Abstract
Objective
This study followed remitted patients from a randomized controlled trial of adults with major depressive disorder (MDD). The aims were to describe rates of recurrence and to evaluate three clinical predictor domains.
Method
Ninety-four treatment naïve patients (50% female; Mage = 38.1; 48.9% White, 30.9% Hispanic) with MDD who had remitted to 12-week monotherapy (escitalopram, duloxetine, or cognitive behavior therapy (CBT)) participated in a 21-month maintenance phase (i.e., continued medication or 3 possible CBT booster sessions per year). Recurrence was assessed quarterly, and the clinical predictors were: two measures of residual depressive symptoms, one measure of lifetime depressive episodes, and two measures of baseline anxiety. Survival analysis models evaluated recurrence rates, and regression models evaluated the predictors.
Results
Among all patients, 15.5% experienced a recurrence, and the survival distributions did not statistically differ among treatments. Residual depressive symptoms on the Hamilton Depression Rating Scale at the end of monotherapy were associated with increased risk for recurrence (Hazard Ratio = 1.31, 95% CI [1.02., 1.67], Wald X2 = 4.41, p = .036), and not having a comorbid anxiety disorder diagnosis at study baseline reduced the risk of recurrence (Hazard Ratio = .31, 95% CI [.10, .94], Wald X2 = 4.28, p = .039).
Conclusions
The study supported the benefits of maintenance treatment for treatment naïve patients remitted to initial monotherapy; nevertheless, remitted patients with a comorbid anxiety disorder diagnosis at the beginning of treatment or residual depressive symptoms after initial treatment were at risk for poorer long-term outcomes.
Keywords: Cognitive Behavior Therapy, Antidepressant Medication, Major Depression, Recurrence
Substantial evidence indicates that antidepressant medications and evidenced-based psychotherapies effectively treat depressed individuals (Cuijpers, van Straten, Warmerdam, & Andersson, 2008; Spielmans, Berman, & Usitalo, 2011). Many such individuals, however, face a significant risk of depressive relapse or recurrence following successful treatment of the acute depressive episode (Craighead & Dunlop, 2014; Judd et al., 2016). Due to the potentially long-term course of major depressive disorder (MDD), clinical guidelines recommend that treatment continue (4–12 months) beyond the point of initial treatment response (APA, 2010; NICE, 2010).
Antidepressant medications (ADMs) are the most widely used treatment for MDD (Olfson & Marcus, 2009), and patients who respond to ADMs and continue to use them appear to have a lower risk of relapse and recurrence than patients who discontinue medications after a treatment response. A meta-analysis (Geddes et al., 2003) of 31 randomized trials of patients who had responded to ADMs found that those randomized to the continued use of ADMs had a significantly lower risk of relapse than patients randomized to ADM discontinuation via pill-placebo. This differential treatment effect appeared to last up to 36 months, although most of the included trials were limited to 12 months of follow-up. A more recent meta-analysis (Glue, Donovan, Kolluri, & Emir, 2010) reached a similar conclusion, as did an extensive review of maintenance trials published by the U.S. Food and Drug Administration (Borges et al., 2014).
Cognitive behavior therapy (CBT), an evidence-based psychotherapy and alternative first-line treatment approach to ADMs, also appears to reduce the risk of relapse and recurrence. An influential meta-analysis (Cuijpers et al., 2013) found that patients initially treated with CBT were less likely to have a relapse or recurrence than patients who were successfully treated with ADMs and then withdrawn from treatment. These authors also reported there was no difference in outcomes between those patients who responded to initial CBT and those patients who responded to initial ADMs and continued to use them during the follow-up.
Despite these promising data on long-term efficacy of maintenance ADMs and CBT, most treatment studies ended their follow-up period after one year (Borges et al., 2014; Cuijpers et al., 2013; Geddes et al., 2003; Glue et al., 2010). This is problematic because the cumulative risk of recurrence increases beyond one year after treatment (Solomon et al., 2000), and practice guidelines recommend that clinicians make treatment decisions that could last beyond one year (APA, 2010; NICE, 2010). Further, because of appropriate a priori research design reasons, the few studies that have directly compared the long-term efficacy of CBT and continuation ADMs for longer than one year of follow-up have, at the end of one year of treatment, withdrawn continuation ADM patients from treatment rather than maintaining them on the effective ADM (e.g., Dobson et al., 2008; Hollon et al., 2005). Consequently, during the second year of follow-up these studies have used naturalistic designs, which involve fewer clinical contact hours and potentially limited treatment efficacy. Thus, there is a need for a study that allows patients to continue using ADMs through a second year of controlled clinical care, and permits comparison to the long-term efficacy of CBT.
The present study addressed the preceding limitations with data from the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study (Dunlop et al., 2012; Dunlop, Kelley, et al., 2017). The PReDICT project was a multi-stage randomized controlled trial for the treatment of MDD in adults. The current analyses include those patients who remitted to 12-week monotherapy (ADMs or CBT) and then participated in a 21-month follow-up during which they could continue treatment; ADM participants could continue to use medication and CBT participants could receive up to three CBT booster sessions per year. The primary aim of the present study was to describe the rates of relapse and recurrence among these patients. Based on the highly-controlled nature of the study protocol and the inclusion of only patients who experienced the optimal initial treatment outcome (i.e., remission), it was expected that rates of recurrence would be lower than reported in previous studies. In addition, it was expected that CBT with occasional booster sessions would have long-term efficacy that was comparable to continuous treatment with ADMs.
Secondary Aim
Leading treatment guidelines recommend that clinicians assess a patient’s risk of relapse and recurrence at the end of initial and continuation treatment (APA, 2010; NICE, 2010). To improve the assessment of patient risk, clinicians need to know which clinical variables reliably predict relapse and recurrence. Three such variables derived from prior relevant studies are: 1) number of lifetime depressive episodes at baseline; 2) baseline co-morbid anxiety; and 3) residual, subthreshold depression symptoms. These variables are cited as predictors of risk in the treatment guidelines (APA, 2010; NICE, 2010) and have also been cited in reviews of cross-sectional and longitudinal community studies (Burcusa & Iacono, 2007) and clinical cohort studies (Craighead & Dunlop, 2014; Hardeveld, Spijker, De Graaf, Nolen, & Beekman, 2010). The secondary aim examined clinical variables that might predict MDD recurrence across treatment modalities. Specifically, it was hypothesized that residual symptoms, number of lifetime depressive episodes, and comorbid baseline anxiety would differentiate patients who experienced a recurrence from those who did not.
Method
Study Overview
PReDICT was a randomized controlled trial aimed at identifying moderators of treatment response among patients who have never previously received treatment for MDD. The study rationale, methods, and design have been described in detail elsewhere (for study protocol see: Dunlop, Binder, et al., 2012). In brief, patients were randomized 1:1:1 to one of three 12-week monotherapies: 1) escitalopram (ESC; 10–20 mg/d), a selective serotonin reuptake inhibitor (SSRI); 2) duloxetine (DUL; 30–60 mg/d), a serotonin-norepinephrine reuptake inhibitor (SNRI); or 3) CBT; 16 one-hour individual sessions. Patients who remitted to their allocated 12-week monotherapy were eligible to enter a 21-month maintenance treatment period.
Participants
The present study included the PReDICT patients who remitted to 12-week monotherapy with no major protocol violations (n = 109) and agreed to participate in the 21-month follow-up (n = 94). Monotherapy remission was defined as a Hamilton Depression Rating Scale 17-item (HAM-D; Hamilton, 1967) score of ≤ 7 at both weeks 10 and 12 of monotherapy treatment. Outcomes from the 12-week monotherapy phase have been described in detail in an earlier report (Dunlop, Kelley, et al., 2017). All participants were assessed and treated under one umbrella clinic located at a university-affiliated outpatient setting and a Spanish-speaking outpatient setting in a large public hospital (Aponte-Rivera et al., 2014). Site was not associated with recurrence, so the data from both sites were combined in the currently reported analyses.
Procedure
Participants were assessed every three months during the 21-month follow-up. These assessment visits consisted of a Longitudinal Interval Follow-up Evaluation (LIFE; Keller et al., 1987) interview and clinical ratings on the HAM-D (Hamilton, 1967) and Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959) by a masked rater, a clinical interview with a psychiatrist, and several self-report measures. Patients continued participating in follow-up until either: 1) 2 years from study baseline; 2) depressive relapse/recurrence; or 3) early termination or lost to follow-up. Participating patients received $50 per follow-up visit.
Patients who remitted to ADM and agreed to participate in follow-up (ESC, n = 34; DUL, n = 37) were encouraged to remain on medication through the first nine months of follow-up, at which point a study psychiatrist discussed the risks and benefits of discontinuing medication. Patients could choose to maintain or stop medications, and patients remained in the follow-up protocol for 12 additional months regardless of their choice. Of the 58 ADM patients who did not leave the study or suffer a recurrence before or at the 9-month follow-up visit (12 months after PReDICT baseline assessment), 17 (29.3%) had stopped their ADM; these patients continued to participate in all remaining follow-up assessments until recurrence, early termination, or the end of the study.
Patients who remitted to CBT monotherapy and agreed to participate in follow-up (n = 23) were offered up to 3 booster sessions during the first 9 months of follow-up and up to three additional booster sessions during the second year of follow-up. All booster sessions were separated by at least 1 month, and an additional “crisis session” was also allowed for patients during each year of follow-up.
Measures
Depression severity and relapse/recurrence
The LIFE (Keller et al., 1987) is a semi-structured interview that tracks the exact dates of the onset and remission of psychiatric disorders, including MDD relapse/recurrence and time survived until relapse/recurrence. The LIFE has demonstrated high interrater reliability for episodic disorders (ICC = .90, Keller et al., 1987). The LIFE interview was conducted at the end of 12-week monotherapy treatment and at each of the seven quarterly visits during the 21-month follow-up. The 17-item HAM-D (Hamilton, 1967; Williams, 1988) is a clinician-rated measure of depressive symptom severity over the past week and is one of the most commonly used measures in psychotherapy and antidepressant medication research. Patients were rated on the HAM-D throughout monotherapy treatment and at each follow-up visit.
Definition of recurrence
The present study followed other investigators (e.g., Jarrett, Minhajuddin, Gershenfeld, Friedman, & Thase, 2013) in referring to relapse and recurrence as the single construct labeled recurrence. The a priori definitions of recurrence used in PReDICT included a patient meeting any one of the four following criteria: 1) meeting criteria for a major depressive episode based on a LIFE score of 3 or greater; 2) a 17-item HAM-D ≥ 14 for two consecutive weeks (patients with an HAM-D ≥ 14 at a follow-up visit were asked to return the following week for an additional rating); 3) a 17-item HAM-D ≥ 14 at any follow-up visit and at which time the patient requested an immediate change in treatment; and 4) high risk of suicide, as determined by the study psychiatrist (Dunlop, Binder, et al., 2012).
Residual symptoms
Residual symptoms were operationalized as the patient’s 17-item HAM-D total score at the end of 12-week monotherapy. Residual symptoms were also measured by patients’ total score on the self-report Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) at the end of 12-week monotherapy.
Number of depressive episodes
The number of episodes was based on data collected at PReDICT baseline via the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1995). Patients were categorized into two groups: patients with less than three lifetime depressive episodes (including the current MDE), and patients with three or more lifetime episodes. This cutoff was selected as there is evidence that MDD becomes a chronic, recurrent disorder after three lifetime episodes (Solomon et al., 2000), and this dichotomous classification has been employed in many studies of recurrence of major depression (Craighead & Dunlop, 2014; Kuyken et al., 2016, Stangier et al., 2013).
Anxiety
Anxiety was operationalized as a patient’s anxiety disorder status (i.e., no/yes) as determined by the PReDICT baseline SCID. Clinician ratings of patient anxiety severity on the Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959) at PReDICT baseline were used as an additional measure of anxiety.
Data Analyses
The following series of preliminary comparisons were performed using Chi-Square tests and Analysis of Variance (ANOVA) on the 7 demographic and 12 clinical variables listed in Table 1. First, eligible patients who agreed to participate in the present study were compared to eligible patients who did not agree to participate, and those patients who did not agree to participate were also compared across treatment groups. Second, to evaluate risk of differential retention of patients from the monotherapy phase to follow-up phase, treatment groups were compared at the beginning of the present study. Because differential retention of participants can affect group equivalence created by initial randomization (e.g., Klein, 1996), conservative probability values (p < .10) were used for the treatment group comparisons per the method used in similar trials (e.g., Dobson et al., 2008; Hollon et al., 2005). Third, patients who terminated the study early (prior to the end of the 21-month follow-up) were compared to all other participating patients (i.e., those who completed the 21-month follow-up and those who suffered a recurrence and were subsequently withdrawn from the study). In addition, patients who terminated the study early were compared across treatment groups.
Table 1.
Demographic and clinical characteristics of participants
| All Patients (N = 94) | CBT (n = 23) | ESC (n = 34) | DUL (n = 37) | |||||||
| Characteristic | M | SD | M | SD | M | SD | M | SD | F | p |
| Age (yrs) | 38.1 | 11.0 | 36.7 | 10.7 | 38.6 | 10.2 | 38.6 | 12.1 | 0.25 | 0.780 |
| Age at first episode (yrs) | 29.0 | 12.7 | 30.3 | 11.5 | 31.2 | 13.2 | 26.1 | 12.6 | 1.63 | 0.202 |
| Current episode duration (wks) | 105.4 | 191.8 | 72.5 | 73.8 | 113.6 | 187.6 | 118.7 | 243.1 | 0.45 | 0.639 |
| Baseline HAM-D | 18.6 | 3.5 | 18.5 | 3.3 | 18.9 | 3.9 | 18.2 | 3.2 | 0.38 | 0.686 |
| Baseline BDI | 21.6 | 6.9 | 22.4 | 7.6 | 21.1 | 6.9 | 21.5 | 6.7 | 0.22 | 0.807 |
| Baseline HAM-A | 14.4 | 4.7 | 15.1 | 4.8 | 15.0 | 4.9 | 13.4 | 4.4 | 1.36 | 0.261 |
| Week 12 HAM-D | 2.8 | 2.2 | 2.7 | 2.3 | 2.4 | 2.0 | 3.2 | 2.2 | 1.35 | 0.264 |
| Week 12 BDI | 2.1 | 2.5 | 2.8 | 2.9 | 1.4 | 2.1 | 2.3 | 2.5 | 2.13 | 0.125 |
| n | % | n | % | n | % | n | % | X2* | p | |
| Sex | 0.07 | 0.965 | ||||||||
| Male | 47 | 50.0 | 11 | 47.8 | 17 | 50.0 | 19 | 51.4 | ||
| Female | 47 | 50.0 | 12 | 52.2 | 17 | 50.0 | 18 | 48.6 | ||
| Race | 5.94 | 0.201 | ||||||||
| White | 46 | 48.9 | 15 | 65.2 | 16 | 47.1 | 15 | 40.5 | ||
| Black | 14 | 14.9 | 1 | 4.3 | 4 | 11.8 | 9 | 24.3 | ||
| Other | 34 | 36.2 | 7 | 30.4 | 14 | 41.2 | 13 | 35.1 | ||
| Ethnicity | 0.57 | 0.752 | ||||||||
| Hispanic | 29 | 30.9 | 7 | 30.4 | 12 | 35.3 | 10 | 27.0 | ||
| Non-Hispanic | 65 | 69.1 | 16 | 69.6 | 22 | 64.7 | 27 | 73.0 | ||
| Married/Cohabitating | 2.16 | 0.340 | ||||||||
| Yes | 54 | 57.4 | 16 | 69.6 | 17 | 50.0 | 21 | 56.8 | ||
| No | 40 | 42.6 | 7 | 30.4 | 17 | 50.0 | 16 | 43.2 | ||
| Employed full-time | 2.07 | 0.356 | ||||||||
| Yes | 45 | 47.9 | 14 | 60.9 | 15 | 44.1 | 16 | 43.2 | ||
| No | 49 | 52.1 | 9 | 39.1 | 19 | 55.9 | 21 | 56.8 | ||
| Anxiety disorder at baseline | 0.31 | 0.858 | ||||||||
| Yes | 31 | 33.0 | 8 | 34.8 | 10 | 29.4 | 13 | 35.1 | ||
| No | 63 | 67.0 | 15 | 65.2 | 24 | 70.6 | 24 | 64.9 | ||
| Lifetime episodes | 3.92 | 0.420 | ||||||||
| 1 | 47 | 50.0 | 13 | 56.5 | 20 | 58.8 | 14 | 37.8 | ||
| 2 | 20 | 21.3 | 5 | 21.7 | 6 | 17.6 | 9 | 24.3 | ||
| ≥3 | 27 | 28.7 | 5 | 21.7 | 8 | 23.5 | 14 | 37.8 | ||
| Chronic episode (≥ 2 yrs) | 27 | 29.0 | 7 | 30.4 | 9 | 26.5 | 11 | 30.6 | 0.17 | 0.918 |
| History of suicide attempt | 6 | 6.4 | 0 | 0.0 | 2 | 5.9 | 4 | 10.8 | 2.41 | 0.279 |
| Insurance status | 1.04 | 0.594 | ||||||||
| Yes | 44 | 47.3 | 13 | 56.5 | 15 | 44.1 | 16 | 44.4 | ||
| No | 49 | 52.7 | 10 | 43.5 | 19 | 55.9 | 20 | 55.6 | ||
Note. CBT = Cognitive Behavioral Therapy; ESC = escitalopram; DUL = duloxetine; HAM-D = 17-item Hamilton Rating Scale for Depression; BDI = Beck Depression Inventory; HAM-A = Hamilton Anxiety Rating Scale
Fisher’s exact test used in analyses with small cells.
For the primary analyses, survival curves and times were estimated using the Kaplan-Meier product limit method (Kaplan & Meier, 1958), and the Mantel-Cox test was used to evaluate differences across treatments (Mantel, 1966). The clinical predictor variables were evaluated using the Kaplan-Meier and Mantel-Cox methods for categorical variables, and the Cox proportional hazard regression model (Cox & Oakes, 1984) for continuous variables. In addition, Cox regressions were used to generate effect sizes (i.e., hazard ratios). Patient endpoints were defined as recurrence; patients lost to follow-up were right censored at the date of their final study visit, and patients who completed the entire follow-up protocol without recurrence were right-censored at 21-months after the end of monotherapy.
All analyses were conducted at a statistical significance of p < .05 (2-tailed), unless otherwise noted, and SPSS 24.0 and R 3.3.2 were used for the analyses. For all analyses including categorical variables, Fisher’s exact test was substituted in analyses with small cells. Additionally, for some analyses, treatment conditions were collapsed to increase statistical power.
Results
Patient Flow and Attrition
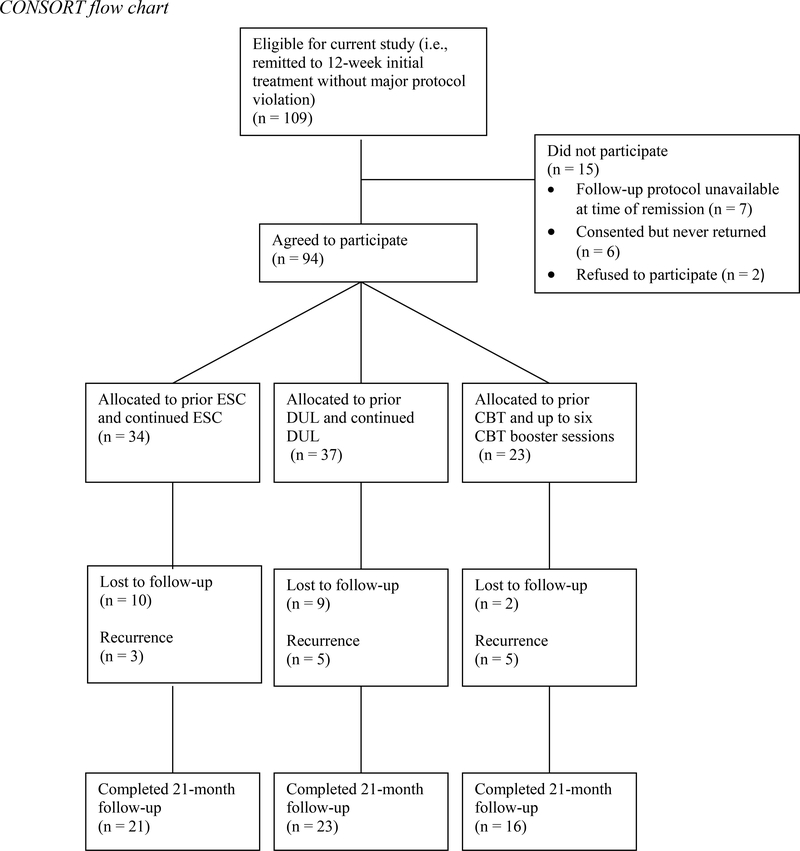
One hundred and nine patients remitted to 12-week monotherapy without any major protocol violations and were eligible to participate in the present study. Fifteen of these patients did not participate in follow-up. Specifically, seven patients were not offered follow-up because that aspect of the study had not been initiated by the time they completed monotherapy; two patients refused to participate; and six patients consented but never returned for a follow-up evaluation. When compared to patients who agreed to participate (n = 94), these 15 non-participating patients were older (Mage = 43.9 vs. 38.1 years old) and more likely to identify as White. When the 7 patients who were not offered follow-up were excluded, the previous differences were no longer significant. Participation rates, excluding the 7 patients who were not offered follow-up, did not differ significantly across treatments (ESC, 100% (34/34); DUL, 90.2% (37/41); and CBT, 85.2% (23/27); Fisher’s exact test, p = .055).
When evaluating the primary sample for this study, i.e., the 94 patients who agreed to participate, the treatment groups did not differ on any of 7 demographic or 12 clinical variables (see Table 1). Of participating patients, 21 (22.3%) left the study before suffering a recurrence or completing the entire 21-month follow-up protocol; the lost to follow-up rates did not differ among treatments (ESC, 29.4% (10/34); DUL, 24.3% (9/37); and CBT, 8.7% (2/23); X2 (2) = 3.53, p = .17).
The complete participant flow is summarized in Figure 1, and the sample characteristics are reported in Table 1.
Figure 1:
CONSORT flow chart
Prevention of Recurrence
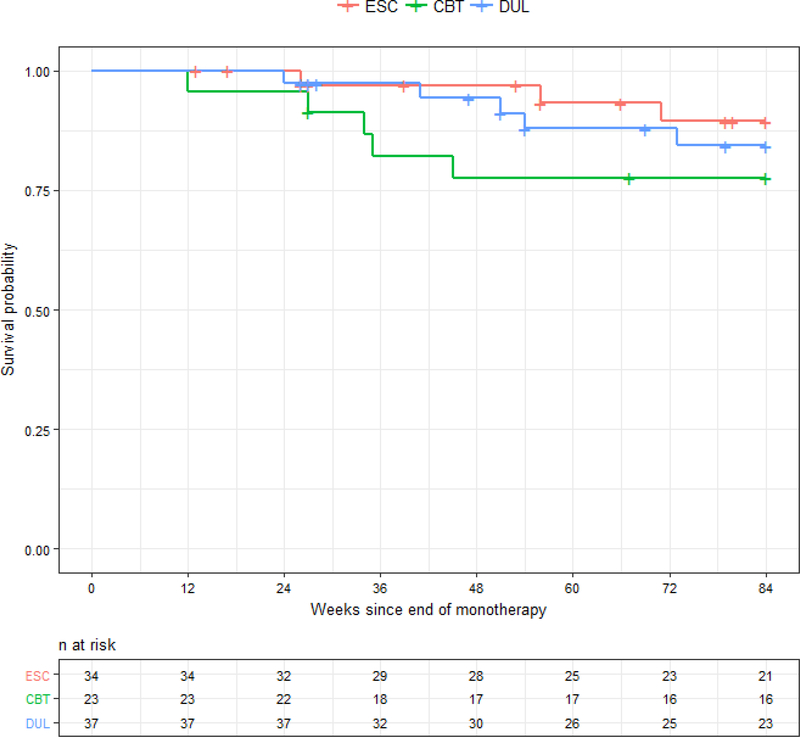
Only 13 patients suffered a recurrence1 (ns = ESC, 3; DUL, 5; CBT, 5), and the survival curves for the entire 21-month follow-up period are presented in Figure 2. The Kaplan-Meier estimated rate of recurrence across all treatment conditions during this period was 15.5%, and estimated rates of recurrence for the treatment groups were: ESC, 10.6%; DUL, 15.7%; and CBT, 22.4%. The survival distributions did not statistically differ across treatment conditions (Mantel-Cox X2 (2) = 1.74, p = .42), when the ADM conditions were collapsed into one group and compared with the CBT condition (estimated recurrence rates: 13.3% vs. 22.4%; Mantel-Cox X2 (1) = 1.46, p = .23), or in any of the pair-wise comparisons.
Figure 2:
Cumulative proportion of participants surviving without depressive relapse/recurrence over 21 months of follow-up
Note. ESC = escitalopram; CBT = cognitive behavior therapy; DUL = duloxetine. The groups did not statistically differ across treatment conditions.
Due to the numerically higher “lost to follow-up rates” in the ADM treatment arm, a series of sensitivity analyses (Shih, 2002) were completed and are described in the supplemental materials. Across all analyses, the estimated combined rate of recurrence across treatment conditions ranged from 17.7% to 21.0%, and the estimated ranges in rates of recurrence for the treatment conditions were: ESC, 13.7% - 20.3%; DUL, 18.0% - 18.4%; and CBT, 22.4% - 27.0%. None of these analyses identified statistically significant differences.
Treatment Utilization
Seventy percent (50/71) of ADM patients continued to use medications as prescribed and monitored by the study psychiatrist until the end of their study participation (i.e., recurrence, early termination, or study termination). Rates of recurrence did not statistically differ between patients who discontinued medication and patients who continued medications (observed recurrence rates: discontinued ADMs, 14.3% (3/21) vs. continued ADMs, 10.0% (5/50); Fisher’s exact test, p = .686). All participating CBT patients (23/23) attended at least one booster session, and the number of booster sessions attended, adjusted for survival time, was not significantly associated with recurrence. Five of the 23 (21.7%) CBT patients utilized a crisis session, and 2 of the 5 (40%) who attended a crisis session experienced a recurrence compared to 3 of 18 (16.7%) who did not attend a crisis session; the small numbers precluded statistical analyses.
Prediction of Recurrence
Residual depressive symptoms on the HAM-D following monotherapy were evaluated via a Cox regression model; the analysis revealed that higher levels of residual symptoms were associated with an increased risk in recurrence and decreased survival time (Hazard Ratio = 1.31, 95% CI [1.02, 1.67], Wald X2 = 4.41, p = .036). Self-reported residual symptoms on the BDI, however, were not significantly associated with recurrence (Hazard Ratio = 1.07, 95% CI [.89, 1.30], Wald X2 = .53, p = .468).
Based on number of lifetime MDEs, patients were categorized into two groups: patients with less than three episodes, and patients with three or more episodes. The observed recurrence rate for patients with fewer than three episodes was 11.9% (8/67), and the observed rate among patients with three or more episodes was 18.5% (5/27). The Kaplan-Meier estimated survival distributions did not statistically differ between groups (Mantel-Cox X2 (1) = .69, p = .408), and having fewer than three lifetime depressive episodes was not significantly associated with a lower risk of recurrence compared to those who had three or more episodes (Hazard Ratio = .63, 95% CI [.21, 1.92], Wald X2 = .67, p = .412).
Patients were divided into two groups based on anxiety disorder status (i.e., no/yes) at PReDICT baseline. The observed recurrence rate for patients without an anxiety disorder at study baseline was 7.9% (5/63), and the observed rate among patients with an anxiety disorder was 25.8% (8/31). The Kaplan-Meier survival distributions were significantly different between the two groups (Mantel-Cox X2 (1) = 4.80, p = .029); not having an anxiety disorder at study baseline was associated with decreased risk of recurrence and longer survival times (Hazard Ratio = .31, 95% CI [.10, .94], Wald X2 = 4.28, p = .039). However, anxiety symptoms on the HAM-A (a continuous variable) at study baseline were not associated with recurrence (Hazard Ratio = 1.01, 95% CI [.90, 1.13], Wald X2 = .03, p = .853). The regression results are summarized in Table 2.
Table 2.
Cox regressions predicting depressive recurrence
| Variables | Parameter Estimate | SE | Wald X2 | p | Hazard Ratio [95% CI] |
|---|---|---|---|---|---|
| Residual Symptoms | |||||
| HAM-D | 0.27 | 0.13 | 4.41 | 0.036 | 1.31 [1.02, 1.67] |
| BDI | 0.07 | 0.10 | 0.53 | 0.468 | 1.07 [.89, 1.30] |
| Lifetime Depressive Episodes | |||||
| <3 vs. ≥3 lifetime MDEs | −0.47 | 0.57 | 0.67 | 0.412 | .63 [.21, 1.92] |
| Baseline Anxiety | |||||
| HAM-A | 0.01 | 0.06 | 0.03 | 0.853 | 1.01 [.90, 1.13] |
| Anxiety Disorder Status (no/yes) | −1.18 | 0.57 | 4.28 | 0.039 | .31 [.10, .94] |
Note. HAM-D = 17-item Hamilton Rating Scale for Depression; BDI = Beck Depression Inventory; MDEs = Lifetime Major Depressive Episodes; HAM-A = Hamilton Anxiety Rating Scale; CI= confidence interval.
An additional model was constructed to evaluate if HAM-D residual symptoms mediated the relationship between baseline anxiety disorder diagnosis and recurrence. The indirect effect of baseline anxiety disorder diagnosis through HAM-D residual symptoms was not statistically significant (p = .49), and the direct effect of anxiety diagnosis, controlling for residual symptoms, was statistically significant (p = .03).
Power Considerations
Ninety-four patients participated in the present study (n: ESC, 34; DUL, 37; CBT, 23), and 60 patients (n: ESC, 21; DUL, 23; CBT, 16) completed the entire 21-month follow-up protocol. Sensitivity analyses revealed that, depending on the comparison and frequency of participant attrition, the study was adequately powered to detect approximately .30 - .50 differences in recurrence rates (e.g., a recurrence rate of 10% in ESC compared to a rate of 40% in DUL).
Discussion
The current results indicated that patients who remitted to their first evidence-based treatment for MDD and who continued to receive maintenance treatment had a low risk of MDD recurrence. The overall recurrence rate of 15.5% was lower than the rates reported for patients followed naturalistically (e.g., Solomon et al., 2000) and for patients who respond to initial ADM treatment and then switched to placebo (Borges et al., 2014; Geddes et al., 2003; Glue et al., 2010). Moreover, the recurrence rates in the current study were lower than rates reported in other large-scale trials with ADM and CBT (Dobson et al., 2008; Hollon et al., 2005; Jarrett et al., 2013; Klein et al., 2004; Shea et al., 1992).
One potential explanation for the low rates of recurrence in the current study is that most prior research followed patients who had responded to initial treatment, whereas the current study only followed patients who had remitted. Given the documented positive relationship between the number of residual symptoms and recurrence (Judd et al., 1998; Paykel et al., 1995), it is reasonable to expect higher rates of recurrence among responders compared to remitted patients because non-remitting responders, by definition, have more residual symptoms at the end of treatment. Indeed, fully remitted patients have better long-term outcomes than patients who do not achieve full remission (Rush, Trivedi, et al., 2006). Another potential explanation is that the PReDICT study excluded patients who had received prior treatment. Patients with a history of failed treatment and patients who have previously responded to treatment but subsequently relapsed or suffered a recurrence may possess characteristics that make them vulnerable to recurrent depressive episodes; both sets of circumstances have resulted in attenuated treatment outcomes (Craighead & Dunlop, 2014).
The similar low recurrence rate among the patients who continued (10%) versus those who discontinued (14.3%) their medication during the follow-up period is particularly notable. In the database of FDA-registered double-blind antidepressant discontinuation trials evaluating 6–12 months of maintenance treatment, recurrence rates among patients continuing on antidepressants versus those who discontinued them varied widely (2–39%, mean:18% versus 14–59%, mean 37%, respectively) (Borges et al., 2014). Thus, the recurrence rate with continued medication in the current analysis is similar to prior studies, but the rate after discontinuation is substantially lower. This low rate of recurrence among patients stopping their antidepressant may stem from: 1) the longer total period of medication treatment prior to its discontinuation in the PReDICT sample (mean = 46 weeks); 2) the low level of residual depressive symptoms due to all included patients having achieved remission during the acute treatment phase; or 3) the study sample being treatment naïve, and therefore less vulnerable to potential tolerance effects of antidepressant medications (Fava & Offidani, 2011).
The results from the current study show that controlled and sustained clinical care can reduce patient risk of recurrence for as long as 21 months of follow-up. This finding is important because the majority of treatment studies have followed patients for one year or less (Borges et al., 2014; Cuijpers et al., 2013; Geddes et al., 2003; Glue et al., 2010). Moreover, this finding provides guidance for clinicians who must frequently make decisions about treatment that may extend beyond one year after initial treatment began (APA, 2010; NICE, 2010).
In terms of relative treatment efficacy, the three conditions produced similar low rates of recurrence; there were no statistically significant differences across conditions. This result is consistent with arguments for the comparable efficacy of initial CBT vs. acute ADMs plus continued ADMs (Cuijpers et al., 2013; Hollon, Stewart, & Strunk, 2006).
The current study also found that residual symptoms on the HAM-D predicted recurrence. This finding is consistent with the aforementioned evidence for residual symptoms predicting recurrence (Judd et al., 1998; Paykel et al., 1995) and with STAR*D findings that residual symptoms predicted relapse or recurrence among remitters to initial treatment (Nierenberg et al., 2010). Residual symptoms may predict relapse/recurrence because they indicate that patients are still suffering somewhat from the index depressive episode, making them more vulnerable to another depressive episode (Judd et al., 2016). This finding has important implications for treatment since many patients end initial treatment with residual symptoms (DeRubeis et al., 2005; Dimidjian et al., 2006; Dunlop, Kelly, et al., 2017; Elkin et al., 1989; Keller et al., 2000). Moreover, evidence that residual symptoms predict recurrence even among remitters supports recent efforts to define more stringent MDD outcome criteria (Dunlop, Holland, Bao, Ninan, & Keller, 2012; Judd et al., 2016).
The current study also found that an anxiety disorder diagnosis at study baseline predicted recurrence. This is consistent with evidence from longitudinal studies that have also found a relationship between anxiety disorder diagnoses and MDD recurrence (Coryell, Endicott, & Keller, 1991; Rao, Hammen, & Daley, 1999; Wilhelm, Parker, Dewhurst-Savellis, & Asghari, 1999). Patients with a comorbid anxiety disorder may suffer from greater exposure to an underlying vulnerability factor for recurrence than patients without comorbid anxiety. For example, these patients may rely on emotion regulation strategies like rumination and avoidance that put them at risk for emotional disorders (Barlow, Allen, & Choate, 2004; Hayes, Wilson, Gifford, Follette, & Strosahl, 1996). This finding is clinically relevant because of the significant comorbidity of depression and anxiety (Kessler et al., 2005; Trivedi et al., 2006). It also suggests the need for modified treatments that differentially target factors that maintain anxiety and depression.
Even though anxiety disorder diagnoses at study baseline predicted recurrence in the current study, baseline anxiety symptom severity on the HAM-A did not. This differs from results from a recent study that found that anxiety symptom severity predicted recurrence in the CBT condition and that an anxiety disorder diagnosis did not predict recurrence in any of the treatment conditions (Forand & DeRubeis, 2013). Moreover, there is evidence that anxiety symptom severity predicts recurrence over and above residual depressive symptoms (Coryell et al., 2012). One potential explanation for the results in the current study is the use of the HAM-A as a measure of anxiety symptoms. Studies that have found a relationship between anxiety symptoms and recurrence have generally used other measures (Coryell et al., 2012; Forand & DeRubeis, 2013). In addition, the HAM-A has been criticized for tapping aspects of depression and missing aspects of anxiety (Koerner, Antony, & Dugas, 2010; Maier, Buller, Philipp, & Heuser, 1988). Despite these discrepancies in the literature, there is accumulating evidence that comorbid anxiety likely impacts acute and maintenance depression treatment outcomes and there may be a need for more specialized treatments tailored for unique pathological pathways or constructs underlying specific subtypes of comorbid anxiety and depression.
Finally, the current study did not find that patients with three or more lifetime depressive episodes had a greater risk of recurrence than patients with two or fewer lifetime episodes. Numerous studies have found that patients with more lifetime depressive episodes are at increased risk of relapse/recurrence (e.g., Bulloch, Williams, Lavorato, & Patten, 2014; Mueller et al., 1999), and lifetime MDEs have been found to moderate treatment outcomes to psychotherapies that specifically target the prevention of MDD relapse and recurrence (e.g., Bockting et al., 2005; Teasdale et al., 2000). Given these discrepancies, it is possible that the current study may not have been sufficiently powered to detect the difference in risk between the two groups. A more plausible interpretation, however, is that participants in the current sample were treatment naïve, for whom the number of lifetime episodes may have a weaker predictive value for recurrence than the degree of success of their initial treatment.
There are several limitations of the current study. First, a key limitation of any follow-up study is the risk of differential retention of patients (Klein, 1996). For the current study, less than thirty percent of all patients initially randomized to treatment were eligible (i.e., remitted to monotherapy) and actually participated in follow-up. Although analyses did not detect differences in participants across treatment groups at the start of follow-up, differing lost to follow-up rates could have biased treatment comparisons. However, lost to follow-up rates in the current study were comparable to those observed in similar studies, and sensitivity analyses did not find that early termination affected the study results. Second, although the sample size in the current study was comparable to that of similar trials, the current study in some instances lacked statistical power to detect small to moderate, yet potentially clinically significant, effects between treatments conditions.
Third, the study sample was treatment naïve, which may limit the study’s generalizability to individuals who are depressed and have failed prior treatments. It is worth noting, however, that remission rates from the monotherapy phase of the PReDICT study were only slightly higher when compared to those of other depression treatment studies (Dunlop, Kelley, et al., 2017), suggesting that treatment efficacy may be comparable between treatment naïve and non-naïve patients. Fourth, patients could choose to end continuation treatment during follow-up, which might have introduced unsystematic change into the study, though most patients continued treatment throughout follow-up.
The current study found low rates of recurrence among patients who remitted to monotherapy and received systematic evaluation and treatment for up to two years. It provides much-needed long-term follow-up information regarding the comparable efficacy of CBT and ADMs, and supports recommendations to make full remission the target of initial treatment (Keller, 2003). The results for recurrence rates support the benefits of continued clinical care (i.e., continuation ADMs or CBT booster sessions) for most patients who remit to initial monotherapy treatment. Nevertheless, patients with a comorbid anxiety disorder diagnosis at the beginning of treatment and those with residual depressive symptoms after initial treatment are at risk for poorer long-term outcomes despite achieving remission.
What is the public health significance of this article?
The results of the current study support the benefits of continued clinical care (i.e., continuation antidepressant medications or cognitive behavior therapy booster sessions) for most treatment naïve patients who remit to initial monotherapy treatment. However, patients with a comorbid anxiety disorder diagnosis or residual depressive symptoms are at risk for poorer long-term outcomes and may need more specialized forms of initial treatment.
Acknowledgments
This work was supported the National Institutes of Health (H.S.M., P50 MH077083; W.E.C., RO1MH080880; B.W.D., K23 MH086690) and the Fuqua family foundations (W.E.C.). Forest Labs and Eli Lilly Inc donated the study medications, escitalopram and duloxetine, respectively, but were otherwise uninvolved in study design, data collection, data analysis, or interpretation of findings.
JCK has no financial disclosures. BWD has received research support from Acadia, Assurex, Axsome, Janssen, the NIH, Otsuka, and Takeda. WEC receives research support from the NIH, is a board member of Hugarheill ehf-an Icelandic company dedicated to the prevention of depression, receives book royalties from John Wiley & Sons, is supported by the Mary and John Brock Foundation, the Fuqua family foundations, is a consultant to the George West Mental Health Foundation and the AIM for Mental Health Scientific Advisory Board, and a member of the Scientific Advisory Board of ADAA. LWC receives research support from the NIH and book royalties from John Wiley & Sons and New Harbinger Publications. CBN has received research support from the NIH and in the past three years has served as a consultant to Xhale, Takeda, SK Pharma, Lilly, Allergan, Mitsubishi Tanaba Pharma Development America, Taisho Pharmaceutical Inc., Lundbeck, Prismic Pharmaceutical, Clintara/Bracket, Sunovion and Total Pain Solutions. He is a stockholder in Xhale, Celgene, Seattle Genetics, Abbvie, and Titan Pharmaceuticals. He has served on the scientific advisory boards of the American Foundation for Suicide Prevention (AFSP), Laureate Institute for Brain Research, Brain and Behavior Research Foundation, Xhale, ADAA, Skyland Trail, Clintara/Bracket, and RiverMend health LLC. He has served on the Board of Directors of the AFSP, Gratitude America, and the ADAA. He has had income sources or equity of $10,000 from the American Psychiatric Publishing, Xhale, and Clintara/Bracket. He holds a patent on the method and devices for transdermal delivery of lithium (US 6,375,990 B1) and the method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay (US 7,148,027 B2). HSM has received consulting fees from St. Jude Medical Neuromodulation and Eli Lilly (2013 only) and intellectual property licensing fees from St. Jude Medical Neuromodulation. Clinicaltrials.gov Identifier:
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Footnote
An analysis also evaluated patients who suffered a “relapse,” defined as meeting relapse criteria at or before the six-month follow-up visit (i.e., nine months from the original PReDICT baseline). Only three patients relapsed during this time period (one in each treatment condition), and Kaplan-Meier estimates of the relapse rates across all treatment conditions was 3.2%. The estimated rates of relapse for the treatment groups were: ESC, 3.1%; DUL, 2.7%; and CBT, 4.3%, and the survival distributions did not statistically differ across any of the comparisons.
Contributor Information
Jamie C. Kennedy, Emory University
Boadie W. Dunlop, Emory University
Linda W. Craighead, Emory University
Charles B. Nemeroff, University of Miami
Helen S. Mayberg, Emory University
W. Edward Craighead, Emory University.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2010). Practice guideline for the treatment of patients with major depressive disorder (3rd ed.). Arlington, VA: American Psychiatric Association. [PubMed] [Google Scholar]
- Aponte‐Rivera V, Dunlop BW, Ramirez C, Kelley ME, Schneider R, Blastos B, ... & Craighead WE (2014). Enhancing Hispanic participation in mental health clinical research: Development of a Spanish‐speaking depression research site. Depression and anxiety, 31(3), 258–267. doi: 10.1002/da.22153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, Allen LB, & Choate ML (2004). Toward a unified treatment for emotional disorders. Behavior Therapy, 35(2), 205–230. 10.1016/S0005-7894(04)80036-4 [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, & Erbaugh J (1961). Beck depression inventory (BDI). Archives of General Psychiatry, 4(6), 561–571. [DOI] [PubMed] [Google Scholar]
- Bockting CLH, Schene AH, Spinhoven P, Koeter MWJ, Wouters LF, Huyser J, & Kamphuis JH (2005). Preventing relapse/recurrence in recurrent depression with cognitive therapy: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 73(4), 647–657. 10.1037/0022-006X.73.4.647 [DOI] [PubMed] [Google Scholar]
- Borges S, Chen Y-F, Laughren TP, Temple R, Patel HD, David PA, … Khin NA (2014). Review of maintenance trials for major depressive disorder: A 25-year perspective from the US Food and Drug Administration. The Journal of Clinical Psychiatry, 75(3), 205–214. 10.4088/JCP.13r08722 [DOI] [PubMed] [Google Scholar]
- Bulloch A, Williams J, Lavorato D, & Patten S (2014). Recurrence of major depressive episodes is strongly dependent on the number of previous episodes. Depression and Anxiety, 31(1), 72–76. 10.1002/da.22173 [DOI] [PubMed] [Google Scholar]
- Burcusa SL, & Iacono WG (2007). Risk for recurrence in depression. Clinical Psychology Review, 27(8), 959–985. 10.1016/j.cpr.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Endicott J, & Keller MB (1991). Predictors of relapse into major depressive disorder in a nonclinical population. The American Journal of Psychiatry, 148(10), 1353–1358. 10.1176/ajp.148.10.1353 [DOI] [PubMed] [Google Scholar]
- Coryell W, Fiedorowicz JG, Solomon D, Leon AC, Rice JP, & Keller MB (2012). Effects of anxiety on the long-term course of depressive disorders. The British Journal of Psychiatry, 200(3), 210 LP–215. 10.1192/bjp.bp.110.081992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR, & Oakes D (1984). Analysis of survival data. London, UK: Chapman & Hall. [Google Scholar]
- Craighead WE, & Dunlop BW (2014). Combination psychotherapy and antidepressant medication treatment for depression: For whom, when, and how. Annual Review of Psychology, 65(1), 267–300. 10.1146/annurev.psych.121208.131653 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, & Andersson G (2013). Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open, 3(4), e002542 10.1136/bmjopen-2012-002542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Warmerdam L, & Andersson G (2008). Psychological treatment of depression: A meta-analytic database of randomized studies. BMC Psychiatry, 8(1), 1–6. 10.1186/1471-244X-8-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, … Gallop R (2005). Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry, 62(4), 409–416. 10.1001/archpsyc.62.4.409 [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, … Jacobson NS (2006). Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology, 74(4), 658–670. 10.1037/0022-006X.74.4.658 [DOI] [PubMed] [Google Scholar]
- Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, … Jacobson NS (2008). Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. Journal of Consulting and Clinical Psychology, 76(3), 468–477. 10.1037/0022-006X.76.3.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Binder EB, Cubells JF, Goodman MM, Kelley ME, Kinkead B, … Mayberg HS (2012). Predictors of remission in depression to individual and combined treatments (PReDICT): Study protocol for a randomized controlled trial. Trials, 13, 106 10.1186/1745-6215-13-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Holland P, Bao W, Ninan PT, & Keller MB (2012). Recovery and subsequent recurrence in patients with recurrent major depressive disorder. Journal of Psychiatric Research, 46(6), 708–715. 10.1016/j.jpsychires.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Kelley ME, Aponte-Rivera V, Kinkead B, Mletzko-Crowe T, . . . Mayberg HS (2017). Effects of patient preferences on outcomes in the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study. American Journal of Psychiatry, 174, 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, … Parloff MB (1989). National Institute of Mental Health Treatment of Depression Collaborative Research Program: General effectiveness of treatments. Archives of General Psychiatry, 46(11), 971–982. 10.1001/archpsyc.1989.01810110013002 [DOI] [PubMed] [Google Scholar]
- Fava GA, & Offidani E (2011). The mechanisms of tolerance in antidepressant action. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(7), 1593–1602. 10.1016/j.pnpbp.2010.07.026 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1995). Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID-I/P, Version 2.0). New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Forand NR, & DeRubeis RJ (2013). Pretreatment anxiety predicts patterns of change in cognitive behavioral therapy and medications for depression. Journal of Consulting and Clinical Psychology, 81(5), 774–782. 10.1037/a0032985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, & Goodwin GM (2003). Relapse prevention with antidepressant drug treatment in depressive disorders: A systematic review. The Lancet, 361(9358), 653–661. 10.1016/S0140-6736(03)12599-8 [DOI] [PubMed] [Google Scholar]
- Glue P, Donovan MR, Kolluri S, & Emir B (2010). Meta-analysis of relapse prevention antidepressant trials in depressive disorders. Australian and New Zealand Journal of Psychiatry, 44(8), 697–705. 10.3109/00048671003705441 [DOI] [PubMed] [Google Scholar]
- Hamilton M (1959). The assessment of anxiety states by rating. British Journal of Medical Psychology, 32, 50–55. 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- Hamilton M (1967). Development of a rating scale for primary depressive illness. The British Journal of Social and Clinical Psychology, 6(4), 278–296. 10.1111/j.2044-8260.1967.tb00530.x [DOI] [PubMed] [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Follette VM, & Strosahl K (1996). Experiential avoidance and behavioral disorders: A functional dimensional approach to diagnosis and treatment. Journal of Consulting and Clinical Psychology, 64(6), 1152–1168. 10.1037/0022-006X.64.6.1152 [DOI] [PubMed] [Google Scholar]
- Hardeveld F, Spijker J, De Graaf R, Nolen WA & Beekman ATF (2010). Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatrica Scandinavica, 122, 184–191. doi.org/ 10.1111/j.1600-0447.2009.01519.x [DOI] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, … Gallop R (2005). Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Archives in General Psychiatry, 62(4), 417–422. 10.1001/archpsyc.62.4.417 [DOI] [PubMed] [Google Scholar]
- Hollon SD, Stewart MO, & Strunk D (2006). Enduring effects for cognitive behavior therapy in the treatment of depression and anxiety. Annual Review of Psychology, 57, 285–315. 10.1146/annurev.psych.57.102904.190044 [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, & Thase ME (2013). Preventing depressive relapse and recurrence in higher risk cognitive therapy responders: a randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatry, 70(11), 1152–1160. 10.1001/jamapsychiatry.2013.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, … Keller MB (1998). A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Archives of General Psychiatry, 55(8), 694–700. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Rush AJ, Coryell WH, Fiedorowicz JG, & Solomon DA (2016). A new empirical definition of major depressive episode recovery and its positive impact on future course of illness. The Journal of Clinical Psychiatry, 77(8), 1065–73. 10.4088/JCP.15m09918. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, & Meier P (1958). Nonparametric estimation from incomplete observations. Journal of the American Statistical Association, 53(282), 457–481. 10.2307/2281868 [DOI] [Google Scholar]
- Keller MB (2003). Past, present, and future directions for defining optimal treatment outcome in depression: Remission and beyond. JAMA: Journal of the American Medical Association, 289(23), 3152–3160. 10.1001/jama.289.23.3152 [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, & Andreasen NC (1987). The Longitudinal Interval Follow-up Evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry, 44(6), 540–548. 10.1001/archpsyc.1987.01800180050009 [DOI] [PubMed] [Google Scholar]
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, … Zajecka J (2000). A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. The New England Journal of Medicine, 342(20), 1462–1470. 10.1056/NEJM200005183422001 [DOI] [PubMed] [Google Scholar]
- Keller MB, Trivedi MH, Thase ME, Shelton RC, Kornstein SG, Nemeroff CB, ... & Hirschfeld RM (2007). The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) Study: Outcomes from the 2-year and combined maintenance phases. The Journal of clinical psychiatry, 68(8), 1246–1256. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62(6), 593–602. 10.1001/archpsyc.62.6.617 [DOI] [PubMed] [Google Scholar]
- Klein DF (1996). Preventing hung juries about therapy studies. Journal of Consulting and Clinical Psychology, 64(1), 81–87. 10.1037/0022-006X.64.1.81 [DOI] [PubMed] [Google Scholar]
- Klein DN, Santiago NJ, Vivian D, Arnow BA, Blalock JA, Dunner DL, . . . Keller MB (2004).Cognitive-behavioral analysis system of psychotherapy as a maintenance treatment for chronic depression. Journal of Consulting and Clinical Psychology, 72(4), 681–688. doi: 10.1037/0022-006X.72.4.681 [DOI] [PubMed] [Google Scholar]
- Koerner M, Antony MM, & Dugas MJ (2010). Limitations of the Hamilton Anxiety Rating Scale as a primary outcome measure in randomized, controlled trials of treatments for generalized anxiety disorder. American Journal of Psychiatry, 167(1), 103–104. 10.1176/appi.ajp.2009.09091264 [DOI] [PubMed] [Google Scholar]
- Kuyken W, Warren FC, Taylor RS, Whalley B, Crane C, Bondolfi G, ... & Segal Z (2016). Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse: an individual patient data meta-analysis from randomized trials. JAMA psychiatry, 73(6), 565–574. doi: 10.1001/jamapsychiatry.2016.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Buller R, Philipp M, & Heuser I (1988). The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders, 14(1), 61–68. 10.1016/0165-0327(88)90072-9 [DOI] [PubMed] [Google Scholar]
- Mantel N (1966). Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports, 50(3), 163–170. [PubMed] [Google Scholar]
- Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, … Maser JD (1999). Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. American Journal of Psychiatry, 156(7), 1000–1006. 10.1176/ajp.156.7.1000 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. (2010). Depression: the treatment and management of depression in adults. (Updated). London, UK: National Institute for Health and Clinical Excellence. [Google Scholar]
- Nierenberg A, Husain M, Trivedi M, Fava M, Warden D, Wisniewski S, . . . Rush A (2010). Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: A STAR*D report. Psychological Medicine, 40(1), 41–50. doi.org/ 10.1017/S0033291709006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, & Marcus SC (2009). National patterns in antidepressant medication treatment. Archives of General Psychiatry, 66(8), 848–856. 10.1001/archgenpsychiatry.2009.81 [DOI] [PubMed] [Google Scholar]
- Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, & Barocka A (1995). Residual symptoms after partial remission: An important outcome in depression. Psychological Medicine, 25(6), 1171–1180. 10.1017/S0033291700033146 [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, & Daley SE (1999). Continuity of depression during the transition to adulthood: A 5-year longitudinal study of young women. Journal of the American Academy of Child & Adolescent Psychiatry, 38(7), 908–915. 10.1097/00004583-199907000-00022 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, … Fava M (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. The American Journal of Psychiatry, 163(11), 1905–1917. 10.1176/appi.ajp.163.11.1905 [DOI] [PubMed] [Google Scholar]
- Shea MT, Elkin I, Imber SD, Sotsky SM, Watkins JT, Collins JF, … Dolan RT (1992). Course of depressive symptoms over follow-up: Findings from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Archives of General Psychiatry, 49(10), 782–787. 10.1001/archpsyc.1992.01820100026006 [DOI] [PubMed] [Google Scholar]
- Shih WJ (2002). Problems in dealing with missing data and informative censoring in clinical trials. Current Controlled Trials in Cardiovascular Medicine, 3(1), 4 10.1186/1468-6708-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller MD, Lavori PW, Shea MT, … Endicott J (2000). Multiple recurrences of major depressive disorder. American Journal of Psychiatry, 157(2), 229–233. 10.1176/appi.ajp.157.2.229 [DOI] [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, & Usitalo AN (2011). Psychotherapy versus second-generation antidepressants in the treatment of depression: A meta-analysis. The Journal of Nervous and Mental Disease, 199(3). 10.1097/NMD.0b013e31820caefb [DOI] [PubMed] [Google Scholar]
- Stangier U, Hilling C, Heidenreich T, Risch AK, Barocka A, Schlösser R, ... & Weck F (2013). Maintenance cognitive-behavioral therapy and manualized psychoeducation in the treatment of recurrent depression: a multicenter prospective randomized controlled trial. American Journal of Psychiatry, 170(6), 624–632. 10.1176/appi.ajp.2013.12060734 [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, & Lau MA (2000). Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology, 68(4), 615–623. 10.1037/0022-006X.68.4.615 [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, … Fava M (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. The American Journal of Psychiatry, 163(1), 28–40. 10.1176/appi.ajp.163.1.28 [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Parker G, Dewhurst-Savellis J, & Asghari A (1999). Psychological predictors of single and recurrent major depressive episodes. Journal of Affective Disorders, 54(1–2), 139–147. 10.1016/S0165-0327(98)00170-0 [DOI] [PubMed] [Google Scholar]
- Williams JW (1988). A structured interview guide for the hamilton depression rating scale. Archives of General Psychiatry, 45(8), 742–747. 10.1001/archpsyc.1988.01800320058007 [DOI] [PubMed] [Google Scholar]