Introduction
Background
Pheochromocytoma is a tumor of the catecholamine-producing cells of the adrenal medulla. The incidence is estimated to be 1–2 cases per 100 000 individuals and they make up approximately 5% of incidental adrenal masses.1
Classical symptoms of pheochromocytomas include headache, episodic perspiration, tachycardia, flushing, nausea, and hypertension, although many tumors can be asymptomatic.1 These tumors can be sporadic or hereditary. 2 Familial cases account for up to 30% of tumors.3 Pheochromocytomas can also occur outside the adrenal gland, known as paragangliomas, in up to 25% of cases.4
About 10% of pheochromocytomas are malignant.5 Although there are several clinical and genetic factors associated with an increased risk of malignancy, at this time, there are no molecular, cellular, or histological criteria that can reliably differentiate benign from malignant disease.5 Therefore, malignancy is defined by the presence of clinical metastases. The most common sites for metastases are lymph nodes, bones, liver, and lungs.6
Evaluation of suspected pheochromocytoma begins with confirming a biochemical disturbance by measuring plasma-free metanephrines or urinary fractionated metanephrines, followed by computed tomography (CT) imaging. Once a pheochromocytoma is confirmed, complete surgical resection of the tumor is advised, preferably via laparoscopic or robot-assisted adrenalectomy. The full details regarding workup and treatment of pheochromocytomas is beyond the scope of this review, but there are recent published guidelines on this topic.7
Following surgery, patients are at risk for tumor persistence and recurrence. Despite an overall good prognosis, the disease can recur in up to 16% of patients within 10 years following surgery.8,9 Recurrences may be local or metastatic and have been reported up to 53 years post-initial resection, making long-term followup essential.10 Extra-adrenal disease, hereditary pheochromocytomas, right-sided tumors, bilateral tumors, and larger tumors are thought to be risk factors for recurrence. Currently, there is no consensus on the proper methodology for followup. There have been no randomized studies addressing optimal followup nor prospective registries to provide higher-quality evidence for this issue. Important clinical questions remain regarding duration of followup and which tests should be used to detect and monitor recurrences.
Objective
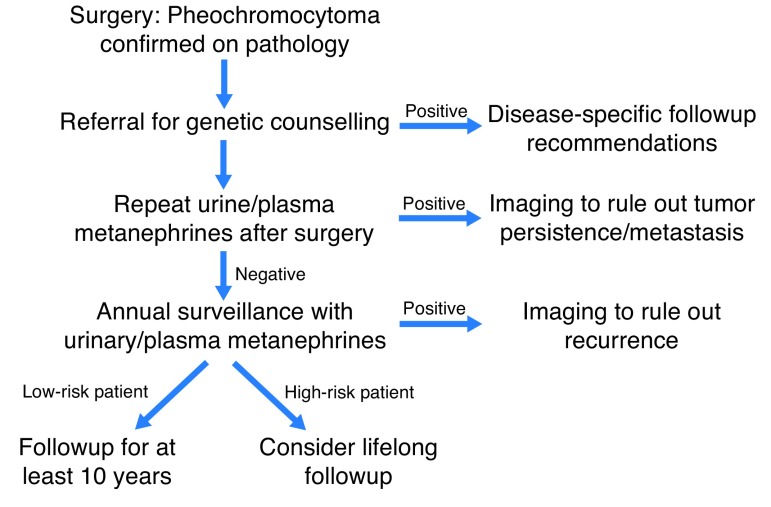
This Best Practice Report (BPR) aims to standardize clinical care regarding the long-term surveillance following surgery for pheochromocytoma, specifically with respect to the duration and monitoring methods (Fig. 1).
Fig. 1.
Algorithm for followup of pheochromocytoma.
Methodology
This BPR was developed using methodology consistent with GRADE. We limited our evidence synthesis to previously published studies examining the long-term followup of surgical patients with pheochromocytoma using PubMed, Medline, and the Cochrane Library database. The bibliographies of relevant articles were searched to avoid exclusion of meaningful articles. In this narrative review, focus was given to systematic reviews, related guidelines, and comparative studies. In particular, a freely accessible clinical practice guideline on this topic was published in 2016 by the European Society of Endocrinology. This guideline addressed similar subject matter and scope that is of interest to the Canadian urology community. Therefore, recommendations from this guideline were endorsed or adapted to a Canadian urology context. Of the 11 recommendations from the European Society of Endocrinology, two were excluded, as they were deemed not applicable and beyond the scope of our review; four were adopted with modifications to better suit a Canadian urologist’s context; and five were adopted without modification. Other statements were based on a systematic review of the literature and one clinical principle was added.
Recommendations
Genetic testing
Pheochromocytomas and paragangliomas (PPGLs) carry a higher degree of heritability than most solid tumors. In the past 15 years, germline mutations in a dozen genes have been identified and it is estimated that approximately 40% of patients carry a causal germline mutation. Hereditary disease linked with pheochromocytomas include neurofibromatosis type 1 (caused by mutations in NF1), multiple endocrine neoplasia type 2 (MEN2; linked with mutations in RET), von Hippel-Lindau disease (associated with mutations in VHL), hereditary paraganglioma (caused by mutations in the SDHx group of genes [SDHA, SDHB, SDHC, SDHD, and SDHAF2]), familial pheochromocytoma (caused by mutations in TMEM127 or MAX), polycythemia paraganglioma syndrome (associated with mutations in EPAS1 [also known as HIF2A]) or hereditary leiomyomatosis and renal cell cancer syndrome (linked with mutations in FH).11
Identification of an underlying mutation is critical in guiding patient management and genetic counselling. For example, patients with mutations in SDHB can develop particularly aggressive and rapidly progressing pheochromocytomas/paragangliomas, as well as other renal cancers and gastrointestinal stromal tumours.11 Furthermore, identification of a hereditary syndrome allows for earlier diagnosis and treatment of pheochromocytomas and other syndromic manifestations in relatives.
Recommendation 1: We recommend all patients with PPGLs be considered for referral for genetic testing (Strong recommendation, Moderate-quality evidence).
Perioperative workup
Traditionally, biochemical testing for pheochromocytomas was done by measurements of urine cathecolamines, often in conjunction with catecholamine metabolites, such as vanillylmandelic acid and metanephrines. Advances in assay technology and our understanding of catecholamine metabolism have led to a newer emphasis on measurements of urine and plasma metanephrines, including normetanephrine and metanephrine, as well as methoxytyramine, a dopamine metabolite. Compared to cathecolamines, measurement of metanephrines is advantageous in detecting tumors that release catecholamines, episodically in low amounts, and they are a more specific marker for catecholamine produced in chromaffin cells and associated tumors.12
Lenders et al provided early evidence that plasma-free metanephrines had a superior diagnostic sensitivity and equivalent specificity compared to the other tests.13 This was supported by a larger National Institutes of Health multi-center cohort study published in 2002 involving over 800 patients, which compared plasma metanephrines vs. plasma catecholamines, urinary cathetholamines, urinary total and fractionated metanephrines, and urinary vanillylmandelic acid.14 They reported a sensitivity of 99% for plasma-free metanephrines vs. a sensitivity of 64–97% for the other biochemical tests. The specificity of plasma-free metanephrines was 89%. Given these findings, they concluded that plasma-free metanephrines provided the best test for excluding or confirming pheochromocytoma. There have been multiple subsequent studies confirming the high diagnostic accuracy for plasma-free metanephrines.
In 2007, Perry et al showed that urine fractionated metanephrines measured by mass spectrometry provided a sensitivity of 97% and specificity of 91% for the diagnosis of pheochromocytomas and paragangliomas, results comparable to those reported of plasma metanephrines in other studies.15 There has not been a direct comparison of plasma metanephrines vs. urinary fractionated metanephrines measured by mass spectrometry, and thus it remains unclear if one test is superior to the other. It is important to highlight that these studies have been done in the diagnostic phase (i.e., pre-resection) and have been extrapolated to be accurate in the setting of monitoring for disease recurrence.
In patients with elevated metanephrines preoperatively, metanephrine determinations should be repeated postoperatively to document complete resection of the tumor. When the postoperative metanephrines fail to normalize, persistent disease should be strongly suspected. Further imaging testing to confirm and locate residual catecholamine-secreting tissue should be undertaken.
Recommendation 2: We suggest repeating plasma and/or 24-hour urinary metanephrines at first postoperative followup to ensure complete resection (Strong recommendation, Low-quality evidence).
Recommendation 3: We suggest monitoring for PPGL recurrence by annually measuring plasma-free metanephrines and/or 24-hour urinary fractionated metanephrines (Strong recommendation, Low-quality evidence).
Duration of followup
In a recent systematic review, Amar et al studied the incidence and factors associated with recurrence or new tumors after complete resection of pheochromocytomas.16 They included 38 studies from 1980–2012 on patients with pheochromocytomas who had complete resection and at least one month of followup. The incidence rates from individual studies were pooled in a meta-analysis.
Of the 38 studies included, there was one randomized control trial and one prospective cohort study, with the rest being retrospective cohorts. There was a total of 2509 patients. The median age of patients was 42 years and median tumor size was 48 mm. The duration of followup ranged from 14–180 months, with a median of 84 months.16
The overall rate of recurrent disease was calculated as 0.95 events/100 person-years (95% confidence interval [CI] 0.68, 1.21), which equates to a five-year cumulative incidence of 4.7%. Of these new events, 22% were new tumors, 23% were local recurrences, and 55% were metastases. The median time from surgery to recurrent disease was 60 months, and ranged from 3–204 months.16
Factors associated with recurrence were also studied. Familial disease was identified as as a main independent risk factors of recurrent disease. The presence of extra-adrenal disease (paraganglioma) was a strong predictor, and younger age and large tumor size were weak predictors of recurrence.16
The European Society of Endocrinology guideline reported results from the European Network for the Study of Adrenal Tumours (ENS@T) database comprised of 1153 patients from six centers.17 A total of 701 patients had complete resection of the primary tumor and documented followup of at least six months. In this database, 34% had a genetic or syndromic disease, median age was 46 years, and median tumor size was 44 mm. Median followup was 54 months.
The risk of recurrence was 10 % over the first five years of followup (new tumors 42%, local recurrences 13%, and metastatic recurrences 45%). The incidence of new events did not decline after five years of followup, but estimates after 10 years of followup were imprecise due to the small numbers of patients for whom data were available. Again, extra-adrenal disease, age <20 years, familial pheochromocytoma, and tumor size >150 mm appeared to correlate with an increased risk of recurrence.17
In both these studies, the incidence of new events is lower than previous estimates. Even after long and uneventful followup, there can be recurrent disease. This appears more common in cases of familial and extra-adrenal disease. There is no tumor size below which there is no risk of a new event nor is there any subgroup in which followup may be safely abandoned.
Recommendation 4: We suggest annual followup for at least 10 years following complete resection to monitor for local or metastatic recurrences or new tumors (Weak recommendation, Very low-quality evidence).
Recommendation 5: We suggest high-risk patients (young, genetic disease, larger tumor and/or a paraganglioma) be offered lifelong annual followup (Weak recommendation, Very low-quality evidence).
Specific conditions
Mild or borderline elevations in metanephrines
Some patients only have a mild or moderate elevation in free plasma metanephrines levels (less than fourfold increase) preoperatively. In this subset, secondary confirmatory testing can be pursued, often facilitated by an endocrinology referral. These include clonidine suppression testing and chromogranin A testing.
Clonidine suppresses norepinephrine production by the sympathetic nervous system but not by pheochromocytomas. Determining normetanephrine levels before and after clonidine administration can help confirm the diagnosis in equivocal cases.18
Chromogranin A determination exists in secretory vesicles of the neuroendocrine and nervous systems and has been shown to be elevated in patients with pheochromocytoma. Determining preoperative chromogranin A levels can help confirm the diagnosis, and in such cases, can also be used for annual postoperative surveillance.19
Recommendation 6: We suggest using annual clonidine suppression or chromogranin A testing for followup of patients with positive preoperative results (Weak recommendation, Very low-quality evidence).
Biochemically negative disease
Pheochromocytomas can rarely be biochemically negative. This appears to occur most frequently in cases of extra-adrenal disease located in the skull base/neck and secondary to mutations in SDHx.20 Evidence suggests these tumors lack the biosynthetic machinery necessary for catecholamine production. In such cases, imaging studies are the principal means of detecting tumors. Such patients remain at risk for the development of biochemically active tumors after initial tumor resection.21
Recommendation 7: We suggest imaging tests be obtained every 1–2 years, in addition to yearly metanephrines for patients with biochemically negative disease (Weak recommendation, Very low-quality evidence).
Imaging
CT and magnetic resonance imaging (MRI) are excellent imaging modalities for characterizing adrenal lesions. Imaging should be sought in patients with elevated metanephrines postoperatively, as well as patients whose metanephrines were normal or not measured preoperatively. A subset of patients with elevated postoperative metanephrines will have no detectable disease on CT or MRI. Historically, metaiodobenzylguanidine (MIBG) scintigraphy has been the preferred test in this setting to localize disease. MIBG is a small-molecule analog of norepinephrine, and when tagged with iodine, is a highly sensitive and specific test for pheochromocytoma.4 More recently, fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET) has emerged for definitive staging in patients with pheochromocytoma. In a study of over 200 patients with adrenal and extra-adrenal pheochromocytoma, 18F-FDG PET had superior test characteristics compared to CT, MRI, and MIBG scintigraphy for almost all patients.22 18F-FDG PET, however, is not widely available and is limited due to cost. The specific indications for each imaging test are beyond the scope of this review but can be found in the 2014 Endocrine Society Clinical Practice Guideline on pheochromocytomas and paragangliomas.7
Recommendation 8: For biochemical recurrence, we suggest CT/MRI as first-line imaging modalities, and 123 I-metaiodobenzylguanidine (MIBG) scintigraphy as second-line (Weak recommendation, Very low-quality evidence).
Malignant pheochromocytoma
Malignant pheochromocytomas are rare. They are associated with significant morbidity due their ability to invade organs and dysregulate the autonomic nervous system.23 Given its rarity, there are few prospective studies investigating potential therapies for the disease, and impact on survival and quality of life is difficult to ascertain. Management is largely guided by retrospective studies’ findings, expert consensus, and clinical experience. Therapies that have been pursued, include surgery, therapeutic 131I-labeled MIBG internal radiotherapy, chemotherapy, targeted therapies, and watchful waiting.23
Recommendation 9: Malignant pheochromocytoma treatment should be discussed in a multidisciplinary setting that includes surgeons, interventional radiologists, endocrinologists, oncologists, and nuclear medicine physicians (Clinical principle).
Conclusions
The risk of recurrence following complete resection of pheochromocytoma is low but significant. Recurrences can occur many years after surgery and are seen more frequently in patients with a genetic disease, young age, large tumor, and extra-adrenal disease. All patients should be considered for genetic testing. Patients should be screened with plasma and urinary metanephrines postoperatively, and yearly thereafter for at least 10 years, and potentially indefinitely for higher-risk patients.
A summary of all BPR recommendations is shown in Table 1.
Table 1.
Summary of recommendations
| Recommendation | Strength of recommendation | Quality of evidence | |
|---|---|---|---|
| 1 | We recommend all patients with PPGLs be considered for referral for genetic testing | Strong | Moderate |
| 2 | We suggest repeating plasma and/or 24-hour urinary metanephrines at first postoperative followup to ensure complete resection. | Strong | Low |
| 3 | We suggest monitoring for PPGL recurrence by annually measuring plasma free metanephrines and/or 24-hour urinary fractionated metanephrines. | Strong | Low |
| 4 | We suggest annual followup for at least 10 years following complete resection to monitor for local or metastatic recurrences or new tumors. | Weak | Very low |
| 5 | We suggest high-risk patients (young, genetic disease, larger tumor, and/or a paraganglioma) be offered lifelong annual followup. | Weak | Very low |
| 6 | We suggest using annual clonidine suppression or chromogranin A testing for followup of patients with positive preoperative results. | Weak | Very low |
| 7 | We suggest imaging tests be obtained every 1–2 years, in addition to yearly metanephrines for patients with biochemically negative disease. | Weak | Very low |
| 8 | For biochemical recurrence, we suggest CT/MRI as first-line imaging modalities, and 123I-metaiodobenzylguanidine (MIBG) scintigraphy as second-line. | Weak | Very low |
| 9 | Malignant pheochromocytoma treatment should be discussed in a multidisciplinary setting including surgeons, interventional radiologists, endocrinologists, oncologists, and nuclear medicine physicians. | Clinical principle | |
CT: computed tomography; MRI magnetic resonance imaging; PPGL: pheochromocytomas and paragangliomas.
Footnotes
Competing interests: Dr. Violette has been a speakers’ bureau member for Janssen and Sanofi (no honoraria). Dr. Tomiak has received speaker honoraria from AstraZeneca. Dr. Izard has received advisory board and consulting fees from Abbvie, Astellas, Ferring, Janssen, and Sanofi; and has participated in clinical trials supported by Astellas, AstraZeneca and Merck. Dr. Rowe has participated in an advisory board meeting for Acerus and has received honoraria from Sanofi. The remaining authors report no competing personal or financial interests related to this work.
Prior to publication, this BPR underwent review by the CUA Guidelines Committee, CUA members at large, the CUAJ Editorial Board, and the CUA Executive Board.
References
- 1.Bravo EL, Tagle R. Pheochromocytoma: State-of-the-art and future prospects. Endocr Rev. 2003;24:539–53. doi: 10.1210/er.2002-0013. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura E, Kaelin WG., Jr Recent insights into the molecular pathogenesis of pheochromocytoma and paraganglioma. Endocr Pathol. 2006;17:97–106. doi: 10.1385/EP:17:2:97. [DOI] [PubMed] [Google Scholar]
- 3.Benn DE, Robinson BG. Genetic basis of pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2006;20:435–50. doi: 10.1016/j.beem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Ilias I, Pacak K. Current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. J Clin Endocrinol Metab. 2004;89:479–91. doi: 10.1210/jc.2003-031091. [DOI] [PubMed] [Google Scholar]
- 5.Lenders JW, Eisenhofer G, Mannelli M, et al. Pheochromocytoma. Lancet. 2005;366:665–75. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 6.Scholz T, Eisenhofer G, Pacak K, et al. Current treatment of malignant pheochromocytoma. J Clin Endocrinol Metab. 2007;92:1217–25. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- 7.Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 8.Amar L, Servais A, Gimenez-Roqueplo AP, et al. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005b;90:2110–6. doi: 10.1210/jc.2004-1398. [DOI] [PubMed] [Google Scholar]
- 9.Plouin PF, Gimenez-Roqueplo AP. Initial workup and long-term followup in patients with pheochromocytomas and paragangliomas. Best Pract Res Clin Endocrinol Metab. 2006a;20:421–34. doi: 10.1016/j.beem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Hamidi O, Young WF, Jr, Iniguez-Ariza NM, et al. Malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. J Clin Endocrinol Metab. 2017;102:3296–305. doi: 10.1210/jc.2017-00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and pheochromocytoma: From genetics to personalized medicine. Nat Rev Endocrinol. 2015;11:101–11. doi: 10.1038/nrendo.2014.188. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhofer G, Peitzsch M. Laboratory evaluation of pheochromocytoma and paraganglioma. Clin Chem. 2014;60:1486–99. doi: 10.1373/clinchem.2014.224832. [DOI] [PubMed] [Google Scholar]
- 13.Lenders JW, Keiser HR, Goldstein DS, et al. Plasma metanephrines in the diagnosis of pheochromocytoma. Ann Intern Med. 1995;123:101–9. doi: 10.7326/0003-4819-123-2-199507150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: Which test is best? JAMA. 2002;287:1427–34. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 15.Perry CG, Sawka AM, Singh R, et al. The diagnostic efficacy of urinary fractionated metanephrines measured by tandem mass spectrometry in detection of pheochromocytoma. Clin Endocrinol (Oxf) 2007;66:703–8. doi: 10.1111/j.1365-2265.2007.02805.x. [DOI] [PubMed] [Google Scholar]
- 16.Amar L, Lussey-Lepoutre C, Lenders JW, et al. Recurrence or new tumors after complete resection of pheochromocytomas and paragangliomas: A systematic review and meta-analysis. Eur J Endocrinol. 2016;175:R135–45. doi: 10.1530/EJE-16-0189. [DOI] [PubMed] [Google Scholar]
- 17.Plouin PF, Amar L, Dekkers OM, et al. European Society of Endocrinology clinical practice guideline for long-term followup of patients operated on for a pheochromocytoma or a paraganglioma. Eur J Endocrinol. 2016;174:G1–0. doi: 10.1530/EJE-16-0033. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhofer G, Goldstein DS, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: How to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003b;88:2656–66. doi: 10.1210/jc.2002-030005. [DOI] [PubMed] [Google Scholar]
- 19.Algeciras-Schimnich A, Preissner CM, Young WF, Jr, et al. Plasma chromogranin A or urine fractionated metanephrines followup testing improves the diagnostic accuracy of plasma fractionated metanephrines for pheochromocytoma. J Clin Endocrinol Metab. 2008;93:91–5. doi: 10.1210/jc.2007-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmers HJ, Pacak K, Huynh TT, et al. Biochemically silent abdominal paragangliomas in patients with mutations in the succinate dehydrogenase subunit B gene. J Clin Endocrinol Metab. 2008;93:4826–32. doi: 10.1210/jc.2008-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gimenez-Roqueplo AP, Caumont-Prim A, Houzard C, et al. Imaging workup for screening of paraganglioma and pheochromocytoma in SDHx mutation carriers: A multicenter prospective study from the PGL.EVA Investigators. J Clin Endocrinol Metab. 2013;98:E162–73. doi: 10.1210/jc.2012-2975. [DOI] [PubMed] [Google Scholar]
- 22.Timmers HJ, Chen CC, Carrasquillo JA, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F- fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012;104:700–8. doi: 10.1093/jnci/djs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baudin E, Habra MA, Deschamps F, et al. Therapy of endocrine disease: treatment of malignant pheochromocytoma and paraganglioma. Eur J Endocrinol. 2014;171:R111–22. doi: 10.1530/EJE-14-0113. [DOI] [PubMed] [Google Scholar]