Abstract
Background
Infective endocarditis (IE) is an infection involving either native or prosthetic heart valves, the endocardial surface of the heart or any implanted intracardiac devices. IE is a rare condition affecting 3–15 patients per 100,000 population. In-hospital mortality rates in patients with IE remain high at around 20% despite treatment advances. There is no consensus recommendation favoring either bioprosthetic valve or mechanical valve implantation in the setting of IE; patient age, co-morbidities and preferences should be considered selecting the replacement prosthesis.
Methods
A systematic review and meta-analysis of studies reporting the outcomes of patients undergoing bioprosthetic or mechanical valve replacement for infective endocarditis with data extracted for overall survival, valve reinfection rates and valve reoperation.
Results
Eleven relevant studies were identified, with 2,336 patients receiving a mechanical valve replacement and 2,057 patients receiving a bioprosthetic valve replacement. There was no significant difference for overall survival between patients treated with mechanical valves and those treated with bioprosthetic valves [hazard ratio (HR) 0.94, 95% confidence interval (CI): 0.73–1.21, P=0.62]. There was no significant difference in reoperation rates between patients treated with a bioprosthetic valve and those treated with a mechanical valve (HR 0.82, 95% CI: 0.34–1.98, P=0.66) and there was no significant difference in the rate of valve reinfection rates (HR 0.95, 95% CI: 0.48–1.89, P=0.89).
Conclusions
The presence of infective endocarditis alone should not influence the decision of which type of valve prosthesis that should be implanted. This decision should be based on patient age, co-morbidities and preferences.
Keywords: Valve replacement, tissue valve, bioprosthesis, bioprosthetic, mechanical, infective endocarditis
Introduction
Infective endocarditis (IE) is an infection involving either native or prosthetic heart valves, the endocardial surface of the heart or any implanted intracardiac devices (1). The intact endocardium is an effective barrier to infection, with the risk of endocarditis rising for individuals with congenital intracardiac defects, acquired degenerative valvular lesions and implanted intra-cardiac prostheses (2); however, it remains an important condition with significant associated morbidity and mortality. IE is a rare condition affecting 3–15 patients per 100,000 population (3-5). Infective endocarditis is commonly diagnosed according to the modified Duke criteria (6) which determines the likelihood of a patient having IE based on organisms detected on blood culture, echocardiographic images and clinical examination. In patients with prosthetic valve replacement, the risk of developing prosthetic endocarditis is 2.2–3.7% (7-9). Aggressive surgical treatment is often required with surgery indicated in up to half of patients with native valve endocarditis (10,11). In-hospital mortality rates in patients with IE remain high at around 20% despite treatment advances (11).
Indications for surgical intervention for left-sided IE are relatively well defined with urgent intervention suggested for patients without adequate control of sepsis despite appropriate antibiotics, for patients with evidence of intra-cardiac abscess formation or other paravalvular extension, for patients with heart failure secondary to valve destruction or fistula formation, and to prevent catastrophic embolic phenomena in the presence of large mobile vegetations (12,13). Where possible, valve repair is preferred over replacement, particularly for right-sided and mitral valve IE. There is no consensus recommendation for either bioprosthetic valve or mechanical valve in the setting of IE and patient age, co-morbidities and preferences should be considered in making the decision for valve selection (12,14).
In the general population, the European Society of Cardiology suggest treatment with mechanical valves under the age of 60 for a valve in the aortic position or under the age 65 for a valve in the mitral position (15). The American Heart Association guidelines recommend a mechanical valve prosthesis in patients under the age of 50 and a bioprosthetic valve prosthesis over the age of 70. In between these ages, patient factors and preferences will determine prosthesis type (16). This meta-analysis was therefore undertaken to evaluate the surgical outcomes comparing mechanical valve replacement and bioprosthetic valve replacement in infective endocarditis.
Methods
Literature search strategy
This meta-analysis was performed in accordance with PRISMA recommendations and guidance. The search strategy was employed to search electronic databases EMBASE, Ovid Medline, the entire Cochrane Central Register of Controlled Trails (CCRCT), Cochrane Database of Systematic reviews (CDSR), the Database of Abstracts of Reviews of Effects (DARE) and the ACP journal club from their inception to 03 March 2019. The search strategy included search terms for “(tissue valve OR bioprosthesis OR biological valve OR bioprosthetic valve OR xenograft OR mechanical valve) AND endocarditis”. The references of previous systematic reviews were assessed to ensure no additional publications were missed.
Selection criteria
Eligibility for inclusion in this systematic review and meta-analysis included papers that assessed the outcomes of patients undergoing conventional valve replacement with a bioprosthetic or mechanical valve prosthesis undertaken in adult patients for the treatment of infective endocarditis. In order to ensure sufficient center experience, papers were only included if more than 15 cases were reported in each arm. English language papers with at least 1-year follow-up were considered for assessment. Studies were excluded if there was inadequate data regarding the outcomes and for patients treated with homograft as a bioprosthesis. If centers reported outcomes of overlapping patient series, then the most contemporary series was analyzed. Conference abstracts, case reports, editorials, expert opinion, reviews and expert opinion were excluded.
Data extraction
For the assessed papers, data was extracted from the reviewed text, tables and figures. Data was extracted independently by two of the authors (CD Flynn, NP Curran) and any discrepancies were reviewed and discussed until consensus was reached. The recorded parameters were: number of cases in series, procedure undertaken, urgency of procedure, average age, average follow-up, early death, late death, valve reinfection, valve reoperation.
Statistical analysis
Meta-analysis of incidence rates of post-operative complications including valve reinfection, reoperation and overall survival. Hazard ratios were calculated from source Kaplan-Meier curves according to Tierney et al. methods (17) with mechanical valve being the reference group. Incidence data was assessed using Comprehensive Meta-analysis v3.3 (Biostat, Englewood, USA). Comparative outcomes were assessed using Review Manager v5.3 (Cochrane Collaboration, Copenhagen, Denmark). Due to the varied patient populations, a random effects model was chosen for analysis. Data heterogeneity was assessed with the Cochrane Q statistic with P value <0.05 being significant and the I2 test statistic an I2 value greater than 50% denoting significant heterogeneity. Publication bias was assessed by generation of funnel plot and assessment using Egger’s test produced with Comprehensive Meta-analysis. Sensitivity analysis was performed using a “leave-one-out” analysis. Individual patient survival data, reinfection and reoperation data were reconstructed using an iterative algorithm that was applied to digitized source Kaplan-Meier curves and subsequently aggregated and graphed (18) using R (R Foundation for Statistical Computing, Vienna, Austria).
Results
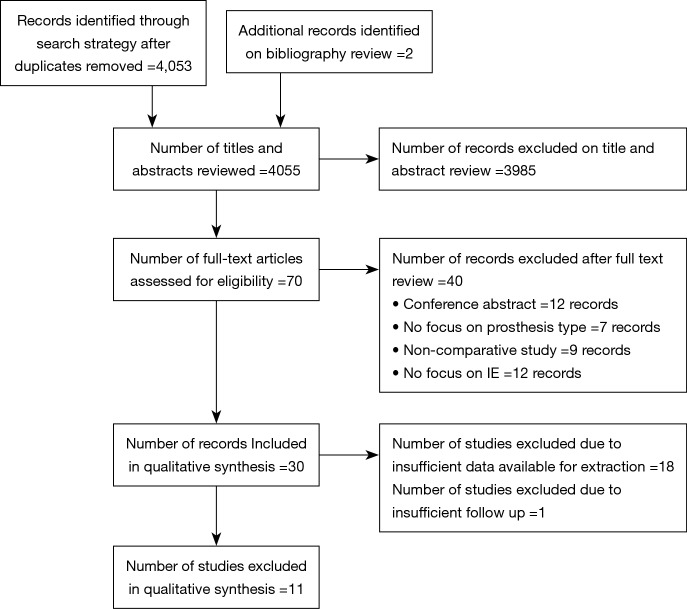
The search strategy revealed 4,053 citations for review after duplications were removed with an additional two studies added for full text review on reviewing reference lists. After full review, 11 papers met the pre-determined inclusion criteria (19-29) (Figure S1). The included publications had a total analyzed patient population of 4,393 patients with 2,336 patients receiving a mechanical valve replacement and 2,057 patients receiving a bioprosthetic valve replacement. The overall study characteristics are detailed in Table 1. An assessment of bias was made for all studies which demonstrated significant risks of bias in all included studies, largely based on study design (Figure S2).
Figure S1.
Study selection. PRISMA flow diagram detailing the systematic review to identify studies reporting the outcomes of bioprosthetic valve replacement compared to mechanical valve replacement for infective endocarditis.
Table 1. Study characteristics.
| Study | Recruitment years | Location of institution | Study design | Patient population | Number of patients (n) | Follow up (median) [range] | Patient age (years) [range] |
|---|---|---|---|---|---|---|---|
| Reul 1989 | 1979–1984 | Houston, Texas (USA) | Single-centre retrospective | NVE and PVE | Total =185 | BV =22 months [6–68 months] | BV =49 [8–77] |
| BV =88 | MV =18 months [6–70 months] | MV =46.6 [11–78] | |||||
| MV =97 | |||||||
| Lytle 1996 | 1975–1992 | Cleveland, Ohio (USA) | Single-centre retrospective (registry data) | PVE | Total =128 | – | – |
| BV =52 | |||||||
| Mv =76 | |||||||
| Moon 2001 | 1964–1995 | Stanford, California (USA) | Single-centre retrospective | Left-sided valve IE (NVE and PVE) | Total =286 | Overall =15.3 years [0–29 years] | Overall =49 |
| BV =221 | |||||||
| MV =65 | |||||||
| Fedoruk 2009 | 1975–2000 | British Columbia, Vancouver (Canada) | Multi-centre retrospective (registry data) | NVE | Total =358 | Overall =5.5±5.5 years | Overall =48.8±15.9 [18–88] |
| BV =189 | BV =6.1±6.1 years | BV =51.6±17.3 | |||||
| MV =169 | MV =4.9±4.5 years | MV =45.6±13.5 | |||||
| Musci 2010 | 1986–2008 | Berlin (Germany) | Single-centre retrospective | PVE | Total =210 | – | – |
| BV =165 | |||||||
| Mv =45 | |||||||
| Nguyen 2010 | 1998–2000 | France | Multicentre prospective | Aortic valve IE (NVE and PVE) | Total =140 | – | Overall =57.9±13.0 |
| BV =31 | BV =63.2±13.6 | ||||||
| MV =109 | MV =57.3±11.6 | ||||||
| Jassar 2012 | 2000–2010 | Philadelphia, Pennsylvania (USA) | Single-centre retrospective | ARR for IE | Total =98 | BV =31.7±28.5 months | BV =62.7±15.1 |
| BV =43 | MV =27.4±22.1 months | MV =51.8±12.7 | |||||
| MV =55 | |||||||
| Leither 2013 | 2004–2007 | USA | Multi-centre retrospective (registry data) | Renal-replacement therapy patients with NVE affecting aortic or mitral valve | Total =1267 | – | – |
| BV =561 | |||||||
| MV =706 | |||||||
| Greason 2014 | 1974–2009 | Rochester, Minnesota (USA) | Single-centre retrospective (registry data) | PVE affecting the MV | Total =36 | Overall =5.7 years [0.6–24 years] | Overall =68 [15.8–82.3] |
| BV =18 | |||||||
| MV =18 | |||||||
| Delahaye 2015 | 2000–2006 | Global | Multicentre prospective | Patients with IE undergoing valve surgery in the acute phase | Total =1,467 | – | Overall =56.6±15.7 |
| MV =917 | BV =61.6±15.2 | ||||||
| MV =917 | MV =53.6±15.2 | ||||||
| Kim 2016 | 2002–2014 | Boston, Massachusetts (USA) | Multicentre prospective | Adult patients with AVE | Total =218 | Overall =29.4 months [4.7–72.6 months] | BV =59.8±14.6 |
| BV =139 | MV =47.2±14.5 | ||||||
| MV =79 |
NVE, native valve endocarditis; PVE, prosthetic valve endocarditis; IE, infective endocarditis; BV, bioprosthetic valve; MV, mechanical valve.
Figure S2.
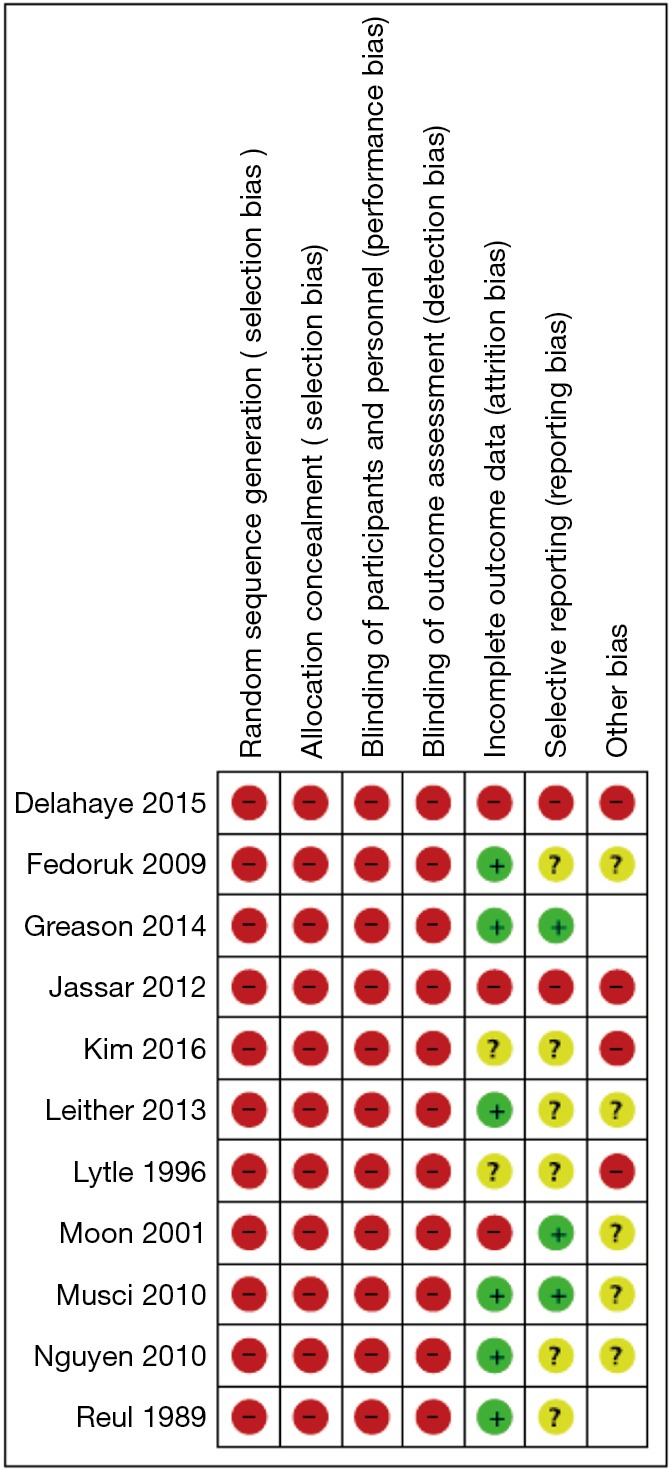
Risk of bias table. Thorough assessment of biases for all studies (red = high risk, yellow = uncertain risk, green = low risk).
The mean age of patients receiving bioprosthetic valves calculated from six studies reporting mean age of patients (19,20,22,23,28,29) was 59.2 years old (95% CI: 58.2–60.1). The mean age of patients receiving mechanical valves was 52.1 years old (95% CI: 51.4–52.9). Overall, patients receiving a mechanical valve were younger by 7.8 years (95% CI: 6.6–9.0, P<0.001).
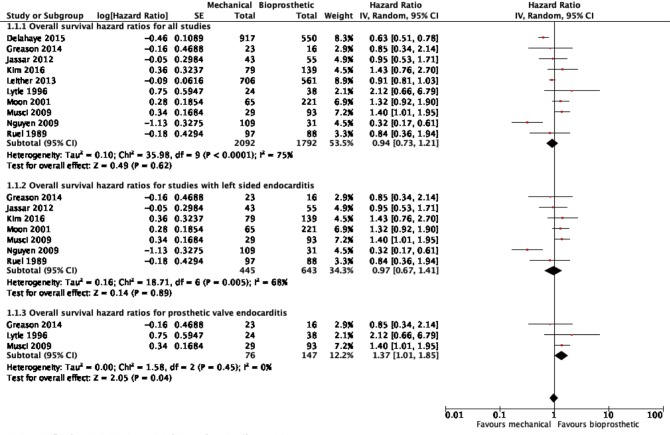
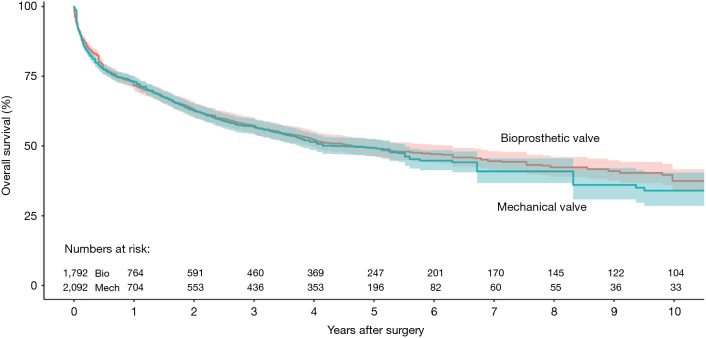
Ten studies reported overall survival data (19,21-29), drawing outcomes data from 3,884 patients (1,792 patients receiving bioprosthetic valves and 2,092 patients receiving mechanical valves). Meta-analysis of derived hazard ratio (HR) demonstrated no significant difference for overall survival between patients treated with mechanical valves and those treated with bioprosthetic valves (HR 0.94, 95% CI: 0.73–1.21; P=0.62) (Figure 1). There was significant heterogeneity Cochrane Q-statistic P value <0.001, I2 =75%. Post-hoc subgroup analysis performed to explore sources of heterogeneity demonstrated that analyzing outcomes of left sided infective endocarditis (21-23,25-29) showed no significant difference in overall survival, regardless of valve type, HR 0.97 (95% CI: 0.67–1.41, P=0.89), there was still significant heterogeneity in data, Cochrane q-statistic P=0.005, I2 =68% (Figure 1). There was a possible benefit for overall survival with bioprosthetic valve replacement in the treatment of prosthetic valve endocarditis (21,25,27) (HR 1.37, 95% CI: 1.01–1.85, P=0.04) (Figure 1). Composite survival curves demonstrated overall survival for bioprosthetic valves of 71.8%, 57.3%, 49.2%, and 37.5% at 1-, 3-, 5-, and 10-year, respectively, and 73.2%, 56.8%, 49.3%, and 34.0% at 1-, 3-, 5-, and 10-year, respectively, for mechanical valves (Figure 2). Leave-one-out sensitivity analysis did not demonstrate any significant impact on overall outcomes with any one study being removed. There was no evidence of publication bias on visual inspection of a funnel plot of the standard error by log hazard ration for overall survival (Figure S3), nor by Egger’s test (P=0.358).
Figure 1.
Forest plot detailing survival outcomes.
Figure 2.
Composite Kaplan-Meyer curve detailing overall survival for patients receiving bioprosthetic and mechanical valve for infective endocarditis (shaded region represents 95% confidence intervals).
Figure S3.
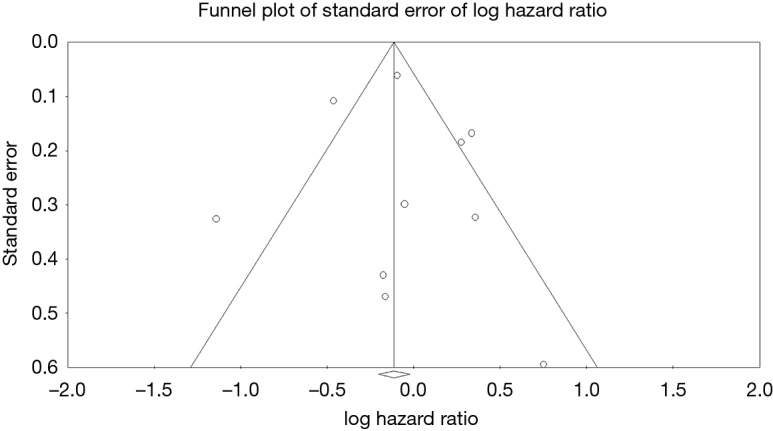
Funnel plot of standard error of log hazard ratio for overall survival.
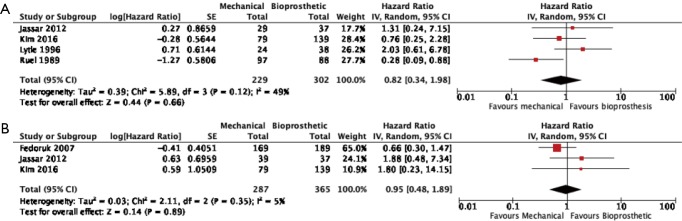
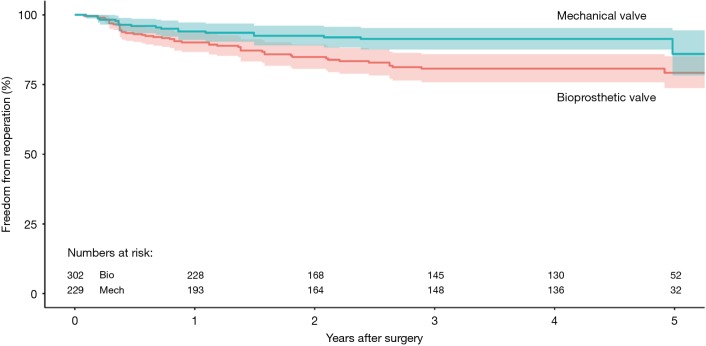
Four studies reported data on reoperation on valve prosthesis (22,23,25,29), drawing outcomes data from 531 patients (302 patients receiving bioprosthetic vales and 229 patients receiving mechanical valves). Meta-analysis of derived HR demonstrated no significant difference between reoperation rates between patients treated with a bioprosthetic valve and those treated with a mechanical valve (HR 0.82, 95% CI: 0.34–1.98, P=0.66) There was moderate heterogeneity with the Cochrane q statistic P=0.12, I2 =49% (Figure 3). Composite survival curves demonstrated freedom from reoperation for bioprosthetic valves of 90.1%, 80.7%, 79.2%, and 56.9% at 1-, 3-, 5-, and 10-year, respectively, and 94.1%, 91.3%, 86.0%, and 75.6% at 1-, 3-, 5-, and 10-year, respectively, for mechanical valves (Figure 4). Leave-one-out sensitivity analysis did not demonstrate any significant difference in overall effect on outcomes. There was no evidence of publication bias on visual inspection of a funnel plot of the standard error by log hazard ration for reoperation rates (Figure S4). Egger’s test was not applied due to the small number of studies being assessed.
Figure 3.
Forest plots detailing outcomes of (A) valve reoperation; (B) valve reinfection.
Figure 4.
Composite Kaplan-Meyer curve detailing freedom from reoperation for patients receiving bioprosthetic and mechanical valve for infective endocarditis (shaded region represents 95% confidence intervals).
Figure S4.
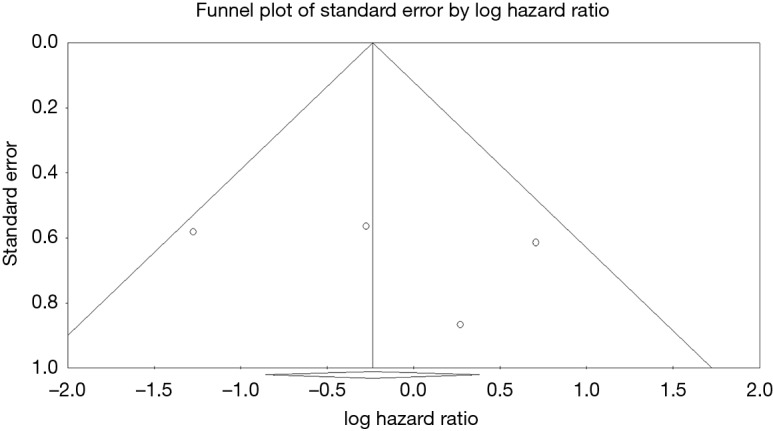
Funnel plot of standard error of log hazard ratio for reoperation rates.
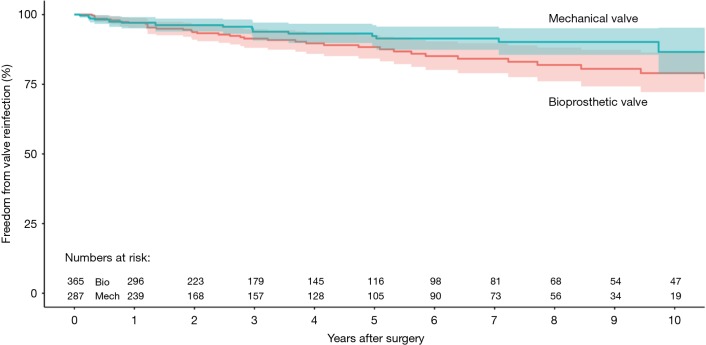
Three studies reported data on valve reinfections (20,22,23) drawing on data from 652 patients (365 patients receiving bioprosthetic valves and 287 patients receiving mechanical valves). Meta-analysis of derived hazard ratios demonstrated no significant difference in the rate of valve reinfection rates (HR 0.95, 95% CI: 0.48–1.89; P=0.89). There was minimal heterogeneity in this small group of studies with the Cochrane Q-statistic P value of 0.35, I2 =5% (Figure 3B). Composite survival curves demonstrated a freedom from valve reinfection for bioprosthetic valves of 97.0%, 91.4%, 88.3%, and 79.0% at 1-, 3-, 5-, and 10-year, respectively, and 97.1%, 93.8%, 92.3%, and 86.6% at 1-, 3-, 5-, and 10-year, respectively, for mechanical valves (Figure 5). Leave-one-out sensitivity analysis did not demonstrate any significant difference in overall effect on outcomes. There was no evidence of publication bias on visual inspection of a funnel plot of the standard error by log hazard ration for valve reinfection rates (Figure S5). Egger’s test was not applied due to the small number of studies being assessed.
Figure 5.
Composite Kaplan-Meyer curve detailing freedom from valve reinfection for patients receiving bioprosthetic and mechanical valve for infective endocarditis (shaded region represents 95% confidence intervals).
Figure S5.
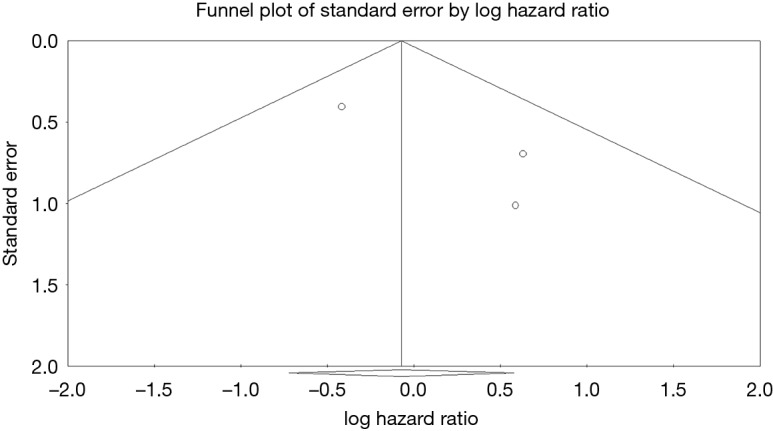
Funnel plot of standard error of log hazard ratio for valve reinfection rates.
Discussion
The evidence supporting indications for valve selection in infective endocarditis is currently limited. This meta-analysis suggests that at present there is no clear evidence that one prosthetic type should be selected over any other, which is nonetheless an important finding. There was significant heterogeneity when reviewing data from all analyses, which may relate to the varied patient populations being examined with variation in valve choices and operations. It was not possible to correct for severity of infective endocarditis, urgency status, concomitant operations or co-morbidities. An important consideration is that two of the three prospective studies (19,28) demonstrated a significant survival advantage for patients receiving mechanical valves, suggesting there is possibly an area of future research to develop more robust evidence.
It may have been expected that bioprosthetic valves would have worse overall survival as this patient cohort was significantly older compared to those receiving mechanical valves. Several studies reported that increasing age was an independent risk factor for overall mortality in patients with endocarditis (19,21,27,28). Nguyen et al. (28) note that advancing age increased the risk for overall 5-year mortality in infective endocarditis (HR 1.03; CI: 1.00–1.05, P=0.037), which was most pronounced in the small number of patients who received a bioprosthetic valve under the age of 65. These patients had a markedly increased 5-year mortality compared to patients receiving a mechanical valve (HR 4.14; 95% CI: 1.27–13.45, P=0.018). However, patients receiving bioprosthetic valves in that study were older overall and had more medical co-morbidities including liver disease (OR 21.86) and severe left ventricle (LV) impairment (OR 14.11), therefore confounding the interpretation of the results. Musci et al. (27) also note age at operation as independent risk factor for early mortality. In contrast to Nguyen et al., Moon et al. (26) found no difference in overall mortality between mechanical and bioprosthetic valves in patients under the age of 60. Unfortunately, there was insufficient data to perform adequate meta-regression to correct for patient age, co-morbidities or pre-operative status in this meta-analysis.
The choice of valve in the general population is determined by balancing the risk of anticoagulation-related and thromboembolic complications that may be associated with mechanical valves versus the risk of structural valve degeneration and the requirement for redo valve surgery for patients receiving bioprosthetic valves. The cut-off for patients receiving a mechanical valve versus a bioprosthetic valve is yet to be firmly established with conflicting evidence regarding long-term outcomes for the two valves. Some evidence refutes an excess of mortality in middle-aged patients receiving bioprosthetic valves, including a large propensity-matched study of non-elderly patients. McClure et al. (30) demonstrated no significant difference in late mortality at 18 years with a cumulative survival estimated at 18 years of 60% for bioprosthetic valve and 51% for mechanical valves, though not unsurprisingly they found an increase in the requirement for reoperation for bioprosthetic valves. Similarly, although Kulick et al. (31) demonstrated no difference in survival for patients aged between 50 to 65 years requiring aortic or mitral valve replacement, mechanical valves were associated with a significant increased risk of thromboembolism (HR 4.1; CI: 1.3–12.7, P=0.01) and an increased risk of reoperation associated with bioprosthetic valves (HR 7.1; CI: 1.8-27.8 P=0.005). However, a recent meta-analysis of five studies by Diaz et al. (32), assessed valve replacement in patients between the age of 50 to 70, demonstrated a statistically significant reduction in long-term mortality for patients receiving mechanical valves (HR 0.86; 95% CI: 0.76–0.97, P=0.01). They did note an increased risk of major bleeding with mechanical valve recipients.
There remains no simple answer for valve choice, particularly in middle aged patients. It appears that the presence of IE alone should not influence the decision outside other patient factors. Newer-generation bioprosthetic valves with novel preservation techniques could optimistically reduce the rate of structural valve degeneration, with ongoing trials currently at 4 years follow-up and no episodes of structural valve degeneration to date (33), although long-term follow-up is required to determine if this truly is an improvement on current technologies. Another addition to the decision-making algorithm is the ever-increasing experience with valve-in-valve transcatheter aortic valve replacement which has offered equivalent mid-term survival rates with reduced early morbidity compared to conventional reoperative surgery (34-36). However, an option for valve-in-valve replacement is questionable for valves smaller than 21 mm, suggesting that expected valve size and the consequences for future management should play a role in prosthesis choice. On the other hand, the latest generation mechanical valves (On-x valve) have been proven to be safe with reduced doses of anticoagulation and have an approved target INR range of 1.5–2.0. This reduction in anticoagulation has significantly reduced major and minor bleeding events without causing a significant increase in thromboemboli (37). This may address some of the major drawbacks associated with mechanical valve replacement.
There are several limitations that must be considered when interpreting results from the present review. Data has been collected from a relatively small number of studies that have been drawn from markedly different patient populations. Furthermore, all studies are at a high risk of selection bias given the non-randomized and non-blinded nature of the study. In particular, many studies have stark differences between the bioprosthetic and mechanical valve patient populations making accurate analysis challenging. Unfortunately, there is insufficient published data to attempt to correct for some of these differences.
Conclusions
This meta-analysis did not identify any evidence of a significant survival advantage, reduction in valve reinfection or reoperation rate for any particular conventional prosthesis choice in the treatment of patients with infective endocarditis. It is clear that evidence guiding our decision making in this area is currently limited and is an area that would benefit from future research. The choice of prosthesis should continue to be made with the conscientious consideration to the patients age, co-morbidities and patient preferences.
Expert opinion: choice of prosthetic valve in infective endocarditis
Zegri-Reiriz, Tauron
In patients with infective endocarditis and indication for surgery, the general recommendations regarding the choice of prosthesis (biological or mechanical) do not differ with respect to the general population. Both have shown similar rates of post-operative mortality and reinfection.
The European guidelines recommend mechanical prostheses for those patients under 60 years in the aortic position and for those under 65 years in the mitral position (12). According to the American Heart Association, mechanical prostheses are recommended in patients <50 years and biological prostheses in <70 years (13).
However, it should be noted that these patients deserve some important considerations. In many cases, they have a serious clinical condition which is the result of uncontrolled infection, acute heart failure or septic embolism.
In this scenario, the risk of bleeding and the implications of anticoagulation in the short and medium term should be carefully evaluated. Thus, some young patients with a high risk of bleeding could benefit from a bioprosthesis if carefully selected.
In worse cases, there is a risk of severe coagulopathy, hepatopathy or in an embolic stroke, due to the risk of hemorrhagic transformation. In this population of critically ill patients who may need mechanical circulatory support, a bioprosthesis would be preferable. On the other hand, a sutureless bioprosthesis could be a reasonable alternative for surgical treatment of prosthetic aortic valve endocarditis with significant involvement of the aortic annulus (38).
These decisions should be individualized in each case, balancing the risk of bleeding due to anticoagulation against the possible benefit of implanting a mechanical prosthesis in young patients.
Expert opinion: choice of valve prosthesis in patients undergoing valve surgery for infective endocarditis
Pettersson, Coselli
In this issue of the Annals of Cardiothoracic Surgery, Flynn and coworkers present a systematic review and meta-analysis comparing outcomes after surgery for left sided infective endocarditis (IE) using mechanical prosthesis versus bioprosthesis. Both choices produced very similar outcomes with slight trends favoring the mechanical valve with regard to freedom from reoperation and risk of recurrent endocarditis; but a possible benefit for overall survival with bioprosthetic valve replacement.
Current AATS Guidelines (39) for surgical treatment of endocarditis makes recommendations related to the choice of replacement valve: valve repair is preferable when possible. When replacement is required and the disease is confined to cusps, leaflets, or valve prosthesis, choice of replacement valve—mechanical or tissue prosthesis should be based on usual criteria.
With regard to risk of recurrent endocarditis the guidelines say: “For patients requiring valve replacement, there is little evidence that risk of recurrent infection is different between mechanical and tissue prostheses.” Also, in Flynn and coworkers’ paper there is no significant difference in risk of recurrent endocarditis, however, in recent registry studies from Sweden and Denmark when comparing incidence of endocarditis following valve replacement of non-infected pathologies, bioprostheses were associated with higher risk of endocarditis than mechanical prostheses (40,41).
The main limitation with Flynn and coworkers’ meta-analysis is that it doesn’t take into consideration all the nuances, reasons for one choice over the other, information about the pathology and complications (e.g., stroke or intracranial bleed), and other factors guiding the surgeons’ and patients’ choice of replacement valve. It is still true that “Patients with IE are often very sick and have suffered strokes, so using allografts or bioprosthetic valves simplifies management and avoids postoperative anticoagulation, lowering the risk of hemorrhagic conversion of strokes and other bleeding complications.” When the disease is invasive and the annulus destroyed, extensive debridement, reconstruction and usually root replacement are required. In this situation many surgeons, including us believe that an allograft is a better choice than a prosthetic valve conduit, be it mechanical or bioprosthetic valve.
Flynn and coworkers should be thanked and congratulated for confirming that current guidelines and practices for choice of prosthetic type for patients with infective endocarditis are adequate and produce very comparable outcomes with both mechanical and bioprosthetic valves.
Expert opinion: individuality2
Misfeld
In the current issue of the Annals of Cardiothoracic Surgery, Flynn et al. present a systematic review and meta-analysis of eleven studies, published between 1989 and 2016, about outcomes of mechanical versus bioprosthetic valve substitutes in infective endocarditis (IE). The analysis did not identify a significant survival advantage, reduction in valve reinfection or reoperation with one of the valve types.
There is no clear agreement on the best prosthetic valve choice, neither for non-infective nor for IE. This is represented by different recommendations of the European and American guidelines (39,42). Despite current recommendations, mechanical valves compared to bioprosthetic valve substitutes are probably used in the ratio 1:10, with more tissue valves being implanted even in younger patients (43,44). Bioprostheses are increasingly implanted not only because anticoagulation and valve noise with mechanical valves remaining important aspects for most patients, but also because interventional options, such as valve-in-valve procedures in tissue valves, have become an attractive second step when biological valves fail (43).
Whatever guidelines recommend, neither the patient, nor the surgeon can foresee, what kind of impact the choice of a valve (biological valve: risk of reoperation, but patient will “forget” about having a valve substitute in between; mechanical valve: valve noise and regular reminder because of regular anticoagulation checks) has for the patient during daily life.
Therefore, it remains an individual decision, which valve type should be used, independent of the underlying disease (valve surgery with/without IE). This individual decision is not only a patient related aspect, but also surgeon related. Patients with IE, especially if complicated by cerebral embolism, will most likely have a tissue valve, despite patient age. On the other hand, surgeon related factors, i.e., not being experienced with the use of allografts, may also influence the type of valve used.
As none of the valve substitutes available for the treatment of IE has been shown to have a clear advantage over the others, surgeons should use the valve substitute they are most familiar and experienced with under the background of patient related factors.
Expert opinion: prosthetic valve endocarditis, still a dilemma
Antunes
In the paper published in this issue of the Annals of Cardiothoracic Surgery, Flynn et al. analyse the incidence of prosthetic valve endocarditis (PVE) in patients with biological and mechanical prostheses. They performed a meta-analysis of studies reporting the outcomes of patients undergoing valve replacement for infective endocarditis (IE), both native and prosthetic. The authors found that at 10-year follow-up, there were no differences in overall survival, and freedom from valve reinfection and reoperation between the two types of prostheses. However, the series included in the study are mostly historical and include both native and prosthetic endocarditis, and many types of prostheses of both groups.
The results are not surprising, but I cannot completely agree with the conclusion derived by the authors that “the presence of infective endocarditis alone should not influence the decision of which type of valve prosthesis that should be implanted”. Although the data is not provided by the authors, the fact is that there was a significant incidence of PVE after valve replacement in IE. There is no consensus with regard to favouring either bioprosthetic or mechanical valve implantation in the setting of IE. Hence, it is not a question of which of these two prostheses but if there are alternatives; and, in my view, there is an alternative, especially useful in the aortic position with significant disruption of the annulus—the homograft.
Several works appear to indicate that homografts may be more resistant to infection than either biological or mechanical prostheses (45,46). My own experience also appears to demonstrate that superiority (47). However, others have shown no difference in the rates of re-infection (23). Implantation of homografts may be technically more demanding and, similarly to bioprostheses, they have the problem of biodegradation. In any case, the pliability of the homograft facilitates the treatment of the extensively disrupted aortic annuli often seen in PVE (48). Some authors have also proposed the use of stentless xenografts for the same indications (49). Finally, the Ross operation has also been advocated in these situations (50), but it is a surgically challenging procedure to be used in an already complex anatomical situation.
Expert opinion: do not radically position yourself in the surgery for infective Endocarditis
Mestres, Quintana
In this issue of the Annals of Cardiothoracic Surgery, Flynn et al. used meta-analysis to understand surgical outcomes comparing mechanical and bioprosthetic valve replacement in infective endocarditis (IE). They found no significant difference in overall survival, reoperation and valve reinfection rates between patients with mechanical or bioprosthetic valves.
The decision of what type of valve to use in the setting of IE has been discussed for over sixty years since the first surgical replacements (51,52), and remains a challenging and controversial topic. In addition to considerations of expected anticoagulation-related events and device reoperations (53), IE is another factor to consider when choosing a prosthesis as this population has higher surgical risk. Current IE guidelines do not support one device over another due to the lack of evidence on superiority (54).
In their analysis, the authors found substantial heterogeneity in the studies and were unable to correct for severity of IE, urgency status, concomitant operations or co-morbidities. There are additional limitations in the Flynn et al. meta-analysis (51). It was impossible to separate by valve positions and type of device. It is difficult to understand if under the term “bioprostheses”, stentless valves and homografts were included. Most homograft implants in IE correspond to the aortic position. Patients implanted with a homograft are at higher risk than those implanted with stented bioprosthesis as they have extravalvular spread of the infection, meaning the infection is more destructive, requiring extensive reconstruction, especially in reoperations for prosthetic valve IE (55,56).
Considering the complexity of IE and the process of choosing a valve, and difficulty in producing high quality clinical data for this surgical population, we should not adopt radical positions, with or without practice recommendations. Guidelines are recommendations, not the law, and all options must be carefully individualized. The treating team makes considered decisions based on preoperative condition, comorbidities, age among other variables, integrating this and acting according to experience, expertise and local resources.
Acknowledgments
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016;387:882-93. 10.1016/S0140-6736(15)00067-7 [DOI] [PubMed] [Google Scholar]
- 2.Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol 2011;8:322. 10.1038/nrcardio.2011.43 [DOI] [PubMed] [Google Scholar]
- 3.Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol 2017;69:325-44. 10.1016/j.jacc.2016.10.066 [DOI] [PubMed] [Google Scholar]
- 4.Hoen B, Alla F, Selton-Suty C, et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 2002;288:75-81. 10.1001/jama.288.1.75 [DOI] [PubMed] [Google Scholar]
- 5.Dzupova O, Machala L, Baloun R, et al. Incidence, predisposing factors, and aetiology of infective endocarditis in the Czech Republic. Scand J Infect Dis 2012;44:250-5. 10.3109/00365548.2011.632643 [DOI] [PubMed] [Google Scholar]
- 6.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633-8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 7.Brennan JM, Edwards FH, Zhao Y, et al. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: Results from the society of thoracic surgeons adult cardiac surgery national database. Circulation 2013;127:1647-55. 10.1161/CIRCULATIONAHA.113.002003 [DOI] [PubMed] [Google Scholar]
- 8.Hellgren L, Granath F, Ekbom A, et al. Biological versus mechanical prosthesis in 3279 patients from the Swedish in-patients register. Scand Cardiovasc J 2011;45:223-8. 10.3109/14017431.2011.571281 [DOI] [PubMed] [Google Scholar]
- 9.Agnihotri AK, McGiffin DC, Galbraith AJ, et al. The prevalence of infective endocarditis after aortic valve replacement. J Thorac Cardiovasc Surg 1995;110:1708-20; discussion 1720-4. [DOI] [PubMed]
- 10.Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation 2010;121:1141-52. 10.1161/CIRCULATIONAHA.108.773598 [DOI] [PubMed] [Google Scholar]
- 11.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463-73. 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 13.Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015;132:1435-86. 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 14.Byrne JG, Rezai K, Sanchez JA, et al. Surgical management of endocarditis: the society of thoracic surgeons clinical practice guideline. Ann Thorac Surg 2011;91:2012-9. 10.1016/j.athoracsur.2011.01.106 [DOI] [PubMed] [Google Scholar]
- 15.Martin AK, Mohananey D, Ranka S, et al. The 2017 European Society of Cardiology (ESC)/European Association of Cardiothoracic Surgeons (EACTS) Guidelines for Management of Valvular Heart Disease-Highlights and Perioperative Implications. J Cardiothorac Vasc Anesth 2018;32:2810-6. 10.1053/j.jvca.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 16.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 17.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. 10.1186/1471-2288-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delahaye F, Chu VH, Altclas J, et al. One-year outcome following biological or mechanical valve replacement for infective endocarditis. Int J Cardiol 2015;178:117-23. 10.1016/j.ijcard.2014.10.125 [DOI] [PubMed] [Google Scholar]
- 20.Fedoruk LM, Jamieson WR, Ling H, et al. Predictors of recurrence and reoperation for prosthetic valve endocarditis after valve replacement surgery for native valve endocarditis. J Thorac Cardiovasc Surg 2009;137:326-33. 10.1016/j.jtcvs.2008.08.024 [DOI] [PubMed] [Google Scholar]
- 21.Greason KL, Thomas M, Steckelberg JM, et al. Outcomes of surgery in the treatment of isolated nonnative mitral valve infective endocarditis. J Thorac Cardiovasc Surg 2014;147:349-54. 10.1016/j.jtcvs.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 22.Jassar AS, Bavaria JE, Szeto WY, et al. Graft selection for aortic root replacement in complex active endocarditis: Does it matter? Ann Thorac Surg 2012;93:480-7. 10.1016/j.athoracsur.2011.09.074 [DOI] [PubMed] [Google Scholar]
- 23.Kim JB, Ejiofor JI, Yammine M, et al. Are homografts superior to conventional prosthetic valves in the setting of infective endocarditis involving the aortic valve? J Thorac Cardiovasc Surg 2016;151:1239-46, 1248.e1-2. [DOI] [PubMed]
- 24.Leither MD, Shroff GR, Ding S, et al. Long-term survival of dialysis patients with bacterial endocarditis undergoing valvular replacement surgery in the United States. Circulation 2013;128:344-51. 10.1161/CIRCULATIONAHA.113.002365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lytle BW, Priest BP, Taylor PC, et al. Surgical treatment of prosthetic valve endocarditis. J Thorac Cardiovasc Surg 1996;111:198-207; discussion 207-10. 10.1016/S0022-5223(96)70417-8 [DOI] [PubMed] [Google Scholar]
- 26.Moon MR, Miller DC, Moore KA, et al. Treatment of endocarditis with valve replacement: The question of tissue versus mechanical prosthesis. Ann Thorac Surg 2001;71:1164-71. 10.1016/S0003-4975(00)02665-5 [DOI] [PubMed] [Google Scholar]
- 27.Musci M, Hubler M, Amiri A, et al. Surgical treatment for active infective prosthetic valve endocarditis: 22-year single-center experience. Eur J Cardiothorac Surg 2010;38:528-38. 10.1016/j.ejcts.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 28.Nguyen DT, Delahaye F, Obadia JF, et al. Aortic valve replacement for active infective endocarditis: 5-year survival comparison of bioprostheses, homografts and mechanical prostheses. Eur J Cardiothorac Surg 2010;37:1025-32. 10.1016/j.ejcts.2009.11.035 [DOI] [PubMed] [Google Scholar]
- 29.Reul GJ, Sweeney MS. Bioprosthetic versus mechanical valve replacement in patients with infective endocarditis. J Card Surg 1989;4:348-51. 10.1111/j.1540-8191.1989.tb00302.x [DOI] [PubMed] [Google Scholar]
- 30.McClure RS, McGurk S, Cevasco M, et al. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: a propensity-matched analysis. J Thorac Cardiovasc Surg 2014;148:1931-9. 10.1016/j.jtcvs.2013.12.042 [DOI] [PubMed] [Google Scholar]
- 31.Kulik A, Bedard P, Lam BK, et al. Mechanical versus bioprosthetic valve replacement in middle-aged patients. Eur J Cardiothorac Surg 2006;30:485-91. 10.1016/j.ejcts.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 32.Diaz R, Hernandez-Vaquero D, Alvarez-Cabo R, et al. Long-term outcomes of mechanical versus biological aortic valve prosthesis: Systematic review and meta-analysis. J Thorac Cardiovasc Surg 2019;158:706-14.e18. 10.1016/j.jtcvs.2018.10.146 [DOI] [PubMed] [Google Scholar]
- 33.Bartus K, Litwinowicz R, Bilewska A, et al. Intermediate-term outcomes after aortic valve replacement with a novel RESILIA(TM) tissue bioprosthesis. J Thorac Dis 2019;11:3039-46. 10.21037/jtd.2019.07.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb JG, Mack MJ, White JM, et al. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Bioprostheses: PARTNER 2 Valve-in-Valve Registry. J Am Coll Cardiol 2017;69:2253-62. 10.1016/j.jacc.2017.02.057 [DOI] [PubMed] [Google Scholar]
- 35.Neupane S, Singh H, Lammer J, et al. Meta-Analysis of Transcatheter Valve-in-Valve Implantation Versus Redo Aortic Valve Surgery for Bioprosthetic Aortic Valve Dysfunction. Am J Cardiol 2018;121:1593-600. 10.1016/j.amjcard.2018.02.054 [DOI] [PubMed] [Google Scholar]
- 36.Silaschi M, Wendler O, Seiffert M, et al. Transcatheter valve-in-valve implantation versus redo surgical aortic valve replacement in patients with failed aortic bioprostheses. Interact Cardiovasc Thorac Surg 2017;24:63-70. 10.1093/icvts/ivw300 [DOI] [PubMed] [Google Scholar]
- 37.Puskas J, Gerdisch M, Nichols D, et al. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg 2014;147:1202-10; discussion 1210-1. 10.1016/j.jtcvs.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 38.Roselló-Díez E, Cuerpo G, Estevez F, et al. Use of thePerceval Sutureless Valve in Active Prosthetic Aortic Valve Endocarditis. Ann Thorac Surg 2018;105:1168-74. 10.1016/j.athoracsur.2017.11.031 [DOI] [PubMed] [Google Scholar]
- 39.Pettersson GB, Coselli JS, Hussain ST, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg 2017;153:1241-1258.e29. 10.1016/j.jtcvs.2016.09.093 [DOI] [PubMed] [Google Scholar]
- 40.Glaser N, Jackson V, Holzmann MJ, et al. Prosthetic valve endocarditis after surgical aortic valve replacement. Circulation 2017;136:329-31. 10.1161/CIRCULATIONAHA.117.028783 [DOI] [PubMed] [Google Scholar]
- 41.Østergaard L, Valeur N, Ihlemann N, et al. Incidence and factors associated with infective endocarditis in patients undergoing left-sided heart valve replacement. Eur Heart J 2018;39:2668-75. 10.1093/eurheartj/ehy153 [DOI] [PubMed] [Google Scholar]
- 42.Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines form the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52:616-64. 10.1093/ejcts/ezx324 [DOI] [PubMed] [Google Scholar]
- 43.Head SJ, Celik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J 2017;38:2183-91. 10.1093/eurheartj/ehx141 [DOI] [PubMed] [Google Scholar]
- 44.Savage EB, Saha-Chaudhuri P, Asher CR, et al. Outcomes and prosthesis choice for active aortic valve infective endocarditis: Analysis of The Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2014;98:806-14. 10.1016/j.athoracsur.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 45.Musci M, Weng Y, Hübler M, et al. Homograft aortic root replacement in native or prosthetic active infective endocarditis: twenty-year single-center experience. J Thorac Cardiovasc Surg 2010;139:665-73. 10.1016/j.jtcvs.2009.07.026 [DOI] [PubMed] [Google Scholar]
- 46.Solari S, Mastrobuoni S, De Kerchove L, et al. Over 20 years experience with aortic homograft in aortic valve replacement during acute infective endocarditis. Eur J Cardiothorac Surg 2016;50:1158-64. 10.1093/ejcts/ezw175 [DOI] [PubMed] [Google Scholar]
- 47.Lopes S, Calvinho P, de Oliveira F, et al. Allograft aortic root replacement in complex prosthetic endocarditis. Eur J Cardiothorac Surg 2007;32:126-30. 10.1016/j.ejcts.2007.01.076 [DOI] [PubMed] [Google Scholar]
- 48.Zwischenberger JB, Shalaby TZ, Conti VR. Viable Cryopreserved Aortic Homograft for Aortic Valve Endocarditis and Annular Abscesses. Ann Thorac Surg 1989;48:365-9; discussion 369-70. 10.1016/S0003-4975(10)62858-5 [DOI] [PubMed] [Google Scholar]
- 49.Schneider AW, Hazekamp MG, Versteegh MI, et al. Stentless bioprostheses: a versatile and durable solution in extensive aortic valve endocarditis. Eur J Cardiothorac Surg 2016;49:1699-704. 10.1093/ejcts/ezv463 [DOI] [PubMed] [Google Scholar]
- 50.Ratschiller T, Sames-Dolzer E, Paulus P. Long-term Evaluation of the Ross Procedure in Acute Infective Endocarditis. Semin Thorac Cardiovasc Surg 2017;29:494-501. 10.1053/j.semtcvs.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 51.Braunwald NS, Cooper T, Morrow AG. Complete replacement of the mitral valve. Successful clinical application of a flexible polyurethane prosthesis. J Thorac Cardiovasc Surg 1960;40:1-11. 10.1016/S0022-5223(19)32638-8 [DOI] [PubMed] [Google Scholar]
- 52.Starr A, Edwards ML. Mitral replacement: clinical experience with a ball-valve prosthesis. Ann Surg 1961;154:726-40. 10.1097/00000658-196110000-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52:616-64. 10.1093/ejcts/ezx324 [DOI] [PubMed] [Google Scholar]
- 54.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 55.Yankah AC, Klose H, Petzina R, et al. Surgical management of acute aortic root endocarditis with viable homograft: 13-year experience. Eur J Cardiothorac Surg 2002;21:260-7. 10.1016/S1010-7940(01)01084-3 [DOI] [PubMed] [Google Scholar]
- 56.Leontyev S, Borger MA, Modi P, et al. Surgical management of aortic root abscess: a 13-year experience in 172 patients with 100% follow-up. J Thorac Cardiovasc Surg 2012;143:332-7. 10.1016/j.jtcvs.2010.10.064 [DOI] [PubMed] [Google Scholar]