Abstract
Background:
Wide variation exists in the timing of atopic dermatitis (AD) disease onset among children. Distinct trajectories of early-onset, mid-onset, and late-onset AD have been previously described.
Objective:
To evaluate longitudinal disease control and persistence with respect to AD onset age.
Methods:
A cohort study was performed using the Pediatric Eczema Elective Registry, a prospective observational cohort of subjects with childhood-onset AD. AD control and persistence are assessed biannually for up to 10 years.
Results:
8,015 subjects with 41,934 person-years of follow-up were included. In longitudinal analyses using generalized linear latent and mixed modelling, older AD onset age was associated with better disease control and less persistent AD. For each additional year of age at AD onset, the adjusted odds ratios for poorer AD control and for persistent AD were 0.93 (95% CI 0.91–0.94) and 0.84 (95% CI 0.80–0.88), respectively. Differences in AD control and persistence among early-, mid-, and late-onset AD were most pronounced from early adolescence onward.
Limitations:
Misclassification bias may arise from using self-reported onset age data. Attrition and missing data in longitudinal studies may introduce bias.
Conclusion:
Early-, mid-, and late-onset pediatric AD appear to be clinically distinct subtypes of the disease.
Keywords: atopic dermatitis, eczema, disease control, disease persistence, early onset, late onset, epidemiology, prognosis
Capsule summary
• The disease course of pediatric atopic dermatitis varies significantly by the timing of its onset, with earlier onset being associated with more longstanding and poorly controlled disease
• Age of disease onset is a useful parameter for risk stratifying and counseling patients with atopic dermatitis
Introduction
Atopic dermatitis (AD) affects up to 20% of children and waxes and wanes in its severity.1, 2 Traditionally, most children were thought to ‘outgrow’ the disease but evidence suggests that AD often persists into adulthood.3–5 While AD most commonly begins in infancy, it may not arise until later in childhood or adolescence.3, 6 This wide variation in timing of disease onset has led to a distinction between early-onset and late-onset AD.5, 7, 8 Our previous work has shown that later onset AD is associated with a lower risk for asthma and seasonal allergies compared to infantile-onset AD.9 Several phenotypes of AD, as distinguished by their temporal disease trajectories, have also recently been identified in European birth cohorts.10, 11 Early-onset, mid-onset, and late-onset AD appear to differ in the presence of active disease over time; however, it is unknown if these groups also differ in the severity of AD. The objective of our study was to evaluate the impact of AD onset age on both longitudinal disease control and persistence in a U.S. cohort.
Methods
We conducted a cohort study using the Pediatric Eczema Elective Registry (PEER), a prospective pediatric AD cohort in the U.S. PEER was designed as a post-marketing safety evaluation of malignancy risk associated with pimecrolimus, a topical calcineurin inhibitor for treating mild to moderate AD. Details of PEER have been previously reported.12 All subjects were 2–17 years old at time of registry enrollment and had a physician-confirmed AD diagnosis. All had used pimecrolimus for ≥6 weeks in the 6-month period preceding enrollment; however, subjects were not required to continue pimecrolimus, and many did not.13 Informed written consent was obtained from subjects upon registry enrollment. The current study was granted exempt status by the University of Pennsylvania Institutional Review Board.
Subjects enrolled in PEER between November 2004 and September 2018 were included. At the time of enrollment (i.e. baseline), subjects or their caregivers provided information on demographics and AD history and treatment, including the age of AD onset. At baseline and every six months thereafter, subjects were surveyed about their AD disease control and treatment use in the preceding 6-month period. Subjects in PEER are followed for up to 10 years.
Age of AD onset was reported by the subject or caregiver as 0–6 months, 6–12 months, or the exact age in years if greater than 12 months. In the primary analysis, AD onset age was considered as a continuous variable, with 0–6 months and 6–12 months being treated as 3 and 9 months, respectively. In secondary analyses, onset age was categorized into ≤2, 3–7, and 8–17 years old, i.e. early-onset, mid-onset, and late-onset AD, respectively. Early-onset AD has traditionally been defined as that beginning before age 2.3, 5, 14–16 While no uniform definitions exist for mid- or late-onset AD, we used an age cut-off of 8 based on a prior study showing that this age best differentiated between patients with and without filaggrin (FLG) mutations, a known genetic risk factor for AD, suggesting that this age may separate different subgroups of AD.17 Our age definitions for early-, mid-, and late-onset AD also align with those recently described by Paternoster et al.10
The primary outcomes were AD disease control and persistence. Disease control was assessed using the following survey question: “During the last 6 months, would you say your child’s skin disease has shown…complete disease control; good disease control; limited disease control; or uncontrolled disease.” This type of patient global assessment has been commonly used in research and has been shown to correlate with the Patient-Oriented Eczema Measure (POEM), a robust patient-reported severity measure.18 AD was defined to be persistent if the subject reported less than complete control and/or any AD medication use, or non-persistent if complete control and no medication use.
To evaluate the relationship between AD onset age and disease control or persistence, we used generalized linear latent and mixed models with random intercepts to estimate subject-specific odds ratios (OR) for persistent AD (binary outcome) and worse disease control (categorized ordinally as complete, good, limited, and uncontrolled) while accounting for repeated measures in each subject. Analyses were adjusted for potential confounders specified a priori, including sex, race/ethnicity, household income, age at enrollment, atopic comorbidities, family history of atopy, baseline AD control, and follow-up duration. We also examined data missingness to assess the assumption of random missingness required for our models. Sensitivity analyses were performed to evaluate the impact of missing data, variable follow-up duration, and enrollment timing on results. Local polynomial smoothed plots using the Epanechnikov kernel function were plotted for control and persistence by year of age and timing of AD onset; plots were constructed for ages up to 25 years due to sparse data on older subjects. Data analysis was performed using Stata (version 14.1, StataCorp, College Station, TX).
Results
In total, 8015 subjects (4273 [53.3%] female) were included in the study. They completed 70,841 follow-up surveys (mean 8.8 [standard deviation 7.4] surveys per subject), comprising 41,934 person-years of follow-up. Subject characteristics are presented in Table I. The median age of subjects upon enrollment was 6.6 (interquartile range [IQR] 3.9–10.4) years. The median age of AD onset was 0.75 (IQR 0.25–3.0) years, with 5770 (72.0%) children having early-onset, 1492 (18.6%) mid-onset, and 712 (8.9%) late-onset AD. Children with mid- or late-onset AD were more likely to be female but less likely to have asthma, seasonal allergies, or family history of atopy (Table I). The early-onset group had slightly longer follow-up (median 5.5 [IQR 1.1–9.9] years) than the mid-onset or late-onset groups (5.1 [IQR 1.0–9.6] and 4.6 [IQR 0.7–9.1] years, respectively). At baseline, subjects with mid- and late-onset AD were more likely to report good or complete control while subjects with early-onset AD were more likely to report limited control or uncontrolled disease (Table I).
Table I.
Baseline characteristics of study participants
| Characteristic | Overall cohort N=8015 |
Early-onset AD N=5770 |
Mid-onset AD N=1492 |
Late-onset AD N=712 |
p* |
|---|---|---|---|---|---|
| Age at enrollment, y, median (IQR) | 6.6 (3.9–10.4) | 5.2 (3.3–8.6) | 8.3 (6.4–10.9) | 13.1 (11.2–15.1) | <0.001* |
| Female sex | 4273 (53.3%) | 2996 (51.9%) | 826 (55.4%) | 427 (60.0%) | <0.001 |
| Race/ethnicity^ | |||||
| White | 2576 (32.1%) | 1895 (32.8%) | 429 (28.8%) | 242 (34.0%) | <0.001 |
| Hispanic | 851 (10.6%) | 554 (9.6%) | 210 (14.1%) | 77 (10.8%) | |
| Black | 4079 (50.9%) | 2966 (51.4%) | 734 (49.2%) | 362 (50.8%) | |
| Asian / Pacific Islander | 254 (3.2%) | 161 (2.8%) | 74 (5.0%) | 18 (2.5%) | |
| American Indian / Alaskan | 49 (0.6%) | 33 (0.6%) | 11 (0.7%) | 5 (0.7%) | |
| Multiracial | 204 (2.6%) | 159 (2.8%) | 34 (2.3%) | 8 (1.1%) | |
| Annual household income | |||||
| $0-$49,999 | 4544 (56.8%) | 3317 (57.6%) | 820 (55.1%) | 380 (53.5%) | <0.001 |
| $50,000-$99,999 | 889 (11.1%) | 680 (11.8%) | 142 (9.5%) | 63 (8.9%) | |
| $100,000+ | 410 (5.1%) | 320 (5.6%) | 58 (3.9%) | 28 (3.9%) | |
| Prefer not to answer | 2161 (27.0%) | 1446 (25.1%) | 469 (31.5%) | 240 (33.8%) | |
| Duration of registry follow-up, y, median (IQR) | 5.3 (1.1–9.9) | 5.5 (1.1–9.9) | 5.1 (1.0–9.6) | 4.6 (0.7–9.1) | <0.001* |
| AD control at baseline | |||||
| Complete | 406 (5.1%) | 255 (4.4%) | 83 (5.6%) | 61 (8.6%) | <0.001 |
| Good | 3797 (47.5%) | 2628 (45.6%) | 789 (53.0%) | 366 (51.5%) | |
| Limited | 3036 (38.0%) | 2305 (40.0%) | 495 (33.2%) | 226 (31.8%) | |
| Uncontrolled | 762 (9.5%) | 575 (10.0%) | 122 (8.2%) | 58 (8.2%) | |
| Duration of AD at enrollment, y, median (IQR) | 4.0 (2.3–7.1) | 4.5 (2.6–7.8) | 3.6 (1.9–6.3) | 2.1 (1.2–3.7) | <0.001 |
| Asthma | 3671 (45.9%) | 2777 (48.2%) | 598 (40.2%) | 281 (39.5%) | <0.001 |
| Seasonal allergies | 5608 (70.1%) | 4110 (71.3%) | 998 (66.9%) | 477 (67.1%) | 0.001 |
| Family history of atopy | 6243 (77.9%) | 4567 (79.2%) | 1112 (74.5%) | 536 (75.3%) | <0.001 |
AD, atopic dermatitis
Fisher’s exact or Kruskal-Wallis test, as appropriate
Non-Hispanic unless otherwise specified or multiracial
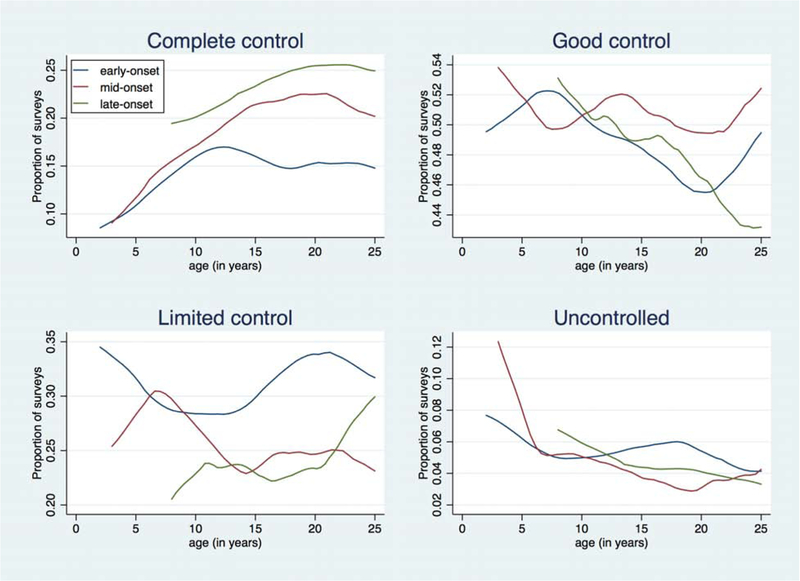
Complete AD control was reported on 16.0% of all surveys, good control on 50.2%, limited control on 28.6%, and uncontrolled disease on 5.2%. In longitudinal analyses, older AD onset age was associated with better control and less persistence (Table II). For each additional year of age at AD onset, the adjusted OR (aOR) for poorer control was 0.93 (95% CI 0.91–0.94); thus, a child whose AD began at age 10 has 44% lower odds of worse control over time compared to one whose AD began at age 2. Similarly, when AD was categorized as early-, mid-, or late-onset, the aORs for worse control were 0.71 (95% CI 0.64–0.80) and 0.51 (95% CI 0.43–0.60) in the mid- and late-onset groups, respectively, compared to the early-onset group (Table II). Plotting AD control by age, we visualized differences across the three onset groups (Figure, A). While all groups increasingly reported complete control with increasing age, the late-onset group was more likely to report complete control than the mid-onset and early-onset groups across all ages, and differences among the three groups were most distinct in the second and third decades of life.
Table II. Multivariable models for worse AD control and persistence AD, with respect to timing of AD onset.
Timing of AD onset is measured as continuous variable in Model 1 and as categorical variable in Model 2.
| Variable | Adjusted OR (95% Cl)* | |||
|---|---|---|---|---|
| Worse AD control^ | Persistent AD | |||
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Timing of AD onset | ||||
| Age of AD onset, per y# | 0.93 (0.91–0.94) | -- | 0.84 (0.80–0.88) | -- |
| Early-onset (≤2 y) | -- | Reference | -- | Reference |
| Mid-onset (3–7 y) | -- | 0.71 (0.64–0.80) | -- | 0.45 (0.34–0.60) |
| Late-onset (8–17 y) | -- | 0.51 (0.43–0.60) | -- | 0.19 (0.12–0.30) |
| Female sex | 1.20 (1.11–1.30) | 1.20 (1.11–1.30) | 1.77 (1.44–2.18) | 1.76 (1.43–2.16) |
| Race/ethnicity+ | ||||
| White | Reference | Reference | Reference | Reference |
| Black | 2.27 (2.06–2.50) | 2.28 (2.07–2.51) | 7.01 (5.45–9.02) | 7.09 (5.51–9.12) |
| Other | 1.60 (1.41–1.81) | 1.62 (1.43–1.83) | 3.36 (2.47–4.58) | 3.50 (2.57–4.78) |
| Annual household income | ||||
| $0-$49,999 | Reference | Reference | Reference | Reference |
| $50,000-$99,999 | 0.72 (0.64–0.83) | 0.73 (0.64–0.83) | 0.53 (0.39–0.73) | 0.53 (0.39–0.73) |
| $100,000+ | 0.62 (0.52–0.75) | 0.62 (0.52–0.75) | 0.51 (0.33–0.77) | 0.51 (0.33–0.77) |
| Prefer not to answer | 0.82 (0.75–0.91) | 0.82 (0.75–0.91) | 0.70 (0.55–0.91) | 0.70 (0.54–0.90) |
| Age at registry enrollment, y | 1.03 (1.02–1.04) | 1.03 (1.02–1.04) | 1.06 (1.03–1.10) | 1.06 (1.03–1.09) |
| Duration of registry follow-up, y | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) | 1.00 (0.96–1.04) | 1.00 (0.97–1.04) |
| AD control at baseline | ||||
| Complete | Reference | Reference | Reference | Reference |
| Good | 4.30 (3.55–5.21) | 4.33 (3.57–5.25) | 2.60 (1.71–3.95) | 2.64 (1.74–4.01) |
| Limited | 19.77 (16.25–24.05) | 19.96 (16.40–24.28) | 7.61 (4.92–11.77) | 7.76 (5.02–12.00) |
| Uncontrolled | 75.65 (60.26–94.98) | 76.36 (60.83–95.85) | 15.92 (9.07–27.94) | 16.26 (9.27–28.53) |
| History of asthma | 1.12 (1.03–1.22) | 1.13 (1.03–1.23) | 1.31 (1.05–1.64) | 1.32 (1.06–1.65) |
| History of seasonal allergies | 1.19 (1.08–1.31) | 1.20 (1.09–1.32) | 1.46 (1.14–1.86) | 1.47 (1.15–1.88) |
| Family history of atopy | 1.15 (1.04–1.27) | 1.16 (1.05–1.28) | 1.19 (0.92–1.54) | 1.21 (0.93–1.57) |
AD, atopic dermatitis; OR, odds ratio
Multivariable generalized linear mixed models also adjusted for repeated surveys and interaction term between survey number and onset age
Ordinally defined as: complete control, good control, limited control, and uncontrolled
Age measured as continuous variable in year
Non-Hispanic white and black; Other includes Hispanic, Asian, Pacific Islander, American Indian/Native Alaskan, and multiracial
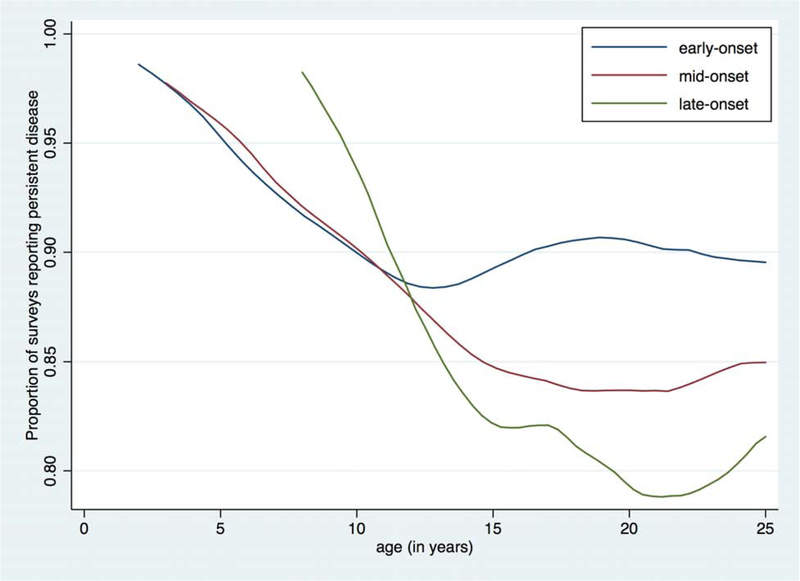
Figure. AD disease control and persistence by year of age and timing of disease onset.
Local polynomial smoothed plots are shown for early-, mid-, and late-onset AD. The y-axis represents the proportion of surveys reporting the specified level of disease control (A) or persistent AD (B) at each age. The x-axis represents age in years.
Persistent AD was reported on 90% of all surveys. In longitudinal analyses, the odds of persistent AD was significantly lower for each additional year of age at AD onset (aOR 0.84 [95% CI 0.80–0.88]) (Table II). A child whose AD began at age 10 thus has a 75% lower odds of persistent disease over time compared to one whose AD began at age 2. When onset age was analyzed categorically, the odds of persistent AD were also lower for mid-onset (aOR 0.45 [95% CI 0.34–0.60]) and late-onset (aOR 0.19 [95% CI 0.12–0.30]) AD relative to early-onset AD (Table II). In all three groups, the proportion of subjects reporting persistent AD generally declined with older age, and differences among the three onset groups were most pronounced from early adolescence onward (Figure, B).
In multivariable models, other statistically significant predictors of worse AD control and persistence were female sex, non-white race, lower income, history of asthma or seasonal allergies, and family history of atopy (Table II).
Response rates to follow-up surveys were 55–70% per survey. The proportion of subjects who did not complete any follow-up surveys was 18.0%, while 47.3% of subjects returned ≤50% of their follow-up surveys. However, 20.8% of subjects completed all surveys. The distributions of missing surveys among the early-, mid-, and late-onset groups were similar though, supporting the assumption that missingness is independent of the exposure (data not shown). Subjects missing ≤50% of their scheduled follow-up surveys and those missing >50% were generally similar in their baseline characteristics (data not shown). Sensitivity analyses of only subjects with ≥80% or 100% completed surveys found similar results to the primary analysis (Table III). Effect estimates were only further strengthened in analyses limited to subjects with ≥6 years of follow-up; 10 years of follow-up; or ≥80% survey completion and ≥6 years of follow-up (Table III).
Table III.
Sensitivity analyses
| Model | Adjusted OR (95% Cl) | |||||
|---|---|---|---|---|---|---|
| Worse AD control^ | Persistent AD | |||||
| Age of AD onset, per y# | Mid-onset* | Late-onset* | Age of AD onset, per y# | Mid-onset* | Late-onset* | |
| Primary model | 0.93 (0.91–0.94) | 0.71 (0.64–0.80) | 0.51 (0.43–0.60) | 0.84 (0.80–0.88) | 0.45 (0.34–0.60) | 0.19 (0.12–0.30) |
| Subjects with ≥80% of surveys completed | 0.91 (0.89–0.94) | 0.66 (0.56–0.78) | 0.41 (0.31–0.54) | 0.80 (0.76–0.85) | 0.33 (0.22–0.48) | 0.13 (0.07–0.23) |
| Subjects with 100% of surveys completed | 0.90 (0.87–0.94) | 0.63 (0.50–0.80) | 0.40 (0.27–0.60) | 0.80 (0.73–0.87) | 0.38 (0.22–0.66) | 0.15 (0.06–0.36) |
| Subjects with ≥6 years of registry follow-up | 0.91 (0.89–0.93) | 0.68 (0.58–0.80) | 0.39 (0.30–0.50) | 0.78 (0.74–0.82) | 0.36 (0.25–0.52) | 0.09 (0.05–0.16) |
| Subjects with 10 years of registry follow-up | 0.90 (0.86–0.93) | 0.72 (0.55–0.93) | 0.34 (0.23–0.50) | 0.72 (0.66–0.79) | 0.35 (0.19–0.65) | 0.04 (0.02–0.10) |
| Subjects with ≥80% of surveys completed and ≥6 years of registry follow-up | 0.90 (0.88–0.93) | 0.67 (0.56–0.81) | 0.36 (0.26–0.49) | 0.77 (0.72–0.83) | 0.34 (0.23–0.52) | 0.08 (0.04–0.15) |
| Subjects who enrolled in PEER within ≤2 years after AD onset | 0.72 (0.60–0.87) | 0.52 (0.38–0.71) | 0.22 (0.11–0.43) | 0.52 (0.33–0.82) | 0.15 (0.07–0.32) | 0.03 (0.01–0.13) |
AD, atopic dermatitis; OR, odds ratio
Ordinally defined as: complete control, good control, limited control, and uncontrolled
Age measured as continuous variable in year
Reference group is early-onset AD
Discussion
In this study of 8,015 children with AD followed into young adulthood, earlier onset AD was associated with worse disease control and greater persistence over time, independent of sociodemographic characteristics and atopic comorbidities. Our findings suggest that AD onset age differentiates clinically distinct forms of the disease. AD subtypes that are partly distinguished by the timing of disease onset have been identified in previous cross-sectional studies using cluster or latent class analysis.19, 20 Two longitudinal studies have described distinct trajectories of AD defined also in part by the age of disease onset. Using data from British and Dutch cohorts, Paternoster et al. identified 6 latent classes of AD from birth to age 16: early-onset-persistent; early-onset-late-resolving; early-onset-early-resolving; mid-onset-resolving; late-onset-resolving; and unaffected/transient.10 Similarly, Roduit et al. defined 4 classes of AD using a rural European birth cohort followed until age 6: early-onset-transient; early-onset persistent; late-onset (after age 2); and never/infrequent.11
Our findings in a U.S. cohort are consistent with these previous observations in European children. We did not further separate early-onset AD into resolving and persistent subtypes, as our objective was to compare the disease course, on average, among early-, mid-, and late-onset AD. We recognize that a subset of patients with early-onset AD experience disease resolution, which our data also support. In our early-onset group, a continuous decline in reports of persistent AD occurs in early childhood and nadirs at age 13, likely reflecting those individuals whose AD resolved; however, the proportion of persistent AD remains fairly stable thereafter, representing those with active AD into adulthood (Figure, B). In contrast to previous birth cohort studies, our study followed children into their 20s, providing insight into older ages. Notably, rates of persistence in early-, mid-, and late-onset AD most clearly separated after age 13 (Figure, B), suggesting that these groups differ more strikingly during adolescence and early adulthood. Additionally, while previous studies only measured any AD activity, we evaluated the level of disease control and found that earlier onset AD also portended worse longitudinal control. Finally, our study was strengthened by the frequency of outcome assessments, thus better capturing the variations inherent to a waxing and waning disorder such as AD.
The identification of distinct AD phenotypes and endotypes is a major focus of ongoing research.5, 21 Variations in the longitudinal course of early-, mid-, and late-onset pediatric AD support the concept that they are distinct subtypes. Previous studies in PEER and other cohorts have also shown that earlier onset AD is associated with a greater risk for asthma, allergic rhinitis, and food allergies and the presence of genetic risk variants for AD.9–11, 17, 19, 22–24 Together, these suggest that the timing of AD onset is driven in part by genetics and is associated with not only AD severity and persistence but also atopic burden overall. However, additional research is needed to understand if, and how, early-, mid-, and late-onset AD differ molecularly or immunologically, and whether they respond differentially to treatment.
There are several potential limitations to note. First, all subjects in PEER were required to have used pimecrolimus, potentially biasing our cohort towards children with more persistent or active disease requiring pharmacologic therapy. It is also possible that subjects with milder or transient AD were less likely to respond to follow-up surveys, thereby also contributing to this potential bias. Nevertheless, pimecrolimus is commonly used for mild-to-moderate AD and its utilization would not be expected to vary by AD onset age. Second, as with all longitudinal studies, attrition and missing data may introduce bias. Sensitivity analyses limited to subjects with longer follow-up and/or less missing data showed similar results however, and subjects with >50% vs. ≤50% missing surveys were fairly similar at baseline. Third, as children could enroll in PEER at any time after AD diagnosis, we do not have information on disease activity between AD onset and registry enrollment, which was 4 years long on average. It is unlikely that children with any particular timing of AD onset were differentially included in PEER based on disease activity prior to registry entry, since subjects and enrolling physicians were unaware of the current study hypothesis. However, it is possible that some children with early-onset, early-resolving AD are underrepresented in PEER, as they may have had AD resolution prior to 2 years of age or were less likely to use pimecrolimus due to age or milder disease, for example. Therefore, our early-onset group is probably more representative of children whose AD remained active after age 2. Similarly, as some children with mid-onset or late-onset AD did not enroll in PEER until a few years after disease onset, it is possible that children with later onset but transient or quickly-resolving AD were also less likely to be included in PEER. Nevertheless, a sensitivity analysis limited to subjects who enrolled within ≤2 years after AD onset was similar to the primary findings (Table III). Finally, AD onset ages were self-reported which may lead to misclassification bias. However, analyses that broadly categorized onset age into early-, mid-, and late-onset AD were consistent with those evaluating age as a continuous variable. Despite these potential limitations, our study is strengthened by the use of a large and diverse AD cohort with frequent disease assessments and relatively long follow-up.
From a clinical perspective, the age of AD onset may be helpful for risk stratifying and counselling patients about their expected disease course. Although more precise subtypes of AD may be identified by combining clinical, genetic, and biomarker data, a simple approach to AD sub-classification remains clinically useful. The timing of disease onset is normally assessed as part of the clinical history for patients with AD while genetic and laboratory tests are not routinely performed. By considering the framework of early-onset or late-onset disease, we can identify those patients at greater risk for persistent or poorly controlled AD and whom may benefit from more intensive treatment or monitoring.
Conclusion
The disease course of AD in children varies significantly by the timing of its onset. AD that begins early in life is associated with more longstanding and poorly controlled disease. Our findings suggest that early-, mid-, and late-onset pediatric AD are clinically distinct disease subtypes, thus making onset age a useful parameter for risk stratifying and counselling patients.
Funding sources:
This study was supported in part by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases T32-AR007465 (Wan) and a Dermatology Foundation Dermatologist Investigator Research Fellowship (Wan). The data source used in this study is the Pediatric Eczema Elective Registry, which is a study funded by Valeant Pharmaceuticals through a grant to Dr. Margolis. No funding sources had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Abbreviations
- aOR
adjusted odds ratio
- AD
atopic dermatitis
- CI
confidence interval
- IQR
interquartile range
- OR
odds ratio
- PEER
Pediatric Eczema Elective Registry
- POEM
Patient-Oriented Eczema Measure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest and financial disclosures: Dr. Wan receives research fellowship funding from Pfizer Inc. (to the Trustees of the University of Pennsylvania) and has received payment for consulting work with Health Union LLC. Dr. Margolis has served on advisory committees for Sanofi/Regeneron and Pfizer Inc. Dr. Yan has served as a consultant to Sanofi/Regeneron, Pfizer Inc, Procter and Gamble, and Ortho Dermatologics/Valeant. Dr. Mitra and Mr. Hoffstad have no relevant conflicts of interest to disclose.
IRB approval: The University of Pennsylvania IRB has exempted this study from review.
References
- 1.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. The Journal of allergy and clinical immunology 2009;124:1251–8.e23. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis : contact, atopic, occupational, drug 2014;25:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy 2015;70:836–45. [DOI] [PubMed] [Google Scholar]
- 4.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA dermatology 2014;150:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieber T, D’Erme AM, Akdis CA, Traidl-Hoffmann C, Lauener R, Schappi G et al. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? The Journal of allergy and clinical immunology 2017;139:S58–s64. [DOI] [PubMed] [Google Scholar]
- 6.Williams HC, Strachan DP. The natural history of childhood eczema: observations from the British 1958 birth cohort study. The British journal of dermatology 1998;139:834–9. [DOI] [PubMed] [Google Scholar]
- 7.Loo EX, Shek LP, Goh A, Teoh OH, Chan YH, Soh SE et al. Atopic Dermatitis in Early Life: Evidence for at Least Three Phenotypes? Results from the GUSTO Study. International archives of allergy and immunology 2015;166:273–9. [DOI] [PubMed] [Google Scholar]
- 8.Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy 2013;68:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan J, Mitra N, Hoffstad OJ, Gelfand JM, Yan AC, Margolis DJ. Variations in risk of asthma and seasonal allergies between early- and late-onset pediatric atopic dermatitis: A cohort study. Journal of the American Academy of Dermatology 2017;77:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paternoster L, Savenije OEM, Heron J, Evans DM, Vonk JM, Brunekreef B et al. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. The Journal of allergy and clinical immunology 2018;141:964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roduit C, Frei R, Depner M, Karvonen AM, Renz H, Braun-Fahrlander C et al. Phenotypes of Atopic Dermatitis Depending on the Timing of Onset and Progression in Childhood. JAMA pediatrics 2017;171:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis DJ, Abuabara K, Hoffstad OJ, Wan J, Raimondo D, Bilker WB. Association Between Malignancy and Topical Use of Pimecrolimus. JAMA dermatology 2015;151:594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoor R, Hoffstad O, Bilker W, Margolis DJ. The frequency and intensity of topical pimecrolimus treatment in children with physician-confirmed mild to moderate atopic dermatitis. Pediatric dermatology 2009;26:682–7. [DOI] [PubMed] [Google Scholar]
- 14.Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. The British journal of dermatology 1994;131:406–16. [DOI] [PubMed] [Google Scholar]
- 15.Mohrenschlager M, Schafer T, Huss-Marp J, Eberlein-Konig B, Weidinger S, Ring J et al. The course of eczema in children aged 5–7 years and its relation to atopy: differences between boys and girls. The British journal of dermatology 2006;154:505–13. [DOI] [PubMed] [Google Scholar]
- 16.Carlsten C, Dimich-Ward H, Ferguson A, Watson W, Rousseau R, Dybuncio A et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2013;110:24–8. [DOI] [PubMed] [Google Scholar]
- 17.Rupnik H, Rijavec M, Korosec P. Filaggrin loss-of-function mutations are not associated with atopic dermatitis that develops in late childhood or adulthood. The British journal of dermatology 2015;172:455–61. [DOI] [PubMed] [Google Scholar]
- 18.Chang J, Bilker WB, Hoffstad O, Margolis DJ. Cross-sectional comparisons of patient-reported disease control, disease severity and symptom frequency in children with atopic dermatitis. The British journal of dermatology 2017;177:e114–e5. [DOI] [PubMed] [Google Scholar]
- 19.Lee E, Lee SH, Kwon JW, Kim YH, Cho HJ, Yang SI et al. Atopic dermatitis phenotype with early onset and high serum IL-13 is linked to the new development of bronchial hyperresponsiveness in school children. Allergy 2016;71:692–700. [DOI] [PubMed] [Google Scholar]
- 20.Seo E, Yoon J, Jung S, Lee J, Lee BH, Yu J. Phenotypes of atopic dermatitis identified by cluster analysis in early childhood. The Journal of dermatology 2018. [DOI] [PubMed] [Google Scholar]
- 21.Thijs JL, Strickland I, Bruijnzeel-Koomen C, Nierkens S, Giovannone B, Csomor E et al. Moving toward endotypes in atopic dermatitis: Identification of patient clusters based on serum biomarker analysis. The Journal of allergy and clinical immunology 2017;140:730–7. [DOI] [PubMed] [Google Scholar]
- 22.Wan J, Mitra N, Hoffstad OJ, Margolis DJ. Influence of FLG mutations and TSLP polymorphisms on atopic dermatitis onset age. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2017;118:737–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe AJ, Angelica B, Su J, Lodge CJ, Hill DJ, Erbas B et al. Age at onset and persistence of eczema are related to subsequent risk of asthma and hay fever from birth to 18 years of age. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology 2017;28:384–90. [DOI] [PubMed] [Google Scholar]
- 24.Dezman K, Korosec P, Rupnik H, Rijavec M. SPINK5 is associated with early-onset and CHI3L1 with late-onset atopic dermatitis. International journal of immunogenetics 2017;44:212–8. [DOI] [PubMed] [Google Scholar]