Abstract
Superwarfarins are long-acting anticoagulant rodenticides developed from warfarin. The mechanism of action is by inhibition of vitamin K epoxide reductase, resulting in the inability of the body to recycle vitamin K. Deficiency of vitamin K thereafter leads to inability for the body to synthesise vitamin K-dependent coagulation factors, factor II, VII, IX, and X, leading to prolonged prothrombin time. Due to the bulky aromatic sidechains, superwarfarins have a much longer half-life when compared to warfarin, and exposure to superwarfarins results in a prolonged period of anticoagulation which can result in clinical bleeding. Diagnosis is straightforward in patients with known history of superwarfarin exposure but has proved difficult for patients who did not report superwarfarin intake. Superwarfarin poisoning should therefore be suspected in all patients with unexplained prolongation of prothrombin time, and can be confirmed by their detection in serum. Treatment for superwarfarin poisoning includes rapid correction of factor deficiencies with either 4-factor prothrombin complex concentrate or fresh frozen plasma in patients with active bleeding, and high dose vitamin K therapy given multiple times per day for a prolonged period of weeks to months.
Introduction
Superwarfarins are long-acting, vitamin K antagonist anticoagulant rodenticides derived from warfarin, in turn a synthetic anticoagulant developed from dicoumarol.1 Compared with warfarin, superwarfarins have very long half-lives and are much more potent in terms of their ability to induce coagulopathy.2–6 Exposure to superwarfarin can be overt or covert. Patients can present with a history of exposure without symptoms, some can present with bleeding symptoms without a clear history of exposure. Mortalities have been reported.7–10 The significant morbidity and mortality from superwarfarin can be contrasted with the highly treatable nature of superwarfarin poisoning with factor replacement and vitamin K therapy, available in most parts of the world.2,4,6,11–13
The diagnosis of superwarfarin poisoning in patients without a clear history of exposure is difficult. Examples include victims of homicide attempts,14,15 the unfortunate users of brodifacoum-laced synthetic cannabinoids in the recent Illinois outbreak,9,16 and a substantial number of patients exposed to superwarfarins of yet uncertain origin in China and Hong Kong.15,17 Worse still, patients with bleeding symptoms such as haematuria, haemoperitoneum, and limb haematoma often are initially cared for by non-toxicologists unfamiliar with superwarfarin poisoning, with the resultant effect that diagnosis was delayed and the management was suboptimal.14,17,18 While detection of superwarfarins in serum confirms the poisoning, this service is not widely available in most parts of the world.7,13,16
The aim of the present mini-review is therefore to raise the awareness of superwarfarin poisoning among clinicians, clinical biochemists and chemical pathologists, such that this highly treatable condition with significant potential for morbidity and mortality does not remain undiagnosed.
The Vitamin K Cycle
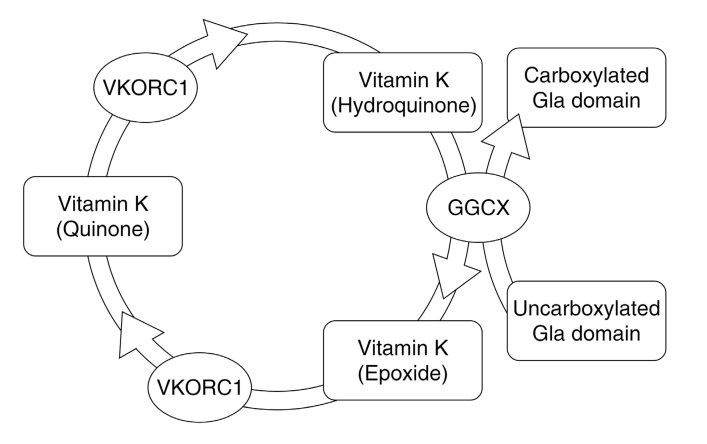
Vitamin K is a collective term describing two classes of naphthoquinone derivatives: phylloquinones and menaquinones; the former are plant in origin, whereas the latter are bacterial in origin (Figure 1). Vitamin K is a fat-soluble vitamin and participates in biochemical processes as electron carriers; in human, vitamin K is a co-factor of gamma-glutamyl carboxylase, which is necessary for post-translational gamma-carboxylation of certain glutamate residues in Gla domain-containing proteins.19,20 The hydroquinone form of vitamin K serves in the process as electron donor, and is oxidised to the epoxide form.21 The epoxide form is cycled back to the hydroquinone by the action of vitamin K epoxide reductase.22 This cycle between the hydroquinone form and the epoxide form is known as the vitamin K cycle (Figure 2).
Figure 1.
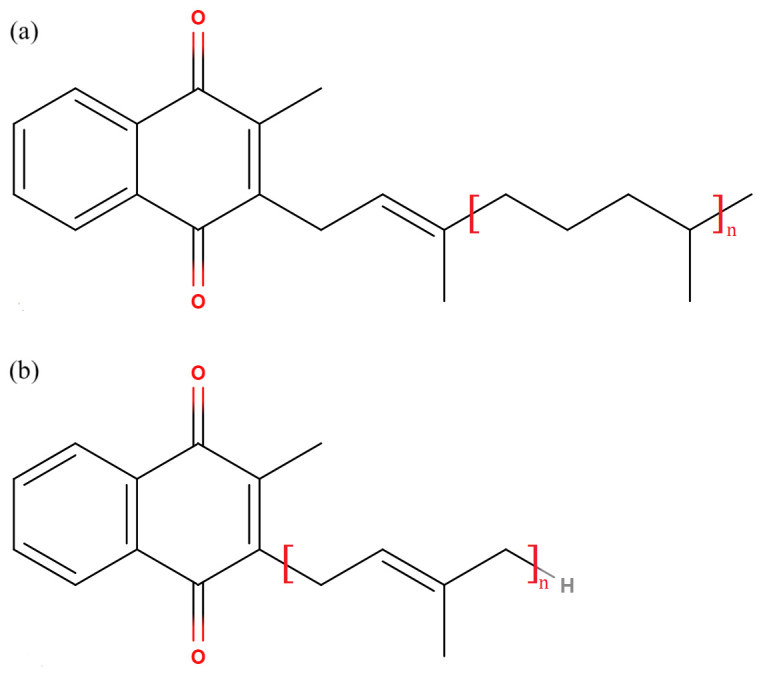
Structure of major vitamin K classes: (a) phylloquinones (e.g. Vitamin K1, n=3), and (b) menaquinones (Vitamin K2, n≥1; Vitamin K3, n=0).
Figure 2.
The vitamin K cycle (VKORC1: vitamin K epoxide reductase; GGCX: gamma-glutamyl carboxylase).
There are many vitamin K-dependent proteins in humans and these include coagulation factors II (prothrombin), VII, IX, and X, as well as the anticoagulants protein C, protein S, and protein Z.23 In the absence of vitamin K hydroquinone, coagulation factors and endogenous anticoagulants produced do not undergo post-translational modification and lack properly functioning Gla domains in these factors.19,24 Functional Gla domains are necessary for binding of calcium ion and stabilisation of the final tertiary structure of the proteins.20,25,26 Despite affecting both coagulation factors and endogenous anticoagulants, the overall effect of vitamin K is that of a procoagulant, the very reason it is named vitamin K (for koagulation, the Scandinavian and German spelling of coagulation).27 Recent evidence cast doubt as to whether properly functioning Gla domains are necessary for the action of prothrombin, and suggested that the pathophysiological mechanism for vitamin K deficiency or antagonism lies in the other factors (VII, IX, and X).28
Apart from the coagulation-related proteins, vitamin K deficiency has been implicated as a factor in osteoporosis, vascular calcification, and insulin resistance, the effects of which are mediated through the Gla proteins osteocalcin, matrix Gla protein, and the signalling protein gas6 (growth arrest specific gene 6).29–31
The Development of Warfarin and Superwarfarins
The discovery of warfarin and its predecessor dicoumarol comes from the Karl Link’s investigation of haemorrhagic disease that occurred when cattle consumed sweet clover hay that was infected by moulds (the haemorrhagic sweet clover disease).32,33 With the help of Wisconsin Alumni Research Foundation, Link and colleagues were able to crystallise and identify the chemical cause of the haemorrhagic sweet clover disease, subsequently known as dicoumarol.34,35 Dicoumarol was subsequently released into clinical use as an anticoagulant.36 Subsequently, more than 150 analogues of dicoumarol were synthesised in an attempt to create a better rodenticide (or ‘better mousetraps’, in the words of Karl Link).33 Compound 42, one of the synthesised analogues, later known as warfarin, was developed as a result.1,33,37 The hydroxycoumarins warfarin, coumachlor, coumafuryl, and coumatetralyl, together with the indandiones diphacinone and chlorophacinone, collectively known as the first generation anticoagulant rodenticides, were developed in the 1950s and early 1960s. These anticoagulant rodenticides dominated the market of rodent control world-wide in the subsequent years.38
This market dominance did not last long with heritable warfarin resistance identified in many species of rats and mice.39 In response to this resistance, superwarfarins were developed.40,41 This led to the development of difenacoum, brodifacoum, and bromadiolone, and these compounds were found to be useful against warfarin-resistant rodents.41–43 These superwarfarins found rapid market acceptance and is reflected in the number of registered products.44 The Table lists commonly encountered anticoagulant rodenticides with their respective molecular formula and molecular weight.
Table.
Commonly encountered anticoagulant rodenticides. Compounds which are not long-acting are marked with an asterisk.
| Class | Compound | Molecular formula | Molecular weight |
|---|---|---|---|
| 4-hydroxycoumarins | Warfarin* | C19H16O4 | 308.34 |
| Coumatetralyl* | C19H16O3 | 292.33 | |
| Bromadiolone | C30H23BrO4 | 527.41 | |
| Brodifacoum | C31H23BrO3 | 523.43 | |
| Difenacoum | C31H24O3 | 444.52 | |
| Difethialone | C31H23BrO2S | 539.49 | |
| Flocoumafen | C33H25F3O4 | 542.54 | |
| Indandiones | Pindone* | C14H14O3 | 230.26 |
| Chlorophacinone | C23H15ClO3 | 374.83 | |
| Diphacinone | C23H16O3 | 340.38 |
The Toxicology of Superwarfarins
The two most commonly encountered superwarfarins in clinical poisoning are bromadiolone and brodifacoum.45,46 Both bromadiolone and brodifacoum are warfarin/4-hydroxycoumarin derivatives.38 In the broad sense of the term used clinically, superwarfarins also encompass long-acting anticoagulant rodenticides which are not warfarin/4-hydroxycoumarin derivatives, for example chlorophacinone and diphacinone, which are indandione derivatives.47 These superwarfarins are all highly lipophilic with extremely long biological half-lives.48 This can be seen by the high octanol:water partition coefficient (log Poct/wat, a measurement of hydrophobicity) of bromadiolone (7.02) and brodifacoum (8.50) in comparison to that of warfarin (2.70) and dicoumarol (1.92).49 They are also much more potent than warfarin: brodifacoum has an IC50 of 0.15 μM towards rat microsomal vitamin K epoxide reductase, compared to 2.2 μM for warfarin.50 The LD50 of brodifacoum and bromadiolone are 0.26 and 1.13 mg/kg respectively, 715 times and 165 times lower than that of warfarin in albino Norway rats.38
Superwarfarins such as bromadiolone and brodifacoum are bound in the liver and remain stable with hepatic half-life of more than 114 days for brodifacoum and ranges from 28 days to 318 days for bromadiolone across different animal species.48,51,52 The plasma half-lives for brodifacoum and bromadiolone were determined to be 91.7 and 33.3 days respectively in rats in a recent study employing liquid chromatography-tandem mass spectrometry based method for quantitative analysis.48 Readers are referred to the excellent review by Horak et al. for a comprehensive review on the toxicokinetics of warfarin and superwarfarins in animals.5 In stark contrast with the vast amount of clinical data available for warfarin,53 human data concerning the pharmacokinetics of superwarfarin remained scarce, with only isolated case reports detailing individual cases of bromadiolone and brodifacoum poisoning.2,3,54–58 The elimination-phase half-life for brodifacoum has been reported to be between 15 and 30 days,3,58 whereas those of bromadiolone have been reported to be between 10 and 24 days.2,57 Enterohepatic recirculation has been suggested as a factor contributing to the very long half-life of superwarfarins.51
Warfarin and superwarfarins act by inhibiting the enzyme vitamin K epoxide reductase, and make it impossible for the body to recycle vitamin K.21,59,60 The enzyme kinetics of vitamin K epoxide reductase following incubation with warfarin have been reported as non-competitive.61 This orthodox concept has since been challenged by recent findings that warfarin competes with vitamin K binding62 as well as trapping the vitamin K epoxide reductase in an intermediate state.63 The exact mechanism by which warfarin and superwarfarins inhibit vitamin K epoxide reductase remains unclear.
Following exposure to superwarfarins, the onset of measurable coagulopathy by prothrombin time depends upon the half-lives of vitamin K-dependent coagulation factors which range from 0.25 days for factor VII to 2.5 days for prothrombin.23 Clinical bleeding as a result of the coagulopathy are later in time course and are most common between 3 and 9 days after an acute exposure.7
Epidemiology of Superwarfarin Poisoning
A review of over 300,000 cases reported to the National Poison Data System in the US showed that a large majority of reported superwarfarin poisoning are accidental (95.6%) and occur in children less than 6 years-of-age, with most cases of superwarfarin exposure due to oral exposure towards rodenticide baits, with commercial rodenticides commonly available in two forms: bait and liquid concentrate.7,46 The bait form is usually at a lower concentration (e.g. bromadiolone at 0.005%) whereas liquid concentrate contains superwarfarin at a much higher concentration (e.g. bromadiolone at 0.5%).64
Superwarfarin poisoning in adults, on the other hand, occurs with deliberate self-harm/suicide, accidental ingestion, occupational exposure with most cases being sporadic in nature.7,14,16,17,65,66 In contrast to the reports in the Western countries, case series from Asia showed a much larger proportion of superwarfarin poisoning occurring in adults, with many cases being suicide attempts, and a large group of patients in whom no possible source of superwarfarin exposure could be elucidated.14,15,17,67
The use of superwarfarin as a poison for homicide or child abuse has been reported in the literature.14,65 In 2011, a radio-controlled explosive device containing lead weights laced with brodifacoum was found and defused.68,69 There are suggestions that similar explosive devices have been employed by Palestinian suicide bombers.70 So far, there have been no reports of explosive injuries associated with superwarfarin poisoning.
The recent association of superwarfarin poisoning with consumption of synthetic cannabinoids have renewed the worry of drugs of abuse-associated superwarfarin poisoning.3,9,71 A major outbreak of synthetic cannabinoid-associated coagulopathy occurred in Illinois, US in 2018, with a total of over 300 persons being affected and a total of 7 deaths between March and October 2018.9,13,16 In the case series by Kelkar et al., brodifacoum was identified as the culprit and was detected in all patients tested, whereas difenacoum, bromadiolone, and warfarin were additionally detected among the patients.16 Limited experimental data suggests that there is no pharmacodynamics interaction between brodifacoum and synthetic cannabinoids, though pharmacokinetic interactions have not been ruled out.72 It remains unclear as to whether the presence of superwarfarins in synthetic cannabinoids represents malicious adulteration or inadvertent contamination.
Clinical Presentation and Laboratory Testing
The signs and symptoms resulting from haemorrhage and bleeding tendencies are the most important clinical features in superwarfarin poisoning. Most patients with accidental superwarfarin exposure do not develop any symptoms.7 For patients with definite history of accidental superwarfarin exposure, a dose of 1 mg of active ingredient, equivalent to 20 g of bait at a concentration of 0.005%, has been suggested as a referral threshold for emergency department assessment.6
Patients with symptomatic superwarfarin exposure present with bleeding-related manifestations such as gross haematuria, mucosal bleeding, gastrointestinal bleeding, soft tissue bleeding, menorrhagia, and haemoptysis, and non-bleeding related symptoms and signs such as abdominal pain, flank pain, and headache.7,14,16,17,73
Rarely, patients with superwarfarin exposure present with clinical features of both thrombosis and bleeding. A 34-year-old woman developed haematuria and later died of brain herniation secondary to thrombosis of superior longitudinal sinus after chlorophacinone poisoning.8 Similarly, cases of deep vein thrombosis together with haematuria have been reported in a patient with brodifacoum poisoning and in another patient with difethialone poisoning.74,75 It has been postulated that the initial phase of superwarfarin poisoning is hypercoagulable, due to deficiencies of vitamin K-dependent endogenous anticoagulants Protein C and Protein S occurring before deficiencies of vitamin K-dependent coagulation factors, similar to that described with the use of warfarin.8,75,76
Prothrombin time and international normalised ratio (INR) are the investigations of choice in identifying the coagulopathy caused by warfarin and superwarfarins.6,77 Further testing on individual coagulation factor levels may identify deficiencies of vitamin K-dependent coagulation factors and may be useful to exclude alternative causes,78 and testing for PIVKA-II and vitamin K epoxide may help to identify patients with vitamin K antagonism.79 Quantitative analysis of superwarfarins is the test of choice for confirmation of superwarfarin poisoning.2,7,12,80 The dangerous practice of using the presence of warfarin and its metabolites in urine to exclude superwarfarin exposure could not be recommended as patients who had co-exposure of superwarfarin(s) and warfarin have been reported in the literature 16,17 and would not be identified by this workflow, and commercial preparations containing warfarin and brodifacoum as a combination rodenticide have been reported.80
The difficulty of diagnosing superwarfarin poisoning lies in those who present without a history of superwarfarin exposure,7,45 with one case series noting a median delay of 6 days to achieve optimal medical treatment for patients without exposure history, reflecting the lack of alertness in entertaining this important differential diagnosis in patients presenting with coagulopathies.17 Superwarfarin poisoning should be suspected in all patients with unexplained bleeding with prolonged prothrombin time.3,7,12,67,70
Detection of Superwarfarin in Biological Matrices
The definitive test for superwarfarin poisoning is direct analysis of superwarfarins in serum.2,7,12,80 Earlier publications used methods such as high performance thin layer chromatography (HPTLC),81 high performance liquid chromatography with fluorescence detection (HPLC-FD)82 and gas chromatography-mass spectrometry (GC-MS).83 Nowadays, liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the preferred technique in the modern clinical toxicology laboratory as it is more sensitive than HPLC-FD and less cumbersome in operation when compared to GC-MS.54,84,85
Published methods for multi-analyte analysis of superwarfarins in biological matrices with LC-MS/MS all utilised liquid-liquid extraction for sample extraction, with acetone, ethyl acetate, and acetonitrile being popular choices.48,54,84,86–91 In recent years, assays using newer sample extraction/pretreatment methods such as ultrasound-assisted liquid-liquid microextraction,92 phospholipid removal93 and supported liquid extraction94 have been reported. The use of C18-based chromatographic column, negative-mode electrospray ionisation, and detection using multiple reaction monitoring mode appears to be near-universal among publications with simultaneous determination of multiple superwarfarins by LC-MS/MS.48,54,84,86–94
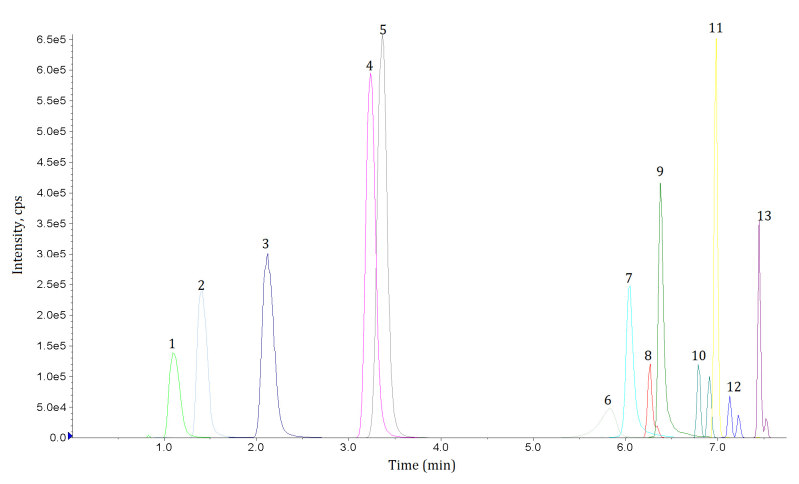
In the present authors’ laboratory, an in-house method based on liquid chromatography-tandem mass spectrometry is used to provide quantitative analysis of bromadiolone and brodifacoum and qualitative analysis of warfarin and superwarfarins in serum as well as qualitative analysis in urine.17 After addition of internal standards (bromadiolone-d5, brodifacoum-d4, and dicoumarol), serum specimens are subject to liquid-liquid extraction with 5% ethanol in ethyl acetate, followed by protein precipitation with trichloroacetic acid. The organic layer of the specimens is then dried, reconstituted and injected into a liquid chromatograph equipped with a C18 column and analytes are detected by negative electrospray ionisation-tandem mass spectrometry operated in multiple reaction monitoring (MRM) mode. The MRM chromatogram of this assay is shown in Figure 3.
Figure 3.
Multiple reaction monitoring (MRM) chromatogram of the in-house assay used in the authors’ laboratory. Legend: (1) warfarin metabolite, (2) coumafuryl, (3) warfarin, (4) coumatetralyl, (5) coumachlor, (6) pindone, (7) diphacinone, (8) bromadiolone, (9) chlorphacinone, (10) difenacoum, (11) flocoumafen, (12) brodifacoum, and (13) difethalone. Note the presence of diastereoisomer present in bromadiolone, difenacoum, brodifacoum, and difethialone.
Treatment for Superwarfarin Poisoning
The treatment of superwarfarin poisoning in a bleeding patient includes vitamin K1 therapy which addresses the underlying vitamin K antagonism, and early and rapid correction of coagulopathy to treat uncontrolled haemorrhage.3,6,7,16,45 On the other hand, the guideline published by the American Association of Poison Control Centers suggested the evaluation of patients with superwarfarin exposure without clinical bleeding with testing for prothrombin time at presentation and after 48–72 hours exposure, and suggested against the administration of vitamin K1 before laboratory evaluation.6 Gastrointestinal decontamination is not considered to be helpful at least for accidental poisoning.95 The use of multiple-dose activated charcoal to arrest enterohepatic circulation has not been successful.6,96
For rapid correction of severe coagulopathy due to vitamin K antagonism, fresh frozen plasma and 4-factor prothrombin complex concentrate have been suggested,7,12,13,16,70 and used in a number of patients suffering from haemorrhage due to superwarfarin poisoning.11,16,97,98 In a number of patients, recombinant activated factor VII was used.14,99 There were no studies directly comparing the effectiveness of fresh frozen plasma versus 4-factor prothrombin complex concentrate. Analogy can be drawn with the reversal of warfarin effect. 4-factor prothrombin complex concentrate has been found to be non-inferior and superior to fresh frozen plasma, when both are co-administered with vitamin K1, in a phase IIIb, open-label, randomised trial: rapid INR reduction (INR ≤1.3 at 30 minutes post-infusion) was achieved in 55% of patients receiving the 4-factor prothrombin complex concentrate, versus 10% of patients in the plasma group.100 On the other hand, in the recent case series by Kelkar et al., the rate of early responses were similar for patients who have been put on adequate oral vitamin K1 therapy, whether or not intravenous vitamin K1, fresh frozen plasma, or both were used.16
Vitamin K1 can be administered orally, subcutaneously, or intravenously. Again drawing from the evidence with warfarin reversal, a meta-analysis comparing the effectiveness of oral, subcutaneous, and intravenous administration concluded that oral and intravenous administration were equivalent, and both are superior to subcutaneous administration, for INR reduction at 24 hours after administration.101 On the other hand, intravenous vitamin K was found to be superior at 4 hours post-administration and equivalent at 24 hours post-administration, at reversing the anticoagulation of warfarin.102 For non-bleeding patients, the earlier onset of the reversal of anticoagulation must be weighed against the risk of anaphalactoid reaction to intravenous vitamin K.103,104
There was no obvious consensus on the optimal dose and frequency of vitamin K1 for patients with superwarfarin poisoning in the literature. The optimal frequency of administration was suggested to be every 6–8 hours.45 In the recent outbreak of synthetic cannabinoid-associated superwarfarin poisoning, patients were treated with vitamin K1 in doses of 100 mg/day, resulting in a monthly drug cost of US$24,000–37,000 per patient although these initially cited prices may be an overestimate due to short-term shortage of drug.16,105–108 Such high cost of vitamin K was not seen in Hong Kong, and is not the case for example with the UK.109
Currently, there are no established guidelines for termination of vitamin K1 therapy in patients with known superwarfarin poisoning. Termination of therapy based on a normal clotting profile obtained while the poisoned patient is still on vitamin K1 can be dangerous.110 A tapering approach with vitamin K1 therapy has been used in the past.65 In many centres, where quantitative measurement of superwarfarins is not routinely available, an approach based on stopping vitamin K1 therapy followed by measurement of coagulation parameters at 48–72 hours have been employed.13,14 While no evidence-based safe concentration levels have been defined for the superwarfarins, bromadiolone and brodifacoum levels of <10 ng/mL have been reported to be associated with normal coagulation and treatment can be terminated safely without exposing patients to the risk of bleeding.3,4
Patients with superwarfarin exposure from an unknown source represent the most difficult group of patients to be treated and followed-up. The difficulty with patients with deliberate self-harm or suicidal intent is obvious and their poor adherence to vitamin K1 therapy is well-reported,14,45 as is the risk of re-exposure.15 On the other hand, where the patients truly do not know about the source of superwarfarin that they are exposed to (which we called ‘hidden superwarfarin poisoning’ in our centre), it is inherently impossible for them to ‘avoid re-exposure’ other than ‘being more careful with what they eat’.
It is suggested that the quantitative analysis of superwarfarins in serum is the first step towards solving the problems surrounding the treatment and follow-up of these patients. This analytical approach takes away the guesswork from the differential diagnoses in a patient with vitamin K-dependent coagulopathy, enables the clinical toxicologist to ascertain re-exposure, and importantly, provides an objective end-point which can be relied upon for termination of treatment without exposing the patient to bleeding risks that stems from stopping vitamin K1.
Conclusions: A Glimpse into the Future
Over the past 20 years, there has been tremendous improvement in the diagnosis and management of superwarfarin poisoning, enabled by better understanding in the pathophysiology of vitamin K antagonism, the pharmacokinetics of vitamin K formulations, the adoption of clinical mass spectrometry for detection of superwarfarins, and the improved care for coagulopathic patient. Superwarfarins have withstood the test of time and the power of natural selection (of rodents) and will remain both in the fields of pest control and the practice of toxicology.
The great difficulty in the diagnosis of superwarfarin poisoning means that there is much that pathologists and scientists can do. It can be as simple as providing suggestions on further investigations based on related test results, for example, deficiency in vitamin K-dependent factors in a patient not on warfarin, or a negative urine toxicology in a coagulopathic patient. Last but not least, bearing in mind the limited availability of such testing services world-wide, we wish to reiterate the importance of providing a quantitative serum assay for superwarfarins to definitively establish the diagnosis, as well as to inform termination of vitamin K1 treatment in patients with superwarfarin poisoning.
Footnotes
Competing Interests: None declared.
References
- 1.Wardrop D, Keeling D. The story of the discovery of heparin and warfarin. Br J Haematol. 2008;141:757–63. doi: 10.1111/j.1365-2141.2008.07119.x. [DOI] [PubMed] [Google Scholar]
- 2.Lo VM, Ching CK, Chan AY, Mak TW. Bromadiolone toxicokinetics: diagnosis and treatment implications. Clin Toxicol (Phila) 2008;46:703–10. doi: 10.1080/15563650701504366. [DOI] [PubMed] [Google Scholar]
- 3.Spahr JE, Maul JS, Rodgers GM. Superwarfarin poisoning: a report of two cases and review of the literature. Am J Hematol. 2007;82:656–60. doi: 10.1002/ajh.20784. [DOI] [PubMed] [Google Scholar]
- 4.Bruno GR, Howland MA, McMeeking A, Hoffman RS. Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann Emerg Med. 2000;36:262–7. doi: 10.1067/mem.2000.108317. [DOI] [PubMed] [Google Scholar]
- 5.Horak KE, Fisher PM, Hopkins B. Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-target Organisms. In: van den Brink NW, Elliott JE, Shore RF, Rattner BA, editors. Anticoagulant Rodenticides and Wildlife. Emerging Topics in Ecotoxicology. Vol. 5. Cham: Springer; 2018. pp. 87–108. [Google Scholar]
- 6.Caravati EM, Erdman AR, Scharman EJ, Woolf AD, Chyka PA, Cobaugh DJ, et al. Long-acting anticoagulant rodenticide poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2007;45:1–22. doi: 10.1080/15563650600795487. [DOI] [PubMed] [Google Scholar]
- 7.King N, Tran M-H. Long-Acting Anticoagulant Rodenticide (Superwarfarin) Poisoning: A Review of Its Historical Development, Epidemiology, and Clinical Management. Transfus Med Rev. 2015;29:250–8. doi: 10.1016/j.tmrv.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Papin F, Clarot F, Vicomte C, Gaulier JM, Daubin C, Chapon F, et al. Lethal paradoxical cerebral vein thrombosis due to suspicious anticoagulant rodenticide intoxication with chlorophacinone. Forensic Sci Int. 2007;166:85–90. doi: 10.1016/j.forsciint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Moritz E, Austin C, Wahl M, DesLauriers C, Navon L, Walblay K, et al. Notes from the field: outbreak of severe illness linked to the vitamin K antagonist brodifacoum and use of synthetic cannabinoids – Illinois, March–April 2018. MMWR Morb Mortal Wkly Rep. 2018;67:607–8. doi: 10.15585/mmwr.mm6721a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruse JA, Carlson RW. Fatal rodenticide poisoning with brodifacoum. Ann Emerg Med. 1992;21:331–6. doi: 10.1016/s0196-0644(05)80900-x. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Kotik V, Fahim G, Alagusundaramoorthy S, Eltawansy SA, Mathis S, et al. Treatment of brodifacoum overdose with prothrombin complex concentrate. Am J Health Syst Pharm. 2016;73:e14–7. doi: 10.2146/ajhp150233. [DOI] [PubMed] [Google Scholar]
- 12.Rubinstein I, Weinberg G, van Breemen R, Hershow RC, Feinstein DL. Treatment for long acting anticoagulant rodenticide poisoning - beyond INR monitoring? Toxicol Commun. 2018;2:59–61. doi: 10.1080/24734306.2018.1500152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arepally GM, Ortel TL. Bad weed: synthetic cannabinoid-associated coagulopathy. Blood. 2019;133:902–5. doi: 10.1182/blood-2018-11-876839. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y-F, Chang C-S, Chung C-Y, Lin H-Y, Wang C-C, Shen M-C. Superwarfarin intoxication: hematuria is a major clinical manifestation. Int J Hematol. 2009;90:170–3. doi: 10.1007/s12185-009-0374-6. [DOI] [PubMed] [Google Scholar]
- 15.Yan H, Zhu L, Zhuo X, Shen M, Xiang P. Anticoagulant rodenticide intoxication in east China: a three-year analysis. Forensic Sci Res. 2016;1:22–7. doi: 10.1080/20961790.2016.1242042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelkar AH, Smith NA, Martial A, Moole H, Tarantino MD, Roberts JC. An outbreak of synthetic cannabinoid–associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216–23. doi: 10.1056/NEJMoa1807652. [DOI] [PubMed] [Google Scholar]
- 17.Ng WY, Ching CK, Chong YK, Ng SW, Cheung WL, Mak TW. Retrospective Study of the Characteristics of Anticoagulant-Type Rodenticide Poisoning in Hong Kong. J Med Toxicol. 2018;14:218–28. doi: 10.1007/s13181-018-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SY, Cho SY, Lee HJ, Suh J-T, Oh SH, Lee W-I, et al. Superwarfarin intoxication of unknown etiology accompanying hemoperitoneum in a patient on fluconazole therapy. Ann Clin Lab Sci. 2010;40:300–3. [PubMed] [Google Scholar]
- 19.Huang M, Rigby AC, Morelli X, Grant MA, Huang G, Furie B, et al. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol. 2003;10:751–6. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- 20.Soriano-Garcia M, Padmanabhan K, de Vos AM, Tulinsky A. The Ca2+ ion and membrane binding structure of the Gla domain of Ca-prothrombin fragment 1. Biochemistry. 1992;31:2554–66. doi: 10.1021/bi00124a016. [DOI] [PubMed] [Google Scholar]
- 21.Oldenburg J, Marinova M, Müller-Reible C, Watzka M. The vitamin K cycle. Vitam Horm. 2008;78:35–62. doi: 10.1016/S0083-6729(07)00003-9. [DOI] [PubMed] [Google Scholar]
- 22.Chu P-H, Huang T-Y, Williams J, Stafford DW. Purified vitamin K epoxide reductase alone is sufficient for conversion of vitamin K epoxide to vitamin K and vitamin K to vitamin KH2. Proc Natl Acad Sci U S A. 2006;103:19308–13. doi: 10.1073/pnas.0609401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brummel-Ziedins K, Mann KG. Molecular Basis of Blood Coagulation. In: Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, et al., editors. Hematology. 7th ed. Elsevier; 2018. pp. 1885–1905.e8. [Google Scholar]
- 24.Stenflo J. Contributions of Gla and EGF-like domains to the function of vitamin K-dependent coagulation factors. Crit Rev Eukaryot Gene Expr. 1999;9:59–88. [PubMed] [Google Scholar]
- 25.Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the calcium ion-bound gamma-carboxyglutamic acid-rich domain of factor IX. Biochemistry. 1995;34:12126–37. doi: 10.1021/bi00038a005. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Darden TA, Freedman SJ, Furie BC, Furie B, Baleja JD, et al. Refinement of the NMR solution structure of the gamma-carboxyglutamic acid domain of coagulation factor IX using molecular dynamics simulation with initial Ca2+ positions determined by a genetic algorithm. Biochemistry. 1997;36:2132–8. doi: 10.1021/bi962250r. [DOI] [PubMed] [Google Scholar]
- 27.Dam H – Nobel Lecture. The discovery of vitamin K, its biological functions and therapeutical application. [Accessed 18 September 2019]. https://www.nobelprize.org/prizes/medicine/1943/dam/lecture/
- 28.Greene LA, Thalji NK, Bradford H, Krishnaswamy S, Camire RM. Prothrombin Membrane Binding and Gla-Dependent Function Are Not Required for Effective Hemostasis In Vivo. Blood. 2015;126:124. [Google Scholar]
- 29.Barrett H, O’Keeffe M, Kavanagh E, Walsh M, O’Connor EM. Is Matrix Gla Protein Associated with Vascular Calcification? A Systematic Review. Nutrients. 2018;10:415. doi: 10.3390/nu10040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bügel S. Vitamin K and bone health in adult humans. Vitam Horm. 2008;78:393–416. doi: 10.1016/S0083-6729(07)00016-7. [DOI] [PubMed] [Google Scholar]
- 31.Arai H, Nagai K, Doi T. Role of growth arrest/specific gene 6 in diabetic nephropathy. Vitam Horm. 2008;78:375–92. doi: 10.1016/S0083-6729(07)00015-5. [DOI] [PubMed] [Google Scholar]
- 32.Link KP. The discovery of dicumarol and its sequels. Circulation. 1959;19:97–107. doi: 10.1161/01.cir.19.1.97. [DOI] [PubMed] [Google Scholar]
- 33.Last JA. The missing link: the story of Karl Paul Link. Toxicol Sci. 2002;66:4–6. doi: 10.1093/toxsci/66.1.4. [DOI] [PubMed] [Google Scholar]
- 34.Campbell HA, Link KP. Studies on the hemorrhagic sweet clover disease: IV. The isolation and crystallization of the hemorrhagic agent. J Biol Chem. 1941;138:21–33. [Google Scholar]
- 35.Stahmann MA, Huebner CF, Link KP. Studies on the hemorrhagic sweet clover disease: V. Identification and synthesis of the hemorrhagic agent. J Biol Chem. 1941;138:513–27. [Google Scholar]
- 36.Barker NW, Hines EA, Jr, Kvale WF, Allen EV. Dicumarol, its action, clinical use and effectiveness as an anticoagulant drug. Am J Med. 1947;3:634–42. doi: 10.1016/0002-9343(47)90209-x. [DOI] [PubMed] [Google Scholar]
- 37.Duxbury BM, Poller L. The oral anticoagulant saga: past, present, and future. Clin Appl Thromb Hemost. 2001;7:269–75. doi: 10.1177/107602960100700403. [DOI] [PubMed] [Google Scholar]
- 38.Hadler MR, Buckle AP. Forty five years of anticoagulant rodenticides - past, present and future trends. Proc Fifteenth Vertebr Pest Conf. 1992 [Google Scholar]
- 39.Greavses JH, Ayres P. Heritable resistance to warfarin in rats. Nature. 1967;215:877–8. doi: 10.1038/215877a0. [DOI] [PubMed] [Google Scholar]
- 40.Hadler MR, Shadbolt RS. Novel 4-hydroxycoumarin anticoagulants active against resistant rats. Nature. 1975;253:275–7. doi: 10.1038/253275a0. [DOI] [PubMed] [Google Scholar]
- 41.Rennison BD, Hadler MR. Field trials of difenacoum against warfarin-resistant infestations of Rattus norvegicus. J Hyg (Lond) 1975;74:449–55. doi: 10.1017/s0022172400046969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redfern R, Gill JE. Laboratory evaluation of bromadiolone as a rodenticide for use against warfarin-resistant and non-resistant rats and mice. J Hyg (Lond) 1980;84:263–8. doi: 10.1017/s0022172400026760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rennison BD, Dubock AC. Field trials of WBA 8119 (PP 581, brodifacoum) against warfarin-resistant infestations of Rattus norvegicus. J Hyg (Lond) 1978;80:77–82. doi: 10.1017/s0022172400053419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisemann JD, Fisher PM, Buckle A, Humphrys S. An International Perspective on the Regulation of Rodenticides. In: van den Brink NW, Elliott JE, Shore RF, Rattner BA, editors. Anticoagulant Rodenticides and Wildlife. Emerging Topics in Ecotoxicology. Vol. 5. Cham: Springer; 2018. pp. 287–318. [Google Scholar]
- 45.Chua JD, Friedenberg WR. Superwarfarin poisoning. Arch Intern Med. 1998;158:1929–32. doi: 10.1001/archinte.158.17.1929. [DOI] [PubMed] [Google Scholar]
- 46.Pringle K, Caupp S, Shi J, Wheeler KK, Spiller HA, Casavant MJ, et al. Analysis of intentional drug poisonings using Ohio Poison Control Center Data, 2002–2014. Clin Toxicol (Phila) 2017;55:652–8. doi: 10.1080/15563650.2017.1309050. [DOI] [PubMed] [Google Scholar]
- 47.Kuijpers EA, den Hartigh J, Savelkoul TJ, de Wolff FA. A method for the simultaneous identification and quantitation of five superwarfarin rodenticides in human serum. J Anal Toxicol. 1995;19:557–62. doi: 10.1093/jat/19.7.557. [DOI] [PubMed] [Google Scholar]
- 48.Vandenbroucke V, Bousquet-Melou A, De Backer P, Croubels S. Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J Vet Pharmacol Ther. 2008;31:437–45. doi: 10.1111/j.1365-2885.2008.00979.x. [DOI] [PubMed] [Google Scholar]
- 49.Gómez-Canela C, Vázquez-Chica A, Lacorte S. Comprehensive characterization of rodenticides in wastewater by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2014;406:345–58. doi: 10.1007/s00216-013-7449-1. [DOI] [PubMed] [Google Scholar]
- 50.Gebauer M. Synthesis and structure-activity relationships of novel warfarin derivatives. Bioorg Med Chem. 2007;15:2414–20. doi: 10.1016/j.bmc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Bachmann KA, Sullivan TJ. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology. 1983;27:281–8. doi: 10.1159/000137881. [DOI] [PubMed] [Google Scholar]
- 52.Eason CT, Fagerstone KA, Eisemann JD, Humphrys S, O’Hare JR, Lapidge SJ. A review of existing and potential New World and Australasian vertebrate pesticides with a rationale for linking use patterns to registration requirements. Int J Pest Manage. 2010;56:109–25. [Google Scholar]
- 53.Holford NH. Clinical pharmacokinetics and pharmacodynamics of warfarin. Understanding the dose-effect relationship. Clin Pharmacokinet. 1986;11:483–504. doi: 10.2165/00003088-198611060-00005. [DOI] [PubMed] [Google Scholar]
- 54.Yan H, Xiang P, Zhu L, Shen M. Determination of bromadiolone and brodifacoum in human blood using LC-ESI/MS/MS and its application in four superwarfarin poisoning cases. Forensic Sci Int. 2012;222:313–7. doi: 10.1016/j.forsciint.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Olmos V, López CM. Brodifacoum poisoning with toxicokinetic data. Clin Toxicol (Phila) 2007;45:487–9. doi: 10.1080/15563650701354093. [DOI] [PubMed] [Google Scholar]
- 56.Jin M-C, Ren Y-P, Xu X-M, Chen X-H. Determination of bromadiolone in whole blood by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Forensic Sci Int. 2007;171:52–6. doi: 10.1016/j.forsciint.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Vindenes V, Karinen R, Hasvold I, Bernard J-P, Mørland JG, Christophersen AS. Bromadiolone poisoning: LC-MS method and pharmacokinetic data. J Forensic Sci. 2008;53:993–6. doi: 10.1111/j.1556-4029.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- 58.Laposata M, Van Cott EM, Lev MH. Case records of the Massachusetts General Hospital. Case 1-2007 – A 40-year-old woman with epistaxis, hematemesis, and altered mental status. N Engl J Med. 2007;356:174–82. doi: 10.1056/NEJMcpc069032. [DOI] [PubMed] [Google Scholar]
- 59.Whitlon DS, Sadowski JA, Suttie JW. Mechanism of coumarin action: significance of vitamin K epoxide reductase inhibition. Biochemistry. 1978;17:1371–7. doi: 10.1021/bi00601a003. [DOI] [PubMed] [Google Scholar]
- 60.Hsia C. Biochemical and Mechanistic Studies of the Interactions Between Vitamin K Antagonists and Vitamin K Epoxide Reductase [Thesis] 2013. [Accessed 18 September 2019]. https://digital.lib.washington.edu/researchworks/handle/1773/21992.
- 61.Bevans CG, Krettler C, Reinhart C, Tran H, Koßmann K, Watzka M, et al. Determination of the warfarin inhibition constant Ki for vitamin K 2,3-epoxide reductase complex subunit-1 (VKORC1) using an in vitro DTT-driven assay. Biochim Biophys Acta. 2013;1830:4202–10. doi: 10.1016/j.bbagen.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 62.Czogalla KJ, Biswas A, Höning K, Hornung V, Liphardt K, Watzka M, et al. Warfarin and vitamin K compete for binding to Phe55 in human VKOR. Nat Struct Mol Biol. 2017;24:77–85. doi: 10.1038/nsmb.3338. [DOI] [PubMed] [Google Scholar]
- 63.Shen G, Cui W, Zhang H, Zhou F, Huang W, Liu Q, et al. Warfarin traps human vitamin K epoxide reductase in an intermediate state during electron transfer. Nat Struct Mol Biol. 2017;24:69–76. doi: 10.1038/nsmb.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Australian Pesticides and Veterinary Medicines Authority. [Accessed 24 June 2019];Public Chemical Registration Information System Search. https://portal.apvma.gov.au/pubcris. [Google Scholar]
- 65.Babcock J, Hartman K, Pedersen A, Murphy M, Alving B. Rodenticide-induced coagulopathy in a young child. A case of Munchausen syndrome by proxy. Am J Pediatr Hematol Oncol. 1993;15:126–30. doi: 10.1097/00043426-199302000-00021. [DOI] [PubMed] [Google Scholar]
- 66.Svendsen SW, Kolstad HA, Steesby E. Bleeding problems associated with occupational exposure to anticoagulant rodenticides. Int Arch Occup Environ Health. 2002;75:515–7. doi: 10.1007/s00420-002-0339-z. [DOI] [PubMed] [Google Scholar]
- 67.Hong J, Yhim H-Y, Bang S-M, Bae SH, Yuh YJ, Yoon S-S, et al. Korean patients with superwarfarin intoxication and their outcome. J Korean Med Sci. 2010;25:1754–8. doi: 10.3346/jkms.2010.25.12.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reuters. Man arrested in Martin Luther King Day bomb plot. [Accessed 25 June 2019]. https://www.reuters.com/article/us-usa-crime-parade/man-arrested-in-martin-luther-king-day-bomb-plot-idUSTRE7287A020110309.
- 69.The Christian Science Monitor. Failed Martin Luther King Day parade bomber gets 32-year sentence. [Accessed 29 June 2019]. https://www.csmonitor.com/USA/Justice/2011/1220/Failed-Martin-Luther-King-Day-parade-bomber-gets-32-year-sentence.
- 70.Feinstein DL, Akpa BS, Ayee MA, Boullerne AI, Braun D, Brodsky SV, et al. The emerging threat of superwarfarins: history, detection, mechanisms, and countermeasures. Ann N Y Acad Sci. 2016;1374:111–22. doi: 10.1111/nyas.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.La Rosa FG, Clarke SH, Lefkowitz JB. Brodifacoum intoxication with marijuana smoking. Arch Pathol Lab Med. 1997;121:67–9. [PubMed] [Google Scholar]
- 72.Sachdev S, Boyd R, Grimsey NL, Connor M. Brodifacoum does not modulate human cannabinoid receptor-mediated hyperpolarization of AtT20 cells or inhibition of adenylyl cyclase in HEK 293 cells. [Accessed 18 September 2019]. https://www.biorxiv.org/content/10.1101/589341v1. [DOI] [PMC free article] [PubMed]
- 73.Anderson SL, Kattappuram RS, Marrs JC, Joseph NM. Intentional brodifacoum ingestion. Am J Med. 2017;130:e27–8. doi: 10.1016/j.amjmed.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 74.De Paula EV, Montalvao SA, Madureira PR, Jose Vieira R, Annichino-Bizzacchi JM, Ozelo MC. Simultaneous bleeding and thrombosis in superwarfarin poisoning. Thromb Res. 2009;123:637–9. doi: 10.1016/j.thromres.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Franco D, Everett G, Manoucheri M. I smell a rat: a case report and literature review of paradoxical thrombosis and hemorrhage in a patient with brodifacoum toxicity. Blood Coagul Fibrinolysis. 2013;24:202–4. doi: 10.1097/MBC.0b013e328358e959. [DOI] [PubMed] [Google Scholar]
- 76.Hirsh J, Dalen JE, Deykin D, Poller L, Bussey H. Oral anticoagulants. Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1995;108(Suppl):231S–46S. doi: 10.1378/chest.108.4_supplement.231s. [DOI] [PubMed] [Google Scholar]
- 77.Hoffman RS, Smilkstein MJ, Goldfrank LR. Evaluation of coagulation factor abnormalities in long-acting anticoagulant overdose. J Toxicol Clin Toxicol. 1988;26:233–48. doi: 10.3109/15563658809000350. [DOI] [PubMed] [Google Scholar]
- 78.Watts RG, Castleberry RP, Sadowski JA. Accidental poisoning with a superwarfarin compound (brodifacoum) in a child. Pediatrics. 1990;86:883–7. [PubMed] [Google Scholar]
- 79.Card DJ, Francis S, Deuchande K, Harrington DJ. Superwarfarin poisoning and its management. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-206360. bcr2014206360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gunja N, Coggins A, Bidny S. Management of intentional superwarfarin poisoning with long-term vitamin K and brodifacoum levels. Clin Toxicol (Phila) 2011;49:385–90. doi: 10.3109/15563650.2011.587126. [DOI] [PubMed] [Google Scholar]
- 81.Berny PJ, Buronfosse T, Lorgue G. Anticoagulant poisoning in animals: a simple new high-performance thin-layer chromatographic (HPTLC) method for the simultaneous determination of eight anticoagulant rodenticides in liver samples. J Anal Toxicol. 1995;19:576–80. doi: 10.1093/jat/19.7.576. [DOI] [PubMed] [Google Scholar]
- 82.Chalermchaikit T, Felice LJ, Murphy MJ. Simultaneous determination of eight anticoagulant rodenticides in blood serum and liver. J Anal Toxicol. 1993;17:56–61. doi: 10.1093/jat/17.1.56. [DOI] [PubMed] [Google Scholar]
- 83.DuVall MD, Murphy MJ, Ray AC, Reagor JC. Case studies on second-generation anticoagulant rodenticide toxicities in nontarget species. J Vet Diagn Invest. 1989;1:66–8. doi: 10.1177/104063878900100118. [DOI] [PubMed] [Google Scholar]
- 84.Dong X, Liang S, Sun H. Determination of seven anticoagulant rodenticides in human serum by ultra-performance liquid chromatography-mass spectrometry. Anal Methods. 2015;7:1884–9. [Google Scholar]
- 85.Guan F, Ishii A, Seno H, Watanabe-Suzuki K, Kumazawa T, Suzuki O. Use of an ion-pairing reagent for high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry determination of anionic anticoagulant rodenticides in body fluids. J Chromatogr B Biomed Sci Appl. 1999;731:155–65. doi: 10.1016/s0378-4347(99)00126-7. [DOI] [PubMed] [Google Scholar]
- 86.Grobosch T, Angelow B, Schönberg L, Lampe D. Acute bromadiolone intoxication. J Anal Toxicol. 2006;30:281–6. doi: 10.1093/jat/30.4.281. [DOI] [PubMed] [Google Scholar]
- 87.Fourel I, Hugnet C, Goy-Thollot I, Berny P. Validation of a new liquid chromatography- tandem mass spectrometry ion-trap technique for the simultaneous determination of thirteen anticoagulant rodenticides, drugs, or natural products. J Anal Toxicol. 2010;34:95–102. doi: 10.1093/jat/34.2.95. [DOI] [PubMed] [Google Scholar]
- 88.Middleberg RA, Homan J. Qualitative identification of rodenticide anticoagulants by LC-MS/MS. In: Langman L, Snozek C, editors. LC-MS in Drug Analysis. Methods in Molecular Biology (Methods and Protocols) Vol. 902. Totowa, NJ: Humana Press; 2012. pp. 139–48. [DOI] [PubMed] [Google Scholar]
- 89.Hernández AM, Bernal J, Bernal JL, Martín MT, Caminero C, Nozal MJ. Analysis of anticoagulant rodenticide residues in Microtus arvalis tissues by liquid chromatography with diode array, fluorescence and mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;925:76–85. doi: 10.1016/j.jchromb.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 90.Zhu L, Yan H, Shen B, Shi Y, Shen M, Xiang P. Determination of bromadiolone and brodifacoum in human hair by liquid chromatography/tandem mass spectrometry and its application to poisoning cases. Rapid Commun Mass Spectrom. 2013;27:513–20. doi: 10.1002/rcm.6477. [DOI] [PubMed] [Google Scholar]
- 91.Qiao Z, Xiang P, Shen B, Shen M, Yan H. Simultaneous Determination of 13 Anticoagulant Rodenticidesin Human Blood by Liquid Chromatography-Tandem Mass Spectrometry and its Application in Three Poisoning Cases. J Forensic Sci. 2018;63:784–92. doi: 10.1111/1556-4029.13613. [DOI] [PubMed] [Google Scholar]
- 92.Yan Z, Li H, Li H, Lai G, Chu J, Guo H, et al. Simultaneous determination of nine anticoagulant rodenticides by ultra-performance liquid chromatography-tandem mass spectrometry with ultrasound-assisted low-density solvent dispersive liquid-liquid microextraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1092:453–8. doi: 10.1016/j.jchromb.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 93.Guo H, Wang J, Wu Y, Liu W, Bu J, Zhao Q. Sensitive and simultaneous determination of nine anticoagulant rodenticides in human blood by UPLC-MS-MS with phospholipid removal pretreatment. J Anal Toxicol. 2018;42:459–66. doi: 10.1093/jat/bky024. [DOI] [PubMed] [Google Scholar]
- 94.Gao X, Li H, Li H, Dong S, Chu J, Guo H, et al. Sensitive determination of nine anticoagulant rodenticides in blood by high resolution mass spectrometry with supported liquid extraction pretreatment. Forensic Sci Int. 2018;292:39–44. doi: 10.1016/j.forsciint.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 95.Ingels M, Lai C, Tai W, Manning BH, Rangan C, Williams SR, et al. A prospective study of acute, unintentional, pediatric superwarfarin ingestions managed without decontamination. Ann Emerg Med. 2002;40:73–8. doi: 10.1067/mem.2002.125449. [DOI] [PubMed] [Google Scholar]
- 96.Donovan JW, Ballard JO, Murphy MJ. Brodifacoum therapy with activated charcoal: effect on elimination kinetics. Vet Hum Toxicol. 1990;32:350. [Google Scholar]
- 97.Booth GS, Mody PZ. Brodifacoum inhalation and its clinical manifestations in a 21-year-old Caucasian man. Lab Med. 2016;47:63–6. doi: 10.1093/labmed/lmv008. [DOI] [PubMed] [Google Scholar]
- 98.Haesloop O, Tillick A, Nichol G, Strote J. Superwarfarin ingestion treated successfully with prothrombin complex concentrate. Am J Emerg Med. 2016;34:116.e1–2. doi: 10.1016/j.ajem.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 99.Zupancić-Salek S, Kovacević-Metelko J, Radman I. Successful reversal of anticoagulant effect of superwarfarin poisoning with recombinant activated factor VII. Blood Coagul Fibrinolysis. 2005;16:239–44. doi: 10.1097/01.mbc.0000169215.70184.56. [DOI] [PubMed] [Google Scholar]
- 100.Goldstein JN, Refaai MA, Milling TJ, Jr, Lewis B, Goldberg-Alberts R, Hug BA, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385:2077–87. doi: 10.1016/S0140-6736(14)61685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dezee KJ, Shimeall WT, Douglas KM, Shumway NM, O’malley PG. Treatment of excessive anticoagulation with phytonadione (vitamin K): a meta-analysis. Arch Intern Med. 2006;166:391–7. doi: 10.1001/.391. [DOI] [PubMed] [Google Scholar]
- 102.Watson HG, Baglin T, Laidlaw SL, Makris M, Preston FE. A comparison of the efficacy and rate of response to oral and intravenous Vitamin K in reversal of over-anticoagulation with warfarin. Br J Haematol. 2001;115:145–9. doi: 10.1046/j.1365-2141.2001.03070.x. [DOI] [PubMed] [Google Scholar]
- 103.Fiore LD, Scola MA, Cantillon CE, Brophy MT. Anaphylactoid reactions to vitamin K. J Thromb Thrombolysis. 2001;11:175–83. doi: 10.1023/a:1011237019082. [DOI] [PubMed] [Google Scholar]
- 104.Yu H-Y, Lin J-L, Fu J-F, Lin J-H, Liu S-H, Weng C-H, et al. Outcomes of patients with rodenticide poisoning at a far east poison center. Springerplus. 2013;2:505. doi: 10.1186/2193-1801-2-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hussain N, Hussain F, Haque D, Saeed S, Jesudas R. An Outbreak of Brodifacoum Coagulopathy Due to Synthetic Marijuana in Central Illinois. Mayo Clin Proc. 2018;93:957–8. doi: 10.1016/j.mayocp.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 106.Mycyk MB. Synthetic Cannabinoid-Associated Coagulopathy. N Engl J Med. 2019;380:100–1. doi: 10.1056/NEJMc1814118. [DOI] [PubMed] [Google Scholar]
- 107.Wang JJ, Howland MA, Biary R. Synthetic Cannabinoid-Associated Coagulopathy. N Engl J Med. 2019;380:101. doi: 10.1056/NEJMc1814118. [DOI] [PubMed] [Google Scholar]
- 108.Kelkar AH, Tarantino MD, Roberts JC. Synthetic Cannabinoid-Associated Coagulopathy. N Engl J Med. 2019;380:101–2. doi: 10.1056/NEJMc1814118. [DOI] [PubMed] [Google Scholar]
- 109.British national formulary. British Medical Association: Royal Pharmaceutical Society of Great Britain; [Accessed 18 September 2019]. https://www.bnf.org. [Google Scholar]
- 110.Underwood EL, Sutton J, Ellis IK, Qualls B, Zamber J, Walker BN. Prolonged coagulopathy after brodifacoum exposure. Am J Health Syst Pharm. 2014;71:639–42. doi: 10.33176/AACB-19-00029. [DOI] [PubMed] [Google Scholar]