Abstract
Psychoactive pollutants, such as antidepressants, are increasingly detected in the environment. Mounting evidence suggests that such pollutants can disrupt the behaviour of non-target species. Despite this, few studies have considered how the response of exposed organisms might be mediated by social context. To redress this, we investigated the impacts of two environmentally realistic concentrations of a pervasive antidepressant pollutant, fluoxetine, on foraging behaviour in fish (Gambusia holbrooki), tested individually or in a group. Fluoxetine did not alter behaviour of solitary fish. However, in a group setting, fluoxetine exposure disrupted the frequency of aggressive interactions and food consumption, with observed effects being contingent on both the mean weight of group members and the level of within-group variation in weight. Our results suggest that behavioural tests in social isolation may not accurately predict the environmental risk of chemical pollutants for group-living species and highlight the potential for social context to mediate the effects of psychoactive pollutants in exposed wildlife.
Keywords: animal behaviour, feeding, fluoxetine, mosquitofish, pharmaceutical pollution, shoal
1. Introduction
Pharmaceutical pollution is widely recognized as an emerging environmental problem [1–3], with over 600 different pharmaceuticals having now been detected in ecosystems globally [2]. Disturbingly, among these pharmaceutical pollutants are large quantities of psychoactive compounds [4]. Given that these drugs are specifically designed to alter mood and behaviour in humans, they also have the potential to do so in exposed wildlife [4,5]. The antidepressant fluoxetine (Prozac®) is one such compound. Fluoxetine is frequently detected in ecosystems globally, at concentrations ranging between less than 0.1–351 ng l−1 [6]. The primary target molecule of fluoxetine (the serotonin transport molecule) is conserved across all vertebrate taxa [7], and, thus, fluoxetine has the potential to affect a diverse array of ecologically important behaviours in wildlife [8,9]. Indeed, there is growing evidence that antidepressants, such as fluoxetine, can disrupt a range of behaviours in non-target species at environmentally relevant concentrations, such as activity [10–13], anxiety [14–16], predator avoidance and escape [17–20], and foraging [21,22]. However, to date, few studies have considered how impacts of psychoactive pollutants might be affected by social context [23,24], and fewer still have directly asked whether impacts seen in social isolation are reflective of those in a social context [25]. This is surprising given that social interactions can play an important role in mediating individual behaviour [26,27]. As a result, for group-living species, behavioural tests performed in social isolation may not accurately predict the environmental risk posed by chemical pollution. Hence, studies of behaviour need to be sensitive to social contexts if we are to fully understand the ecological impacts of chemical exposure on social species.
Here, we tested the effects of fluoxetine exposure on foraging behaviour in wild-caught female mosquitofish (Gambusia holbrooki), both individually and when part of a small group (three fish). The nominal (i.e. desired) concentrations for the low- and high-fluoxetine treatments were 30 and 300 ng l−1, respectively. The lower fluoxetine concentration represents a level commonly detected in aquatic ecosystems, while the high concentration represents more heavily polluted ecosystems [6].
2. Material and methods
Female mosquitofish used in this study (n = 445) were collected from a wild population (37°54′28″ S, 145°08′16″ E) and transported to Monash University, where they were acclimated to laboratory conditions for one month. Water samples previously taken from this site revealed no fluoxetine contamination (analysis performed using liquid chromatography–mass spectrometry; J Fick 2016, unpublished data). Here, we used only one sex to control for any effects of sexual behaviour that may have confounded the results [28,29]. Female mosquitofish were used as they show a stronger tendency to form shoals than males [28,30]. After acclimation to laboratory conditions, fish were randomly allocated to one of three treatments for 28 days (unexposed, low-fluoxetine or high-fluoxetine). A 28-day exposure was selected as the effects of fluoxetine can be time dependent, taking two to four weeks to manifest in humans [31,32]. For all three treatments, fish were held in flow-through systems (24 h cycling), with eight tanks per treatment (24 tanks total; housing approximately 20 fish per tank) over the 28 days. Exposure was performed following previously established protocols (see electronic supplementary material, S1.1; [33–36]). During the exposure, gas chromatography coupled to tandem mass spectrometry was used for analytical verification of fluoxetine concentrations (described in [36]). During both the laboratory acclimation and exposure period, fish were maintained in aged carbon-filtered freshwater (pH: 6.9–7.9) under a 24 h light : dark cycle (7.00–18.00 light), and fed ad libitum once daily with commercial fish food (Otohime Hirame larval diet). To standardize hunger levels, fish were not fed for 24 h prior to the start of each trial.
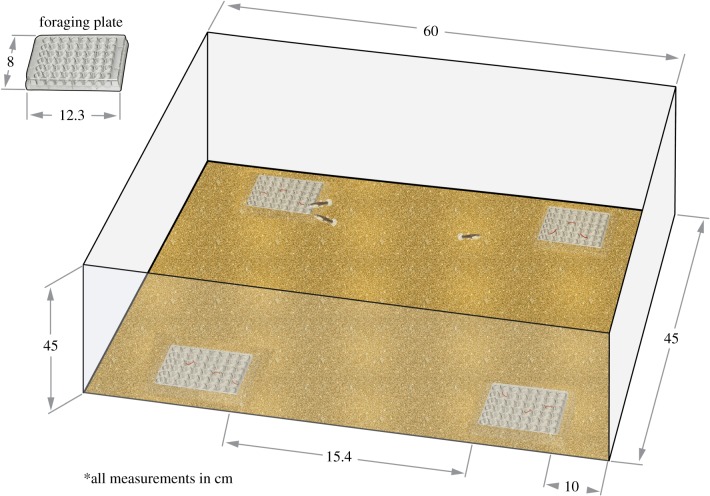
After the 28-day exposure protocol, fish performed a novel foraging trial either individually or as part of a group of three [37]. All foraging trials, regardless of treatment, were performed in aged carbon-filtered freshwater from the same source that supplied the flow-through exposure systems (aerated and heated in large reservoirs). Trial arenas (121.5 l; 60 × 45 × 45 cm; length × width × height; figure 1) were filled to a depth of 10 cm with water (i.e. 27 l), had a sand substrate and four foraging plates (12.3 × 8 × 2 cm), each of which contained 48 wells. Foraging plates were positioned in each corner of the arena, 10 cm from the edge. Before mosquitofish were introduced, chironomid larvae (i.e. prey items), were distributed equally across each of the foraging plates in shallow wells. Shallow wells were used to ensure that fish were actively engaging in food discovery and foraging behaviour [37]. Twelve and 36 chironomid larvae were placed in the arena of individual and group foraging trials, respectively. This was done to balance the ratio of prey items to fish across individual and group trials. To ensure that prey items were recognized as a food source, chironomid larvae were introduced into fish diets over the week prior to the behavioural experiments. Prior to the start of each trial, fish were acclimated inside the foraging arena within an opaque cylinder (one fish per cylinder; 7.5 cm diameter) for 5 min. After acclimation, the cylinders were remotely removed, and the individual or group was left to explore the foraging arena for 20 min. Upon completion of each trial, the foraging arenas were emptied and refilled with aged carbon-filtered freshwater. Fish behaviour was video-recorded from above and later scored blind to experimental treatment (i.e. video identification tags did not contain experimental treatment information) using open-source event-logging software (BORIS v. 7.4.7; [38]). Specifically, for each fish, the time taken to first consume a prey item, and the total number of prey items consumed, was recorded. In addition, for group trials, the total number of aggressive feeding interactions was recorded as the number of times group members attempted to steal food from one another. For individual trials, a total of 113 fish completed the foraging experiment (n: control = 38, low-fluoxetine = 38 and high-fluoxetine = 37). For group trials, a total of 103 groups of fish (i.e. groups of three) completed the foraging experiment (n: control = 34, low-fluoxetine = 34 and high-fluoxetine = 35). The required sample size per treatment group was estimated based on previous experiments investigating the effects of fluoxetine on fish behaviour (Martin et al. [16,19,34]; Fursdon et al. [35]). Following behavioural trials, all fish were measured for weight (±0.0001 g) and standard length (±0.01 mm). These measures were used to calculate a body condition index for each fish by producing a least-squares regression of fish weight against standard length, with condition index being the residual distance from this regression line.
Figure 1.
Experimental arena. (Online version in colour.)
Data were analysed using R v. 3.5.1 [39]. For a full description of statistical methods, see electronic supplementary material, S2, as well as tables S1, S3 and S5 for details of model parameters. Briefly, all models used for individual trials included exposure treatment, fish weight and their interaction term as fixed effects. Group trial models included exposure treatment, the mean weight of the three group members, the standard deviation of the group members’ weights and their interaction terms as fixed effects. Weight was selected as a covariate because it is a biologically meaningful predictor of both the effects of fluoxetine exposure and behaviour [16,34]. To account for possible tank effects, exposure tank ID (i.e. exposure tank number 1–24) was included in all models as a random effect. Fish morphometrics (i.e. weight, length and condition) were compared across treatment groups using fish from both individual and group trials. Time to event, continuous and count data were tested using Cox proportional hazard mixed effect models (COXME), linear mixed effect models (LME) and generalized linear mixed models (GLMM), respectively. Cox proportional hazard models were selected to analyse latency data because these models are specifically designed to test the effects of a treatment or set of treatments (i.e. fixed factors) on the time a specified event takes to occur (i.e. survival analysis). Furthermore, these models deal with censored data (i.e. instances where fish did not consume a prey item) and can simultaneously assess the effect of several covariates on the dependent variable.
3. Results
(a). Analytical verification of fluoxetine concentrations
The mean measured concentrations for the low- and high-fluoxetine treatments during the 28-day exposure period were 18.19 ± 4.98 ng l−1 (n = 32) and 214.69 ± 38.89 ng l−1 (n = 32). For all control samples, fluoxetine was not detected (i.e. under detection limit; less than 2 ng l−1, n = 16).
(b). Individual trials
For the time taken to first consume a prey item, there was no significant interaction between exposure treatment and fish weight, nor was there a main effect of exposure treatment or fish weight (COXME; all p > 0.05; electronic supplementary material, tables S1 and S2 and figure S1). Similarly, there was no significant interaction between exposure treatment and fish weight, nor was there a main effect of exposure treatment or fish weight on the total number of prey items consumed (nbGLM; all p > 0.05; electronic supplementary material, tables S1 and S2 and figure S2).
(c). Group trials
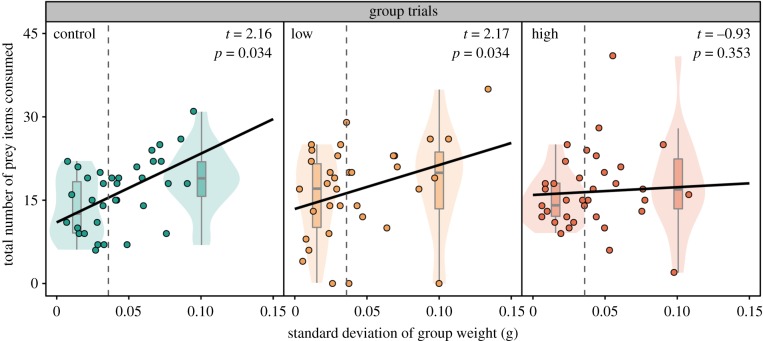
There were no significant interactions or main effects detected on the average time for group members to first consume a prey item (COXME; all p > 0.05; electronic supplementary material, table S3 and figure S3). For the total number of prey items consumed, there was a significant interaction between the standard deviation in group weight and exposure treatment (GLMM; F = 6.40, p = 0.041; figure 2). For unexposed and low-fluoxetine-exposed fish, there was a significant positive effect of standard deviation in group weight on the number of prey items consumed (GLMM; unexposed: t = 2.16, p = 0.034, low: t = 2.17, p = 0.034), although no such relationship was detected in high-exposed fish (GLMM; t = –0.93, p = 0.353).
Figure 2.
Total number of prey consumed plotted against standard deviation of group weight (g) for control (n = 34), low-fluoxetine (n = 34) and high-fluoxetine groups (n = 35). Dashed grey lines show the median standard deviation of group weight (0.0359 g). To represent the interaction between standard deviation of group weight, within each treatment, violin plots represent groups of small (less than 0.0359 g; left side of the dashed grey line) and large (greater than 0.0359 g; right side of the dashed grey line) weight variability. (Online version in colour.)
We found a significant interaction between group mean weight and exposure treatment (nbGLMM; F = 3.79, p = 0.026; electronic supplementary material, figure S4), as well as between standard deviation in group weight and exposure treatment (GLMM; F = 4.39, p = 0.017; electronic supplementary material, figure S5), on the number of aggressive interactions performed during foraging. Specifically, for unexposed fish, variability in group weight was positively associated with foraging aggression (nbGLMM; z = 2.05, p = 0.040), while mean group weight was negatively associated with aggression (nbGLMM; z = –2.16, p = 0.031), although the latter appears to be driven by a few groups with high aggression and low mean weight (electronic supplementary material, figure S4). By contrast, for both low- and high-exposed fish, there was no significant effect of either mean group weight (nbGLMM; low: z = 1.09, p = 0.275; high: z = –0.89, p = 0.374) or standard deviation in group weight (nbGLMM; low: z = –1.34, p = 0.180; high: z = 0.08, p = 0.940) on foraging aggression.
(d). Fish morphology
There was no significant difference between fish weight, standard length or condition index across the exposure treatment groups (LME; all p > 0.05; electronic supplementary material, table S3).
4. Discussion
We found that the effect of fluoxetine exposure on fish foraging behaviour was dependent on the social context. For solitary fish, fluoxetine at environmentally relevant concentrations had no significant effect on foraging. This is consistent with previous studies that have similarly employed field-relevant concentrations (i.e. less than 540 ng l−1; [18,40]), whereas at higher concentrations, fluoxetine has been reported to reduce foraging [41–44]. In combination, these findings suggest that fluoxetine exposure at field-detected levels is not sufficient to alter the foraging behaviour of solitary individuals.
In contrast to the results for solitary fish, we found that fluoxetine affected foraging dynamics in social trials. Firstly, fluoxetine exposure disrupted the relationship between the total number of prey consumed and standard deviation in group weight. Specifically, for unexposed and low-fluoxetine-exposed fish, standard deviation in fish weight was a positive predictor of the number of prey items consumed (i.e. groups with larger variation in body weight consumed more prey). However, this relationship was not present in high-fluoxetine-exposed fish. Importantly, this effect was not generated by differences in the standard deviation of group weight across treatments, as there was no significant difference in weight variability among group members across treatments (LME: F = 0.09, p = 0.912; electronic supplementary material, table S7 and figure S6). Secondly, fluoxetine exposure disrupted body weight-dependent aggressive interactions during feeding. For unexposed fish, mean group weight negatively predicted the number of aggressive interactions during feeding, while standard deviation in group weight positively predicted aggressive interactions. The increase in aggression and competition associated with greater heterogeneity in weight among group members likely led to the increased food consumption observed, as has been shown in Brook trout (Salvelinus fontinalis; [45]) and group-living spiders (Stegodyphus dumicola; [46]). However, these body weight-dependent foraging dynamics were disrupted in fluoxetine-exposed fish. For fish in the low- and high-fluoxetine treatments, neither mean group weight nor standard deviation in group weight significantly predicted the number of aggressive interactions during foraging. Fluoxetine has previously been shown to affect aggressive behaviours, including reduced conspecific chasing behaviour in Arabian killifish (Aphanius dispar; [23]) and lowered aggression in Siamese fighting fish (Betta splendens; [47,48]). It is possible, therefore, that fluoxetine, through its anxiolytic effects, disturbed weight-dependent aggressive interactions during foraging, which, in turn, disrupted the relationship between group weight variability and foraging behaviour.
Following the 28-day exposure, we did not detect an effect of fluoxetine on fish morphology (i.e. length, weight or condition). A fluoxetine-induced decrease in condition index has previously been reported in fish [36,41,42,49] although, typically, such effects are reported at dosages higher than those employed here [36]. Given that the effects of fluoxetine can take two to three weeks to manifest [31,32], it is possible that, over a longer duration, shifts in social foraging behaviour could result in shifts in morphology.
In summary, the effects of fluoxetine reported here were dependent on the social context in which fish were tested. We report evidence of a group-specific effect of fluoxetine exposure on foraging behaviour and aggressive interactions during foraging, while no change in behaviour was seen in solitary individuals. Our results suggest that social context may be an important, but underappreciated, factor influencing the ecological impacts of chemical pollutants on wildlife.
Supplementary Material
Supplementary Material
Supplementary Material
Ethics
All experiments were approved by and conducted in accordance with, the Biological Sciences Animal Ethics Committee of Monash University (permit no.: BSCI/2016/21).
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ns1rn8pnh [50].
Authors' contributions
J.M.M., M.S., M.G.B., V.N.-R., D.K.D. and B.B.M.W. conceived and designed the experiments, which J.M.M. and H.T. carried out. Video and statistical analyses were performed by J.M.M. and H.T. The manuscript was drafted by J.M.M., V.N.-R., D.K.D. and B.B.M.W. All authors contributed critically to drafts and gave final approval for publication. All authors agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Australian Government Research Training Program Scholarships (to J.M.M. and M.G.B.) and the Australian Research Council (DP130100385 and DP160100372; both to B.B.M.W.).
References
- 1.Boxall ABA, et al. 2012. Pharmaceuticals and personal care products in the environment: what are the big questions? Environ. Health Perspect. 120, 1221–1229. ( 10.1289/ehp.1104477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Küster A, Adler N. 2014. Pharmaceuticals in the environment: scientific evidence of risks and its regulation. Phil. Trans. R. Soc. B 369, 20130587 ( 10.1098/rstb.2013.0587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhardt ES, Rosi EJ, Gessner MO. 2017. Synthetic chemicals as agents of global change. Front Ecol. Environ. 15, 84–90. ( 10.1002/fee.1450) [DOI] [Google Scholar]
- 4.Calisto V, Esteves VI. 2009. Psychiatric pharmaceuticals in the environment. Chemosphere 77, 1257––1274. ( 10.1016/j.chemosphere.2009.09.021) [DOI] [PubMed] [Google Scholar]
- 5.Sehonova P, Svobodova Z, Dolezelova P, Vosmerova P, Faggio C. 2018. Effects of waterborne antidepressants on non-target animals living in the aquatic environment: a review. Sci. Total Environ. 631–632, 789–794. ( 10.1016/j.scitotenv.2018.03.076) [DOI] [PubMed] [Google Scholar]
- 6.Mole RA, Brooks BW. 2019. Global scanning of selective serotonin reuptake inhibitors: occurrence, wastewater treatment and hazards in aquatic systems. Environ. Pollut. 250, 1019–1031. ( 10.1016/j.envpol.2019.04.118) [DOI] [PubMed] [Google Scholar]
- 7.Caveney S, Cladman W, Verellen L, Donly C. 2006. Ancestry of neuronal monoamine transporters in the Metazoa. J. Exp. Biol. 209, 4858–4868. ( 10.1242/jeb.02607) [DOI] [PubMed] [Google Scholar]
- 8.Fong PP. 2001. Antidepressants in aquatic organisms: a wide range of effects. In Pharmaceuticals and personal health care products in the environment: scientific and regulatory issues (eds Daughton CG, Jones-Lepp TL), pp. 264–281, ACS Symposium Series 791 Washington, DC: American Chemical Society. [Google Scholar]
- 9.McDonald DM. 2017. An AOP analysis of selective serotonin reuptake inhibitors (SSRIs) for fish. Comp. Biochem. Physiol. C 197, 19– 31 ( 10.1016/j.cbpc.2017.03.007) [DOI] [PubMed] [Google Scholar]
- 10.Meijide FJ, Da Cuna RH, Prieto JP, Dorelle LS, Babay PA, Lo Nostro FL. 2018. Effects of waterborne exposure to the antidepressant fluoxetine on swimming, shoaling and anxiety behaviors of the mosquitofish Gambusia holbrooki. Ecotoxicol. Environ. Saf. 163, 646–655. ( 10.1016/j.ecoenv.2018.07.085) [DOI] [PubMed] [Google Scholar]
- 11.Nielsen SV, Kellner M, Henriksen PG, Olsen H, Hansen SH, Baatrup E. 2018. The psychoactive drug escitalopram affects swimming behaviour and increases boldness in zebrafish (Danio rerio). Ecotoxicology 27, 485–497. ( 10.1007/s10646-018-1920-x) [DOI] [PubMed] [Google Scholar]
- 12.de Farias NO, et al. 2019. Exposure to low concentration of fluoxetine affects development, behaviour and acetylcholinesterase activity of zebrafish embryos. Comp. Biochem. Physiol. C 215, 1–8. ( 10.1016/j.cbpc.2018.08.009) [DOI] [PubMed] [Google Scholar]
- 13.Huang IJ, Sirotkin HI, McElroy AE. 2019. Varying the exposure period and duration of neuroactive pharmaceuticals and their metabolites modulates effects on the visual motor response in zebrafish (Danio rerio) larvae. Neurotoxicol. Teratol. 72, 39–48. ( 10.1016/j.ntt.2019.01.006) [DOI] [PubMed] [Google Scholar]
- 14.Dzieweczynski TL, Kane JL, Campbell BA, Lavin LE. 2016a. Fluoxetine exposure impacts boldness in female Siamese fighting fish, Betta splendens. Ecotoxicology 25, 69–79. ( 10.1007/s10646-015-1568-8) [DOI] [PubMed] [Google Scholar]
- 15.Dzieweczynski TL, Campbell BA, Kane JL. 2016b. Dose-dependent fluoxetine effects on boldness in male Siamese fighting fish. J. Exp. Biol. 219, 797–804. ( 10.1242/jeb.132761) [DOI] [PubMed] [Google Scholar]
- 16.Martin JM, et al. 2019. Antidepressants in surface waters: fluoxetine influences mosquitofish anxiety-related behavior at environmentally relevant levels. Environ. Sci. Technol. 53, 6035–6043. ( 10.1021/acs.est.9b00944) [DOI] [PubMed] [Google Scholar]
- 17.Painter MM, Buerkley MA, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL. 2009. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 28, 2677–2684. ( 10.1897/08-556.1) [DOI] [PubMed] [Google Scholar]
- 18.Weinberger J, Klaper R. 2014. Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow). Aquat. Toxicol. 151, 77–83. ( 10.1016/j.aquatox.2013.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin JM, Saaristo M, Bertram MG, Lewis PJ, Coggan T L, Clarke BO, Wong BBM. 2017. The psychoactive pollutant fluoxetine compromises antipredator behavior in fish. Environ. Pollut. 222, 592–599. ( 10.1016/j.envpol.2016.10.010) [DOI] [PubMed] [Google Scholar]
- 20.Saaristo M, McLennan A, Johnstone CP, Clarke B, Wong BBM. 2017. Impacts of the antidepressant fluoxetine on the anti-predator behavior of wild guppies (Poecilia reticulata). Aquat. Toxicol. 183, 38–45. ( 10.1016/j.aquatox.2016.12.007) [DOI] [PubMed] [Google Scholar]
- 21.De Castro-Catala N, Munoz I, Riera JL, Ford AT. 2017. Evidence of low dose effects of the antidepressant fluoxetine and the fungicide prochloraz on the behavior of the keystone freshwater invertebrate Gammarus pulex. Environ. Pollut. 231, 406–414. ( 10.1016/j.envpol.2017.07.088) [DOI] [PubMed] [Google Scholar]
- 22.Peters JR, Granek EF, de Rivera CE, Rollins M. 2017. Prozac in the water: chronic fluoxetine exposure and predation risk interact to shape behaviors in an estuarine crab. Ecol. Evol. 7, 9151–9161. ( 10.1002/ece3.3453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry MJ. 2013. Effects of fluoxetine on the swimming and behavioral responses of the Arabian killifish. Ecotoxicology 22, 425–432. ( 10.1007/s10646-012-1036-7) [DOI] [PubMed] [Google Scholar]
- 24.Barry MJ. 2014. Fluoxetine inhibits predator avoidance behavior in tadpoles. Toxicol. Environ. Chem. 96, 641–649. ( 10.1080/02772248.2014.966713) [DOI] [Google Scholar]
- 25.Pelli M, Connaughton VP. 2015. Chronic exposure to environmentally-relevant concentrations of fluoxetine (Prozac) decreases survival, increases abnormal behaviors, and delays predator escape responses in guppies. Chemosphere 139, 202–209. ( 10.1016/j.chemosphere.2015.06.033) [DOI] [PubMed] [Google Scholar]
- 26.Delgado MD, Miranda M, Alvarez SJ, Gurarie E, Fagan WF, Penteriani V, di Virgilio A, Morales JM. 2018. The importance of individual variation in the dynamics of animal collective movements. Phil. Trans. R. Soc. B 373, 20170008 ( 10.1098/rstb.2017.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planas-Sitja I, Nicolis SC, Sempo G, Deneubourg JL. 2018. The interplay between personalities and social interactions affects the cohesion of the group and the speed of aggregation. PLoS ONE 13, e0201053 ( 10.1371/journal.pone.0201053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilastro A, Benetton S, Bisazza A. 2003. Female aggregation and male competition reduce costs of sexual harassment in the mosquitofish Gambusia holbrooki. Anim. Behav. 65, 1161–1167. ( 10.1006/anbe.2003.2118) [DOI] [Google Scholar]
- 29.Arrington JJ, Tharnan KRJ, Rettig JE, Smith GR. 2009. Foraging behavior of male and female mosquitofish (Gambusia affinis) in single- and mixed-sex groups. J. Freshw. Ecol. 24, 327–329. ( 10.1080/02705060.2009.9664299) [DOI] [Google Scholar]
- 30.Martin RG. 1975. Sexual and aggressive behavior, density and social structure in a natural population of mosquitofish, Gambusia affinis holbrooki . Copeia 3, 445–454. ( 10.2307/1443641) [DOI] [Google Scholar]
- 31.Gardier AM, Malagie I, Trillat AC, Jacquot C, Artigas F. 1996. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundam. Clin. Pharmacol. 10, 16–27. ( 10.1111/j.1472-8206.1996.tb00145.x) [DOI] [PubMed] [Google Scholar]
- 32.Hensler JG. 2003. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sci. 72, 1665–1682. ( 10.1016/s0024-3205(02)02482-7) [DOI] [PubMed] [Google Scholar]
- 33.Saaristo M, Tomkins P, Allinson M, Allinson G, Wong BBM. 2013. An androgenic agricultural contaminant impairs female reproductive behaviour in a freshwater fish. PLoS ONE 8, e62782 ( 10.1371/journal.pone.0062782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin JM, Bertram MG, Saaristo M, Ecker TE, Hannington SL, Tanner JL, Michelangeli M, O'Bryan MK, Wong BBM. 2019. Impact of the widespread pharmaceutical pollutant fluoxetine on behavior and sperm traits in a freshwater fish. Sci. Total Environ. 650, 1771–1778. ( 10.1016/j.scitotenv.2018.09.294) [DOI] [PubMed] [Google Scholar]
- 35.Fursdon JB, Martin JM, Bertram MG, Lehtonen TK, Wong BBM. 2019. The pharmaceutical pollutant fluoxetine alters reproductive behavior in a fish independent of predation risk. Sci. Total Environ. 650, 642–652. ( 10.1016/j.scitotenv.2018.09.046) [DOI] [PubMed] [Google Scholar]
- 36.Bertram MG, Ecker TE, Wong BBM, O'Bryan MK, Baumgartner JB, Martin JM, Saaristo M. 2018. The antidepressant fluoxetine alters mechanisms of pre- and post-copulatory sexual selection in the eastern mosquitofish (Gambusia holbrooki). Environ. Pollut. 238, 238–247. ( 10.1016/j.envpol.2018.03.006) [DOI] [PubMed] [Google Scholar]
- 37.Bertram MG, Saaristo M, Martin JM, Ecker TE, Michelangeli M, Johnstone CP, Wong BBM. 2018. Field-realistic exposure to the androgenic endocrine disruptor 17 beta-trenbolone alters ecologically important behaviours in female fish across multiple contexts. Environ. Pollut. 243, 900–911. ( 10.1016/j.envpol.2018.09.044) [DOI] [PubMed] [Google Scholar]
- 38.Friard O, Gamba M. 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. ( 10.1111/2041-210X.12584) [DOI] [Google Scholar]
- 39.R Development Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 40.Mennigen JA, Sassine J, Trudeau VL, Moon TW. 2010. Waterborne fluoxetine disrupts feeding and energy metabolism in the goldfish Carassius auratus. Aquat. Toxicol. 100, 128–137. ( 10.1016/j.aquatox.2010.07.022) [DOI] [PubMed] [Google Scholar]
- 41.Stanley JK, Ramirez AJ, Chambliss CK, Brooks BW. 2007. Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere 69, 9–16. ( 10.1016/j.chemosphere.2007.04.08) [DOI] [PubMed] [Google Scholar]
- 42.Gaworecki KM, Klaine SJ. 2008. Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat. Toxicol. 88, 207–213. ( 10.1016/j.aquatox.2008.04.011) [DOI] [PubMed] [Google Scholar]
- 43.Mennigen JA, Harris EA, Chang JP, Moon TW, Trudeau VL. 2009. Fluoxetine affects weight gain and expression of feeding peptides in the female goldfish brain. Regul. Pept. 155, 99–104. ( 10.1016/j.regpep.2009.01.001) [DOI] [PubMed] [Google Scholar]
- 44.Hedgespeth ML, Nilsson PA, Berglund O. 2014. Ecological implications of altered fish foraging after exposure to an antidepressant pharmaceutical. Aquat. Toxicol. 151, 84–87. ( 10.1016/j.aquatox.2013.12.011) [DOI] [PubMed] [Google Scholar]
- 45.Grant JWA. 1990. Aggressiveness and the foraging behavior of young-of-the-year brook charr (Salvelinus fontinalis). Can. J. Fish. Aquat. Sci. 47, 915–920. ( 10.1139/f90-105) [DOI] [Google Scholar]
- 46.Amir N, Whitehouse MEA, Lubin Y. 2000. Food consumption rates and competition in a communally feeding social spider, Stegodyphus dumicola (Eresidae). J. Arachnol. 28, 195–200. ( 10.1636/0161-8202(2000)028[0195:FCRACI]2.0.CO;2) [DOI] [Google Scholar]
- 47.Dzieweczynski TL, Hebert OL. 2012. Fluoxetine alters behavioral consistency of aggression and courtship in male Siamese fighting fish, Betta splendens. Physiol. Behav. 107, 92–97. ( 10.1016/j.physbeh.2012.06.007) [DOI] [PubMed] [Google Scholar]
- 48.Forsatkar MN, Nematollahi MA, Amiri BM, Huang WB. 2014. Fluoxetine inhibits aggressive behaviour during parental care in male fighting fish (Betta splendens, Regan). Ecotoxicology 23, 1794–1802. ( 10.1007/s10646-014-1345-0) [DOI] [PubMed] [Google Scholar]
- 49.Latifi T, Forsatkar MN, Nematollahi MA. 2015. Reproduction and behavioral responses of convict cichlid, Amatitlania nigrofasciata to fluoxetine. J. Fish. Aquat. Sci. 10, 111–120. ( 10.3923/jfas.2015.111.120) [DOI] [Google Scholar]
- 50.Martin JM, Saaristo M, Tan H, Bertram MG, Nagarajan-Radha V, Dowling DK, Wong BBM. 2019. Data from: Field-realistic antidepressant exposure disrupts group foraging dynamics in mosquitofish Dryad Digital Repository. ( 10.5061/dryad.ns1rn8pnh) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Martin JM, Saaristo M, Tan H, Bertram MG, Nagarajan-Radha V, Dowling DK, Wong BBM. 2019. Data from: Field-realistic antidepressant exposure disrupts group foraging dynamics in mosquitofish Dryad Digital Repository. ( 10.5061/dryad.ns1rn8pnh) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ns1rn8pnh [50].