Abstract
Antiretroviral therapy (ART) should be started as soon as possible after HIV diagnosis. Recommended starting ART regimens in patients with any baseline viral load include bictegravir plus tenofovir alafenamide (TAF)/emtricitabine (FTC), dolutegravir (DTG) plus abacavir/lamivudine, DTG plus TAF (or TDF)/FTC, or DTG plus 3TC. Initial laboratory evaluation includes CD4+ cell count, plasma HIV-1 RNA, and testing for HIV reverse transcriptase and protease resistance mutations. ART regimens do not need to be altered for virologic blips due to release of virus from chronically latently infected cells in patients otherwise exhibiting viral suppression. Patients with continuously undetectable viral load on ART pose virtually no risk of transmitting infection through sexual contact. This article is based on a case-based presentation by Michael S. Saag, MD, at the 2018 Clinical Conference at the National Ryan White Conference on HIV Care & Treatment in December 2018 and intended for clinicians who are new to HIV disease management.
Keywords: HIV, antiretroviral, initial ART, InSTI, integrase inhibitor, nRTI, NNRTI, protease inhibitor, PI, viral load, CD4+ count, HIV RNA
Initial ART
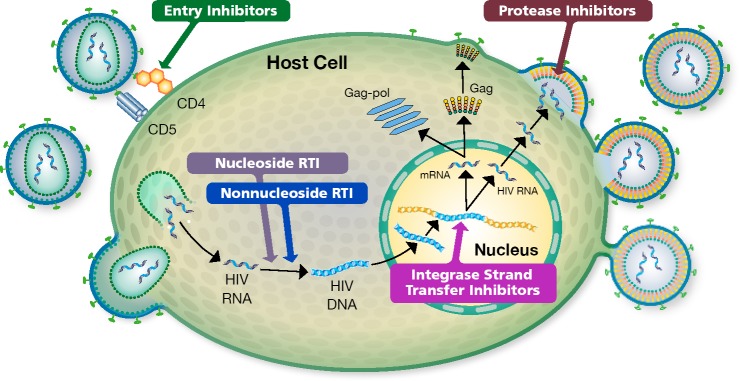
Two important resources for use of antiretroviral therapy (ART) are the updated guidelines from the International Antiviral Society-USA (IAS-USA) and from the US Department of Health and Human Services (DHHS).1,2 The goal of therapy is to block HIV replication (Figure 1).
Figure 1.
HIV Life Cycle. The goal of ART is to block replication of HIV. This is accomplished through protection of uninfected cells from becoming infected, via blockage of 1 or more of several steps in the HIV life cycle, as illustrated above. RTI indicates reverse transcriptase inhibitor.
ART should be started as soon as possible after diagnosis of HIV infection. This includes rapid start on the day of diagnosis, unless the patient is not ready to commit to starting therapy. Further, ART should be started as soon as possible (but within 2 weeks) after diagnosis of most HIV-related opportunistic diseases. The objective of starting as soon as possible is to effectively suppress the virus, which reduces immunologic damage in the patient and reduces likelihood of forward transmission (Figure 1). Less time between diagnosis and the initiation ART also increases the likelihood that patients will show up for their first clinic visit to initiate treatment.
Structural barriers to initiating ART and HIV care should be removed. Before starting ART, laboratory monitoring should include: CD4+ cell count; plasma HIV-1 RNA; hepatitis A, B, and C virus serologies; serum chemistries; estimated creatinine clearance rate; complete blood cell count; urine glucose and protein; sexually transmitted infection (STI) screening; and fasting lipid levels. In regions with an incidence of tuberculosis greater than 1%, a tuberculosis test should be performed at baseline. All patients should undergo testing for reverse transcriptase and protease resistance mutations. HLA-B∗5701 and chemokine receptor 5 (CCR5) tropism testing results must be confirmed prior to initiating therapy with either abacavir or maraviroc, respectively.
Chemistries should be drawn before beginning ART, but treatment may be started before results are available. NNRTIs (due to possible transmitted resistance) and abacavir (without HLAB∗5701 results) should not be used for rapid ART start. It should be noted that if ART is to be started immediately in the clinic, with no information yet available on viral load, CD4+ count, viral genotype, or HLAB∗5701 status, initial treatment is basically limited to a tenofovir-based regimen with an unboosted integrase strand transfer inhibitor (InSTI) or boosted darunavir. The presence of the M184I/V mutation, which confers resistance to lamivudine and emtricitabine, has been shown not to compromise the recommended regimens.
Case 1
Figure 1. HIV Life Cycle. The goal of ART is to block replication of HIV. This is accomplished through protection of uninfected cells from becoming infected, via blockage of 1 or more of several steps in the HIV life cycle, as illustrated above. RTI indicates reverse transcriptase inhibitor.
Consider the case of a 48-year-old man presenting with newly diagnosed HIV infection. He is asymptomatic. His initial plasma HIV RNA level is 28,000 copies/mL and CD4+ cell count is 650/μL. Other laboratory findings are normal, he is HLA-B∗5701-positive, and HIV genotyping shows wild-type virus. He has no prior notable medical history and his renal function is normal. He is willing to start ART if the clinician thinks it is advisable to do so. What ART regimen should the patient be started on?
Initial regimens recommended by IAS-USA and DHHS consist of: bictegravir plus tenofovir alafenamide (TAF)/emtricitabine (FTC), dolutegravir (DTG) plus abacavir/lamivudine, and DTG plus TAF/FTC, with DHHS also recommending raltegravir plus tenofovir (TAF or TDF)/FTC. The InSTI in these regimens is the anchor drug with the regimen backbones consisting of 2 nRTIs. In the case of the current patient, the regimen containing abacavir should not be used, since the patient is HLA-B∗5701-positive. A regimen of DTG plus_lamivudine, although not specifically recommended in either guidelines (yet), is an acceptable regimen in this case owing to the absence of baseline nRTI resistance mutations and recently pre sented 96-week data showing non-inferiority to standard InSTI-plus-2 nRTI regimens.3,4 A recent study showed no difference in attaining viral load levels below 50 copies/mL with bictegravir/TAF/FTC, DTG/abacavir/lamivudine, or DTG/TAF/FTC.5,6
The difference between tenofovir disoproxil fumarate (TDF) and TAF should be noted. TDF is formulated as a 300 mg tablet and TAF as a 25 mg tablet. Higher plasma levels of TDF than TAF are needed to get sufficient amounts of tenofovir into cells. The higher plasma levels required for TDF are associated with increased adverse events. The use of TDF in regimens with boosted protease inhibitors (eg, boosted darunavir) appears to increase the adverse effects of TDF on bone and renal function.7,8
Case 2
Now, consider the case of a 48-year-old man presenting with newly diagnosed HIV infection who is asymptomatic except for weight loss and fatigue and has a high initial viral load (760,000 copies/mL) and low CD4+ count (21 cells/μL). His other laboratory values are within normal limits and his HLA-B∗5701 is negative. HIV genotype is wild-type. He has no notable past medical history, has normal renal function, and is willing to start ART if the clinician thinks it advisable. What ART regimen should the patient be started on?
Use of rilpivirine is not recommended in this setting because it is not going to be active at viral loads above 100,000 copies/mL. Regimens with DTG/lamivudine work well at viral load levels for individuals with between 100,000 and 500,000 copies/mL in the absence of resistance mutations, although there might be some hesitancy in using it in those patients with CD4+ counts below 200 cells/μL. Abacavir has been considered not to be a good choice if viral load is greater than 100,000 copies/mL; however, that rule of thumb is not relevant when abacavir is paired with DTG (rather than with a boosted PI or an NNRTI). In the study2 cited above among patients with viral load greater than 100,000 copies/mL, there was little difference in achieving viral load below 50 copies/mL with the bictegravir/TAF/FTC, DTG/abacavir/lamivudine, and DTG/TAF/FTC regimens.5
Table 1 shows the ART regimens that are likely to be used most in initial treatment. Doravirine is a recently approved NNRTI with broad activity, including against reverse transcriptase inhibitor-resistant virus. Rilpivirine is still a good choice for many patients when the viral load is below 100,000 copies/mL. Table 2 lists recommended initial regimens if InSTIs are not available.
Table 1.
Antiretroviral Regimens for Initial Treatment for Most Patients
Integrase strand transfer inhibitor-based regimens
|
Protease inhibitor-based regimens
|
Nonnucleoside reverse transcriptase inhibitor-based regimens
|
TDF may be substituted for TAF if added cost or reimbursement restrictions exist.
Table 2.
Recommended Initial Regimens if an Integrase Strand Transfer Inhibitor is Not Available.
|
Adapted from Saag et al, JAMA, 20181
In monitoring patients after starting ART it is important to note that several antiretroviral drugs, such as dolutegravir, bictegravir, and ritonavir, are associated with an increase (eg, of 0.1–0.15 mg/dL) in serum creatinine level at a week after starting treatment in most patients. Like DTG, bictegravir inhibits the organic cation transporter 2 (OCT2) enzyme in the proximal renal tubule, resulting in a small increase in serum creatinine due to blockade of creatinine secretion; this has no effect on actual glomerular filtration rate.
Case 3
Consider the case of a 30-year-old woman with newly diagnosed HIV infection. She is asymptomatic, has initial viral load of 128,000 copies/mL, and CD4+ count of 350 cells/μL. Her other laboratory findings are within normal limits and the HLA-B∗5701 is negative. HIV genotyping shows reverse transcriptase mutations M184V and K103N. She has no notable prior medical history, no children, and does not plan to become pregnant. She is willing to start ART if her clinician thinks it advisable. What regimen should she be started on?
In this case the presence of M184V and K103N takes most NNRTI drugs off the table. The exception would be doravirine, which should maintain activity. The use of InSTI-and PI-based regimens remains the same. However, the presence of M184V removes the option of DTG plus lamivudine, which is contraindicated when this mutation is present. Moreover, TAF or TDF are preferentially used here over abacavir because the M184V partially weakens the activity of abacavir.
Recommendations for Switching ART for Virologic Failure
Recommendations for switching ART in cases of virologic failure have remained fairly constant for the past several years. Virologic failure should be confirmed and, if resistance is identified, there should be prompt switch to another active regimen. Recommended regimens after initial ART has failed are as follows:
Dolutegravir or bictegravir plus 2 nRTIs (with at least 1 active as determined by genotype) after initial treatment failure with a regimen that contains an NNRTI
A boosted PI plus 2 nRTIs (with at least 1 active nRTI) for initial treatment failure of an InSTI-containing regimen
Dolutegravir plus at least 1 fully active other agent may be effective in the setting of raltegravir or elvitegravir resistance. Dolutegravir should be dosed twice daily in this setting. Of note, bictegravir should not be used here because it cannot be dosed twice daily owing to the fixed-dose formulation with TAF/emtricitabine
Recommended Laboratory Monitoring
The IAS-USA-recommended laboratory assessments and monitoring across the HIV continuum of care1 are shown in Table 3.
Table 3.
International Antiviral Society-USA Recommendations for Laboratory Assessments and Monitoring Across the HIV Continuum of Care.
| Test | At HIV Diagnosis | During ART | At Virologic Failure |
|---|---|---|---|
| HIV RNA level | ✓ | Within the first 6 weeks of starting ART or a new ART regimen, then every 3 months until <50 copies/mL for 1 year, then every 6 months | ✓ |
| CD4+ cell count | ✓ | Every 6 months until >250/μL for 1 year then stop as long as virus is suppressed | ✓ |
| HIV RT-pro genotype | ✓ | ✓ | |
| HIV integrase genotype | If failing ART regimen included an InSTI | ||
| Viral tropism | Each time before the start of ART that includes maraviroc | ||
| HLA-B∗5701 | ✓ (before initiating abacavir; just once) | ✓ (if considering abacavir and not determined previously) | |
| Safety testing | ✓ | ✓ | ✓ |
| Coinfection (STIs, tuberculosis, hepatitis, Pap test) | ✓ | ✓ | |
| Health maintenance | ✓ | ✓ |
Adapted from Saag et al, JAMA, 20181
Abbreviations: ART indicates antiretrovial therapy; InSTI, integrase strand transfer inhibitor; STI, sexually transmitted infection.
After starting ART, once the HIV RNA level is below 50 copies/mL, viral load should be monitored every 3 months until virus is suppressed for at least a year. Then, monitoring can be reduced to every 6 months if the patient maintains adherence. CD4+ cell count should be monitored every 6 months until counts are greater than 250/μL for at least 1 year with concomitant viral suppression. It is now recommended that CD4+ counts not be monitored thereafter unless virologic suppression is lost. CD4+ cell count monitoring is expensive and it is thought that little useful information is gained from continued routine monitoring in such a setting so long as viral suppression is maintained.
If a patient is found to have viral load above 50 copies/mL, testing should be repeated within 4 weeks and the patient reassessed for adherence and medication tolerability. Measurement of viral load at 4 to 6 weeks after starting a new ART regimen is recommended. The test should be repeated within 4 weeks if HIV RNA level remains above the limit of quantification 24 weeks after starting new treatment or if rebound above 50 copies/mL occurs. Tropism testing should be performed at the time of virologic failure of a CCR5 inhibitor.
Age- and risk-appropriate screening for STIs at various anatomical sites should continue, with testing for anal or cervical dysplasia, sexually transmitted infections (especially syphilis), and monitoring for general health and medication toxicity continued on a regular basis.
Table 4.
HIV Transmission According to Sexual Behavior Reported By the HIV-Seronegative Partners Over 2700 Couple Years of Follow Up.
| Linked transmission | Upper 95%CI | CYFU | CLS acts | |
|---|---|---|---|---|
| Any sex | 0 | 0.23 | 1596 | 76991 |
| Anal sex | 0 | 0.24 | 1546 | 70743 |
| Insertive anal sex | 0 | 0.27 | 1345 | 52 572 |
| Receptive anal sex with ejaculation | 0 | 0.57 | 652 | 20770 |
| Receptive anal sex without ejaculation | 0 | 0.43 | 867 | 23153 |
| Any sex with a STI | 0 | 2.74 | 135 | 6301 |
Abbreviations: CI, confidence interval; CYFU, couple years of follow up; CLS, condomless sex acts; STI, sexually transmitted disease
Regimens in Individuals Who Are Pregnant or Considering Pregnancy
Case 4
Consider the case of a 30-year-old woman presenting with newly diagnosed asymptomatic HIV infection. She is 2.5 months pregnant, with her first pregnancy. Her initial viral load is 28,000 copies/mL and CD4+ cell count is 650/μL. Other laboratory values are within normal limits; she is HLA-B∗5701-negative. Genotyping shows wild-type virus. She had no prior notable medical history and is willing to start ART if her clinician thinks it advisable. On what ART regimen should she be started?
In patients who are pregnant or anticipate becoming pregnant, additional considerations related to potential teratogenicity, placental transfer of drugs, and medication tolerability during pregnancy come into play. Most of the drugs recommended for use as initial regimens, such as rilpivirine, efavirenz, ritonavir boosted-atazanavir or -darunavir, or raltegravir are all acceptable anchor drugs for ARV regimens in pregnant women.
Abacavir, TDF, emtricitabine and lamivudine are all acceptable nRTI drugs for use in pregnancy. However, insufficient data exist regarding the use of TAF, bictegravir, and cobicistat, and these drugs should be avoided until more data emerge regarding their efficacy and safety in the setting of pregnancy.
The use of DTG is controversial. In southern Africa, where dolutegravir was widely adopted as a component of initial therapy for all patients living with HIV, a signal of approximately 4-fold increase in neural tube defects (NTDs) occurring in infants born to mothers taking DTG-based regimens during the first trimester (of note, effect on neural tube development was noted when DTG was initiated after 8 weeks gestation owing to the timing of neural tube closure approximately week 6 of gestation). Follow-up data obtained over the subsequent 2 years following the initial reports of NTD have shown a reduction in incidence toward those reported among mothers not taking DTG. Further data are needed, but it seems likely that the initial signal of increased incidence of NTD is dissipating as higher numbers of mothers have been followed.10 Of note, the mechanism of NTD appears related to relatively low maternal folate levels.11 Therefore, use of folate supplementation is suggested for all women taking DTG who are contemplating pregnancy or who are in their first trimester of pregnancy.
Should Regimens be Changed When Low Level Detectable Virus Is Present?
Case 5
Consider the case of a 55-year-old man referred for evaluation. He was diagnosed 18 years ago with HIV infection, with initial viral load of 936,000 copies/mL and CD4+ count of 70 cells/μL. His current viral load is 85 copies/mL, after a prior level of 62 copies/mL, and CD4+ count is 525cells/μL. His initial treatment was nelfinavir/stavudine/lamivudine. He has subsequently received ritonavir-boosted lopinavir plus TDF/emtricitabine, efavirenz plus
TDF/emtricitabine, and is now on DTG plus darunavir/cobicistat/lamivudine. No historical resistance tests are available. Given the detectable viral load, should the ART regimen be changed?
In such a situation, there is little rationale for altering the patient's ART regimen. There is no clear indication that any regimen would do better or even that adding another drug (eg, TDF or TAF) would result in a change in viral load. These blips in viral load are related to residual viral reservoir cells—or chronically latently infected cells—that get stimulated occasionally to ‘spit out’ some virus. Viral load is directly proportional to the number of cells in the body that are producing virus. In patients who have very high viral loads prior to treatment, a large number of reservoir cells are established, with viral blips occurring over time even in the context successful suppression of all de novo infection in vivo. It is estimated that these chronically latently infected cells have a half life on the order of 80 months. No change in ART is going to affect this phenomenon until curative therapy is developed. However, an increase in viral load to greater than 200 copies/mL, for example, is cause for concern regarding potential virologic failure and should be investigated.
Sexual Transmission Risk for Patients with Undetectable HIV RNA
A patient with continuously undetectable viral load has zero, or virtually zero, likelihood of sexually transmitting infection to an uninfected partner. As shown in Figure 1, a recent study showed no linked transmissions of infection from infected to HIV-seronegative partners over the course of approximately 77,000 sex acts over nearly 1600 couple-years of follow up.11
Footnotes
Presented by Dr Saag in December 2018. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Saag in September 2019.
Financial affiliations in the past 12 months: Dr Saag has received research grants and support awarded to his institution from Gilead Sciences, Inc, Merck, and ViiV Healthcare.
References
- 1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA panel. JAMA. 2018; 320(4):379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. Accessed on August 8, 2018.
- 3. Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019; 393(10167):143–155. [DOI] [PubMed] [Google Scholar]
- 4. Cahn P, Sierra Madera J, et al. Durable efficacy of dolutegravir (DTG) plus lamivudine (3TC) in antiretroviral treatment-naive adults with HIV-1 infection – 96-week results from the GEMINI studies. [Abstract WEAB0404LB.] Poster presented at: 10th IAS Conference on HIV Science (IAS 2019); July 21–24, 2019; Mexico City, Mexico.
- 5. Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a doubleblind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390;2063–2072. [DOI] [PubMed] [Google Scholar]
- 6. Sax PE, DeJesus E, Crofoot G, et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;4(4):e154–e160. [DOI] [PubMed] [Google Scholar]
- 7. Goicoechea M, Liu S, Best B, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008; 197(1):102–108. [DOI] [PubMed] [Google Scholar]
- 8. Hill A, Hughes SL, Gotham D, Pozniak AL. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Erad. 2018;4(2):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019; 393(10189):2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zash R, et al. Neural tube defects by antiretroviral and HIV exposure in the Tsepamo Study, Bostwana. [Abstract MOAX-0105LB.] Poster presented at: 10th IAS Conference on HIV Science (IAS 2019); July 21–24, 2019; Mexico City, Mexico.
- 11. Cabrera RM, Souder JP, Steele JW, Yeo l, Tukeman G, Gorelick DA, Finnell RH. The antagonism of foliate receptor by dolutegravir: developmental toxicity reduction by supplemental folic acid. AIDS. 2019;33;1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]