | ↓1 | − | (Plante et al., 2003) | |||
| Acute stress GCs | Birds | White-crowned sparrow (Zonotrichia leucophrys gambelii) | Free-living population | 4 months at start | − | ↓ | ↑ | − | (Romero and Wingfield, 1999) |
| House sparrow (Passer domesticus) | Same individual pre- capture | 5 days | − | − | − | ↓ | (Lattin et al., 2012) | ||
| Amphibians | Eastern red-spotted newt (Notophthalmus viridescens) | Free-living population | >1 year | ↑ | ↑ | − | (Berner et al., 2013) | ||
| Fish | Winter flounder (Pseudopleuronectes americanus) (juvenile) | Free-living population | 2 months at start | − | − | (Plante et al., 2003) | |||
| T | Mammals | Armadillos (Dasypus novemcinctus) | Free-living population | 2 weeks–3 years | − | − | − | − | (Czekala et al., 1980) |
| Amphibians | Green frog (Rana esculenta) (♀) | Same individual pre-capture | 3 days | ↓ | − | − | (Zerani et al., 1991) | ||
| Amphibian: green frog (Rana esculenta) (♂) | Same individual pre-capture | 3 days | ↓ | ↓ | ↓ | (Zerani et al., 1991) | |||
| E2 | Amphibians | Amphibian: green frog (Rana esculenta) (♀) | Same individual pre-capture | 3 days | ↓ | ↓ | ↓ | (Zerani et al., 1991) | |
| Amphibian: green frog (Rana esculenta) (♂) | Same individual pre-capture | 3 days | − | − | ↓ | (Zerani et al., 1991) | |||
Arrows indicate direction of change in captive animals relative to an established free-living level. Captive and wild measurements were taken at the same time of year. GCs glucocorticoids; T testosterone; E2 estradiol.
1Delay in acquiring wild baseline.
Other physiological consequences of captivity
Some studies, primarily in marine mammals, reported the effects of captivity on thyroid hormone. Unfortunately, there is not a consistent impact. For example, one study of beluga whales reported that thyroid hormone decreased over the first few days of captivity, but increased to a long-term stable level by day 11 (Orlov et al., 1991), whereas another study reported that thyroid hormone decreased within the first few days and remained low throughout 10 weeks of captivity (St Aubin and Geraci, 1988). Similarly, rehabilitated harbor seal juveniles held in captivity for 4 months had lower thyroid hormone than free-living juveniles (Trumble et al., 2013). In contrast, long-term captive harbor porpoises had the same thyroid hormone levels as wild populations (Siebert et al., 2011) and in female brushtail possums, thyroid hormone was elevated from Weeks 6–13, the same period when the animals were regaining weight they had lost in captivity (Baker et al., 1998). Clearly, more work is needed to determine the effect of captivity on thyroid hormone regulation.
Anatomical changes may also occur in captivity. Mountain chickadees showed remarkable reduction in hippocampal volume after 4 months of captivity (LaDage et al., 2009), an effect mimicked by black-capped chickadees after 4–6 weeks in captivity (Tarr et al., 2009). In neither species was the telencephalon affected—the effect was localized to the part of the brain involved in location-based memory tasks. This effect persisted even when the environment was enriched to include memory tasks (LaDage et al., 2009).
Captivity can lead to various pathologies. In a histological study of mouse lemurs that died spontaneously in captivity, lesions in the kidney were strongly correlated with captivity duration and with adrenal size (Perret, 1982). The investigator also concluded that cardiac disease may result from chronic adrenomedullary stimulation, although they did not measure hormone concentrations directly (Perret, 1982). Similarly, herring gulls developed amyloid deposits in the blood vessels of their spleens after 28 days in captivity (Hoffman and Leighton, 1985). There may be many more hidden anatomical changes resulting from captivity, but few studies have looked for them.
Finally, recent data indicates that captivity can have profound effects at the DNA level. Bringing house sparrows into captivity resulted in an approximately doubling of DNA damage in red blood cells (Gormally et al., 2019). The impact of this damage on the individual remains to be determined.
Amelioration of captivity stress
Captivity can cause a wide array of physiological changes in wild animals that are consistent with chronic stress and are likely to be detrimental to health. However, can anything be done to prevent these changes? Is there a way to protect animals from the negative consequences of captivity stress? While this is not an exhaustive review of the solutions that have been tried, we offer some ideas that have been attempted to relieve symptoms of chronic stress due to captivity conditions.
Adjusting the physical conditions of captivity may be one of the simplest ways to reduce symptoms of chronic stress. Transferal from outdoors cages to indoors cages led to reduced reproductive hormones and behaviors in long term captive European starlings (Dickens and Bentley, 2014) and to weight loss and reduced immune function in water voles (Moorhouse et al., 2007). Cage size and density are also important for the development of chronic stress. High density housing during the initial captivity period resulted in elevated GCs compared to low density housing in flounders (Nester Bolasina, 2011) and wedge sole (Herrera et al., 2016). However, reducing density by caging animals individually can have negative consequences, particularly in social species. Housing brushtail possums in groups eliminated the infection, weight loss and mortality that were seen when the animals were caged individually (McLeod et al., 1997). In male brown headed cowbirds, adding a female to the cage (previously solo housed) resulted in reduced plasma GCs, as well as increased testicular regrowth in photostimulated males (Dufty Jr and Wingfield, 1986).
Many animals benefit from the use of behavioral enrichments to reduce abnormal behaviors that develop in captivity (reviewed in Mason et al., 2007). Enrichments have become standard practice in zoo environments and situations where animals are held long-term or bred in captivity. Enrichments consist of providing animals with the means and motivation to practice a full range of natural behaviors, such as foraging opportunities, exercise opportunities and places to bathe or dust bathe. Even in temporary or laboratory conditions, environmental enrichments can be relatively easy to supply. However, we were unable to find any papers where the physiological benefits of enrichment techniques were specifically tested in newly captured animals. Using these techniques to accelerate the adjustment to captivity would be an exciting avenue for future research.
Lighting conditions may be very important for visual species. European starlings show more behavioral signs of chronic stress under fluorescent lights with a low flicker rate than a high flicker rate (Evans et al., 2012), but the low flicker rate does not elicit a GC response (Greenwood et al., 2004). Ultraviolet-deficient lighting resulted in higher baseline GCs in European starlings, although immediately after capture, this stressor may be too subtle to make a difference compared with the other stressors of captivity (Maddocks et al., 2002). Temperature conditions should also be carefully considered, particularly for poikilotherms. Warm conditions during the initial transfer to captivity resulted in high mortality in sardines (Marcalo et al., 2008) and higher GCs in cane toads (Narayan et al., 2012).
Overall, by matching captivity conditions as closely as possible to conditions in the wild, with roomy cages, exposure to naturalistic lighting and temperature conditions and animal densities kept relatively low, many animals will be better able to adjust to captivity and may have reduced chronic stress as a result. However, naturalistic housing conditions may be impractical for many situations. Furthermore, some stressors associated with captivity may be unavoidable. For example, nearly any visual or auditory contact with handlers resulted in a heart rate increase in two red-shouldered hawks (Patton et al., 1985). Therefore, in some cases, it might be beneficial to use pharmaceuticals to reduce symptoms of chronic stress.
Tranquilizers or sedatives are perhaps the most obvious drug classes to consider using in newly-captured animals. However, these may not be particularly effective at eliminating chronic stress symptoms. A long-acting neuroleptic did not result in many physiological changes in newly-caught otters (Fernandez-Moran et al., 2004). Tranquilizers did not impact any physiological variable in newly caught impala (Knox et al., 1990) or red-necked wallabies (Holz and Barnett, 1996), although they reduced behavioral agitation to human approach and handling in the later study. Similarly, a long-acting tranquilizer changed behavior but not heart rate response to human approach in captive wildebeest (Laubscher et al., 2016). The anxiolytic and sedative diazepam did not affect GCs, heart rate, heart rate variability or activity in house sparrows during the first week of captivity (unpublished personal data). Overall, tranquilizers and sedatives do not appear to have long-term physiological benefits in captive animals. However, they may be useful in the short term. For example, by reducing physical agitation, they may prevent animals from injuring themselves during transport (e.g. in nurse sharks being moved into captivity; Smith, 1992) or during necessary handling by humans (e.g. in red-necked wallabies; Holz and Barnett, 1996).
Another strategy for pharmaceutical reduction of symptoms of chronic stress may be to chemically block the hormones of the stress response. The chemical agent mitotane causes a reversible chemical adrenalectomy, which drastically reduces circulating GCs (Sanderson, 2006). In house sparrows treated with mitotane immediately upon capture, baseline and stress induced GCs were drastically reduced during the initial captivity period, but recovered to the level of untreated birds by Day 10 of captivity (Breuner et al., 2000). We investigated the effects of mitotane treatment during the first 7 days of captivity in house sparrows and found that it reduced resting heart rate even when it did not cause the expected dramatic decrease in GC (unpublished personal data). The adrenomedullary response can also be pharmaceutically reduced by blocking the receptors of epinephrine and norepinephrine. We used alpha- and beta-blockers (which interfere with binding of epinephrine and norepinephrine to their receptors) during the first week to block chronic captivity stress in house sparrows. We found that while the beta-blocker propranolol had no effect on heart rate, it did prevent the increase in baseline GCs that we typically see in newly-captured members of this species (Fischer and Romero, 2016).
The persistence of captivity effects after release
The physiological changes caused by captivity can persist even after animals have been released back into the wild. Chukar partridges that were held in captivity 10 days and then released to a new location than where they had originated had lasting changes to their GC regulation (decreased negative feedback for at least 30 days, Dickens et al., 2009a). Red foxes that were kept in captivity for 2 to 8 weeks were less likely to establish a stable territory upon release than foxes that were caught and immediately released (Tolhurst et al., 2016). River otters kept in captivity for 10 months had lower survival than otters not kept in captivity (Ben-David et al., 2002). The captivity effect was strong enough that crude oil ingestion (mimicking the state of oiled otters in rehabilitation) had no further effect on survival (Ben-David et al., 2002). Rehabilitated barn owls (Fajardo et al., 2000) and guillemots (Wernham et al., 1997) had much shorter life expectancies than wild birds.
However, captivity may not necessarily have lasting negative impacts. In Grevy’s zebra, fecal GC metabolites were elevated in captivity, but decreased back to the wild norm quickly after release (Franceschini et al., 2008). Similarly, released Eastern Bettongs decreased GC metabolites after release from a period of over 30 days of captivity (Batson et al., 2017). Hermann’s tortoises kept in captivity for 2–8 years following an injury showed no difference in movement, thermoregulation or body condition compared to free-living animals after release to the wild (Lepeigneul et al., 2014). Captivity up to 3 months did not affect survival in Stellar’s sea lions (Shuert et al., 2015). Captivity may even have positive effects in some cases. For example, hedgehogs were more likely to survive a translocation event if they were held in captivity for greater than 1 month compared to those held <6 days (Molony et al., 2006).
Whether an animal will be permanently negatively impacted by captivity or not may depend on the captivity conditions, species, time of year, method of release or individual effects. Wild rabbits held for 2, 4, 6 or 8 weeks in quarantine before release did not differ in survival probability (Calvete et al., 2005). In another study in that species, GCs did not change over the course of a quarantine period, but animals with higher plasma and fecal GCs were more likely to survive, even though they had worse body condition (Cabezas et al., 2007). Saddlebacks were more likely to survive post-release when they had a robust GC response to a standardized acute stressor (Adams et al., 2010). Therefore, captivity may have more profound effects on survival if it negatively and permanently changes GC regulation.
Conclusions
Captivity can cause weight loss, persistent changes in baseline and integrated GCs, changes in the immune system and reproductive suppression. These effects can last for months or years in some species, indicating that some species may never truly adjust to captivity conditions. The welfare implications of chronic captivity stress are obvious, and zoos and other institutions that hold animals in captivity long-term generally have strategies in place to minimize captivity stress. Breeding facilities (for conservation, research and agriculture/fisheries) are particularly invested in reducing chronic captivity stress, given its profound impact on the reproductive system. Figure 3 indicates that many species may continue to have elevated GCs months or years after capture, while Figs 2 and 4 suggest that most animals will recover from the weight loss and elevated N or H:L ratios caused by the initial transfer to captivity. Given that weight loss and changes to N or H:L ratio are affected by GCs, it is possible that with continuing high GC concentrations, sensitivity to these hormones decreases in captive animals. The reproductive system tends to be negatively impacted by captivity, presumably because of elevated GC hormones. The negative effects of captivity are species-specific, some species adjust to captivity while others do not (see also Mason, 2010).
A captive animal may be physiologically quite different than a wild animal (Calisi and Bentley, 2009). Therefore, the confounding effects of captivity must be considered in physiological studies using captive wild animals, even when stress is not the focus of research. Animals that are held in captivity for research might respond quite differently to a range of experimental treatments than a wild, free-living individual would. For example, environmental contaminants had different effects on wild and captive sea otters (Levin et al., 2007), and experimentally induced chronic stress caused a change in fecal GCs in free-living but not captive European starlings (Cyr and Romero, 2008).
The existing literature indicates that the effects of captivity on physiology are inconsistent. Some of the differences between animals that adjust and do not adjust to captivity might be explained by life-history features of the different species (see Mason, 2010). For example, captive predators that have large ranges in nature tend to show more behavioral anomalies and more infant mortality than those that naturally have smaller ranges (Clubb and Mason, 2003). However, it may be possible to improve the physiological outcome for newly-captured animals by adjusting the season of capture, improving and enriching housing, allowing for an appropriate adjustment period, and possibly by the careful use of pharmaceuticals. Captivity stress will continue to be a factor in captive animal research, and the conditions, timing and duration of captivity must be considered as experiments are designed and interpreted.
Unfortunately, the results of this literature review do not suggest useful overall and/or generalized guidelines to wildlife managers. The overall picture is that wild animals acclimate to captivity in a highly species-specific manner. However, the most important conclusion from this review is that collecting multiple measures of physiology, rather than restricting studies to a single measure (e.g. GC concentrations), will provide a better picture of how well an individual or species is, or is not, coping with introduction to captivity.
Funding
This work was supported by the U.S. National Science Foundation [grant number IOS-1655269 to L.M.R.].
References
- Adams NJ, Farnworth MJ, Rickett J, Parker KA, Cockrem JF (2011) Behavioural and corticosterone responses to capture and confinement of wild blackbirds (Turdus merula). Appl Anim Behav Sci 134: 246–255. [Google Scholar]
- Adams NJ, Parker KA, Cockrem JF, Brunton DH, Candy EJ (2010) Corticosterone responses and post-release survival in translocated North Island saddlebacks (Philesturnus rufusater) in New Zealand. Emu 110: 296–301. [Google Scholar]
- Aldridge RD, Arackal AA (2005) Reproductive biology and stress of captivity in male brown treesnakes (Boiga irregularis) on Guam. Aust J Zool 53: 249–256. [Google Scholar]
- Baker ML, Gemmell E, Gemmell RT (1998) Physiological changes in brushtail possums, Trichosurus vulpecula, transferred from the wild to captivity. J Exp Zool 280: 203–212. [DOI] [PubMed] [Google Scholar]
- Barry M, Cockrem JF, Brunton DH (2010) Seasonal variation in plasma corticosterone concentrations in wild and captive adult Duvaucel's geckos (Hoplodactylus duvaucelii) in New Zealand. Aust J Zool 58: 234–242. [Google Scholar]
- Batson WG, Gordon IJ, Fletcher DB, Portas TJ, Manning AD (2017) The effect of pre-release captivity on the stress physiologyof a reintroduced population of wild eastern bettongs. J Zool 303: 311–319. [Google Scholar]
- Begg D, Kemp R, Griffin F (2004) Normal levels of immunocompetence in possums (Trichosurus vulpecula) exposed to different laboratory housing conditions post capture. Immunol Cell Biol 82: 253–256. [DOI] [PubMed] [Google Scholar]
- Ben-David M, Blundell GM, Blake JE (2002) Post-release survival of river otters: effects of exposure to crude oil and captivity. J Wildlife Manage 66: 1208–1223. [Google Scholar]
- Berner NJ, Heil LA, Romero LM (2013) Seasonal variation in corticosterone in free-living and captive eastern red-spotted newts Notophthalmus viridescens viridescens. J Herpetol 47: 466–470. [Google Scholar]
- Bisson IA, Butler LK, Hayden TJ, Romero LM, Wikelski MC (2009) No energetic cost of anthropogenic disturbance in a songbird. Proc R Soc B Biol Sci 276: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolasina SN. (2011) Stress response of juvenile flounder (Paralichthys orbignyanus, Valenciennes 1839), to acute and chronic stressors. Aquaculture 313: 140–143. [Google Scholar]
- Bonacic C, Macdonald DW (2003) The physiological impact of wool-harvesting procedures in vicunas (Vicugna vicugna). Anim Welf 12: 387–402. [Google Scholar]
- Bosson CO, Palme R, Boonstra R (2009) Assessment of the stress response in Columbian ground squirrels: laboratory and field validation of an enzyme immunoassay for fecal cortisol metabolites. Physiol Biochem Zool 82: 291–301. [DOI] [PubMed] [Google Scholar]
- Bourke RE, Brock J, Nakamura RM (1987) A study of delayed capture mortality syndrome in skipjack tuna, Katsuwonus pelamis (1). J Fish Dis 10: 275–287. [Google Scholar]
- Breuner CW, Jennings DH, Moore MC, Orchinik M (2000) Pharmacological adrenalectomy with mitotane. Gen Comp Endocrinol 120: 27–34. [DOI] [PubMed] [Google Scholar]
- Buehler DM, Piersma T, Tieleman BI (2008) Captive and free-living red knots Calidris canutus exhibit differences in non-induced immunity that suggest different immune strategies in different environments. J Avian Biol 39: 560–566. [Google Scholar]
- Cabezas S, Blas J, Marchant TA, Moreno S (2007) Physiological stress levels predict survival probabilities in wild rabbits. Horm Behav 51: 313–320. [DOI] [PubMed] [Google Scholar]
- Calisi RM, Bentley GE (2009) Lab and field experiments: are they the same animal? Horm Behav 56: 1–10. [DOI] [PubMed] [Google Scholar]
- Calvete C, Angulo E, Estrada R, Moreno S, Villafuerte R (2005) Quarantine length and survival of translocated European wild rabbits. J Wildlife Manage 69: 1063–1072. [Google Scholar]
- Clearwater SJPN. (1997) The response to capture and confinement stress of plasma cortisol, plasma sex steroids and vitellogenic oocytes in the marine teleost, red gurnard. J Fish Biol 50: 429–441. [Google Scholar]
- Clubb R, Mason G (2003) Captivity effects on wide-ranging carnivores. Nature 425: 473–474. [DOI] [PubMed] [Google Scholar]
- Coburn S, Salman M, Rhyan J, Keefe T, McCollum M (2010) Comparison of endocrine response to stress between captive-raised and wild-caught bighorn sheep. J Wildlife Manage 74: 532–538. [Google Scholar]
- Cyr NE, Romero LM (2008) Fecal glucocorticoid metabolites of experimentally stressed captive and free-living starlings: implications for conservation research. Gen Comp Endocrinol 158: 20–28. [DOI] [PubMed] [Google Scholar]
- Czekala NM, Hodges JK, Gause GE (1980) Lasley BL, Annual circulating testosterone levels in captive and free-ranging male armadillos (Dasypus novemcinctus). J Reprod Fertil 59: 199–204. [DOI] [PubMed] [Google Scholar]
- Dale DC, Fauci AS, Guerry D, Wolff SM (1975) Comparison of agents producing a neutrophilic leukocytosis in man. Hydrocortisone, prednisone, endotoxin, and etiocholanolone. J Clin Investig 56: 808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson GW, Thorarensen HT, Lokman M, Davie PS (1997) Stress of capture and captivity in kahawai Arripis trutta (Bloch and Schneider) (Perciformes: Arripidae). Comp Biochem Physiol A 118: 1405–1410. [Google Scholar]
- Davies S, Rodriguez NS, Sweazea KL, Deviche P (2013) The effect of acute stress and long-term corticosteroid administration on plasma metabolites in an urban and desert songbird. Physiol Biochem Zool 86: 47–60. [DOI] [PubMed] [Google Scholar]
- Davis AK, Maerz JC (2008) Comparison of hematological stress indicators in recently captured and captive paedomorphic mole salamanders, Ambystoma talpoideum. Copeia 2008:613–617. [Google Scholar]
- Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22: 760–772. [Google Scholar]
- Day TD, O'Connor CE (2000) Behavioural adaptation of brushtail possums (Trichosurus vulpecula) to captivity. Anim Welf 9: 413–420. [Google Scholar]
- de Assis VR, Titon SCM, Barsotti AMG, Titon B, Gomes FR (2015) Effects of acute restraint stress, prolonged captivity stress and transdermal corticosterone application on Immunocompetence and plasma levels of corticosterone on the cururu toad (Rhinella icterica). Plos One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS. (2002) Stress-induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun 16: 785–798. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS (1997) Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun 11: 286–306. [DOI] [PubMed] [Google Scholar]
- Dickens MJ, Bentley GE (2014) Stress, captivity, and reproduction in a wild bird species. Horm Behav 66: 685–693. [DOI] [PubMed] [Google Scholar]
- Dickens MJ, Delehanty DJ, Romero LM (2009a) Stress and translocation: alterations in the stress physiology of translocated birds. Proc R Soc Biol Sci Ser B 276: 2051–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, Earle K, Romero LM (2009b) Initial transference of wild birds to captivity alters stress physiology. Gen Comp Endocrinol 160: 76–83. [DOI] [PubMed] [Google Scholar]
- Dickens MJ, Romero LM (2009) Wild European starlings (Sturnus vulgaris) adjust to captivity with sustained sympathetic nervous system drive and a reduced fight-or-flight response. Physiol Biochem Zool 82: 603–610. [DOI] [PubMed] [Google Scholar]
- Dickens MJ, Romero LM (2013) A consensus endocrine profile for chronically stressed wild animals does not exist. Gen Comp Endocrinol 191: 177–189. [DOI] [PubMed] [Google Scholar]
- Dufty AM Jr, Belthoff JR (1997) Corticosterone and the stress response in young western screech-owls: effects of captivity, gender, and activity period. Physiol Zool 70: 143–149. [DOI] [PubMed] [Google Scholar]
- Dufty AM Jr, Wingfield JC (1986) Temporal patterns of circulating luteinizing and steroid hormones in a brood parasite the brown-headed cowbird, Molothrus-Ater I. Males. J Zool Ser A 208: 191–204. [Google Scholar]
- Evans JE, Smith EL, Bennett ATD, Cuthill IC, Buchanan KL (2012) Short-term physiological and behavioural effects of high- versus low-frequency fluorescent light on captive birds. Anim Behav 83: 25–33. [Google Scholar]
- Ewenson EL, Zann RA, Flannery GR (2001) Body condition and immune response in wild zebra finches: effects of capture, confinement and captive-rearing. Naturwissenschaften 88: 391–394. [DOI] [PubMed] [Google Scholar]
- Fajardo I, Babiloni G, Miranda Y (2000) Rehabilitated and wild barn owls (Tyto alba): dispersal, life expectancy and mortality in Spain. Biol Conserv 94: 287–295. [Google Scholar]
- Fanson KV, Wielebnowski NC, Shenk TM, Lucas JR (2012) Comparative patterns of adrenal activity in captive and wild Canada lynx (Lynx canadensis). J Comp Physiol B 182: 157–165. [DOI] [PubMed] [Google Scholar]
- Fernandez-Moran J, Saavedra D, De La JLR, Manteca-Vilanov X (2004) Stress in wild-caught Eurasian otters (Lutra lutra): effects of a long-acting neuroleptic and time in captivity. Anim Welf 13: 143–149. [Google Scholar]
- Fischer CP, Rao R, Romero LM (2017) Exogenous and endogenous corticosterone in feathers. J Avian Biol 48: 1301–1309. [Google Scholar]
- Fischer CP, Romero LM (2016) The use of α- or β-blockers to ameliorate the chronic stress of captivity in the house sparrow (Passer domesticus). Conserv Physiol 4(1):cow049; doi:10.1093/conphys/cow049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer CP, Wright-Lichter J, Romero LM (2018) Chronic stress and the introduction to captivity: how wild house sparrows (Passer domesticus) adjust to laboratory conditions. Gen Comp Endocrinol 259: 85–92. [DOI] [PubMed] [Google Scholar]
- Flugge G. (1996) Alterations in the central nervous alpha(2)-adrenoceptor system under chronic psychosocial stress. Neuroscience 75: 187–196. [DOI] [PubMed] [Google Scholar]
- Fokidis HB, Hurley L, Rogowski C, Sweazea K, Deviche P (2011) Effects of captivity and body condition on plasma corticosterone, locomotor behavior, and plasma metabolites in curve-billed thrashers. Physiol Biochem Zool 84: 595–606. [DOI] [PubMed] [Google Scholar]
- Franceschini MD, Rubenstein DI, Low B, Romero LM (2008) Fecal glucocorticoid metabolites as an indicator of stress during translocation and acclimation in an endangered large mammal, the Grevy’s zebra. Anim Conserv 11: 263–269. [Google Scholar]
- Franzman AWT, Thorne ET (1970) Physiologic values in wild bighorn sheep (Ovis canadensis canadensis) at capture, after handling, and after captivity. J Am Vet Med Assoc 157: 647–650. [PubMed] [Google Scholar]
- Gardiner KJ, Hall AJ (1997) Diel and annual variation in plasma cortisol concentrations among wild and captive harbor seals (Phoca vitulina). Can J Zool 75: 1773–1780. [Google Scholar]
- Gobbetti A, Zerani M (1996) Possible mechanism for the first response to short captivity stress in the water frog, Rana esculenta. J Endocrinol 148: 233–239. [DOI] [PubMed] [Google Scholar]
- Gormally BMG, Fuller R, McVey M, Romero LM (2019) DNA damage as an indicator of chronic stress: correlations with corticosterone and uric acid. Comp Biochem Physiol A Mol Integr Physiol 227: 116–122. [DOI] [PubMed] [Google Scholar]
- Greenwood VJ, Smith EL, Goldsmith AR, Cuthill IC, Crisp LH, Walter-Swan MB, Bennett ATD (2004) Does the flicker frequency of fluorescent lighting affect the welfare of captive European starlings? Appl Anim Behav Sci 86: 145–159. [Google Scholar]
- Gross WB, Siegel HS (1983) Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis 27: 972–979. [PubMed] [Google Scholar]
- Grutter AS, Pankhurst NW (2000) The effects of capture, handling, confinement and ectoparasite load on plasma levels of cortisol, glucose and lactate in the coral reef fish Hemigymnus melapterus. J Fish Biol 57: 391–401. [Google Scholar]
- Hamalainen A, Heistermann M, Fenosoa ZS, Kraus C (2014) Evaluating capture stress in wild gray mouse lemurs via repeated fecal sampling: method validation and the influence of prior experience and handling protocols on stress responses. Gen Comp Endocrinol 195: 68–79. [DOI] [PubMed] [Google Scholar]
- Hare JF, Ryan CP, Enright C, Gardiner LE, Skyner LJ, Berkvens CN, Anderson WG (2014) Validation of a radioimmunoassay-based fecal corticosteroid assay for Richardson's ground squirrels Urocitellus richardsonii and behavioural correlates of stress. Curr Zool 60: 591–601. [Google Scholar]
- Herrera M, Rodiles A, Sanchez B, Lopez JM, de La E (2016) Physiological stress responses to captivity in early developmental stages of the wedge sole Dicologoglossa cuneata (Moreau). Aquacult Res 47: 732–740. [Google Scholar]
- Hoffman AM, Leighton FA (1985) Hemograms and microscopic lesions of herring-gulls during captivity. J Am Vet Med Assoc 187: 1125–1128. [PubMed] [Google Scholar]
- Holz P, Barnett JEF (1996) Long-acting tranquilizers: their use as a management tool in the confinement of free-ranging red-necked wallabies (Macropus rufogriseus). J Zoo Wildl Med 27: 54–60. [Google Scholar]
- Imanaga Y, Nyuji M, Amano M, Takahashi A, Kitano H, Yamaguchi A, Matsuyama M (2014) Characterization of gonadotropin-releasing hormone and gonadotropin in jack mackerel (Trachurus japonicus): comparative gene expression analysis with respect to reproductive dysfunction in captive and wild fish. Aquaculture 428: 226–235. [Google Scholar]
- Jepsen EM, Ganswindt A, Ngcamphalala CA, Bourne AR, Ridley AR, McKechnie AE (2019) Non-invasive monitoring of physiological stress in an afrotropical arid-zone passerine bird, the southern pied babbler. Gen Comp Endocrinol 276: 60–68. [DOI] [PubMed] [Google Scholar]
- Johnstone CP, Reina RD, Lill A (2012) Interpreting indices of physiological stress in free-living vertebrates. J Comp Physiol B 182: 861–879. [DOI] [PubMed] [Google Scholar]
- Jones SM, Bell K (2004) Plasma corticosterone concentrations in males of the skink Egernia whitii during acute and chronic confinement, and over a diel period. Comp Biochem Physiol 137A: 105–113. [DOI] [PubMed] [Google Scholar]
- Kagira JM, Ngotho M, Thuita JK, Maina NW, Hau J (2007) Hernatological changes in vervet monkeys (Chlorocebus aethiops) during eight months' adaptation to captivity. Am J Primatol 69: 1053–1063. [DOI] [PubMed] [Google Scholar]
- Knox CM, Hattingh J, Raath JP (1990) The effect of tranquilizers on the immediate responses to repeated capture and handling of boma-kept impala. Comp Biochem Physiol C 95: 247–251. [Google Scholar]
- Kock RA, Mihok SRO, Wambua J, Mwanzia J, Saigawa K (1999) Effects of translocation on hematologic parameters of free-ranging black rhinoceros (Diceros bicornis michaeli) in Kenya. J Zoo Wildl Med 30: 389–396. [PubMed] [Google Scholar]
- Krams I, Vrublevska J, Cirule D, Kivleniece I, Krama T, Rantala MJ, Kaasik A, Horak P, Sepp T (2013) Stress, behaviour and immunity in wild-caught wintering great tits (Parus major). Ethology 119: 397–406. [Google Scholar]
- Kuhlman JR, Martin LB (2010) Captivity affects immune redistribution to skin in a wild bird. Funct Ecol 24: 830–837. [Google Scholar]
- LaDage LD, Roth TC, Fox RA, Pravosudov VV (2009) Effects of captivity and memory-based experiences on the hippocampus in mountain chickadees. Behav Neurosci 123: 284–291. [DOI] [PubMed] [Google Scholar]
- Landsman RE. (1993) The effects of captivity on the electric organ discharge and plasma-hormone levels in Gnathonemus petersii (Mormyriformes). J Comp Physiol A 172: 619–631. [DOI] [PubMed] [Google Scholar]
- Larcombe SD, Tregaskes CA, Coffey J, Stevenson AE, Alexander LG, Arnold KE (2015) Oxidative stress, activity behaviour and body mass in captive parrots. Conserv Physiol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattin CR, Bauer CM, de R, Romero LM (2012) Hypothalamus-pituitary-adrenal axis activity and the subsequent response to chronic stress differ depending upon life history stage. Gen Comp Endocrinol 178: 494–501. [DOI] [PubMed] [Google Scholar]
- Lattin CR, Romero LM (2014) Chronic exposure to a low dose of ingested petroleum disrupts corticosterone receptor signalling in a tissue-specific manner in the house sparrow (Passer domesticus). Conserv Physiol 2: cou058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubscher LL, Hoffman LC, Pitts NI, Raath JP (2016) The effect of a slow-release formulation of zuclopenthixol acetate (Acunil (R)) on captive blue wildebeest (Connochaetes taurinus) behavior and physiological response. J Zoo Wildl Med 47: 514–522. [DOI] [PubMed] [Google Scholar]
- Lepeigneul O, Ballouard JM, Bonnet X, Beck E, Barbier M, Ekori A, Buisson E, Caron S (2014) Immediate response to translocation without acclimation from captivity to the wild in Hermann's tortoise. Eur J Wildlife Res 60: 897–907. [Google Scholar]
- Levin M, Leibrecht H, Mori C, Jessup D, De S (2007) Immunomodulatory effects of organochlorine mixtures upon in vitro exposure of peripheral blood leukocytes differ between free-ranging and captive southern sea otters (Enhydra lutris). Vet Immunol Immunopathol 119: 269–277. [DOI] [PubMed] [Google Scholar]
- Lilly AA, Mehlman PT, Higley JD (1999) Trait-like immunological and hematological measures in female rhesus across varied environmental conditions. Am J Primatol 48: 197–223. [DOI] [PubMed] [Google Scholar]
- Linklater WL, MacDonald EA, Flamand JRB, Czekala NM (2010) Declining and low fecal corticoids are associated with distress, not acclimation to stress, during the translocation of African rhinoceros. Anim Conserv 13: 104–111. [Google Scholar]
- Lombardo MP, Thorpe PA (2009) Captivity affects sperm production, testes size and beak color in house sparrows (Passer domesticus). Int Stud Sparrows 33: 5–16. [Google Scholar]
- Love AC, Lovern MB, DuRant SE (2017) Captivity influences immune responses, stress endocrinology, and organ size in house sparrows (Passer domesticus). Gen Comp Endocrinol 252: 18–26. [DOI] [PubMed] [Google Scholar]
- Maddocks SA, Goldsmith AR, Cuthill IC (2002) Behavioural and physiological effects of absence of ultraviolet wavelengths on European starlings Sturnus vulgaris. J Avian Biol 33: 103–106. [Google Scholar]
- Mahmoud IY, Guillette LJ, McAsey ME, Cady C (1989) Stress-induced changes in serum testosterone, estradiol-17β and progesterone in the turtle, Chelydra serpentina. Comp Biochem Physiol A 93: 423–427. [Google Scholar]
- Marcalo A, Pousao-Ferreira P, Mateus L, Correia JHD, Stratoudakis Y (2008) Sardine early survival, physical condition and stress after introduction to captivity. J Fish Biol 72: 103–120. [Google Scholar]
- Marra PP, Lampe KT, Tedford BL (1995) Plasma corticosterone levels in two species of Zonotrichia sparrows under captive and free-living conditions. Wilson Bull 107: 296–305. [Google Scholar]
- Martin LB. (2009) Stress and immunity in wild vertebrates: timing is everything. Gen Comp Endocrinol 163: 70–76. [DOI] [PubMed] [Google Scholar]
- Martin LB, Kidd L, Liebl AL, Coon CAC (2011) Captivity induces hyper-inflammation in the house sparrow (Passer domesticus). J Exp Biol 214: 2579–2585. [DOI] [PubMed] [Google Scholar]
- Mason G, Clubb R, Latham N, Vickery S (2007) Why and how should we use environmental enrichment to tackle stereotypic behaviour? Appl Anim Behav Sci 102: 163–188. [Google Scholar]
- Mason GJ. (2010) Species differences in responses to captivity: stress, welfare and the comparative method. Trends Ecol Evol 25: 713–721. [DOI] [PubMed] [Google Scholar]
- Mathies T, Felix TA, Lance VA (2001) Effects of trapping and subsequent short-term confinement stress on plasma corticosterone in the brown treesnake (Boiga irregularis) on Guam. Gen Comp Endocrinol 124: 106–114. [DOI] [PubMed] [Google Scholar]
- McCormick GL, Shea K, Langkilde T (2015) How do duration, frequency, and intensity of exogenous CORT elevation affect immune outcomes of stress? Gen Comp Endocrinol 222: 81–87. [DOI] [PubMed] [Google Scholar]
- McLeod BJ, Thompson EG, Crawford JL, Shackell GH (1997) Successful group housing of wild-caught brushtail possums (Trichosurus vulpecula). Anim Welf 6: 67–76. [Google Scholar]
- McPhee ME, Carlstead K (2010) The importance of maintaining natural behaviors in captive mammals In Kleiman DG, Thompson KV, Baer CK, eds, Wild Mammals in Captivity: Principles and Techniques for Zoo Management. University of Chicago Press, Chicago, pp. 303–313. [Google Scholar]
- Mellish JE, Calkins DG, Christen DR, Horning M, Rea LD, Atkinson SK (2006) Temporary captivity as a research tool: comprehensive study of wild pinnipeds under controlled conditions. Aquat Mamm 32: 58–65. [Google Scholar]
- Miller NW, Tripp MR (1982) The effect of captivity on the immune response of the killifish, Fundulus heteroclitus L. J Fish Biol 20: 301–308. [Google Scholar]
- Millet S, Bennett J, Lee KA, Hau M, Klasing KC (2007) Quantifying and comparing constitutive immunity across avian species. Dev Comp Immunol 31: 188–201. [DOI] [PubMed] [Google Scholar]
- Molony SE, Dowding CV, Baker PJ, Cuthill IC, Harris S (2006) The effect of translocation and temporary captivity on wildlife rehabilitation success: an experimental study using European hedgehogs (Erinaceus europaeus). Biol Conserv 130: 530–537. [Google Scholar]
- Moore MC, Thompson CW, Marler CA (1991) Reciprocal changes in corticosterone and testosterone levels following acute and chronic handling stress in the tree lizard, Urosaurus ornatus. Gen Comp Endocrinol 81: 217–226. [DOI] [PubMed] [Google Scholar]
- Moorhouse TP, Gelling M, McLaren GW, Mian R, Macdonald DW (2007) Physiological consequences of captive conditions in water voles (Arvicola terrestris). J Zool 271: 19–26. [Google Scholar]
- Morales MH, Sanchez EJ (1996) Changes in vitellogenin expression during captivity-induced stress in a tropical anole. Gen Comp Endocrinol 103: 209–219. [DOI] [PubMed] [Google Scholar]
- Morgan KN, Tromborg CT (2007) Sources of stress in captivity. Appl Anim Behav Sci 102: 262–302. [Google Scholar]
- Narayan E, Hero JM (2011) Urinary corticosterone responses and haematological stress indicators in the endangered Fijian ground frog (Platymantis vitiana) during transportation and captivity. Aust J Zool 59: 79–85. [Google Scholar]
- Narayan EJ, Cockrem JF, Hero JM (2011) Urinary corticosterone metabolite responses to capture and captivity in the cane toad (Rhinella marina). Gen Comp Endocrinol 173: 371–377. [DOI] [PubMed] [Google Scholar]
- Narayan EJ, Cockrem JF, Hero JM (2012) Effects of temperature on urinary corticosterone metabolite responses to short-term capture and handling stress in the cane toad (Rhinella marina). Gen Comp Endocrinol 178: 301–305. [DOI] [PubMed] [Google Scholar]
- Nester Bolasina S. (2011) Stress response of juvenile flounder (Paralichthys orbignyanus, Valenciennes 1839), to acute and chronic stressors. Aquaculture 313: 140–143. [Google Scholar]
- Nikoo M, Falahatkar B, Alekhorshid M, Haghi BN, Asadollahpour A, Dangsareki MZ, Langroudi HF (2010) Physiological stress responses in kutum Rutilus frisii kutum subjected to captivity. Int Aquat Res 2: 55–60. [Google Scholar]
- Olsen DE, Seabloom RW (1973) Adrenocortical response to captivity in Microtus pennsylvanicus. J Mammal 54: 779–781. [PubMed] [Google Scholar]
- Orlov MM, Mukhlya AM, Postoyanova NI, Kazanova NV (1991) Biochemical adaptation of cetaceans in captivity. J Evol Biochem Physiol 27: 331–336. [Google Scholar]
- Pankhurst NW, Sharples DF (1992) Effects of capture and confinement on plasma cortisol concentrations in the snapper, Pagrus auratus. Aust J Mar Freshw Res 43: 345–356. [Google Scholar]
- Patton KT, Croonquist MJ, Crawford WC (1985) Management-related stress in the red-shouldered hawk. Zoo Biol 4: 235–243. [Google Scholar]
- Peinado VI, Fernandezarias A, Zabala JL, Palomeque J (1995) Effect of captivity on the blood composition of Spanish ibex (Capra pyrenaica hispanica). Vet Rec 137: 588–591. [PubMed] [Google Scholar]
- Perret M. (1982) Influence of captivity and social-groups on lesser mouse lemur physiology.1. Stress-effects in Microcebus murinus. Folia Primatol 39: 63–114. [DOI] [PubMed] [Google Scholar]
- Peters G, Delventhal H, Klinger H (1980) Physiological and morphological effects of social stress in the eel, (Anguilla anguilla L). Arch Fischereiwiss 302: 157–180. [Google Scholar]
- Piersma T, Koolhaas A, Dekinga A, Gwinner E (2000) Red blood cell and white blood cell counts in sandpipers (Philomachus pugnax, Calidris canutus): effects of captivity, season, nutritional status, and frequent bleedings. Can J Zool 78: 1349–1355. [Google Scholar]
- Piersma T, Ramenofsky M (1998) Long-term decreases of corticosterone in captive migrant shorebirds that maintain seasonal mass and moult cycles. J Avian Biol 29: 97–104. [Google Scholar]
- Plante S, Audet C, Lambert Y, de la J (2003) Comparison of stress responses in wild and captive winter flounder (Pseudopleuronectes americanus Walbaum) broodstock. Aquacult Res 34: 803–812. [Google Scholar]
- Quispe R, Villavicencio CP, Addis E, Wingfield JC, Vasquez RA (2014) Seasonal variations of basal cortisol and high stress response to captivity in Octodon degus, a mammalian model species. Gen Comp Endocrinol 197: 65–72. [DOI] [PubMed] [Google Scholar]
- Rangel-Negrin A, Alfaro JL, Valdez RA, Romano MC, Serio-Silva JC (2009) Stress in Yucatan spider monkeys: effects of environmental conditions on fecal cortisol levels in wild and captive populations. Anim Conserv 12: 496–502. [Google Scholar]
- Rich EL, Romero LM (2005) Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am J Physiol Regul Integr Comp Physiol 288: R1628–R1636. [DOI] [PubMed] [Google Scholar]
- Rideout BA, Gause GE, Benirschke K, Lasley BL (1985) Stress-induced adrenal changes and their relation to reproductive failure in captive 9-banded armadillos (Dasypus novemcinctus). Zoo Biol 4: 129–137. [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav 55: 375–389. [DOI] [PubMed] [Google Scholar]
- Romero LM, Reed JM (2005) Collecting baseline corticosterone samples in the field: is under three minutes good enough? Comp Biochem Physiol Part A Mol Integr Physiol 140: 73–79. [DOI] [PubMed] [Google Scholar]
- Romero LM, Wingfield JC (1999) Alterations in hypothalamic-pituitary-adrenal function associated with captivity in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii). Comp Biochem Physiol 122B: 13–20. [DOI] [PubMed] [Google Scholar]
- Romero LM, Wingfield JC (2016) Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope. Oxford University Press, New York [Google Scholar]
- Ruiz G, Rosenmann M, Novoa FF, Sabat P (2002) Hematological parameters and stress index in rufous-collared sparrows dwelling in urban environments. Condor 104: 162–166. [Google Scholar]
- Sanchez-Sarmiento AM, Zwarg T, Fernandes-Santos RC, Guimaraes-Luiz T, Genoy-Puerto A, Matushima ER (2015) Hematological parameters and the variations resulting from stress of Alouatta caraya during a wildlife rescue program in Brazil. Am J Primatol 77: 246–253. [DOI] [PubMed] [Google Scholar]
- Sanderson JT. (2006) The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci 94: 3–21. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress-responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Sepp T, Sild E, Horak P (2010) Hematological condition indexes in greenfinches: effects of captivity and diurnal variation. Physiol Biochem Zool 83: 276–282. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166: 869–887. [DOI] [PubMed] [Google Scholar]
- Shuert C, Horning M, Mellish JA (2015) The effect of novel research activities on long-term survival of temporarily captive Steller Sea lions (Eumetopias jubatus). Plos One 10:e0141948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert U, Pozniak B, Hansen KA, Nordstrom G, Teilmann J, van N, Vossen A, Dietz R (2011) Investigations of thyroid and stress hormones in free-ranging and Captive Harbor porpoises (Phocoena phocoena): a pilot study. Aquat Mamm 37: 443–453. [Google Scholar]
- Smith MFL. (1992) Capture and transportation of elasmobranchs, with emphasis on the gray nurse shark (Carcharias taurus). Aust J Mar Freshwater Res 43: 325–343. [Google Scholar]
- Sparkman AM, Bronikowski AM, Williams S, Parsai S, Manhart W, Palacios MG (2014) Physiological indices of stress in wild and captive garter snakes: correlations, repeatability, and ecological variation. Comp Biochem Physiol A Mol Integr Physiol 174: 11–17. [DOI] [PubMed] [Google Scholar]
- St Aubin DJ, Geraci JR (1988) Capture and handling stress suppresses circulating levels of thyroxine T4 and triiodothyronine T3 in beluga whales Delphinapterus leucas. Physiol Zool 61: 170–175. [Google Scholar]
- St Aubin DJ, Geraci JR (1989) Adaptive changes in hematologic and plasma chemical constituents in captive beluga whales, Delphinapterus leucas. Can J Fish Aquat Sci 46: 796–803. [Google Scholar]
- Stead-Richardson E, Bradshaw D, Friend T, Fletcher T (2010) Monitoring reproduction in the critically endangered marsupial, Gilbert's potoroo (Potorous gilbertii): preliminary analysis of faecal oestradiol-17 beta, cortisol and progestagens. Gen Comp Endocrinol 165: 155–162. [DOI] [PubMed] [Google Scholar]
- Steyn DG. (1975) The effects of captivity stress on the blood chemical values of the chacma baboon Papio ursinus. Lab Anim 9: 111–120. [DOI] [PubMed] [Google Scholar]
- Suleman MA, Wango E, Sapolsky RM, Odongo H, Hau J (2004) Physiologic manifestations of stress from capture and restraint of free-ranging male african green monkeys (Cercopithecus aethiops). J Zoo Wildl Med 35: 20–24. [DOI] [PubMed] [Google Scholar]
- Sykes KL, Klukowski M (2009) Effects of acute temperature change, confinement and housing on plasma Corticosterone in water snakes, Nerodia sipedon (Colubridae: Natricinae). J Exp Zool A 311A: 172–181. [DOI] [PubMed] [Google Scholar]
- Tarr BA, Rabinowitz JS, Imtiaz MA, DeVoogd TJ (2009) Captivity reduces hippocampal volume but not survival of new cells in a food-storing bird. Dev Neurobiol 69: 972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titon SCM, Assis VR, Titon B, Cassettari BD, Fernandes P, Gomes FR (2017) Captivity effects on immune response and steroid plasma levels of a Brazilian toad (Rhinella schneideri). J Exp Zool A 327: 127–138. [DOI] [PubMed] [Google Scholar]
- Titon SCM, Titon Junior B, Assis VR, Kinker GS, Fernandes PACM, Gomes FR (2018) Interplay among steroids, body condition and immunity in response to long-term captivity in toads. Sci Rep 8: 17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst B, Grogan A, Hughes H, Scott D (2016) Effects of temporary captivity on ranging behaviour in urban red foxes (Vulpes vulpes). Appl Anim Behav Sci 181: 182–190. [Google Scholar]
- Trumble SJ, O'Neil D, Cornick LA, Gulland FMD, Castellini MA, Atkinson S (2013) Endocrine changes in harbor seal (Phoca vitulina) pups undergoing rehabilitation. Zoo Biol 32: 134–141. [DOI] [PubMed] [Google Scholar]
- Tyrrell C, Cree A (1994) Plasma corticosterone concentrations in wild and captive juvenile tuatara (Sphenodon punctatus). New Zeal J Zool 21: 407–416. [Google Scholar]
- Van der Weyd LK, Martin GB, Paris MCJ (2016) Monitoring stress in captive and free-ranging African wild dogs (Lycaon pictus) using faecal glucocorticoid metabolites. Gen Comp Endocrinol 226: 50–55. [DOI] [PubMed] [Google Scholar]
- Vera F, Antenucci CD, Zenuto RR (2011) Cortisol and corticosterone exhibit different seasonal variation and responses to acute stress and captivity in tuco-tucos (Ctenomys talarum). Gen Comp Endocrinol 170: 550–557. [DOI] [PubMed] [Google Scholar]
- Vitousek MN, Taff CC, Ryan TA, Zimmer C (2019) Stress resilience and the dynamic regulation of glucocorticoids. Integr Comp Biol . [DOI] [PubMed] [Google Scholar]
- Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL (2000) A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120: 260–275. [DOI] [PubMed] [Google Scholar]
- Weiss SL, Jennings DH, Moore MC (2002) Effect of captivity in semi-natural enclosures on the reproductive endocrinology of female lizards. Gen Comp Endocrinol 128: 238–246. [DOI] [PubMed] [Google Scholar]
- Wernham C, Peach WJ, Browne SJ (1997) Survival rates of rehabilitated guillemots. British Trust for Ornithology. Thetford, Norfolk, UK.
- West DB, York B (1998) Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr 67: 505S–512S. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD (1998) Ecological bases of hormone-behavior interactions: the ‘emergency life history stage’. Integr Comp Biol 38: 191–206. [Google Scholar]
- Wingfield JC, Smith JP, Farner DS (1982) Endocrine responses of white-crowned sparrows to environmental stress. Condor 84: 399–409. [Google Scholar]
- Zerani M, Amabili F, Mosconi G, Gobbetti A (1991) Effects of captivity stress on plasma steroid levels in the green frog, Rana esculenta, during the annual reproductive cycle. Comp Biochem Physiol 98A: 491–496. [Google Scholar]


