Polar warming will have widespread near-term consequences for sea level rise, extreme weather, plants, animals, and humans.
Abstract
Over the past decade, the Arctic has warmed by 0.75°C, far outpacing the global average, while Antarctic temperatures have remained comparatively stable. As Earth approaches 2°C warming, the Arctic and Antarctic may reach 4°C and 2°C mean annual warming, and 7°C and 3°C winter warming, respectively. Expected consequences of increased Arctic warming include ongoing loss of land and sea ice, threats to wildlife and traditional human livelihoods, increased methane emissions, and extreme weather at lower latitudes. With low biodiversity, Antarctic ecosystems may be vulnerable to state shifts and species invasions. Land ice loss in both regions will contribute substantially to global sea level rise, with up to 3 m rise possible if certain thresholds are crossed. Mitigation efforts can slow or reduce warming, but without them northern high latitude warming may accelerate in the next two to four decades. International cooperation will be crucial to foreseeing and adapting to expected changes.
INTRODUCTION
Earth has warmed by approximately 0.8°C since the late 19th century, while the Arctic has warmed by 2° to 3°C over the same period (Fig. 1A) (1). Conversely, the Antarctic has experienced more pronounced interannual and decadal variation in mean annual temperature anomalies than the Arctic, with no obvious upward trend in the last two decades (Fig. 1A). Spatially, observed warming has been markedly heterogeneous in both regions during the more recent instrumental satellite record (since 1986), with both warming and spatial variability in warming having increased more for the Arctic than the Antarctic over the past 13 years (Fig. 1B) (2, 3). Therefore, despite similarities in defining characteristics such as pronounced seasonality and the year-round presence of ice and snow, these two regions may face different futures in response to ongoing warming.
Fig. 1. Temperature trends and variability for the Arctic and Antarctic regions.
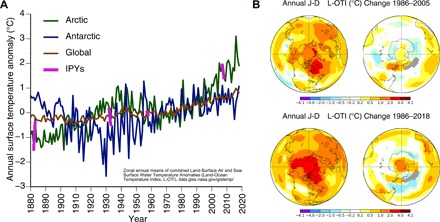
(A) Annual mean anomalies of the combined Land-Ocean Temperature Index (L-OTI) for the Arctic (64°N to 90°N), Antarctic (64°S to 90°S), and globe between 1880 and 2018 (zonal data bins defined by data acquired at https://data.giss.nasa.gov relative to the mean period 1951–1980). Temperature anomalies for the Arctic during each of the four IPYs, the first of which was based in the Arctic, are highlighted in purple. (B) Annual [January to December (J-D)] mean temperature change (°C) in the Northern (left) and Southern (right) hemispheres for 1986–2005 (upper) and 1986–2018 (lower) relative to the mean period of 1951–1980. Generated from the NASA/Goddard Institute for Space Studies (GISS) online plotting tool (2); the GISS analysis is based on updated Global Historical Climatology Network v3/SCAR (2, 3) and updates to Analysis (v3).
Having arrived at the 10th anniversary of the Fourth International Polar Year (IPY), a milestone that intensified focus on observed and expected changes in the polar regions, we review key environmental and ecological impacts of warming over the past decade. We also review ancillary effects of polar warming at lower latitudes, for which evidence has mounted recently. Over the past decade alone, the Arctic has warmed by 0.75°C relative to the mean for 1951–1980, while the Antarctic has remained comparatively stable (2009–present; Fig. 1A). Our emphasis is on consideration of consequences for atmospheric, cryospheric, and biospheric changes in the polar regions, as Earth continues to approach 2°C global mean warming (Table 1). Hence, we first consider the expected magnitude and pace of warming in the Arctic and Antarctic under two carbon emissions futures: Representative Concentration Pathway (RCP) 8.5 and RCP4.5 scenarios. We then outline potential consequences of such warming on the basis of recent observed changes in both regions. While our retrospective assessments of warming to date (Fig. 1) refer to temperature anomalies relative to the period covered by the instrumental record (1880–2018) (2) and a baseline mean period (1951–1980), our projections of expected warming are presented relative to the Intergovernmental Panel on Climate Change (IPCC) standard baseline period (1981–2005) (4).
Table 1. Summary of key concerns or vulnerabilities to atmospheric, cyrospheric, and biospheric components of the Arctic and Antarctic highlighted by recent developments in polar research.
| System component | Key concerns or vulnerabilities |
| Atmosphere | More rapid mean annual warming to 2°C above baseline in both polar regions compared to the globe as a whole |
| Winter warming up to 7°C in the Arctic and 3°C in the Antarctic with 2°C global mean warming | |
| Potential for more extreme weather at lower latitudes, including drought and heat waves | |
| Cryosphere | Possible acceleration of arctic sea-ice decline |
| Development of an ice-free Arctic Ocean during summer within the next few decades | |
| Rapid loss of land ice from the Greenland Ice Sheet and Thwaites Glacier contributing to global sea level rise | |
| Biosphere | Sea-ice decline contributing to loss of habitat for ice-dependent marine mammals |
| Altered timing of seasonal species interactions | |
| Warming-related species’ range shifts and invasions | |
| Gradual or sudden declines in populations of large herbivores and reciprocal effects on tundra vegetation diversity and productivity | |
| Pronounced increase in methane emissions | |
| Threats to maintenance of traditional livelihoods of indigenous people of the north |
The most recent generation of general circulation models in the Coupled Model Intercomparison Project Phase 5 (CMIP5) indicates that the Arctic is expected to continue to warm much more rapidly than lower latitudes, even under the moderate carbon mitigation trajectory characterized by the RCP4.5 scenario. The Arctic is expected to achieve an additional 2°C annual mean warming above the 1981–2005 baseline approximately 25 to 50 years before the globe as a whole under the business-as-usual (RCP8.5) and moderate mitigation (RCP4.5) scenarios, respectively (Fig. 2, A and B). The Antarctic, in contrast, is expected to lag slightly a 2°C global mean warming under the business-as-usual scenario (Fig. 2C) but reach 2°C annual mean warming slightly earlier than the globe under the moderate mitigation scenario (Fig. 2D). Under both scenarios, Antarctic warming is expected to outpace global mean warming only during austral late autumn and winter months (Fig. 2, C and D).
Fig. 2. Approximate year by which the 2°C warming threshold is reached for the Arctic and Antarctic compared to the globe as a whole.
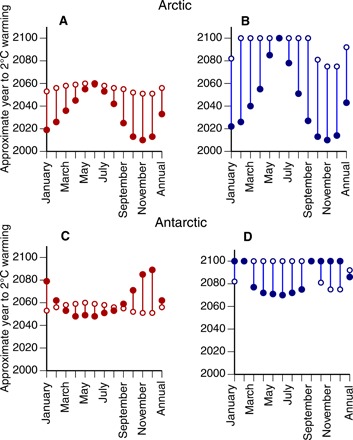
Expected time to 2°C warming above the 1981–2005 mean under RCP8.5 (red) and RCP4.5 (blue) for the globe (open circles) compared to the Arctic [solid circles; (A and B)] and Antarctic [solid circles; (C and D)]. Means of 36 CMIP5 ensemble runs by Overland et al. (1) are shown. In (B) and (D), symbols positioned at year 2100 indicate that 2°C warming could be at 2100 or later.
The Arctic may experience as much as 4°C mean annual warming and 7°C warming in late boreal autumn, when a 2°C global mean warming above the 1981–2005 mean is reached, regardless of which RCP scenario is considered (Fig. 3, solid circles) (1). Particularly notable is the 13°C Arctic warming projected for boreal late autumn months by the end of the 21st century under a business-as-usual scenario (RCP8.5) (1). Annual mean warming in the Antarctic is expected to reach approximately 2°C under both scenarios, with slightly greater warming possible under RCP8.5 during the austral autumn and early winter (Fig. 3, open circles). Hence, mitigation of carbon emissions with a target of constraining global annual mean warming to 2°C may not constrain the annual mean warming in the Arctic or Antarctic to below 2°C. However, mitigation of carbon emissions can delay the crossing of the 2°C annual mean warming threshold for the Arctic, as suggested by the difference in time to annual mean 2°C warming between the RCP4.5 and RCP8.5 scenarios in Fig. 2.
Fig. 3. Greater warming likely in the Arctic and Antarctic with 2°C global warming.
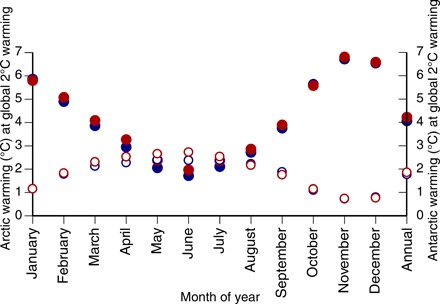
Expected magnitude of monthly and mean annual warming above the 1981–2005 mean in the Arctic (solid circles) and Antarctic (open circles) with 2°C global warming under RCP8.5 (red) and RCP4.5 (blue) according to 36 CMIP5 ensemble runs by Overland et al. (1).
Recognizing the urgency of the magnitude and pace of ongoing and expected future warming in the polar regions, we present below a series of eight urgent considerations spurred by developments over the past decade. These are followed by a brief, concluding overview of international agreements in the Arctic and Antarctic as exemplars for cooperative scientific and political engagement that is likely necessary for addressing the complexities of expected climate-related changes in the polar regions. Our objectives are to catalyze consideration of potential consequences of a 2°C warmer world for the polar regions and to thereby inform policy considerations of these consequences. A key emergent feature of this synthesis is that direct comparisons of ongoing and expected changes in the Arctic and Antarctic are rendered difficult by the relative inaccessibility and data scarcity of the Antarctic compared to the Arctic. This disparity is especially evident in our capacity to anticipate expected changes to terrestrial ecosystems in the Antarctic. We stress that this synthesis is not intended as a comprehensive review of recent and growing emphases in polar research, some notable examples of which include arctic ozone dynamics (5, 6), Southern Ocean heat uptake from the atmosphere (7), and associations between Southern Ocean warming and ice sheet dynamics on land (8).
HOW RAPIDLY IS ARCTIC SEA ICE DIMINISHING, AND WHAT ARE LIKELY TO BE THE MOST PRESSING ECOLOGICAL CONSEQUENCES OF CONTINUED SEA-ICE LOSS?
One of the major potential consequences of rapid and pronounced arctic warming is the development of an ice-free summer Arctic Ocean (9), which will have large-scale environmental consequences that reach beyond the northern high latitudes. During the past four decades of consistent satellite observations, Arctic sea-ice cover has undergone significant reductions in extent (10), the proportion of perennial versus first-year ice, the age of that perennial ice (11), and thickness (12) as well as shifts toward an earlier onset of spring snow melt on sea ice across much of the Arctic (13). Recent reconstructions of sea ice back to 1850 using historical observations (ship reports, airplane surveys, historical ice charts, and whaling log reports) (14) show that contemporary sea-ice loss is unprecedented in the record period (Fig. 4). In contrast, Antarctic sea-ice extent increased slightly between 1978 and 2015 (15), although record or near-record minima were observed in the austral autumns of 2017 and 2018 (16).
Fig. 4. Declining Northern Hemisphere sea-ice extent.
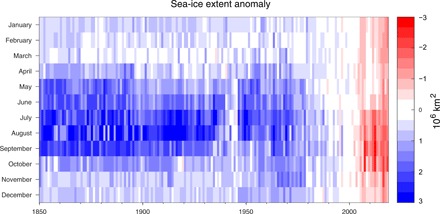
Northern Hemisphere monthly sea-ice extent anomalies (relative to 1981–2010) from 1850 to 2018 updated after the Walsh et al. (14) dataset. Image credit: A. Barrett.
Arctic sea-ice loss encompasses all calendar months, with the largest trends in late summer and the smallest in winter. Yet, while the largest rates of decline still occur during September (~−83,000 km2/year from 1979 to 2018 or − 12.9% per decade relative to the 1981–2010 mean), every month has displayed a negative linear trend for the past 40 years, and May and November 2016 were the most anomalous months recorded, falling nearly 4 SDs below the 1981–2010 mean (17).
Successive record minimum arctic sea-ice extents have occurred in the past decade (10, 18). While external forcing from increasing concentrations of CO2 plays a dominant role in the long-term decline and thinning of sea ice (19, 20), several feedback processes and internal climate variability have contributed to persistence of recent low summer extents. Record minima in 2007 and 2012 are clear examples of extreme events in which atmospheric circulation patterns during summer played a substantial role (21, 22). An ice-free summer Arctic Ocean may be realized within a few decades, as the pace of observed ice loss has exceeded some model projections under both RCP8.5 and RCP4.5 scenarios (1). The linear relationship between observed September sea-ice extent and climate models with increased cumulative atmospheric CO2 (19) suggests that ice extent will drop below 1 million km2 with an additional 800 Gt of CO2 (17). At current emission rates of 35 to 40 Gt year−1, this will occur within the next 20 to 25 years.
Research addressing impacts of ongoing and accelerating arctic sea-ice loss on sea-ice–dependent marine organisms (23), as well as for components of adjacent terrestrial systems, has seen increasing focus in the past decade (24, 25). Among the clearest examples of these impacts are those extending across the arctic marine food web from shifts in the timing of algal blooms (26) and increases in Arctic Ocean primary productivity, which cascades to zooplankton and vertebrates (23, 27, 28). Loss of sea ice broadly affects arctic marine mammal (AMM) movements, feeding, and life history events (29, 30). In turn, these impacts cascade to human communities that rely on AMMs for nutritional, cultural, and economic reasons.
For polar bears (Ursus maritimus), current and projected loss of optimal habitat (31) has been associated with reduced on-ice foraging and longer periods on land (32, 33). Recent work on ice habitat loss indicates demographic and physiological consequences for polar bears, such as reduced survival or abundance (34), increased energetic demands of travel over less stable sea ice or open water (35, 36), and nutritional stress from summertime fasting (37). Recent sea-ice loss has also affected ice-dependent pinnipeds, with large land-based haul-outs of Pacific walrus (Odobenus rosmarus divergens) in the absence of summer sea ice, resulting in trampling deaths (38).
Shifts toward earlier timing of spring sea-ice breakup have also driven increased mortality among harp (Pagophilus groenlandicus) and ringed (Pusa hispida) seal pups (39, 40). In addition, phytoplankton blooms have shifted earlier in the year in areas of the Arctic Ocean where the timing of sea-ice melt has advanced (41). In some areas, cetaceans have experienced what are likely short-term benefits due to increased primary and secondary production, such as increased body condition in bowhead whales (Balaena mysticetus) (42), and range expansion opening previously unavailable habitat for bowheads and sub-Arctic whales (43, 44). Broadly, sea-ice loss is expected to affect species assemblages and interactions in the Arctic, with an influx of sub-Arctic species and the potential for increased competition with endemic Arctic species (45).
Crucially, a recent circumpolar review of AMM population status identified large data gaps on population structure, abundance, and population trend (29). However, stabilization and reduction of atmospheric greenhouse gas concentrations have emerged as the most important conservation actions for AMMs. Throughout their range, most AMM stocks and populations are subject to subsistence harvest by Native people in the Arctic (29), so declines in AMM populations will likely affect these human populations. And even under intermediate RCP4.5 and RCP6.0 scenarios, large-scale impacts to ice-dependent AMMs are virtually certain; specifically, reductions in abundance coupled with range shifts and impacts to life history. Furthermore, expected increases in human activity in marine and coastal zones in an ice-free Arctic in summer, such as offshore oil and gas drilling or trans-Arctic shipping, are likely to result in cumulative negative impacts on AMMs (46–48). Improved monitoring, especially for data-deficient species such as Atlantic and Pacific walrus, will be important for improving AMM population status updates critical for ongoing development of adaptive management and conservation policy.
HOW WILL ARCTIC WARMING AFFECT WEATHER AT LOWER LATITUDES?
Environmental consequences of continued Arctic warming are unlikely to be limited to the northern high latitudes. The past decade has witnessed an increase in the occurrence of unusually hot summers in Europe and the most extreme heat wave on record: the 2010 Russian heat wave in which 55,000 heat-related deaths were estimated (49). Although large uncertainties remain, recent developments in atmospheric science indicate that anthropogenic warming (50), and in particular Arctic amplification of warming associated with sea-ice loss (49), may increase the probability of occurrence of Northern Hemisphere mid-latitude summer weather extremes. Weaker poleward summer temperature gradients resulting from Arctic amplification of warming leads, e.g., to a weaker jet stream (51), while amplification of planetary (“Rossby”) waves through the process of quasi-resonant amplification (QRA) is likely leading to a more meandering jet stream (52). Together, these factors are ostensibly contributing to an increase in persistent mid-latitude summer weather extremes—i.e., historic droughts, floods, and heat waves—in recent years, highlighted by the unprecedented weather extremes of summer 2018. Arctic warming also likely affects mid-latitude winter weather patterns. Although still debated (53), there is growing evidence (54–56) that Arctic amplification of warming in winter may be weakening the winter jet stream and the polar vortex, potentially increasing the frequency of continental cold-air winter outbreaks such as those seen during the winter of 2018/2019. These high-latitude impacts are in addition to other (e.g., tropical) impacts on mid-latitude weather dynamics.
An increase in occurrence of QRA during the satellite era (1979–2011) coincides with a measure of Arctic amplification of warming (52). Most recently (57), a specific observational-based fingerprint was developed for QRA conditions based on anomalous zonal-mean surface temperature profiles. Examination of the trend in this fingerprint in both long-term historical observations and the CMIP5 climate historical model simulations revealed consistent evidence for an increase in QRA conditions tied to anthropogenic warming. These QRA events are expected to become more frequent with continued Arctic warming (58).
The California drought of 2011–2017 has also recently been linked to changing arctic conditions (59). A continued decline in Arctic sea-ice extent, and the associated increase in Arctic sea surface temperatures, could affect the Northern Hemisphere jet stream in such a way as to direct winter storms north of California, leading to decreased snowpack and rainfall and exacerbated drought conditions (60). Moreover, recent modeling (61) has strengthened the proposed link between anthropogenic climate change and the type of high-pressure “ridging” pattern that is responsible for the poleward diversion of storm tracks over the western United States.
WHAT ARE THE CONSEQUENCES OF CONTINUED POLAR WARMING FOR LAND ICE LOSS AND SEA LEVEL RISE?
Additional lower-latitude environmental consequences of high-latitude warming relate to expected continued loss of land ice and resultant sea level rise. The most recent IPCC end of the 21st-century projection is approximately 0.5-m global sea level rise even under mitigation scenario RCP4.5 (62). The rise is attributable mostly to thermal expansion of ocean water and melting mountain glaciers, with smaller contributions from increasing ice sheet flow and meltwater runoff of the Greenland Ice Sheet (GIS), West Antarctic Ice Sheet (WAIS), and East Antarctic Ice Sheet (EAIS). Semiempirical models suggest an approximately 70% greater projected rise in sea level (62). The IPCC-projected contributions from ice sheets have increased since 2001, with some other assessments giving still higher ranges (63), and the process-based IPCC projections of future warming show accelerating ice mass loss (64). Hence, stronger warming, such as that projected under RCP8.5, is likely to cause an even larger sea level rise.
Warming above a “survival” threshold, previously estimated as approximately 1° to 4°C above preindustrial, may cause loss of most of the GIS over the following centuries or longer (62). Related modeling experiments reveal that seasonal sea-ice loss increases ice sheet mass loss and lowers the ice sheet survival threshold (65). However, GIS melting may slow the Atlantic meridional overturning circulation (66), perhaps helping to cool and stabilize the GIS. Past (67) and ongoing (68) warming has driven rapid GIS retreat along deep fjords. At least some of the past fjord retreats were triggered by ice-shelf thinning and loss and proceeded by iceberg calving from tidewater (nonfloating) cliffs (68). Recent increased physical understanding of tidewater calving processes suggests that the GIS is at least somewhat more sensitive to warming than modeled (69). Notably, recent work indicates that the GIS experienced one or more extensive and persistent deglaciations within the last ~1.2 million years when paleoclimatic records show only slightly warmer conditions than observed recently (70). This recent research suggests great GIS sensitivity to warming.
In contrast to the GIS, major mass loss over the coming decades from surface runoff is not expected for Antarctica under RCP4.5 or greater emissions (62). However, ongoing mass loss was recently triggered when warmer ocean waters thinned ice shelves, reducing their buttressing effect, allowing for faster flow of nonfloating ice into the ocean [reviewed in (71)]. Sufficient warming to trigger GIS-type ice-shelf loss and tidewater-calving retreat could contribute substantially to sea level rise in the next ~100 years especially from WAIS, even if iceberg calving is limited to rates already exceeded locally in GIS, owing to the much wider WAIS calving front that could develop (72, 73). In addition, because WAIS could produce higher cliffs with less drag from fjord sides than in the GIS, and thus greater stress imbalances driving calving, even faster sea level rise is possible (71).
Within the WAIS, Thwaites Glacier has undergone notably rapid ice loss and appears particularly vulnerable to accelerated ice loss with increased ice-shelf basal melt. In a recent comparison of two simplified model scenarios representing “constant climate” and “warming climate,” Thwaites Glacier collapsed in 80% of constant climate experiments and in 100% of warming climate experiments (74). Collapse of Thwaites Glacier and other Antarctic sources could contribute more than 3 m to global sea level rise over a time span that is poorly characterized but could be less than a century following initiation if ice-shelf loss and cliff retreat become important (72, 75). Further warming could extend these processes into marine basins of EAIS, potentially adding an additional 12 m or more of sea level rise further in the future (72). Geoengineering solutions have been proposed (76), but grave difficulties remain.
Recent work (77, 78) suggests that past ice sheet fluctuations can be modeled without invoking ice-shelf loss and subsequent cliff failure, favoring models that give smaller or slower sea level rise than calculated by some studies (72), but essentially all ice that flows into the ocean ends in calving cliffs. Ice-shelf loss has been observed in several cases with subsequent flow acceleration (75), so models lacking cliff physics are omitting known processes that are critical to ice loss. Uncertainties are very large on many aspects of this topic, including poor knowledge of the threshold warming of ocean or atmosphere needed to trigger major ice-shelf loss for vulnerable drainages. Large, rapid sea level rise under strong warming thus remains possible but unproven.
HOW WILL BIOLOGICAL SEASONALITY, AND THE TIMING OF SPECIES INTERACTIONS, RESPOND TO CONTINUED WARMING?
Phenological responses to climate change have been most pronounced at northern high latitudes, and recent work shows that shifts in phenology are even more extreme than previously expected, likely because of a nonlinear increase in warming with latitude (79). Across the Arctic, recent phenological shifts in plants have resulted in longer growing seasons and shorter flowering seasons (80, 81). Recent meta-analyses have also revealed greater sensitivity of leaf emergence and flowering phenology to warming at colder than at warmer sites across the Arctic (82), suggesting a potential for phenological homogenization across large spatial extents. Plant landscape-scale and community-level phenological responses have consequences for higher trophic levels, and change in synchrony among interacting species has seen increasing focus over the past decade, especially in the context of negative consequences for consumer species of phenological mismatch with resource species (83). For example, shorter flowering seasons have recently been associated with declines in flies, a major group of arctic pollinators (Fig. 5) (84). In another recent example, rates of chick growth slowed in some high-arctic breeding shorebirds that experienced reduced synchrony between chick hatching and the timing of peak availability of forage insects (85).
Fig. 5. Reduced pollinator abundance following shorter overlap with flowering duration.
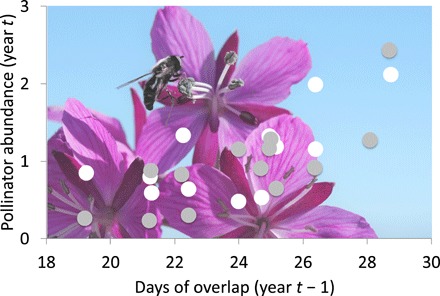
Association between current-year pollinator abundance and the number of days of overlap between pollinator presence and community-wide flowering during the previous year at Zackenberg, Greenland (1996–2009). White symbols denote muscid fly abundance, and gray symbols denote chironomid fly abundance. Modified from Høye et al. (84). Background photo of syrphid fly (Diptera) on dwarf fireweed (Chamerion latifolium) in Greenland. Photo credit: C. Urbanowicz.
In contrast, some species may benefit from earlier onset of the annual plant growing season. Muskoxen (Ovibos moschatus), which typically produce offspring before the onset of spring green-up, may experience increasing trophic match as green-up timing advances (86, 87). In northeast Greenland, increasing abundance of muskoxen has been associated with a longer plant growing season related to summer warming (88). In west Greenland, where the length of the plant growing season has also increased (89), muskox abundance has increased nearly steadily since 2002 (87). The degree to which these phenological responses reflect adaptation to changing environmental conditions or phenological plasticity is unclear, but threshold responses of phenology to climate suggest that limits to plasticity are becoming apparent (90).
HOW WILL CONTINUED ARCTIC WARMING AFFECT TUNDRA HERBIVORES, ESPECIALLY SPECIES OF SOCIOECONOMIC IMPORTANCE?
In arctic systems, large herbivores, particularly caribou/reindeer (both Rangifer tarandus), integrate critical cultural, socioeconomic, and resource value with pronounced capacity to influence ecosystem dynamics. These reasons warrant improved understanding of the effects of continued warming on tundra herbivores, as well as of reciprocal feedbacks between herbivores and ecosystem structure and function in the Arctic. Multiple recent studies indicate that herbivores can mediate responses to warming of key ecosystem properties, affecting carbon uptake (91, 92), landscape-scale vegetation cycles (93), surface albedo (94), and plant diversity (95, 96). Reciprocally, declines in North American caribou populations have recently been linked to changes in tundra shrub cover associated with declining sea-ice extent (97).
In some arctic plant communities, the stature of tundra vegetation has increased with warming (98). However, in some cases, arctic herbivores hinder plants from growing taller and thus prevent the competitive exclusion of low-growing plants (96). This can result in a positive effect of warming on species richness in the presence of herbivores but a negative effect in the absence of herbivores (96). Herbivores are thus important in preserving biodiversity in a warming Arctic, and this role will likely become more important with additional warming (95).
The region extending from northern Fennoscandia through West Siberia, the world’s major semidomesticated reindeer herding region, has received increasing focus over the past decade on research into consequences of climatic warming and associated extreme weather (99, 100). On the Yamal Peninsula, where reindeer abundance totals approximately 340,000 animals managed by 6000 fully nomadic indigenous Nenets herders (101), summer warming has been associated with increasing deciduous shrub growth (99, 102). In the same region, winter warming and stronger and more extensive rain-on-snow events have led to ice-encrusted rangelands and catastrophic mass starvation of reindeer (100). The region’s largest recorded reindeer mortality episode occurred in 2013–2014, when an estimated 61,000 animals died in the Yamal Peninsula alone (Fig. 6) (100). Nonetheless, some recent work indicates that rain-on-snow events may not be a ubiquitous factor in dynamics of Rangifer populations across their distribution (103). On Svalbard, where rain-on-snow events also occur, reindeer in separate populations have increased in abundance over the past several decades (104, 105). Hence, single-population responses to extreme events may not inform genus- or species-level responses to long-term climatic trends (87, 103).
Fig. 6. Extensive reindeer mortality in West Siberia.

Semidomesticated reindeer belonging to Nenets herders frozen in position from the most extensive and severe rain-on-snow event on record for Yamal Peninsula, West Siberia, in which at least 61,000 animals died of starvation during winters of 2013–2014. Photo credit: R. Serotetto.
Research during the past decade has also indicated that Yamal Nenets herders are concerned about their ability to mutually coexist with rapidly expanding natural gas development on their ancient tundra reindeer rangelands (106). Catastrophic herd mortality during the winters of 2006–2007 and 2013–2014 added another element to the suite of risks that tundra nomads face during their annual migrations. If cyclic synoptic weather patterns have indeed shifted in response to regional warming, then these will have implications for long-distance migration of large reindeer herds in the context of rapidly expanding natural gas extraction.
WILL ANTARCTIC ECOSYSTEMS BE VULNERABLE TO INVASIONS OR STATE SHIFTS UNDER WARMING?
In the five decades following the International Geophysical Year (1957–1958), Antarctica has warmed in excess of 0.1°C per decade (107). However, long-term terrestrial ecosystem research in Antarctica did not begin until the early 1990s. Hence, we have a limited record of contemporary Antarctic ecosystem response to climate change. Recently, increasing focus on Antarctica’s McMurdo dry valleys (MDVs) ecosystem has emphasized its responsiveness to changes in physical boundary conditions and linked internal states of its components (Fig. 7). During a decadal cooling period (1987–2000), the MDV experienced reduced glacial meltwater streamflow generation, thickening ice covers, lowering lake levels, and drier soils (108). The associated biological communities responded with decreasing populations of soil invertebrates, declining stream biomass, and reduced lake primary productivity (109). This decadal cooling pattern ended in 2002 with an austral summer of high solar radiation and warm temperatures, increasing glacial melt and hydrological connectivity between soils, streams, and lakes. The following decade showed no discernible pattern in summer air temperatures or solar radiation (108). Over this decade, the ecosystem showed a prolonged response to the “flood year” of 2002, with increased stream flows, thinning lake ice, increased lake levels, increased stream and lake productivity, and increased populations of soil invertebrates. These decade-long responses in varying directions and magnitudes to marked pulse events may be representative of potential future state shifts resulting from rapid or abrupt changes in climate.
Fig. 7. Contrasting patterns of connectivity among components of an Antarctic ecosystem.
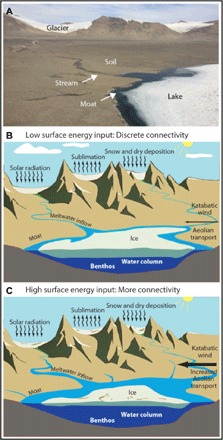
Controls on (A) the McMurdo dry valleys of Antarctica during (B) an austral summer of low surface energy input (solar radiation, conduction from air temperatures, etc.) and during (C) an austral summer of high surface energy input to the landscape. These physical changes to the system have direct implications for biological communities in each part of the ecosystem. Image credit: E. Parrish.
The future biodiversity and functioning of Antarctic terrestrial and freshwater ecosystems are closely coupled to the climatic changes expected to occur in Antarctica. The retreat of ice associated with near-future warming will expose previously unavailable habitats (110) that can be colonized by local, and potentially by invasive, species (111, 112). Some penguin species have already begun to move to previously unused breeding grounds in response to changes along the Antarctic Peninsula (113), and future warming may drive additional range shifts of penguins in the Southern Ocean (114). Recent work also emphasizes that the Southern Ocean may serve as a conduit for, rather than barrier to, biological invasions of Antarctica under future warming (115), with notable implications for infectious disease introductions (116). The MDV region, in particular, is expected to warm substantially in the coming decades (117). Under prolonged warming, there may likely be consistent enhanced physical connectivity across the MDV landscape (i.e., Fig. 7C), one potential outcome of which is the spatial homogenization of communities and resource status among landscape units. Antarctica, with its low-biodiversity ecosystems and physical systems responsive to small changes in the energy budget, may be one of the best places to untangle the complex biological and physical interactions that will determine high-latitude ecosystem function under future climate change (108, 118).
HOW WILL METHANE FLUXES RESPOND TO PERMAFROST THAWING?
Methane (CH4) has approximately 30× the heat-trapping capacity of CO2, and globally, terrestrial wetlands are the largest single source of atmospheric CH4, with current annual emissions estimated at 140 to 280 Tg CH4 year−1 (119). Moreover, northern wetlands store more than 50% of global soil organic carbon due to slow organic carbon decomposition rates resulting from wet surface conditions and low temperatures (120).
Future climatic warming at high latitudes could substantially increase net CH4 emissions from wetlands and permafrost degradation, serving as a positive feedback to warming of the global climate system (121). Similarly, increased net primary production, vascular plant species composition, and soil water content could enhance methanogenesis (the microbial basis of wetland methane production) and thereby CH4 emissions (122). One of the largest uncertainties in wetland CH4 estimates is how wetland extent or total inundated area will change with future warming (123). One recent study indicates that under business-as-usual emissions, total wetland area increases by 13%, and global CH4 emissions nearly double relative to current levels (124). For high-latitude wetlands, higher temperatures, winter thawing, and a consequent increase in soil moisture content are expected to be the primary drivers of elevated emissions (124, 125).
Emission scenarios span changes between a modest increase of 10 Tg CH4 year−1 to more than 50 Tg CH4 year−1 for far northern natural terrestrial methane emissions through the year 2080 with a 2°C global temperature increase (126, 127). Although increases in methane emissions in excess of 50 Tg CH4 year−1 represent extreme scenarios, these projections do not consider possible abrupt changes or accelerating trends with future warming. Given the potential for decomposition of large stocks of organic soil carbon, these changes could be an important factor in the future. In addition to these uncertainties, many processes not well represented in current models, including hydrology, lake dynamics, and permafrost dynamics, are likely to affect future arctic methane emissions and deserve increased focus (128).
HOW WILL CONTINUED ARCTIC WARMING AFFECT TUNDRA PRIMARY PRODUCTIVITY?
Improved focus on understanding heterogeneity in and drivers of tundra vegetation productivity and responses to expected warming will be critical to resolving questions of net ecosystem carbon storage and release as the Arctic warms (129). While early, coarse-scale satellite evidence inferred widespread tundra greening (130), recent disparities between positive and negative trends in tundra productivity across arctic sites have been detected (131). Although productivity responses of deciduous shrubs to warming have been greatest in wet and warm arctic sites (132, 133), a recent gradual loss of temperature signal in remotely sensed productivity at the pan-Arctic level (134) and in site-based plots (135) coincides with a deceleration to the initial greening trends (131). Despite this, the area of tundra that has greened over the satellite era is 20 times the area that has browned (136). Because site-based terrestrial Arctic research is spatially clustered, a few key locations account for a disproportionate amount of local evidence, biasing the study of mechanisms behind Arctic vegetation trends (137, 138). We thus advise inclusion of information from a greater variety of sites across the Arctic.
The study of drivers other than temperature of tundra vegetation dynamics has recently been reinvigorated. Recent warming has begun to relax strong thermal limitation on terrestrial primary productivity in the Arctic, shifting its control to additional factors such as moisture or nutrient limitation (139, 140). Recent attention has also shifted to cold-season controls, extreme events (141), snow depth (142), and the indirect influence of sea-ice decline through local and regional weather and climate (24, 25). The compound local/regional effects of many such abiotic drivers will likely continue to result in the emergence of browning signals (143). Understanding tundra productivity responses to future climate change should be improved through the maintenance of long-term ecological monitoring and manipulation sites; expansion of site-based studies to the widest possible range of habitats within the Arctic; and increasing the spatial, temporal, and spectral resolutions in remote sensing [e.g., (144)], with a focus on addressing sensor disagreement through calibration/ground truthing across scales (145, 146).
Considerations of scale will similarly improve predictions and help resolve seemingly contradictory responses to warming between tundra productivity and plant phenology. While plot-scale data indicate greater phenological sensitivity to warming at higher-latitude sites (79, 82), satellite and plot-scale measures of green-up show little to no advance in the faster warming high Arctic (132, 147). To what extent these contradictions are scale-dependent ecological patterns or artifacts of mismatches in methodologies and precision remains unclear (136). New sources of data—from ground (148), drone (149), and satellite-based sensors—offer opportunities to address these uncertainties. The research potential of these emerging approaches will be maximized by careful integration with, rather than replacement of, existing monitoring strategies.
Anticipating near-term changes: The importance of international agreements and cooperation
Ongoing and possible future atmospheric, cryospheric, and biospheric changes such as those reviewed here in response to expected warming in the polar regions cannot be addressed effectively by any single nation in isolation. Similarly, the challenges that will inevitably arise from increasing access to the polar regions and global pressure for resources cannot be managed unilaterally. Existing monitoring programs, such as the U.S. Arctic Observing Network and the British Antarctic Survey, are comparatively well developed in the polar regions. Maintaining and expanding these efforts will provide considerable value in scenario planning and policy development in anticipation of ongoing climate change and associated impacts (150). Despite uncertainties concerning precise mechanisms linking large-scale abiotic and ecological dynamics in, e.g., the Arctic, calls have already arisen for multinational cooperation and policy shifts in anticipation of further changes (25, 48, 151). Existing multinational agreements provide encouraging exemplars of the nature of engagement and cooperation likely necessary for mitigation and adaptation as Earth inches toward 2°C mean warming.
The Antarctic Treaty, for instance, was drafted following the Third IPY by 12 nations participating in research in the region, and its signatories have since grown to 53 nations. At the time of its formalization in 1961, the treaty was a hallmark of geopolitical peace agreements and scientific foresight, openness, and cooperation. However, increasing risks of intrusion by private vessels, and pressure to exploit Antarctic fisheries and mineral resources, have led to calls for changes to the processes by which the treaty might undergo modification (152). Although one recent analysis reported that most topics discussed during annual Antarctic Treaty Consultative Meetings (ATCM) between 1998 and 2011 focused on protected areas, environmental issues, and tourism, it also found that a small subset of the treaty’s signatories exert the greatest influence on the ATCM agenda (153).
The Arctic is experiencing an increase in shipping, tourism, and natural resource extraction facilitated by easier access and propelled by global demand (48, 154). Interest in shorter routes and reduced transit times over the next few decades (151) has prompted investment in infrastructure and international partnerships (154). These developments have prompted reviews of existing agreements and research partnerships in the Arctic to assess their adequacy in light of changing conditions. No comprehensive agreement like the Antarctic Treaty covers the Arctic. However, several relevant treaties, agreements, and collaborations are in place. Three recent examples of increased international cooperation include (i) Arctic Science Ministerial meetings in Washington, D.C. (in 2016) and in Berlin, Germany (in 2018), with 26 nations and the European Union combining efforts to pool resources and capacity for arctic science; (ii) The Agreement on Enhancing International Arctic Scientific Cooperation, negotiated under the auspices of the Arctic Council and put into effect in 2018; and (iii) The Agreement to Prevent Unregulated High Seas Fisheries in the Central Arctic Ocean (155), a rare example of application of the precautionary principle. Given that very little is known about the marine resources and ecosystem conditions of the region beyond the national jurisdictions of the Arctic nations, it would be impossible to manage a commercial fishery in that region on a sustainable basis. These developments are encouraging and reflect increasing awareness of the rapid rate of change in the Arctic and the critical need to understand how those changes are affecting the region and the world.
The close of the Fourth IPY saw the publication of syntheses calling for increased international, multidisciplinary research collaboration to improve the prospects of foreseeing and mitigating consequences of future warming in the polar regions (118). Although these and other examples demonstrate some progress toward that goal over the ensuing decade, more can be done by the nations of the world to work together to advance meaningful scientific cooperation in the polar regions. In the absence of efforts to curb or reduce carbon emissions over the next two to four decades, warming, especially in the northern high latitudes, is likely to accelerate (1). Given the implications of this warming, it is essential to also accelerate efforts to better understand, prepare for, and be able to address the environmental, ecological, and societal changes that will result from continued high-latitude warming.
Acknowledgments
We thank H. Levy, R. Dunbar, and anonymous reviewers for helpful comments and discussion. Funding: Funding was provided by grants from the U.S. National Science Foundation (NSF OPP 1738934 to R.B.A., NSF OPP 1637708 to M.N.G. and R.A.V., and NSF OPP 1748052 and NSF OPP 1836774 to E.P.), the Academy of Finland (decision no. 256991) and JPI Climate (no. 291581) (to B.C.F), the National Geographic Society (CP-061R-17 to J.T.K.), the Natural Environment Research Council (NE/L011859/1 to M.M.-F.), the Swedish Research Council (2017-04515 to J.O.), the U.S. National Aeronautics and Space Administration (NASA grant NNX16AJ92G S03 to J.C.S.), and the Joint Institute for the Study of the Atmosphere and Ocean (JISAO) under the U.S. National Oceanographic and Atmospheric Administration (NOAA cooperative agreement NA15OAR4320063, contribution no. 2018-0181) and the NOAA Pacific Marine Environmental Laboratory (contribution no. 4900) (to M.W.). Author contributions: E.P conceived, outlined, and coordinated the review. All authors contributed to writing, discussion of content changes, and editing of previous drafts. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- 1.Overland J. E., Wang M., Walsh J. E., Stroeve J. C., Future arctic climate changes: Adaptation and mitigation time scales. Earths Future 2, 68–74 (2014). [Google Scholar]
- 2.GISTEMPTeam, NASA Goddard Institute for Space Studies (2018); https://science.gsfc.nasa.gov/earth/giss/.
- 3.Hansen J., Ruedy R., Sato M., Lo K., Global surface temperature change. Rev. Geophys. 48, 2010RG000345 (2010). [Google Scholar]
- 4.Intergovernmental Panel on Climate Change, Climate Change 2014: Impacts, Adaptation, and Vulnerability, in Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, C. B. Field, V. R. Barros, D. J Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, M. Chatterjee, K. L. Ebi, Y. O. Estrada, R. C. Genova, B. Girma, E. S. Kissel, A. N. Levy, S. MacCracken, P. R. Mastrandrea, L. L. White, Eds. (Cambridge Univ. Press, 2014), pp. 1132. [Google Scholar]
- 5.Ivy D. J., Solomon S., Calvo N., Thompson D. W. J., Observed connections of Arctic stratospheric ozone extremes to Northern Hemisphere surface climate. Environ. Res. Lett. 12, 024004 (2017). [Google Scholar]
- 6.Manney G. L., Santee M. L., Rex M., Livesey N. J., Pitts M. C., Veefkind P., Nash E. R., Wohltmann I., Lehmann R., Froidevaux L., Poole L. R., Schoeberl M. R., Haffner D. P., Davies J., Dorokhov V., Gernandt H., Johnson B., Kivi R., Kyrö E., Larsen N., Levelt P. F., Makshtas A., McElroy C. T., Nakajima H., Parrondo M. C., Tarasick D. W., von der Gathen P., Walker K. A., Zinoviev N. S., Unprecedented Arctic ozone loss in 2011. Nature 478, 469–475 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Shi J.-R., Xie S.-P., Talley L. D., Evolving relative importance of the Southern Ocean and North Atlantic in anthropogenic ocean heat uptake. J. Climate 31, 7459–7479 (2018). [Google Scholar]
- 8.Sangiorgi F., Bijl P. K., Passchier S., Salzmann U., Schouten S., McKay R., Cody R. D., Pross J., van de Flierdt T., Bohaty S. M., Levy R., Williams T., Escutia C., Brinkhuis H., Southern Ocean warming and Wilkes Land ice sheet retreat during the mid-Miocene. Nat. Commun. 9, 317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M., Overland J. E., A sea ice free summer Arctic within 30 years? Geophys. Res. Lett. 36, 10.1029/2009GL037820 (2009). [Google Scholar]
- 10.Stroeve J. C., Serreze M. C., Holland M. M., Kay J. E., Malanik J., Barrett A. P., The Arctic’s rapidly shrinking sea ice cover: A research synthesis. Clim. Change 110, 1005–1027 (2012). [Google Scholar]
- 11.Maslanik J., Stroeve J., Fowler C., Emery W., Distribution and trends in Arctic sea ice age through spring 2011. Geophys. Res. Lett. 38, (2011). [Google Scholar]
- 12.Lindsay R., Schweiger A., Arctic sea ice thickness loss determined using subsurface, aircraft, and satellite observations. Cryosphere 9, 269–283 (2015). [Google Scholar]
- 13.Stroeve J. C., Markus T., Boisvert L., Miller J., Barrett A., Changes in Arctic melt season and implications for sea ice loss. Geophys. Res. Lett. 41, 1216–1225 (2014). [Google Scholar]
- 14.J. E. Walsh, W. L. Chapman, F. Fetterer, Gridded Monthly Sea Ice Extent and Concentration (National Snow and Ice Data Center, 2015).
- 15.Comiso J. C., Gersten R. A., Stock L. V., Turner J., Perez G. J., Cho K., Positive trend in the Antarctic sea ice cover and associated changes in surface temperature. J. Climate 30, 2251–2267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NSIDC, National Snow and Ice Data Center (2018); https://nsidc.org/.
- 17.Stroeve J., Notz D., Changing state of Arctic sea ice across all seasons. Environ. Res. Lett. 13, 103001 (2018). [Google Scholar]
- 18.Serreze M. C., Stroeve J., Arctic sea ice trends, variability and implications for seasonal ice forecasting. Philos. Trans. A Math. Phys. Eng. Sci. 373, 20140159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Notz D., Stroeve J., Observed Arctic sea-ice loss directly follows anthropogenic CO2 emission. Science 354, 747–750 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Stroeve J. C., Kattsov V., Barrett A., Serreze M., Pavlova T., Holland M., Meier W. N., Trends in Arctic sea ice extent from CMIP5, CMIP3 and observations. Geophys. Res. Lett. 39, 10.1029/2012GL052676 (2012). [Google Scholar]
- 21.Stroeve J. C., Serreze M., Drobot S., Gearheard S., Holland M., Maslanik J., Meier W., Scambos T., Arctic sea ice extent plummets in 2007. EOS Transactions 89, 13–14 (2008). [Google Scholar]
- 22.Zhang L., Guo H., Ji L., Lei L., Wang C., Yan D., Li B., Li J., Vegetation greenness trend (2000 to 2009) and the climate controls in the Qinghai-Tibetan Plateau. J. Appl. Remote Sens. 7, 073572 (2013). [Google Scholar]
- 23.Post E., Implications of earlier sea ice melt for phenological cascades in arctic marine food webs. Food Webs 13, 60–66 (2017). [Google Scholar]
- 24.Macias-Fauria M., Karlsen S. R., Forbes B. C., Disentangling the coupling between sea ice and tundra productivity in Svalbard. Sci. Rep. 7, 8586 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macias-Fauria M., Post E., Effects of sea ice on Arctic biota: An emerging crisis discipline. Biol. Lett. 14, 20170702 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tedesco L., Vichi M., Scoccimarro E., Sea-ice algal phenology in a warmer Arctic. Sci. Adv. 5, eaav4830 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.K. E. Frey, K. R. Arrigo, R. R. Gradinger, Arctic Ocean primary productivity, in Arctic Report Card: Update for 2011 (National Oceanic and Atmospheric Administration, 2011).
- 28.Arrigo K. R., Perovich D. K., Pickart R. S., Brown Z. W., van Dijken G. L., Lowry K. E., Mills M. M., Palmer M. A., Balch W. M., Bahr F., Bates N. R., Benitez-Nelson C., Bowler B., Brownlee E., Ehn J. K., Frey K. E., Garley R., Laney S. R., Lubelczyk L., Mathis J., Matsuoka A., Mitchell B. G., Moore G. W. K., Ortega-Retuerta E., Pal S., Polashenski C. M., Reynolds R. A., Schieber B., Sosik H. M., Stephens M., Swift J. H., Massive phytoplankton blooms under Arctic sea ice. Science 336, 1408 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Laidre K. L., Stern H., Kovacs K. M., Lowry L., Moore S. E., Regehr E. V., Ferguson S. H., Wiig Ø., Boveng P., Angliss R. P., Born E. W., Litovka D., Quakenbush L., Lydersen C., Vongraven D., Ugarte F., Arctic marine mammal population status, sea ice habitat loss, and conservation recommendations for the 21st century. Conserv. Biol. 29, 724–737 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovacs K. M., Moore S., Overland J. E., Lydersen C., Impacts of changing sea-ice conditions on Arctic marine mammals. Mar. Biodiversity 41, 181–194 (2011). [Google Scholar]
- 31.Durner G. M., Douglas D. C., Nielson R. M., Amstrup S. C., McDonald T. L., Stirling I., Mauritzen M., Born E. W., Wiig Ø., DeWeaver E., Serreze M. C., Belikov S. E., Holland M. M., Maslanik J., Aars J., Bailey D. A., Derocher A. E., Predicting 21st-century polar bear habitat distribution from global climate models. Ecol. Monogr. 79, 25–58 (2009). [Google Scholar]
- 32.Atwood T. C., Marcot B. G., Douglas D. C., Amstrup S. C., Rode K. D., Durner G. M., Bromaghin J. F., Forecasting the relative influence of environmental and anthropogenic stressors on polar bears. Ecosphere 7, e01370 (2016). [Google Scholar]
- 33.Rode K. D., Wilson R. R., Regehr E. V., St. Martin M., Douglas D. C., Olson J., increased land use by chukchi sea polar bears in relation to changing sea ice conditions. PLOS ONE 10, e0142213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bromaghin J. F., McDonald T. L., Stirling I., Derocher A. E., Richardson E. S., Regehr E. V., Douglas D. C., Durner G. M., Atwood T., Amstrup S. C., Polar bear population dynamics in the southern Beaufort Sea during a period of sea ice decline. Ecol. Appl. 25, 634–651 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Durner G. M., Douglas D. C., Albeke S. E., Whiteman J. P., Amstrup S. C., Richardson E., Wilson R. R., Ben-David M., Increased Arctic sea ice drift alters adult female polar bear movements and energetics. Glob. Chang. Biol. 23, 3460–3473 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Pagano A. M., Durner G. M., Rode K. D., Atwood T. C., Atkinson S. N., Peacock E., Costa D. P., Owen M. A., Williams T. M., High-energy, high-fat lifestyle challenges an Arctic apex predator, the polar bear. Science 359, 568–572 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Whiteman J. P., Harlow H. J., Durner G. M., Anderson-Sprecher R., Albeke S. E., Regehr E. V., Amstrup S. C., Ben-David M., Summer declines in activity and body temperature offer polar bears limited energy savings. Science 349, 295–298 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Jay C. V., Fischbach A. S., Kochnev A. A., Walrus areas of use in the Chukchi Sea during sparse sea ice cover. Mar. Ecol. Prog. Ser. 468, 1–13 (2012). [Google Scholar]
- 39.Iacozza J., Ferguson S. H., Spatio-temporal variability of snow over sea ice in western Hudson Bay, with reference to ringed seal pup survival. Polar Biol. 37, 817–832 (2014). [Google Scholar]
- 40.Stenson G. B., Hammill M. O., Can ice breeding seals adapt to habitat loss in a time of climate change? Ices J. Marine Sci. 71, 1977–1986 (2014). [Google Scholar]
- 41.Kahru M., Brotas V., Manzano-Sarabia M., Mitchell B. G., Are phytoplankton blooms occurring earlier in the Arctic? Glob. Chang. Biol. 17, 1733–1739 (2011). [Google Scholar]
- 42.George J. C., Druckenmiller M. L., Laidre K. L., Suydam R., Person B., Bowhead whale body condition and links to summer sea ice and upwelling in the Beaufort Sea. Prog. Oceanogr. 136, 250–262 (2015). [Google Scholar]
- 43.Heide-Jørgensen M. P., Laidre K. L., Quakenbush L. T., Citta J. J., The Northwest Passage opens for bowhead whales. Biol. Lett. 8, 270–273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore S. E., Is it ‘boom times’ for baleen whales in the Pacific Arctic region? Biol. Lett. 12, 20160251 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laidre K. L., Heide-Jørgensen M. P., Spring partitioning of Disko Bay, West Greenland, by Arctic and Subarctic baleen whales. Ices J. Marine Sci. 69, 1226–1233 (2012). [Google Scholar]
- 46.Reeves R. R., Ewins P. J., Agbayani S., Heide-Jørgensen M. P., Kovacs K. M., Lydersen C., Suydam R., Elliott W., Polet G., van Dijk Y., Blijleven R., Distribution of endemic cetaceans in relation to hydrocarbon development and commercial shipping in a warming Arctic. Mar. Policy 44, 375–389 (2014). [Google Scholar]
- 47.Williams T. M., Blackwell S. B., Richter B., Sinding M. H. S., Heide-Jorgensen M. P., Paradoxical escape responses by narwhals (Monodon monoceros). Science 358, 1329–1331 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Post E., Brodie J., Anticipating novel conservation risks of increased human access to remote regions with warming. Clim. Chang. Responses 2, 2 (2015). [Google Scholar]
- 49.Coumou D., Di Capua G., Vavrus S., Wang L., Wang S., The influence of Arctic amplification on mid-latitude summer circulation. Nat. Commun. 9, 2959 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swain D. L., Langenbrunner B., Neelin J. D., Hall A., Increasing precipitation volatility in twenty-first-century California. Nat. Clim. Change 8, 427–433 (2018). [Google Scholar]
- 51.Coumou D., Lehmann J., Beckmann J., The weakening summer circulation in the Northern Hemisphere mid-latitudes. Science 348, 324–327 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Coumou D., Petoukhov V., Rahmstorf S., Petri S., Schellnhuber H. J., Quasi-resonant circulation regimes and hemispheric synchronization of extreme weather in boreal summer. Proc. Natl. Acad. Sci. U.S.A. 111, 12331–12336 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun L., Perlwitz J., Hoerling M., What caused the recent “Warm Arctic, cold continents” trend pattern in winter temperatures? Geophys. Res. Lett. 43, 5345–5352 (2016). [Google Scholar]
- 54.Francis J. A., Vavrus S. J., Evidence linking Arctic amplification to extreme weather in mid-latitudes. Geophys. Res. Lett. 39, (2012). [Google Scholar]
- 55.Francis J. A., Varvus S. J., Evidence linking rapid Arctic warming to mid-latitude weather patterns. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 373, 20140170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mori M., Kosaka Y., Watanabe M., Nakamura H., Kimoto M., A reconciled estimate of the influence of Arctic sea-ice loss on recent Eurasian cooling. Nat. Clim. Change 9, 123–129 (2019). [Google Scholar]
- 57.Mann M. E., Rahmstorf S., Kornhuber K., Steinman B. A., Miller S. K., Coumou D., Influence of anthropogenic climate change on planetary wave resonance and extreme weather events. Sci. Rep. 7, 45242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mann M. E., Rahmstorf S., Kornhuber K., Steinman B. A., Miller S. K., Petri S., Coumou D., Projected changes in persistent extreme summer weather events: The role of quasi-resonant amplification. Sci. Adv. 4, eaat3272 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffin D., Anchukaitis K. J., How unusual is the 2012-2014 California drought? Geophys. Res. Lett. 41, 9017–9023 (2014). [Google Scholar]
- 60.Sewall J. O., Sloan L. C., Disappearing Arctic sea ice reduces available water in the American west. Geophys. Res. Lett. 31, 10.1029/2003GL019133 (2004). [Google Scholar]
- 61.Swain D. L., Tsiang M., Haugen M., Singh D., Charland A., Rajaratnam B., Diffenbaugh N., The extraordinary California drought of 2013/14: Character, context, and the role of climate change. Bull. Am. Meteorol. Soc. 95, S3–S7 (2014). [Google Scholar]
- 62.J. A. Church, P. U. Clark, A. Cazenave, J. M. Gregory, S. Jevrejeva, A. Levermann, M. A. Merrifield, G. A. Milne, R. S. Nerem, P. D. Nunn, A. J. Payne, W. T. Pfeffer, D. Stammer, A. S. Unnikrishnan, 2013, Sea level change, in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, T. F. Stocker et al., Eds. (Cambridge Univ. Press, 2013). [Google Scholar]
- 63.Oppenheimer M., Alley R. B., How high will the seas rise? Science 354, 1375–1377 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Shepherd A., Nowicki S., Improvements in ice-sheet sea-level projections. Nature Clim. Change 7, 672–674 (2017). [Google Scholar]
- 65.Koenig S. J., DeConto R. M., Pollard D., Impact of reduced Arctic sea ice on Greenland ice sheet variability in a warmer than present climate. Geophys. Res. Lett. 41, 3933–3942 (2014). [Google Scholar]
- 66.Rahmstorf S., Box J. E., Feulner G., Mann M. E., Robinson A., Rutherford S., Schaffernicht E. J., Exceptional twentieth-century slowdown in Atlantic Ocean overturning circulation. Nat. Clim. Change 5, 475–480 (2015). [Google Scholar]
- 67.Roberts D. H., Rea B. R., Lane T. P., Schnabel C., Rodes A., New constraints on Greenland ice sheet dynamics during the last glacial cycle: Evidence from the Uummannaq ice stream system. J. Geophys. Res. Earth Surface 118, 519–541 (2013). [Google Scholar]
- 68.Joughin I., Smith B. E., Howat I. M., Floricioiu D., Alley R. B., Truffer M., Fahnestock M., Seasonal to decadal scale variations in the surface velocity of Jakobshavn Isbrae, Greenland: Observation and model-based analysis. J. Geophys. Res. 117, 10.1029/2011JF002110 (2012). [Google Scholar]
- 69.Bamber J. L., Siegert M. J., Griggs J. A., Marshall S. J., Spada G., Paleofluvial mega-canyon beneath the central greenland ice sheet. Science 341, 997–999 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Schaefer J. M., Finkel R. C., Balco G., Alley R. B., Caffee M. W., Briner J. P., Young N. E., Gow A. J., Schwartz R., Greenland was nearly ice-free for extended periods during the Pleistocene. Nature 540, 252–255 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Alley R. B., Anandakrishnan S., Christianson K., Horgan H. J., Muto A., Parizek B. R., Oceanic forcing of ice-sheet retreat: West Antartica and more. Annu. Rev. Earth Planet. Sci. 43, 207–231 (2015). [Google Scholar]
- 72.DeConto R. M., Pollard D., Contribution of Antarctica to past and future sea-level rise. Nature 531, 591–597 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Pollard D., Chang W., Haran M., Applegate P., DeConto R., Large ensemble modeling of the last deglacial retreat of the West Antarctic Ice Sheet: Comparison of simple and advanced statistical techniques. Geosci. Model Dev. 9, 1697–1723 (2016). [Google Scholar]
- 74.Wolovick M. J., Moore J. C., Stopping the flood: Could we use targeted geoengineering to mitigate sea level rise? Cryosphere 12, 2955–2967 (2018). [Google Scholar]
- 75.Scambos T. A., Bell R. E., Alley R. B., Anandakrishnan S., Bromwich D. H., Brunt K., Christianson K., Creyts T., Das S. B., DeConto R., Dutrieux P., Fricker H. A., Holland D., MacGregor J., Medley B., Nicolas J. P., Pollard D., Siegfried M. R., Smith A. M., Steig E. J., Trusel L. D., Vaughan D. G., Yager P. L., How much, how fast?: A science review and outlook for research on the instability of Antarctica’s Thwaites Glacier in the 21st century. Global Planet. Change 153, 16–34 (2017). [Google Scholar]
- 76.Moore J. C., Gladstone R., Zwinger T., Wolovick M., Geoengineer polar glaciers to slow sea-level rise. Nature 555, 303–305 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Edwards T. L., Brandon M. A., Durand G., Edwards N. R., Golledge N. R., Holden P. B., Nias I. J., Payne A. J., Ritz C., Wernecke A., Revisiting Antarctic ice loss due to marine ice-cliff instability. Nature 566, 58–64 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Golledge N. R., Keller E. D., Gomez N., Naughten K. A., Bernales J., Trusel L. D., Edwards T. L., Global environmental consequences of twenty-first-century ice-sheet melt. Nature 566, 65–72 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Post E., Steinman B. A., Mann M. E., Acceleration of phenological advance and warming with latitude over the past century. Sci. Rep. 8, 3927 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ernakovich J. G., Hopping K. A., Berdanier A. B., Simpson R. T., Kachergis E. J., Steltzer H., Wallenstein M. D., Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob. Chang. Biol. 20, 3256–3269 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Prevéy J. S., Rixen C., Rüger N., Høye T. T., Bjorkman A. D., Myers-Smith I. H., Elmendorf S. C., Ashton I. W., Cannone N., Chisholm C. L., Clark K., Cooper E. J., Elberling B., Fosaa A. M., Henry G. H. R., Hollister R. D., Jónsdóttir I. S., Klanderud K., Kopp C. W., Lévesque E., Mauritz M., Molau U., Natali S. M., Oberbauer S. F., Panchen Z. A., Post E., Rumpf S. B., Schmidt N. M., Schuur E., Semenchuk P. R., Smith J. G., Suding K. N., Totland Ø., Troxler T., Venn S., Wahren C.-H., Welker J. M., Wipf S., Warming shortens flowering seasons of tundra plant communities. Nat. Ecol. Evol. 3, 45–52 (2019). [DOI] [PubMed] [Google Scholar]
- 82.Prevéy J., Vellend M., Rüger N., Hollister R. D., Bjorkman A. D., Myers-Smith I. H., Elmendorf S. C., Clark K., Cooper E. J., Elberling B., Fosaa A. M., Henry G. H. R., Høye T. T., Jónsdóttir I. S., Klanderud K., Lévesque E., Mauritz M., Molau U., Natali S. M., Oberbauer S. F., Panchen Z. A., Post E., Rumpf S. B., Schmidt N. M., Schuur E. A. G., Semenchuk P. R., Troxler T., Welker J. M., Rixen C., Greater temperature sensitivity of plant phenology at colder sites: Implications for convergence across northern latitudes. Glob. Chang. Biol. 23, 2660–2671 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Kerby J. T., Post E., Advancing plant phenology and reduced herbivore production in a terrestrial system associated with sea ice decline. Nat. Commun. 4, 2514 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Høye T. T., Post E., Schmidt N. M., Trojelsgaard K., Forchhammer M. C., Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nat. Clim. Change 3, 759–763 (2013). [Google Scholar]
- 85.McKinnon L., Picotin M., Bolduc E., Juillet C., Bety J., Timing of breeding, peak food availability, and effects of mismatch on chick growth in birds nesting in the High Arctic. Canadian J. Zool. 90, 961–971 (2012). [Google Scholar]
- 86.Kerby J., Post E., Capital and income breeding traits differentiate trophic match-mismatch dynamics in large herbivores. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120484 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.E. Post, Ecology of Climate Change: The Importance of Biotic Interactions (Princeton Univ. Press, 2013).
- 88.Mortensen L. O., Moshøj C., Forchhammer M. C., Density and climate influence seasonal population dynamics in an Arctic ungulate. Arctic Antarctic Alpine Res. 48, 523–530 (2016). [Google Scholar]
- 89.Post E., Beyen E., Bøving P. S., Higgins R. C., John C., Kerby J., Pedersen C., Watts D. A., Unusual late July observation of a fledgling Lapland longspur in low arctic Greenland following the cool spring of 2018. Arctic Sci. 6, 161–166 (2019). [Google Scholar]
- 90.Iler A. M., Høye T. T., Inouye D. W., Schmidt N. M., Nonlinear flowering responses to climate: Are species approaching their limits of phenological change? Philos. Trans. R. Soc. B Biol. Sci. 368, 20120489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Väisänen M., Ylänne H., Kaarlejärvi E., Sjögersten S., Olofsson J., Crout N., Stark S., Consequences of warming on tundra carbon balance determined by reindeer grazing history. Nat. Clim. Change 4, 384–388 (2014). [Google Scholar]
- 92.Cahoon S. M. P., Sullivan P. F., Post E., Welker J. W., Large herbivores limit CO2 uptake and suppress carbon cycle responses to warming in West Greenland. Glob. Chang. Biol. 18, 469–479 (2012). [Google Scholar]
- 93.Olofsson J., Tommervik H., Callaghan T. V., Vole and lemming activity observed from space. Nat. Clim. Change 2, 880–883 (2012). [Google Scholar]
- 94.te Beest M., Sitters J., Menard C. B., Olofsson J., Reindeer grazing increases summer albedo by reducing shrub abundance in Arctic tundra. Environ. Res. Lett. 11, 125013 (2016). [Google Scholar]
- 95.Post E., Erosion of community diversity and stability by herbivore removal under warming. Proc. R. Soc. B Biol. Sci. 280, 10.1098/rspb.2012.2722 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaarlejärvi E., Eskelinen A., Olofsson J., Herbivores rescue diversity in warming tundra by modulating trait-dependent species losses and gains. Nat. Commun. 8, 2283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fauchald P., Park T., Tømmervik H., Myneni R., Hausner V. H., Arctic greening from warming promotes declines in caribou populations. Sci. Adv. 3, e1601365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bjorkman A. D., Myers-Smith I. H., Elmendorf S. C., Normand S., Rüger N., Beck P. S. A., Blach-Overgaard A., Blok D., Cornelissen J. H. C., Forbes B. C., Georges D., Goetz S. J., Guay K. C., Henry G. H. R., HilleRisLambers J., Hollister R. D., Karger D. N., Kattge J., Manning P., Prevéy J. S., Rixen C., Schaepman-Strub G., Thomas H. J. D., Vellend M., Wilmking M., Wipf S., Carbognani M., Hermanutz L., Lévesque E., Molau U., Petraglia A., Soudzilovskaia N. A., Spasojevic M. J., Tomaselli M., Vowles T., Alatalo J. M., Alexander H. D., Anadon-Rosell A., Angers-Blondin S., te Beest M., Berner L., Björk R. G., Buchwal A., Buras A., Christie K., Cooper E. J., Dullinger S., Elberling B., Eskelinen A., Frei E. R., Grau O., Grogan P., Hallinger M., Harper K. A., Heijmans M. M. P. D., Hudson J., Hülber K., Iturrate-Garcia M., Iversen C. M., Jaroszynska F., Johnstone J. F., Jørgensen R. H., Kaarlejärvi E., Klady R., Kuleza S., Kulonen A., Lamarque L. J., Lantz T., Little C. J., Speed J. D. M., Michelsen A., Milbau A., Nabe-Nielsen J., Nielsen S. S., Ninot J. M., Oberbauer S. F., Olofsson J., Onipchenko V. G., Rumpf S. B., Semenchuk P., Shetti R., Collier L. S., Street L. E., Suding K. N., Tape K. D., Trant A., Treier U. A., Tremblay J.-P., Tremblay M., Venn S., Weijers S., Zamin T., Boulanger-Lapointe N., Gould W. A., Hik D. S., Hofgaard A., Jónsdóttir I. S., Jorgenson J., Klein J., Magnusson B., Tweedie C., Wookey P. A., Bahn M., Blonder B., van Bodegom P. M., Bond-Lamberty B., Campetella G., Cerabolini B. E. L., Stuart Chapin F. III, Cornwell W. K., Craine J., Dainese M., de Vries F. T., Díaz S., Enquist B. J., Green W., Milla R., Niinemets Ü., Onoda Y., Ordoñez J. C., Ozinga W. A., Penuelas J., Poorter H., Poschlod P., Reich P. B., Sandel B., Schamp B., Sheremetev S., Weiher E., Plant functional trait change across a warming tundra biome. Nature 562, 57–62 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Macias-Fauria M., Forbes B. C., Zetterberg P., Kumpula T., Eurasian Arctic greening reveals teleconnections and the potential for structurally novel ecosystems. Nat. Clim. Change 2, 613–618 (2012). [Google Scholar]
- 100.Forbes B. C., Kumpula T., Meschtyb N., Laptander R., Macias-Fauria M., Zetterberg P., Verdonen M., Skarin A., Kim K. Y., Boisvert L. N., Stroeve J. C., Bartsch A., Sea ice, rain-on-snow and tundra reindeer nomadism in Arctic Russia. Biol. Lett. 12, 20160466 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anonymous, “Report on the socio-economic situation of the Yamalskii District in the year 2016 (Yar Sale, Yunnan Observatories, Department of Economics, Municipal Administration, in Russian, 2017).
- 102.Liljedahl A. K., Boike J., Daanen R. P., Fedorov A. N., Frost G. V., Grosse G., Hinzman L. D., Iijma Y., Jorgenson J. C., Matveyeva N., Necsoiu M., Raynolds M. K., Romanovsky V. E., Schulla J., Tape K. D., Walker D. A., Wilson C. J., Yabuki H., Zona D., Pan-Arctic ice-wedge degradation in warming permafrost and its influence on tundra hydrology. Nat. Geosci. 9, 312–318 (2016). [Google Scholar]
- 103.Tyler N. J. C., Climate, snow, ice, crashes, and declines in populations of reindeer and caribou (Rangifer tarandus L.). Ecol. Monogr. 80, 197–219 (2010). [Google Scholar]
- 104.Tyler N. J. C., Forchhammer M. C., Øritsland N. A., Nonlinear effects of climate and density in the dynamics of a fluctuating population of reindeer. Ecology 89, 1675–1686 (2008). [DOI] [PubMed] [Google Scholar]
- 105.Albon S. D., Irvine R. J., Halvorsen O., Langvatn R., Loe L. E., Ropstad E., Veiberg V., van der Wal R., Bjørkvoll E. M., Duff E. I., Hansen B. B., Lee A. M., Tveraa T., Stien A., Contrasting effects of summer and winter warming on body mass explain population dynamics in a food-limited Arctic herbivore. Glob. Chang. Biol. 23, 1374–1389 (2016). [DOI] [PubMed] [Google Scholar]
- 106.Forbes B. C., Stammler F., Kumpula T., Meschtyb N., Pajunen A., Kaarlejärvi E., High resilience in the Yamal-Nenets social–ecological system, West Siberian Arctic, Russia. Proc. Natl. Acad. Sci. U.S.A. 106, 22041–22048 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steig E. J., Schneider D. P., Rutherford S. D., Mann M. E., Comiso J. C., Shindell D. T., Warming of the Antarctic ice-sheet surface since the 1957 International Geophysical Year. Nature 457, 459–462 (2009). [DOI] [PubMed] [Google Scholar]
- 108.Gooseff M. N., Barrett J. E., Adams B. J., Doran P. T., Fountain A. G., Lyons W. B., McKnight D. M., Priscu J. C., Sokol E. R., Takacs-Vesbach C., Vandegehuchte M. L., Virginia R. A., Wall D. H., Decadal ecosystem response to an anomalous melt season in a polar desert in Antarctica. Nat. Ecol. Evol. 1, 1334–1338 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Fountain A. G., Saba G., Adams B., Doran P., Fraser W., Gooseff M., Obryk M., Priscu J. C., Stammerjohn S., Virginia R. A., The impact of a large-scale climate event on Antarctic ecosystem processes. Bioscience 66, 848–863 (2016). [Google Scholar]
- 110.Lee J. R., Raymond B., Bracegirdle T. J., Chadès I., Fuller R. A., Shaw J. D., Terauds A., Climate change drives expansion of Antarctic ice-free habitat. Nature 547, 49–54 (2017). [DOI] [PubMed] [Google Scholar]
- 111.Chown S. L., Clarke A., Fraser C. I., Cary S. C., Moon K. L., McGeoch M. A., The changing form of Antarctic biodiversity. Nature 522, 427–434 (2015). [DOI] [PubMed] [Google Scholar]
- 112.Duffy G. A., Coetzee B. W. T., Latombe G., Akerman A. H., McGeoch M. A., Chown S. L., Barriers to globally invasive species are weakening across the Antarctic. Diver. Distrib. 23, 982–996 (2017). [Google Scholar]
- 113.Schofield O., Ducklow H. W., Martinson D. G., Meredith M. P., Moline M. A., Fraser W. R., How Do Polar Marine Ecosystems Respond to Rapid Climate Change? Science 328, 1520–1523 (2010). [DOI] [PubMed] [Google Scholar]
- 114.Cristofari R., Liu X., Bonadonna F., Cherel Y., Pistorius P., le Maho Y., Raybaud V., Stenseth N. C., le Bohec C., Trucchi E., Climate-driven range shifts of the king penguin in a fragmented ecosystem. Nat. Clim. Change 8, 245–251 (2018). [Google Scholar]
- 115.Fraser C. I., Morrison A. K., Hogg A. M. C., Macaya E. C., van Sebille E., Ryan P. G., Padovan A., Jack C., Valdivia N., Waters J. M., Antarctica’s ecological isolation will be broken by storm-driven dispersal and warming. Nat. Clim. Change 8, 704–708 (2018). [Google Scholar]
- 116.Grimaldi W. W., Seddon P. J., Lyver P. O., Nakagawa S., Tompkins D. M., Infectious diseases of Antarctic penguins: Current status and future threats. Polar Biol. 38, 591–606 (2015). [Google Scholar]
- 117.Walsh J. E., A comparison of Arctic and Antarctic climate change, present and future. Antarct. Sci. 21, 179–188 (2009). [Google Scholar]
- 118.Post E., Forchhammer M. C., Bret-Harte M. S., Callaghan T. V., Christensen T. R., Elberling B., Fox A. D., Gilg O., Hik D. S., Hoye T. T., Ims R. A., Jeppesen E., Klein D. R., Madsen J., McGuire A. D., Rysgaard S., Schindler D. E., Stirling I., Tamstorf M. P., Tyler N. J. C., van der Wal R., Welker J., Wookey P. A., Schmidt N. M., Aastrup P., Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355–1358 (2009). [DOI] [PubMed] [Google Scholar]
- 119.Meng L., Hess P. G. M., Mahowald N. M., Yavitt J. B., Riley W. J., Subin Z. M., Lawrence D. M., Swenson S. C., Jauhiainen J., Fuka D. R., Sensitivity of wetland methane emissions to model assumptions: Application and model testing against site observations. Biogeosciences 9, 2793–2819 (2012). [Google Scholar]
- 120.Hugelius G., Tarnocai C., Broll G., Canadell J. G., Kuhry P., Swanson D. K., The Northern Circumpolar Soil carbon database: Spatially distributed datasets of soil coverage and soil carbon storage in the northern permafrost regions. Earth Syst. Sci. Data 5, 3–13 (2013). [Google Scholar]
- 121.Arctic Monitoring and Assessment Programme (AMAP), Snow, Water, Ice and Permafrost in the Arctic (SWIPA) 2017 (AMAP, 2017), pp. 269.
- 122.Ström L., Tagesson T., Mastepanov M., Christensen T. R., Presence of Eriophorum scheuchzeri enhances substrate availability and methane emission in an Arctic wetland. Soil Biol. Biochem. 45, 61–70 (2012). [Google Scholar]
- 123.T. R. Christensen, K. van Huissteden, T. Sachs, in AMAP Assessment 2015: Methane as an Arctic climate forcer (Arctic Monitoring and Assessment Programme, 2015), pp. 15–26.
- 124.Zhang Z., Zimmermann N. E., Stenke A., Li X., Hodson E. L., Zhu G., Huang C., Poulter B., Emerging role of wetland methane emissions in driving 21st century climate change. Proc. Natl. Acad. Sci. U.S.A. 114, 9647–9652 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.T. R. Christensen, in Methane and climate change, D. Reay, P. Smith, A. van Amstel, Eds. (Earthscan, 2010), pp. 27–41.
- 126.Zhang W., Jansson C., Miller P. A., Smith B., Samuelsson P., Biogeophysical feedbacks enhance the Arctic terrestrial carbon sink in regional Earth system dynamics. Biogeosciences 11, 5503–5519 (2014). [Google Scholar]
- 127.Christensen T. R., Arora V. K., Gauss M., Höglund-Isaksson L., Parmentier F.-J. W., Tracing the climate signal: Mitigation of anthropogenic methane emissions can outweigh a large Arctic natural emission increase. Sci. Rep. 9, 1146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.T. R. Christensen, S. Rsgaard, J. Bendsten, B. Else, R. N. Glud, K. van Huissteden, J. E. Vonk, Arctic carbon cycling in AMAP, in Snow, Water, Ice and Permafrost in the Arctic (SWIPA) 2017 (Arctic Monitoring and Assessment Programme, 2017), pp. 203–218.
- 129.McGuire A. D., Lawrence D. M., Koven C., Clein J. S., Burke E., Chen G., Jafarov E., MacDougall A. H., Marchenko S., Nicolsky D., Peng S., Rinke A., Ciais P., Gouttevin I., Hayes D. J., Ji D., Krinner G., Moore J. C., Romanovsky V., Schädel C., Schaefer K., Schuur E. A. G., Zhuang Q., Dependence of the evolution of carbon dynamics in the northern permafrost region on the trajectory of climate change. Proc. Natl. Acad. Sci. U.S.A. 115, 3882–3887 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Raynolds M. K., Walker D. A., Epstein H. E., Pinzon J. E., Tucker C. J., A new estimate of tundra-biome phytomass from trans-Arctic field data and AVHRR NDVI. Remote Sens. Lett. 3, 403–411 (2011). [Google Scholar]
- 131.Epstein H., Tundra greenness. Bull. Am. Meteorol. Soc. 98, S145–S147 (2017). [Google Scholar]
- 132.Myers-Smith I. H., Elmendorf S. C., Beck P. S. A., Wilmking M., Hallinger M., Blok D., Tape K. D., Rayback S. A., Macias-Fauria M., Forbes B. C., Speed J. D. M., Boulanger-Lapointe N., Rixen C., Lévesque E., Schmidt N. M., Baittinger C., Trant A. J., Hermanutz L., Collier L. S., Dawes M. A., Lantz T. C., Weijers S., Jørgensen R. H., Buchwal A., Buras A., Naito A. T., Ravolainen V., Schaepman-Strub G., Wheeler J. A., Wipf S., Guay K. C., Hik D. S., Vellend M., Climate sensitivity of shrub growth across the tundra biome. Nat. Clim. Change 5, 887–891 (2015). [Google Scholar]
- 133.Elmendorf S. C., Henry G. H. R., Hollister R. D., Björk R. G., Bjorkman A. D., Callaghan T. V., Collier L. S., Cooper E. J., Cornelissen J. H. C., Day T. A., Fosaa A. M., Gould W. A., Grétarsdóttir J., Harte J., Hermanutz L., Hik D. S., Hofgaard A., Jarrad F., Jónsdóttir I. S., Keuper F., Klanderud K., Klein J. A., Koh S., Kudo G., Lang S. I., Loewen V., May J. L., Mercado J., Michelsen A., Molau U., Myers-Smith I. H., Oberbauer S. F., Pieper S., Post E., Rixen C., Robinson C. H., Schmidt N. M., Shaver G. R., Stenström A., Tolvanen A., Totland Ø., Troxler T., Wahren C. H., Webber P. J., Welker J. M., Wookey P. A., Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecol. Lett. 15, 164–175 (2012). [DOI] [PubMed] [Google Scholar]
- 134.Piao S. L., Nan H., Huntingford C., Ciais P., Friedlingstein P., Sitch S., Peng S., Ahlström A., Canadell J. G., Cong N., Levis S., Levy P. E., Liu L., Lomas M. R., Mao J., Myneni R. B., Peylin P., Poulter B., Shi X., Yin G., Viovy N., Wang T., Wang X., Zaehle S., Zeng N., Zeng Z., Chen A., Evidence for a weakening relationship between interannual temperature variability and northern vegetation activity. Nat. Commun. 5, 5108 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Kremers K. S., Hollister R. D., Oberbauer S. F., Diminished response of Arctic plants to warming over time. PLOS ONE 10, e0116586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park T., Ganguly S., Tømmervik H., Euskirchen E. S., Høgda K. A., Karlsen S. R., Brovkin V., Nemani R. R., Myneni R. B., Changes in growing season duration and productivity of northern vegetation inferred from long-term remote sensing data. Environ. Res. Lett. 11, 084001 (2016). [Google Scholar]
- 137.Metcalfe D. B., Hermans T. D. G., Ahlstrand J., Becker M., Berggren M., Björk R. G., Björkman M. P., Blok D., Chaudhary N., Chisholm C., Classen A. T., Hasselquist N. J., Jonsson M., Kristensen J. A., Kumordzi B. B., Lee H., Mayor J. R., Prevéy J., Pantazatou K., Rousk J., Sponseller R. A., Sundqvist M. K., Tang J., Uddling J., Wallin G., Zhang W., Ahlström A., Tenenbaum D. E., Abdi A. M., Patchy field sampling biases understanding of climate change impacts across the Arctic. Nat. Ecol. Evol. 2, 1443–1448 (2018). [DOI] [PubMed] [Google Scholar]
- 138.Martin A. C., Jeffers E. S., Petrokofsky G., Myers-Smith I., Macias-Fauria M., Shrub growth and expansion in the Arctic tundra: An assessment of controlling factors using an evidence-based approach. Environ. Res. Lett. 12, 085007 (2017). [Google Scholar]
- 139.Forchhammer M., Sea-ice induced growth decline in Arctic shrubs. Biol. Lett. 13, 20170122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gamm C. M., Sullivan P. F., Buchwal A., Dial R. J., Young A. B., Watts D. A., Cahoon S. M. P., Welker J. M., Post E., Declining growth of deciduous shrubs in the warming climate of continental western Greenland. J. Ecol. 106, 640–654 (2018). [Google Scholar]
- 141.Bjerke J. W., et al. , Understanding the drivers of extensive plant damage in boreal and Arctic ecosystems: Insights from field surveys in the aftermath of damage. Sci. Total Environ. 599, 1965–1976 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Pedersen S. H., Tamstorf M. P., Abermann J., Westergaard-Nielsen A., Lund M., Skov K., Sisgaard C., Mylius M. R., Hansen B. U., Liston G. E., Schmidt N. M., Spatiotemporal characteristics of seasonal snow cover in Northeast Greenland from in situ observations. Arctic Antarctic Alpine Res. 48, 653–671 (2016). [Google Scholar]
- 143.Bjerke J. W., Rune Karlsen S., Arild Høgda K., Malnes E., Jepsen J. U., Lovibond S., Vikhamar-Schuler D., Tømmervik H., Record-low primary productivity and high plant damage in the Nordic Arctic Region in 2012 caused by multiple weather events and pest outbreaks. Environ. Res. Lett. 9, 084006 (2014). [Google Scholar]
- 144.Bratsch S., Epstein H., Buchhorn M., Walker D., Landes H., Relationships between hyperspectral data and components of vegetation biomass in Low Arctic tundra communities at Ivotuk, Alaska. Environ. Res. Lett. 12, 025003 (2017). [Google Scholar]
- 145.Jiang C. Y., Ryu Y., Fang H., Myneni R., Claverie M., Zhu Z., Inconsistencies of interannual variability and trends in long-term satellite leaf area index products. Glob. Chang. Biol. 23, 4133–4146 (2017). [DOI] [PubMed] [Google Scholar]
- 146.I. H. Myers-Smith, J. T. Kerby, G. K.Phoenix, J. W. Bjerke, H. E.Epstein, J. J. Assmann, C. John, L. Andreu-Hayles, S. Angers-Blodin, P. S. A. Beck, L. T. Berner, U. S. Bhatt, A. D. Bjorkman, D. Blok, A. Bryn, C. T. Christiansen, J. Hans C. Cornelissen, A. M. Cunliffe, S. C. Elmendorf, B. C. Forbes, S. J.Goetz, R. D. Hollister, R. de Jong, M. M. Loranty, M. Macias-Fauria, K. Maseyk, S. Normand, J. Olofsson, T. C. Parker, F.-J. W. Parmentier, E. Post, G. Schaepman-Strub, F. Stordal, P. F. Sullivan, H. J. D.Thomas, H. Tømmervik, R. Treharne, C. E.Tweedie, D. A. Walker, M. Wilmking, S. Wipf, Complexity revealed in the greening of the Arctic. (2019); 10.32942/osf.io/mzyjk. [DOI]
- 147.Wang X., Piao S., Xu X., Ciais P., MacBean N., Myneni R. B., Li L., Has the advancing onset of spring vegetation green-up slowed down or changed abruptly over the last three decades? Glob. Ecol. Biogeogr. 24, 621–631 (2015). [Google Scholar]
- 148.Richardson A. D., Tracking seasonal rhythms of plants in diverse ecosystems with digital camera imagery. New Phytol. 222, 1742–1750 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Helman D., Land surface phenology: What do we really ‘see’ from space? Sci. Total Environ. 618, 665–673 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Schindler D. E., Hilborn R., Prediction, precaution, and policy under global change. Science 347, 953–954 (2015). [DOI] [PubMed] [Google Scholar]
- 151.J. W. Greenert, U.S. Navy Arctic Roadmap 2014–2030 (Department of the Navy, 2014).
- 152. Reform the Antarctic Treaty. Nature 558, 161 (2018). [DOI] [PubMed] [Google Scholar]
- 153.Sánchez R., A brief analysis of countries’ patterns of participation in the Antarctic Treaty Consultative Meetings (1998-2011); towards leveling the playing field? Polar Rec. 52, 686–697 (2016). [Google Scholar]
- 154.Stephenson S. R., Smith L. C., Agnew J. A., Divergent long-term trajectories of human access to the Arctic. Nat. Clim. Change 1, 156–160 (2011). [Google Scholar]
- 155.Hoag H., Nations agree to ban fishing in Arctic Ocean for at least 16 years. Science, aar6437 (2017). [Google Scholar]


