Abstract
Objective:
Nearly 80% of cancer patients struggle with insomnia, which is associated with decreased heart rate variability (HRV) and quality of life (QOL).. The aim of this secondary analysis was to evaluate the possible effects of Brief Behavioral Therapy for Cancer-Related Insomnia (BBT-CI), delivered during chemotherapy visits, on QOL and HRV in patients with breast cancer (BC).
Methods:
QOL and HRV data were obtained during a pilot clinical trial assessing the feasibility and effects of BBT-CI on insomnia. A total of 71 BC patients (mean age=52.5 years) were assigned randomly to either BBT-CI or a healthy eating control intervention (HEAL). BBT-CI and HEAL were delivered over six weeks (two face-to-face sessions plus four phone calls) by trained staff at four NCI-funded Community Oncology Research Program clinics. QOL was measured with the Functional Assessment of Cancer Therapy (FACT-G) and HRV with the Firstbeat device at baseline and after intervention.
Results:
There were significant improvements in QOL after intervention for BBT-CI (FACT-G, p=.009; FACT-B, p=.016; ANCOVA) and five-minute supine HRV measures (SDNN, p=.005; rMSSD, p=.004; HF, p=.009; ANCOVA) compared with HEAL.
Conclusions:
Patients randomized to BBT-CI showed improvements in QOL and HRV, providing support for BBT-CI’s possible benefit when delivered in the community oncology setting by trained staff. A more definitive efficacy trial of BBT-CI is currently being planned with sufficient statistical power to evaluate the intervention’s clinical utility.
Keywords: short-term heart rate variability, quality of life, brief behavioral therapy, breast neoplasms, multicenter study, randomized controlled trial
Background
Approximately 80% of cancer patients undergoing chemotherapy or radiation experience insomnia at some point during the course of their treatments (Palesh et al., 2010). Insomnia has been associated with a number of side effects including higher levels of fatigue, depression, pain, and reduced overall quality of life (QOL) (Palesh et al., 2013; Palesh et al 2012). Insomnia often results in or causes dysregulation of other major systems (Levenson, Kay, & Buysse, 2015), potentially explaining the numerous side effects that accompany it. For example, autonomic nervous system dysregulation is commonly seen in patients with insomnia. Specifically, many patients with insomnia have a decreased arousal threshold (hyperarousal), which can be understood as a predominance of sympathetic rather than parasympathetic activity, making sleep initiation and continuation more difficult. Although the mechanisms behind falling and staying asleep are complex (Miglis, 2016; Somers, Dyken, Mark, & Abboud, 1993), autonomic dysfunction is common in cancer patients (Lakoski, Jones, Krone, Stein, & Scott, 2015). Thus, an imbalance between sympathetic and parasympathetic activation may be related to the development and maintenance of insomnia in breast cancer (Palesh et al., 2008).
Optimal autonomic nervous system (ANS) functioning has been linked to improved overall health and resilience. ANS dysregulation, on the other hand, has been associated with worse psychological (e.g., emotional, Williams et al., 2015) and physical health, often resulting in reduced overall QOL (Thayer & Sternberg, 2006 Van Gestel et al., 2011). Furthermore, ANS dysregulation has been implicated in the development of malignancies, in part due to the role of the ANS in immune functioning (Mravec et al., 2006; Rosas-Ballina & Tracey, 2009; Tracey, 2002), and is associated with earlier mortality in some cancer patients (De Couck, Mravec, & Gidron, 2012; Giese-Davis et al., 2015; Kim et al., 2010; Scheiber et al., 2018). Despite the close associations between insomnia, ANS dysregulation, and reduced QOL, few studies have examined their relationships in patients undergoing treatment.
ANS functioning and, more specifically, vagal activity can be measured through analysis of heart rate variability (HRV) (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996), and has been associated with various health outcomes (Ernst, 2017). Higher HRV is associated with better glucose regulation, better hypothalamic–pituitary–adrenal (HPA) axis function, decreased inflammation, decreased risk of stroke, reduced risk of cardiovascular disease (CVD), and decreased all-cause mortality (Liao, Carnethon, Evans, Cascio, & Heiss, 2002; Thayer & Fischer, 2009; Thayer & Lane, 2007; Thayer & Sternberg, 2006). Chemotherapy (Poreba et al., 2014) can decrease HRV and cause significant decreases in heart rate variance and turbulence, which is in turn associated with multiple cardiovascular diseases and poorer outcomes as well as decreased survival (Giese-Davis, et al., 2015).
Historically, HRV was calculated using 24-hour recordings on a Holter electrocardiography device, and this instrument remains to be the gold standard for HRV measurement (Akintola, van de Pol, Bimmel, Maan, & van Heemst, 2016). However, depending on the parameters of interest (i.e., SDNN, rMSSD, HF, LF, LF/HF), five-minute recordings provide sufficient data (Ernst, 2017) and other devices display sufficient accuracy for capturing acute HRV within a five-minute window (Parak & Korhonen, 2014). Consecutive heart beats are commonly characterized by R wave to R wave (RR) or normal beat to normal beat (NN) intervals, and the standard deviation of all RR intervals (SDNN) is the most frequently used parameter; SDNN exhibits the cyclic components responsible for HRV (Laborde, Mosley, & Thayer, 2017). R-R intervals refer to the time in milliseconds between sequential R waves of the ECG. The R wave is a reflection of the heart’s ventricular contraction. The square root of the mean of successive NN intervals (rMSSD) and high frequency (HF) parameters reflect vagal activity, whereas low frequency (LF) parameters reflect a mix of sympathetic and vagal activity (Laborde, et al., 2017). The ratio of LF spectral power to HF spectral power reflects sympathovagal balance (Laborde, et al., 2017). SDNN requires 6–120 seconds of recording, while rMSSD, HF, and LF require five minutes (Ernst, 2017; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996).
Existing randomized clinical trials (RCTs) utilizing behavioral interventions for insomnia (e.g., cognitive behavioral or mindfulness-based therapies) were primarily conducted in cancer survivors with chronic insomnia (Epstein & Dirksen, 2007; Espie et al., 2008; Fiorentino et al., 2010; Garland et al., 2014; Savard, Simard, Ivers, & Morin, 2005). One behavioral study conducted in cancer patients did not observe long-term positive effects on insomnia (Berger, et al., 2009). Diagnosis of cancer and associated treatments are catalysts for the development or worsening of insomnia symptoms and other side effects (Costa et al., 2014; Fleming, Gillespie, & Espie, 2010; Palesh et al., 2012; Savard, Hervouet, & Ivers, 2013). Treating insomnia with pharmacological agents during cancer therapies (e.g., chemotherapy) is complicated due to possible interaction effects. Moreover, given patients’ limited time and resources, existing psychotherapy approaches can be impractical. Intervening early, without adding unnecessary burden to the patient, can help prevent the development of chronic or more severe insomnia symptoms and may improve physiological and psychological wellbeing. We created a behavioral intervention for insomnia specifically tailored to cancer patients undergoing active treatment, Brief Behavioral Therapy for Cancer-Related Insomnia (BBT-CI) which we pilot-tested in the National Cancer Institute (NCI) Community Oncology Research Program (NCORP). The primary outcomes of the pilot RCT study showed that BBT-CI was feasible and acceptable and could be delivered in the community oncology setting by the clinic’s staff (e.g., nurses). Our study provided proof-of-concept regarding BBT-CI’s possible effects on insomnia symptoms compared to the time- and attention-matched control condition (Palesh et al. 2018). We now report on its effects on ANS functioning, as measured by HRV, and overall QOL in this secondary outcome study. We hypothesized that the successful reduction of insomnia may also have a positive impact on HRV and overall QOL.
Methods
BBT-CI Intervention and Study Sample
Palesh et al. (2018) provide a summary of results from the pilot randomized trial of the BBT-CI intervention as well as a more detailed description of the study methods. Here we provide a brief overview of the sample, recruitment procedures, randomization and intervention. This article describes the results of a secondary analysis of that study.
The RCT of the feasibility and possible effects of BBT-CI intervention was conducted between February 2014 and December 2015 out of the University of Rochester Cancer Center (URCC) NCORP Research Base in NCORP Rochester, New York in the United States. Participants were recruited at four participating NCORP affiliates; no participants were recruited at the URCC itself.
Patients were included if they were 1) at least 21-years-old; 2) female with newly diagnosed breast cancer (stages I-IIIA); 3) receiving chemotherapy with at least six weeks of chemotherapy treatments remaining; 4) reporting sleep problems as indicated by a minimum score of eight on the insomnia severity index (ISI); and 5) able to speak and understand English. Patients were excluded if they 1) regularly took sleep medications other than melatonin (vs. as needed; patients who took prescription sleep aids or any other sleep aids occasionally were not excluded from the study); 2) had a diagnosis of Stage IV breast cancer; 3) suffered from sleep apnea or restless leg syndrome; 4) had a diagnosis of a severe mental illness; 5) could not abstain from taking anxiolytics at least four hours before each intervention session; 6) had an existing heart condition requiring an implant; or, 7) had irregular sleep-wake cycles due to shift-work or other work schedules. Each site’s institutional review board [Metro Minnesota, MN; Wichita, KS; Hematology Oncology Associates, NY; and Southeast Cancer Control Consortium (SCCC, comprising VA, NC, SC, and GA)] approved this study. All procedures performed involving human participants were in accordance with the ethical standards of the University of Rochester Office for Human Subject Protection and the Declaration of Helsinki (World Medical Association, 1964) and its later amendments or comparable ethical standards. Informed consent was obtained from all individuals before randomization.
Recruitment
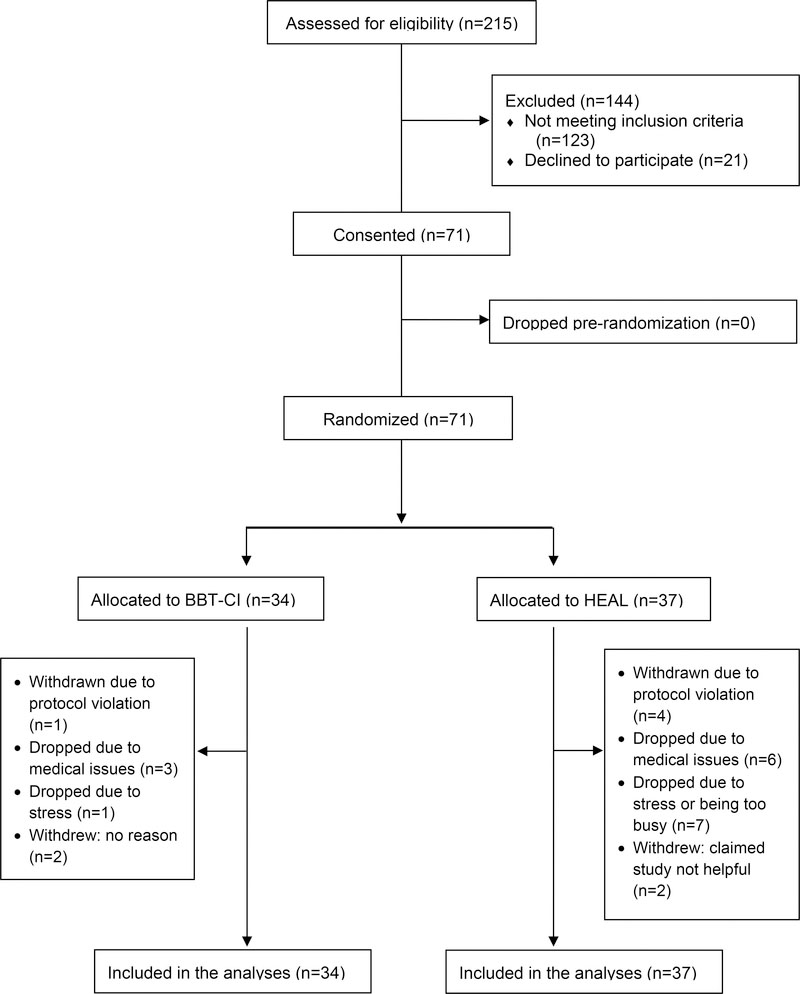
Clinical research assistants screened and enrolled participants. Of the 92 eligible patients, 21 refused to participate due to a lack of interest, feeling too overwhelmed, or their physician advising against participation. The remaining 71 patients participated in the study, yielding a recruitment rate of 77%. Figure 1 provides the CONSORT diagram of the flow of participants through the study.
Figure 1.
Recruitment CONSORT Diagram: Feasibility and Acceptability of Brief Behavioral Therapy for Cancer-Related Insomnia (BBT-CI)
Randomization and Intervention
The 71 consenting participants were randomly assigned to either the BBT-CI treatment arm or the Healthy Eating Education Learning (HEAL) control group. Participants were stratified by NCORP site and ISI severity score to ensure equal distribution of severity of sleep problems in both arms (ISI score of 8–15 = “moderate” and 16–28 = “severe”). A random allocation sequence was computer-generated using the Mersenne Twister algorithm by the URCC NCORP Research Base which provided the research team with randomization assignment. At the time of intervention assignment, only the participant, intervener, and biostatistician knew of the allocation to BBT-CI or HEAL. All other assessors gathering clinical data remained blinded. Regardless of arm assignment, both treatment arms involved two face-to-face sessions and four phone calls with an assigned intervener. The face-to-face sessions were conducted during the participants’ infusion appointment in the infusion center of the oncology clinics.
The BBT-CI treatment arm included 31 breast cancer patients with sleep difficulties. The BBT-CI intervention closely resembled cognitive behavioral therapy for insomnia (CBT-I) principles modified to fit the needs of breast cancer patients undergoing chemotherapy. Specifically, BBT-CI was tailored in several ways. First, it was delivered during infusion while patients underwent chemotherapy, thus minimizing patient travel time and burden. Second, as it may not be safe to restrict sleep in cancer patients, it did not employ sleep restriction but instead was focused on stimulus control. Third, BBT-CI also provided education about how cancer and cancer treatments can contribute to the development of insomnia and circadian dysfunction, and addressed how side effects from treatments such as hot flashes can adversely impact sleep.
The control intervention, HEAL, had no behavioral sleep intervention elements but followed the same format as the BBT-CI intervention: two face-to-face sessions and four 15-minute phone calls. The HEAL intervention was also matched on time and attention to the BBT-CI arm. HEAL’s content was provided by the National Cancer Institute and included nutritional education and suggestions for symptom management related to commonly experienced chemotherapy side effects, including nausea, diarrhea, hydration, constipation, and anorexia (Berger, et al., 2009; Berger, Kuhn, Farr, Lynch et al., 2009).
Interveners and Training
Participating staff members from the four treatment centers were trained in both BBT-CI and HEAL interventions. In total, 16 interveners were trained by the principal investigator and designated research staff by phone, for an average of 12 sessions. Interveners were provided training manuals, videos, and other resources. After completion of the training, the PI certified interveners based on practice session. Supervisors reviewed recordings of the face-to-face sessions with patients and ensured interveners and participants completed a BBT-CI protocol adherence checklist. The checklists and auditory recordings confirmed that interveners adhered to the intervention protocol and delivered 81% of the intervention components successfully (Palesh et al., 2018).
Measures
Heart Rate Variability.
HRV was measured using the Firstbeat® device, an ambulatory heart rate monitor that measures two time domains and three frequency domains of HRV. The Firstbeat® device’s RR intervals are as accurate as standard clinical ECG-derived RR intervals during rest and various physical activities (Parak & Korhonen, 2014). This device has been successfully used in a number of research studies and has been shown to be a reliable and valid detector of HRV (Parak & Korhonen, 2014; Sztajzel, 2004). Time domains included 1) SDNN and 2) rMSSD. Frequency domains included 1) HF, 2) LF, and 3) LF/HF. Recordings were gathered under consistent conditions to minimize variability caused by potential confounding variables such as movement and pace of breathing (i.e., patients were breathing naturally while maintaining a constant posture – relaxed and sitting). The data were analyzed using Kubios software, version 2.2. HRV data were screened for outlier or artifact RR intervals. Data that were determined clearly to be artifacts at the beginning or end of the recording session were trimmed from the dataset (Kubios HRV, 2.2, University of Kuopio, Kuopio, Finland). Outliers and minor artifacts were smoothed using threshold levels, from low to strong, depending on their severity (Kubios HRV, 2.2, University of Kuopio, Kuopio, Finland). Interpolation was used to fill in for artifacts prior to analyses. We recorded 20 minutes of data on the Firstbeat® devices, well over the five minutes needed to calculate the desired HRV indices reliably. Twenty staff members from the four participating sites were trained on using the Firstbeat® device.
Quality of Life.
Multidimensional QOL was assessed with the Functional Assessment of Cancer Therapy-Breast Cancer (FACT-B, Version 4). FACT-B is a 37-item self-report measure developed specifically to assess QOL across multiple dimensions in cancer patients. It consists of the FACT-General (FACT-G) (Cella et al., 1993) and a breast cancer specific subscale (Brady et al., 1997). The FACT-B is a well-validated scale that takes about 15 minutes to complete (Cella & Nowinski, 2002). In addition to a QOL total score index, the FACT-B yields five wellbeing subscale scores (physical, social/family, emotional, functional, and additional breast carcinoma concerns) (Brady, et al., 1997).
Statistical Analysis
Power analysis.
The study sample size was powered based on the feasibility outcomes based on the BBT-CI pilot clinical trial. To assess the feasibility and reliability of the BBT-CI intervention at 80% power, at least 30 participants per treatment arm were needed, based on a one-sided test with a 5% alpha significance level. With an anticipated 15% attrition rate, the aim was to recruit 70 participants in total, 35 for the BBT-CI arm and 35 for the HEAL control arm.
Analytical strategy.
Analysis of Covariance (ANCOVA) was used to assess the group differences of mean post-pre change for each of the outcomes with the baseline as the covariate adjusting for stratification factors (NCORP site and baseline insomnia level). Stratification factors were retained in the models if they were statistically significant at the 0.05 level. A term for Baseline*Arm Interaction was included to accommodate possible dependency of efficacy on baseline. This interaction was retained only when it was significant at the 0.05 level. If the interaction was found to be significant, the authors estimated the arm differences in mean change at different baseline levels.
Missing Data.
The Jamshidian and Jalal’s Test (2010) was used to test the missing completely at random assumption based on the sleep and distress outcomes, which were representative of the missing value patterns for HRV and QOL (Palesh, et al., 2018). The test was not significant at the 0.05 level. Hence, it was concluded that analyses of data from participants who completed post-intervention assessments did not involve bias due to missing data.
Results
All participants were female breast cancer patients with a mean (±SD) age of 52.5±9.8 years (range 29–73 years), mostly white, non-Hispanic/Latino, and married or living with a long-term partner; approximately half used occasional sleep aids (Table 1). Thirty-four participants were randomized to the BBT-CI treatment arm, and 37 participants to HEAL. At baseline, there were no significant differences in terms of demographics or clinical characteristics between the two treatment arms. A larger number of HEAL arm participants discontinued the study during the intervention.
Table 1.
Demographics Table
| Variable | Total (n=71) | BBT-CI (n=34) | HEAL (n=37) |
|---|---|---|---|
| Age, y, Mean (SD) | 52.5 (9.8) | 50.9 (7.9) | 53.8 (11.2) |
| Range | 29–73 | 33–67 | 29–73 |
| Race, N (%) | |||
| White | 68 (96%) | 33 (97%) | 35 (96%) |
| Black | 2 (3%) | 1 (3%) | 1 (2%) |
| Asian | 1 (1%) | 0 (0%) | 1 (2%) |
| Marital Status, N (%) | |||
| Married/Long-Term Partner | 48 (68%) | 28 (83%) | 20 (56%) |
| Divorced | 11 (15%) | 3 (9%) | 8 (22%) |
| Single | 4 (6%) | 2 (7%) | 2 (5%) |
| Widowed | 1 (1%) | 0 (0%) | 1 (1%) |
| No answer | 7 (10%) | 1 (1%) | 6 (16%) |
| Usage of Sleep Aids, N (%) | |||
| Yes | 30 (43%) | 18 (53%) | 12 (32%) |
| No | 29 (41%) | 14 (41%) | 15 (41%) |
| No Answer | 12 (16%) | 2 (6%) | 10 (27%) |
| Sleep Severity, Mean (SD) | |||
| Prior To Cancer Diagnosis | 3.5 (2.2) | 3.6 (2.1) | 3.4 (2.2) |
| Since Cancer Diagnosis | 6.9 (1.5) | 7.0 (1.6) | 6.7 (1.4) |
| Last Seven Days | 6.7 (1.7) | 6.8 (1.6) | 6.6 (1.8) |
| Entry ISI, N (%) | |||
| Moderate | 36 (51%) | 16 (47%) | 20 (55%) |
| Severe | 35 (49%) | 18 (53%) | 17 (45%) |
| Stages of Disease, N (%) | |||
| Stage I | 14 (20%) | 7 (21%) | 7 (18%) |
| Stage II | 39 (54%) | 20 (58%) | 19 (52%) |
| Stage III | 16 (23%) | 7 (21%) | 9 (25%) |
| Unknown | 2 (3%) | 0 (0%) | 2 (5%) |
The majority of participants in the BBT-CI arm (25 of 34) completed at least five of the six BBT-CI sessions, reflecting a 73.5% adherence rate. Lastly, intervention checklist and recordings confirmed that interveners delivered 80.7% of intervention components successfully (Palesh, et al., 2018). Any deviations to the protocol, tracked via study logs, included changes in the date of the intervention due to patient travel/unavailability, illness, or cancellation of chemotherapy.
Table 2 summarizes the mean and standard deviation (SD) change scores and ANCOVA results for all HRV indexes for both BBT-CI and HEAL. Mean scores indicate that HRV indexes remained stable for the BBT-CI arm, but decreased for HEAL. At post-intervention, there were significant group differences on several HRV indexes. Specifically, results revealed BBT-HEAL = 5.562, p = .0047 for SDNN; BBT-HEAL = 5.177, p = .0042 for rMSSD; and, BBT-HEAL = 1.200, p = .0094 for HF. No differences were found between the two study arms for LF/HF or for average heart rate after the intervention. Eight of the BBT-CI participants did not show SDNN improvements as compared to only one of the HEAL group.
Table 2.
Measures of HRV Mean (SD) Change Scores and ANCOVA Results for BBT-CI and HEAL Arms at Pre- and Post-Intervention
| BBT-CI | HEAL | ANCOVA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% Confidence
Interval |
95% Confidence
Interval |
95% Confidence
Interval |
|||||||||||||
| Pre- and Post-Intervention Differences | Mean Change (SD) | t | Estimate | Lower Bound | Upper Bound | Mean Change (SD) | t | Estimate | Lower Bound | Upper Bound | Estimate | SE | p | Lower Bound | Upper Bound |
| SDNN | 0.41 (6.60) | 0.23 | 0.06 | −3.40 | 4.22 | −4.76 (4.78) | −2.99 | −0.73 | −8.43 | −1.08 | 5.56 | 2.22 | <0.01 | 0.93 | 10.20 |
| rMSSD | 0.94 (5.53) | 0.63 | 0.20 | −2.26 | 4.13 | −3.40 (2.72) | −3.75 | −1.11 | −5.49 | −1.31 | 5.18 | 1.91 | <0.01 | 1.19 | 9.16 |
| HF Power | 0.09 (0.83) | 0.40 | 0.12 | −0.39 | 0.57 | −1.04 (1.17) | −2.67 | −1.33 | −1.94 | −0.14 | 1.20 | 0.42 | <0.01 | 0.33 | 2.07 |
| LF/HF | 0.39 (1.98) | 0.73 | 0.13 | −0.75 | 1.53 | 1.42 (3.40) | 1.25 | 0.52 | −1.20 | 4.03 | −1.00 | 1.14 | 0.39 | −3.39 | 1.38 |
| Mean Heart Rate | 0.18 (9.71) | 0.07 | 0.01 | −5.43 | 5.79 | 8.26 (12.89) | 1.92 | 0.91 | −1.65 | 18.16 | −7.91 | 4.68 | 0.11 | −17.67 | 1.86 |
Note: SDNN = Standard Deviation of all Normal to R-R Intervals; rMSSD = Root Mean Square of Successive Differences; HF = High Frequency Power; LF/HF = Low Frequency/High Frequency Power Ratio
Changes in FACT-B and HRV indexes were moderately correlated with one another: (SDNN: r = .44, p < .05; rMSSD: r = .57, p < .01; and HF: r = .55, p < .01), suggesting an important relationship between HRV and QOL. Table 3 summarizes the means and SDs of the FACT-B (QOL) total score index and subscale scores. Means suggest that participants in the BBT-CI arm had higher QOL than those in HEAL at post-intervention.
Table 3.
FACT-B Means (SDs) for BBT-CI and HEAL Arms at Pre- and Post-Intervention (n=71)
| Variable | Pre | Post | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Summary Scores | |||||
| FACT-B | |||||
| BBT-CI | 98.4 | 15.37 | 101.96 | 15.66 | |
| HEAL | 94.6 | 16.34 | 93.99 | 14.91 | |
| FACT-G Total | |||||
| BBT-CI | 74.9 | 12.09 | 78.21 | 12.72 | |
| HEAL | 74.0 | 11.57 | 72.74 | 11.51 | |
| Trial Outcome Index | |||||
| BBT-CI | 56.35 | 10.68 | 59.59 | 11.05 | |
| HEAL | 53.89 | 14.56 | 54.55 | 12.22 | |
| Subscale Scores | |||||
| Physical Well-being | |||||
| BBT-CI | 17.53 | 4.50 | 18.50 | 5.30 | |
| HEAL | 17.04 | 6.41 | 18.00 | 5.99 | |
| Emotional Well-being | |||||
| BBT-CI | 18.53 | 3.65 | 19.46 | 3.77 | |
| HEAL | 16.93 | 3.68 | 17.60 | 3.71 | |
| Social/Family Well-being | |||||
| BBT-CI | 23.50 | 3.84 | 22.91 | 3.93 | |
| HEAL | 23.77 | 2.68 | 21.84 | 4.31 | |
| Functional Well-being | |||||
| BBT-CI | 15.32 | 4.36 | 17.34 | 4.64 | |
| HEAL | 16.26 | 4.50 | 15.30 | 4.19 | |
| Additional Concerns | |||||
| BBT-CI | 23.50 | 5.09 | 23.75 | 4.81 | |
| HEAL | 20.59 | 6.07 | 21.25 | 5.37 | |
Note: FACT-B = FACT-G Total + Additional Concerns. FACT G Total = Physical Well-being + Emotional Well-being + Social/Family Well-being + Functioning Well-being. Trial Outcome Index = Physical Well-being + Functional Well-being + Additional Concerns
Table 4 summarizes the ANCOVA results. FACT-B scores improved among participants in the BBT-CI arm while those in HEAL worsened (BBT-HEAL = 6.896, p = .016) after intervention. The functional wellbeing subscale also improved among BBT-CI participants after the -intervention (BBT-HEAL=3.198, p =.004). There were no significant differences for social/family wellbeing, emotional wellbeing, physical wellbeing, or additional breast carcinoma concern subscales after intervention.
Table 4.
FACT-B Mean (SD) Change Scores and ANCOVA Results for BBT-CI and HEAL Arms at Pre- and Post-Intervention (n=71)
| Pre-Post Change | ANCOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% Confidence
Interval |
95% Confidence
Interval |
||||||||||
| Variable | Mean Change | SD | p | Lower Bound | Upper Bound | Estimate | SE | p | Lower Bound | Upper Bound | |
| Summary Scores | |||||||||||
| FACT-B | 6.90 | 2.75 | 0.02 | 1.35 | 12.44 | ||||||
| BBT-CI | 3.92 | 10.88 | 0.07 | −0.30 | 8.14 | ||||||
| HEAL | −2.71 | 7.93 | 0.01 | −6.42 | 1.01 | ||||||
| FACT-G Total | 6.48 | 2.38 | 0.01 | 1.68 | 11.27 | ||||||
| BBT-CI | 3.60 | 8.89 | 0.04 | 0.15 | 7.05 | ||||||
| HEAL | −3.11 | 7.41 | 0.08 | −6.57 | 0.36 | ||||||
| Trial Outcome Index | 4.67 | 2.23 | 0.04 | 0.18 | 9.16 | ||||||
| BBT-CI | 3.48 | 8.68 | 0.04 | 0.11 | 6.84 | ||||||
| HEAL | −1.05 | 7.79 | 0.55 | −4.70 | 2.60 | ||||||
| Subscale Scores | |||||||||||
| Physical Well-being | 0.66 | 1.35 | 0.63 | −2.07 | 3.38 | ||||||
| BBT-CI | 1.11 | 4.63 | 0.22 | −0.69 | 2.90 | ||||||
| HEAL | 0.35 | 5.56 | 0.87 | −2.25 | 2.95 | ||||||
| Emotional Well-being | 0.92 | 0.87 | 0.29 | −0.82 | 2.67 | ||||||
| BBT-CI | 0.64 | 2.50 | 0.18 | −0.33 | 1.61 | ||||||
| HEAL | 0.10 | 3.66 | 0.90 | −1.61 | 1.81 | ||||||
| Social/Family Well-being | 1.47 | 0.89 | 0.10 | −0.32 | 3.26 | ||||||
| BBT-CI | −0.20 | 2.61 | 0.69 | −1.21 | 0.82 | ||||||
| HEAL | −1.76 | 3.59 | 0.04 | −3.44 | −0.08 | ||||||
| Functioning Well-being | 3.20 | 1.04 | <0.01 | 1.10 | 5.29 | ||||||
| BBT-CI | 2.05 | 3.91 | 0.01 | 0.53 | 3.56 | ||||||
| HEAL | −1.80 | 3.62 | 0.04 | −3.50 | −0.11 | ||||||
| Additional Concerns | 0.73 | 1.03 | 0.48 | −1.33 | 2.80 | ||||||
| BBT-CI | 0.32 | 4.02 | 0.68 | −1.24 | 1.88 | ||||||
| HEAL | 0.30 | 3.44 | 0.61 | −1.21 | 2.01 | ||||||
Note: FACT-B = FACT-G Total + Additional Concerns. FACT G Total = Physical Well-being + Emotional Well-being + Social/Family Well-being + Functioning Well-being. Trial Outcome Index = Physical Well-being + Functional Well-being + Additional Concerns
Table 5 summarizes the measures of HRV mean and standard deviation before and after the intervention. Results indicate that SDNN, LF/HF, and heart rate declined over time in both groups, while rMSSD increased over time in both groups. HF power (ln) increased in the BBT-CI group while decreasing in the HEAL group.
Table 5.
Measures of HRV Mean (SD) for BBT-CI and HEAL Arms at Pre- and Post-intervention
| BBT-CI | HEAL | |||
|---|---|---|---|---|
| Measure | Pre Mean (SD) | Post Mean (SD) | Pre Mean (SD) | Post Mean (SD) |
| SDNN | 17.93 (7.12) | 17.63 (6.12) | 16.29 (6.48) | 11.62 (6.44) |
| rMSSD | 12.82 (4.58) | 13.55 (5.15) | 10.28 (3.07) | 7.37 (3.45) |
| HFpower(ln) | 4.12 (.75) | 4.20 (.81) | 3.75 (.78) | 2.84 (1.37) |
| LF/HF | 3.40 (2.92) | 3.41 (3.86) | 4.06 (2.73) | 4.98 (3.94) |
| Heart Rate | 87.06 (15.27) | 87.44 (15.18) | 87.68(9.05) | 95.14 (13.26) |
Note: SDNN = Standard Deviation of all Normal to R-R Intervals; rMSSD = Root Mean Square of Successive Differences; HF = High Frequency Power; LF/HF = Low Frequency/High Frequency Power Ratio
Discussion
The results of this study showed that, over the course of chemotherapy, several measures of HRV remained stable in breast cancer patients who received the BBT-CI but declined among patients in the HEAL control condition. In addition, patients randomized to BBT-CI showed significant improvement in overall QOL and functional well-being compared to patients who were randomized to HEAL. The primary outcome of this study showed that BBT-CI was feasible and acceptable to both cancer patients and clinic staff. The secondary outcomes reported here suggest that the reduction in insomnia symptoms may be associated with better HRV indexes and improved QOL, but need to be replicated in a study with a larger number of participants.
Previous RCTs have demonstrated that pharmacological (Jansson et al., 1999), exercise (Sandercock, Bromley, & Brodie, 2005; Selig et al., 2004), and biofeedback (Nolan et al., 2005) interventions can improve HRV in patients with cardiovascular diseases. In contrast, few studies of factors that affect HRV have been conducted in cancer patients. Of note is a single three-arm controlled, quasi-experimental study of an exercise intervention that showed positive preliminary effects on HRV and QOL in cancer patients during and after treatments (Niederer et al., 2013). The exercise intervention was intensive, and required participants to engage in a 16-week exercise plan, meeting for 1 hr each week and exercising at home three to five times each week. The intensity of the exercise intervention might have made it difficult for most cancer patients to participate (Niederer, et al., 2013), potentially reducing generalizability of findings. Similarly, an RCT utilizing an aerobic exercise intervention as part of a 28-day inpatient rehabilitation program for lung cancer patients following treatment found positive effects on HRV and QOL (Riesenberg & Lubbe, 2010).
Overall, the time and effort associated with the existing exercise-based HRV interventions seem beneficial for cancer patients who are able to participate in such programs. Results of the present study suggest several strengths of the BBT-CI intervention that may reach a larger range of patients: 1) it is deliverable by trained nurses and clinical research assistants in a community setting; 2) it can be effective without adding unnecessary burden to patients typically experienced during CBT-I (e.g., frequency of sessions, travel to a psychologist’s office, lengthy sessions, components irrelevant or harmful to cancer patients); and 3) it can prevent decreases in HRV and it can enhance QOL in cancer patients undergoing chemotherapy.
A number of RCTs have shown positive effects on QOL following yoga in cancer patients and survivors (Chandwani et al., 2010; Moadel et al., 2007; Mustian et al., 2010; Mustian et al., 2009; Vadiraja et al., 2009). Previous literature has reported an association between HRV and mental and physical wellbeing, both of which are important markers of overall QOL (Thayer, Ahs, Fredrikson, Sollers, & Wager, 2012). To our knowledge, the present study is the first to report the possibly beneficial effects of a behavioral intervention on a physiological outcome variable (HRV) and a clinical status variable (QOL) in breast cancer patients actively undergoing chemotherapy. Consistent with past research, the current study also found significant correlations between HRV indexes and overall QOL. Results of this study suggest that improving behavioral symptoms can also have a positive effect on physiological outcomes. In fact, HRV may provide an objective measure of patients’ clinical status (e.g., overall QOL) which is not based on self-report.
Behavioral interventions similar to BBT-CI, such as CBT have been shown to be beneficial to cancer survivors for relieving depression, anxiety, and pain, as well as improving physical functioning and QOL (Osborn, Demoncada, & Feuerstein, 2006). In addition, a cancer diagnosis—a life-threatening illness—can result in post-traumatic stress disorder (PTSD) and hypervigilance to threat; CBT has been shown to be a moderately effective treatment for cancer-related PTSD (Foa, 2000, Kangas, Henry, & Bryant, 2002). However, less is known about cancer-specific PTSD disorder and treatment. Thus, results of the BBT-CI intervention seem consistent with other reports that describe CBT as effective in treating many different emotional disorders, requiring relatively little effort and resources, with few, if any, side effects. It would be very advantageous for CBT and other validated behavioral interventions to be available to all cancer patients so that they can prevent and treat a variety of persistent cancer- and treatment-related symptoms.
This study has many strengths, including randomization, separation of intervention and data collection, a strong control condition, a standardized treatment protocol and training program, and the application of both a self-report and objective measure of physical and psychological wellbeing. Its robust research methodology allows for replication of the results. In addition, this study included four different community oncology network clinics across the US, enhancing the generalizability of findings. However, results of the study should not be interpreted without taking limitations into consideration. First, the primary goal of this RCT was to test the feasibility and acceptability of BBT-CI on insomnia in breast cancer patients undergoing chemotherapy. Measuring the effects of HRV and QOL was a secondary aim. Second, the present study was based on a small sample size RCT focused on feasibility; a study with a larger number of participants is needed to confirm these findings. Third, a differential dropout rate exists between the two randomization arms, not uncommon in studies of this kind. Based on intervener and participant feedback, dropout rate differences may be improved by better tailoring nutritional guidelines for sleep and/or enhancing intervener comfort levels with control arm materials. Additionally, significant improvements in QOL total scores (FACT-B/FACT-G) may be driven by improvements found on the functional wellbeing subscale. Future research should investigate the specific aspects of functional wellbeing that improve when symptoms of insomnia are reduced. Another limitation of our study is the relatively short HRV recording. Future research studies should replicate findings using longer recording times. While the measure used (Firstbeat ®) is acceptable when participants are in a resting state, future researchers must be cautious when using this device for participants that are not static. Finally, future research should include males and individuals with other types of cancers to address the generalizability of these results.
In sum, the findings herein provide proof-of-concept regarding possible effects of an innovative behavioral intervention for insomnia that can be delivered by trained nurses and research staff in community oncology clinics. If confirmed, the BBT-CI intervention might reduce insomnia symptoms. It may also affect HRV positively, and improve overall QOL in breast cancer patients actively undergoing chemotherapy. A definitive efficacy trial is currently being planned in a multicenter setting that would allow us to draw conclusions about BBT-CI’s clinical utility.
Acknowledgments
The authors would like to extend special thanks to the cancer patients as well as the research staff of the URCC NCORP Research Base and at each of the NCORPs affiliates who recruited and followed participants in this study. We thank the staff of the University of Rochester Medical Center/Wilmot Cancer Institute PEAK Human Performance Clinical Research Lab, especially Michelle Porto, Ann Colasurdo, Dr. Po-Ju Lin, and Haley Forrest. In addition, we would like to thank Carl Deguzman for the assistance with the training sessions and Briana Saravanabavanandhan for her critical review of the manuscript. We thank the NCI and the Division of Cancer Prevention for their funding support of this project. All authors declare that they have no financial or non-financial competing interests.
Funding: NCI R21 CA185678, NCI R01 CA181659, NCI U10 CA037420, NCI K07CA168886-01A1.
Footnotes
TRIAL REGISTRATION: ClinicalTrials.gov Identifier: NCT02002533
References
- Akintola AA, van de Pol V, Bimmel D, Maan AC, & van Heemst D (2016). Comparative Analysis of the Equivital EQ02 Lifemonitor with Holter Ambulatory ECG Device for Continuous Measurement of ECG, Heart Rate, and Heart Rate Variability: A Validation Study for Precision and Accuracy. Front Physiol, 7, 391. doi: 10.3389/fphys.2016.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AM, Kuhn BR, Farr LA, Von Essen SG, Chamberlain J, Lynch JC, & Agrawal S (2009). One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. Journal of Clinical Oncology, 27(35), 6033–6040. doi: 10.1200/JCO.2008.20.8306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AM, Kuhn BR, Farr LA, Lynch JC, Agrawal S, Chamberlain J, Von Essen SG Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology. 2009. June;18(6): 634–46. doi: 10.1002/pon.1438 [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Waugh R, Georgiades A, … Hinderliter A (2005). Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. Journal of the American Medical Association, 293(13), 1626–1634. doi: 10.1001/jama.293.13.1626 [DOI] [PubMed] [Google Scholar]
- Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, … Shiomoto G (1997). Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol, 15(3), 974–986. doi: 10.1200/JCO.1997.15.3.974 [DOI] [PubMed] [Google Scholar]
- Cella D, & Nowinski CJ (2002). Measuring quality of life in chronic illness: the functional assessment of chronic illness therapy measurement system. Archives of Physical Medicine and Rehabilitation, 83(12 Suppl 2), S10–17. doi: 10.1053/apmr.2002.36959 [DOI] [PubMed] [Google Scholar]
- Cella D, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, … Harris J (1993). The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol, 11(3), 570–579. doi: 10.1200/JCO.1993.11.3.570 [DOI] [PubMed] [Google Scholar]
- Chandwani KD, Thornton B, Perkins GH, Arun B, Raghuram NV, Nagendra HR, … Cohen L (2010). Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. Journal of the Society for Integrative Oncology, 8(2), 43–55. [PubMed] [Google Scholar]
- Costa AR, Fontes F, Pereira S, Goncalves M, Azevedo A, & Lunet N (2014). Impact of breast cancer treatments on sleep disturbances - A systematic review. Breast, 23(6), 697–709. doi: 10.1016/j.breast.2014.09.003 [DOI] [PubMed] [Google Scholar]
- De Couck M, Mravec B, & Gidron Y (2012). You may need the vagus nerve to understand pathophysiology and to treat diseases. Clinical Science, 122(7–8), 323–328. doi: 10.1042/Cs20110299 [DOI] [PubMed] [Google Scholar]
- Epstein DR, & Dirksen SR (2007). Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncology Nursing Forum, 34(5), E51–59. doi: 10.1188/07.ONF.E51-E59 [DOI] [PubMed] [Google Scholar]
- Ernst G (2017). Hidden Signals-The History and Methods of Heart Rate Variability. Front Public Health, 5, 265. doi: 10.3389/fpubh.2017.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, … Paul J (2008). Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. Journal of Clinical Oncology, 26(28), 4651–4658. doi: 10.1200/Jco.2007.13.9006 [DOI] [PubMed] [Google Scholar]
- Fiorentino L, McQuaid JR, Liu L, Natarajan L, He F, Cornejo M, … Ancoli-Israel S (2010). Individual cognitive behavioral therapy for insomnia in breast cancer survivors: a randomized controlled crossover pilot study. Nature and Science of Sleep, 2, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming L, Gillespie S, & Espie CA (2010). The development and impact of insomnia on cancer survivors: a qualitative analysis. Psychooncology, 19(9), 991–996. doi: 10.1002/pon.1652 [DOI] [PubMed] [Google Scholar]
- Foa EB Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry. 2000;61 Suppl 5:43–8; discussion 49-51. [PubMed] [Google Scholar]
- Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, & Campbell TS (2014). Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. Journal of Clinical Oncology, 32(5), 449–457. doi: 10.1200/JCO.2012.47.7265 [DOI] [PubMed] [Google Scholar]
- Giese-Davis J, Wilhelm FH, Tamagawa R, Palesh O, Neri E, Taylor CB, … Spiegel D (2015). Higher vagal activity as related to survival in patients with advanced breast cancer: an analysis of autonomic dysregulation. Psychosom Med, 77(4), 346–355. doi: 10.1097/PSY.0000000000000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidian M, & Jalal S (2010). Tests of Homoscedasticity, Normality, and Missing Completely at Random for Incomplete Multivariate Data. Psychometrika, 75(4), 649–674. doi: 10.1007/s11336-010-9175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson K, Hagerman I, Ostlund R, Karlberg KE, Nylander E, Nyquist O, & Dahlstrom U (1999). The effects of metoprolol and captopril on heart rate variability in patients with idiopathic dilated cardiomyopathy. Clinical Cardiology, 22(6), 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas M, Henry JL, & Bryant RA (2002). Posttraumatic stress disorder following cancer. A conceptual and empirical review. Clin Psychol Rev, 22(4), 499–524. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim JA, Choi YS, Kim SH, Lee JY, & Kim YE (2010). Heart rate variability and length of survival in hospice cancer patients. Journal of Korean Medical Science, 25(8), 1140–1145. doi: 10.3346/jkms.2010.25.8.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde S, Mosley E, & Thayer JF (2017). Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research - Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front Psychol, 8, 213. doi: 10.3389/fpsyg.2017.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoski SG, Jones LW, Krone RJ, Stein PK, & Scott JM (2015). Autonomic dysfunction in early breast cancer: Incidence, clinical importance, and underlying mechanisms. [Research Support, N.I.H., Extramural Review]. American Heart Journal, 170(2), 231–241. doi: 10.1016/j.ahj.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lent L, Hahn E, Eremenco S, Webster K, & Cella D (1999). Using cross-cultural input to adapt the Functional Assessment of Chronic Illness Therapy (FACIT) scales. Acta Oncologica, 38(6), 695–702. [DOI] [PubMed] [Google Scholar]
- Levenson JC, Kay DB, & Buysse DJ (2015). The pathophysiology of insomnia. Chest, 147(4), 1179–1192. doi: 10.1378/chest.14-1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao DP, Carnethon M, Evans GW, Cascio WE, & Heiss G (2002). Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes - The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes, 51(12), 3524–3531. doi: DOI 10.2337/diabetes.51.12.3524 [DOI] [PubMed] [Google Scholar]
- Miglis MG (2016). Autonomic dysfunction in primary sleep disorders. Sleep Medicine, 19, 40–49. doi: 10.1016/j.sleep.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Moadel AB, Shah C, Wylie-Rosett J, Harris MS, Patel SR, Hall CB, & Sparano JA (2007). Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: Effects on quality of life. Journal of Clinical Oncology, 25(28), 4387–4395. doi: 10.1200/Jco.2006.06.6027 [DOI] [PubMed] [Google Scholar]
- Mravec B, Gidron Y, Kukanova B, Bizik J, Kiss A, & Hulin I (2006). Neural-endocrine-immune complex in the central modulation of tumorigenesis: facts, assumptions, and hypotheses. Journal of Neuroimmunology, 180(1–2), 104–116. doi: 10.1016/j.jneuroim.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Mustian KM, Palesh O, Sprod L, Peppone LJ, Heckler CE, Yates JS, … Morrow GR (2010). Effect of YOCAS yoga on sleep, fatigue, and quality of life: A URCC CCOP randomized, controlled clinical trial among 410 cancer survivors. Journal of Clinical Oncology, 28(15), 9013. doi: 10.1200/jco.2010.28.15_suppl.9013 [DOI] [Google Scholar]
- Mustian KM, Sprod LK, Palesh OG, Peppone LJ, Janelsins MC, Mohile SG, & Carroll J (2009). Exercise for the management of side effects and quality of life among cancer survivors. Current sports medicine reports, 8(6), 325–330. doi: 10.1249/JSR.0b013e3181c22324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer D, Vogt L, Thiel C, Schmidt K, Bernhorster M, Lungwitz A, … Banzer W (2013). Exercise effects on HRV in cancer patients. International Journal of Sports Medicine, 34(1), 68–73. doi: 10.1055/s-0032-1314816 [DOI] [PubMed] [Google Scholar]
- Nolan RP, Kamath MV, Floras JS, Stanley J, Pang C, Picton P, & Young QR (2005). Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. American Heart Journal, 149(6), 1137.e1131–1137.e1137. doi: 10.1016/j.ahj.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Osborn RL, Demoncada AC, & Feuerstein M (2006). Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. Int J Psychiatry Med, 36(1), 13–34. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L [DOI] [PubMed] [Google Scholar]
- Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, & Innominato PF (2013). Sleep disruption in breast cancer patients and survivors. Journal of the National Comprehensive Cancer Network, 11(12), 1523–1530. doi: 10.6004/jnccn.2013.0179 [DOI] [PubMed] [Google Scholar]
- Palesh O, Peppone L, Innominato PF, Janelsins M, Jeong M, Sprod L, Savard J, Rotatori M, Kesler S, Telli M, Mustian K Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012. December 17;4:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesh O, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, … Morrow GR (2010). Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. Journal of Clinical Oncology, 28(2), 292–298. doi: 10.1200/JCO.2009.22.5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesh O, Scheiber C, Kesler S, Janelsins MC, Guido JJ, Heckler C, Cases MG, Miller J, Chrysson NG, Mustian KM. Feasibility and acceptability of brief behavioral therapy for cancer-related insomnia: effects on insomnia and circadian rhythm during chemotherapy: a phase II randomised multicentre controlled trial. Br J Cancer. 2018. August;119(3):274–281. doi: 10.1038/s41416-018-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesh O, Scheiber C, Kesler S, Janelsins MC, Guido JJ, Heckler C, Cases MG, Miller J, Chrysson NG, Mustian KM Feasibility and acceptability of brief behavioral therapy for cancer-related insomnia: effects on insomnia and circadian rhythm during chemotherapy: a phase II randomised multicentre controlled trial. Br J Cancer. 2018. August;119(3):274–281. doi: 10.1038/s41416-018-0154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesh O, Zeitzer JM, Conrad A, Giese-Davis J, Mustian KM, Popek V, … Spiegel D (2008). Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. Journal of Clinical Sleep Medicine, 4(5), 441–449. [PMC free article] [PubMed] [Google Scholar]
- Parak J, & Korhonen I (2014). Accuracy of Firstbeat Bodyguard 2 beat-to-beat heart rate monitor. Finland. [Google Scholar]
- Poreba M, Poreba R, Gac P, Usnarska-Zubkiewicz L, Pilecki W, Piotrowicz E, … Sobieszczanska M (2014). Heart rate variability and heart rate turbulence in patients with hematologic malignancies subjected to high-dose chemotherapy in the course of hematopoietic stem cell transplantation. Ann Noninvasive Electrocardiol, 19(2), 157–165. doi: 10.1111/anec.12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenberg H, & Lubbe AS (2010). In-patient rehabilitation of lung cancer patients--a prospective study. Supportive Care in Cancer, 18(7), 877–882. doi: 10.1007/s00520-009-0727-y [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, & Tracey KJ (2009). The neurology of the immune system: neural reflexes regulate immunity. Neuron, 64(1), 28–32. doi: 10.1016/j.neuron.2009.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandercock GR, Bromley PD, & Brodie DA (2005). Effects of exercise on heart rate variability: inferences from meta-analysis. Medicine & Science in Sports & Exercise, 37(3), 433–439. [DOI] [PubMed] [Google Scholar]
- Savard J, Hervouet S, & Ivers H (2013). Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology, 22(6), 1381–1388. doi: 10.1002/pon.3150 [DOI] [PubMed] [Google Scholar]
- Savard J, Simard S, Ivers H, & Morin CM (2005). Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. Journal of Clinical Oncology, 23(25), 6097–6106. doi: 10.1200/JCO.2005.12.513 [DOI] [PubMed] [Google Scholar]
- Scheiber C, Johnston L, Packer M, Gevirtz R, Edwards KS, & Palesh O (2018). Heart Rate Variability Markers as Correlates of Survival in Recipients of Hematopoietic Cell Transplantation. Oncology nursing forum, 45(2), 250–259. doi: 10.1188/18.ONF.250-259 [DOI] [PubMed] [Google Scholar]
- Selig SE, Carey MF, Menzies DG, Patterson J, Geerling RH, Williams AD, … Hare DL (2004). Moderate-intensity resistance exercise training in patients with chronic heart failure improves strength, endurance, heart rate variability, and forearm blood flow. Journal of Cardiac Failure, 10(1), 21–30. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Mark AL, & Abboud FM (1993). Sympathetic-nerve activity during sleep in normal subjects. New England Journal of Medicine, 328(5), 303–307. doi: 10.1056/NEJM199302043280502 [DOI] [PubMed] [Google Scholar]
- Sztajzel J (2004). Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Medical Weekly, 134(35–36), 514–522. doi: 2004/35/smw-10321 [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation, 93(5), 1043–1065. doi: 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36(2), 747–756. doi: 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Fischer JE (2009). Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J Intern Med, 265(4), 439–447. doi: 10.1111/j.1365-2796.2008.02023.x [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2007). The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol, 74(2), 224–242. doi: 10.1016/j.biopsycho.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Sternberg E (2006). Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci, 1088, 361–372. doi: 10.1196/annals.1366.014 [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2002). The inflammatory reflex. Nature, 420(6917), 853–859. doi: 10.1038/nature01321 [DOI] [PubMed] [Google Scholar]
- Vadiraja HS, Rao MR, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, … Rao N (2009). Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complementary Therapies in Medicine, 17(5–6), 274–280. doi: 10.1016/j.ctim.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Van Gestel AJ, Kohler M, Steier J, Teschler S, Russi EW, & Teschler H (2011). Cardiac autonomic dysfunction and health-related quality of life in patients with chronic obstructive pulmonary disease. Respirology, 16(6), 939–946. doi: 10.1111/j.1440-1843.2011.01992.x [DOI] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, & Thayer JF (2015). Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Frontiers in Psychology, 6, 1–8. doi: 10.3389/fpsyg.2015.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]