Abstract
Introduction
Natesto®, testosterone nasal gel (TNG) is an intranasal testosterone therapy (TTh) used to restore testosterone levels and improve symptoms of hypogonadism. Treatment requires application two (bid) or three (tid) times daily. The Treatment Satisfaction Questionnaire for Medication (TSQM) and a Patient Preference and Use (PPU) questionnaire were used to obtain patient feedback on the use of TNG and compare it to experience with topical TTh.
Methods
The study enrolled 24 TTh-naive (TThN) and 93 TTh-experienced (TThE) hypogonadal men. Treatment lasted up to 120 days, with titration at day 90 to determine the most appropriate dose for restoration of testosterone levels (11 mg bid or tid). Patient satisfaction and symptom changes were measured at days 0, 30, 60, 90, and 120. The PPU questionnaire was performed at study entry and study completion.
Results
Symptoms improved from baseline (30.6) to day 90 (35.1) (p<0.0001; +15%), consistent with testosterone replacement. TNG increased scores for effectiveness (+20%), convenience (+30%), and global satisfaction (+3%) as compared to their previous topical TTh. TThE patients reported ease of use, convenience, efficacy/ effectiveness, and travel friendliness as “likes” of TNG therapy. Overall, 67.2% of patients agreed or strongly agreed that they preferred TNG over topical TTh and 59% sought a prescription to continue treatment with TNG.
Conclusions
Patients switching from topical TTh to TNG reported significant improvements in symptoms and patient satisfaction compared to their previous topical TTh. Patients also reported a significant improvement in convenience with TNG despite two to three times daily application. Preference, satisfaction, and convenience may translate to better treatment compliance.
Introduction
A variety of testosterone replacement therapy (TTh) options are available to patients to treat hypogonadism. These include intramuscular injections, topical gels and patches, buccal patches, nasal gel, and in some countries oral capsules. 1 Patients and physicians weigh the advantages and disadvantages of each when making a choice that best fits the therapeutic needs, preferences, safety/tolerances, and lifestyle. Factors include convenience, cost, potential adverse local (irritation) or systemic (cardiovascular, hematocrit) reactions, transference, administration, smell/odor, and physician recommendations.2–8
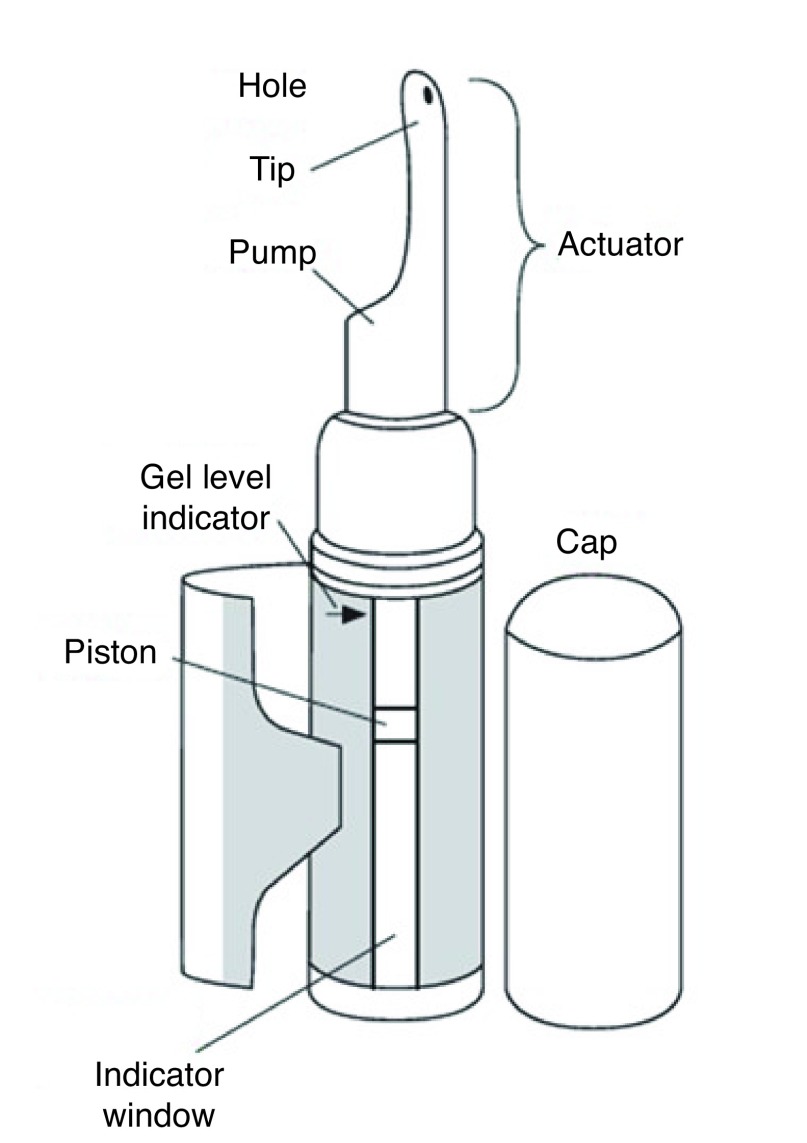
Nasal delivery with Natesto®, a 4.5% testosterone nasal gel (TNG), is a new and unique route of administering testosterone. 9 TNG provides a peak-and-trough (ultradian) serum profile of testosterone, which, like the natural circadian rhythm of endogenous testosterone production, is not steady state.10–12 The resulting serum profile provided by twice daily (bid) dosing from the metered-dose pump dispenser (Fig. 1) is believed to provide a unique combination of advantages related to safety and efficacy, including: 1) no-touch administration that avoids transference; 2) fast/ easy self-administration requiring only about 10 seconds per dose; 3) very low risk of experiencing supraphysiological testosterone even at the maximum recommended daily dose due to a divided dose;13 and 4) retention of gonadotropin feedback with potential retention of sperm count and the possibility of lessening of testicular atrophy.14 Perceived disadvantages of TNG may include repeat dosing and the nasal route of administration.
Fig. 1.
Testosterone nasal gel provides a peak-and-trough (ultradian) serum profile of testosterone via twice-daily dosing from a metered-dose pump dispenser.
The My-T study was designed to address two questions related to treatment with TNG: 1) Given the proven safety of the maximum recommended daily dose, can titration be performed based on symptom improvement, using total testosterone (TT) levels after dose adjustment to confirm restoration of levels for safety and efficacy? 2) What are patients’ perceptions of dosing with TNG relative to more widely prescribed topical TTh medication? In this paper, we present the results of the second question.
This study primarily recruited hypogonadal males who were taking a topical TTh medication for hypogonadism and were willing to switch for a period of up to 120 days. Patient satisfaction data was captured just prior to TNG initiation (baseline) for TTh-experience (E) patients and compared to the results after completing the treatment period in order to provide quantitative and qualitative information on efficacy, effectiveness, satisfaction, and preferences.
Methods
Study population
Patients were adult hypogonadal males (<65 years of age) with documented TT levels ≤10.4 nmol/L. Approximately 75% of patients enrolled were receiving treatment with a topical TTh for at least three months prior to the study and were on active treatment at the time of inclusion. Approximately 25% of patients were treatment-naive (TTHN).
Study design
The My-T study (NCT02937740) was a multicenter, single-arm intervention study treating hypogonadal males with TNG for up to 120 days with potential dose adjustment at day 90. This study was conducted under CTA issued by Health Canada (September 15, 2016), in accordance with guidelines set forth by the International Conference on Harmonisation (ICH) Guidelines for Good Clinical Practice (GCP), and in accordance with ethical principles that have their origins in the Declaration of Helsinki regarding treatment of human patients in a study.
The study assessed patients’ symptoms using the Quantitative Androgen Deficiency for Aging Males Questionnaire (qADAM)15 and measured patient treatment satisfaction using the Treatment Satisfaction Questionnaire for Medication (TSQM). The TSQM version 9 is a nine-item validated instrument with domains for effectiveness (three items), convenience (three items), and global satisfaction (three items).16 Domain scores are the sum of values of questions in each domain expressed as a percentage of the maximal possible score. The TSQM was administered on days 0, 30, 60, 90, and 120, and analyses were performed using analysis of variance (ANOVA). Missing data points were assessed using the Last Observation Carried Forward (LOCF) methodology and statistical analyses, as deemed appropriate. The primary efficacy analyses were performed using the intent-to-treat (ITT) LOCF population. Changes in TSQM domains were analyzed using a Shapiro-Wilk normality test. Based on results, p values were calculated using a paired t-test (two-sided) for normally distributed data or a Wilcoxon signed-rank test (two-sided) for non-normally distributed data. All patients completed an internally developed Patient Preference and Use (PPU) questionnaire. The questionnaire asked patients to designate a degree of importance (from very important to not important at all), agreement (strongly agree to strongly disagree), asked qualitative questions about patients’ likes and dislikes, and recorded their willingness to switch to TNG, which was confirmed by phone 30 days after completion of the treatment period. Normality of patient response data was determined using the Shapiro-Wilk normality test. P values were calculated using paired t-test (two-sided) for normally distributed data or Wilcoxon matched pairs signed-rank test (two-sided) for non-normally distributed data. Analyses were performed on the complete study population and stratified by dosage or prior TTh experience. P values were not adjusted for multiple comparisons.
Results
Table 1 provides the demographic information for the 117 patients who were enrolled from 11 Canadian sites. Age, weight, height, age at diagnosis, and racial breakdown were similar between TThN and TThE patients. Of these, 93 (79.5%) were on topical testosterone treatment for at least the previous three months; 24 patients (20.5%) were TThN. All subjects received the starting dose of 22.0 mg daily (11 mg bid). The dose was adjusted, as necessary, at day 90 to manage symptoms. One hundred patients (100; 85%) were included in the ITT population, including 77 (77.0%) TThE patients. Seventy-seven (77.0%) patients were in the normal TT range at study completion, with a mean serum TT of 19.4 nmol/L. Seventy (70.0%) patients stayed on the 22.0 mg dose, while 30 (30.0%) were up-titrated to the 33.0 mg dose. Seventy-eight patients (66.7%) completed the study in its entirety. Eighteen patients withdrew consent, five patients withdrew consent due to an adverse event, 10 patients were lost to followup, five patients were discontinued due to an adverse reaction, and one patient withdrew for other reasons.
Table 1.
Summary of demographic data (ITT analysis)
| ITT | |||
|---|---|---|---|
|
| |||
| Parameter | All patients (n=100) | TThN (n=23) | TThE (n=77) |
| Age, mean (SD) | 52.8 (9.0) | 52.7 (11.6) | 52.9 (8.2) |
| Weight, kg mean (SD) | 102.7 (24.6) | 104.0 (20.4) | 102.3 (25.8) |
| Height, cm mean (SD) | 176.4 (6.3) | 176.0 (7.7) | 176.6 (5.9) |
| BMI, kg/m2 mean (SD) | 32.9 (7.6) | 33.5 (6.0) | 32.8 (8.0) |
| Age of hypogonadism diagnosis, mean (SD) | 49.3 (9.6) | 51.4 (11.1) | 48.7 (9.0) |
| Baseline/historical TT, nmol/L mean (SD) | 6.9 (2.6) | 6.6 (2.1) | 7.0 (2.8) |
| Race, n (%) | |||
| Caucasian | 87 (87.0) | 20 (87.0) | 67 (87.0) |
| Black | 1 (1.0) | 0 (0.0) | 1 (1.3) |
| Asian | 5 (5.0) | 1 (4.3) | 4 (5.2) |
| Hispanic | 1 (1.0) | 0 (0.0) | 1 (1.3) |
| Middle-Eastern | 5 (5.0) | 1 (4.3) | 4 (5.2) |
| Other | 1 (1.0) | 1 (4.3) | 0 (0.0) |
BMI: body mass index; ITT: intent to treat; SD: standard deviation; TT: total testosterone; TThE: testosterone therapy-experienced; TThN: testosterone therapy-naive.
Table 2 provides absolute values for TSQM domain responses for all patients. For TThE patients (n=69), TSQM was given at day 0 and represented patient assessment of the topical medication at study initiation as a reference point for comparison. TThN patients, who were not on active treatment, were not administered the TSQM at day 0. In general, there was an increase in all domains of treatment satisfaction, which was the strongest between baseline (day 0) and day 30, the first followup visit after starting TNG. The mean values for all three TSQM domains from day 30 to study endpoint were comparable between TThE and TThN patients, indicating that both groups reported similar TNG treatment satisfaction.
Table 2.
Mean TSQM values by domain for the ITT LOCF population all patients
| Cohort | n | Domain | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Effectiveness | Convenience | Global satisfaction | ||||||||||||||
|
| ||||||||||||||||
| D0 | D30 | D60 | D90 | EP | D0 | D30 | D60 | D90 | EP | D0 | D30 | D60 | D90 | EP | ||
| TThE bid | 52 | 44.9 | 51.6* | 52.9* | 52.8* | NA | 56.7 | 70.6* | 71.8* | 73.1* | NA | 50.4 | 51.5 | 49.0 | 47.9 | NA |
| TThE tid | 17 | 37.3 | 53.1* | 52.6* | 50.0* | 59.2* | 56.9 | 73.9* | 76.1* | 76.8* | 76.1* | 40.8 | 50.8 | 53.4* | 53.4* | 52.9* |
| TThE all | 69 | 43.0 | 52.0* | 52.8* | 52.1* | 54.3* | 56.8 | 71.4* | 72.9* | 74.0* | 73.8* | 48.0 | 51.3 | 50.1 | 49.3 | 49.2 |
| TThN bid | 10 | NA | 58.3 | 57.2 | 65.0 | NA | NA | 63.9 | 66.7 | 67.8 | NA | NA | 57.1 | 55.7 | 60.0 | NA |
| TThN tid | 13 | NA | 44.0 | 44.9 | 41.0 | 45.3 | NA | 73.5 | 79.1 | 76.9 | 75.6 | NA | 52.2 | 52.2 | 45.1 | 46.7 |
| TThN all | 23 | NA | 50.2 | 50.2 | 51.4 | 53.9 | NA | 6.93 | 73.7 | 72.9 | 72.2 | NA | 54.3 | 53.7 | 51.6 | 52.5 |
Statistically significant change from day 0 (p<0.05).
bid: twice daily; D: day; EP: endpoint (value at the last visit study visit [D90 or D120]); ITT: intent to treat; LOCF: last observation carried forward; NA: not applicable; tid: three times daily; TSQM: Treatment Satisfaction Questionnaire for Medication; TThE: testosterone therapy-experienced; TThN: testosterone therapy-naive.
Tables 3 and 4 provide a summary of changes from baseline for each of the TSQM domains for patients stratified by dose at study completion. Of the 60 TThE patients who completed the study at the bid dose, there were significant increases from baseline in the effectiveness (+9.6 [standard deviation (SD) 25.9]; p=0.020) and convenience domains (+18.9 [SD 21.4]; p<0.0001) at study conclusion, but significance was not achieved for global satisfaction (−0.6 [SD 33.0]; p=0.8966) (Table 3). For TThE patients completing the study at the three times daily (tid) dose (n=15), there was a statistically significant (p=0.044) increase from baseline in the effectiveness domain from 37.3 (SD 18.1) to 50.0 (SD 23.3) at day 90 while receiving the bid dose, and another increase to 58.9 (SD 15.7) on day 120 after up-titration to tid dosing. Similarly, the convenience domain showed a significant increase (p=0.0049) from 56.9 (SD 22.7) at baseline to 76.8 (SD 16.7) through day 90, which was followed by a modest increase to 78.1 (SD 16.3) at day 120. The statistically significant (p=0.0252) increase from baseline in the global satisfaction domain from 40.8 (SD 19.4) at baseline to 53.4 (SD 25.3) at day 90 remained essentially unchanged (52.9 [SD 21.9]) at day 120.
Table 3.
TThE bid dose – TSQM domains
| RAW | LOCF | |||
|---|---|---|---|---|
|
| ||||
| Day 0 (n=60) | Day 90 (n=47) | Day 0 (n=52) | Day 90 (n=52) | |
| Effectiveness | ||||
| n (missing) | 55 (5) | 45 (2) | 52 (0) | 52 (0) |
| Mean (SD) | 45.3 (20.2) | 56.2 (22.4) | 44.9 (20.6) | 52.8 (23.5) |
| Median | 44.4 | 61.1 | 44.4 | 55.6 |
| Q1, Q3 | 33.3, 61.1 | 38.9, 72.2 | 33.3, 61.1 | 33.3, 66.7 |
| Min, max | 0.0, 100.0 | 0.0, 100.0 | 0.0, 100.0 | 0.0, 100.0 |
| Paired change from day 0 | ||||
| n (missing) | 43 (4) | 52 (0) | ||
| Mean (SD) | 9.6 (25.9) | 7.9 (26.1) | ||
| Median | 5.6 | 5.6 | ||
| Q1, Q3 | −11.1, 33.3 | −11.1, 25.0 | ||
| Min, max | −33.3, 66.7 | −33.3, 66.7 | ||
| p | 0.0200 (1) | 0.0333 (1) | ||
| Convenience | ||||
| n (missing) | 55 (5) | 46 (1) | 52 (0) | 52 (0) |
| Mean (SD) | 56.5 (20.9) | 73.2 (17.8) | 56.7 (21.2) | 73.1 (18.3) |
| Median | 55.6 | 75.0 | 55.6 | 75.0 |
| Q1, Q3 | 44.4, 66.7 | 61.1, 83.3 | 44.4, 66.7 | 63.9, 83.3 |
| Min, max | 11.1, 100.0 | 33.3, 100.0 | 11.1, 100.0 | 27.8, 100.0 |
| Paired change from day 0 | ||||
| n (missing) | 44 (3) | 52 (0) | ||
| Mean (SD) | 18.9 (21.4) | 16.3 (23.9) | ||
| Median | 19.4 | 16.7 | ||
| Q1, Q3 | 0.0, 33.3 | 0.0, 33.3 | ||
| Min, max | −22.2, 83.3 | −55.6, 83.3 | ||
| p | <0.0001 (1) | <0.0001 (1) | ||
| Global satisfaction | ||||
| n (missing) | 55 (5) | 46 (1) | 52 (0) | 52 (0) |
| Mean (SD) | 50.6 (23.8) | 50.8 (26.0) | 50.4 (24.4) | 47.9 (25.9) |
| Median | 50.0 | 57.1 | 50.0 | 53.6 |
| Q1, Q3 | 42.9, 64.3 | 21.4, 71.4 | 39.3, 64.3 | 21.4, 71.4 |
| Min, max | 0.0, 100.0 | 0.0, 92.9 | 0.0, 100.0 | 0.0, 92.9 |
| Paired change from day 0 | ||||
| n (missing) | 44 (3) | 52 (0) | ||
| Mean (SD) | −0.6 (33.0) | −2.5 (31.9) | ||
| Median | −7.1 | −7.1 | ||
| Q1, Q3 | −25.0, 17.9 | −25.0, 14.3 | ||
| Min, max | −64.3, 85.7 | −64.3, 85.7 | ||
| p | 0.8966 (1) | 0.5788 (1) | ||
LOCF: last observation carried forward; SD: standard deviation; TSQM: Treatment Satisfaction Questionnaire for Medication; TThE: testosterone therapy-experienced.
Table 4.
Most important symptom for all patients (n=92)
| n (%) | |
|---|---|
| Difficulty achieving/maintaining an erection | 26 (28.3) |
| Low sex drive | 23 (25.0) |
| Fatigue/loss of energy | 23 (25.0) |
| Mood changes/irritability | 5 (5.4) |
| Increased body fat | 5 (5.4) |
| Decline in general feeling of well-being | 3 (3.3) |
| Depression/depressed mood | 3 (3.3) |
| Difficulty falling asleep/staying asleep | 2 (2.2) |
| Inability to concentrate | 1 (1.1) |
| Decreased physical activity/vitality | 1 (1.1) |
Patients were asked about their most important symptom in a multiple-choice question on the PPU questionnaire (n=100). There was no significant difference in responses when stratified by prior TTh experience (data not shown). Results of the combined population are shown in Table 4. Patients reported difficulty achieving/maintaining an erection (28.3%) and fatigue/loss of energy (25.0%) and sex drive (25.0%) as the most important symptoms to treat.
Patients were also asked to provide a list of both what they “liked” and “disliked” about TNG. Patients’ “likes” that occurred most frequently included: ease of use (>45 responses), convenience (12), efficacy (10) or effectiveness, and travel friendliness (10). “Dislikes” that patients reported 10 or more times included: nasal drip (17), feeling/discomfort (12), and smell (10). Rarely did patients indicate “frequency of use” as a dislike (one response), and this was not reported as a reason for discontinuation. The relative importance of likes and dislikes, or how this impacted choice or preference, was not probed directly; yet, 64% of all TThE patients agreed/ strongly agreed that they preferred nasal testosterone over topical medication. Also, 30 days after completing the study treatment, a followup call determined that 59% of patients sought a prescription in order to continue medicating with TNG, while 22% of patients were still considering it.
Discussion
Hypogonadal patients who seek TTh have a wide choice of medical preparations for treatment, including pellets intramuscular injections, topical, patch, oral, buccal, and nasal. In general, all of these preparations are considered safe and effective. While independent surveys show fairly good overall rates of satisfaction (>70% patients),17 only 18.63% of first-time TTh users in the U.S. refilled their prescription within three months.18 Given the various treatment options, it is perhaps central to better understand what factors are important to patients when selecting one option over another, as well as issues that may impact patient preference and patient compliance.
Patient-reported outcomes (PRO) or questionnaires have been used to improve treatment paradigms.19,20 There is evidence suggesting that integrating PROs in clinical settings improves communication between patients and clinicians, allowing for positive effects on patient care, outcomes, and compliance.21 The ability to evaluate and document changes in symptoms and patient satisfaction is of significant clinical value, specifically when it comes down to patient choice and preference, therefore, contributing to a higher quality of care.22 Quantification of treatment satisfaction with the TSQM is a pertinent, validated, and reliable tool to understand and compare treatment modalities. In prior studies, TSQM correlated with patient compliance and medication adherence with good consistency.16,23–25
The majority of patients (75%) enrolled in this study had prior topical TTh experience and were able to compare their prior treatment to the nasal delivery form of TTh using the TSQM. A comparison of dosing recommendations for TNG and topical preparations is shown in Table 5.
Table 5.
Side-by-side comparison of TTh topical products
| TNG | Topical transdermal gel | |
|---|---|---|
| Testosterone per day (starting dose) | 22 mg | 50 mg Androgel1 50 mg Testim2 |
| Delivery mechanism | Nasal applicator | Spread by hand to upper arms/shoulders and/or right and left abdomen |
| Black box warning | None | Transference risk |
| Applications per day | 2 or 3 | 1 |
| Time required for a single application | 10 seconds | 8–10 minutes |
| Application notes | NA | Includes application, drying and estimated cleanup times required to reduce transference risks Area must be continually covered and/or product removed if skin-to-skin contact with treated area and another person is anticipated |
Androgel (testosterone gel) product monograph. BGP Pharma ULC. Canada. January 8, 2016.
Testim (testosterone gel) product monograph. Paladin Labs, Inc. Canada. February 3, 2017.
TNG: testosterone nasal gel; TTh: testosterone therapy.
While TThE patients were modestly satisfied with their topical medication, as shown by TSQM and qADAM scores at study entry, qADAM domains (particularly energy level, libido, and strength/endurance), as well as the TSQM domain for effectiveness and convenience (p <0.05) were all statistically higher at study completion. Overall, qADAM and TSQM results suggest that TNG is effective in achieving patients’ primary goal of therapy and symptom relief.
TThE patients also found TNG to be significantly more convenient compared to their prior topical medication. It is noteworthy that an increase in daily dose from bid to tid did not negatively impact patient responses in either the effectiveness or convenience domains. Lastly, while TThN patients did not have prior experience with TTh at baseline, mean endpoint values in each of the TSQM domains were comparable to those of experienced patients, indicating that prior exposure to a topical testosterone product is not necessary to observe satisfaction with TNG.
Possible weaknesses in the study include a small subset of naive subjects in the trial, which did not allow complete statistical analysis of this group. The main goal of this analysis was to provide a comparison of nasal and topical medications, which is a perspective that naive patients lack. Yet, endpoints for the TThN subset were similar to the larger populations, suggesting that satisfaction with TNG and preferences are similar for all patients. The discontinuation rate in this study was greater than observed in prior experiences with TNG, which may have affected the quantitative results reported, yet, there remains a significant proportion of subjects who completed the study and who clearly perceived benefits from access to TNG. The PPU questionnaire was not a validated instrument and used multiple-choice and open-ended questions that provide useful feedback that was not quantified, but rather provided indicators. There may be a bias in terms of patient selection in the TThE patient group, as some patients may have been unhappy with their previous topical TTh prior to entering this study. Patients who were very satisfied with their topical TTh may have been less likely to enter the study. However, the baseline qADAM mean was >30, indicating that patients in this study felt their condition at an average level of symptom relief on topical TTh, while the mean baseline TSQM convenience scores were over 50, suggesting patients found the topical product to be “somewhat easy” to use.
Conclusions
TNG restored testosterone levels to normal range and improved symptoms for patients in this study. For patients switching from a topical medication to TNG, effectiveness, convenience, and symptoms all demonstrated significant improvements, indicating that the divided-dose, ultradian profile with TNG is at least as, and possibly more, efficacious than the steady state profile provided by topical testosterone. When followed up 30 days post-treatment, the majority of patients in this study reported having made the switch to TNG as their preferred choice of medication for testosterone replacement.
Furthermore, patient satisfaction improved significantly on TNG relative to a topical TTh. The convenience domain of the TSQM showed the strongest improvements. Patients overwhelmingly liked the “ease of use/application” of TNG (the most frequent response to any like or dislike). Convenience was not impacted by increasing dose frequency from bid to tid. Repeat dosing, which can at times be a compliance risk, was actually preferred in this study, suggesting that TNG’s 10-second, no-touch application method is a real and significant improvement relative to the up to 20-minute once-daily application of a topical gel.
Acknowledgments
Editorial assistance in the preparation of this manuscript was provided by Christina Sanguinetti.
Footnotes
See related commentary on page 390
Competing interests: Dr. Lee has been an advisory board member for Acerus and Paladin; a speakers’ bureau member for Acerus, Astellas, and Paladin; has received honoraria from Acerus, Astellas, Paladin, and Pfizer; and has participated in clinical trials supported by Acerus. Dr. Brock has been an advisory board and speakers’ bureau member for, and has received honoraria from Acerus, Lilly, and Pfizer; and holds investments in Boston Scientific and Pfizer. Dr. Barkin has been an advisory board member for Acerus; has received various speaking and study grants; and has participated in the My-T and Natesto Phase 4 trials supported by Acerus. Dr. Bryson, Dr. Gronksi, and Dr. Ormsby are all employees of Acerus.
Funding: This study was supported by Acerus Pharmaceuticals.
This paper has been peer-reviewed.
References
- 1.Men’s Health Review Panel. Men’s Health Guidelines for Family Medicine. 1st ed. MUMS Guideline Clearinghouse; 2017. [Google Scholar]
- 2.Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–98. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- 3.Dean JD, Carnegie C, Rodzvilla J, et al. Long-term effects of Testim®1% testosterone gel in hypogonadal men. Rev Urol. 2005;7:87–94. [PMC free article] [PubMed] [Google Scholar]
- 4.Pastuszak A, Gomez L, Scovell J, et al. Comparison of the effects of testosterone gels, injections, and pellets on serum hormones, erythrocytosis, lipids, and prostate-specific antigen. Sex Med. 2015;3:165–73. doi: 10.1002/sm2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang GY, Gu YQ, Wang XH, et al. A pharmacokinetic study of injectable testosterone undecanoate in hypogonadal men. J Androl. 1998;19:761–8. [PubMed] [Google Scholar]
- 6.Hajjar RR, Kaiser FE, Morley JE. Outcomes of long-term testosterone replacement in older hypogonadal males: A retrospective analysis. J Clin Endocrinol Metab. 1997;82:3793–6. doi: 10.1210/jcem.82.11.4387. [DOI] [PubMed] [Google Scholar]
- 7.Malkin CJ, Pugh PJ, West JN, et al. Testosterone therapy in men with moderate severity heart failure: A double-blind, randomized, placebo-controlled trial. Eur Heart J. 2006;27:57–64. doi: 10.1093/eurheartj/ehi443. [DOI] [PubMed] [Google Scholar]
- 8.Kühnert B, Byrne M, Simoni M, et al. Testosterone substitution with a new transdermal, hydroalcoholic gel applied to scrotal or non-scrotal skin: A multicenter trial. Eur J Endocrinol. 2005;153:317–26. doi: 10.1530/eje.1.01964. [DOI] [PubMed] [Google Scholar]
- 9.NATESTO® (testosterone nasal gel 4.5%) product monograph. Acerus Biopharma Inc; Nov 27, 2017. [Google Scholar]
- 10.Ohdo S. Chronopharmacology focused on biological clock. Drug Metab Pharmacokin. 2007;22:3–14. doi: 10.2133/dmpk.22.3. [DOI] [PubMed] [Google Scholar]
- 11.Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: Timing is everything. Horm Behav. 2006;49:557–74. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lincoln GA, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals — a unifying hypothesis. J Endocrinol. 2003;179:1–13. doi: 10.1677/joe.0.1790001. [DOI] [PubMed] [Google Scholar]
- 13.Rogol AD, Tkachenko N, Bryson N. Natesto®, a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology. 2016;4:46–54. doi: 10.1111/andr.12137. [DOI] [PubMed] [Google Scholar]
- 14.Ramasamy R. Effects of NATESTO® on reproductive hormones and semen parameters: A prospective clinical trial. Paper presented at: American Society for Reproductive Medicine (ASRM); Oct 2018; Denver, CO, USA. [Google Scholar]
- 15.Mohamed O, Freundlich RE, Dakik HK, et al. The quantitative ADAM questionnaire: A new tool in quantifying the severity of hypogonadism. Int J Impotence Res. 2010;22:20–4. doi: 10.1038/ijir.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. doi: 10.1186/1477-7525-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovac JR, Rajanahally S, Smith RP, et al. Patient satisfaction with testosterone replacement therapies: The reasons behind the choices. J Sex Med. 2014;11:553–62. doi: 10.1111/jsm.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465–6. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: A review of the options and considerations. Qual Life Res. 2012;21:1305–14. doi: 10.1007/s11136-011-0054-x. [DOI] [PubMed] [Google Scholar]
- 20.Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307:1583–4. doi: 10.1001/jama.2012.500. [DOI] [PubMed] [Google Scholar]
- 21.Valderas JM, Kotzeva A, Espallargues M, et al. The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Qual Life Res. 2008;17:179–93. doi: 10.1007/s11136-007-9295-0. [DOI] [PubMed] [Google Scholar]
- 22.Gelhorn HL, Bodhani AR, Wahala LS, et al. Development of the Hypogonadism Impact of Symptoms Questionnaire Short Form: Qualitative research. J Sex Med. 2016;13:1729–36. doi: 10.1016/j.jsxm.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Cobden DS, Niessen LW, Barr CE, et al. Relationships among self-management, patient perceptions of care, and health economic outcomes for decision-making and clinical practice in type 2 diabetes. Value Health. 2010;13:138–47. doi: 10.1111/j.1524-4733.2009.00587.x. [DOI] [PubMed] [Google Scholar]
- 24.Pollack MF, Purayidathil FW, Bolge SC, et al. Patient-reported tolerability issues with oral antidiabetic agents: Associations with adherence; treatment satisfaction and health-related quality of life. Diabetes Res Clin Pract. 2010;87:204–10. doi: 10.1016/j.diabres.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Biderman A, Noff E, Harris SB, et al. Treatment satisfaction of diabetic patients: what are the contributing factors? Fam Pract. 2009;26:102–8. doi: 10.1093/fampra/cmp007. [DOI] [PubMed] [Google Scholar]