Abstract
Very preterm infants (≤31 weeks gestational age) are at high risk for brain injury and delayed development. Applying functional connectivity and graph theory methods to resting state MRI data (fcMRI), we tested the hypothesis that preterm infants would demonstrate alterations in connectivity measures both globally and in specific networks related to motor, language and cognitive function, even when there is no anatomical imaging evidence of injury. Fifty-one healthy full-term controls and 24 very preterm infants without significant neonatal brain injury, were evaluated at term-equivalent age with fcMRI. Preterm subjects showed lower functional connectivity from regions associated with motor, cognitive, language and executive function, than term controls. Examining brain networks using graph theory measures of functional connectivity, very preterm infants also exhibited lower rich-club coefficient and assortativity but higher small-worldness and no significant difference in modularity when compared to term infants. The findings provide evidence that functional connectivity exhibits deficits soon after birth in very preterm infants in key brain networks responsible for motor, language and executive functions, even in the absence of anatomical lesions. These functional network measures could serve as prognostic biomarkers for later developmental disabilities and guide decisions about early interventions.
Keywords: Premature Infant, Neonatal, Brain, Imaging, Connectivity, fMRI
Introduction
Preterm birth is a serious health problem, often resulting in significant morbidity and mortality and later cognitive and motor impairments (Botting et al. 1998; Hack 2009; Mansson and Stjernqvist 2014). Brain injury and altered brain development related to preterm birth have been well described using conventional and advanced magnetic resonance imaging (MRI), but predicting later adverse neurodevelopmental outcomes based on neonatal structural MRI remains difficult (Van’t Hooft et al. 2015; Parikh 2016). Functional connectivity analysis of functional MRI (fMRI) data shows promise as a sophisticated technique to elucidate more subtle adverse effects of preterm birth on the developing brain.
Numerous studies have demonstrated the cognitive deficits associated with preterm birth, including decreased memory and attention skills (Kerr-Wilson et al. 2012; de Kieviet et al. 2012). Even preterm infants with normal IQ remain at high risk for school failure, possibly due to deficits in executive function as compared to full-term peers (Edgin et al. 2008; Burnett, et al. 2015). One in three children born prematurely also show signs of language delay even before school age (Foster-Cohen et al. 2010); motor impairments and cerebral palsy are also common (Woodward et al. 2009; Moore et al. 2012; Synnes et al. 2015). These difficulties persist through adolescence and young adulthood (Breeman et al. 2015). Previous studies evaluating alterations of functional brain networks soon after birth in preterm infants suggest a mechanism for these numerous issues (Doria et al., 2010; Smyser et al. 2010; Smyser et al. 2014). Few studies have directly related network alterations in the neonatal period to later outcomes, Rogers et al has related neonatal amygdala connectivity to later social processing at 2 years (Rogers et al. 2017).
The large majority of studies of functional brain connectivity in children born prematurely have reported the effects of preterm birth on motor, language, and cognitive development in older children, several years after preterm birth, and at school age (Wilke et al. 2014; Degnan et al. 2015; Lee et al. 2011). Consequently, we are beginning to recognize the causal relationship between deficits in connectivity within brain networks that persist into school age in children born prematurely and neurocognitive deficits associated with these networks, even in the absence of anatomical lesions (Rowlands et al. 2016). Presumably, these infants exhibit microscopic/microstructrual injury and/or delayed brain maturation soon after birth (Volpe JJ, 2009; Chau V et al. 2013). The investigation of the effect of preterm birth on functional connectivity during the neonatal period remains poorly understood. However, recent investigations have demonstrated a relationship between alternations in structural connectivity (i.e. white matter development) and poor cognitive and motor outcomes in children born prematurely (Fischi-Gómez et al. 2015; Liu et al. 2010; Chau V et al. 2013; Parikh, 2016). The rationale for the current study is to identify deficits in functional connectivity in brain networks that exist soon after birth in infants born prematurely, that may underlie motor and cognitive deficits that emerge as the children develop. By examining specific networks that are now known to control sensorimotor, language and executive functions in older children and adults, we can identify potential sources of future deficits in these domains in infants born prematurely, providing a potential biomarker for poor outcomes as well as an indicator of children who may benefit most from early interventions.
More recently, graph theory has been applied to fMRI data from the human brain to compute complex network maps (Bullmore and Sporns 2009). Graph theory is an area of mathematics that can provide quantitative information about networks on both a local and global scale. It has been extensively applied to characterize topological properties of functional brain networks in healthy adults. Small-worldness (Salvador et al. 2005; Achard et al. 2006), modularity, rich-club coefficient (van den Heuvel et al. 2011, 2012; Cao et al. 2014) and assortativity, are topological characteristics of functional brain networks that have been shown to change significantly during development (Bullmore and Sporns 2012; Di Martino et al. 2014) raising the question of how they emerge and change in the preterm population.
In this study, we investigated functional connectivity and network measures using graph theory in very preterm infants without significant structural brain injury and healthy full-term control infants using resting state fMRI data collected at term-equivalent age. We hypothesize that preterm and full-term infants will differ in global network measures as well as specific network measures related to motor, language, and executive function, with decreased connectivity in the preterm group during the neonatal period. This general hypothesis will be tested by examining both whole brain and network-specific brain connectivity differences between pre-term and full term infants based on fMRI data obtained within 2 weeks of term equivalent age.
Materials and Methods
Participants
For this cross-sectional study, we included 24 very preterm infants (≤31 weeks gestational age) without significant brain injury on anatomic MRI from a longitudinal cohort study of very preterm infants cared for in the neonatal intensive care unit at Nationwide Children’s Hospital. We compared these infants to a control population of 51 healthy full-term infants (38–41 completed weeks gestation), also conducted at Nationwide Children’s. Baseline demographic and clinical outcome data for the preterm and full-term cohorts are included in Table 1. Term control infants were recruited from two well-baby nurseries at The Ohio State University Medical Center and Riverside Methodist Hospital. Infants with known structural congenital central nervous system anomalies, congenital chromosomal anomalies, or congenital cyanotic cardiac defects were excluded. Additionally, we excluded full-term infants that were small or large for gestational age and those with a history of perinatal distress or complications. None of the term infants exhibited any signs of parenchymal brain injury or delayed development on anatomic MRI at term.
Table 1.
Baseline demographic and clinical outcome data for the preterm and full-term cohorts.
| Clinical Variable | Preterm (N=24) | Full-term (N=51) |
|---|---|---|
| Gestational age, mean (SD), weeks | 28.6 (2.6) | 39.5 (0.9) |
| Birth weight, mean (SD), grams | 1156 .5 (430.2) | 3371.5 (297.2) |
| Sex, male | 14 (58.0%) | 26 (51%) |
| Antenatal steroids | 83.3% | 0% |
| Low Apgar score at 5 minutes, ≤5 | 12.5% | 0% |
| Necrotizing enterocolitis or spontaneous intestinal perforation | 12.5% | 0% |
| Sepsis, culture-positive | 4.2% | 0% |
| Retinopathy of prematurity – stage 3 or higher or with plus disease | 12.5% | 0% |
| Bronchopulmonary dysplasia¶ | 41.7% | 0% |
Bronchopulmonary dysplasia was defined as any oxygen requirement at 36 weeks postmenstrual age
The Nationwide Children’s Hospital Institutional Review Board approved this study and written parental informed consent was obtained for every subject prior to imaging.
MRI acquisition
Full term infants (mean postmenstrual age at scan 40 weeks, range 40-42 weeks) and preterm infants (mean postmenstrual age at scan 39.8 weeks, range 39-41 weeks) were scanned on a 3T Siemens Skyra scanner using a 32-channel head coil during natural sleep. Previously we have found it feasible to obtain high quality fMRI data from sleeping infants and toddlers. (Patel et al. 2007; Tan et al. 2013; Deshpande et al. 2016; Merhar et al. 2016) It is not ethical to use sedation exclusively for research involving healthy, therefore sleep is a good option to minimize motion during MRI scanning in infants for research (Dehaene-Lambertz et al. 2002). Inducing and maintaining natural sleep is a challenging element to add to any imaging experimental protocol. Nevertheless, development of these protocols has taken place at a few sites, including ours (Dehaene-Lambertz et al. 2002; Anderson et al. 2001; Redcay et al. 2007; Vannest et al. 2014). Previous work in our laboratory demonstrates that fMRI is capable of imaging the status of auditory cortex and subsequent processing in response to sound stimulation (Holland et al. 2004; Patel et al. 2007). Indeed, auditory response is one of the last cortical functions to shut down under the influence of sleep or anesthesia and auditory responsiveness is used by anesthesiologists to assess level of sedation (Malviya et al. 2006; Malviya et al. 2007). Therefore a passive auditory stimulation paradigm was a natural choice to evaluate functional connectivity for this study in infants (Cheour et al. 2004). We probed language networks using a well-characterized task (Karunanayaka et al. 2007; Schmithorst et al. 2006): passively listening to stories (Story Listening Task). Given the similar (though attenuated) brain activation patterns we observed in the sleeping infants relative to our previous studies in sleeping (Merhar et al. 2016; Vannest et al. 2014; Wilke et al. 2003), sedated (Patel et al. 2007; Holland et al. 2004; DiFrancesco et al. 2013) and awake children (Szaflarski et al. 2012; Karunanayaka et al. 2007; Vannest et al. 2009; Holland et al. 2007) using this task, we have a good measure of confidence that the BOLD effect is somewhat preserved in the sleeping infant cohorts studied here. Similar arguments apply to our assessment of sensory motor function in this study.
Although, objective measurements of the depth of sleep were not performed for this study, the acquisition of the resting state fMRI was begun when infants were already in a deep stage of sleep to insure that they would not be awakened by the scanner noise which as a A-weighted sound pressure level (SLP-A) of approximately 104 dB for the resting state fMRI protocol. A more detailed analysis of the literature on fMRI connectivity and natural sleep is provided later in the Discussion.
High-resolution anatomical T1-and T2-weighted images were acquired for alignment of fMRI data and for inspection of brain anatomy. T1-weighted 3D MPRAGE images were acquired using TE=2.9ms, TR=2130ms, 1×1×1 mm3 voxel size, flip angle=12°, and FOV=174×192 mm2. T2-weighted images were obtained with TE=147ms, TR=9500ms, 0.93×0.93×1.1 mm3 voxel size, flip angle=150°, and FOV=180×180 mm2. The resting state fMRI data was collected from a gradient echo planar sequence with TE=30ms, TR=3000ms, FOV=212×212 mm2, matrix size=104×104, slice thickness 2.5mm and 150 measurements.
Prior to quantitative analysis of resting state fMRI data for connectivity using graph measures, all anatomical, T1 and T2-weighted, images were reviewed by a pediatric neuroradiologist and a neonatologist skilled in reading neonatal MRI scans. No subjects with evidence of defects in white and/or grey matter were included in the subsequent analysis because anatomical lesions provide separate evidence for predicting poor cognitive or motor outcome and are also likely to affect brain connectivity.
Data analysis
SPM was used for pre-processing of the resting state functional imaging data. The functional image series were corrected for head motion by realigning all images to the mean of all functional volumes. Although the neonates were asleep during the fMRI procedures, head motion was still problematic and was corrected as described in detail in the section below. The mean functional image was co-registered to a corresponding T2-weighted high-resolution image. T2-weighted images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using the UNC neonatal tissue probability maps (Shi et al. 2010). Tools developed for segmentation of adult brain images do not work as well in neonatal brain images due to reduced contrast between gray matter and white matter that arises from the higher water content and limited myelination in the neonatal brain. Consequently, we utilized a neonatal brain atlas developed specifically to address this low contrast condition by introducing age appropriate prior probability estimates (Shi et al. 2010). As a final step, the images were normalized to the neonatal template (Shi et al. 2011), re-sliced to a voxel size 2×2×2mm, and smoothed with a full width at half maximum (FWHM) Gaussian Smoothing kernel of 6mm. T1-weighted images in neonates do not provide sufficient contrast for segmentation and corregistration and were not used in our analysis.
ROI-to-ROI Functional Connectivity
To test our hypothesis that preterm and full-term infants will differ in specific network measures related to motor, language, and executive function, during the neonatal period, we used ROI-to-ROI connectivity methods to examine connectivity between all brain regions. The assessment of functional connectivity for pre-processed resting state data was carried out using the Conn functional connectivity toolbox (Whitfield-Gabrieli and Nieto-Castanon 2012). Conn is a Matlab-based toolbox for the computation, display and analysis of functional connectivity in fMRI (fcMRI). The residual BOLD time-series was extracted from gray matter voxels. The resting state fMRI time series from each voxel was corrected using a strict noise reduction method called aCompCor, which removed the principal components attributed to white matter and cerebrospinal fluid signals (Behzadi et al. 2007) and eliminated the need for a global signal regression (Chai et al. 2012; Murphy et al. 2009). In addition, subject-specific six motion parameter time-series and their first derivatives, and extreme outlier volumes detected with the threshold 1mm in ART (http://www.nitrc.org/projects/artifact_detect/) were also introduced as potential confounds. ART is a graphic tool for automatic and manual detection of global mean and motion outliers in fMRI data. In addition, the residual BOLD time-series in each voxel was band-pass filtered at 0.008 to 0.08 Hz to focus on low frequency fluctuations (Fox et al. 2005).
Following these temporal preprocessing steps, an ROI-to-ROI functional connectivity analysis was performed by grouping voxels into the 90 ROIs defined specifically for the neonatal brain. These 90 ROIs are defined objectively using a neonatal brain image atlas, neonate.aal (Automated Anatomical Labeling) (Shi et al. 2011). The correlation matrix among these ROIs is then estimated for each subject by computing Pearson’s correlation coefficients between each ROI time-series and the time-series of all other ROIs. The correlation values for each subject were Fisher transformed to normally distributed scores and used in subsequent analysis.
The normalized correlation coefficients were averaged within each group for each region and correlation matrices were created for each group for all 90 ROIs. Additionally, a two-sample t-test was run between the groups for each ROI-ROI pair, to examine whether differences in connectivity between full-term and preterm groups were significant for any of the 3960 possible connections. For this analysis an FDR-corrected threshold p<0.05, was used. All ROIs that reached this level of difference between preterm and full-term infants were then defined as seed regions for further connectivity analysis within the specific networks shown in Figure 1-3 (5 sensory/motor, 2 auditory/language, 6 cognitive/executive function,). These ROIs are listed in Table 2 and identified as the black circles on the connectivity plots in Figures 1, 2 and 3.
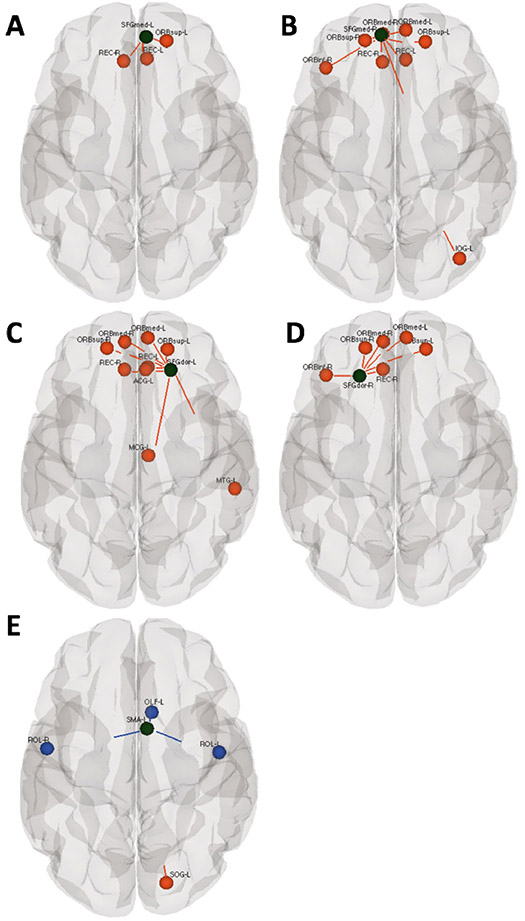
Figure 1:
Group differences between preterm and full-term neonate for ROI-to-ROI functional connectivity with motor area seed regions. Black circles represent the regions that reached the group level difference in A-C) Superior frontal gyrus (medial), B-D) Superior frontal gyrus (dorsal), E) Supplementary motor area. Red colors indicate ROIs where the full-term group exhibit greater functional connectivity than the preterm group while blue lines indicate increased functional connectivity to motor area seed ROI for the preterm group relative to the full-term group. Connectivity differences are shown at a threshold of p<0.05 corrected for multiple comparisons using false discovery rate.
Figure 3:
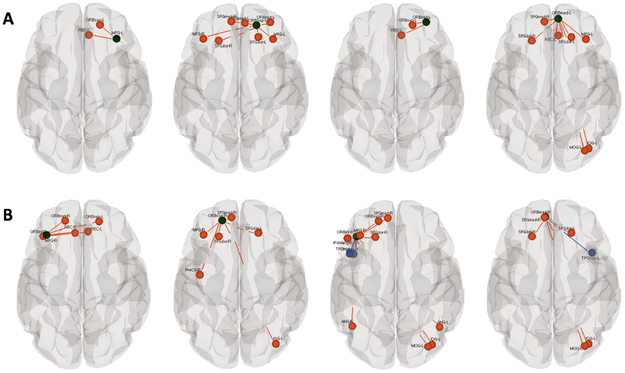
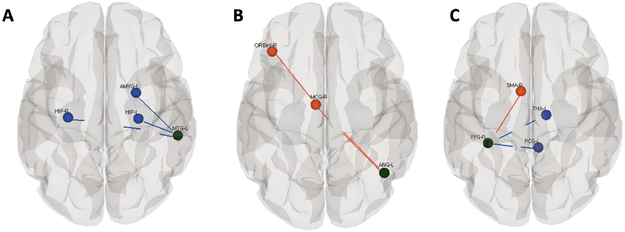
Group differences between preterm and full-term neonate for ROI-to-ROI functional connectivity with cognitive, executive function area seed regions in: A) Left Middle frontal gyrus and Orbitofrontal cortex, B) Right Middle frontal gyrus and Orbitofrontal cortex .Black circles represent the regions that reached the group level difference.Red colors represent connection where the full-term group had greater functional connectivity and blue lines represent the preterm group had greater functional connectivity with a threshold of p<0.05 corrected for multiple comparisons using false discovery rate.
Table 2.
All regions with significant group differences in functional connectivity are listed by anatomical area and network, along with the t value and p value for the between group difference and MNI coordinates of the center of each ROI.
| Region | t value | p value | (x, y, z) |
|---|---|---|---|
| Sensory/motor network | |||
| Superior frontal gyrus (medial) left SFGmed-L | 2.33 | 0.011 | (3, 28, 17) |
| Superior frontal gyrus (medial) right SFGmed-R | 2.52 | 0.011 | (− 6, 29, 17) |
| Superior frontal gyrus (dorsal) left SFGdor-L | 3.11 | 0.0009 | (11, 19, 26) |
| Superior frontal gyrus (dorsal) right SFGdor-R | 3.20 | 0.0008 | (− 14, 16, 27) |
| Supplementary motor area left SMA-L | 2.62 | 0.0044 | (3, − 1, 38) |
| Language network | |||
| Middle temporal gyrus left MTG-L | 1.92 | 0.039 | (37, − 23, 2) |
| Angular gyrus left ANG-L | 1.82 | 0.0435 | (32, − 39, 26) |
| Fusiform gyrus right FFG-R | 2.31 | 0.0118 | (− 18, − 28, − 14) |
| Executive function/working memory network | |||
| Middle frontal gyrus left MFG-L | 1.75 | 0.0455 | (21, 19, 21) |
| Middle frontal gyrus right MFG-R | 1.75 | 0.0455 | (− 24, 18, 19) |
| Orbitofrontal cortex (superior) left ORBsup-L | 2.67 | 0.0037 | (9, 25, − 12) |
| Orbitofrontal cortex (superior) right ORBsup-R | 2.58 | 0.005 | (− 10, 25, − 13) |
| Orbitofrontal cortex (middle) left ORBmid-L | 1.48 | 0.0141 | (18, 30, − 8) |
| Orbitofrontal cortex (inferior) right ORBinf-R | 3.25 | 0.0006 | (− 24, 16, − 10) |
| Orbitofrontal cortex (medial) left ORBmed-L | 3.09 | 0.001 | (3, 31, − 8) |
| Orbitofrontal cortex (medial) right ORBmed-R | 2.76 | 0.0028 | (− 5, 28, − 8) |
Figure 2:
Group differences between preterm and full-term neonate for ROI-to-ROI functional connectivity with language area seed regions. Black circles represent the regions that reached the group level difference in A) Left Middle Temporal Gyrus, B) Left Angular Gyrus, and C) Right Fusiform Gyrus. Red colors indicate ROIs where the full-term group exhibit greater functional connectivity than the preterm group while blue lines indicate increased functional connectivity to language area seed ROI for the preterm group relative to the full-term group. Connectivity differences are shown at a threshold of p<0.05 corrected for multiple comparisons using false discovery rate.
Graph theory processing
To test our hypothesis that preterm and full-term infants would differ in global network measures during the neonatal period, we use graph theory to examine whole brain network connectivity based on global and local topological measures. The unweighted covariance matrix for these 90 ROIs for each subject from the Conn functional connectivity toolbox described above was used as input to the graph theory network analysis in the Brain Connectivity Toolbox (https://sites.google.com/site/bctnet/). Both global and regional topological properties were investigated using the graph theory parameters: global efficiency, local efficiency, degree, betweenness centrality, assortativity, small-worldness, modularity and rich-club coefficient. These parameters characterize the properties of the network and the efficiency of information transfer within it. Graph theory parameters were tested for differences between the groups on a regional basis using the same 90 ROIs defined above in the correlation analysis. The specific graph theory parameters we examined are defined as follows.
Global efficiency is defined as the average inverse shortest path length between all pairs of nodes (Latora and Marchiori 2001). Global efficiency quantifies how efficiently information is exchanged over the network for a system in which each node sends information concurrently along the network. A high global efficiency indicates the capacity to transfer information in a network in which each node can send information simultaneously. Local efficiency is computed as the shortest path length between nodes i and j containing neighbors of node k. In contrast to global efficiency, local efficiency represents the capacity to transfer information within the neighbors of a given node, and a high local efficiency reflects efficient information transfer in the immediate neighborhood of each node (Lo et al. 2010). Betweenness centrality of a node is the fraction of shortest paths between all other pairs of nodes in the network that actually pass through the node of interest (Freeman 1978). This measure has been used to identify the most central nodes in a network, which reflects the importance of the specific node in transferring information to other nodes. A node with high betweenness centrality is crucial to efficient communication Node degree is defined as the number of links connected to the node. Modularity (Q) quantifies the degree to which the network may be partitioned into a subdivision that has higher connections with each other than with the rest of the network (Newman, 2006). Assortativity quantifies how often nodes with a certain degree tend to link to other nodes with the same or similar degree (Newman 2002).
Small-worldness was quantified using clustering coefficient and characteristic path length (Watts and Strogate, 1998). Clustering coefficient (C) is the fraction of the node’s neighbors that are also neighbors of each other. Characteristic path length (L) is defined as the average of the shortest path length between all pairs of nodes. Small-worldness (σ) of a network was defined by the ratio of normalized clustering coefficient (γ) and and normalized characteristic path length (λ)
where γ=C/Crand and λ=L/Lrand (Watts and Strogatz, 1998). The clustering coefficient and characteristic path length of a random network, Crand and Lrand, were the average of the values calculated from 100 randomized models. It should be noted that a network shows small-worldness if λ > 1, σ> 1 and λ≈1.
Rich-club organization was calculated over a range of degree k after removing all nodes with degree ≤k. The rich-club coefficient ∅(k) for any k is the ratio of connections between the remaining nodes E and the total number of possible connections for a fully connected network with the same number of nodes N. The rich-club coefficient ∅(k) was computed as follows:
For comparison across individuals rich-club coefficient was normalized by the average rich-club coefficient over 1000 random networks ∅rand(k),
A normalized rich-club clustering coefficient ∅norm(k) > 1 over a range of k indicates rich-club organization in the network (van den Heuvel and Sporns, 2011).
Measures of network degree, global efficiency, local efficiency and betweenness centrality (BC), were computed for every region by means of graph theory analysis implemented in the Brain Connectivity Toolbox. Additionally, assortativity, small-worldness, modularity and rich-club coefficient were computed for the entire brain using a cost threshold. Cost is a measure of the proportion of connections for each ROI in relation to all connections in the network.
A two-sample t-test was used to compare graph measures between full-term and preterm groups to determine if these parameters differed significantly over the entire brain or within any of the 90 ROIs tested. Regions where any of these parameters differed significantly are tabulated (Table 3) and plotted (Figure 5).
Table 3.
Regions with significant group differences in regional graph theory measures.
| Region | t value | dof | p value | (x, y, z) |
|---|---|---|---|---|
| (A) Global efficiency | ||||
| Superior frontal gyrus (medial) right SFGmed-R | 3.64 | 73 | 0.045297 | (− 6, 29, 17) |
| Superior frontal gyrus (medial) left SFGmed-L | 3.08 | 73 | 0.048219 | (3, 28, 17) |
| Superior frontal gyrus (dorsal) left SFGdor-L | 3.38 | 73 | 0.048219 | (11, 19, 26) |
| Orbitofrontal cortex (superior) left ORBsup-L | 3.13 | 73 | 0.048219 | (9, 25, − 12) |
| Heschl gyrus left HES-L | 3.02 | 73 | 0.048219 | (26, − 15, 9) |
| Superior temporal gyrus left STG-L | 2.91 | 73 | 0.048219 | (36, − 14, 8) |
| Superior temporal gyrus right STG-R | 2.98 | 73 | 0.048219 | (− 37, − 19, 5) |
| Middle frontal gyrus left MFG-L | 2.97 | 73 | 0.048219 | (21, 19, 21) |
| Precentral gyrus right-PreCG-R | 2.91 | 73 | 0.048219 | (− 26, − 7, 32) |
| (B) Degree | ||||
| Superior frontal gyrus (medial) right SFGmed-R | 3.89 | 73 | 0.019611 | (− 6, 29, 17) |
| Superior frontal gyrus (dorsal) left SFGdor-L | 3.59 | 73 | 0.019611 | (11, 19, 26) |
| Orbitofrontal cortex (superior) left ORBsup-L | 3.56 | 73 | 0.019611 | (9, 25, − 12) |
| Heschl gyrus left HES-L | 3.35 | 73 | 0.028869 | (26, − 15, 9) |
| Superior frontal gyrus (medial) left SFGmed-L | 3.18 | 73 | 0.038471 | (3, 28, 17) |
| Superior temporal gyrus left STG-L | 3.00 | 73 | 0.047593 | (36, − 14, 8) |
| Orbitofrontal cortex (superior) right ORBsup-R | 2.96 | 73 | 0.047593 | (− 10, 25, − 13) |
| Middle frontal gyrus left MFG-L | 2.95 | 73 | 0.047593 | (21, 19, 21) |
Regions are listed by anatomical designation along with relevant statistical measures between groups and MNI coordinates at the center of each ROI.
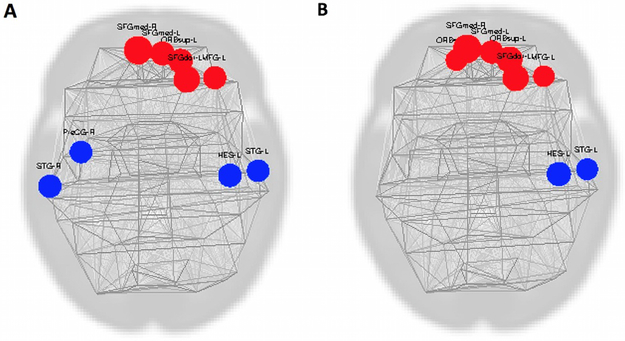
Figure 5:
Group difference maps. Red indicates that the full-term group had greater Global Efficiency (A) or Degree (B) compared with the preterm group while blue colors represent the converse with the preterm group exhibiting increases relative to the full-term babies. A threshold of p<0.05 was used for all maps, with correction for multiple comparisons using false discovery rate.
Finally, to determine the influence of post-menstrual age and numbers of days post-delivery, both graph theory measures and ROI-ROI connectivity values were entered into a general linear model (GLM) to analyze these effects in full-term and preterm babies.
Results
Table 1 summarizes the demographics and, clinical characteristics of the infants included in this study. The mean gestational age at birth for full term infants was 39.5 weeks (±0.9, range 38-41 weeks, median 39 weeks) and the mean gestational age at birth for preterm infants was 28.6 weeks (±2.6, range 24-31 weeks, median 29 weeks). High resolution T2-weighted and T1-weighted, MPRAGE MRI scans of the participants in both the preterm and full-term cohorts did not exhibit evidence of defects or lesions in white and/or grey matter.
The mean (SD) age of the mothers of the preterm group was 31.4 years (4.8) and for healthy term controls mean maternal age was 28.2 years (5.7) (P=0.02). Although this mean difference of 3 years is statistically significant, it is not clinically significant in terms of fetal gestation and is unlikely to affect brain development of the fetus. Five mothers of preemies (20.8%) and one mother of a term control infant (2.0%) stated they smoked during pregnancy (NS). Each of these smokers stated they smoked less than a half a pack per day. Two mothers of preemies admitted to using street drugs (8.3%). Both stated they used marijuana only. None of the moms of healthy term controls admitted to using street drugs. One mother of a preterm infant (4.2%) and 11 mothers of term controls (20.6%) stated they drank alcohol during pregnancy (NS). All but one of these mothers said they stopped once they learned they were pregnant; the other mother (of a term control infant) said she drank infrequently during her pregnancy (<2 alcoholic drinks per week).
Our hypothesis that preterm and full-term infants will differ in brain connectivity in specific networks related to motor, language, and executive function during the neonatal period was initially tested using whole brain analysis to identify any brain regions that differed significantly between the groups based on the two-sample t-test described above. Based on the ROI to ROI connectivity analysis we found that preterm infants differed from full-term infants in the expected brain networks. These differences are listed in Table 2 and shown as black circles in Figure 1-3. No other ROIs or networks exhibited significant differences in brain connectivity based on the statistical thresholds defined by our rigorous methodology. All of these regions fall within motor, language and cognitive networks as we hypothesized based on known behavioral outcomes from preterm birth as outlined in the Introduction. No other significant differences in brain connectivity were identified among the 90 ROIs tested. Using ROIs where significant differences were detected as seeds regions in a seed-to-ROI connectivity analysis, we further explored connectivity differences between the groups in each network.
The sensory/motor network exhibited decreased connectivity of the medial and dorsal superior frontal gyrus (SFGmed, SFGdor) (premotor area) (Picard and Strick 2001) bilaterally and left supplementary motor area (SMA) (Goldberg 1985) to other areas of the brain as compared with the term group. The sensory/motor seed ROIs are listed in Table 2 and indicated in black in Figures 1 A-E, with connections that are stronger in the full-term infants than in preterm neonates highlighted in red. Conversely, increased functional connectivity was found in the preterm group relative to the full-term group between the seed region in the left SMA and left olfactory (OLF) and rolandic operculum (ROL) bilaterally; highlighted in blue in Fig 1E.
We also found differences in expressive and semantic language networks between preterm and full-term babies. The specific regional differences we found within the language network are listed in Table 2 and indicated by the black circles in Figure 2. Preterm infants showed decreased connectivity in expressive language regions (Friederici and Gierhan 2013; Vigneau et al. 2006) in the left angular gyrus (ANG) (word reading and comprehension) and right fusiform gyrus (FFG) (object recognition and reading) as compared with term controls; highlighted in red in Fig 2B and 2C. However, increased connectivity in preterm relative to full-term infants was found between the left middle temporal gyrus (MTG) (semantic processing) and hippocampus (HIP) bilaterally and amygdala (AMYG) in the left hemisphere as indicted by the blue circles and lines in Fig 2A. Preterm infants also showed increased connection between right FFG and left thalamus (THA) and posterior cingulate gyrus (PCG) (Fig 2C-blue).
Connectivity within cognitive and executive function networks also differed between the groups of infants as indicated by the black cricles in Figure 3A and 3B. Coordinates of these seed regions are listed in Table 2. Strikingly, we found decreased connectivity for the preterm group between the orbitofrontal cortex (ORB) (cognitive, executive function) (Kringelbach 2005) as well as middle frontal gyrus (MFG) (working memory) (Fan et el. 2005) and multiple brain regions bilaterally (Fig 3 A and B -red). We also found increased connectivity for the preterm group between the right inferior ORB and right superior temporal pole (TPO) and right middle temporal pole (TPO) and between the right medial ORB and left superior TPO (Fig 3B – blue).
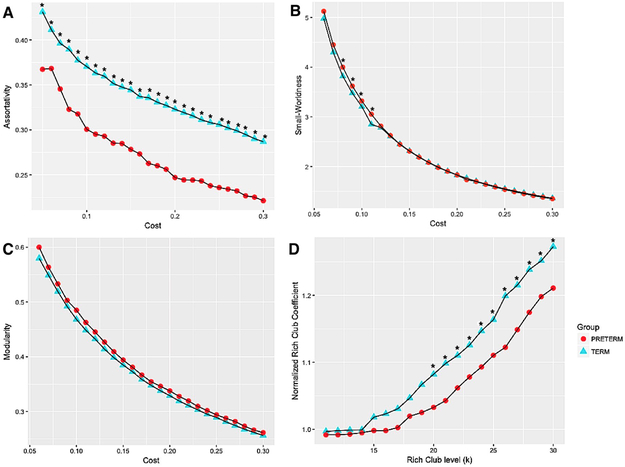
Using graph theory analysis over the entire brain, we also found some significant differences between preterm and full-term infants. Assortativity was significantly decreased in preterm infants (p<0.05) across the full range of cost function values tested (Fig 4A). Differences in small-worldness (σ > 1) were not significant over a range of cost threshold (0.06 to 0.3), but at the cost level of 0.08-0.11, preterm infants showed higher small-worldness (p<0.05) (Fig 4B). Modularity (Q) was not significantly different between preterm and full term infants (Fig 4C) over the entire range of cost levels we tested of 0.06-0.3. Fig 4D shows that preterm infants exhibit characteristic rich-club organization (∅norm(k) > 1) for the range of k = 18 to k = 30 whereas full term infants exhibit characteristic rich-club organization (∅norm(k) > 1) for the range of k = 15 to k = 30. Rich-club organization was significantly reduced in preterm infants for values of the Rich-club level >15 (p<0.05).
Figure 4:
Comparison of global network measures between preterm and full term born infants plotted as a function of the cost threshold or rich club level (k) used to estimate the parameter: A) Small-Worldness, B) Modularity C) Rich-club Coefficient D) Assortativity. (*) indicates the difference is significant at p<0.05 corrected for multiple comparisons using false discovery rate.
Finally, we present region specific group differences in graph theory parameters in Figure 5. By examining all 90 ROIs and all of the topological measures we calculated, we constructed the ball and stick representations shown in Figure 5, of the specific areas where differences occur in the graphical measures between groups. These ROIs are also listed in Table 3. Differences are highlighted showing full-term infants with increased global efficiency and degree in frontal networks (SFGmed, SFGdor, MFG, and superior ORB) (Fig 5A and B-red). Preterm infants exhibit increased global efficiency relative to full term in auditory, language networks (left Heschl’s gyrus-HES, superior temporal gyrus-STG) and primary motor networks (precentral gyrus-PreCG) and increased degree in auditory and language networks (HES, STG) (Fig 5A and B-blue, respectively). Local efficiency and betweenness centrality were not significantly different between preterm and full term infants.
Regression analysis for connectivity measures with gestational age at birth and post-menstrual age at MRI scan did not return any significant influence from these factors on the differences between full-term and preterm babies for either the ROI-to-ROI or whole brain analysis for any of the measures we calculated.
Discussion
The aim of this study was to identify topological differences in brain networks associated with very preterm birth, during the neonatal period of development that exist even in the absence of anatomical lesions and that might lead to the poor outcomes often observed in these children later during infancy and childhood. In particular, we focused on network measures and functional connectivity alterations in regions related to language, motor, cognitive and executive functions because these domains have been shown to be adversely affected in children born preterm as compared to their term counterparts. Alterations in network functional connectivity measures were found in the preterm group in all of the networks we hypothesized. Remarkably, although we began our analysis with a full set of 90 ROIs covering the entire brain and full range of brain networks, differences between the preterm and full-term infants were limited to the hypothesized networks.
Consistent with our hypothesis of decreased network connectivity in preterm infants, we found alterations in neural connectivity for motor regions in preterm infants (Fig 1). Furthermore, we found clear evidence of pervasive decreased connectivity in language and executive function pathways in the brains of very preterm infants at term-equivalent age, as compared to healthy full-term neonates (Figs 2 & 3). However, our data also highlight selected pathways of enhanced connectivity in the preterm group in each of these networks. Understanding why this occurs and how it affects long-term development could provide important insights about the optimal conditions and trajectories for environmental stimulation of preterm infants.
Importantly, we documented decreased functional connectivity from the left ANG and right FFG (Fig 2B & C) in the preterm group. However, the preterm group had increased connectivity from the left MTG and right FFG to several other areas of the brain (Fig 2A & C). Additionally, preterm infants had higher total number of connected links to the left STG than full term infants (Fig 5B). Similar findings were noted in preterm children at school age and during adolescence, with increased connectivity in Wernicke’s area in the left STG (left Brodmann’s area 22) using task-based fMRI (Gozzo et al. 2009; Myers et al. 2010). These data suggest either the engagement of a broader network of phonologic processing of language or a delay of semantic processing of language in preterm infants. Additionally, preterm born adults showed increased whole brain connectivity in left and right temporal parietal language regions (Scheinost et al. 2012). In addition to demonstrating increased connectivity, a recent functional connectivity study showed that infants born preterm showed decreased lateralization of connectivity in left frontal temporal language areas when compared the term born infants (Kwon et al.2015). It is notable that in this study, the connections to reading and comprehension areas of the developing language network in the IFG, ANG and FFG appear to suffer from decreased network connectivity in preterm infants while semantic processing elements of the language network in the MTG seem to exhibit increased functional connectivity in the preterm group relative to the full-term infants. Considering possible explanations for this apparently inconsistent finding we are forced to consider the relative developmental advantage of allocating neural resources to receptive language processing in the newborn infant. Is it possible that enhanced connectivity between receptive language areas of the brain with attention and striatal and motor pathways conveys a developmental advantage in a preterm infant that warrants selective allocation of resources to this pathway? Alternatively, the increased connectivity may simply reflect the impact on brain development of the exposure of preterm infants to types and amounts of increased auditory stimuli that do not match the “expected” stimuli (i.e. in utero environment) for a given GA (Lawrence et al. 2010; Lubsen et al. 2011; Ment et al. 2006; Schafer et al. 2009).
Reduced connectivity was also found between regions related to cognitive and executive functions in the ORB and MFG bilaterally (Fig 3). We believe that these findings provide strong support for previous studies (Smyser et al. 2014, Doria et al. 2010) in which preterm infants demonstrated reduced covariance in fronto-parietal networks suggests that functional connections are less complex in preterm infants with attentional networks forming at different rates with gestation.
Using network measures from graph theory that reflect whole brain functional organization we demonstrated that preterm birth consistently alters network topology globally and that such effects are detectable by term-equivalent age. Comparing preterm and full-term infants at term-equivalent age, preterm infants showed reduced rich-club coefficient, assortativity and no significant difference in modularity (Fig 4A, D &C). Rich-club coefficient identifies the tendency for high degree nodes to be more densely connected among themselves than with other nodes. This is directly consistent with a recent finding that rich-club organization and assortativity lowered in preterm infants but modularity is not significantly different between preterm and full term infants (Scheinost et al. 2015). Although a recent study (Scheinost et al. 2015) suggests reduced clustering coefficient in preterms, small-worldness was increased in preterm infants in our study, indicating increased clustering coefficient. Since network measures are very sensitive to cost threshold, we repeated the analysis across a range of cost thresholds that might effect results. Results remain reliable across a range of cost values. Furthermore, preterm infants had lower global efficiency and degree in frontal regions including premotor and cognitive function areas. However, connectivity within the auditory network appears to be enhanced in the preterm group. For this network, preterm infants exhibited increased global efficiency and degree; findings that are consistent with the seed-based analysis presented in Figs 1-3.
As mentioned previously, a likely explanation for the increased ROI to ROI connectivity in the motor area in preterm infants (Fig 1 – blue) could be the earlier and additional time of exposure to sensorimotor stimulation ex-utero in the preterm group. At the time of MR imaging, preterm infants had been exposed to 9-16 weeks of direct sensory and motor stimulation that was not experienced by full term infants. Hence, it is conceivable that preterm infants formed additional connections to primary auditory and sensorimotor brain regions that are not present in the full-term infants. However, evidence of later speech and language delays as well as sensory and motor impairments in preterm children suggests that this early enrichment of network connections may not be complemented by projections of these areas to secondary brain regions necessary for temporal sequencing, coordination, association and other functions essential to optimal motor and cognitive function.
Increased functional connectivity in preterm relative to full term infants from left but not right SMA could arise from several factors. As noted below, sleep tends to attenuate the BOLD fMRI signal, resulting in decreased functional connectivity. Therefore it is possible that the absence of bilateral differences in SMA connectivity within the motor network could arise from the decreased signal to noise of the fMRI signal due to sleep. Given the prevalence of right hand dominance in humans, it is also possible that left SMA is already becoming more strongly connected to other sensory, motor, visual and other brain regions than the right hemisphere analog. There is some evidence supporting this suggestion in adult subjects (Sarfeld et al. 2012)
The alterations in network characteristics revealed by this study clearly demonstrate that differences in brain networks associated with preterm birth can be observed as early as term-equivalent age. Our results reflect regional and network-based differences in connectivity measures in preterm infants at term-equivalent age. These findings provide a possible explanation for later impairments in sensorimotor, cognitive, and behavioral function commonly seen in children who are born very prematurely (Lawrence et al. 2010; Lubsen et al. 2011; Ment et al. 2006; Schafer et al. 2009; Gozzo et al. 2009). The networks we examined are associated with sensorimotor and cognitive neural systems known to reflect a delay or alteration in maturation in preterm infants. Documentation of the developmental trajectory of the observed network alterations is an important first step towards determining the best way to improve network connectivity and developmental outcomes in terms of timing and methodology (Doria et al. 2010; He and Parikh. 2016; Keunen et al. 2017). As such, these results may inform the development of intervention strategies to minimize the long-term impact of premature birth on sensorimotor and cognitive outcomes. Alternatively, network measures could be further developed and used as early surrogate outcome measures for neonatal neuroprotective interventions (e.g., erythropoietin), rather than waiting for 2+ years for neurodevelopmental outcomes.
It is important to note that the differences in connectivity we have identified between preterm and full-term infants are not due to differences in head motion during the resting state fMRI scans. Differences in motion between groups have been shown to influence estimates of functional connectivity differences from resting state fMRI data (Power et al. 2015; Garrison et al. 2015; Power et al. 2013; Power et al. 2012). Motion effects have been shown to differentially decrease long-range connectivity, including characteristic path length and global efficiency while apparently causing increases in local efficiency and betweenness centrality. In our study, fMRI scans in both groups of infants were performed during natural sleep. When we compared preterm and full-term infants, there were no significant differences in motion (p=0.2839) as indicated by the root mean square (RMS) displacement and also we did not detect a significant effect of motion on the parameter estimates.
There are several limitations of the current study. Given the rapid brain development that occurs during the neonatal period, it is possible that slight differences in the post-menstrual age at the time of the MRI scans could have influenced the findings of connectivity differences between groups (See Table 1). We therefore examined the influence of post-menstrual age, and did not detect a significant effect of post-menstrual age on any of the connectivity parameters. Perhaps the major limitation of this report on brain network alterations during the neonatal period in preterm infants is that longer-term outcome measures are not yet available on the cohorts of infants included in the study. Ideally the participants would be followed longitudinally and sensorimotor and cognitive outcomes would be measured in each child at key developmental age points during preschool, kindergarten and school age years. However, this pilot study was not designed to provide longitudinal outcomes and most participants are still too young to provide reliable cognitive measures.
As noted, all of the resting state fMRI scans for this study were completed while the infants were sleeping. Ethical standards prohibit the use of medication-induced sedation for research involving children, so natural sleep is the only viable condition under which the youngest children can be engaged in fMRI research (Dehaene-Lambertz et al. 2002). Functional brain connectivity during sleep has been studied extensively and the findings are somewhat inconsistent. For example, significant effect of resting state connectivity was detected using EEG as well as FMRI during deep sleep (Dang-Vu et al. 2008) Similarly, some reduction has been observed in functional connection within default network during deep sleep (Horovitz et al. 2008). On the other hand, a recent work has shown that functional connectivity within attention, default and executive networks and primary sensory networks was maintained from wakefulness to light sleep (Larson-Prior et al. 2009). A recent study in infants has reported no significant differences between functional connectivity in deep sleep and sedation (Doria et al. 2010). In our experience sleep and sedation both have the impact of attenuating the BOLD fMRI signal itself as well as selectively influencing connectivity in specific brain networks mediating attention and consciousness. (Wilke et al. 2003; DiFrancesco et al. 2013) However, we have also demonstrated that it is feasible to obtain robust and meaningful brain activation maps from sleeping and/or sedated infants and toddlers. (Patel et al. 2007; Tan et al. 2013; Deshpande et al. 2016; Merhar et al. 2016)
Recently Laumann et al. have concluded that “resting-state BOLD correlations do not primarily reflect moment-to-moment changes in cognitive content. Rather, resting-state BOLD correlations may predominantly reflect processes concerned with the maintenance of the long-term stability of the brain's functional organization” (Laumann et al. 2016) Given this background and remaining uncertainty, we emerge with some confidence that connectivity within the predefined networks we have selected for this study remain somewhat intact during natural sleep, with some level of attenuation. Sleep is likely to reduce the significance of ROI-ROI correlations and resulting functional connectivity and differences between groups. However, this suggests that our results would be stronger in a wakeful state if that could be achieved in this young cohort. Note too that although sleep is likely to alter BOLD fMRI signal and spatial correlations underlying functional connectivity measures, in our study both the pre-term and full term infants were asleep. Therefore differences between brain networks that emerge between cohorts are not likely to be due to the influence of sleep since both groups were in a similar state of consciousness.
Finally, the sample size for the preterm cohort of 24 infants is relatively small. This limits the significance of some findings. With a larger sample size, we would expect to find more pervasive differences in brain networks and additional graph measures that exhibit significant differences on a smaller scale. However, the current study provides evidence that such network alterations exists during the earliest stages of development and therefore provide important support for further exploration of graph theory measures as predictive biomarkers.
In conclusion, we found that resting-state networks are already well established early in development in very preterm neonates, but that functional connectivity and network characteristics in preterm infants differ from those of full-term infants by term-equivalent age. Using functional connectivity analysis and graph theory, we also demonstrated significant differences in key brain networks between preterm and full-term infants, even at this early stage in development. In future studies we plan to examine long-term neurodevelopmental outcomes in preterm infants to validate early functional connectivity and network measures as early prognostic biomarkers for preterm birth outcomes.
Acknowledgments
Funding
This study was funded in part by the Research Institute at Nationwide Children’s and the National Institutes of Neurological Disorders and Stroke under NIH grant 5R01-NS094200 to PI: Nehal Parikh, DO, MS.
Footnotes
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Nationwide Children’s Hospital (Columbus, Ohio, USA) Institutional Review Board approved this study and written parental informed consent was obtained for every subject prior to imaging.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E (2006) A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. Journal of Neuroscience 26 (1):63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, Schneider KC, Gore JC, Ment LR (2001) Neonatal auditory activation detected by functional magnetic resonance imaging. Magnetic resonance imaging, 19:1–5 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37 (1):90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botting N, Powls A, Cooke RW, Marlow N (1998) Cognitive and educational outcome of very-low-birthweight children in early adolescence. Dev Med Child Neurol 40 (10):652–660 [DOI] [PubMed] [Google Scholar]
- Breeman LD, Jaekel J, Baumann N, Bartmann P, Wolke D (2015) Preterm cognitive function into adulthood. Pediatrics 136 (3):415–423 [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience 10 (3):186–198 [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2012) The economy of brain network organization. Nature Reviews Neuroscience 13 (5):336. [DOI] [PubMed] [Google Scholar]
- Burnett AC, Scratch SE, Lee KJ, Cheong J, Searle K, Hutchinson E, De Luca C, Davey M-A, Roberts G, Doyle LW (2015) Executive Function in Adolescents Born< 1000 g or< 28 Weeks: A Prospective Cohort Study. Pediatrics 135 (4):e826–e834 [DOI] [PubMed] [Google Scholar]
- Cao M, Wang J-H, Dai Z-J, Cao X-Y, Jiang L-L, Fan F-M, Song X-W, Xia M-R, Shu N, Dong Q (2014) Topological organization of the human brain functional connectome across the lifespan. Developmental cognitive neuroscience 7:76–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Öngür D, Whitfield-Gabrieli S (2012) Anticorrelations in resting state networks without global signal regression. Neuroimage 59 (2):1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP (2013) Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 81 (24):2082–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheour M, Imada T, Taulu S, Ahonen A, Salonen J, Kuhl P (2004) Magnetoencephalography is feasible for infant assessment of auditory discrimination. Experimental Neurology, 190:44–51 [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G (2008) Spontaneous neural activity during human slow wave sleep. Proceedings of the National Academy of Sciences 105 (39):15160–15165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kieviet JF, van Elburg RM, Lafeber HN, Oosterlaan J (2012) Attention problems of very preterm children compared with age-matched term controls at school-age. The Journal of pediatrics 161 (5):824–829. doi: 10.1016/j.jpeds.2012.05.010 [DOI] [PubMed] [Google Scholar]
- Degnan AJ, Wisnowski JL, Choi S, Ceschin R, Bhushan C, Leahy RM, Corby P, Schmithorst VJ, Panigrahy A (2015) Alterations of resting state networks and structural connectivity in relation to the prefrontal and anterior cingulate cortices in late prematurity. Neuroreport 26 (1):22–26 [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L (2002) Functional neuroimaging of speech perception in infants. science, 298:2013–2015 [DOI] [PubMed] [Google Scholar]
- Deshpande AK, Tan L, Lu LJ, Altaye M, Holland SK (2016) fMRI as a Preimplant Objective Tool to Predict Postimplant Oral Language Outcomes in Children with Cochlear Implants. Ear and hearing, 37:e263–e272 [DOI] [PubMed] [Google Scholar]
- DiFrancesco MW, Robertson SA, Karunanayaka P, Holland SK (2013) BOLD fMRI in infants under sedation: comparing the impact of pentobarbital and propofol on auditory and language activation. Journal of Magnetic Resonance Imaging, 38:1184–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, Craddock RC, Luna B, Leventhal BL, Zuo X-N (2014) Unraveling the miswired connectome: a developmental perspective. Neuron 83 (6):1335–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG (2010) Emergence of resting state networks in the preterm human brain. Proceedings of the National Academy of Sciences 107 (46):20015–20020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgin JO, Inder TE, Anderson PJ, Hood KM, Clark CA, Woodward LJ (2008) Executive functioning in preschool children born very preterm: relationship with early white matter pathology. Journal of the International Neuropsychological Society 14 (01):90–101 [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI (2005) The activation of attentional networks. Neuroimage 26 (2):471–479 [DOI] [PubMed] [Google Scholar]
- Fischi-Gómez E, Vasung L, Meskaldji D-E, Lazeyras F, Borradori-Tolsa C, Hagmann P, Barisnikov K, Thiran J-P, Hüppi PS (2015) Structural brain connectivity in school-age preterm infants provides evidence for impaired networks relevant for higher order cognitive skills and social cognition. Cerebral cortex 25 (9):2793–2805 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America 102 (27):9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster-Cohen SH, Friesen MD, Champion PR, Woodward LJ (2010) High prevalence/low severity language delay in preschool children born very preterm. Journal of Developmental & Behavioral Pediatrics 31 (8):658–667 [DOI] [PubMed] [Google Scholar]
- Freeman LC (1978) Centrality in social networks conceptual clarification. Social networks 1 (3):215–239 [Google Scholar]
- Friederici AD, Gierhan SM (2013) The language network. Current Opinion in Neurobiology 23 (2):250–254 [DOI] [PubMed] [Google Scholar]
- Garrison KA, Scheinost D, Finn ES, Shen X, Constable RT (2015) The (in) stability of functional brain network measures across thresholds. Neuroimage 118:651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G (1985) Supplementary motor area structure and function: review and hypotheses. Behavioral and brain Sciences 8 (4):567–588 [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, Schneider KC, Peterson BS, Rajeevan N, Makuch RW (2009) Alterations in neural connectivity in preterm children at school age. Neuroimage 48 (2):458–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M (2009) Adult outcomes of preterm children. Journal of Developmental & Behavioral Pediatrics 30 (5):460–470 [DOI] [PubMed] [Google Scholar]
- He L, Parikh NA (2016) Brain functional network connectivity development in very preterm infants: The first six months. Early Human Development 98:29–35 [DOI] [PubMed] [Google Scholar]
- Holland S, Choo D, Ret J, Hilbert L, Dunn R, Schmithorst V (2004) fMRI of severe to profoundly hearing-impaired infants and toddlers under sedation In: International Congress Series. Elsevier, pp 383–386. [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema J-M, Karunanayaka PR, Schmithorst VJ, Yuan W, Plante E, Byars AW (2007) Functional MRI of language lateralization during development in children. International journal of audiology, 46:533–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH (2008) Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG‐fMRI study. Human brain mapping 29 (6):671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanayaka PR, Holland SK, Schmithorst VJ, Solodkin A, Chen EE, Szaflarski JP, Plante E (2007) Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage, 34:349–360 [DOI] [PubMed] [Google Scholar]
- Keunen K, Counsell SJ, Benders MJ (2017) The emergence of functional architecture during early brain development. NeuroImage [DOI] [PubMed] [Google Scholar]
- Kerr-Wilson C, Mackay D, Smith G, Pell J (2012) Meta-analysis of the association between preterm delivery and intelligence. Journal of public health 34 (2):209–216 [DOI] [PubMed] [Google Scholar]
- Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience 6 (9):691. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Scheinost D, Lacadie C, Sze G, Schneider KC, Dai F, Constable RT, Ment LR (2015) Adaptive mechanisms of developing brain: Cerebral lateralization in the prematurely-born. NeuroImage 108:144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME (2009) Cortical network functional connectivity in the descent to sleep. Proceedings of the National Academy of Sciences 106 (11):4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M (2001) Efficient behavior of small-world networks. Physical review letters 87 (19):198701. [DOI] [PubMed] [Google Scholar]
- Laumann TO, Snyder AZ, Mitra A, Gordon EM, Gratton C, Adeyemo B, Gilmore AW, Nelson SM, Berg JJ, Greene DJ (2016) On the stability of BOLD fMRI correlations. Cerebral cortex 27(10):4719–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence EJ, McGuire PK, Allin M, Walshe M, Giampietro V, Murray RM, Rifkin L, Nosarti C (2010) The very preterm brain in young adulthood: the neural correlates of verbal paired associate learning. The Journal of pediatrics 156 (6):889–895 [DOI] [PubMed] [Google Scholar]
- Lee JD, Park H-J, Park ES, Oh M-K, Park B, Rha D-W, Cho S-R, Kim EY, Park JY, Kim CH (2011) Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain 134 (4):1199–1210 [DOI] [PubMed] [Google Scholar]
- Liu Y, Balériaux D, Kavec M, Metens T, Absil J, Denolin V, Pardou A, Avni F, Van Bogaert P, Aeby A (2010) Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: a diffusion tensor imaging and probabilistic tractography study. Neuroimage 51 (2):783–788 [DOI] [PubMed] [Google Scholar]
- Lo C-Y, Wang P-N, Chou K-H, Wang J, He Y, Lin C-P (2010) Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer’s disease. Journal of Neuroscience 30 (50):16876–16885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubsen J, Vohr B, Myers E, Hampson M, Lacadie C, Schneider KC, Katz KH, Constable RT, Ment LR (2011) Microstructural and functional connectivity in the developing preterm brain In: Seminars in perinatology,. 1 Elsevier, pp 34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malviya S, Voepel-Lewis T, Tait AR (2006) A comparison of observational and objective measures to differentiate depth of sedation in children from birth to 18 years of age. Anesthesia & Analgesia, 102:389–394 [DOI] [PubMed] [Google Scholar]
- Malviya S, Voepel-Lewis T, Tait AR, Watcha MF, Sadhasivam S, Friesen RH (2007) Effect of age and sedative agent on the accuracy of bispectral index in detecting depth of sedation in children. Pediatrics, 120:e461–e470. [DOI] [PubMed] [Google Scholar]
- Månsson J, Stjernqvist K (2014) Children born extremely preterm show significant lower cognitive, language and motor function levels compared with children born at term, as measured by the Bayley‐III at 2.5 years. Acta Paediatrica 103 (5):504–511 [DOI] [PubMed] [Google Scholar]
- Ment LR, Peterson BS, Vohr B, Allan W, Schneider KC, Lacadie C, Katz KH, Maller-Kesselman J, Pugh K, Duncan CC (2006) Cortical recruitment patterns in children born prematurely compared with control subjects during a passive listening functional magnetic resonance imaging task. The Journal of pediatrics 149 (4):490–498. e492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhar SL, Gozdas E, Tkach JA, Harpster KL, Schwartz TL, Yuan W, Kline-Fath BM, Leach JL, Altaye M, Holland SK (2016) Functional and structural connectivity of the visual system in infants with perinatal brain injury. Pediatric research, 80:43. [DOI] [PubMed] [Google Scholar]
- Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, Marlow N (2012) Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. Bmj 345:e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009) The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44 (3):893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers EH, Hampson M, Vohr B, Lacadie C, Frost SJ, Pugh KR, Katz KH, Schneider KC, Makuch RW, Constable RT (2010) Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage 51 (4):1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME (2006) Modularity and community structure in networks. Proceedings of the national academy of sciences 103 (23):8577–8582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME (2002) Assortative mixing in networks. Physical review letters 89 (20):208701. [DOI] [PubMed] [Google Scholar]
- Parikh NA (2016) Advanced neuroimaging and its role in predicting neurodevelopmental outcomes in very preterm infants In: Seminars in Perinatology. vol 8 Elsevier, pp 530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AM, Cahill LD, Ret J, Schmithorst V, Choo D, Holland S (2007) Functional magnetic resonance imaging of hearing-impaired children under sedation before cochlear implantation. Archives of Otolaryngology–Head & Neck Surgery, 133:677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL (2001) Imaging the premotor areas. Current opinion in neurobiology 11 (6):663–672 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59 (3):2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2013) Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE (2015) Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105:536–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Kennedy DP, Courchesne E (2007) fMRI during natural sleep as a method to study brain function during early childhood. Neuroimage, 38:696–707 [DOI] [PubMed] [Google Scholar]
- Rogers CE, Sylvester CM, Mintz C, Kenley JK, Shimony JS, Barch DM, Smyser CD (2017) Neonatal Amygdala Functional Connectivity at Rest in Healthy and Preterm Infants and Early Internalizing Symptoms. Journal of the American Academy of Child & Adolescent Psychiatry 56 (2):157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands MA, Scheinost D, Lacadie C, Vohr B, Li F, Schneider KC, Constable RT, Ment LR (2016) Language at rest: A longitudinal study of intrinsic functional connectivity in preterm children. NeuroImage: Clinical 11:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E (2005) Neurophysiological architecture of functional magnetic resonance images of human brain. Cerebral cortex 15 (9):1332–1342 [DOI] [PubMed] [Google Scholar]
- Sarfeld AS, Diekhoff S, Wang LE, Liuzzi G, Uludağ K, Eickhoff SB, Fink GR, Grefkes C (2012) Convergence of human brain mapping tools: neuronavigated TMS parameters and fMRI activity in the hand motor area. Human brain mapping, 33:1107–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, Pugh KR, Makuch RW, Reiss AL, Constable RT (2009) Alterations in functional connectivity for language in prematurely born adolescents. Brain 132 (3):661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Benjamin J, Lacadie C, Vohr B, Schneider KC, Ment LR, Papademetris X, Constable RT (2012) The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity. Neuroimage 62 (3):1510–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Shen X, Lacadie C, Schneider KC, Dai F, Ment LR, Constable RT (2015) Preterm birth alters neonatal, functional rich club organization. Brain Structure and Function:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E (2006) Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. Neuroimage, 29:254–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Fan Y, Tang S, Gilmore JH, Lin W, Shen D (2010) Neonatal brain image segmentation in longitudinal MRI studies. Neuroimage 49 (1):391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap P-T, Fan Y, Gilmore JH, Lin W, Shen D (2010) Construction of multi-region-multi-reference atlases for neonatal brain MRI segmentation. Neuroimage 51 (2):684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap P-T, Wu G, Jia H, Gilmore JH, Lin W, Shen D (2011) Infant brain atlases from neonates to 1-and 2-year-olds. PloS one 6 (4):e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ (2010) Longitudinal analysis of neural network development in preterm infants. Cerebral cortex:bhq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ (2014) Resting-state network complexity and magnitude are reduced in prematurely born infants. Cerebral cortex:bhu251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synnes A, Anderson PJ, Grunau RE, Dewey D, Moddemann D, Tin W, Davis PG, Doyle LW, Foster G, Khairy M (2015) Predicting severe motor impairment in preterm children at age 5 years. Archives of disease in childhood:archdischild-2014–307695 [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Altaye M, Rajagopal A, Eaton K, Meng X, Plante E, Holland SK (2012) A 10-year longitudinal fMRI study of narrative comprehension in children and adolescents. Neuroimage, 63:1188–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Chen Y, Maloney TC, Caré MM, Holland SK, Lu LJ (2013) Combined analysis of sMRI and fMRI imaging data provides accurate disease markers for hearing impairment. NeuroImage: Clinical, 3:416–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. Journal of Neuroscience 31 (44):15775–15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goñi J, Sporns O (2012) High-cost, high-capacity backbone for global brain communication. Proceedings of the National Academy of Sciences 109 (28):11372–11377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest J, Rajagopal A, Cicchino ND, Franks-Henry J, Simpson SM, Lee G, Altaye M, Sroka C, Holland SK, Consortium CA (2014) Factors determining success of awake and asleep magnetic resonance imaging scans in nonsedated children. Neuropediatrics, 45:370–377. [DOI] [PubMed] [Google Scholar]
- Vannest JJ, Karunanayaka PR, Altaye M, Schmithorst VJ, Plante EM, Eaton KJ, Rasmussen JM, Holland SK (2009) Comparison of fMRI data from passive listening and active‐response story processing tasks in children. Journal of Magnetic Resonance Imaging, 29:971–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Hooft J, van der Lee JH, Opmeer BC, Aarnoudse-Moens CS, Leenders AG, Mol BWJ, de Haan TR (2015) Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Systematic reviews 4 (1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve P-Y, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N (2006) Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30 (4):1414–1432 [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology 8 (1):110–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH (1998) Collective dynamics of ‘small-world’networks. nature 393 (6684):440–442 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity 2 (3):125–141 [DOI] [PubMed] [Google Scholar]
- Wilke M, Hauser TK, Krägeloh‐Mann I, Lidzba K (2014) Specific impairment of functional connectivity between language regions in former early preterms. Human brain mapping 35 (7):3372–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Ball WS (2003) Language processing during natural sleep in a 6-year-old boy, as assessed with functional MR imaging. American journal of neuroradiology, 24:42–44 [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Moor S, Hood KM, Champion PR, Foster-Cohen S, Inder TE, Austin NC (2009) Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Archives of Disease in Childhood-Fetal and Neonatal Edition 94 (5):339–344 [DOI] [PubMed] [Google Scholar]