Abstract
The 2019 edition of the IAS-USA drug resistance mutations list updates the Figure last published in January 2017. The mutations listed are those that have been identified by specific criteria for evidence and drugs described. The Figure is designed to assist practitioners in identifying key mutations associated with resistance to antiretroviral drugs, and therefore, in making clinical decisions regarding antiretroviral therapy.
Keywords: HIV, antiretroviral, drug resistance, therapy, mutations
The 2019 edition of the International Antiviral Society-USA (IAS-USA) drug resistance mutations list updates the Figure last published in January 2017.1 In this update:
2 integrase strand transfer inhibitors (InSTIs), bictegravir and cabotegravir, and the nonnucleoside reverse transcriptase inhibitor (NNRTI), doravirine, were added to the Figure.
Bictegravir (formerly GS-9883) was approved by the US Food and Drug Administration (FDA) in February 2018 as part of a fixed-dose combination of bictegravir/emtricitabine/tenofovir alafenamide for the treatment of HIV-infected, treatmentnaive individuals or to replace an antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA below 50 copies/mL) on a stable antiretroviral regimen for at least 3 months with no history of treatment failure and no known substitutions associated with resistance to the individual components of the fixed-dose combination.2
Cabotegravir (formerly S/GSK-1265744) is an investigational drug that has been filed for approval at the FDA as part of a fixed-dose, long-acting combination of cabotegravir/rilpivirine for the treatment of HIV-infection in adults who are virologically suppressed and have no resistance to the individual components of the combination.3
Doravirine (formerly MK-1439) was approved by the FDA in August 2018 for the treatment of HIV-infected, treatment-naive individuals in combination with other antiretroviral drugs.4
Several changes were made to drugs already on the Figure. On the lopinavir/ritonavir bar, mutations at positions 50, 54, and 84 were changed to boldface to indicate recognition as major mutations rather than minor mutations.5–7 The G118R mutation was added to the bar for the InSTI dolutegravir.8,9
For antiretroviral drugs that are no longer recommended, the bars are listed at the bottom of the class and are shaded in gray.
Methods
The IAS-USA Drug Resistance Mutations Group is an independent, volunteer panel of experts charged with delivering accurate, unbiased, and evidence-based information on drug resistance-associated mutations for HIV clinical practitioners. The group reviews new data on HIV drug resistance to maintain a current list of mutations associated with clinical resistance to HIV-1. This list includes mutations that may contribute to a reduced virologic response to a drug.
The group considers only data that have been published or have been presented at a scientific conference. Table 1 provides the list of amino acids and the abbreviations used. Drugs that have been approved by the US Food and Drug Administration and are generally recommended, as well as any drugs available in expanded access programs are included (listed in alphabetic order by drug class). Drugs that are no longer recommended are listed at the bottom of the class and are shaded in gray. User notes provide additional information. Although the Drug Resistance Mutations Group works to maintain a complete and current list of these mutations, it cannot be assumed that the list presented here is exhaustive.
Table 1.
Amino acids and their abbreviations.
| Alanine | A |
| Cysteine | C |
| Aspartate | D |
| Glutamate | E |
| Phenylalanine | F |
| Glycine | G |
| Histidine | H |
| Isoleucine | I |
| Lysine | K |
| Leucine | L |
| Methionine | M |
| Asparagine | N |
| Proline | P |
| Glutamine | Q |
| Arginine | R |
| Serine | S |
| Threonine | T |
| Valine | V |
| Tryptophan | W |
| Tryosine | Y |
The magnitude of the reduction in susceptibility conferred by drug resistance mutations varies widely, and is modulated by the genetic context of the HIV sequence in which the mutation occurs. Despite the fact that mutations result in a spectrum of degrees of resistance, mutations have been arbitrarily designated as major (bolded) or minor (not bolded) (see Figure 1). Those defined as major tend to occur earlier during treatment failure and generally confer larger reductions in susceptibility. Those defined as minor tend to accrue after the emergence of a major mutation, confer some incremental resistance, may occur as well as polymorphisms in wild-type virus, and in some cases do not reduce susceptibility but restore replication fitness to viruses with resistance mutations that impair fitness. In general, a major mutation should raise concern that a drug is at least partially compromised; a minor mutation on its own may not raise such a concern, but it should add concern in the presence of other mutations.
Figure 1.
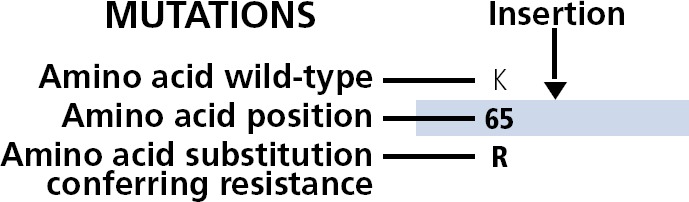
Display of the Figure Bar: Amino acid position, wild type, mutation conferring resistance, and indication of insertion mutation.
Identification of Mutations
The mutations listed are those that have been identified by 1 or more of the following criteria: (1) in vitro passage experiments or validation of contribution to resistance by using site-directed mutagenesis; (2) susceptibility testing of laboratory or clinical isolates; (3) nucleotide sequencing of viruses from patients in whom the drug is failing; (4) association studies between genotype at baseline and virologic response in patients exposed to the drug.
The development of more recently approved drugs that cannot be tested as monotherapy precludes assessment of the impact of resistance on antiretroviral activity that is not seriously confounded by activity of other drug components in the background regimen. Readers are encouraged to consult the literature and experts in the field for clarification or more information about specific mutations and their clinical impact. Polymorphisms associated with impaired treatment responses that occur in otherwise wildtype viruses should not be used in epidemiologic analyses to identify transmitted HIV-1 drug resistance. Consequently, only some of the resistance mutations depicted on the Figure can be used to identify transmitted drug resistance.10
Clinical Context
The Figure is designed for practitioners to use in identifying key mutations associated with antiretroviral drug resistance and in making therapeutic decisions. In the context of making clinical decisions regarding antiretroviral therapy, evaluating the results of HIV-1 genotypic testing includes: (1) assessing whether the pattern or absence of a pattern in the mutations is consistent with the patient's history of antiretroviral therapy; (2) recognizing that in the absence of current drug treatment that is conferring selection pressure, resistant strains may be present at levels below the limit of detection of the test (analyzing stored samples, collected under selection pressure, could be useful in this setting); and (3) recognizing that virologic failure of a first-line regimen typically involves HIV-1 isolates with resistance to only 1 or 2 of the drugs in the regimen. In this setting, resistance emerges most commonly to lamivudine or emtricitabine, nonnucleoside analogue reverse transcriptase inhibitors, or first generation InSTIs (elvitegravir, raltegravir).
The absence of detectable viral resistance after treatment failure may result from any combination of the following factors: the presence of drug-resistant minority viral populations, a prolonged interval between the time of antiretroviral drug discontinuation and genotypic testing, nonadherence to medications, laboratory error, lack of current knowledge of the association of certain mutations with drug resistance, the occurrence of relevant mutations outside the regions targeted by routine resistance assays, drug-drug interactions leading to subtherapeutic drug levels, and possibly compartmental issues, indicating that drugs may not reach optimal levels in specific cellular or tissue reservoirs.
For more in-depth reading and an extensive reference list, see the 2018 IAS-USA panel recommendations for resistance testing11 and 2018 IAS-USA panel recommendations for antiretroviral therapy.12 Updates are posted periodically at www.iasusa.org.
Comments
Please send your evidence-based comments, including relevant reference citations, to journal@iasusa.org.
Reprint Requests
The Drug Resistance Mutations Group welcomes interest in the Figure as an educational resource for practitioners and encourages dissemination of the material to as broad an audience as possible. However, permission is required to reprint the Figure and no alterations in format or content can be made.
Requests to reprint the material should include the name of the publisher or sponsor, the name or a description of the publication in which the material will be reprinted, the funding organization(s), if applicable, and the intended audience. Requests to make any minimal adaptations of the material should include the former, plus a detailed explanation of the adaptation(s) and, if possible, a copy of the proposed adaptation. To ensure the integrity of the Figure, IAS-USA policy is to grant permission for only minor, pre-approved adaptations of the Figure (eg, an adjustment in size). Minimal adaptations only will be considered; no alterations of the content of the Figure or user notes will be permitted.
Permission will be granted only for requests to reprint or adapt the most current version of the Figure as they are posted at www.iasusa.org. Because scientific under-standing of HIV drug resistance evolves and the goal of the Drug Resistance Mutations Group is to maintain the most up-to-date compilation of mutations for HIV clinicians and researchers, publication of out-of-date figures is counterproductive. If you have any questions about reprints or adaptations, please contact IAS-USA.
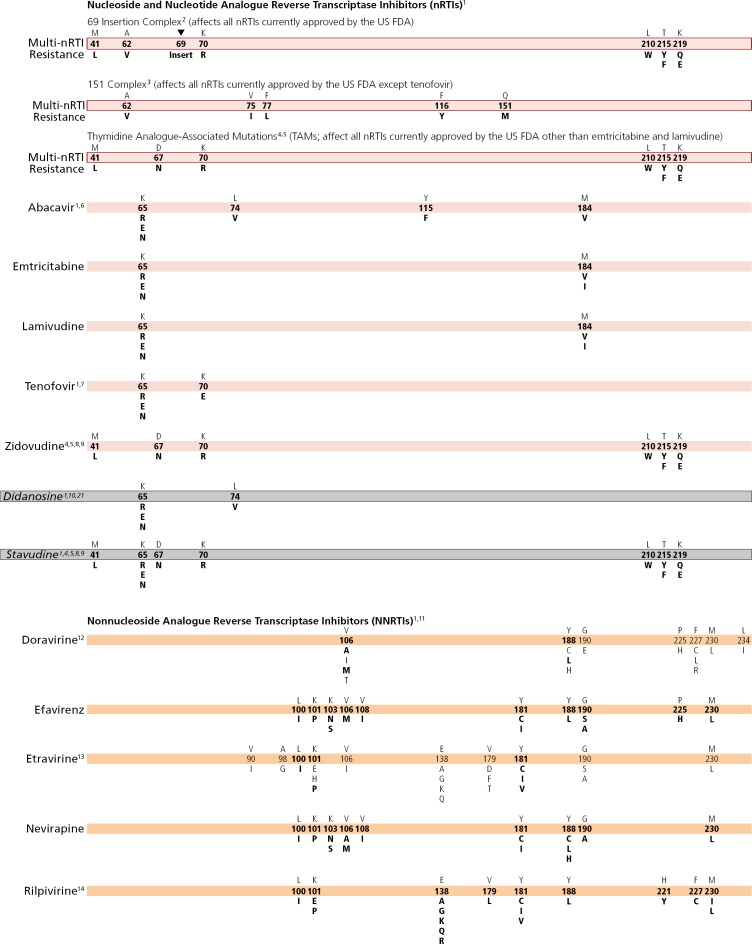
MUTATIONS IN THE REVERSE TRANSCRIPTASE GENE ASSOCIATED WITH RESISTANCE TO REVERSE TRANSCRIPTASE INHIBITORS
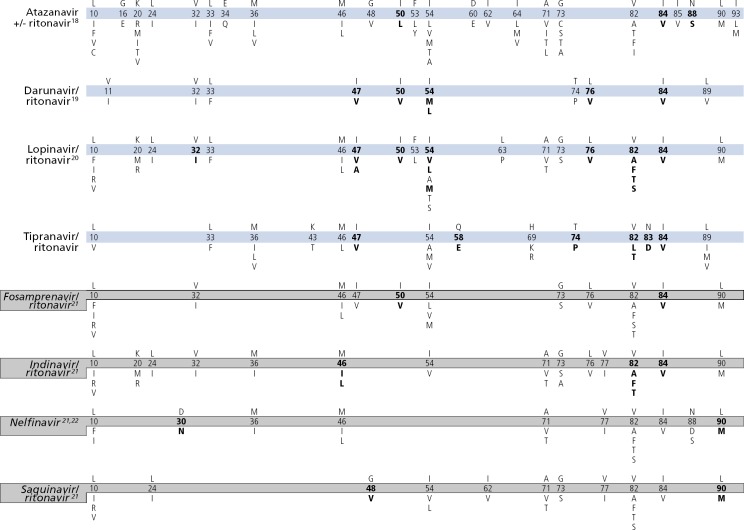
MUTATIONS IN THE PROTEASE GENE ASSOCIATED WITH RESISTANCE TO PROTEASE INHIBITORS(PIs)15,16,17
MUTATIONS IN THE ENVELOPE GENE ASSOCIATED WITH RESISTANCE TO ENTRY INHIBITORS
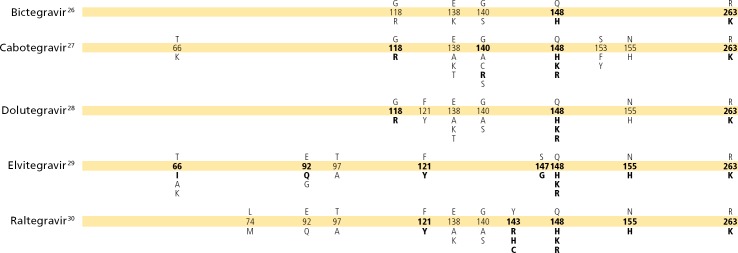
MUTATIONS IN THE INTEGRASE GENE ASSOCIATED WITH RESISTANCE TO INTEGRASE STRAND TRANSFER INHIBITORS25
User Notes
1. Mutations at the C-terminal reverse transcriptase domains (amino acids 293–560) outside of regions depicted on the Figure Bar may contribute to nucleoside (or nucleotide) analogue reverse transcriptase inhibitor (nRTI) and nonnucleoside analogue reverse transcriptase inhibitors (NNRTI) HIV-1 drug resistance. The clinical relevance of these connection domain mutations arises mostly in conjunction with thymidine analogue-associated mutations (TAMs) and M184V and they have not been associated with increased rates of virologic failure of etravirine or rilpivirine in clinical trials.1–3 K65E/N/R variants are reported in patients experiencing treatment failure with tenofovir (meaning tenofovir disoproxil fumarate [TDF] or tenofovir alafenamide [TAF]) stavudine, or didanosine. The K65R/N variants may be selected by tenofovir, didanosine, abacavir, or stavudine and are associated with decreased viral susceptibility to these drugs.4–8 65R may be more easily selected in subtype C clades.9 K65E usually occurs in mixtures with wild-type virus. Patient-derived viruses with K65E and site-directed mutations replicate very poorly in vitro; as such, no susceptibility testing can be performed.10,11 Some nRTI mutations, like T215Y and H208Y,12 may lead to viral hypersusceptibility to nonnucleoside reverse transcriptase inhibitors (NNRTIs), including etravirine,13 in nRTI-treated individuals. The presence of these mutations may improve subsequent virologic response to NNRTI-containing regimens (nevirapine or efavirenz) in NNRTI-naive individuals,14–18 although no clinical data exist for improved response to etravirine in NNRTI-experienced individuals.
2. The 69 insertion complex consists of a substitution at codon 69 (typically T69S) and an insertion of 2 or more amino acids (S-S, S-A, S-G, or others). The 69 insertion complex is associated with resistance to all nRTIs currently approved by the US Food and Drug Administration (FDA) when present with 1 or more TAMs at codons 41, 210, or 215.4 Some other amino acid changes from the wild-type T at codon 69 without the insertion may be associated with broad nRTI resistance.
3. Tenofovir retains activity against the Q151M complex of mutations.4 Q151M is the most important mutation in the complex (ie, the other mutations in the complex [A62V, V75I, F77L, and F116Y] in isolation may not reflect multidrug resistance). Since no differences in resistance patterns have been observed between TDF and TAF, both drugs are referred to as “tenofovir” on the Figure Bar.19 recommended with tenofovir or zidovudine in the presence of TAMs. The degree to which cross-resistance is observed depends on the specific mutations and number of mutations involved.21–24
5. Although reverse transcriptase changes associated with the E44D and V118I mutations may have an accessory role in increased resistance to nRTIs in the presence of TAMs, their clinical relevance is very limited.25–27
6. The M184V mutation alone does not appear to be associated with a reduced virologic response to abacavir in vivo. When associated with TAMs, M184V increases abacavir resistance.5,28
7. The presence of K65R is associated with a reduced virologic response to tenofovir.4 A reduced response also occurs in the presence of 3 or more TAMs inclusive of either M41L or L210W.4 The presence of TAMs or combined treatment with zidovudine prevents the emergence of K65R in the presence of tenofovir.29–31
8. The presence of M184V appears to delay or prevent emergence of TAMs.32 This effect may be overcome by an accumulation of TAMs or other mutations.
9. The T215A/C/D/E/G/H/I/L/N/S/V substitutions are revertant mutations at codon 215 that confer increased risk of virologic failure of zidovudine or stavudine in antiretroviral-naive patients.33,34 The T215Y mutant may emerge quickly from one of these mutations in the presence of zidovudine or stavudine.35
10. The presence of 3 of the following mutations—M41L, D67N, L210W, T215Y/F, K219Q/E—is associated with resistance to didanosine.36 The presence of K70R or M184V alone does not decrease virologic response to didanosine.37 However, the mutations depicted on the Figure Bar cannot be considered comprehensive because little relevant research has been reported in recent years to update the resistance and cross-resistance patterns for this drug.
11. There is no evidence for the utility of efavirenz, nevirapine, or rilpivirine in patients with NNRTI resistance.38
12. Doravirine is active in vitro against variants containing the common NNRTI mutations K103N, E138K, Y181C, and G190A.39,40 Doravirine selects for mutations at positions 106, 108, 227, and 234, with more than 1 mutation usually required for substantial levels of resistance.41 Mutations V106A, Y188L, and M230L are associated with a 10- or greater fold reduced susceptibility to doravirine. V106A and Y188L have also been selected in vivo.42,43 In 1 clinical isolate, G190E was associated with about 20-fold reduced susceptibility to doravirine.40 Furthermore, the double and triple mutants V106A and F227L; V106A and L234I; V106A and F227L and L234I; and V106A and 190A and F227L, are all associated with substantial resistance to doravirine.39,41,44
13. Resistance to etravirine has been extensively studied only in the context of co-administration with ritonavir-boosted darunavir. There, mutations associated with virologic outcome were assessed and their relative weights (or magnitudes of impact) assigned. In addition, phenotypic cutoff values were calculated, and assessments of genotypephenotype correlations from a large clinical database have determined relative importance of the various mutations. These 2 approaches are in agreement for many, but not all, mutations and weights.45–47 The single mutations L100I, K101P, and Y181C/I/V have high relative weights with regard to reduced susceptibility and reduced clinical response compared with other mutations.48,49 The presence of K103N alone does not affect etravirine response.49 Accumulation of several mutations results in greater reductions in susceptibility and virologic response than do single mutations.50–52
14. Fifteen mutations have been associated with decreased rilpivirine susceptibility (K101E/P, E138A/G/K/Q/R, V179L, Y181C/I/V, H221Y, F227C, and M230I/L).53–55 A 16th mutation, Y188L, reduces rilpivirine susceptibility 6 fold. The K101P and Y181I/V mutations reduce rilpivirine susceptibility approximately 50 fold and 15 fold, respectively, but are not commonly observed in patients receiving rilpivirine.56–58 Mutations at position 138 (most notably E138A) may occur as natural polymorphisms, especially in non-B subtype virus.59 The K101E, E138K, and Y181C mutations, each of which reduces rilpivirine susceptibility 2.5 fold to 3 fold, occur commonly in patients receiving rilpivirine. E138K and to a lesser extent K101E usually occur in combination with the nRTI resistance-associated mutation M184I, which alone does not reduce rilpivirine susceptibility. When M184I is combined with E138K or K101E, rilpivirine susceptibility is reduced about 7 fold and 4.5 fold, respectively.58,60–62 The combinations of reverse transcriptase-associated mutations L100I plus K103N/S and L100I plus K103R plus V179D were strongly associated with reduced susceptibility to rilpivirine. However, for isolates harboring the K103N/R/S or V179D as single mutations, no reduction in susceptibility was detected.55,63
15. Often, several mutations are necessary to substantially impact virologic response to a ritonavir-boosted protease inhibitor (PI).64
16. Mutations in Gag cleavage sites may confer or contribute to resistance to PIs and may even emerge before mutations in protease.65 A large proportion of virus samples from patients with confirmed virologic failure on a PI-containing regimen is not found to have PI resistance-associated mutations.
17. Ritonavir is not listed separately, as it is currently used only at low doses as a pharmacologic booster of other PIs.
18. Several mutations are associated with atazanavir resistance. Their impacts differ, with I50L, I84V, and N88S having the greatest effect. Mutations that are selected during unboosted atazanavir are not different from those selected during boosted atazanavir, but the relative frequency of mutations may differ. Higher atazanavir levels obtained with ritonavir boosting increase the number of mutations required for loss of activity. The presence of M46I plus L76V might increase susceptibility to atazanavir when no other related mutations are present.66
19. Virologic response to ritonavir-boosted darunavir correlates with baseline susceptibility and the presence of several specific PI resistance-associated mutations. Reductions in response are associated with increasing numbers of the mutations indicated on the Figure Bar. The negative impact of the protease mutations I47V, I54M, T74P, and I84V and the positive impact of the protease mutation V82A on virologic response to ritonavir-boosted darunavir were shown independently in 2 data sets.67,68 Some of these mutations appear to have a greater effect on susceptibility than others (eg, I50V vs V11I). The presence at baseline of 2 or more of the substitutions V11I, V32I, L33F, I47V, I50V, I54L/M, T74P, L76V, I84V, or L89V was associated with a decreased virologic response to ritonavir-boosted darunavir.69
20. Virologic response to ritonavir-boosted lopinavir is affected by the presence of 3 or more of the following amino acid substitutions in protease at baseline: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V. In addition, the combination of 47A/V with V32I is associated with high-level resistance.66,70–76 I50V is only occasionally selected in vivo but has a clear impact on susceptibility.77–79 Subtype C patterns with M46L, I54V, L76V, and V82A are frequently observed in patients receiving ritonavir-boosted lopinavir.
21. The mutations depicted on the Figure Bar cannot be considered comprehensive because little relevant research has been reported in recent years to update the resistance and cross-resistance patterns for this drug.
22. In some nonsubtype-B HIV-1, D30N is selected less frequently than are other PI resistance-associated mutations.80
23. Resistance to enfuvirtide is associated primarily with mutations in the first heptad repeat (HR1) region of the gp41 envelope gene. However, mutations or polymorphisms in other regions of the env (eg, the HR2 region or those yet to be identified), as well as coreceptor usage and density, may affect susceptibility to enfuvirtide.81–83
24. The activity of CC chemokine receptor 5 (CCR5) antagonists is limited to patients with virus that use only CCR5 for entry (R5 virus). Viruses that use both CCR5 and CXC chemokine receptor 4 (CXCR4; termed dual/mixed [D/M] virus) or only CXCR4 (X4 virus) do not respond to treatment with CCR5 antagonists. Virologic failure of these drugs is frequently associated with outgrowth of D/M or X4 virus from a preexisting minority population present at levels below the limit of assay detection. Mutations in HIV-1 gp120 that allow the virus to bind to the drug-bound form of CCR5 have been described in viruses from some patients whose virus remained R5 after virologic failure of a CCR5 antagonist. Most of these mutations are found in the V3 loop, the major determinant of viral tropism.84 There is as yet no consensus on specific signature mutations for CCR5 antagonist resistance, so they are not depicted on the Figure Bar. Some CCR5 antagonist-resistant viruses selected in vitro have shown mutations in gp41 without mutations in V3;85 the clinical significance of such mutations is not yet known.
25. In site-directed mutants and clinical isolates, the mutation F121Y has a profound effect on susceptibility to elvitegravir and raltegravir and to a lesser extent to dolutegravir. R263K can be selected in vivo during treatment with dolutegravir and raltegravir and results in a 2-to 5-fold reduction in susceptibility to dolutegravir, elvitegravir, and raltegravir.86–91 263K has been selected in vitro under pressure with bictegravir and cabotegravir.92
26. Bictegravir is a second-generation integrase strand transfer inhibitor (InSTI), like dolutegravir, with higher genetic barrier to resistance than raltegravir and elvitegravir. Bictegravir has only been studied in detail in treatment-naive individuals and those with suppressed viremia (<50 HIV-RNA copies/mL) who have been on stable antiretroviral therapy for at least 3 months without a history of treatment failure and without relevant resistance to bictegravir or its coformulated drugs. Susceptibility studies in vitro and in animal models93 show that mutations G118R and R263K confer 14-fold and 6-fold increases in 50% effective concentration (EC50), whereas the G140S and Q148H combination of mutations decreases HIV-1 susceptibility to bictegravir 4.8 fold. Bictegravir dose-escalation tissue culture experiments also showed the selection of the M50I and R263K mutations. In combination with Q148 and G140A mutations, E138K reduces bictegravir susceptibility 10 fold.94 Bictegravir is coformulated with TAF/emtricitabine, which may protect the drug from mutations such as those observed during virologic failure of dolutegravir monotherapy.93–97
27. Cabotegravir is an investigational, longacting InSTI. In clinical trials, Q148R (fold changes, 5.2–9.4) and G140R (fold change, 6.7) have been observed particularly in HIV-1 A1 subtype harboring the L74I integrase polymorphism.93,98–101 The G118R mutation has been selected in macaques receiving cabotegravir (long-acting) for pre-exposure prophylaxis during acute simian/human immunodeficiency virus infection.102
28. Several mutations are required in HIV integrase to confer high-level resistance to dolutegravir.103 Cross-resistance studies with raltegravir- and elvitegravir-resistant viruses indicate that Q148H/R and G140S in combination with mutations L74I/M, E92Q, T97A, E138A/K, G140A, or N155H are associated with 5-fold to 20-fold reduced dolutegravir susceptibility104 and reduced virologic suppression in patients.105–108
29. Seven elvitegravir codon mutations have been observed in InSTI treatment-naive and -experienced patients in whom therapy is failing.109–115 T97A, which may occur as a polymorphism,116 results in only a 2-fold change in elvitegravir susceptibility and may require additional mutations for resistance.114,115 The sequential use of elvitegravir and raltegravir (in either order) is not recommended because of cross-resistance between these drugs.114
30. Raltegravir failure is associated with integrase mutations in at least 3 distinct, but not exclusive, genetic pathways defined by 2 or more mutations including (1) a mutation at Q148H/K/R, N155H, or Y143R/H/C; and (2) 1 or more additional minor mutations. Minor mutations described in the Q148H/K/R pathway include L74M plus E138A, E138K, or G140S. The most common mutational pattern in this pathway is Q148H plus G140S, which also confers the greatest loss of drug susceptibility. Mutations described in the N155H pathway include this major mutation plus either L74M, E92Q, T97A, E92Q plus T97A, Y143H, G163K/R, V151I, or D232N.117 The Y143R/H/C mutation is uncommon.118–122 E92Q alone reduces susceptibility to elvitegravir more than 20 fold and causes limited (<5 fold) cross-resistance to raltegravir.123–125 N155H mutants tend to predominate early in the course of raltegravir failure, but are gradually replaced by viruses with higher resistance, often bearing mutations G140S plus Q148H/R/K, with continuing raltegravir treatment.
Footnotes
Financial affiliations in the past 12 months: Dr Calvez has served as an advisor or consultant to and has received research grants from Bristol-Myers Squibb, Johnson & Johnson, ViiV Healthcare, and Gilead Sciences, Inc, and is a founder of SkinDermic Pharma. Dr Ceccherini-Silberstein has been a consultant to ViiV Healthcare, Bristol-Myers Squibb, and Merck Sharp & Dohme, Inc, and has received research grants from ViiV Healthcare, Gilead Sciences, Inc, and Merck Sharp & Dohme, Inc. Dr Charpentier serves as an advisor for ViiV Healthcare, Gilead Sciences, Inc, Janssen Therapeutics, and Merck Sharp & Dohme, Inc, and has received research grants from ViiV Healthcare. Dr Günthard has served as a consultant to Merck & Co, Inc, ViiV Healthcare, Sandoz, Teva Pharmaceutical Industries, and Gilead Sciences, Inc, and has received research grants from Gilead Sciences, Inc. Dr Paredes has received research grants from and has served as an advisor for ViiV Healthcare, Gilead Sciences, Inc, and Merck Sharp & Dohme, Inc. Dr Richman has been a consultant to Antiva Biosciences, Gilead Sciences, Inc, and Viriome, Inc. Dr Shafer has received research grants from Janssen Therapeutics, Vela Diagnostics, and InSilixa, Inc, and consulting fees from Abbott Diagnostics. Dr Wensing has served on advisory boards for ViiV Healthcare, Merck & Co, Inc, Janssen Therapeutics, and Gilead Sciences, Inc, and has received research or unrestricted educational grants from Janssen Therapeutics, ViiV Healthcare, Merck & Co, Inc, and Gilead Sciences, Inc.
Funding/Support: This work was funded by IAS-USA. No commercial company or government funding was used to support the effort. Panel members are not compensated.
The authors thank Jose Francisco for administrative support for the work.
Contributor Information
Annemarie M. Wensing, (Group Chair), University Medical Center Utrecht, The Netherlands and University of the Witwatersrand, Johannesburg, South Africa.
Vincent Calvez, Pierre et Marie Curie University and Pitié-Salpêtrière Hospital, Paris, France.
Francesca Ceccherini-Silberstein, University of Rome Tor Vergata, Rome, Italy.
Charlotte Charpentier, Paris Diderot University and Bichat-Claude Bernard Hospital, France.
Huldrych F. Günthard, University Hospital Zurich and Institute of Medical Virology, University of Zurich, Switzerland.
Roger Paredes, HIV Unit and IrsiCaixa AIDS Research Institute, Hospital Universitari Germans Trias i Pujol, Badalona, Spain.
Robert W. Shafer, Stanford University Medical School, California.
Douglas D. Richman, (Group Vice Chair), Veterans Affairs San Diego Healthcare System and University of California San Diego..
References
- 1. Wensing AM, Calvez V, Gunthard HF, et al. 2017 Update of the Drug Resistance Mutations in HIV-1. Top Antivir Med. 2017; 24(4):132–133. [PMC free article] [PubMed] [Google Scholar]
- 2. Gilead Sciences Inc. BIKTARVY [prescribing information]. 2018. Foster City, CA, Gilead Sciences, Inc. [Google Scholar]
- 3. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101): 1499–1510. [DOI] [PubMed] [Google Scholar]
- 4. Merck & Co Inc. Doravirine [prescribing information]. 2018. Whitehouse Station, NJ, Merck. [Google Scholar]
- 5. Lam E, Parkin NT. Amprenavir resistance imparted by the I50V mutation in HIV-1 protease can be suppressed by the N88S mutation. Clin Infect Dis. 2003; 37(9):1273–1274. [DOI] [PubMed] [Google Scholar]
- 6. Rhee SY, Taylor J, Fessel WJ, et al. HIV-1 protease mutations and protease inhibitor cross-resistance. Antimicrob Agents Chemother. 2010;54(10):4253–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hermans LE, Steegen K, ter Heine R, et al. PI drug-level testing as screening tool for drug resistance in 2nd-line ART failure. Top Antivir Med. 2019;27(1s):169s. [Google Scholar]
- 8. Smith SJ, Zhao XZ, Burke TR Jr., Hughes SH. Efficacies of cabotegravir and bictegravir against drug-resistant HIV-1 inte grase mutants. Retrovirology. 2018;15(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quashie PK, Mesplede T, Han YS, et al. Biochemical analysis of the role of G118R-linked dolutegravir drug resistance substitutions in HIV-1 integrase. Antimicrob Agents Chemother. 2013;57(12): 6223–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pingen M, Nijhuis M, de Bruijn JA, Boucher CA, Wensing AM. Evolutionary pathways of transmitted drug-resistant HIV-1. J Antimicrob Chemother. 2011;66(7):1467–1480. [DOI] [PubMed] [Google Scholar]
- 11. Gunthard HF, Calvez V, Paredes R, et al. Human immunodeficiency virus drug resistance: 2018 recommendations of the International Antiviral Society-USA panel. Clin Infect Dis. 2018;67:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA panel. JAMA. 2018;320(4):379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to the User Notes
- 1. von Wyl V, Ehteshami M, Demeter LM, et al. HIV-1 reverse transcriptase connection domain mutations: dynamics of emergence and implications for success of combination antiretroviral therapy. Clin Infect Dis. 2010; 51(5):620–628. [DOI] [PubMed] [Google Scholar]
- 2. Gupta S, Vingerhoets J, Fransen S, et al. Connection domain mutations in HIV-1 reverse transcriptase do not impact etravirine susceptibility and virologic responses to etravirine-containing regimens. Antimicrob Agents Chemother. 2011;55(6):2872–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rimsky L, Van Eygen V, Vingerhoets J, Leijskens E, Picchio G. Reverse transcriptase connection domain mutations were not associated with virological failure or phenotypic resistance in rilpivirine-treated patients from the ECHO and THRIVE Phase III trials (week 96 analysis). Antivir Ther. 2012;17(Suppl 1):A36. [Google Scholar]
- 4. Miller MD, Margot N, Lu B, et al. Genotypic and phenotypic predictors of the magnitude of response to tenofovir disoproxil fumarate treatment in antiretroviral-experienced patients. J Infect Dis. 2004;189(5):837–846. [DOI] [PubMed] [Google Scholar]
- 5. Harrigan PR, Stone C, Griffin P, et al. Resistance profile of the human immunodeficiency virus type 1 reverse transcriptase inhibitor abacavir (1592U89) after monotherapy and combination therapy. CNA2001 Investigative Group. J Infect Dis. 2000;181(3):912–920. [DOI] [PubMed] [Google Scholar]
- 6. Winters MA, Shafer RW, Jellinger RA, Mamtora G, Gingeras T, Merigan TC. Human immunodeficiency virus type 1 reverse transcriptase genotype and drug susceptibility changes in infected individuals receiving dideoxyinosine monotherapy for 1 to 2 years. Antimicrob Agents Chemother. 1997;41(4): 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Svarovskaia ES, Margot NA, Bae AS, et al. Low-level K65R mutation in HIV-1 reverse transcriptase of treatment-experienced patients exposed to abacavir or didanosine. JAIDS. 2007; 46(2):174–180. [DOI] [PubMed] [Google Scholar]
- 8. Hawkins CA, Chaplin B, Idoko J, et al. Clinical and genotypic findings in HIV-infected patients with the K65R mutation failing firstline antiretroviral therapy in Nigeria. JAIDS. 2009;52(2):228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brenner BG, Oliveira M, Doualla-Bell F, et al. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. AIDS. 2006;20:F9–F13. [DOI] [PubMed] [Google Scholar]
- 10. Fourati S, Visseaux B, Armenia D, et al. Identification of a rare mutation at reverse transcriptase Lys65 (K65E) in HIV-1-infected patients failing on nucleos(t)ide reverse transcriptase inhibitors. J Antimicrob Chemother. 2013;68(10):2199–2204. [DOI] [PubMed] [Google Scholar]
- 11. Chunduri H, Crumpacker C, Sharma PL. Reverse transcriptase mutation K65N confers a decreased replication capacity to HIV-1 in comparison to K65R due to a decreased RT processivity. Virology. 2011;414(1):34–41. [DOI] [PubMed] [Google Scholar]
- 12. Clark SA, Shulman NS, Bosch RJ, Mellors JW. Reverse transcriptase mutations 118I, 208Y, and 215Y cause HIV-1 hypersusceptibility to non-nucleoside reverse transcriptase inhibitors. AIDS. 2006;20(7):981–984. [DOI] [PubMed] [Google Scholar]
- 13. Picchio G, Vingerhoets J, Parkin N, Azijn H, de Bethune MP. Nucleoside-associated mutations cause hypersusceptibility to etravirine. Antivir Ther. 2008;13(Suppl 3):A25. [Google Scholar]
- 14. Shulman NS, Bosch RJ, Mellors JW, Albrecht MA, Katzenstein DA. Genetic correlates of efavirenz hypersusceptibility. AIDS. 2004; 18(13): 1781–1785. [DOI] [PubMed] [Google Scholar]
- 15. Demeter LM, DeGruttola V, Lustgarten S, et al. Association of efavirenz hypersusceptibility with virologic response in ACTG 368, a randomized trial of abacavir (ABC) in combination with efavirenz (EFV) and indinavir (IDV) in HIV-infected subjects with prior nucleoside analog experience. HIV Clin Trials. 2008;9(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haubrich RH, Kemper CA, Hellmann NS, et al. The clinical relevance of non-nucleoside reverse transcriptase inhibitor hypersusceptibility: a prospective cohort analysis. AIDS. 2002;16(15):F33–F40. [DOI] [PubMed] [Google Scholar]
- 17. Tozzi V, Zaccarelli M, Narciso P, et al. Mutations in HIV-1 reverse transcriptase potentially associated with hypersusceptibility to non-nucleoside reverse-transcriptase inhibitors: effect on response to efavirenz-based therapy in an urban observational cohort. J Infect Dis. 2004;189(9):1688–1695. [DOI] [PubMed] [Google Scholar]
- 18. Katzenstein DA, Bosch RJ, Hellmann N, et al. Phenotypic susceptibility and virological outcome in nucleoside-experienced patients receiving three or four antiretroviral drugs. AIDS. 2003;17(6):821–830. [DOI] [PubMed] [Google Scholar]
- 19. Margot N, Cox S, Das M, McCallister S, Miller MD, Callebaut C. Infrequent development of drug resistance in HIV-1-infected treatment-naive subjects after 96 weeks of treatment with elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide or elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate. Antivir Ther. 2017;22(5):443–446. [DOI] [PubMed] [Google Scholar]
- 20. Whitcomb JM, Parkin NT, Chappey C, Hellman NS, Petropoulos CJ. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J Infect Dis. 2003;188(7):992–1000. [DOI] [PubMed] [Google Scholar]
- 21. Larder BA, Kemp SD. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989;246(4934):1155–1158. [DOI] [PubMed] [Google Scholar]
- 22. Kellam P, Boucher CA, Larder BA. Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contributes to the development of high-level resistance to zidovudine. Proc Natl Acad Sci USA. 1992; 89(5):1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calvez V, Costagliola D, Descamps D, et al. Impact of stavudine phenotype and thymidine analogues mutations on viral response to stavudine plus lamivudine in ALTIS 2 ANRS trial. Antivir Ther. 2002;7(3):211–218. [PubMed] [Google Scholar]
- 24. Kuritzkes DR, Bassett RL, Hazelwood JD, et al. Rate of thymidine analogue resistance mutation accumulation with zidovudine- or stavudine-based regimens. JAIDS. 2004;36(1): 600–603. [DOI] [PubMed] [Google Scholar]
- 25. Romano L, Venturi G, Bloor S, et al. Broad nucleoside-analogue resistance implications for human immunodeficiency virus type 1 reverse-transcriptase mutations at codons 44 and 118. J Infect Dis. 2002;185(7):898–904. [DOI] [PubMed] [Google Scholar]
- 26. Walter H, Schmidt B, Werwein M, Schwingel E, Korn K. Prediction of abacavir resistance from genotypic data: impact of zidovudine and lamivudine resistance in vitro and in vivo. Antimicrob Agents Chemother. 2002;46(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mihailidis C, Dunn D, Pillay D, Pozniak A. Effect of isolated V118I mutation in reverse transcriptase on response to first-line antiretroviral therapy. AIDS. 2008;22(3):427–430. [DOI] [PubMed] [Google Scholar]
- 28. Lanier ER, Ait-Khaled M, Scott J, et al. Antiviral efficacy of abacavir in antiretroviral therapy-experienced adults harbouring HIV-1 with specific patterns of resistance to nucleoside reverse transcriptase inhibitors. Antivir Ther. 2004;9(1):37–45. [DOI] [PubMed] [Google Scholar]
- 29. Parikh UM, Zelina S, Sluis-Cremer N, Mellors JW. Molecular mechanisms of bidirectional antagonism between K65R and thymidine analog mutations in HIV-1 reverse transcriptase. AIDS. 2007;21(11):1405–1414. [DOI] [PubMed] [Google Scholar]
- 30. Parikh UM, Barnas DC, Faruki H, Mellors JW. Antagonism between the HIV-1 reverse-transcriptase mutation K65R and thymidine-analogue mutations at the genomic level. J Infect Dis. 2006;194(5):651–660. [DOI] [PubMed] [Google Scholar]
- 31. von Wyl V, Yerly S, Böni J, et al. Factors associated with the emergence of K65R in patients with HIV-1 infection treated with combination antiretroviral therapy containing tenofovir. Clin Infect Dis. 2008;46(8):1299–1309. [DOI] [PubMed] [Google Scholar]
- 32. Kuritzkes DR, Quinn JB, Benoit SL, et al. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine (3TC) versus zidovudine (ZDV) versus ZDV plus 3TC in previously untreated patients. AIDS. 1996;10(9):975–981. [DOI] [PubMed] [Google Scholar]
- 33. Violin M, Cozzi-Lepri A, Velleca R, et al. Risk of failure in patients with 215 HIV-1 revertants starting their first thymidine analog-containing highly active antiretroviral therapy. AIDS. 2004;18(2):227–235. [DOI] [PubMed] [Google Scholar]
- 34. Chappey C, Wrin T, Deeks S, Petropoulos CJ. Evolution of amino acid 215 in HIV-1 reverse transcriptase in response to intermittent drug selection. Antivir Ther. 2003;8:S37. [Google Scholar]
- 35. Garcia-Lerma JG, MacInnes H, Bennett D, Weinstock H, Heneine W. Transmitted human immunodeficiency virus type 1 carrying the D67N or K219Q/E mutation evolves rapidly to zidovudine resistance in vitro and shows a high replicative fitness in the presence of zidovudine. J Virol. 2004;78(14):7545–7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marcelin AG, Flandre P, Pavie J, et al. Clinically relevant genotype interpretation of resistance to didanosine. Antimicrob Agents Chemother. 2005;49(5):1739–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molina JM, Marcelin AG, Pavie J, et al. Didanosine in HIV-1-infected patients experiencing failure of antiretroviral therapy: a randomized placebo-controlled trial. J Infect Dis. 2005;191(6):840–847. [DOI] [PubMed] [Google Scholar]
- 38. Antinori A, Zaccarelli M, Cingolani A, et al. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res Hum Retroviruses. 2002;18(12):835–838. [DOI] [PubMed] [Google Scholar]
- 39. Feng M, Wang D, Grobler JA, Hazuda DJ, Miller MD, Lai MT. In vitro resistance selection with doravirine (MK-1439), a novel nonnucleoside reverse transcriptase inhibitor with distinct mutation development pathways. Antimicrob Agents Chemother. 2015; 59(1):590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai MT, Xu M, Ngo W, et al. Characterization of doravirine-selected resistance patterns from participants in treatment-naive phase 3 clinical trials. Poster presented at: 22nd International AIDS Conference; July 23–27, 2018; Amsterdam, The Netherlands.
- 41. Lai MT, Feng M, Falgueyret JP, et al. In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2014; 58(3):1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Orkin C, Squires KE, Molina JM, et al. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis. 2019; 68(4):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Molina JM, Squires K, Sax PE, et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVEFORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV. 2018;5(5):e211–e220. [DOI] [PubMed] [Google Scholar]
- 44. Smith SJ, Pauly GT, Akram A, et al. Rilpivirine and doravirine have complementary efficacies against NNRTI-resistant HIV-1 mutants. JAIDS. 2016;72(5):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benhamida J, Chappey C, Coakley E, Parkin NT. HIV-1 genotype algorithms for prediction of etravirine susceptibility: novel mutations and weighting factors identified through correlations to phenotype. Antivir Ther. 2008;13(Suppl 3):A142. [Google Scholar]
- 46. Coakley E, Chappey C, Benhamida J, et al. Biological and clinical cut-off analyses for etravirine in the PhenoSense HIV assay. Antivir Ther. 2008;13(Suppl 3):A134. [Google Scholar]
- 47. Vingerhoets J, Tambuyzer L, Azijn H, et al. Resistance profile of etravirine: combined analysis of baseline genotypic and phenotypic data from the randomized, controlled Phase III clinical studies. AIDS. 2010;24(4):503–514. [DOI] [PubMed] [Google Scholar]
- 48. Haddad M, Stawiski E, Benhamida J, Coakley E. Improved genotypic algorithm for predicting etravirine susceptibility: comprehensive list of mutations identified through correlation with matched phenotype. Poster presented at: 17th Conference on Retroviruses and Opportunistic Infections (CROI); February 16–19, 2010; San Francisco, CA.
- 49. Etravirine [prescribing information]. 2013. Titusville, NJ, Janssen Therapeutics. [Google Scholar]
- 50. Scherrer AU, Hasse B, Von Wyl V, et al. Prevalence of etravirine mutations and impact on response to treatment in routine clinical care: the Swiss HIV Cohort Study (SHCS). HIV Med. 2009;10(10):647–656. [DOI] [PubMed] [Google Scholar]
- 51. Tambuyzer L, Nijs S, Daems B, Picchio G, Vingerhoets J. Effect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirine. JAIDS. 2011;58(1):18–22. [DOI] [PubMed] [Google Scholar]
- 52. Tudor-Williams G, Cahn P, Chokephaibulkit K, et al. Etravirine in treatment-experienced, HIV-1-infected children and adolescents: 48-week safety, efficacy and resistance analysis of the phase II PIANO study. HIV Med. 2014; 15(9):513–524. [DOI] [PubMed] [Google Scholar]
- 53. Rilpivirine [prescribing information]. 2015. Titusville, NJ, Janssen Therapeutics. [Google Scholar]
- 54. Azijn H, Tirry I, Vingerhoets J, et al. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother. 2010;54(2):718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Picchio GR, Rimsky LT, Van Eygen V, et al. Prevalence in the USA of rilpivirine resistance-associated mutations in clinical samples and effects on phenotypic susceptibility to rilpivirine and etravirine. Antivir Ther. 2014;19(8): 819–823. [DOI] [PubMed] [Google Scholar]
- 56. Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011; 378(9787):229–237. [DOI] [PubMed] [Google Scholar]
- 57. Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011; 378(9787):238–246. [DOI] [PubMed] [Google Scholar]
- 58. Rimsky L, Vingerhoets J, Van Eygen V, et al. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. JAIDS. 2012;59(1):39–46. [DOI] [PubMed] [Google Scholar]
- 59. Hofstra LM, Sauvageot N, Albert J, et al. Transmission of HIV drug resistance and the predicted effect on current first-line regimens in Europe. Clin Infect Dis. 2016;62(5):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kulkarni R, Babaoglu K, Lansdon EB, et al. The HIV-1 reverse transcriptase M184I mutation enhances the E138K-associated resistance to rilpivirine and decreases viral fitness. JAIDS. 2012;59(1):47–54. [DOI] [PubMed] [Google Scholar]
- 61. Hu Z, Kuritzkes DR. Interaction of reverse transcriptase (RT) mutations conferring resistance to lamivudine and etravirine: effects on fitness and RT activity of human immunodeficiency virus type 1. J Virol. 2011; 85(21):11309–11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu HT, Asahchop EL, Oliveira M, et al. Compensation by the E138K mutation in HIV-1 reverse transcriptase for deficits in viral replication capacity and enzyme processivity associated with the M184I/V mutations. J Virol. 2011;85(21):11300–11308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Haddad M, Napolitano LA, Frantzell A, et al. Combinations of HIV-1 reverse transcriptase mutations L100I + K103N/S and L100I + K103R + V179D reduce susceptibility to rilpivirine. Poster presented at: 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September 10–13, 2013; Denver, CO.
- 64. Hirsch MS, Günthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47(2):266–285. [DOI] [PubMed] [Google Scholar]
- 65. Fun A, Wensing AM, Verheyen J, Nijhuis M. Human Immunodeficiency Virus Gag and protease: partners in resistance. Retrovirology. 2012;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Young TP, Parkin NT, Stawiski E, et al. Prevalence, mutation patterns, and effects on protease inhibitor susceptibility of the L76V mutation in HIV-1 protease. Antimicrob Agents Chemother. 2010;54(11):4903–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. De Meyer S, Descamps D, Van Baelen B, et al. Confirmation of the negative impact of protease mutations I47V, I54M, T74P and I84V and the positive impact of protease mutation V82A on virological response to darunavir/ritonavir. Antivir Ther. 2009;14(Suppl 1):A147. [Google Scholar]
- 68. Descamps D, Lambert-Niclot S, Marcelin AG, et al. Mutations associated with virological response to darunavir/ritonavir in HIV-1-infected protease inhibitor-experienced patients. J Antimicrob Chemother. 2009;63(3): 585–592. [DOI] [PubMed] [Google Scholar]
- 69. Darunavir [prescribing information]. 2015. Titusville, NJ, Janssen Therapeutics. [Google Scholar]
- 70. Masquelier B, Breilh D, Neau D, et al. Human immunodeficiency virus type 1 genotypic and pharmacokinetic determinants of the virological response to lopinavir-ritonavir-containing therapy in protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2002;46(9):2926–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kempf DJ, Isaacson JD, King MS, et al. Identification of genotypic changes in human immunodeficiency virus protease that correlate with reduced susceptibility to the protease inhibitor lopinavir among viral isolates from protease inhibitor-experienced patients. J Virol. 2001;75(16):7462–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lopinavir/ritonavir [prescribing information]. 2015. Abbott Park, IL, AbbVie Inc. [Google Scholar]
- 73. Mo H, King MS, King K, Molla A, Brun S, Kempf DJ. Selection of resistance in protease inhibitor-experienced, human immunodeficiency virus type 1-infected subjects failing lopinavir- and ritonavir-based therapy: mutation patterns and baseline correlates. J Virol. 2005;79(6):3329–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Friend J, Parkin N, Liegler T, Martin JN, Deeks SG. Isolated lopinavir resistance after virological rebound of a ritonavir/lopinavir-based regimen. AIDS. 2004;18(14):1965–1966. [DOI] [PubMed] [Google Scholar]
- 75. Kagan RM, Shenderovich M, Heseltine PN, Ramnarayan K. Structural analysis of an HIV-1 protease I47A mutant resistant to the protease inhibitor lopinavir. Protein Sci. 2005; 14(7):1870–1878.15937277 [Google Scholar]
- 76. KALETRA (lopinavir and ritonavir) [prescribing information]. 2018. Abbott Park, IL, AbbVie Inc. [Google Scholar]
- 77. Lam E, Parkin NT. Amprenavir resistance imparted by the I50V mutation in HIV-1 protease can be suppressed by the N88S mutation. Clin Infect Dis. 2003;37(9):1273–1274. [DOI] [PubMed] [Google Scholar]
- 78. Rhee SY, Taylor J, Fessel WJ, et al. HIV-1 protease mutations and protease inhibitor cross-resistance. Antimicrob Agents Chemother. 2010;54(10):4253–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hermans LE, Steegen K, ter Heine R, et al. PI drug-level testing as screening tool for drug resistance in 2nd-line ART failure. Top Antivir Med. 2019;27(1s):169s. [Google Scholar]
- 80. Gonzalez LM, Brindeiro RM, Aguiar RS, et al. Impact of nelfinavir resistance mutations on in vitro phenotype, fitness, and replication capacity of human immunodeficiency virus type 1 with subtype B and C proteases. Antimicrob Agents Chemother. 2004;48(9):3552–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Reeves JD, Gallo SA, Ahmad N, et al. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci USA. 2002;99(25):16249–16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reeves JD, Miamidian JL, Biscone MJ, et al. Impact of mutations in the coreceptor binding site on human immunodeficiency virus type 1 fusion, infection, and entry inhibitor sensitivity. J Virol. 2004;78(10):5476–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xu L, Pozniak A, Wildfire A, et al. Emergence and evolution of enfuvirtide resistance following long-term therapy involves heptad repeat 2 mutations within gp41. Antimicrob Agents Chemother. 2005;49(3):1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maraviroc [prescribing information]. 2015. Research Triangle Park, NC, ViiV Healthcare. [Google Scholar]
- 85. Anastassopoulou CG, Ketas TJ, Sanders RW, Klasse PJ, Moore JP. Effects of sequence changes in the HIV-1 gp41 fusion peptide on CCR5 inhibitor resistance. Virology. 2012; 428(2):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Malet I, Gimferrer AL, Artese A, et al. New raltegravir resistance pathways induce broad cross-resistance to all currently used integrase inhibitors. J Antimicrob Chemother. 2014;69(8): 2118–2122. [DOI] [PubMed] [Google Scholar]
- 87. Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–708. [DOI] [PubMed] [Google Scholar]
- 88. Quashie PK, Mesplede T, Han YS, et al. Characterization of the R263K mutation in HIV-1 integrase that confers low-level resistance to the second-generation integrase strand transfer inhibitor dolutegravir. J Virol. 2012;86(5): 2696–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Souza Cavalcanti J, Minhoto LA, de Paula Ferreira JL, da Eira M, de Souza Dantas DS, de Macedo Brigido LF. In-vivo selection of the mutation F121Y in a patient failing raltegravir containing salvage regimen. Antiviral Res. 2012;95(1):9–11. [DOI] [PubMed] [Google Scholar]
- 90. Margot NA, Hluhanich RM, Jones GS, et al. In vitro resistance selections using elvitegravir, raltegravir, and two metabolites of elvitegravir M1 and M4. Antiviral Res. 2012;93(2):288–296. [DOI] [PubMed] [Google Scholar]
- 91. Brenner BG, Lowe M, Moisi D, et al. Subtype diversity associated with the development of HIV-1 resistance to integrase inhibitors. J Med Virol. 2011;83(5):751–759. [DOI] [PubMed] [Google Scholar]
- 92. Oliveira M, Ibanescu RI, Anstett K, et al. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology. 2018;15(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hassounah SA, Alikhani A, Oliveira M, et al. Antiviral activity of bictegravir and cabotegravir against integrase inhibitor-resistant SIVmac239 and HIV-1. Antimicrob Agents Chemother. 2017;61(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tsiang M, Jones GS, Goldsmith J, et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother. 2016;60(12): 7086–7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sax PE, Pozniak A, Montes ML, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GSUS-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073–2082. [DOI] [PubMed] [Google Scholar]
- 96. Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GSUS-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390:2063–2072. [DOI] [PubMed] [Google Scholar]
- 97. Sax PE, DeJesus EG, Crofoot GE, et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;4(4):e154–e160. [DOI] [PubMed] [Google Scholar]
- 98. Margolis DA, Brinson CC, Smith GH, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15(10):1145–1155. [DOI] [PubMed] [Google Scholar]
- 99. Smith SJ, Zhao XZ, Burke TR Jr., Hughes SH. Efficacies of cabotegravir and bictegravir against drug-resistant HIV-1 integrase mutants. Retrovirology. 2018;15(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Orkin C, Arasteh K, Hernandez-Mora MG, et al. Long-acting cabogravir + rilpivirine for HIV maintenance: FLAIR week 48 results. Top Antivir Med. 2019;27(1s):52s. [Google Scholar]
- 101. Radzio-Basu J, Council O, Cong ME, et al. Drug resistance emergence in macaques administered cabotegravir long-acting for pre-exposure prophylaxis during acute SHIV infection. Nat Commun. 2019;10(1):2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Radzio J, Council O, Cong M, et al. Resistance emergence in Macaques administered cabotegravir LA during acute infection [Abstract 84]. Top Antivir Med. 2017;25(1s):33s. [Google Scholar]
- 103. Frantzell A, Petropoulos C, Huang W. Dolutegravir resistance requires multiple primary mutations in HIV-1 integrase. Top Antivir Med. 2015;23(e-1):51. [Google Scholar]
- 104. Kobayashi M, Yoshinaga T, Seki T, et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011; 55(2):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–743. [DOI] [PubMed] [Google Scholar]
- 106. Eron JJ, Clotet B, Durant J, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Seki T, Suyama-Kagitani A, Kawauchi-Miki S, et al. Effects of raltegravir or elvitegravir resistance signature mutations on the barrier to dolutegravir resistance in vitro. Antimicrob Agents Chemother. 2015;59(5):2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. DeAnda F, Hightower KE, Nolte RT, et al. Dolutegravir interactions with HIV-1 integrase-DNA: structural rationale for drug resistance and dissociation kinetics. PLoS One. 2013;8(10):e77448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Goodman D, Hluhanich R, Waters J, et al. Integrase inhibitor resistance involves complex interactions among primary and second resistance mutations: a novel mutation L68V/I associates with E92Q and increases resistance. Antivir Ther. 2008;13(Suppl 3):A15. [Google Scholar]
- 110. Waters J, Margot N, Hluhanich R, et al. Evolution of resistance to the HIV integrase inhibitor (INI) elvitegravir can involve genotypic switching among primary INI resistance patterns. Fort Myers, FL. Antivir Ther. 2009; 14(Supp 1):A137. [Google Scholar]
- 111. Doyle T, Dunn DT, Ceccherini-Silbers tein F, et al. Integrase inhibitor (INI) genotypic resistance in treatment-naive and raltegravir-experienced patients infected with diverse HIV-1 clades. J Antimicrob Chemother. 2015;70(11):3080–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, doubleblind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–2448. [DOI] [PubMed] [Google Scholar]
- 113. DeJesus E, Rockstroh J, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835): 2429–2438. [DOI] [PubMed] [Google Scholar]
- 114. Abram ME, Hluhanich RM, Goodman DD, et al. Impact of primary elvitegravir resistance-associated mutations in HIV-1 integrase on drug susceptibility and viral replication fitness. Antimicrob Agents Chemother. 2013; 57(6):2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. White K, Kulkarni R, Miller MD. Analysis of early resistance development at the first failure timepoint in elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate-treated patients. J Antimicrob Chemother. 2015;70(9): 2632–2638. [DOI] [PubMed] [Google Scholar]
- 116. Scherrer AU, Yang WL, Kouyos RD, et al. Successful prevention of transmission of integrase resistance in the Swiss HIV Cohort Study. J Infect Dis. 2016;214(3):399–402. [DOI] [PubMed] [Google Scholar]
- 117. Hazuda DF, Miller MD, Nguyen BY, Zhao J, for the P005 Study Team. Resistance to the HIV-integrase inhibitor raltegravir: analysis of protocol 005, a phase II study in patients with triple-class resistant HIV-1 infection. Antivir Ther. 2007;12:S10. [Google Scholar]
- 118. Gatell JM, Katlama C, Grinsztejn B, et al. Long-term efficacy and safety of the HIV integrase inhibitor raltegravir in patients with limited treatment options in a Phase II study. JAIDS. 2010;53(4):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fransen S, Gupta S, Danovich R, et al. Loss of raltegravir susceptibility by human immunodeficiency virus type 1 is conferred via multiple nonoverlapping genetic pathways. J Virol. 2009;83(22):11440–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hatano H, Lampiris H, Fransen S, et al. Evolution of integrase resistance during failure of integrase inhibitor-based antiretroviral therapy. J Acquir Immune Defic Syndr. 2010; 54(4):389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wittkop L, Breilh D, Da Silva D, et al. Virological and immunological response in HIV-1-infected patients with multiple treatment failures receiving raltegravir and optimized background therapy, ANRS CO3 Aquitaine Cohort. J Antimicrob Chemother. 2009;63(6): 1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Armenia D, Vandenbroucke I, Fabeni L, et al. Study of genotypic and phenotypic HIV-1 dynamics of integrase mutations during raltegravir treatment: a refined analysis by ultra-deep 454 pyrosequencing. J Infect Dis. 2012;205(4):557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359(4):355–365. [DOI] [PubMed] [Google Scholar]
- 124. Malet I, Delelis O, Valantin MA, et al. Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro. Antimicrob Agents Chemother. 2008;52(4):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Blanco JL, Varghese V, Rhee SY, Gatell JM, Shafer RW. HIV-1 integrase inhibitor resistance and its clinical implications. J Infect Dis. 2011;203(9):1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]