Abstract
The visual system must organize dynamic input into useful percepts across time, balancing between stability and sensitivity to change. The temporal integration window (TIW) has been hypothesized to underlie this balance: If two or more stimuli fall within the same TIW, they are integrated into a single percept; those that fall in different windows are segmented (Arnett & Di Lollo, 1979; Wutz, Muschter, van Koningsbruggen, Weisz, & Melcher, 2016). Visual TIWs have been studied in adults, showing average windows of 65 ms (Wutz et al., 2016); however, it is unclear how windows develop through early childhood. Here we measured TIWs in 5- to 7-year-old children and adults, using a variant of the missing dot task (Di Lollo, 1980; Wutz et al. 2016), in which integration and segmentation thresholds were measured within the same participant, using the same stimuli. Participants saw a sequence of two displays separated by an interstimulus interval (ISI) that determined the visibility of a visual search target. Longer ISIs increased the likelihood of detecting a segmentation target (but decreased detection for the integration target) although shorter ISIs increased the likelihood of detecting the integration target (but decreased detection of the segmentation target). We could then estimate the TIW by measuring the point at which these two functions intersect. Children's TIWs (M = 68 ms) were comparable to adults' (M = 73 ms) with no appreciable age trend within our sample, indicating that TIWs reach adult levels by approximately 5 years of age.
Keywords: temporal integration, temporal segmentation, visual development, temporal processing, children
Introduction
Temporal processing and the TIW
The visual system is presented with a flow of sensory information that must be organized, over time, into meaningful objects, scenes, and events (Rucci, Ahissar, & Burr, 2018). The temporal integration window (TIW)—the period in which visual input is combined into a singular percept—has proven a useful construct for characterizing and comparing temporal processing between individuals (Arnett & Di Lollo, 1979; Wutz et al., 2016). If two events fall within the same TIW, they are integrated; if they fall in different windows, they are segmented. Shorter TIWs, then, facilitate temporal resolution and sensitivity to rapid change, and longer TIWs facilitate the integration of information and thereby certainty about objects and events (Blake & Lee, 2005; Rüter, Marcille, Sprekeler, Gerstner, & Herzog, 2012; Wutz & Melcher, 2013, 2014; Zimmermann, Morrone, & Burr, 2013). Differences in TIWs can influence high-level cognitive and perceptual processes that require well-adapted timing, such as object individuation (Drewes, Zhu, Wutz, & Melcher, 2015; Wutz & Melcher, 2014), visual working memory (Wutz & Melcher, 2013, 2014), apparent motion (Fairhall, Albi, & Melcher, 2014; Honey et al., 2012), action sequence perception (Faivre & Koch, 2014), language processing (Hillock-Dunn, Grantham, & Wallace, 2016), action planning (Hommel, Müsseler, Aschersleben, & Prinz, 2001), and pragmatic aspects of communication, such as interactional synchrony (Trevarthen & Daniel, 2005). Because of the fundamental role temporal processing plays in visual perception, it is important to understand the trajectory of how TIWs change over development.
Temporal processing is often assessed through simultaneity judgments. For example, two-flash fusion tasks measure the minimum temporal lag at which two spatially coincident flashes can be discriminated from a single flash (Samaha & Postle, 2015) or the minimum temporal lag at which two stimuli, flashed side by side, are perceived as simultaneous (Falter, Elliott, & Bailey, 2012). These procedures are not ideal for children, however, because it would be challenging to determine whether observed differences (say between age groups or between children and adults) are caused by temporal processing differences per se or differences in general performance factors, such as motivation or response execution, or response bias. For example, if one were measuring the accuracy with which a participant could discriminate one flash from two flashes as a function of lag, then lapses in motivation would lead to worse performance, leading to an (erroneous) misestimate of temporal thresholds. To minimize these challenges, we adapted a variant of the missing element task (Di Lollo, 1980; Wutz et al., 2016) to measure both integration and segmentation within the same participant (5- to 7-year-old children and adults) using the same stimuli and task. Participants were presented with two frames separated by a blank interstimulus interval (ISI) and instructed to locate an integration target (which is visible only if the two frames are integrated over time—facilitated by shorter ISIs) or a segmentation target (which is visible only if the two frames are segmented over time—facilitated by longer ISIs), allowing us to pinpoint the TIW by measuring the “crossover point” at which these two functions intersect. General performance factors, then, may influence the overall performance (i.e., the height of this crossover point) but not the estimated duration of the TIW (i.e., the left–right positioning of the crossover on the time axis). We hypothesized that TIWs might be longer—a tendency toward integration—in very young children although with rapid maturation (O. J. Braddick & Atkinson, 2009). Our results show that children's TIWs are actually already indistinguishable from adults' (68 ms and 73 ms, respectively) by 5–7 years of age.
The development of temporal integration/segmentation
Temporal integration and segmentation occurs at many levels throughout the brain, wherever information must be processed across different time scales from Bloch's law in the retina (Gorea, 2015) to episodic memory in the medial temporal structures (Nyberg, McIntosh, Houle, Nilsson, & Tulving 1996). For early vision, the most comprehensive way to characterize temporal processing is to measure, analogously to the contrast sensitivity function for spatial vision, the temporal contrast sensitivity function (tCSF). The tCSF is determined by measuring the contrast transients necessary to detect flicker at various temporal frequencies. For adults, the tCSF is a bandpass function that peaks at 5–10 Hz (Wooten, Renzi, Moore, & Hammond, 2010), and although showing much lower overall contrast sensitivity (in which sensitivity is reduced at lower frequencies and reaches adult-like levels by 7 years), even infants (as young as 3 months) have a tCSF that is nearly identical in shape and tuning to adults' (Dobkins, Anderson, & Lia, 1999; Dobkins & Teller, 1996; Ellemberg, Lewis, Liu, & Maurer, 1999; Hartmann & Banks, 1992).1 This low-level, retinal process is mature within the first few months of life (around 3 months of age), suggesting that temporal limitations across development reflect higher-level processes that integrate (and segment) pattern and form across time, independent of low-level flicker fusion and perhaps with a different developmental course. In the end, though, these low- and high-level processes are thought to work together to create a coherent percept across time (Holcombe, 2009; Wutz & Melcher, 2014).
For the present study, we focus on form and pattern integration, a fundamental but higher-level process that assembles spatial information (edges, textures, contours) into objects and scenes that can inform decisions and actions (VanRullen, 2016). Motion and shape perception are relatively well developed early on (around 3–4 months; O. Braddick & Atkinson, 2007; O. J. Braddick & Atkinson, 2009; Gunn et al., 2002), and the ability to integrate both motion and shape information (integrating partial features that are in motion behind a slit into a global dynamic shape) is present by around 5 months (Imura & Shirai, 2014). In that task, infants were presented with an image that moved behind a narrow slit. Subsequently, in a preferential looking test, 5-month-old infants (and older) looked longer at a novel object as opposed to the one that had been presented behind the slit, showing that they had appreciated and familiarized to its global form. However, the development of the temporal parameters governing how an infant can integrate and segment a continuous stream of visual information is not well specified.
Most developmental work has focused on multisensory temporal integration: the period in which information from multiple senses (typically, vision and audition) is bound together in time and perceived as originating from the same event or perceived as occurring simultaneously (Wallace & Stevenson, 2014). Multisensory perception depends on whether two stimuli fall within a particular temporal window with audiovisual and visual-tactile integration typically involving windows of around 30–80 ms (for a review, see Vroomen & Keetels, 2010). Previous work suggests that multisensory TIWs vary depending on stimuli and task with the narrowest windows for simple flashes and beeps and the broadest for more complex stimuli, such as speech. Hillock-Dunn et al. (2016) used the McGurk effect to measure the development of the TIW for more complex, audiovisual speech in which the binding or integration of audio and visual information (reflecting two different sounds) results in the perception of a different sound. This effect is reduced as you increase the asynchrony between the audio and visual information. They found no differences in the TIW across the tested age range (7- to 24-year-olds), suggesting that the TIW for audiovisual speech information is adult-like by 7 years old. It has been suggested that the TIW for nonlinguistic stimuli, such as noise bursts and visual flashes, develops later. Chen, Shore, Lewis, and Maurer (2016) measured whether participants (adults and children aged 5–11 years) judged audiovisual stimuli as simultaneous while varying the stimulus onset asynchrony, finding that the TIW for simple noise bursts and visual flashes reached adult levels by 9 years of age. More generally, it has been argued that multisensory TIWs are shaped by experience and learned associations, relying on low-level physical characteristics, such as spatiotemporal information in early development and later relying on goal- or task-related multisensory interactions (Hillock-Dunn et al., 2016; Murray, Lewkowicz, Amedi, & Wallace, 2016).2 These results helped inform our hypothesis that unisensory, visual TIWs narrow over development, reaching adult levels in early childhood.
Within the visual modality, there has been limited work investigating the development of temporal processing, and that work has typically measured either integration or segmentation in isolation with adults and children split across studies and often tested with different stimuli, making it challenging to infer developmental trends between children and adults. For instance, Hogben, Rodino, Clark, and Pratt (1995) measured integration in 8- to 10-year-olds using the missing dot task, in which a two-frame sequence of a 4 × 4 matrix of dots with one dot missing in one frame was separated by a blank ISI with matrix size also varying across trials (large matrix: 60 min dot separation; small matrix: 12 min). Participants were required to locate the missing element (the missing element is visible only when two frames are combined). They found thresholds (the rate at which two or more stimuli are combined into a coherent percept—in this case, the rate at which two frames are combined to make the missing dot target visible) of approximately 59 ms for the large matrix and 15 ms for the small matrix. In that study, the goal was to compare typically developing children with those diagnosed with dyslexia (the results did not differ significantly between groups). In an earlier study, Di Lollo and Hogben (1987) had measured temporal integration in adults, using the same paradigm, with matrix sizes ranging from 24 to 72 mins. At the size most comparable to the small-matrix condition in the Hogben et al. study of children, integration thresholds were at 20 ms, closely matching the value of 15 ms measured in that study. This is consistent with integration being adult-like in 8- to 10-year-olds. As well, using a similar missing dot task, Arnett and Di Lollo (1979) found that temporal integration in 7- to 13-year-olds did not change across age with integration thresholds of 45–50 ms. However, in a later study (using the same task), thresholds increased incrementally within a broader age range that included older adults (58–70 years old), increasing to 80 ms in the oldest participants (Di Lollo, Arnett, & Kruk, 1982).
Although those studies investigated temporal integration, the capacity to combine information over time, there has been only one study aimed at describing the development of rapid visual segmentation processes. Farzin, Rivera, and Whitney (2011) conducted a comparison of visual segmentation in typically developing infants and adults. They showed infants (between 6 and 15 months) and adults a 2 × 2 matrix of temporally alternating regions of light and dark with one region alternating in counter-phase to the others. When segmented, the featural-temporal asynchrony and the oddball polarity of the region become clear. They measured the fastest rate at which this segmentation still occurred (as measured by preferential looking to the oddball region) for each group. The results showed that infants had significantly longer threshold rates of approximately 1,000–2,000 ms for 6- and 15-month-olds, respectively, versus 100 ms for adults. To ensure that temporal differences were not caused by infants' inability to detect low-level flicker (changes in luminance), they conducted a second experiment showing that infants (6–15 months) can detect a flicker rate of 10 Hz, suggesting that the limitation on the ability to segment the array reflected a higher-level process with a different developmental time course. In contrast to the findings with older children, this work on infants suggests that visual temporal resolution is lower but increasing dramatically over the first 1–2 years of life.
In the current study, we chose to focus on 5- to 7-year-olds, who are in between the ages studied in Farzin et al. (2011; 6- to 15-month-olds) and those studied by Di Lollo, Hogben and colleagues (Arnett & Di Lollo 1979; Hogben et al., 1995; 7- to 13-year-olds). As well, 5-year-olds are the youngest children who can reliably follow instructions in this kind of psychophysical task.
The current study
The goal of this study was to investigate the development of visual TIWs. Toward that end, we measured both integration and segmentation in a group of 5- to 7-year-old children and a control group of adults. Although adults were likely to have better performance on both tasks, we looked to the crossover point between the integration and segmentation performance curves; the point at which integration transitions to segmentation as the delay between visual events is increased. Using the crossover point as an estimate of visual temporal resolution helps correct for differences in performance that might accompany a group that is better at psychophysical tasks. This time point reflects the critical ISI at which the target is perceived, which has been widely used in the classic studies by Di Lollo and colleagues (Arnett & Di Lollo, 1979; Di Lollo & Hogben, 1987; Hogben et al., 1995) as an estimate of temporal integration. This is an estimate of the “temporal resolution” of visual perception, showing the rate at which the visual system parses oncoming sensory input into discrete objects and events (Ronconi & Melcher, 2017; Samaha & Postle, 2015).
Methods
Participants
In this study, 38 typically developing, 5- to 7-year-old children were tested (age range: 5.01–6.99 years, mean age = 5.88, SD = 0.59, 17 females). Families were recruited from the UMass Boston Baby Lab's database, which contains thousands of families from the greater Boston area and is based on birth records. In addition, 22 adults (age range: 19–29 years, mean age = 22.43, SD = 3.32, 19 females) were tested and recruited from the UMass Boston student body. All participants had normal or corrected-to-normal vision and no first-degree relatives with color blindness.
Informed consent was obtained by all child participants' caregivers, and compensation was given for their participation. The experimental protocol was approved by the institutional review board at the University of Massachusetts Boston.
Apparatus
Stimuli were generated using MATLAB R2016a (MathWorks, Natick, MA) and Psychtoolbox (Brainard, 1997; Kleiner et al., 2007) and run on a Samsung CFG70 monitor with a display resolution of 1,920 × 1,080, a 1-ms moving picture response time, and a 7.7-ms 20-80-20 pixel response time (these specifications met or exceeded those of the Samsung 2233RZ, which fared well in monitor tests tailored to visual psychophysics; Wang & Nikolić, 2011). Participants were seated approximately 57 cm from the display monitor, next to the experimenter, in a quiet testing room.
Stimuli and procedure
All experimental stimuli and procedures were identical for children and adults. On each trial, participants were presented with a rapid sequence of two complementary displays (Display A and Display B), each exposed twice in an ABAB pattern. Each display was presented for 17 ms and separated by a brief ISI of 17, 33, 83, or 117 ms (Figure 1). The number of A/B displays were held constant, and duration of the display exposure varied with ISI (117, 167, 317, and 417 ms with ISIs 17, 33, 83, 117, respectively). This design was adopted from Wutz et al. (2016). The ISI values were chosen to bookend the TIW found in previous work with adults (∼65 ms) and to add an assumedly easier condition (the 17-ms ISI for integration and the 117-ms ISI for segmentation) while still staying within the bounds of biologically plausible TIWs. Display A contained seven dark gray (25 cd/m2) circles and one half circle randomly positioned on a visible 4 × 4 grid (25 cd/m2; subtending 8.6° × 8.6° of visual angle) against a lighter gray background (69 cd/m2). Display B contained the same type of elements but with the circles occupying previously empty locations in the virtual grid and, importantly, with the half circle occupying the same location as in Display A but with a complementary orientation (adapted from the odd element task; Wutz et al., 2016). The half circle constituted the segmentation target and was visible only if the two displays were segmented in time such that the two half circles were separated rather than integrated into a full circle. Across the two displays, only 15 out of the 16 locations on the grid were occupied, yielding an empty space (randomly positioned from trial to trial). This constituted the empty-space integration target (similar to the missing dot task; Di Lollo, 1980). In order to correctly localize the integration target, Displays A and B had to be perceptually combined across time or else that particular empty space would be lost among all the other empty spaces in each individual display. Essentially, this constituted a single-feature visual search task (Treisman & Gelade, 1980; Wolfe & Horowitz, 2017) in which the likelihood of perceiving either target was directly influenced by the duration of the ISI: longer ISIs increased the likelihood of perceiving the segmentation target (but decreased the visibility of the integration target), and shorter ISIs increased the likelihood of perceiving the integration target (but decreased the visibility of the segmentation target; see Sharp, Melcher, & Hickey, 2018; Wutz, Melcher, & Samaha, 2018; Wutz et al., 2016).
Figure 1.
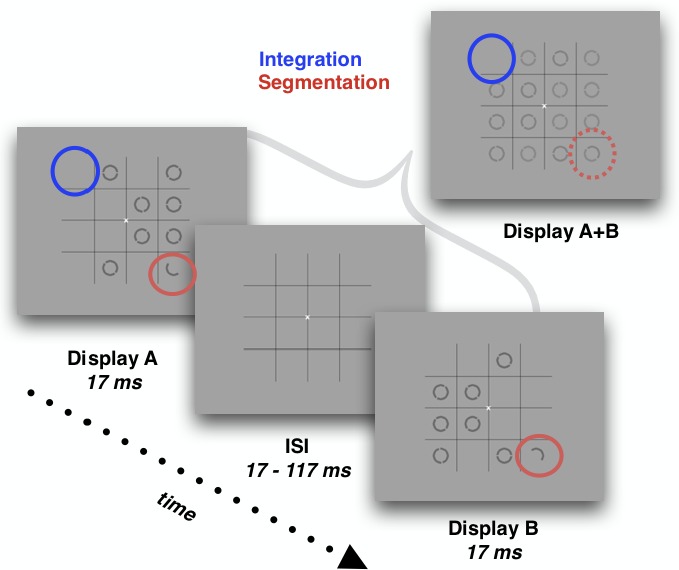
Test trial sequence for adults and children. Participants were instructed to locate either the half-circle segmentation target (highlighted here by the red solid circles) or the empty-space integration target (indicated by the solid blue circle). The segmentation target is visible only to the extent that Displays A and B are visually segmented, and the integration target is visible only to the extent that Displays A and B are visually integrated. An ISI separated Displays A and B, directly influencing the likelihood of integrating or segmenting the displays and thereby driving the detectability of the respective targets. In each trial, two AB pairs were presented.
Practice trials and lapse rate estimation
Participants first completed a five-trial practice block for integration and for segmentation. These practice trials served to familiarize the participant with each task, trial sequence, and response. They were designed to minimize temporal processing demands per se by having a much longer overall exposure duration by using six AB-paired exposures instead of the two used in test trials. Importantly, only extreme values of the ISI were used to favor integration (17 ms ISI) or segmentation (167 ms ISI). It is well known that children face various challenges when performing in psychophysical paradigms designed for adults, leading to a “lapse rate” (an error rate due to the overhead of task participation that lowers asymptotic performance) that is higher than adults' (Manning, Jones, Dekker, & Pellicano, 2018). Success in the present task—reliably identifying the location of the integration or segmentation target—requires sufficient task understanding, response execution (willingness and ability to properly point out the target's perceived location), searchability (i.e., the extent to which the target pops out), and gaze/engagement synchronization to the brief exposures of the displays; all contribute to the lapse rate. Because the lapse rate is taken to be unrelated to experimental variables of interest, accounting for it is important in order to make fairer comparisons (Koldewyn, Whitney, & Rivera, 2010; Manning et al., 2018). Practice trials allowed us to account for the lapse rate by providing an estimate of asymptotic performance that could then be used to normalize test-trial performance. Not unexpectedly, the adult group was at ceiling, 100% correct on both the integration and segmentation practice blocks. However, although both 5- and 6-year-olds were able to achieve near-ceiling performance on segmentation practice, 97.4% and 96.8% correct, respectively, they had much higher lapse rates on integration practice, 56% and 76% correct, respectively.3 (We speculate that it was the uniquely short exposures of the integration trials that were particularly challenging for some, especially younger, children—the shorter the exposure, the greater the impact of an errant gaze or lapse of attention.) In our main analysis (see Data analysis), participants' performance over the test trials in the main experiment for integration and segmentation was normalized by dividing it by the pretest practice performance of the relevant group.
Test trials
The test session consisted of two blocks of integration trials and two blocks of segmentation trials (with run order alternating and starting block counterbalanced across participants). Each 15-trial block contained five trials at each of three ISIs (17, 33, and 87 ms for integration trials and 33, 87, and 117 ms for segmentation trials, randomized within the block). For each participant, then, across the two blocks for a particular condition, there were 10 trials at each of the three ISIs. Depending on condition, participants were instructed to locate (by pointing) either the integration target or the segmentation target. The experimenter then recorded the chosen location via mouse click. If the chosen location was correct, it was accompanied by a “horn” sound effect and an incorrect choice by a neutral “bonk” sound. In either case, further feedback was given by highlighting the correct location of the target with a green square within the location grid. The experimenter advanced to the next trial when the participant was ready. Children were rewarded with stickers after each completed test block.
Data analysis
Individual performance was measured by calculating the proportion of trials in which a participant correctly selected the integration or segmentation target out of the set of 10 trials for a particular condition and ISI. In this way, a percentage correct score was calculated for each of the three ISIs (17, 33, and 87 ms) for integration trials and the three ISIs (33, 87, and 117 ms) for segmentation trials. Following that, data were normalized by practice trial performance in order to account for the higher lapse rates of children (see Practice trials and lapse rate estimation). For our main analysis, we averaged across participants to produce estimates for integration and segmentation performance as a function of ISI for the adult (N = 22) and child (N = 38) groups. The intersection of the least squares linear fits for the integration and segmentation functions allow for estimation of the group's TIW crossover. Our main contrast between groups was based on a comparison of the confidence intervals around the crossover points (Filliben & McKinny, 1972). Assessing age-related trends in the TIW required estimating crossovers for individual participants. Similarly to the group-level analysis, the intersection of the least squares linear fits of the integration and segmentation functions provided an estimate of each participant's crossover. However, one significant challenge to an individual-level crossover analysis is that, because of the low trial counts, performance estimates and, therefore, estimated crossovers have high variability. This meant that, for some participants, the crossover estimate could have been deemed an outlier. Instead of excluding these values from the correlation analyses, we opted to include all values and instead use a robust statistic, the Kendall rank correlation, which is based on medians and relatively insensitive to outliers (Kendall, 1948).
Results
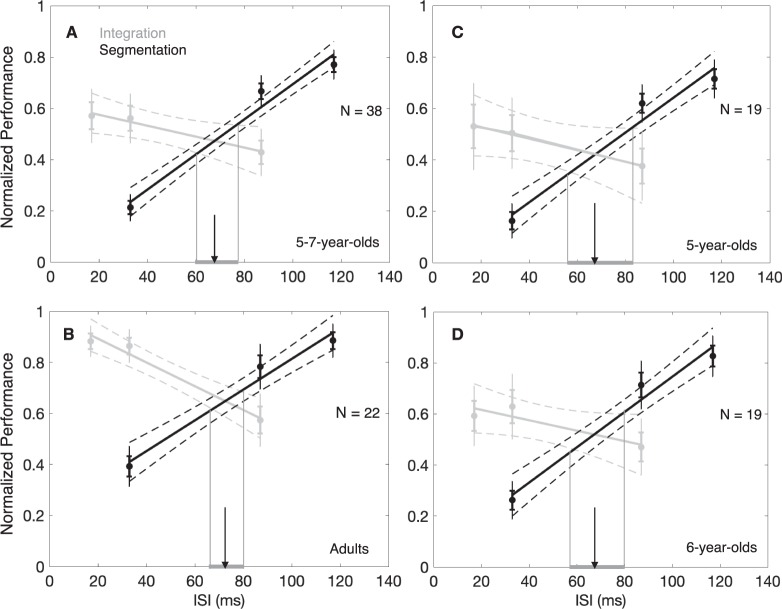
The crossover point of the integration and segmentation functions for children (Figure 2A, gray and black lines, respectively) was found at 68 ms at a performance of 48% correct. For adults (Figure 2B, gray and black lines, respectively), the TIW was estimated to be 73 ms at a performance of 65% correct. Both values are comparable to the 65-ms TIW estimate found in Wutz et al. (2016). For both children and adults, we computed 95% confidence intervals around the least squares fits (Filliben & McKinny, 1972). There was considerable overlap between the confidence regions for the child (59.70–77.55 ms) and adult (65.76–80.26 ms) groups. We also performed a median split, group-level analysis, based on age. The below-median “5-year-old” age group (N = 19, mean age = 5.37 years) had a TIW of 67.5 ms (42% correct; Figure 2C), and the above-median “6-year-old” group (N = 19, mean age = 6.33 years) had a TIW of 67.3 ms (52% correct; Figure 2D). Again, confidence intervals around these TIWs (56.16–83.10 ms and 56.92–80.09 ms for 5- and 6-year-olds, respectively) had near total overlap. Alongside these group-level analyses, we performed an individual-level analysis for each child participant (N = 38, age range 5–7 years) to further assess the potential relationship between age and TIW. We found no significant relationship between an individual child participant's age and the child's individual crossover (τb = 0.04, p = 0.71; Figure 3). Taken together with the group-level analyses, we find little evidence for a developmental trend within our population.
Figure 2.
Performance (proportion correct) in the integration (gray) and segmentation (black) tasks (normalized by pretest practice trials; see Practice trials and lapse rate estimation) as a function of ISI. The point at which these two functions intersect defined the crossover point, that is, our estimate for the length of the TIW. (A) The child group (N = 38) had a TIW of 68 ms (at 47% correct), and (B) the adult group (N = 22) had a TIW of 73 ms (at 65% correct). (C) The below-median age group (5-year-olds) had a TIW of 67.5 ms (at 42% correct), and (D) the above-median group (6-year-olds) had a TIW of 67.3 ms (at 52% correct). Error bars indicate one SEM and whiskers the 95% confidence intervals. Dotted lines show confidence intervals around the least squares fits of the data. The arrow indicates the TIW. The shaded segment of the x-axis shows the 95% confidence interval around the estimate of the TIW.
Figure 3.
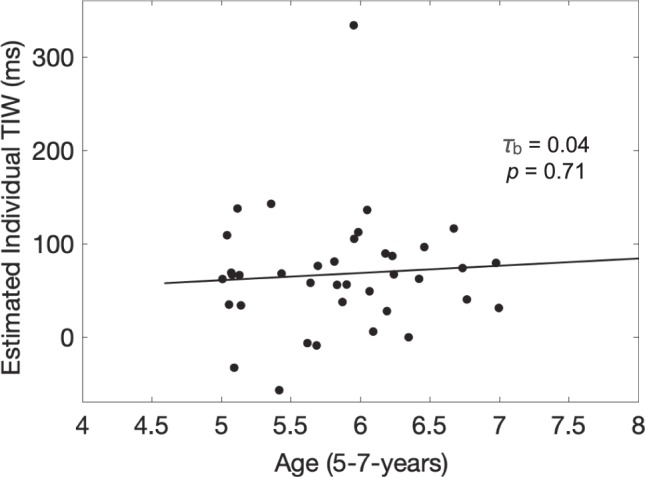
Kendall rank correlation between TIWs and age for individual children (N = 38). There was no significant age effect on the TIW from 5 to 7 years of age (τb = 0.04, p = 0.71). Tau represents the correlation coefficient, and a Theil-Sen line is shown for reference (the Theil-Sen estimator is a nonparametric alternative to the least squares regression line for linear trends; Wilcox, 2005).
There have been reports that gender may influence temporal processing (e.g., adult males having higher temporal frequency thresholds than females in an attentional tracking task for instance; Roudaia & Faubert, 2017). To assess this, we grouped the child data by gender and estimated group crossovers as we did in the other analyses. Here, we found no difference in TIW (or performance) between female (N = 17) and male (N = 21) children with TIWs of 65 ms (at 47% correct) and 71 ms (at 48% correct), respectively. Both values are well within the 95% confidence intervals around the estimated TIWs (52.76–81.12 ms for males and 60.72–85.14 ms for females).
We also performed an individual-level analysis to evaluate the relationship between a participant's age and overall task performance (average percentage correct over all timing conditions). Here we saw a moderate, significant relationship between age and performance (τb = 0.37, p = 0.001; Figure 4).
Figure 4.
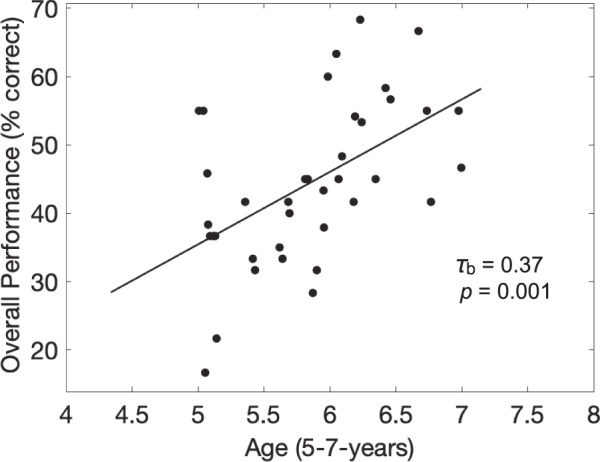
Kendall rank correlation between overall performance and age for individual children (N = 38). There is a significant age effect on overall performance (τb = 0.37, p = 0.001). Tau represents the correlation coefficient, and a Theil-Sen line is shown for reference.
Discussion
We estimated the duration of visual TIWs in typically developing 5- to 7-year-old children and adults by measuring both integration and segmentation using the same stimuli and task and within the same participants. We found that TIWs were adult-like in children at approximately 68 ms. This value was comparable to the value of 73 ms we found for adults and replicates values from adult work using a similar paradigm (Wutz et al., 2016). Although we found a significant age effect on overall performance, which likely reflects increases in overall task compliance and visual search abilities (e.g., a developmental increase in processing speed during a visual search task; Lobaugh, Cole, & Rovet, 1998), we did not find a significant effect of age on the TIW.
Developmental studies up until now have measured only temporal integration and segmentation separately, using different paradigms and stimuli across different age groups. Di Lollo, Hogben, and colleagues measured integration in children between 7 and 13 years of age (Arnett & Di Lollo, 1979; Hogben et al., 1995) and found thresholds that were similar to adults. However, the use of different stimuli and temporal parameters made it challenging to directly compare between age groups. Farzin et al. (2011) measured segmentation in 6- to 15-month-olds and found a decrease in thresholds (narrowing windows) within this age range. In this study, we measured temporal processing in an age group that fell between those studies. Even though we found adult-like TIWs in the 5- to 7-year-old age range, in line with the results of Farzin et al., we would still expect that younger children (under 5 years of age) would have broader TIWs if tested with this same paradigm.
With broader TIWs, an observer is more likely to integrate across longer snapshots, gathering more information about a scene or event, gaining certainty about identity and interpretation, and with narrower TIWs, an observer is more likely to segment shorter snapshots, thereby discriminating changes that occur closely in time. Understanding the temporal resolution of the visual system across development (as measured by the TIW), then, informs how younger children interact with and learn from their environment. Although temporal precision may be reduced in younger children, it may be adequate (or even adaptive) for their range of cognitive and motor processes. We can only speculate at this stage, but broader TIWs may be more advantageous for younger children in that, with more time, they are better able to assess featural properties, such as color and shape, and to better individuate and identify objects. That said, there is a speed–accuracy trade-off with which gathering more information over time comes at the cost of possibly missing rapid dynamic changes. Indeed, the narrowing of TIWs through development (reaching adult levels around 5 years of age) may align with, and facilitate, the emergence of particular cognitive abilities, such as acquiring reading skills or interactional synchrony for pragmatic aspects of communication.
Because of the fundamental role temporal processing plays in visual perception, future work should look not only at how TIWs change over typical development, but also in populations diagnosed with neurodevelopmental disorders, such as autism; potential temporal processing differences may affect downstream social and cognitive processes. For instance, there is growing evidence for alterations in multisensory temporal integration windows in developmental disorders, including developmental dyslexia (for review, Hahn et al., 2014) and autism spectrum disorder (for review, Kawakami et al., 2018; Stevenson et al., 2014; Zhou et al., 2018). A key question is whether these differences are specific to the need to integrate across different sensory modalities or, rather, reflect a more general alteration in temporal aspects of sensory processing. In the later case, differences might be found in unisensory (auditory or visual) temporal integration windows as well. The current study provides a method for investigating such differences in visual temporal integration, building on the finding of adult-like levels of visual temporal integration by the age of 5–7 years old.
Development of brain mechanisms underlying the TIW
Most theories of visual temporal integration propose at least two or three stages of processing at different time scales, likely organized in a hierarchical fashion (Holcombe, 2009; Pöppel, 2009; Ronconi, Oosterhof, Bonmassar, & Melcher, 2017; Wutz & Melcher, 2014). (Similar arguments have been made about temporal windows in audition that support spoken language; Hillock-Dunn et al., 2016). Temporal limits of vision can be categorized into at least two groups, in which fast processing includes integration of low-level features (e.g., changes in luminance), and slow processing includes integration of high-level features (e.g., the featural properties of an object; Holcombe, 2009; Wutz & Melcher, 2014). These processes have a different developmental time course. CFF (the rate at which an intermittent light stimulus appears steady), a low-level retinal process, matures early on and reaches adult-like levels within the first few months of life (Dobkins & Teller, 1996; Rasengane et al., 1997; Regal, 1981). In contrast, temporal processing of higher-level processes develops later. For example, Farzin et al. (2011) found that infants (6–15 months) had increased segmentation thresholds compared to adults (1,000–2,000 ms vs. 100 ms) when having to discriminate alternating patterns of light and dark squares, yet their flicker thresholds were much lower, around 100 ms. These results suggest that temporal segmentation of patterns reflects a separate mechanism from low-level flicker fusion and with a different developmental time course. Combined with evidence that higher-level visual processing, such as auditory processing, seems to include multiple independent stages (Ronconi et al., 2017), this raises the question of whether even these higher-level stages mature along different developmental trajectories.
The narrowing of temporal windows to become more adult-like over early development is consistent with brain structural maturation. Recent work has shown that efficiency of processing has been implicated as a neural correlate of TIWs (Wutz & Melcher, 2014; Wutz et al., 2016). Processing efficiency is characterized by communication between lower and higher brain regions and feedback processes, which is supported by an increase in white matter myelination and network structure as a result of synaptic pruning. Thus, infants and young children with cortical regions that are less myelinated have lower efficiency (Dubois et al., 2008) that could lengthen TIWs. It has also been shown that younger (3-year-old) children show less processing efficiency (increased inspection times in an object recognition task) and decreased myelination in the visual cortices as compared to older (5-year-old) children (Chevalier et al., 2015). Our results, then, suggest that the ongoing structural maturation is sufficient for adult-like temporal processing by 5–7 years of age.
Supporting this, it has been shown that peak alpha frequency approaches adult levels (around 10 Hz) by 4 years of age, whereas peak alpha is very low (or is not found) in infants (Miskovic et al., 2015; Smith, 1941). A link has been shown between peak alpha frequency, measured with EEG and MEG, and the speed of temporal integration and segmentation (Ronconi & Melcher, 2017; Samaha & Postle, 2015; Wutz et al., 2018): Information tends to be integrated if it falls within the same alpha cycle and segmented if it falls in a different alpha cycle. (Neural oscillations in the occipital alpha band cycle have also been linked to how one strategically parses the visual environment given certain task demands with alpha frequencies increasing when a task requires segmentation and decreasing when instead integration is beneficial, highlighting top-down modulation of perceptual processes; Wutz et al., 2018). This suggests that the brain maturation involved in alpha network coherence has neared completion in early childhood, consistent with the lack of developmental change in temporal processing from 5 years of age to adulthood found in this study.
Acknowledgments
Preliminary data from this study were presented at the Annual Meeting of the Vision Sciences Society (Freschl, Melcher, Kaldy, & Blaser, 2018). This project was supported by a grant from the National Institute of Mental Health (R21MH117787) to EB, ZK, and DM. We thank Sangya Dhungana and other members of the UMass Boston Baby Lab for their help with participant recruitment and the families that participated in this study.
Commercial relationships: none.
Corresponding author: Julie Freschl.
Email: julie.freschl001@umb.edu.
Address: Department of Psychology, University of Massachusetts Boston, Boston, MA, USA.
Footnotes
In this context, the critical flicker frequency (CFF)—the rate at which an intermittent light stimulus appears steady to the human observer—can be thought of as testing just a single point on the tCSF: the maximum temporal frequency cutoff as opposed to the full curve. Developmental studies measuring CFF thresholds have shown that within the first few months of life, CFF frequency approaches adult levels (Dobkins & Teller, 1996; Rasengane, Allen, & Manny, 1997; Regal, 1981).
Differences in the tuning of TIWs, particularly for multisensory integration, have also been linked to a number of developmental disorders. Such differences have been reported for developmental dyslexia (reviewed in Hahn, Foxe, & Molholm, 2014) and autism spectrum disorder (reviewed in Stevenson et al., 2014; Kawakami, Uono, Otsuka, Zhao, & Toichi, 2018; and Zhou et al., 2018). These results suggest a possible link between the tuning of TIWs during development and the acquisition of skills for language and social interaction. Thus, a better understanding of the developmental time course of temporal integration is important for characterizing normal and altered sensory processing and its effects on cognition.
It is worth noting that the odds of guessing correctly, chance, is 1/16 (6.3%).
References
- Arnett J. L, Di Lollo V. Visual information processing in relation to age and to reading ability. Journal of Experimental Child Psychology. (1979);27(1):143–152. doi: 10.1016/0022-0965(79)90066-3. [DOI] [PubMed] [Google Scholar]
- Blake R, Lee S.-H. The role of temporal structure in human vision. Behavioral and Cognitive Neuroscience Reviews. (2005);4(1):21–42. doi: 10.1177/1534582305276839. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J. Development of brain mechanisms for visual global processing and object segmentation. Progress in Brain Research. (2007);164:151–168. doi: 10.1016/S0079-6123(07)64008-4. [DOI] [PubMed] [Google Scholar]
- Braddick O. J, Atkinson J. Infants' sensitivity to motion and temporal change. Optometry and Vision Science. (2009);86(6):577–582. doi: 10.1097/OPX.0b013e3181a76e84. [DOI] [PubMed] [Google Scholar]
- Brainard D. H. The Psychophysics Toolbox. Spatial Vision. (1997);10(4):433–436. [PubMed] [Google Scholar]
- Chen Y.-C, Shore D. I, Lewis T. L, Maurer D. The development of the perception of audiovisual simultaneity. Journal of Experimental Child Psychology. (2016);146:17–33. doi: 10.1016/j.jecp.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Kurth S, Doucette M. R, Wiseheart M, Deoni S. C. L, Dean D. C, 3rd, LeBourgeois M. K. Myelination is associated with processing speed in early childhood: Preliminary insights. PLoS One. (2015);10(10):e0139897. doi: 10.1371/journal.pone.0139897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lollo V. Temporal integration in visual memory. Journal of Experimental Psychology, General. (1980);109(1):75–97. doi: 10.1037/0096-3445.109.1.75. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Arnett J. L, Kruk R. V. Age-related changes in rate of visual information processing. Journal of Experimental Psychology, Human Perception and Performance. (1982);8(2):225–237. doi: 10.1037/0096-1523.8.2.225. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Hogben J. H. Suppression of visible persistence as a function of spatial separation between inducing stimuli. Perception & Psychophysics. (1987);41(4):345–354. doi: 10.3758/bf03208236. [DOI] [PubMed] [Google Scholar]
- Dobkins K. R, Anderson C. M, Lia B. Infant temporal contrast sensitivity functions (tCSFs) mature earlier for luminance than for chromatic stimuli: Evidence for precocious magnocellular development? Vision Research. (1999);39(19):3223–3239. doi: 10.1016/s0042-6989(99)00020-6. [DOI] [PubMed] [Google Scholar]
- Dobkins K. R, Teller D. Y. Infant motion: Detection (M:D) ratios for chromatically defined and luminance-defined moving stimuli. Vision Research. (1996);36(20):3293–3310. doi: 10.1016/0042-6989(96)00069-7. [DOI] [PubMed] [Google Scholar]
- Drewes J, Zhu W, Wutz A, Melcher D. Dense sampling reveals behavioral oscillations in rapid visual categorization. Scientific Reports. (2015);5:16290. doi: 10.1038/srep16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Soarès C, Cointepas Y, Le Bihan D, Hertz-Pannier L. Microstructural correlates of infant functional development: Example of the visual pathways. The Journal of Neuroscience. (2008);28(8):1943–1948. doi: 10.1523/JNEUROSCI.5145-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellemberg D, Lewis T. L, Liu C. H, Maurer D. Development of spatial and temporal vision during childhood. Vision Research. (1999);39(14):2325–2333. doi: 10.1016/s0042-6989(98)00280-6. [DOI] [PubMed] [Google Scholar]
- Fairhall S. L, Albi A, Melcher D. Temporal integration windows for naturalistic visual sequences. PLoS One. (2014);9(7):e102248. doi: 10.1371/journal.pone.0102248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre N, Koch C. Temporal structure coding with and without awareness. Cognition. (2014);131(3):404–414. doi: 10.1016/j.cognition.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Falter C. M, Elliott M. A, Bailey A. J. Enhanced visual temporal resolution in autism spectrum disorders. PLoS One. (2012);7(3):e32774. doi: 10.1371/journal.pone.0032774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Rivera S. M, Whitney D. Time crawls: The temporal resolution of infants' visual attention. Psychological Science. (2011);22(8):1004–1010. doi: 10.1177/0956797611413291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliben J. J, McKinny J. E. Confidence limits for the abscissa of intersection of two linear regressions. Journal of Research of the National Bureau of Standards, Section B: Mathematical Sciences. (1972);76B:179–192. doi: 10.6028/jres.076b.013. [DOI] [Google Scholar]
- Freschl J, Melcher D, Kaldy Z, Blaser E. Visual Temporal Integration Windows are adult-like in typically developing 5-7-year-old children. Poster presented at the Annual Meeting of the Vision Sciences Society; St. Pete Beach, FL: (2018). May 18–23, 2018. [Google Scholar]
- Gorea A. A refresher of the original Bloch's law paper (Bloch, July 1885) I-Perception. (2015);6(4):2041669515593043. doi: 10.1177/2041669515593043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn A, Cory E, Atkinson J, Braddick O, Wattam-Bell J, Guzzetta A, Cioni G. Dorsal and ventral stream sensitivity in normal development and hemiplegia. Neuroreport. (2002);13(6):843–847. doi: 10.1097/00001756-200205070-00021. [DOI] [PubMed] [Google Scholar]
- Hahn N, Foxe J. J, Molholm S. Impairments of multisensory integration and cross-sensory learning as pathways to dyslexia. Neuroscience and Biobehavioral Reviews. (2014);47:384–392. doi: 10.1016/j.neubiorev.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E. E, Banks M. S. Temporal contrast sensitivity in human infants. Vision Research. (1992);32(6):1163–1168. doi: 10.1016/0042-6989(92)90018-e. [DOI] [PubMed] [Google Scholar]
- Hillock-Dunn A, Grantham D. W, Wallace M. T. The temporal binding window for audiovisual speech: Children are like little adults. Neuropsychologia. (2016);88:74–82. doi: 10.1016/j.neuropsychologia.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Hogben J. H, Rodino I. S, Clark C. D, Pratt C. A comparison of temporal integration in children with a specific reading disability and normal readers. Vision Research. (1995);35(14):2067–2074. doi: 10.1016/0042-6989(94)00278-t. [DOI] [PubMed] [Google Scholar]
- Holcombe A. O. Seeing slow and seeing fast: Two limits on perception. Trends in Cognitive Sciences. (2009);13(5):216–221. doi: 10.1016/j.tics.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Hommel B, Müsseler J, Aschersleben G, Prinz W. The theory of event coding (TEC): A framework for perception and action planning. The Behavioral and Brain Sciences. (2001);24(5):849–878. doi: 10.1017/s0140525x01000103. discussion 878–937. [DOI] [PubMed] [Google Scholar]
- Honey C. J, Thesen T, Donner T. H, Silbert L. J, Carlson C. E, Devinsky O, Hasson U. Slow cortical dynamics and the accumulation of information over long timescales. Neuron. (2012);76(2):423–434. doi: 10.1016/j.neuron.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Shirai N. Early development of dynamic shape perception under slit-viewing conditions. Perception. (2014);43(7):654–662. doi: 10.1068/p7606. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Uono S, Otsuka S, Zhao S, Toichi M. Everything has its time: Narrow temporal windows are associated with high levels of autistic traits via weaknesses in multisensory integration. Journal of Autism and Developmental Disorders. (2018) doi: 10.1007/s10803-018-3762-z. [DOI] [PubMed]
- Kendall M. G. Rank correlation methods. Oxford, England: Griffin; (1948). [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What's new in Psychtoolbox-3. Perception. (2007);36(14):1–16. [Google Scholar]
- Koldewyn K, Whitney D, Rivera S. M. The psychophysics of visual motion and global form processing in autism. Brain. (2010);133(Pt 2):599–610. doi: 10.1093/brain/awp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh N. J, Cole S, Rovet J. F. Visual search for features and conjunctions in development. Canadian Journal of Experimental Psychology. (1998);52(4):201–212. doi: 10.1037/h0087293. [DOI] [PubMed] [Google Scholar]
- Manning C, Jones P. R, Dekker T. M, Pellicano E. Psychophysics with children: Investigating the effects of attentional lapses on threshold estimates. Attention, Perception, & Psychophysics. (2018);80(5):1311–1324. doi: 10.3758/s13414-018-1510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Ma X, Chou C.-A, Fan M, Owens M, Sayama H, Gibb B. E. Developmental changes in spontaneous electrocortical activity and network organization from early to late childhood. NeuroImage. (2015);118:237–247. doi: 10.1016/j.neuroimage.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. M, Lewkowicz D. J, Amedi A, Wallace M. T. Multisensory processes: A balancing act across the lifespan. Trends in Neurosciences. (2016);39(8):567–579. doi: 10.1016/j.tins.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, McIntosh A. R, Houle S, Nilsson L. G, Tulving E. Activation of medial temporal structures during episodic memory retrieval. Nature. (1996 Apr 25);380(6576):715–717. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]
- Pöppel E. Pre-semantically defined temporal windows for cognitive processing. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. (2009);364(1525):1887–1896. doi: 10.1098/rstb.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasengane T. A, Allen D, Manny R. E. Development of temporal contrast sensitivity in human infants. Vision Research. (1997);37(13):1747–1754. doi: 10.1016/s0042-6989(96)00300-8. [DOI] [PubMed] [Google Scholar]
- Regal D. M. Development of critical flicker frequency in human infants. Vision Research. (1981);21(4):549–555. doi: 10.1016/0042-6989(81)90100-0. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Melcher D. Alpha oscillation phase determines the timing of perception: Evidence from sensory entrainment. Journal of Vision. (2017);17(10):726. https://doi.org/10.1167/17.10.726 Abstract. [Google Scholar]
- Ronconi L, Oosterhof N. N, Bonmassar C, Melcher D. Multiple oscillatory rhythms determine the temporal organization of perception. Proceedings of the National Academy of Sciences, USA. (2017);114(51):13435–13440. doi: 10.1073/pnas.1714522114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudaia E, Faubert J. Different effects of aging and gender on the temporal resolution in attentional tracking. Journal of Vision. (2017);17(11):1–16.:1. doi: 10.1167/17.11.1. https://doi.org/10.1167/17.11.1 PubMed] [ Article. [DOI] [PubMed] [Google Scholar]
- Rucci M, Ahissar E, Burr D. Temporal coding of visual space. Trends in Cognitive Sciences. (2018);22(10):883–895. doi: 10.1016/j.tics.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüter J, Marcille N, Sprekeler H, Gerstner W, Herzog M. H. Paradoxical evidence integration in rapid decision processes. PLoS Computational Biology. (2012);8(2):e1002382. doi: 10.1371/journal.pcbi.1002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha J, Postle B. R. The speed of alpha-band oscillations predicts the temporal resolution of visual perception. Current Biology. (2015);25(22):2985–2990. doi: 10.1016/j.cub.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P, Melcher D, Hickey C. Endogenous attention modulates the temporal window of integration. Attention, Perception & Psychophysics. (2018);80(5):1214–1228. doi: 10.3758/s13414-018-1506-y. [DOI] [PubMed] [Google Scholar]
- Smith J. R. The frequency growth of the human alpha rhythms during normal infancy and childhood. The Journal of Psychology. (1941);11(1):177–198. [Google Scholar]
- Stevenson R. A, Siemann J. K, Schneider B. C, Eberly H. E, Woynaroski T. G, Camarata S. M, Wallace M. T. Multisensory temporal integration in autism spectrum disorders. The Journal of Neuroscience. (2014);34(3):691–697. doi: 10.1523/JNEUROSCI.3615-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. M, Gelade G. A feature-integration theory of attention. Cognitive Psychology. (1980);12(1):97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Trevarthen C, Daniel S. Disorganized rhythm and synchrony: Early signs of autism and Rett syndrome. Brain & Development. (2005);27(Suppl. 1):S25–S34. doi: 10.1016/j.braindev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- VanRullen R. Perceptual cycles. Trends in Cognitive Sciences. (2016);20(10):723–735. doi: 10.1016/j.tics.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Vroomen J, Keetels M. Perception of intersensory synchrony: A tutorial review. Attention, Perception & Psychophysics. (2010);72(4):871–884. doi: 10.3758/APP.72.4.871. [DOI] [PubMed] [Google Scholar]
- Wallace M. T, Stevenson R. A. The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia. (2014);64:105–123. doi: 10.1016/j.neuropsychologia.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Nikolić D. An LCD monitor with sufficiently precise timing for research in vision. Frontiers in Human Neuroscience. (2011);5:85. doi: 10.3389/fnhum.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox R. R. Introduction to robust estimation and hypothesis testing. Amsterdam: Academic Press, an imprint of Elsevier; (2005). [Google Scholar]
- Wolfe J. M, Horowitz T. S. Five factors that guide attention in visual search. Nature Human Behaviour. (2017);1:0058. doi: 10.1038/s41562-017-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten B. R, Renzi L. M, Moore R, Hammond B. R. A practical method of measuring the human temporal contrast sensitivity function. Biomedical Optics Express. (2010);1(1):47–58. doi: 10.1364/BOE.1.000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A, Melcher D. Temporal buffering and visual capacity: The time course of object formation underlies capacity limits in visual cognition. Attention, Perception & Psychophysics. (2013);75(5):921–933. doi: 10.3758/s13414-013-0454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A, Melcher D. The temporal window of individuation limits visual capacity. Frontiers in Psychology. (2014);5:952. doi: 10.3389/fpsyg.2014.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A, Melcher D, Samaha J. Frequency modulation of neural oscillations according to visual task demands. Proceedings of the National Academy of Sciences, USA. (2018);115(6):1346–1351. doi: 10.1073/pnas.1713318115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A, Muschter E, van Koningsbruggen M. G, Weisz N, Melcher D. Temporal integration windows in neural processing and perception aligned to saccadic eye movements. Current Biology. (2016);26(13):1659–1668. doi: 10.1016/j.cub.2016.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.-Y, Cai X.-L, Weigl M, Bang P, Cheung E. F. C, Chan R. C. K. Multisensory temporal binding window in autism spectrum disorders and schizophrenia spectrum disorders: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. (2018);86:66–76. doi: 10.1016/j.neubiorev.2017.12.013. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Morrone M. C, Burr D. C. Spatial position information accumulates steadily over time. The Journal of Neuroscience. (2013);33(47):18396–18401. doi: 10.1523/JNEUROSCI.1864-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]