Abstract
Soluble epoxide hydrolase (sEH) inhibitors are potential drugs for several diseases. Adamantyl ureas are excellent sEH inhibitors but have limited metabolic stability. Herein, we report the effect of replacing the adamantane group by alternative polycyclic hydrocarbons on sEH inhibition, solubility, permeability and metabolic stability. Compounds bearing smaller or larger polycyclic hydrocarbons than adamantane yielded all good inhibition potency of the human sEH (0.4 ≤ IC50 ≤ 21.7 nM), indicating that sEH is able to accommodate inhibitors of very different size. Human liver microsomal stability of diamantane containing inhibitors is lower than that of their corresponding adamantane counterparts.
Keywords: Adamantane, Inhibitor, Isocyanate, Soluble epoxide hydrolase, Urea
1. Introduction
Arachidonic acid (AA), a polyunsaturated fatty acid, plays important roles in cellular signaling as a second messenger and is also a precursor for a wide variety of lipid mediators that are involved in many physiological and pathophysiological processes. The first step in the biosynthesis of these mediators, known as eicosanoids or oxylipins, is an oxidation, which can be catalyzed by cyclooxygenases (COX), lipoxygenases (LOX), and cytochrome P450 enzymes.1 Most research on AA derivatives has focused on prostaglandins, processed by COX, and leukotrienes, originated from LOX. Both types of metabolites are potent inflammatory mediators and, consequently, several pharmaceuticals have been produced to alleviate inflammatory conditions. These included non-selective COX-1 and COX-2 inhibitors (e.g., ibuprofen, indomethacin), selective COX-2 inhibitors (e.g., celecoxib, etoricoxib), and 5-LOX inhibitors (e.g., zileuton).2–4
Comparatively, the third pathway remains relatively unexplored. Cytochromes P450 enzymes transform AA to various biologically active compounds, including epoxyeicosatrienoic acids (EETs).5,6 EETs are reported to exhibit anti-inflammatory and anti-nociceptive properties and are involved in the regulation of blood pressure and cellular stress.7–11 Soluble epoxide hydrolase (sEH, EPHX2, E.C. 3.3.2.3), a member of the α/β-hydrolase fold family of enzymes, catalyzes the hydrolysis of EETs to the corresponding dihydroxyeicosatrienoic acids (DHETs), reducing the beneficial activities of EETs.12–15 The inhibition of sEH in vivo by potent, selective inhibitors results in an increase of the concentration of the EETs, reducing blood pressure and inflammatory and pain states, thereby suggesting that sEH inhibitors may serve as novel agents for treating hypertension, inflammatory diseases, pain and, more recently, neurodegenerative diseases.16–21
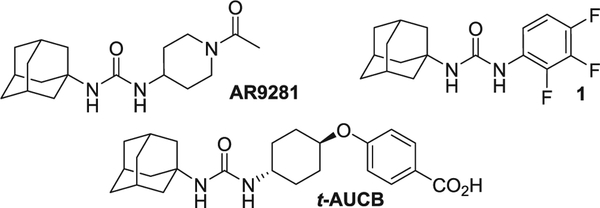
X-ray crystallographic studies revealed that sEH has an active site with a catalytic triad at the corner of an L-shaped hydrophobic pocket. The triad includes a nucleophilic aspartic acid, which attacks the epoxide carbon-highly polarized by hydrogen bonds with two tyrosine residues, and a histidine-aspartic acid pair, which activates the hydrolysis of the acyl-enzyme intermediate.22 Therefore, lipophilic groups such as cyclohexyl or adamantyl are commonly present in potent sEH inhibitors in order to stablish hydrophobic interactions with the pocket. In fact, the first sEH inhibitor to enter in clinical trials was AR9281, an adamantyl urea (Fig. 1).23 Specifically, hundreds of sEH inhibitors featuring a common structure of Ad-NH-C(O)-NH-R, where Ad is adamantan-1-yl and R is alkyl, aryl or heterocyclyl groups, have been synthesized and, subsequently, evaluated in several in vivo models (Fig. 1).23–35 However, the poor metabolic stability of some adamantane containing ureas could limit their usefulness to treat patients.36 Notwithstanding the high potency generally associated to adamantane-derived sEH inhibitors, alternative polycyclic hydrocarbons have been scarcely evaluated.
Fig. 1.
Adamantyl-based sEH inhibitors.
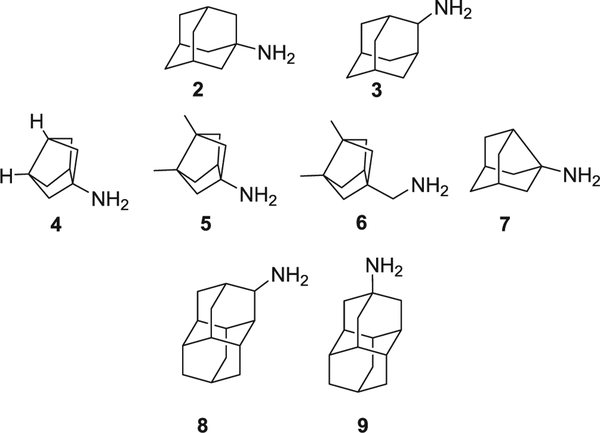
In this work, a series of ring-contracted and ring-expanded analogs of three potent adamantane sEH inhibitors, AR9281 (IC50 = 7.0 nM),23 t-AUCB (IC50 = 0.5 nM),37 and 1 (IC50 = 0.4 nM),38 were synthesized and pharmacologically evaluated in order to test if alterations in the size of the lipophilic unit attached to the urea significantly impact its potency toward the human sEH (Figs. 1 and 2) as well as influencing solubility, permeability and metabolic stability.
Fig. 2.
Polycyclic amines used in this study.
2. Results and discussion
2.1. Chemistry
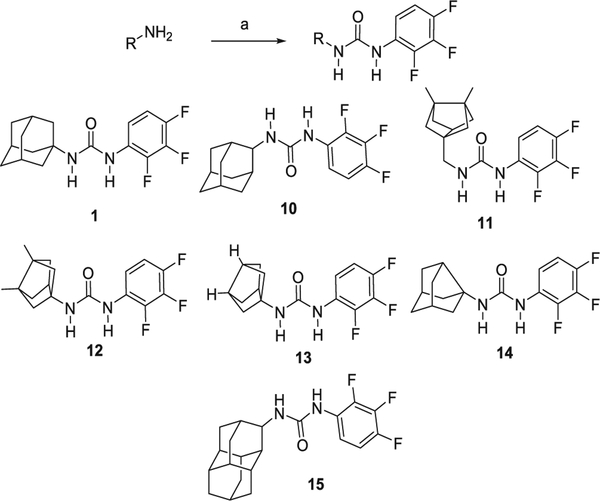
Adamantyl ureas are typically synthesized by the reaction of adamantyl isocyanates with a primary amine. Alternatively, the reaction of amantadine (1-adamantylamine) with an isocyanate also furnishes adamantyl ureas. Taking into account that 2,3,4-trifluorophenylisocyanate is a commercially available compound, for the preparation of the analogs of urea 1, we reacted this isocyanate with four different amines, 4–7, featuring smaller polycyclic rings than adamantane. Bisnoradamantane amines 4, 5 and 6 were synthesized following reported procedures,39,40 while noramantadine 7 is commercially available. For comparative purposes, we also synthesized, using the same reaction, urea 1 and its isomer 10 (Scheme 1).
Scheme 1.
Synthesis of analogs containing a trifluorophenyl unit. Reagents and conditions: (a) 2,3,4-trifluorophenylisocyanate, Et3N, anh. DCM, overnight.
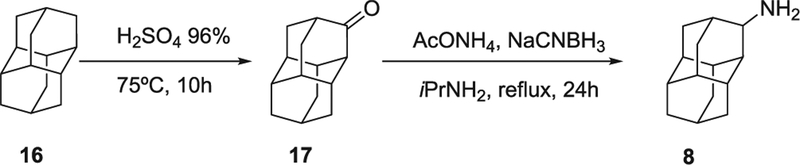
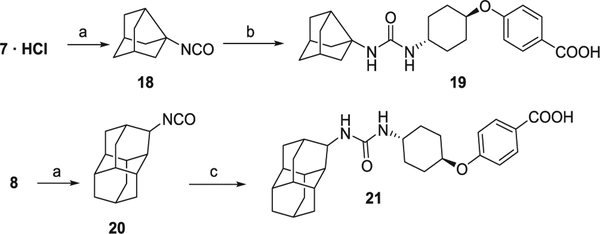
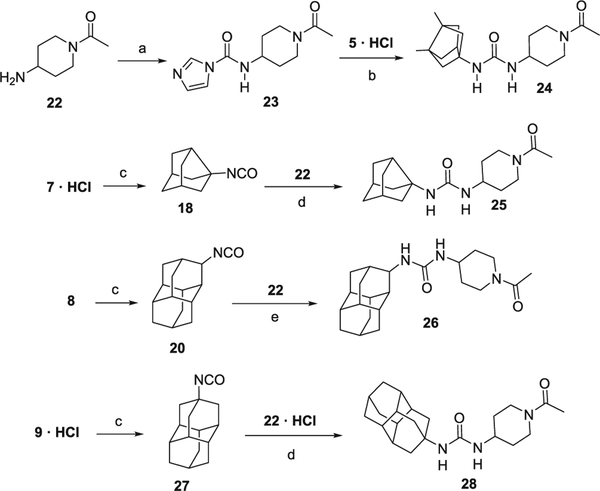
In order to obtain the ring-expanded analog 15, we started from diamantanamine 8, which was synthesized in two steps from commercially available diamantane, 16. Oxidation of 16 with sulfuric acid followed by a reductive amination of ketone 17 by ammonium acetate and NaCNBH3 led to amine 8 (Scheme 2). For the synthesis of the ring-contracted and ring-expanded analogs of t-AUCB and AR9281, we first prepared the required isocyanate by the reaction of the corresponding polycyclic amine with triphosgene. The reaction of these isocyanates with either t-4-[(4-aminocyclohexyl)oxy]benzoic acid (Scheme 3) or N-acetyl-4-aminopiperidine, 22 (Scheme 4), furnished the desired compounds. An alternative procedure was employed for the synthesis of urea 24, involving the activation of 22 with 1,1′-carbonyldiimidazole (Scheme 4).
Scheme 2.
Synthesis of diamantanamine 8.
Scheme 3.
Synthesis of t-AUCB analogs. Reagents and conditions: (a) triphosgene, NaHCO3, DCM, 30 min; (b) t-4-[(4-aminocyclohexyl)oxy]benzoic acid, Et3N, DCM, 30 °C, overnight; (c) t-4-[(4-aminocyclohexyl)oxy]benzoic acid, Et3N, DMF, 50 °C, 3 days.
Scheme 4.
Synthesis of AR9281 analogs. Reagents and conditions: (a) 1,1′-carbonyldiimidazole, 1,2-dichloroethane, 50 °C, 21 h; (b) Et3N, CHCl3, 50 °C, 24 h; (c) triphosgene, Et3N, DCM, 30 min; (d) DCM, Et3N, overnight; (e) DCM, overnight.
2.2. sEH inhibition and structure-activity relationships
The potency of the new compounds as human soluble epoxide hydrolase inhibitors was tested using a previously reported sensitive fluorescent-based assay (Table 1).41
Table 1.
Inhibitory activities against the human and murine sEH.
| Entry | Compound | IC50 (nM) human sEHa | IC50 (nM) murine sEHa |
|---|---|---|---|
| 1 | 1 | 0.4 | NDb |
| 2 | 10 | 0.4 | ND |
| 3 | 11 | 0.4 | ND |
| 4 | 12 | 0.5 | ND |
| 5 | 13 | 3.2 | ND |
| 6 | 14 | 3.3 | ND |
| 7 | 15 | 8.0 | ND |
| 8 | t-AUCB | 0.5 | ND |
| 9 | 19 | 8.6 | ND |
| 10 | 21 | 0.5 | ND |
| 11 | AR9281 | 8.0 | 3.0 |
| 12 | 24 | 6.5 | 3.3 |
| 13 | 25 | 21.7 | ND |
| 14 | 26 | 3.4 | 5.0 |
| 15 | 28 | 7.2 | 1.8 |
Reported IC50 values are the average of three replicates. The fluorescent assay as performed here has a standard error between 10 and 20% suggesting that differences of two-fold or greater are significant. Because of the limitations of the assay it is difficult to distinguish among potencies < 0.5 nM.41
ND: not determined.
Within the series of the 2,3,4-trifluophenyl inhibitors, sequential ring contraction from adamantanes 1 or 10 to noradamantane 14 and to bisnoradamantane 13 resulted in a decrease of the inhibitory potency (compare entries 1 and 2 vs 5 and 6, Table 1), likely because of the reduction of hydrophobic interactions between the ring-contracted moiety and the lipophilic pocket of the enzyme and the increase of desolvation energy to transfer the molecule from the solution state to the receptor cavity. This reduction in potency was also observed in the other two series of sEH inhibitors (compare entry 11 vs 13, and entry 8 vs 9, Table 1).
Nevertheless, the inhibitory potency was restored by the introduction of two methyl groups in the bridgehead positions of the bisnoradamantane moiety (compare entries 1 and 2 vs entries 3 and 4, and entry 11 vs entry 12, Table 1), probably because the addition of the methyl groups compensates the reduction in size from the adamantane to the bisnoradamantane scaffold. Furthermore, the results showed that the introduction of a methylene unit between the hydrophobic moiety and the urea does not affect the potency of the compounds (compare entry 3 vs 4, Table 1).
Taking into account that the reduction of the polycyclic moiety from adamantane to bisnoradamantane led, within the three series of inhibitors, to a reduction of the potency, we wondered if the opposite was true. That is, whether an increase in the size of the lipophilic unit of the inhibitor would lead to more potent compounds.
With the aim of exploring the ring-expanded analogs, the adamantane ring was replaced by the much larger diamantane moiety. Somehow surprisingly, considering the substantial increase in size and previous consideration of the adamantyl group as the marginal biggest group as the N-substituent for sEH inhibitors,42 diamantane ureas 26 and 28 showed IC50 values in the same range as that of AR9281 (compare entry 11 vs entries 14 and 15). Considering that 26 was slightly more potent than its isomer 28, we synthesized two further analogs derived from diamantane 8, i.e., the new ureas 15 and 21, analogs of inhibitors 1 and t-AUCB, respectively. In line with the aforementioned results, diamantane derivative 21 showed to be as potent as t-AUCB (compare entry 8 vs 10, Table 1). However, within the trifluorophenyl series, the diamantane derivative 15 was considerably less potent than adamantane derivatives 1 or 10 (compare entries 1 and 2 vs 7, Table 1). The dissimilar behavior of 15 compared with 21 and 26 could be due to an opposite binding orientation of 15 compared to that of 21 and 26, as observed previously for a different series of sEH inhibitors.43
Typically, steric parameters have stronger effects on the potency of inhibitors against murine sEH rather than on the human sEH.32,41,43,44 For example, it has recently been reported that the progressive introduction of one, two or three methyl groups in the bridgehead positions of the adamantane unit of t-AUCB did not lead to significant changes in the IC50 values against the human enzyme, while leading to a gradual increase in the IC50 values against the murine enzyme.32 However, when we tested the inhibition of the murine sEH by AR9281 and three analogs (24, 26 and 28), we did not find significant differences between their activities in human and murine species (Table 1).
2.3. Microsomal stability
It is known that the adamantane nucleus is prone to rapid metabolism in vivo giving rise to a variety of inactive hydroxylated derivatives. This results in low drug concentrations in blood and short in vivo half-life. Metabolism studies have shown that the bridgehead hydroxylation (tertiary carbon) is favored over the secondary carbon positions, producing water-soluble hydroxyadamantane derivatives in the liver, which are then easily excreted.45 Additionally, metabolic studies showed that liver microsomes from phenobarbital-treated rats readily metabolize diamantane to mono-, di- and possibly tri-hydroxy derivatives.46,47 It is also known that several diamantanes are cytochromes P450 inhibitors.48,49
Considering the aforementioned metabolism liability of the adamantane and diamantane scaffolds, we assessed the in vitro stability of some representative new ureas in human microsomes in order to examine the impact of the different hydrophobic units in their metabolic stabilities.
As anticipated, diamantane derivatives were extremely labile compounds, with their adamantane counterparts being considerably more stable (compare entries 1 vs 6, 7 vs 9, and 10 vs 13 and 14, Table 2). These results are in the line with what was expected, since diamantane moiety features more tertiary carbon atoms than the adamantane ring, which are prone to be hydroxylated.
Table 2.
Solubility and microsomal stability of the new compounds.
| Entry | Compound | Microsomal stabilitya | Solubility (μM)b | Lipophilicityc |
|---|---|---|---|---|
| 1 | 1 | 34.3% | 57 | 4.1 |
| 2 | 10 | 19.4% | NDd | 3.9 |
| 3 | 12 | 30.0% | 66 | 4.1 |
| 4 | 13 | 41.9% | 82 | 3.5 |
| 5 | 14 | 30.2% | 65 | 3.8 |
| 6 | 15 | 0.0% | 18 | 4.5 |
| 7 | t-AUCB | 93.5% | 60 | 3.7 |
| 8 | 19 | 64.6% | 76 | 3.4 |
| 9 | 21 | 14.6% | 7 | 3.9 |
| 10 | AR9281 | 80.1% | > 100 | 2.1 |
| 11 | 24 | 59.3% | > 100 | 2.2 |
| 12 | 25 | 88.7% | ND | 1.8 |
| 13 | 26 | 0.0% | 86 | 2.5 |
| 14 | 28 | 2.6% | 85 | 2.6 |
Percentage of remaining compound after 60 min of incubation with human microsomes obtained from Tebu-Xenotech in the presence of NADP at 37 °C. Metabolism of testosterone was used as a positive control for metabolism (22.4% remaining compound).
Solubility in a 1% DMSO: 99% PBS buffer solution, see experimental section for details.
Lipophilicity refers to the consensus log Po/w value calculated using the SwissADME program50 for five predictive log Po/w models (iLOGP, XLOGP3, WLOGP, MLOGP and SILICOS-IT).
ND: not determined.
Finally, the bisnoradamantane and the noradamantane units seem to have similar (compare entries 1 vs 3 and 4, and 1 vs 5 and 10 vs 12, Table 2) or somehow reduced (compare entries 7 vs 8 and 10 vs 11, Table 2) microsomal stability than adamantane.
2.4. Solubility and lipophilicity
In order to assess the impact of the polycyclic scaffold in the solubility of the inhibitors, we experimentally determined their solubility in a 1% DMSO: 99% PBS buffer solution.
As expected, within the trifluorophenylurea series, the solubility highly increases from diamantane 15 to adamantane 1 (18 and 57 μM, respectively, Table 2) and then, slightly further increases to the noradamantane 14 and to the bisnoradamantane 13 (65 and 82 μM, respectively, Table 2). In fact, the diamantane derivatives were dramatically less soluble than their adamantane, noradamantane or bisnoradamantane counterparts (compare entries 6 vs 1, 3, 4 and 5, entries 9 vs 7 and 8 and entries 13 and 14 vs 10 and 11, Table 2). Finally, considering the right-hand side of the inhibitors, the acetylpiperidine derivatives were the more soluble compounds, with the two other series having similar solubility.
Of note, the experimental solubility values showed a good correlation with the calculated lipophilicity values (see Table 2), the more soluble AR9281 analogs being the compounds with the lowest lipophilicity. As expected, for any given right-hand side unit, the diamantane derivatives showed always the higher lipophilicity.
2.5. Permeability
In order to evaluate the permeability of selected inhibitors, the Caco-2 cell permeability model was used in this study. Apparent permeability values (Papp) were determined from the amount permeated through the Caco-2 cell membranes at both apical-basolateral (A-B) and basolateral-apical (B-A) direction (Table 3).
Table 3.
Permeability in the Caco-2 cell line of selected sEH inhibitors.
| Entry | Compound | Papp A→B (nm/s) | Papp B→A (nm/s) | ERa |
|---|---|---|---|---|
| 1 | 1 | 11.8 ± 1.3 | 1.8 ± 0 | 0.2 ± 0 |
| 2 | 10 | 2.3 ± 0.1 | 7.3 ± 0.3 | 3.2 ± 0.2 |
| 3 | 15 | 16.2 ± 2.4 | 5.4 ± 0.1 | 0.3 ± 0.1 |
| 4 | t-AUCB | 1.9 ± 0.2 | 210.3 ± 53.7 | 111 ± 34.5 |
| 5 | 21 | 3.1 ± 0.3 | 67.5 ± 2.4 | 22.2 ± 1.9 |
| 6 | 24 | 159.2 ± 2.8 | 180.2 ± 31.6 | 1.1 ± 0.2 |
| 7 | 26 | 156.2 ± 13.6 | 146.9 ± 19.6 | 1.0 ± 0.2 |
| 8 | 28 | 208.3 ± 20.2 | 191.6 ± 38.5 | 0.9 ± 0.1 |
The efflux ratio was calculated as ER = (Papp B → A)/(Papp A → B). See the experimental section for further details. Permeability of estrone-3-sulfate and colchicine were used as references.
Of note, the size of the lipophilic unit of the sEH inhibitors seems to be of little relevance regarding permeability, as evidenced through the comparison within the three series of inhibitors: the trifluorophenyl derivatives (compare entries 1 and 2 vs 3, Table 3), the benzoic acid derivatives (compare entries 4 vs 5, Table 3) and the acetylpiperidine derivatives (compare entries 6–8, Table 3). Regarding the right-hand side of the ureas, acetylpiperidine derivatives were endowed with the best permeability, while the trifluorophenyl compounds 1, 10 and 15 displayed much lower permeability. As expected, benzoic acid derivatives t-AUCB and 21 were the less permeable compounds (Table 3).
3. Conclusions
Overall, it seems clear that the catalytic center of the sEH enzyme can accommodate polycycles of different sizes, ranging from the small bisnoradamantane moiety to the very large diamantane group. Notwithstanding this, it appears, particularly within the t-AUCB and AR9281 derivatives, that the replacement of the adamantane by larger polycyclic rings, such as the diamantanes, is better than the replacement by smaller ones.
Of note, although the present results highlight the interest of diamondoids as tools for investigating the size-limit of inhibitors,51 the low solubility and the high metabolic lability of these derivatives severely limits their potential use in medicinal chemistry.52
4. Experimental section
4.1. Chemistry
4.1.1. General
Commercially available reagents and solvents were used without further purification unless stated otherwise. Preparative normal phase chromatography was performed on a CombiFlash Rf 150 (Teledyne Isco) with pre-packed RediSep Rf silica gel cartridges. Thin-layer chromatography was performed with aluminum-backed sheets with silica gel 60 F254 (Merck, ref. 1.05554), and spots were visualized with UV light and 1% aqueous solution of KMnO4. Melting points were determined in open capillary tubes with a MFB 595010M Gallenkamp. 400 MHz 1H and 100.6 MHz 13C NMR spectra were recorded on a Varian Mercury 400 or on a Bruker 400 Avance III spectrometers. 500 MHz 1H NMR spectra were recorded on a Varian Inova 500 spectrometer. The chemical shifts are reported in ppm (δscale) relative to internal tetramethylsilane, and coupling constants are reported in Hertz (Hz). Assignments given for the NMR spectra of selected new compounds have been carried out on the basis of DEPT, COSY 1H/1H (standard procedures), and COSY 1H/13C (gHSQC and gHMBC sequences) experiments. IR spectra were run on Perkin-Elmer Spectrum RX I, Perkin-Elmer Spectrum TWO or Nicolet Avatar 320 FT-IR spectrophotometers. Absorption values are expressed as wave-numbers (cm−1); only significant absorption bands are given. High-resolution mass spectrometry (HRMS) analyses were performed with an LC/MSD TOF Agilent Technologies spectrometer. The elemental analyses were carried out in a Flash 1112 series Thermofinnigan elemental microanalyzator (A5) to determine C, H, N and S. The structure of all new compounds was confirmed by elemental analysis and/or accurate mass measurement, IR, 1H NMR and 13C NMR. The analytical samples of all the new compounds, which were subjected to pharmacological evaluation, possessed purity ≥95% as evidenced by their elemental analyses.
4.1.2. Diamantan-3-one (17)
Diamantane, 16 (600 mg, 3.19 mmol), was suspended in conc. H2SO4 96% (5 mL). The mixture was stirred at 75 °C for 10 h. The reaction mixture was cooled to room temperature and poured on ice. This aqueous solution was extracted with diethyl ether (3 × 50 mL). The combined organic phases were dried over anh. Na2SO4 and filtered. Evaporation of the organics gave a white solid (494 mg). This residue was dissolved in DCM (20 mL) 25 g of neutrum alumina were added and the solvent was evaporated obtaining a white solid. Then, hexane (25 mL) was added, the suspension was stirred for 5 min and was filtrated (×2). Diethyl ether (50 mL) was added, the suspension was stirred for 5 min and it was filtrated. Evaporation of the organics gave 17 as a white solid (440 mg, 69% yield). The spectroscopic data coincide with those described in the bibliography.53,54
4.1.3. Diamantane-3-amine (8)
Diamantan-3-one, 17 (583 mg, 2.88 mmol) was dissolved in IPA (6 mL), followed by the addition of AcONH4 (3.33 g, 43.22 mmol). The mixture was stirred at reflux for 1 h. Then, NaCNBH3 (1.26 g, 20.17 mmol) was added. The reaction mixture was stirred at reflux for 24 h. The dark solution was cooled down to room temperature and 10 N NaOH was added until basic pH to quench the reaction. This mixture was extracted with DCM (3 × 50 mL) and the combined organic phases were dried over anh. Na2SO4 and filtered. Evaporation of the organics gave a white solid (580 mg) which was dissolved in EtOAc and extracted with 2 N HCl. The aqueous layer was basified with 5 N NaOH until basic pH and extracted with EtOAc. The combined organic phases were dried over anh. Na2SO4 and filtered. Evaporation of the organics in vacuo gave 8 as a white solid (390 mg, 66% yield). 1H NMR (400 MHz, CDCl3) δ: 1.50 (dt, J = 12.8 Hz; J′ = 3.2 Hz, 1H), 1.61–1.83 (complex signal, 15H), 1.92–1.98 (complex signal, 2H), 2.91 (t, J = 2.8 Hz, 1H, 3-H). 13C NMR (100.6 MHz, CDCl3) δ: 26.4 (CH), 31.1 (CH), 31.7 (CH2), 32.4 (CH), 36.6 (CH), 37.0 (CH), 37.6, 37.7, 37.8, 38.83 and 38.0 (1 CH and 4 CH2), 43.9 (CH), 56.4 (CH). HRMS-ESI+ m/z [M+H]+ calcd for [C14H21N+H]+: 204.1747, found: 204.1753.
4.1.4. General procedure for the synthesis of the ureas 1 and 10–15
In a round-bottom flask equipped with a stir bar under nitrogen atmosphere the appropriate amine hydrochloride (1.2 mmol) was added to anh. DCM (~110 mM). To this suspension 2,3,4-trifluorophenyl isocyanate (1 mmol) followed by triethylamine (7 mmol) was added. The reaction mixture was stirred at room temperature overnight. Then the solvent was removed in vacuo and the resulting crude was purified by column chromatography.
4.1.5. 1-(1-Adamantyl)-3-(2,3,4-trifluorophenyl)urea (1)
From adamantan-1-amine hydrochloride (2·HCl) (162 mg) and following the general procedure a crude was obtained. Column chromatography (SiO2, Hexane/Ethyl Acetate mixture) followed by evaporation in vacuo of the appropriate fractions gave the urea 1 (280 mg, quantitative yield). The analytical sample was obtained by crystallization from methanol. The spectroscopic data coincide with those described in the bibliography.37
4.1.6. 1-(2-Adamantyl)-3-(2,3,4-trifluorophenyl)urea (10)
From adamantan-2-amine hydrochloride (3·HCl) (166 mg) and following the general procedure a crude was obtained. Column chromatography (SiO2, Hexane/Ethyl Acetate mixture) followed by evaporation in vacuo of the appropriate fractions gave the urea 10 (270 mg, 94% yield). The analytical sample was obtained by crystallization from EtOAc/pentane. The spectroscopic data were identical to those previously published.37
4.1.7. 1-[(3,7-Dimethyl(tricyclo[3.3.0.03,7]oct-1-yl)methyl]-3-(2,3,4-trifluorophenyl)urea (11)
From (3,7-dimethyltricyclo[3.3.0.03,7]octan-1-yl)methanamine hydrochloride (6·HCl) (50 mg) and following the general procedure a crude was obtained. Column chromatography (SiO2, Hexane/Ethyl Acetate mixture) followed by concentration in vacuo of the appropriate fractions gave the urea 11 (82 mg, 98% yield) as a white solid, mp 133–134 °C. IR (ATR) ν: 3323, 2952, 2881, 1637, 1621, 1570, 1510, 1474, 1292, 1244, 1177, 1045, 1001, 985, 809, 794, 755, 682, 653 cm−1. 1H NMR (500 MHz, CD3OD) δ: 1.17 (s, 6H, C3(7)-CH3), 1.32–1.38 (complex signal, 4H, 4(6)-Ha and 2(8)-Ha), 1.42 (d, J = 7.5 Hz, 2H, 2(8)-Hb), 1.59 (dd, J = 8 Hz, J′ = 3 Hz, 2H, 4(6)-Hb), 2.10 (t, J = 3 Hz, 1H, 5-H), 3.42 (s, 2H, CH2-N), 7.01 (m, 1H, 5′-H), 7.75 (m, 1H, 6′-H). 13C NMR (125.7 MHz, CD3OD) δ: 17.1 [CH3, C3(7)-CH3], 42.7 (CH, C5), 43.8 [CH2, CH2-N], 48.5 [C, C3(7)], 52.3 (C,C1), 54.9 [CH2, C4(6)], 57.2 [CH2, C2(8)], 112.3 (CH, dd, 2JC-F = 17.7 Hz, 3JC-F = 3.9 Hz, C5′), 116.7 (CH, C6′), 127.0 (C, d, 2JC-F = 6 Hz, C1′), 141.5 (C, dt, 1JC-F = 247 Hz, 2JC-F = 15 Hz, C3′), 143.6 (C, dd, 1JC-F = 246 Hz, 2JC-F = 12 Hz, C4′), 147.5 (C, dd, 1JC-F = 243 Hz, 2JC-F = 9 Hz, C2′), 157.8 (C, CO). MS (DIP), m/z (%); significant ions: 338 (M+, 1), 149 [(C11H17)+, 43], 148 (86), 147 [(C6H4F3N)+, 42], 136 (19), 135 [(C10H15)+, 100], 119 (18), 107 (56), 106 (15), 105 (15), 93 (42), 91 (28), 79 (16), 77 (16). Elemental analysis: Calculated for C18H21F3N2O: C 63.89, H 6.26, N 8.28. Found: C 63.83, H 6.52, N 8.26.
4.1.8. 1-(3,7-Dimethyl(tricyclo[3.3.0.03,7]oct-1-yl))-3-(2,3,4-trifluorophenyl)urea (12)
From 3,7-dimethyltricyclo[3.3.0.03,7]octan-1-amine hydrocloride (5·HCl) (61 mg) and following the general procedure a crude was obtained. Column chromatography (SiO2, Hexane/Ethyl Acetate mixture) followed by evaporation in vacuo of the appropriate fractions gave the urea 12 (50 mg, 47% yield) as a white solid, mp 174–175 °C. IR (ATR) ν: 3335, 2957, 2930, 2882, 2158, 2005, 1686, 1656, 1637, 1621, 1565, 1509, 1471, 1308, 1289, 1242, 1204, 1165, 1154, 1118, 1081, 1064, 1020, 1009, 964, 946, 816, 796, 719, 694, 678, 657 cm−1. 1H NMR (400 MHz, CDCl3) δ: 1.15 [s, 6H, 3(7)-CH3], 1.39 [dd, J = 8.4 Hz, J′ = 3.6 Hz, 2H, 4(6)-Ha], 1.64 [dd, J = 7.6 Hz, J′ = 3.6 Hz, 2H, 2(8)-Ha], 1.76 [dd, J = 8.4 Hz, J′ = 3.0 Hz, 2H, 4(6)-Hb], 1.82 [d, J = 7.6 Hz, 2H, 2(8)-Hb], 2.38 (t, J = 3.0 Hz, 1H, 5-H), 5.47 (broad s, 1H, 1-NH), 6.75 (broad s, 1H, 3-NH), 6.89 (m, 1H, 5′-H), 7.80 (m, 1H, 6′-H). 13C NMR (100.6 MHz, CDCl3) δ: 16.5 [CH3, C3(7)-CH3], 44.8 (CH, C5), 46.2 [C, C3(7)], 53.1 [CH2, C4(6)], 57.5 [CH2, C2(8)], 61.8 (C, C1), 111.4 (CH, dd, 2JC-F = 17.7 Hz, 3JC-F = 3.8 Hz, C5′), 115.0 (CH, t, 3JC-F = 5 Hz, C6′), 124.8 (C, dd, 2JC-F = 8 Hz, 3JC-F = 3.4 Hz, C1′), 139.7 (C, ddd, 1JC-F = 249 Hz, 2JC-F = 16.3 Hz, 2JC-F = 13.7 Hz, C3′), 142.1 (C, ddd, 1JC-F = 244 Hz, 2JC-F = 11.9 Hz, 3JC-F = 3.2 Hz, C4′), 141.4 (C, ddd, 1JC-F = 245 Hz, 2JC-F = 10 Hz, 3JC-F = 2.8 Hz, C2′), 154.6 (C, CO). MS (EI), m/z (%); significant ions: 324 (M+, 8), 268 (15), 148 (44), 147 [(C6H4F3N)+, 100], 146 (23), 136 (17), 134 (84), 122 (89), 121 (54), 120 (40), 119 (81), 110 (20), 109(51), 108 (50), 107 (28), 106 (16), 105 (21), 96 (17), 95 (70), 94 (54), 93 (52), 92 (18), 91 (44), 81 (17), 80 (18), 79 (37), 77 (33), 67 (24), 55 (16), 41 (23). Elemental analysis: Calculated for C17H19F3N2O: C 62.95, H 5.90, N 8.64. Found: C 63.12, H 6.17, N 8.48.
4.1.9. 1-(Tricyclo[3.3.0.03,7]oct-1-yl)-3-(2,3,4-trifluorophenyl)urea (13)
From tricyclo[3.3.0.03,7]octan-1-amine hydrochloride (4·HCl) (19 mg) and following the general procedure a crude was obtained. Column chromatography (SiO2, Hexane/Ethyl Acetate mixture) followed by evaporation in vacuo of the appropriate fractions gave the urea 13 (24 mg, 66% yield) as a white solid, mp 185–186 °C. IR (ATR) ν: 3331, 3105, 2970, 2943, 2894, 2159, 1656, 1640, 1620, 1563, 1510, 1467, 1318, 1288, 1244, 1204, 1171, 1107, 1076, 1065, 1017, 979, 823, 800, 764, 723, 710, 690, 668, 645, 620 cm−1. 1H NMR (400 MHz, CDCl3) δ: 1.51 [d, J = 8.8 Hz, 2H, 4(6)-Ha], 1.74 [complex signal, 6H, 4(6)-Ha, 2(8)-H2], 2.34 [broad s, 2H, 3(7)-H], 2.42 (m, 1H, 5-H), 5.42 (broad s, 1H, 1-NH), 6.67 (broad s, 1H, 3-NH), 6.90 (m, 1H, 5′-H), 7.82 (m, 1H, 6′-H). 13C NMR (100.6 MHz, CDCl3) δ: 32.8 [CH, C3(7)], 43.0 (CH, C5), 46.6 [CH2, C4(6)], 51.1 [CH2, C2(8)], 61.6 (C, C1), 111.4 (CH, dd, 2JC-F = 17.7 Hz, 3JC-F = 3.7 Hz, C5′), 115.1 (CH, t, 3JC-F = 5.6 Hz, C6′), 124.8 (C, dd, 2JC-F = 8 Hz, 3JC-F = 3.5 Hz, C1′), 139.7 (C, dt, 1JC-F = 245 Hz, 2JC-F = 15 Hz, C3′), 142.2 (C, dd, 1JC-F = 225 Hz, 2JC-F = 12 Hz, C4′), 146.5 (C, dd, 1JC-F = 246 Hz, 2JC-F = 10 Hz, Ar-C2′), 154.6 (C, CO). MS (DIP), m/z (%); significant ions: 296 (M+, 34), 268 (14), 267 (24), 254 (22), 147 [(C6H4F3N)+, 77], 146 (23), 119 (14), 95 (29), 94 (1 0 0), 82 (29), 81 (62), 80(20), 79(18). Elemental analysis: Calculated for C15H15F3N2O: C 60.81, H 5.10, N 9.45. Found: C 60.87, H 5.34, N 9.19.
4.1.10. 1-(Tricyclo[3.3.1.03,7]non-3-yl)-3-(2,3,4-trifluorophenyl)urea (14)
From tricyclo[3.3.1.03,7]nonyl-3-amine hydrochloride (7·HCl) (100 mg) and following the general procedure, a yellow solid was obtained (191 mg). Column chromatography (Hexane/Ethyl Acetate mixture) gave urea 14 as a white solid (52 mg, 57% yield), mp 192–193 °C. IR (ATR) ν: 661, 757, 798, 956, 1005, 1021, 1052, 1083, 1101, 1155, 1176, 1248, 1287, 1325, 1382, 1429, 1470, 1509, 1563, 1633, 1656, 2346, 2852, 2925, 3343 cm−1. 1H NMR (400 MHz, CDCl3) δ: 1.54–1.69 [complex signal, 4H, 9-H2 and 6(8)-Hax], 1.92 [dd, J = 10.0 Hz, J′ = 2.8 Hz, 2H, 2(4)-Hax], 2.01–2.10 [complex signal, 4H, 6(8)-Heq and 2(4)-Heq], 2.25 [broad singlet, 2H, 1(5)-H], 2.40 [tt, J = 6.8 Hz, J′ = 2.5 Hz, 1(7)-H], 7.00 (m, 1H, 5′-H), 7.69 (m, 1H, 6′-H). 13C NMR (100.6 MHz, CDCl3) δ: 35.9 (CH2, C9), 38.8 (CH, C1 and C5), 44.3 (CH2, C6 and C6), 44.8 (CH, C7), 49.9 (CH2, C2 and C4), 65.3 (C, C3), 112.2 (CH, dd, 2JC-F = 18 Hz, 3JC-F = 4 Hz, C5′), 116.8 (CH, C6′), 126.9 (C, dd, J = 3 Hz, J′ = 8 Hz, C1′), 139.9 (C, dt, 1JC-F = 247.4, 2JC-F = 14, C3′), 146–148 (complex signal, C4′ and C2′), 156.8 (C, CO). Elemental analysis: Calcd for C16H17F3N2O·0.25 MeOH: C 61.31, H 5.70, N 8.80. Found C 61.51, H 5.94, N 8.55.
4.1.11. 1-(Diamant-3-yl)-3-(2,3,4-trifluorophenyl)urea (15)
From diamantane-3-amine (8) (160 mg) and following the general procedure, a solid was obtained (291 mg). Column chromatography (Hexane/Dichloromethane mixture) gave the urea 15 (72 mg, 24% yield) as a white solid, mp 199–200 °C. IR (ATR) ν: 678, 806, 1002, 1025, 1088, 1210, 1249, 1289, 1467, 1513, 1564, 1623, 1671, 1862, 1933, 1997, 2107, 2198, 2357, 2413, 2903 cm−1. 1H NMR (400 MHz, CDCl3) δ: 1.55–1.84 (complex signal, 17 diamantane-H), 3.87 (m, 1H, 3 diamantane-H), 5.57 (broad s, 1H, 1-NH), 6.90 (m, 1H, 5′-H), 7.09 (broad s, 1H, 3-NH), 7.74 (m, 1H, 6′-H). 13C NMR (100.6 MHz, CDCl3) δ: 26.1 (CH), 30.1 (CH), 32.3 (CH), 32.7 (CH2), 36.3 (CH), 36.6 (CH), 37.2, 37.3, 37.35, 37.4 and 37.5 (1 CH and 4 CH2), 37.7 (CH2) 41.5 (CH, C4), 55.2 (CH, C3), 111.5 (CH, dd, 2JC-F = 18 Hz, 3JC-F = 4 Hz, C5′), 115.1 (CH, C6′), 125.0 (C, dd, 2JC-F = 8 Hz, 3JC-F = 3 Hz, C1′), 141.1 (C, dt, 1JC-F = 247, 2JC-F = 16, C3′), 146.5 (C, dm, 1JC-F = 246 Hz, C4′), 146.9 (C, dm, 1JC-F = 248 Hz, C2′), 154.6 (C, CO). Elemental analysis: Calcd for C21H23F3N2O·0.25 CH2Cl2: C 64.18, H 5.96, N 7.04. Found: C 64.40, H 6.35, N 6.71.
4.1.12. Tricyclo[3.3.1.03,7]nonyl-3-isocyanate (18)
Tricyclo[3.3.1.03,7]nonyl-3-amine hydrochloride (7·HCl) (750 mg, 4.33 mmol) was suspended in DCM (52 mL) and aq. NaHCO3 (22 mL) was added. Under argon atmosphere, the mixture was stirred and cooled to 4 °C on an ice bath. Immediately, trisphosgene (642 mg, 2.16 mmol) was added. The mixture was stirred at 4 °C for 30 min. The 2 phases were separated and the organic layer was washed with brine (2 × 30 mL). The organic phase was dried over anh. Na2SO4 and filtered. Evaporation in vacuo of the organics gave 18 as a yellowish oil (360 mg, 51% yield) which was used in the next step without further purification.
4.1.13. 4-((Trans-4-(3-(tricyclo[3.3.1.03,7]non-3-yl)ureido)cyclohexyl) oxy)benzoic acid (19)
Under argon atmosphere, tricyclo[3.3.1.03,7]nonyl-3-isocyanate (18) (250 mg, 1.53 mmol) was dissolved in anh. DCM (16 mL). 4-[(trans-4-aminocyclohexyl)oxy]benzoic acid55 hydrochloride (497 mg, 1.83 mmol) and Et3N (619 mg, 6.12 mmol) were added. The reaction mixture was stirred at 30 °C overnight. Water (60 mL) was added to the resulting mixture and two phases were separated. The aqueous phase was washed with DCM (2 × 50 mL) and acidified until pH = 2 with 5 N HCl. This acid solution was extracted with DCM (5 × 30 mL) and the organic layer was dried over anh. Na2SO4, filtered and evaporated. The residue was dissolved in EtOAc, washed with 2 N HCl, dried over anh. Na2SO4, filtered and evaporated. The organics were evaporated to afford a yellowish oil (70 mg, 12% yield). Urea 19 was obtained by crystallization from hot MeOH as white solid, mp 262–263 °C. IR (ATR) ν: 3377, 3334, 2926, 2862, 1683, 1628, 1605, 1556, 1508, 1456, 1421, 1382, 1326, 1304, 1248, 1163, 1129, 1119, 1096, 1008, 953, 902, 847, 775, 698, 636, 599 cm−1. 1H NMR (400 MHz, CD3OD) δ: 1.36 (dq, J = 3.2 Hz, J′ = 13.2 Hz, 2H, 3′(5′)-Hax], 1.51–1.66 [complex signal, 6H, 2′(6′)-Hax, 9″-H2 and 6″(8″)-Hax], 1.86 [dd, J = 10.0 Hz, J′ = 2.8 Hz, 2H, 2″(4″)-Hax], 1.98–2.05 [complex signal, 6H, 6″(8″)-Heq and 3′(5′)-Heq and 2″(4″)-Heq], 2.13 [dd, J = 4 Hz, J′ = 12.8 Hz, 2H, 2′(6′)-Heq], 2.22 [broad s, 2H, 1″(5″)-H], 2.34 (t, J = 6.8 Hz, 1H, 7″-H), 4.39 (m, 1H, 1′-H), 6.96 [d, J = 9.2 Hz, 2H, 3(5)-H], 7.93 [d, J = 8.8 Hz, 2H, 2(6)-H]. 13C NMR (100.6 MHz, CD3OD) δ: 29.1 [CH2, C2′(6′)], 29.6 [CH2, C3′(5′)], 33.9 (CH2, C9″), 36.7 [CH, C1″(5″)], 42.3 [CH2, C6″(8″)], 42.9 (CH, C7″), 46.8 (CH, C4′), 48.2 [CH2, C2″(4″)], 63.3 (C, C3″), 74.0 (CH, C1′), 114.2 [CH, C3(5)], 121.2 (C, C1), 130.6 [CH, C2(6)], 157.9, (C, CO), 161.3 (C, C4), 166.5 (CO2H). HRMS-ESI− m/z [M−H]− calcd for [C23H30N2O4−H]−: 397.2133, found: 397.2147.
4.1.14. Diamantane-3-isocyanate (20)
Triphosgene (110 mg, 0.368 mmol) was added in a single portion to a solution diamantane-3-amine (8) (150 mg, 0.73 mmol) in DCM (10.5 mL) and saturated NaHCO3 solution (4.5 mL). The resulting biphasic mixture was stirred at room temperature for 30 min. Then, the two phases were separated and the organic layer was washed with brine, dried over anh. Na2SO4 and filtered. Evaporation in vacuo provided the isocyanate 20 as a white solid (152 mg, 90% yield), which was used in the next step without further purification.
4.1.15. 4-((Trans-4-(3-(diamantan-3-yl)ureido)cyclohexyl)oxy)benzoic acid (21)
4-[(Trans-4-aminocyclohexyl)oxy]benzoic acid hydrochloride55 (196 mg, 0.720 mmol) is dissolved in DMF (5 mL) and diamantane-3-isocyanate (20) (150 mg, 0.65 mmol) was added followed by Et3N (145 mg, 1.44 mmol). The reaction mixture was stirred at 50 °C for 3 days. The suspension was filtrated and the solvent was evaporated to obtain a brown solid (315 mg) which was dissolved in DCM and washed with 2 N HCl (2 × 20 mL). The organic layer was dried over anh. Na2SO4, filtered and evaporated. The resulting residue was crystallized from hot DCM affording the urea 21 (111 mg, 37% yield) as a white solid, mp 229–230 °C. IR (ATR) ν: 633, 695, 770, 845, 1031, 1052, 1088, 1163, 1243, 1312, 1504, 1568, 1599, 1710, 1956, 2020, 2237, 2346, 2496, 2868, 2904 cm−1. 1H NMR (400 MHz, CD3OD) δ: 1.36 (dq, J = 3.2 Hz, J′ = 13.2 Hz, 2H, 3′(5′)-Hax], 1.52–1.63 [complex signal, 3H, 2′(6′)-Hax and 1 diamantane-H], 1.67–1.91 (complex signal, 17 diamantane-H), 2.02 [dd, J = 4.4 Hz, J′ = 13.2 Hz, 2H, 3′(5′)-Heq], 2.13 [dd, J = 3.6 Hz, J′ = 13.2 Hz, 2H, 2′(6′)-Heq], 3.58 (m, 1H, 4-H′), 3.73 (t, J = 2.8 Hz, 1H, 3″-H), 4.39 (m, 1H, 1′-H), 6.95 [d, J = 8.8 Hz, 2H, 3(5)-H], 7.94 [d, J = 8.8 Hz, 2H, 2(6)-H]. 13C NMR (100.6 MHz, CD3OD) δ: 27.7 (CH), 31.1 [CH2, C2′(6′)], 31.7 [CH2, C3′(5′)], 31.8 (CH), 33.4 (CH), 33.6 (CH2), 38.0 (CH), 38.2, 38.6, 38.74, 38.77, 38.93, 38.95, 39.0, 43.3 (CH), 48.9 (CH, C4′), 55.2 (CH, C3″), 76.0 (CH, C1′), 111.4 (C, C1), 116.1 [CH, C3(5)], 132.8 [CH, C2(6)], 159.9, (C, CO), 163.0 (C, C4), 170.3 (CO2H). Elemental analysis: Calcd for C28H36N2O4·0.1 CH2Cl2: C 71.34, H 7.71, N 5.92. Found: C 71.36, H 7.85, N 5.70.
4.1.16. N-(1-Acetylpiperidin-4-yl)-1H-imidazole-1-carboxamide (23)
N,N′-carbonyldiimidazole (400 mg, 2.46 mmol) was suspended in anh. 1,2-dichloroethane (15 mL) under nitrogen. Then 1-acetyl-4-aminopiperidine (22) (250 mg, 1.76 mmol) was added and the reaction mixture was heated to 50 °C for 21 h. With an external ice bath, the mixture was cooled down for 30 min. The resulting solid was collected by filtration in vacuo and washed with 1,2-DCE (20 mL) affording 23 (312 mg, 75% yield) as a white solid, mp 191–193 °C. IR (ATR) ν: 3216, 3118, 3038, 2918, 2342, 2074, 1709, 1613, 1542, 1479, 1463, 1441, 1369, 1358, 1320, 1281, 1272, 1233, 1195, 1137, 1111, 1090, 1068, 1053, 1001, 984, 974, 916, 902, 859, 799, 748, 652 cm−1. 1H NMR (400 MHz, CDCl3) δ: 1.37 (complex signal, 2H, 3-Hax, 5-Hax), 1.99 (dm, J = 12.8 Hz, J′ = 4 Hz, 1H) and 2.21 (dm, J = 12.8 Hz, J′ = 4 Hz, 1H) (3′-Heq and 5′-Heq), 2.09 (s, 3H, COCH3), 2.69 (ddd, J = 13 Hz, J′ = 2.6 Hz, 1H) and 3.21 (ddd, J = 13 Hz, J′ = 2.6 Hz, 1H) (2′-Hax and 6′-Hax), 3.86 (dm, J = 13.6 Hz, 1H) and 4.67 (dm, J = 13.6 Hz, 1H) (2′- H eq and 6′-Heq), 4.10 (m, 1H, 4′-H), 7.06 (dd, J = 1.6 Hz, J′ = 0.8 Hz, 1H, 4-H), 7.29 (broad d, J = 7.6 Hz, 1H, NH), 7.60 (dd, J = 1.6 Hz, J′ = 1.2 Hz, 1H, 5-H), 8.29 (dd, J = 1.2 Hz, J′ = 0.8 Hz, 1H, 2-H). 13C NMR (100.6 MHz, CDCl3) δ: 21.5 (CH3, COCH3), 31.4 and 33.1 (CH2, C3′ and C5′), 40.9 and 45.6 (CH2, C2′ and C6′), 48.2 (CH, C4′), 116.2 (CH, C5), 130.3 (CH, C4), 136.2 (CH, C2), 148.5 (C, NHCNH), 169.2 (C, COCH3). MS (DIP), m/z (%); significant ions: 169 (10), 168 (100), 153 (19), 126 (53), 125 (31), 85 (19), 84 (42), 83 (20), 82 (23), 81 (21), 68 (98), 57 (40), 56 (56), 55 (16). HRMS-ESI+ m/z [M+H]+ calcd for [C11H16N4O2+H]+: 237.1346, found: 237.1345.
4.1.17. 1-(1-Acetylpiperidin-4-yl)-3-(3,7-dimethyl(tricyclo[3.3.0.03,7] octa-1-yl)urea (24)
In a round bottom flask equipped with a condenser apparatus and magnetic stirrer a solution of 3,7-dimethyltricyclo[3.3.0.03,7]octan-1-amine hydrochloride (5·HCl) (68 mg, 0.36 mmol) in chloroform (5 mL) was prepared, to which was added N-(1-acetylpiperidin-4-yl)-1H-imidazole-1-carboxamide (23) (172 mg, 0.73 mmol) followed by triethylamine (0.06 mL, 0.40 mmol). The solution was heated to 50 °C for 25 h, whereupon the reaction mixture was tempered to room temperature and evaporated in vacuo to dryness (384 mg). Purification by column chromatography (SiO2, Dichloromethane/Methanol mixture) afforded 24 (90 mg, 77% yield) as a white solid, mp 165–167 °C. IR (ATR) ν: 3359, 3244, 2947, 2878, 2170, 2034, 1960, 1613, 1556, 1477, 1443, 1371, 1318, 1264, 1227, 1151, 1096, 1033, 978, 717, 639 cm−1. 1H NMR (400 MHz, CDCl3) δ: 1.11 [s, 6H, 3′(7′)-CH3], 1.22 [complex signal, 2H, 2(6)-Ha], 1.34 [dd, J = 8.2 Hz, J′ = 3.4 Hz, 2H, 4′(6′)-Ha], 1.54 [dd, J = 7.4 Hz, J′ = 3.4 Hz, 2H, 2′(8′)-Ha], 1.70 [dd, J = 8.2 Hz, J′ = 2.6 Hz, 2H, 4′(6′)-Hb], 1.75 [d, J = 7.6 Hz, 2H, 2′(8′)-Hb], 1.90 and 2.03 (complex signal, 2H, 3-Hax and 5-Hax), 2.07 (s, 3H, COCH3), 2.27 (t, J = 2.6 Hz, 1H, 5′-H), 2.75 (dt, J = 14.0 Hz, J′ = 2.8 Hz, 1H, 2-Hax or 6-Hax), 3.14 (dt, J = 11.2 Hz, J′ = 2.8 Hz, 2H, 6-Hax or 2-Hax), 3.73 (broad d, J = 13 Hz, 2H, 6-Heq or 2-Heq), 3.83 (m, 1H, 4-H), 4.42 (broad d, J = 13 Hz, 2H, 2-Heq or 6-Heq), 4.79 (d, J = 7.6 Hz, 1H, 1-NH), 5.18 (broad s, 1H, 3-NH). 13C NMR (100.6 MHz, CDCl3) δ: 16.5 [CH3, C3′(7′)-CH3], 21.4 (CH3, COCH3), 33.6 [CH2, C3(5)], 40.6 (CH2, C2), 44.7 (CH, C5′), 45.3 (CH2, C6), 46.1 [C, C3′(7′)], 46.9 (CH, C4), 53.2 [CH2, C4′(6′)], 57.6 [CH2, C2′(8′)], 61.7 (C, C1′), 157.4 (C, CO urea), 169.0 (C, COCH3). MS (DIP), m/z (%); significant ions: 278 (10), 277 (58), 263 (20), 178 (10), 169 (25), 151 (18), 150 (22), 148 (25), 143 (100), 136 (26), 135 (43), 134 (31), 127 (16), 126 (23), 125 (17), 123 (11), 122 (86), 121 (29), 119 (25), 110 (22), 109 (86), 108 (48), 96 (18), 95 (62), 94 (29), 93 (16), 91 (15), 84 (31), 83 (16), 82 (33), 80 (11), 79 (12), 77 (11), 67 (11), 57 (13), 56 (25), 55 (17). Elemental analysis: Calculated for C18H29N3O2: C 67.68, H 9.15, N 13.15. Calculated for C18H29N3O2·1.0 H2O: C 64.07, H 9.26, N 12.45. Found: C 64.00, H 9.31, N 12.40.
4.1.18. 1-(1-Acetylpiperidin-4-yl)-3-(tricyclo[3.3.1.03,7]non-3-yl)urea (25)
Under argon atmosphere, tricyclo[3.3.1.03,7]nonane-3-isocyanate (18) (360 mg, 2.20 mmol) was dissolved in anh. DCM (10 mL). 1-acetyl-4-aminopiperidine (375 mg, 2.64 mmol) and Et3N (445 mg, 4.40 mmol) were added. The reaction mixture was stirred at room temperature overnight. The solvent was removed in vacuo and the residue was dissolved in EtOAc and washed with 2 N HCl. The organics were dried over anh. Na2SO4, filtered and evaporated in vacuo affording a white yellowish solid which was washed with acetone and EtOAc affording the urea 25 as a yellowish solid (240 mg, 36% yield), mp 164–165 °C. IR (ATR) ν: 638, 705, 783, 860, 904, 974, 992, 1059, 1139, 1230, 1269, 1318, 1361, 1429, 1555, 1620, 1659, 2351, 2919, 3328 cm−1. 1H NMR (400 MHz, CDCl3) δ: 1.16–1.29 (complex signal, 2H, 4-Hax and 5-Hax), 1.47–1.61 [complex signal, 4H, 9′-H2 and 6′(8′)-Hax], 1.80 [dd, J = 10.0 Hz, J′ = 2.8 Hz, 2H, 2′(4′)-Hax], 1.84–2.01 [complex signal, 6H, 6′(8′)-Heq, 2′(4′)-Heq, 4-Heq and 5-Heq], 2.07 (s, 3H, 8-H), 2.23 [broad singlet, 2H, 1′(5′)-H], 2.34 (t, J = 6.8 Hz, 1H, 7′-H), 2.74 (dt, J = 11.2 Hz, J′ = 2.4 Hz, 1H, 2-Hax or 6-Hax), 3.13 (dt, J = 12 Hz, J′ = 2.4 Hz, 1H, 6-Hax or 2-Hax), 3.71–3.85 (complex signal, 2H, 2-Heq or 6-Heq and 4-H), 4.43 (d, J = 13.6 Hz, 1H, 6-Heq or 2-Heq), 4.89 (d, J = 8.0 Hz, 1H, NH), 5.11 (s, 1H, NH). 13C NMR (100.6 MHz, CDCl3) δ: 21.4 (CH3, COCH3), 32.5 (CH2, C4 or C5), 33.7 (CH2, C5 or C4), 34.8 (CH2, C9′), 37.3 [CH, C1′(5′)], 40.7 (CH2, C2 or C6), 43.4 [CH2, C6′(8′)], 43.7 (CH, C7′), 45.4 (CH2, C6 or C2), 46.7 (CH, C4), 49.30 and 49.32 (CH2, C2′ and C4′), 64.1 (C, C3′), 157.1 (CO, urea), 169.0 (CO, COCH3). Elemental analysis: Calcd for C17H27N3O2·0.15C5H12: C 67.42, H 9.18, N 13.29. Found: C 66.38, H 9.00, N 13.05.
4.1.19. 1-(1-Acetylpiperidin-4-yl)-3-(diamant-3-yl)urea (26)
Diamantane-3-isocyanate (20) (155 mg, 0.67 mmol) was dissolved in DCM (3 mL) and 1-acetyl-4-aminopiperidine (115 mg, 0.811 mmol) dissolved in DCM (2 mL) was added. The mixture was stirred at room temperature overnight. Evaporation of the solvent gave a white solid (272 mg). Column chromatography (Dichloromethane/Methanol mixtures) gave the urea 26 (160 mg, 65% yield) as a white solid, mp 230–231 °C. IR (ATR) ν: 669, 727, 770, 808, 862, 917, 989, 1047, 1136, 1240, 1319, 1364, 1453, 1560, 1629, 1794, 1855, 1893, 1944, 1977, 2051, 2102, 2153, 2209, 2270, 2352, 2418, 2545, 2596, 2734, 2877, 3020, 3071, 3275, 3316, 3494, 3566, 3688 cm−1. 1H NMR (400 MHz, CD3OD) δ: 1.32 [complex signal, 2H, 3(5)-Hax], 1.66–1.82 (complex signal, 16H, diamantane-H), 1.85–1.98 (complex signal, 4H, 3-Heq, 5-Heq, 2 diamantane-H), 2.10 (s, 3H, COCH3), 2.91 (dt, J = 11.2 Hz, J′ = 2.8 Hz, 1H, 2-Hax or 6-Hax), 3.25 (dt, J = 11.2 Hz, J′ = 3.2 Hz, 2H, 6-Hax or 2-Hax), 3.71–3.78 (complex signal, 2H, 4-H and 1′-H), 3.85 (dt, J = 14 Hz, J′ = 2.4 Hz, 2H, 6-Heq or 2-Heq), 4.29 (dt, J = 13 Hz, J′ = 2.8 Hz, 2H, 2-Heq or 6-Heq). 13C NMR (100.6 MHz, CD3OD) δ: 21.2 (CH3, COCH3), 27.7 (CH), 31.8 (CH), 33.3 (CH2, C3 or C5), 33.4 (CH2), 33.6 (CH2), 34.1 (CH2, C5 or C3), 38.0, 38.1, 38.6, 38.7 (2 carbon), 38.91, 38.94 and 39.0 (3 CH2 and 5 CH, diamantane signals), 41.6 (CH2, C2 or C6), 43.3 (CH, C4′), 46.3 (CH2, C6 or C2), 47.7 (CH, C4), 55.9 (CH, C3′), 159.7 (C, CO urea), 171.5 (C, COCH3). HRMS-ESI+ m/z [M+H]+ calcd for [C22H33N3O2+H]+: 372.2646, found: 372.2644.
4.1.20. Diamantane-4-isocyanate (27)
Diamantane-4-amine hydrochloride (9·HCl) (110 mg, 0.458 mmol) was suspended in DCM (2 mL) and aq. NaHCO3 was added, followed by triphosgene (68 mg, 0.23 mmol). The biphasic mixture was stirred at room temperature for 30 min. The two phases were separated and the organic layer was washed with brine. The organics were dried over anh. Na2SO4, filtered and evaporated until 1 mL. The solution of isocyanate (27) in DCM was used in the next step without further purification.
4.1.21. 1-(1-Acetylpiperidin-4-yl)-3-(diamant-4-yl)urea (28)
To the solution of diamantane-4-isocyanate (27) (105 mg, 0.46 mmol) in DCM (1 mL) from the previous step is added 1-acetyl-4-aminopiperidine hydrochloride (22·HCl) (98 mg, 0.55 mmol) and DCM (1 mL), followed by Et3N. The mixture was stirred at room temperature overnight. DCM was added to the mixture and it was washed with 2 N HCl (30 mL). The organics were dried over anh. Na2SO4, filtered and evaporated to obtain a residue (48 mg). Column chromatography (Dichloromethane/Methanol mixture) gave the desired urea (28) (33 mg, 21% overall yield) as a beige solid, mp 195–196 °C. IR (ATR): ν: 3364, 2906, 2881, 2847, 1686, 1601, 1550, 1480, 1462, 1444, 1429, 1374, 1348, 1322, 1305, 1267, 1221, 1138, 1105, 1048, 1002, 986, 976, 918, 613, 597, 576 cm−1. 1H NMR (400 MHz, CDCl3) δ: 1.19 (complex signal, 2H, 3-Hax and 5-Hax), 1.69–1.78 (complex signal, 10H, diamantane-H), 1.83–1.94 (complex signal, 11H, 3-Heq, 5-Heq, 9 diamantane-H), 2.08 (s, 3H, COCH3), 2.73 (dt, J = 11.6 Hz, J′ = 3.2 Hz, 1 H, 2-Hax or 6-Hax), 3.13 (dt, J = 11.6 Hz, J′ = 3.2 Hz, 2H, 6-Hax or 2-H ax), 3.71–3.85 (complex signal, 2H, 4-H and 6-Heq or 2-Heq), 4.36 (broad s, NH, urea), 4.42–4.50 (complex signal, 2H, 2-Heq or 6-Heq and NH). 13C NMR (100.6 MHz, CDCl3) δ: 21.4 (CH3, COCH3), 25.6 (CH, C9′), 32.4 (CH2, C3 or C5), 33.6 (CH2, C5 or C3), 36.6 (CH), 37.4 (CH2), 38.7 (CH), 40.7 (CH2, C2 or C6), 43.0 (CH2), 45.4 (CH2, C6 or C2), 46.9 (CH, C4), 49.8 (C, C4′), 156.6 (C, CO urea), 169.0 (C, COCH3). HRMS-ESI+ m/z [M+H]+ calcd for [C22H33N3O2+H]+: 372.2646, found: 372.2657.
4.2. Solubility
A 10 mM stock solution of the compound was serially diluted in 100% DMSO and 1 μL of this solution was added to a 384-well UV-transparent plate (Greiner) containing 99 μL of PBS. The plate was incubated at 37 °C for 2 h and the light scattering was measured in a Nephelostar Plus reader (BMG LABTECH). The data was fitted to a segmented linear regression for measuring the compound solubility.
4.3. Microsomal stability
The human microsomes employed were purchased from Tebu-Xenotech. The compound was incubated at 37 °C with the microsomes in a 50 mM phosphate buffer (pH = 7.4) containing 3 mM MgCl2, 1 mM NADP, 10 mM glucose-6-phosphate and 1 U/mL glucose-6-phosphate-dehydrogenase. Samples (75 μL) were taken from each well at 0, 10, 20, 40 and 60 min and transferred to a plate containing 4 °C 75 μL acetonitrile and 30 μL of 0.5% formic acid in water were added for improving the chromatographic conditions. The plate was centrifuged (46,000g, 30 min) and supernatants were taken and analyzed in a UPLC-MS/MS (Xevo-TQD, Waters) by employing a BEH C18 column and an isocratic gradient of 0.1% formic acid in water: 0.1% formic acid acetonitrile (60:40). The metabolic stability of the compounds was calculated from the logarithm of the remaining compounds at each of the time points studied.
4.4. Permeability
The Caco-2 cells were cultured to confluency, trypsinized and seeded onto a filter transwell inserted at a density of ~10,000 cells/well in DMEM cell culture medium. Confluent Caco-2 cells were subcultured at passages 58–62 and grown in a humidified atmosphere of 5% CO2 at 37 °C. Following an overnight attachment period (24 h after seeding), the cell medium was replaced with fresh medium in both the apical and basolateral compartments every other day. The cell monolayers were used for transport studies 21 days post seeding. The monolayer integrity was checked by measuring the transepithelial electrical resistance (TEER) obtaining values ≥500 Ω/cm2. On the day of the study, after the TEER measurement, the medium was removed and the cells were washed twice with pre-warmed (37 °C) Hank’s Balanced Salt Solution (HBSS) buffer to remove traces of medium. Stock solutions were made in dimethyl sulfoxide (DMSO), and further diluted in HBSS (final DMSO concentration 1%). Each compound and reference compounds (Colchicine, E3S) were all tested at a final concentration of 10 μM. For A → B directional transport, the donor working solution was added to the apical (A) compartment and the transport media as receiver working solution was added to the basolateral (B) compartment. For B → A directional transport, the donor working was added to the basolateral (B) compartment and transport media as receiver working solution was added to the apical (A) compartment. The cells were incubated at 37 °C for 2 h with gentle stirring.
At the end of the incubation, samples were taken from both donor and receiver compartments and transferred into 384-well plates and analyzed by UPLC-MS/MS. The detection was performed using an ACQUITY UPLC/Xevo TQD System. After the assay, Lucifer yellow was used to further validate the cell monolayer integrity, cells were incubated with LY 10 μM in HBSS for 1 h at 37 °C, obtaining permeability (Papp) values for LY of ≤10 nm/s confirming the well-established Caco-2 monolayer.
Supplementary Material
Acknowledgments
This work was funded by the Spanish Ministerio de Economía, Industria y Competitividad (Grant SAF2017-82771-R to S.V.), the European Regional Development Fund (ERDF), the Xunta de Galicia (GRC2014/011 and ED431C2018-21) and the Generalitat de Catalunya (2017 SGR 106). S.C., E.V. and R.L. acknowledge PhD fellowships from the Universitat de Barcelona (APIF grant), the Institute of Biomedicine of the University of Barcelona (IBUB), and the Spanish Ministerio de Educacion, Cultura y Deporte (FPU grant), respectively. This work was supported in part by the NIEHS Grant R01 ES002710 (to B.D.H.) and NIEHS Superfund Research Program P42 ES004699.
Footnotes
Appendix A. Supplementary data
Supplementary data (1H and 13C NMR spectra of the compounds) to this article can be found online at https://doi.org/10.1016/j.bmc.2019.115078.
References
- 1.Meirer K, Steinhilber D, Proschak E Basic Clin Pharmacol Toxicol 2014;114:83–91. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasekharan NV, Simmons DL Genome Biol 2004;5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrignani P, Patrono C Biochim Biophys Acta 1851;2015:422–432. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn H, Banthiya S, van Leyen K Biochim Biophys Acta 2015;185:308–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeldin DC J Biol Chem 2001;276:36059–126062. [DOI] [PubMed] [Google Scholar]
- 6.Christmas P Adv Pharmacol 2015;74:163–192. [DOI] [PubMed] [Google Scholar]
- 7.Imig JD. Hypertension 2015;65:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Mäki-Petäjä K, Cheriyan J, McEniery C, Wilkinson IB Br J Clin Pharmacol 2015;80:28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imig JD Adv Pharmacol 2016;77:105–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan F, Roman RJ J Am Soc Nephrol 2017;28:2845–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamieson KL, Endo T, Darwesh AM, Samokhvalov V, Seubert JM Pharmacol Ther 2017;179:47–83. [DOI] [PubMed] [Google Scholar]
- 12.Arand M, Grant DF, Beetham JK, Friedberg F, Oesch F, Hammock BD FEBS Lett 1994;338:251–256. [DOI] [PubMed] [Google Scholar]
- 13.Oesch F Xenobiotica 1973;3:305–340. [DOI] [PubMed] [Google Scholar]
- 14.Harris TR, Hammock BD Gene 2013;526:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming I Pharmacol Rev 2014;66:1106–1140. [DOI] [PubMed] [Google Scholar]
- 16.Imig JD, Hammock BD Nat Rev Drug Discov 2009;8:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZH, Davis BB, Jiang DQ, Zhao TT, Xu DY Curr Vasc Pharmacol 2013;11:105–111. [PubMed] [Google Scholar]
- 18.Kodani SD, Hammock BD Drug Metab Dispos 2015;43:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillarisetti S, Khanna I Drug Discov Today 2015;20:1382–1390. [DOI] [PubMed] [Google Scholar]
- 20.Wagner KM, McReynolds CB, Schmidt WK, Hammock BD Pharmacol Ther 2017;180:62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarriello S, Tuazon JP, Corey S, et al. Prog Neurobiol 2019;172:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez GA, Morisseau C, Hammock BD, Christianson DW Biochemistry 2004;43:4716–4723. [DOI] [PubMed] [Google Scholar]
- 23.Jones PD, Tsai H-J, Do ZN, Morisseau C, Hammock BD Bioorg Med Chem Lett 2006;16:5212–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen HC, Hammock BD J Med Chem 2012;55:1789–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim I-H, Nishi K, Kasagami T, et al. Bioorg Med Chem Lett 2012;22:5889–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S-X, Cao B, Morisseau C, Jin Y, Long Y-Q, Hammock BD Med Chem Commun 2012;3:379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim I-H, Lee I-H, Nishiwaki H, Hammock BD, Nishi K Bioorg Med Chem 2014;22:1163–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burmistrov V, Morisseau C, Lee KSS, et al. Bioorg Med Chem Lett 2014;24:2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burmistrov V, Morisseau C, Danilov D, et al. Bioorg Med Chem Lett 2015;25:5514–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim I-H, Park Y-K, Nishiwaki H, Hammock BD, Nishi K Bioorg Med Chem 2015;23:7199–7210. [DOI] [PubMed] [Google Scholar]
- 31.Burmistrov VV, Butov GM, Karlov DS, et al. Russ J Bioorg Chem 2016;42:404–414. [Google Scholar]
- 32.Burmistrov V, Morisseau C, Harris TR, Butov GM, Hammock BD Bioorg Chem 2018;76:510–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burmistrov V, Morisseau C, Pitushkin D, et al. Bioorg Med Chem Lett 2018;28:2302–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burmistrov VV, Butov GM Russ J Org Chem 2018;54:1307–1312. [Google Scholar]
- 35.D’yachenko VS, Danilov DV, Shkineva TK, Vatsadze IA, Burmistrov VV, Butov GM Chem Heterocycl Compd 2019;55:129–134. [Google Scholar]
- 36.Tsai H-J, Hwang SH, Morisseau C, et al. Eur J Pharm Sci 2010;40:222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang SH, Tsai H-J, Liu J-Y, Morisseau C, Hammock BD J Med Chem 2007;50:3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown JR, North EJ, Hurdle JG, et al. Bioorg Med Chem 2011;19:5585–5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoover JRE. US3496228; 1970.
- 40.Camps P, Duque MD, Vázquez S, et al. Bioorg Med Chem 2008;16:9925–9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones PD, Wolf NM, Morisseau C, Whetstone P, Hock B, Hammock BD Anal Biochem 2005;343:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang SH, Morisseau C, Do Z, Hammock BD Bioorg Med Chem Lett 2006;16:5773–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gómez GA, Morisseau C, Hammock BD, Christianson DW Protein Sci 2006;15:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amano Y, Yamaguchi T, Tanabe E Bioorg Med Chem 2014;22:2427–2434. [DOI] [PubMed] [Google Scholar]
- 45.Liu J-Y, Tsai H-J, Morisseau C, et al. Biochem Pharmacol 2015;98:718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodek P, Janščák P, Anzenbacher P, Burkhard J, Janků J, Vodička L Xenobiotica 1988;18:1109–1118. [DOI] [PubMed] [Google Scholar]
- 47.Hodek P, Burkhard J, Janků J Gen Physiol Biophys 1995;14:225–239. [PubMed] [Google Scholar]
- 48.Bořek-Dohalská L, Hodek P, Stiborová M Collect Czech Chem Commun 2000;65:122–132. [Google Scholar]
- 49.Hodek P, Bořek-Dohalská L, Sopko B, et al. J Enzyme Inhib Med Chem 2005;20:25–33. [DOI] [PubMed] [Google Scholar]
- 50.Daina A, Michielin O, Zoete V Sci Rep 2017;7:42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meggers E Angew Chem Int Ed 2011;50:2442–2448. [DOI] [PubMed] [Google Scholar]
- 52.Schwertfeger H, Fokin AA, Schreiner PR Angew Chem Int Ed 2008;47:1022–1036. [DOI] [PubMed] [Google Scholar]
- 53.Courtney T, Johnston DE, McKervey MA, Rooney JJ J Chem Soc Perkin Trans 1972;1:2691–2696. [Google Scholar]
- 54.Gund TM, Nomura M, Schleyer PVR J Org Chem 1974;39:2987–2994. [Google Scholar]
- 55.Xier L, Ochterski JW, Gao Y, et al. WO 2007016496; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.