Abstract
Pseudomonas aeruginosa is the most common pathogenic gram-negative bacteria causing corneal ulcers globally. In severe cases, often after trauma and eye injury, corneal destruction progresses rapidly and may be completed within 24–48 h causing blindness. In our preliminary work, we have established an ultrasensitive polyaniline (PANI)/gold nanoparticles (Au NPs)/indium tin oxide (ITO) modified sensor for rapid detection of pyocyanin (PYO) in P. aeruginosa infections with a linear range from 238 μM to 1.9 μM and a detection limit of 500 nM. In the present study, we evaluated the efficiency of the established modified electrochemical sensor in the diagnosis of P. aeruginosa in 50 samples collected from patients suffering from corneal ulcers. The obtained results were compared with the results gained by the screen-printed electrode, conventional techniques, automated identification method, and the amplification of the 16 s rRNA gene by PCR as a gold standard test for P. aeruginosa identification. We have found that the electrochemical detection of PYO by square wave voltammetry technique using PANI/Au NPs modified ITO electrode was the only technique showing 100% agreement with the molecular method in sensitivity, specificity, positive and negative predictive values when compared with the SPE, conventional and automated methods.
Subject terms: Biochemistry, Biotechnology, Microbiology
Introduction
A bacterial corneal ulcer is an infection and inflammation of the cornea, producing impaired vision, light sensitivity, pain, and tears or discharge from the eye. Corneal destruction progresses rapidly and in severe cases, may be completed within 24–48 h, causing blindness, especially with highly virulent bacteria1. Predisposing factors for corneal ulcers include ocular surface diseases, ocular trauma, and the extensive use of contact lenses. Among a wide range of bacteria that cause corneal ulcers, P. aeruginosa is the most frequent and the most pathogenic. It can cause corneal perforation in less than 24 h from the onset of infection2. P. aeruginosa may acquire multidrug resistance, posing a significant challenge to its eradication with antimicrobial agents3. Hence, early identification of the bacterium is vital to avoid the establishment of chronic infection4.
Although using culture media for bacterial identification is a well-established technique in microbiology laboratories, it is not quantitative and takes 24 h or more to provide results5. Polymerase chain reaction (PCR) identification is nowadays available in an increasing number of laboratories. Still, PCR requires extensive sample preparation, uses expensive reagents, and is time-consuming6.
P. aeruginosa is known to produce a blue-green redox-active phenazine pigment known as pyocyanin (PYO) that can kill competing bacteria and mammalian cells by generating reactive oxygen intermediates7. P. aeruginosa produces large amounts of PYO during the early colonization phase8. It is exclusively produced by P. aeruginosa, making it potentially an excellent diagnostic biomarker9.
Electrochemical approaches are superior to the spectrophotometric methods of PYO detection in being more sensitive, cheap, fast, and direct methods of detecting PYO5. Hereafter, they became popular alternatives to the usual optical or separation based analytical techniques. Additionally, Alatraktchi and her collaborators have described an electrochemical method for selective identification and quantification of PYO10. The scanning electrochemical microscopy (SECM) was also used to observe PYO at the surface of biofilms with high resolution11. Voltammetric techniques were efficiently employed to detect and quantify PYO in human fluid samples5,12,13.
Gold nanostructures modified electrodes are now widely used as biosensors because of the exceptional electrical and optical characteristics and affinity to bind with biomolecules14–24. Likewise, the use of the Au modified ITO electrode has led to fast and ultrasensitive detection of some multidrug-resistant bacteria such as E. coli and Staph. aureus20. Likewise, polyaniline (PANI) is one of the promising conducting polymers25 that has gained much interest in recent electrochemical sensors researches26,27, with good conductivity, stability, and easy to prepare.
Hybridization of PANI with nanomaterials could endow great promise in the sensors field due to the enhancement of its electrical conductivity in addition to its capability to act as a scaffold for immobilization of the biological species12,13. However, none of the previously mentioned electroanalytical techniques for the PYO detection have employed PANI/Au NPs modified indium tin oxide (ITO) as an electrode-based electrochemical sensor.
Up to date, it is still challenging to discover novel and more sensitive approaches for rapid and accurate diagnosis of P. aeruginosa infections, which will aid in fast treatment decisions, particularly in cases of corneal ulcers. Recently, we have established a PANI/Au NPs/ITO modified electrode as a rapid ultra-sensitive technique for the detection of PYO in P. aeruginosa infections28. The PANI/Au NPs/ITO modified electrode with positive charges on its surface was selected to monitor PYO that is bearing a negative charge and hence could enhance the mass transfer rate based on the electrostatic attraction force.
The aim of the current study was to compare the results of the electrochemical biosensors using the established PANI/Au NPs/ITO modified electrode with the screen-printed electrode (SPE) and with the conventional, automated and molecular methods for P. aeruginosa detection in cases of corneal ulcers.
Materials and Methods
Fifty patients with corneal ulcers were enrolled in this study. They were 27 females and 23 males. Their mean age was 50.5 ± 7 years. The study was conducted in the Medical Microbiology & Immunology Department, Faculty of Medicine, Assiut University, from November 2018 to February 2019. Electrochemical detection was done in the Chemistry department, Faculty of Science, Assiut University. Clinical diagnosis of corneal ulcers was performed in the Department of Ophthalmology, Assiut University Hospital using the fluorescein stain and slit-lamp examination. Patients who started antimicrobial therapy were excluded from this study.
Sample collection
Corneal ulcer samples were collected from the patients by an ophthalmologist under complete aseptic conditions using a sterile cotton swab by swabbing the base of the corneal ulcer using gentle pressure with enough pressure to indent the cornea slightly. If the cornea was significantly thinned, less pressure was applied to avoid perforation29. The swab was placed in LB broth transport media (BIO BASIC INC, Canada) and was incubated at 37 °C for 24 h. LB is a rich medium, allowing rapid and high growth rates of different microorganisms and significantly higher concentrations of PYO were detected in the LB cultures30. The final pH of the LB broth culture at room temperature is found to be 7.4 (the physiological pH). Afterward, for the phenotypic identification of P. aeruginosa, all collected samples were subjected to cultures, biochemical tests, and automated system based identification. Electrochemical biosensors were used to measure PYO. Confirmation of diagnosis was done by the amplification of the 16 s rRNA gene of P. aeruginosa.
Electrochemical identification of P. aeruginosa in corneal ulcer samples
The Autolab potentiostat instrument (Metrohm Model 663 VA, Netherlands) was used for all electrochemical experiments. It was connected to a three-electrode system; Nova software was used to control the system at room temperature. Two types of 3- electrode electrochemical cell systems were used for the electrochemical measurements:
Voltammetric cell that consisted of a platinum (Pt) wire as a counter electrode (CE), a reference electrode (RE) of silver/silver chloride Ag/AgCl and PANI/Au NPs modified ITO electrode as a working electrode (WE). The three electrodes were connected to a potentiostat. Preparation of PANI/Au NPs modified ITO electrodes was done as was described in our earlier study28. SEM (JOEL-JSM-5400LV) was used to investigate the morphology of the Au NPs modified ITO and PANI/Au NPs modified ITO electrodes.
Disposable SPE (SPE C110, DropSens, Spain), which consisted of carbon as working (4 mm diameter) and counter electrodes, and silver as a RE. SPE was connected to potentiostat by a connector (DSC, DropSens, Spain).
The electrochemical detection of P. aeruginosa was done by adding 10 ml of the overnight LB broth culture of the sample in the electrochemical cell when using PANI/Au NPs modified ITO electrode. Also, 10 µl of overnight LB broth culture of the sample was pipetted into the detector well of the SPE. The surface of the SPE was first subjected to chemically pretreatment based on immersing the SPE in a solution of NaOH (3 M) for 1 h, then rinsed with DIW and finally dried at 120 °C.
The electrochemical measurement was done using both the cyclic voltammetry (CV) scans (at a potential extending from −0.4 V to 0.0 V and scan rate 0.05 V/s) and the square wave voltammetry (SWV) scans were performed at frequency = 15 Hz, pulse amplitude 0.05 V, step potential = 0.004 V, initial potential = −0.7 V and final potential = 0.0 V.
Isolation and identification of the bacterial isolates by the conventional methods
After the overnight culture in LB broth, all samples were sub-cultured on several selective culture media and incubated at 37 °C for 24 h. The P. aeruginosa isolates were presumptively recognized by the routine tests, including colony morphology, pigment production, and biochemical tests. Suspected colonies of P. aeruginosa on nutrient agar (large, opaque, irregular colonies with blue-green pigment), blood agar (smooth, large, flat colonies, pigment diffuses in the medium giving dark greenish-blue color and may produce diffuse hemolysis) and on MacConkey’s agar (pale showing non-lactose fermenter colonies) were further identified as Gram-negative, non-spore forming rods31 and oxidase-positive; showing deep purple color within 5–10 seconds of a single colony transfer onto oxidase disc (HiMedia Laboratories Ltd., India)32. P. aeruginosa also showed alkaline butt (red butt) and alkaline slant (red slant) when stabbed onto Triple sugar iron (TSI) (HiMedia Laboratories Ltd., India). P. aeruginosa isolates were citrate positive; changing the color of Simmon’s Citrate to the blue33. If no pigment was present after incubation for 48 h, further tests were used to identify the isolate completely34.
Antimicrobial susceptibility testing
The antimicrobial susceptibility of the isolated P. aeruginosa isolates was done by the Kirby-Bauer disc diffusion method35, according to the CLSI guidelines, 201836. The following antimicrobial discs were used (Hi-Media Laboratories Ltd., India): Amikacin (30 µg), Piperacillin (100 µg), Ceftriaxone (30 µg), Ciprofloxacin (5 µg), Cefoperazone-Sulbactam (25 µg), Imipenem (10 µg) and Meropenem (10 µg).
Automated identification of the P. aeruginosa isolates
Pure Pseudomonas isolates were further characterized by the automated ID & Ast system for microbial identification and antibiotic susceptibility testing, MA120 (Render group, China), compliant with the manufacturer instructions.
PCR amplification of the 16s rRNA gene of P. aeruginosa isolated strains
DNA was extracted by the boiling method37. The primer set used to amplify the 16 s rRNA gene of P. aeruginosa was as follows: Forward (5′GGGGGATCTTCGGACCTCA3′) and reverse (5′TCCTTAGAGTGCCCACCCG3′)38. PCR was performed using the SimpliAmp thermal cycler (Applied Biosystems, USA). The amplification procedure was done, as was previously described38. Briefly, initial denaturation was for 2 minutes at 95 °C. Then 25 cycles were completed, each consisting of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 50 s. Subsequently, a final extension of 5 minutes at 72 °C was applied. PCR products were resolved on 2% agarose gels with ethidium bromide and imaged with the EZ imager (Bio-Rad), where a band was visualized at the expected size of 956 base pair.
Ethical approval
Written informed consent for the sample and data collection was obtained from each patient. It is worth to advise that all patients included in the study were able to sign the written informed consent by themselves because there was no single case that suffered from corneal ulcers in both eyes. Moreover, some patients experienced blurry vision but only in one eye. The local Ethical Committee of the Faculty of Medicine Assiut University appraised and accepted the study protocol in accordance with the latest revision of the Declaration of Helsinki (IRB NO. 17300293).
Results
Electrochemical detection of P. aeruginosa in corneal ulcer samples
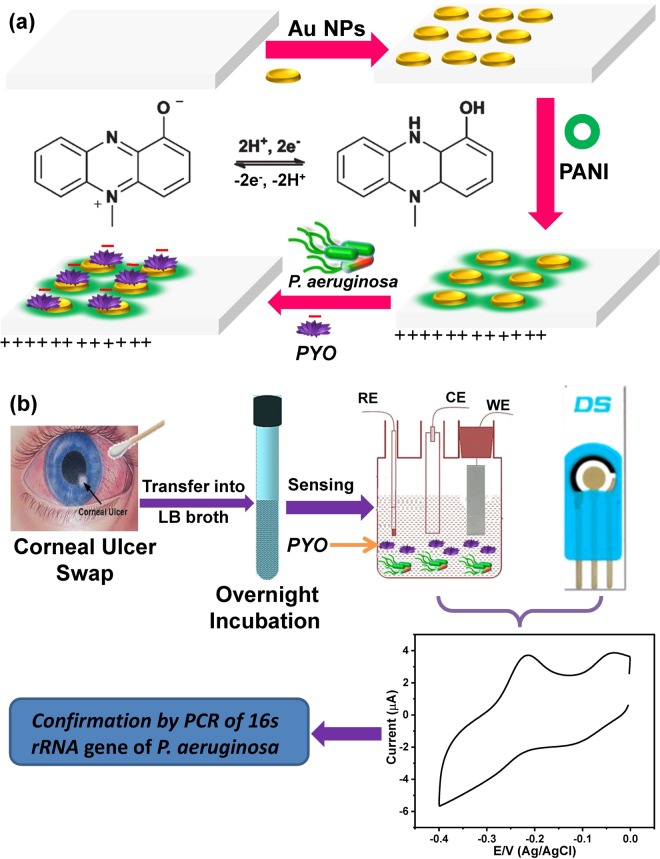
Here, PANI/Au NPs/ITO modified electrode with positive charges on the surface of the polymer layer was selected to monitor the negatively charged PYO molecules, and hence this electrostatic attraction force could enhance the rate of the mass transfer, as represented in Fig. (1a). To investigate the capability of both PANI/Au NPs modified ITO and the SPE electrodes, we have collected 50 corneal ulcer samples and studied their electrochemical behaviors based on CV and SWV techniques, as revealed in Fig. (1b).
Figure 1.
(a) The schematic diagram for fabrication of PANI/Au NPs/ITO electrode and the interaction between PANI and PYO and (b) the schematic diagram for electrochemical sensing of P. aeruginosa by using either PANI/Au NPs/ITO or SPE electrodes, the eye image was used form this website, https://www.pinterest.com/pin/7529524362981604/.
Using PANI/Au NPs modified ITO electrode
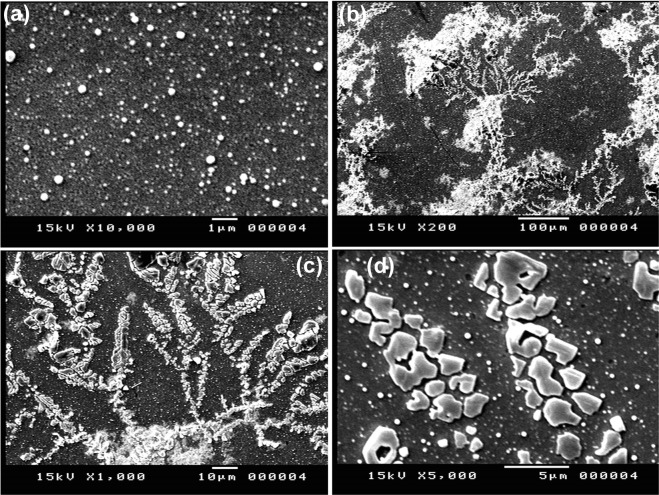
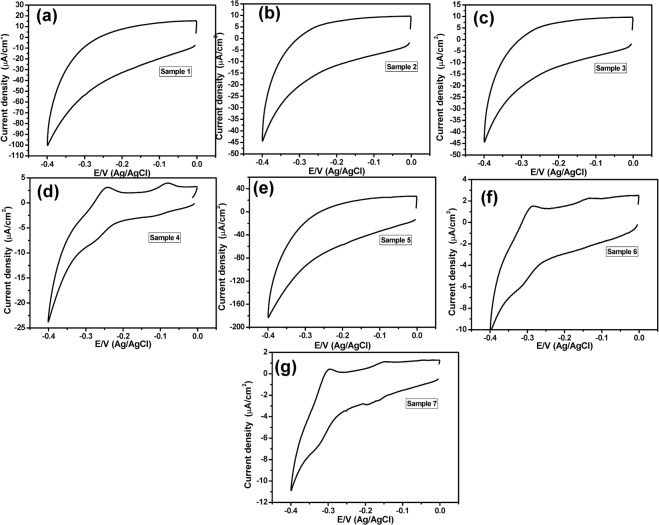
The PANI/Au NPs modified ITO electrode was prepared according to our previous study28. Figure (2a) showed the SEM image of the Au NPs/ITO that indicated the formation of nanodots with an average size of about 95 nm. On the other hand, the morphology of the obtained PANI/Au NPs/ITO electrode was studied by using SEM images (Fig. (2b–d)), which confirmed the fabrication of a tree-like structure of PANI layer over the Au NPs. Figure (3a) represented a background CV response with no redox peaks detected within the PYO potential window (−0.4 V to 0.0 V). This behavior was obtained in 44 samples.
Figure 2.
(a) SEM image of Au NPs modified ITO electrode and (b–d) SEM images of the PANI/Au NPs/ITO electrode.
Figure 3.
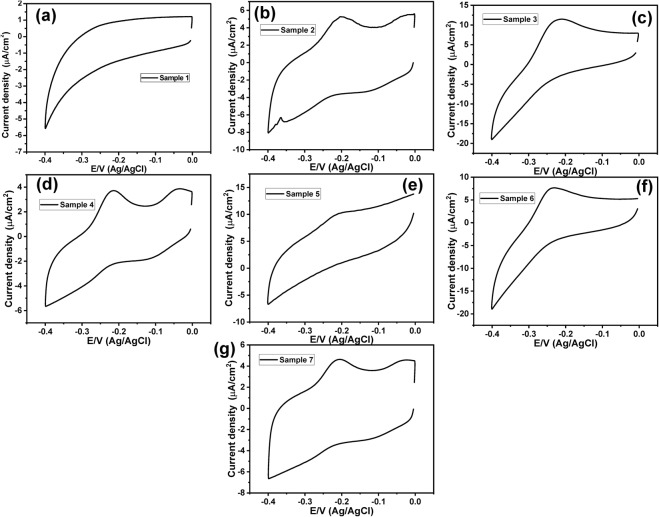
(a) Cyclic voltammogram of a negative sample and (b–g) Cyclic voltammograms of positive samples using PANI/Au NPs modified ITO electrode within a potential window (−0.4 V to 0.0 V), at a scan rate of 50 mV/sec.
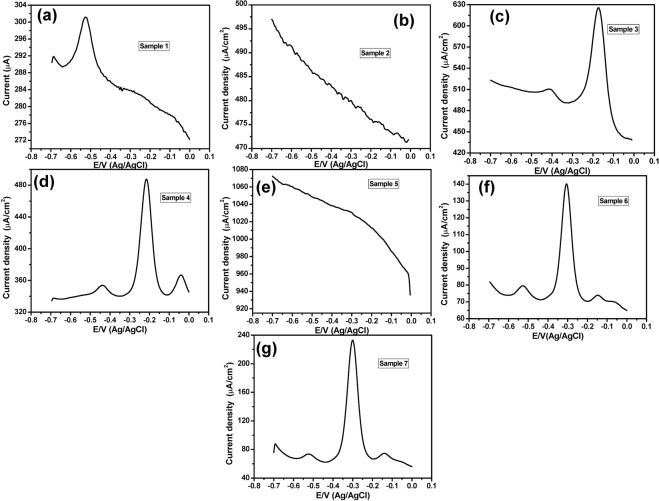
On the other hand, Fig. (3b–g) showed positive CV responses of positive samples, which represented an oxidation peak at about −0.23 V with different current intensities (a specific and unique peak of PYO). This was obtained only in 6 samples. To get more accurate and sensitive results we have applied the SWV technique instead of the CV technique. Figure (4a–g) represented the SWV responses from −0.7 V to 0.0 V of positive samples that showed an oxidation peak at −0.23 V. While Fig. (4h) showed the SWV response with no detectable redox peak within the PYO potential window (−0.7 V to 0.0 V) that distinguishes the negative sample.
Figure 4.
(a–g) SWV of positive samples, and (h) SWV of a negative sample, using PANI/Au NPs modified ITO electrode and in a potential window (−0.7 V to 0.0 V).
The electrochemical results obtained by using the PANI/Au NPs modified ITO electrode were summarized in Table (1), which indicated that among the 50 tested corneal ulcer samples, 7/50 samples (14%) were electrochemically positive using SWV technique, while 6/50 samples (12%) were electrochemically positive using CV technique. The remaining samples were electrochemically negative.
Table 1.
Comparison between the electrochemical detection results of P. Aeruginosa positive corneal ulcer samples using the PANI/Au NPs modified ITO electrode and the screen-printed electrode (SPE).
| Sample number | PANI/Au NPs modified ITO | SPE | ||
|---|---|---|---|---|
| CV | SWV | CV | SWV | |
| 1 | −ve | +ve | −ve | +ve |
| 2 | +ve | +ve | −ve | −ve |
| 3 | +ve | +ve | −ve | +ve |
| 4 | +ve | +ve | +ve | +ve |
| 5 | +ve | +ve | −ve | −ve |
| 6 | +ve | +ve | +ve | +ve |
| 7 | +ve | +ve | +ve | +ve |
Using the SPE sensor
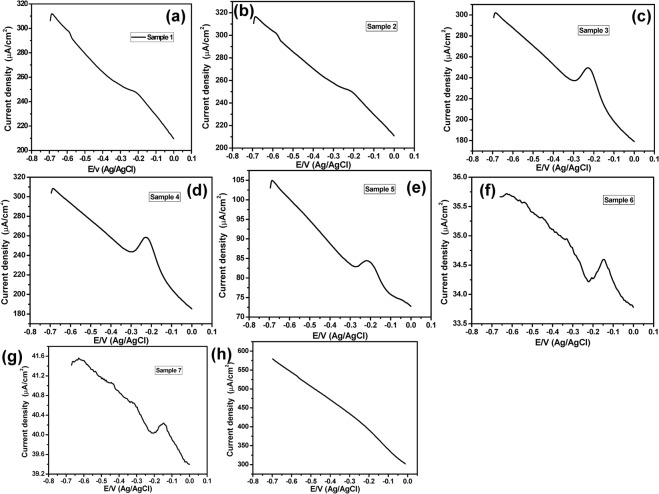
To validate the efficiency of the PANI/Au NPs modified ITO electrode for monitoring the PYO in corneal ulcer samples, SPE was used as a traditional electrode for detecting the PYO in the 50 samples instead of PANI/Au NPs modified ITO electrode under the same conditions. Figure (5a–c,e) showed the CV responses of negative samples within the PYO potential window (−0.4 V to 0.0 V) that didn’t show any redox peak. Furthermore, the CV responses of positive samples using SPE sensor that represented an oxidation peak at about −0.30 V were shown in Fig. (5d,f,g).
Figure 5.
Cyclic voltammograms of (a–c,e) negative samples, and (d,f,g) positive samples, using SPE sensor within a potential window (−0.4 V to 0.0 V).
Moreover, the SWV technique was also used to study the performance of SPE in detecting PYO. For 45 samples, no oxidation peaks could be observed within the potential range from −0.7 V to 0.0 V as shown in Fig. (6b,e) which means these 45 samples are negative samples. Furthermore, the SWV responses of four samples (samples# 3, 4, 6 and 7) have shown a set of oxidation peaks including the characteristic oxidation peak of PYO molecules at about −0.22 V (Fig. 6c,d,f,g) and hence theses samples are positive samples. On the other hand, the SWV of the sample# 1 showed a strong oxidation peak at about −0.52 V in addition to a very weak and broad peak at about −0.25 (Fig. 6a), so this sample could be considered as a positive sample. The above data were tabled in comparison with the PANI/Au NPs modified ITO electrode in Table 1. Among the 50 corneal ulcer samples, 5/50 samples (10%) were electrochemically positive using the SWV technique, while 3/50 samples (6%) were electrochemically positive using the CV technique. It is worth to note that although the SW voltammograms of the SPE positive samples have shown sharper oxidation current peaks in comparison with those observed at the PANI/Au NPs/ITO electrode, the oxidation peak was detectable only in five samples in case of using the SPE while the PANI/Au NPs/ITO electrode detected easily the oxidation peak in 7 samples. Thus, the PANI/Au NPs/ITO electrode is more accurate and selective for PYO detection.
Figure 6.
(a,c,d,f,g) The square wave voltammograms of positive samples, and (b,e) the SWV responses of negative samples, using the SPE sensor within a potential window (−0.7 V to 0.0 V).
In order to study the kinetics of the electrode reaction, we have measured the half-peak width of the SW voltammograms based on a previously reported method39. Figure 7 showed the forward and backward SW voltammograms of PYO at a frequency (f) of 15 Hz, amplitude (Esw) = 0.05 V, and potential increment (ΔE) = 0.004 V. From Fig. 7 we found that the peak potential (ΔEp) = −216 mV, peak current (Ψp) = 301 µA and the half-peak width (ΔEp/2) = 65 mV.
Figure 7.
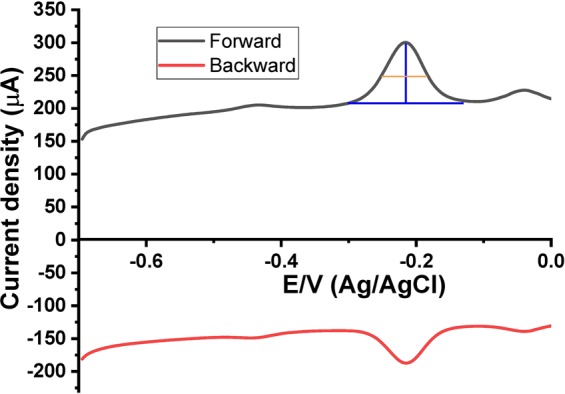
The forward and backward SW voltammograms of PYO.
The dimensionless electrode kinetic parameter (K) was calculated from Eq. (1).
| 1 |
So, ln(K) = −25.85, and hence K = 3.25
The standard rate constant of electron transfer (ks) was calculated from Eq. (2).
| 2 |
where, D (cm2.s−1) is the diffusion coefficient.
So, ks = 0.00315 cm.s−1
Isolation and phenotypic identification of Pseudomonas aeruginosa by conventional bacteriologic identification methods
All collected samples were investigated by using the conventional bacteriologic identification methods and the results were summarized in Table 2. Of the 50 corneal ulcer samples, 30/50 (60%) were due to bacterial infections, while the other 20/50 (40%) cases were negative for bacterial growth. Of the bacterial isolates, 14/30 (46.7%) were Staphylococcus spp. [11/30 (36.7%) were coagulase-positive while 3/30 (10%) were coagulase-negative], 2/30 (6.7%) were Streptococci. On the other hand, 9/30 (30%) were Pseudomonas spp. [5/30 (16.7%) were P. aeruginosa while 4/30 (13.3%) were non-aeruginosa] and 5/30 (16.7%) were Klebsiella. All the seven P. aeruginosa isolates were resistant to ceftriaxone, four were resistant to ciprofloxacin, three resistant to imipenem, meropenem, cefoperazone-sulbactam and piperacillin and two were resistant to amikacin.
Table 2.
Phenotypic identification of P. aeruginosa in the corneal ulcer samples.
| Bacterial pathogens | # of isolates | Percentage of isolates |
|---|---|---|
| Staphylococcus spp. | 14 | 46.7 (%) |
| Coagulase +ve | 11 | 36.7 (%) |
| Coagulase −ve | 3 | 10 (%) |
| Streptococcus pneumoniae | 2 | 6.7 (%) |
| Pseudomonas spp. | 9 | 30 (%) |
| P. Aeruginosa | 5 | 16.7 (%) |
| Non Aeruginosa | 4 | 13.3 (%) |
| Klebsiella | 5 | 16.7 (%) |
Automated identification of the P. aeruginosa isolates
Six of the nine Pseudomonas spp. isolates were of the aeruginosa spp., while 3/9 where P. fluorescence by the automated ID & Ast system.
PCR amplification of the 16 s rRNA gene of P. aeruginosa
All Pseudomonas spp. isolates were tested by PCR. Figures S1–S3 showed the gel electrophoresis of the PCR-amplified products for the detection of the 16 s rRNA gene for nine samples, which indicated that 7/9 isolates were having the 16 s rRNA gene of P. aeruginosa.
Sensitivity and specificity of the electrochemical, conventional and automated methods compared with PCR amplification of 16 s rRNA gene for the detection of P. aeruginosa in corneal ulcer samples
A summary of the results of the different methods for the identification of P. aeruginosa was illustrated in Table (3). All P. aeruginosa isolates identified by PCR (100%) were also positive by the SWV using PANI/Au NPs modified ITO. Meanwhile, 6/7 (85.7%) previous isolates were positive by both the CV using PANI/Au NPs modified ITO and the automated ID & Ast system. Only 5/7 (71.4%) isolates were positive by both conventional methods and SWV using SPE. CV using SPE could only detect 3/7 isolates (42.9%) of P. aeruginosa.
Table 3.
Summary of the results of P. aeruginosa detection by electrochemical, phenotypic, automated and 16 s rRNA gene PCR methods.
| Detection method | Number of positive samples | Percentage |
|---|---|---|
| PCR amplification of 16 s rRNA gene | 7 | 100% |
| SWV using PANI/Au NPs modified ITO | 7 | 100% |
| CV using PANI/Au NPs modified ITO | 6 | 85.7% |
| The automated ID & Ast system | 6 | 85.7% |
| SWV using SPE | 5 | 71.4% |
| Phenotypic methods | 5 | 71.4% |
| CV using SPE | 3 | 42.9% |
CV cyclic voltammetry scans, SWV square wave voltammetry scans, SPE screen-printed electrode.
Percentages are calculated from P. Aeruginosa positive samples by PCR amplification of 16s rRNA gene.
Sensitivities and specificities of the electrochemical methods and conventional methods for the detection of P. aeruginosa in corneal ulcer samples compared with PCR amplification of the 16 s rRNA gene of P. aeruginosa were reported in Table (4). SWV using PANI/Au NPs modified ITO has shown 100% sensitivity, specificity, positive and negative predictive values. The remaining methods have only revealed 100% specificities and positive predictive values. The CV using PANI/Au NPs modified ITO and the automated ID & Ast system have shown better sensitivities (85.7%) than both the SWV using SPE and the conventional identification methods (71.4%) followed by the CV using SPE (42.9%). Similarly, higher negative predictive values were obtained by the CV using PANI/Au NPs modified ITO and the automated ID & Ast system (66.6%) followed by both SWV using SPE and the phenotypic identification methods (50%) then by the CV using SPE (33.3%).
Table 4.
Sensitivities and specificities of the electrochemical, phenotypic and automated identification methods of P. aeruginosa compared with PCR amplification of the 16s rRNA gene.
| PANI/Au NPs/ITO electrode | Screen printed electrode | Phenotypic methods | Automated Method | |||
|---|---|---|---|---|---|---|
| CV | SWV | CV | SWV | |||
| Sensitivity | 85.7% | 100% | 42.9% | 71.4% | 71.4% | 85.7% |
| Specificity | 100% | 100% | 100% | 100% | 100% | 100% |
| PPV | 100% | 100% | 100% | 100% | 100% | 100% |
| NPV | 66.6% | 100% | 33.3% | 50% | 50% | 66.6% |
CV cyclic voltammetry scans, SWV square wave voltammetric scans, PPV positive predictive value, NPV negative predictive value.
Discussion
The bacterial corneal disease remains the leading cause of blindness globally, and it often occurs following trauma or eye injury. Typically corneal ulcer is characterized by red-eye, mild to severe ocular discharges, pain, and reduced vision40. Pascolini and Mariotti, 2012 reported that corneal opacities, caused by an infectious corneal ulcer, are the fourth leading cause of blindness worldwide and represent 10% of visual impairment, particularly in developed countries41. In the United States, infectious keratitis is often associated with contact lens wear42; however, in developing countries, it is commonly associated with ocular trauma occurring during farming43. In India, approximately 2 million people develop a corneal ulcer every year44. Unfortunately, corneal damage can occur within 24 h, especially with the serious infections caused by highly virulent opportunistic pathogens, frequently as P. aeruginosa45.
The ubiquitous gram-negative bacteria P. aeruginosa is multidrug-resistant and frequently identified in biofilms46. It causes severe infections in immune-compromised patients47 that are difficult to eradicate with antibiotics48. Delays in obtaining results of the conventional identification methods influence both patient care, and the ability to decide an appropriate antibiotic therapy. Moreover, in spite of the improvements in the rapid molecular techniques, the use of cultures remains the standard practice in laboratories48. These days, there is an urgent need to find rapid and precise approaches for bacterial identification because the culture procedure can take from 24 to 48 h to get results. Early diagnosis of P. aeruginosa is a highly required goal in hospitals that consequently will improve patients care outcomes.
PYO is a redox-active molecule produced by P. aeruginosa49 and is considered an excellent biomarker for P. aeruginosa that could be used as a selective alternative for direct detection. Great efforts were made to expand the applications of the electrochemical technologies in health, environment, and security. A variety of printed sensors and biosensors have been developed within the last few years50–52, to improve the routine diagnostic tools. Preceding studies have described the electrochemical detection of PYO in P. aeruginosa identification5,10,30,53–55. Still, none of the previous electrochemical detection methods have been used to identify P. aeruginosa in corneal ulcers, and none of them tested the use of the ITO electrode as a working electrode for the detection of PYO.
In our preliminary work28, we have established the design and operation of PANI/Au NPs/ITO electrode which has shown a fast, 100% sensitivity and specificity and excellent selectivity for PYO at extremely low concentrations in the presence of interfering substances like ascorbic acid, uric acid, and glucose. We used the scanning electron microscope and CV to characterize the fabricated electrode. The detection limit of PYO was 500 nM, with a linear detection range from 238 µM to 1.9 µM. The modified electrode had a four-fold enhanced performance compared with the published results of the SPE.
In this study, we evaluated the efficiency of the modified ITO sensor on the detection of P. aeruginosa in 50 corneal ulcer samples and compared the results with the SPE sensor, conventional and automated identification methods using the PCR amplification of 16 s rRNA gene as a gold standard test for P. aeruginosa identification. P. aeruginosa was the most commonly isolated gram-negative bacteria in this study. We have found that 7/50 samples were positive by PCR and SWV using PANI/Au NPs modified ITO electrode. While six of those seven samples (85.7%) were positive by the automated system and by the CV using PANI/Au NPs modified ITO electrode, 5/7 (71.4%) samples were positive by the conventional phenotypic methods and by SWV using SPE and 3/7 (42.9%) by the CV using SPE. All positive samples had cyclic voltammograms with a unique oxidation peak of PYO at −0.23 V when using PANI/Au NPs modified ITO electrode while −0.30 V when using SPE. This small potential shift in the location of the PYO peak may be attributed to the limited stability of the silver reference electrode of the disposable SPE sensor5.
Isolation of pathogens is still done by the conventional culture methods in a large number of laboratories because it is convenient and manageable. Nevertheless, culture media and biochemical tests are not entirely specific and sometimes fail to offer probable identification, especially up to the species level. Therefore, additional verification of species identity is frequently needed, which is time and money consuming56. Two of our corneal ulcer samples were not considered P. aeruginosa by the phenotypic identification methods as they did not produce a visible pigment on the culture media. Meanwhile, these two samples were positive by PCR amplification of the 16 s rRNA gene of P. aeruginosa. It was recorded earlier that not all strains of P. aeruginosa can produce pigment on the culture medium34. These strains may have produced PYO, but its amount was too small to be seen on the culture medium, but the highly sensitive SWV technique using PANI/Au NPs/ITO electrode was capable of detecting these limited amounts of PYO.
Different non-culture based approaches are currently used to provide rapid bacterial identification and antibiotic susceptibility simultaneously56. But in these methods, it is impossible to process directly patient samples57, and there is a great need for a pure culture of the bacteria and long processing time56. Despite that, in our study, the automated system has shown better sensitivity in detecting Pseudomonas spp. than the conventional methods; one case was misinterpreted for P. fluorescence instead of P. aeruoginosa.
As far as we know, it is the first study to evaluate the use of electrochemical sensors in the detection of P. aeruginosa in corneal ulcers. We have demonstrated that the electrochemical detection of PYO by the SWV technique using PANI/Au NPs modified ITO electrode was the only technique showing 100% agreement with the molecular method in sensitivity, specificity, positive and negative predictive values compared with the SPE, conventional and automated methods. The modified electrode was able to discriminate between P. aeruginosa, Staphylococcus spp., Streptococci, and Klebsiella. This endorses our previous findings28 that using the modified electrode allows fast, accurate, and selective detection of P. aeruginosa in mixed bacterial cultures without the need for isolation of pure colonies of bacteria and in the presence of interfering molecules.
The result obtained by the modified sensor was better than that of the disposable electrochemical sensor used by Sismaet and his collaborators54 for rapid screening for P. aeruginosa in fluids collected from chronic wounds, which demonstrated a sensitivity of 71% and specificity of 57%58. SWV technique showed higher sensitivity to PYO than CV technique. These results were attributed to the drawbacks of the CV, which introduce a huge capacitive background current (or noise). This noise makes it difficult to recognize the faradaic current, which is the desired signal and rigorously disturbs the signal to noise ratio. Since SWV is a pulsed technique, it can discriminate against the charging current and eliminate this drawback. For this reason, the SWV technique can detect a very low concentration of PYO in the clinical sample.
In summary, we have studied the electrochemical capability of the developed electrochemical sensor for the detection of PYO by SWV technique in 50 samples collected from patients suffering from corneal ulcers. The obtained results indicated that the electrochemical detection of PYO by SWV technique using PANI/Au NPs modified ITO electrode was the only technique showing 100% agreement with the molecular method in sensitivity, specificity, positive and negative predictive values when compared with the SPE, conventional and automated methods (phenotypic and 16 s rRNA gene PCR methods). Therefore, PANI/Au NPs modified ITO electrode is recommended as a fast, cheap, accurate, and selective PYO biomarker sensor based on the SWV technique for the detection of P. aeruginosa in cases of corneal ulcer.
Supplementary information
Acknowledgements
The authors would like to express their special gratitude to Professor Sherine A. Aly, professor of Medical Microbiology and Immunology, for providing the EZ imager needed to visualize the PCR products. We also would like to thank Dr. Marwa O. M. Aly for her kind help in sample collection. This study was funded by a grant from the Faculty of Medicine, Grant Office, Assiut University # 2016/12/29-007 to Dr. EL-Badawy O. This support is appreciatively acknowledged.
Author contributions
Marwa M. Khalifa performed the experiments. Statistical analysis of data. Wrote the initial draft of the manuscript and managed the literature searches. Amal A. Elkhawaga. Formulation of the research question and statement of hypothesis. Conceived and designed the experiments. Participated in data analysis and interpretation. Critical review and revision of the final manuscript. Asmaa M. Zahran conceived and designed the experiments. Participated in data analysis and interpretation. Critical review and revision of the final manuscript. Mona A. Hassan coordination of research activities. Critical review and revision of the final manuscript. Ahmed M. Fathalla recruited patients, carried out the clinical investigations and collected patients’ clinical data. Critical review and revision of the final manuscript. Waleed A. El-Said formulation of the research question and statement of hypothesis. Conceived and designed the experiments. Participated in data analysis and interpretation. Critical review and revision of the final manuscript. Omnia El-Badawy formulation of the research question and statement of hypothesis. Conceived and designed the experiments. Obtaining funding participated in data analysis and interpretation. Wrote the initial draft of the manuscript and managed the literature searches.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-54667-0.
References
- 1.Janumala, H., Sehgal, P. K. & Mandal, A. B., Bacterial keratitis-causes, symptoms and treatment. Keratitis (2012).
- 2.Gebremariam TT. Bacteriology and Risk Factors of Bacterial Keratitis in Jimma, Southwest Ethiopia. Ethiop Med J. 2015;53(4):191–7. [PubMed] [Google Scholar]
- 3.Stover CK, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959–64. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 4.Stuart B, Lin JH, Mogayzel PJ., Jr. Early eradication of Pseudomonas aeruginosa in patients with cystic fibrosis. Paediatr Respir Rev. 2010;11(3):177–84. doi: 10.1016/j.prrv.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster TA, Sismaet HJ, Conte JL, Chan IP, Goluch ED. Electrochemical detection of Pseudomonas aeruginosa in human fluid samples via pyocyanin. Biosens Bioelectron. 2014;60:265–70. doi: 10.1016/j.bios.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Andrews JM. Determination of minimum inhibitory concentrations. Antimicrob Chemother. 2001;48(Suppl 1, (suppl_1)):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 7.Cezairliyan B, et al. Identification of Pseudomonas aeruginosa phenazines that kill Caenorhabditis elegans. PLoS Pathog. 2013;9(1):e1003101. doi: 10.1371/journal.ppat.1003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fothergill JL, Winstanley C. The role of quorum sensing in chronic cystic fibrosis Pseudomonas aeruginosa infections. FEMS Microbiology Letters. 2009;290(1):1–9. doi: 10.1111/j.1574-6968.2008.01394.x. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich LE, Price‐Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Molecular microbiology. 2006;61(5):1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 10.Alatraktchi FA, Johansen HK, Molin S, Svendsen WE. Electrochemical sensing of biomarker for diagnostics of bacteria-specific infections. Nanomedicine (Lond) 2016;11(16):2185–95. doi: 10.2217/nnm-2016-0155. [DOI] [PubMed] [Google Scholar]
- 11.Connell JL, Kim J, Shear JB, Bard AJ, Whiteley M. Real-time monitoring of quorum sensing in 3D-printed bacterial aggregates using scanning electrochemical microscopy. Proceedings of the National Academy of Sciences. 2014;111(51):18255–18260. doi: 10.1073/pnas.1421211111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharp D, Gladstone P, Smith RB, Forsythe S, Davis J. Approaching intelligent infection diagnostics: Carbon fibre sensor for electrochemical pyocyanin detection. Bioelectrochemistry. 2010;77(2):114–9. doi: 10.1016/j.bioelechem.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Alatraktchi FA, Breum Andersen S, Krogh Johansen H, Molin S, Svendsen WE. Fast selective detection of pyocyanin using cyclic voltammetry. Sensors. 2016;16(3):408. doi: 10.3390/s16030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Said WA, Lee J-H, Oh B-K, Choi J-W. 3-D nanoporous gold thin film for the simultaneous electrochemical determination of dopamine and ascorbic acid. Electrochemistry Communications. 2010;12(12):1756–1759. doi: 10.1016/j.elecom.2010.10.015. [DOI] [Google Scholar]
- 15.El-Said WA, Kim T-H, Kim H, Choi J-W. Detection of effect of chemotherapeutic agents to cancer cells on gold nanoflower patterned substrate using surface-enhanced Raman scattering and cyclic voltammetry. Biosensors and Bioelectronics. 2010;26(4):1486–1492. doi: 10.1016/j.bios.2010.07.089. [DOI] [PubMed] [Google Scholar]
- 16.El-Said WA, Kim T-H, Kim H, Choi J-W. Three-dimensional mesoporous gold film to enhance the sensitivity of electrochemical detection. Nanotechnology. 2010;21(45):455501. doi: 10.1088/0957-4484/21/45/455501. [DOI] [PubMed] [Google Scholar]
- 17.Jung M, El-Said WA, Choi J-W. Fabrication of gold nanodot arrays on a transparent substrate as a nanobioplatform for label-free visualization of living cells. Nanotechnology. 2011;22(23):235304. doi: 10.1088/0957-4484/22/23/235304. [DOI] [PubMed] [Google Scholar]
- 18.Kim TH, El-Said WA, An JH, Choi JW. ITO/gold nanoparticle/RGD peptide composites to enhance electrochemical signals and proliferation of human neural stem cells. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9(3):336–44. doi: 10.1016/j.nano.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 19.El-Said WA, Choi J-W. Electrochemical Biosensor consisted of conducting polymer layer on gold nanodots patterned Indium Tin Oxide electrode for rapid and simultaneous determination of purine bases. Electrochimica Acta. 2014;123:51–57. doi: 10.1016/j.electacta.2013.12.144. [DOI] [Google Scholar]
- 20.Lv X, et al. Rapid and ultrasensitive electrochemical detection of multidrug-resistant bacteria based on nanostructured gold coated ITO electrode. ACS Appl Mater Interfaces. 2014;6(14):11025–31. doi: 10.1021/am5016099. [DOI] [PubMed] [Google Scholar]
- 21.El‐Said WA, Abd El‐Hameed K, Abo El‐Maali N, Sayyed HG. Label‐free Electrochemical Sensor for Ex‐vivo Monitoring of Alzheimer’s Disease Biomarker. Electroanalysis. 2017;29(3):748–755. doi: 10.1002/elan.201600467. [DOI] [Google Scholar]
- 22.Qiu Z, Shu J, Liu J, Tang D. Dual-channel photoelectrochemical ratiometric aptasensor with up-converting nanocrystals using spatial-resolved technique on homemade 3D printed device. Analytical chemistry. 2018;91(2):1260–1268. doi: 10.1021/acs.analchem.8b05455. [DOI] [PubMed] [Google Scholar]
- 23.Zeng R, et al. Palindromic molecular beacon based Z-scheme BiOCl-Au-CdS photoelectrochemical biodetection. Analytical chemistry. 2019;91(3):2447–2454. doi: 10.1021/acs.analchem.8b05265. [DOI] [PubMed] [Google Scholar]
- 24.Luo Z, Zhang L, Zeng R, Su L, Tang D. Near-infrared light-excited core–core–shell UCNP@ Au@ CdS upconversion nanospheres for ultrasensitive photoelectrochemical enzyme immunoassay. Analytical chemistry. 2018;90(15):9568–9575. doi: 10.1021/acs.analchem.8b02421. [DOI] [PubMed] [Google Scholar]
- 25.Wei D, Ivaska A. Electrochemical biosensors based on polyaniline. Chemia analityczna. 2006;51:839–852. [Google Scholar]
- 26.Zhang B, He Y, Liu B, Tang D. Nickel-functionalized reduced graphene oxide with polyaniline for non-enzymatic glucose sensing. Microchimica Acta. 2015;182(3-4):625–631. doi: 10.1007/s00604-014-1366-7. [DOI] [Google Scholar]
- 27.Zeng R, Luo Z, Zhang L, Tang D. Platinum nanozyme-catalyzed gas generation for pressure-based bioassay using polyaniline nanowires-functionalized graphene oxide framework. Analytical chemistry. 2018;90(20):12299–12306. doi: 10.1021/acs.analchem.8b03889. [DOI] [PubMed] [Google Scholar]
- 28.Elkhawaga, A. A.; Khalifa, M. M.; El-badawy, O. H. B.; Hassan, M. A.; El-Said, W. A. Rapid and highly sensitive detection of pyocyanin biomarker in different <em>Pseudomonas aeruginosa</em> infections using gold nanoparticles modified sensor. 616797 (2019) [DOI] [PMC free article] [PubMed]
- 29.Robinson J, Ellen J, Hadel B, Lighthizer N. Collecting a corneal culture. Review of Optometry. 2016;153(4):64–71. [Google Scholar]
- 30.Santiveri CR, Sismaet HJ, Kimani M, Goluch ED. Electrochemical Detection of Pseudomonas aeruginosa in Polymicrobial Environments. ChemistrySelect. 2018;3(11):2926–2930. doi: 10.1002/slct.201800569. [DOI] [Google Scholar]
- 31.Al-Kobaisi MF, Jawetz M. & Adelberg’s Medical Microbiology: 24(th) Edition. Sultan Qaboos Univ Med J. 2007;7(3):273–275. [Google Scholar]
- 32.Isenberg, H. D., Clinical microbiology procedures handbook. American Society of Microbiology (1992).
- 33.Mackie, T. J. Mackie & McCartney practical medical microbiology. Harcourt Health Sciences (1996).
- 34.Reyes E, Bale MJ, Cannon WH, Matsen JM. Identification of Pseudomonas aeruginosa by pyocyanin production on Tech agar. Journal of clinical microbiology. 1981;13(3):456–458. doi: 10.1128/jcm.13.3.456-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauer A, Kirby W, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. American journal of clinical pathology. 1966;45(4_ts):493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 36.CLSI, Performance standards for antimicrobial susceptibility testing. In Clinical and Laboratory Standards Institute 28th, Ed. Vol. informational supplement M100 (2018).
- 37.Caylan R, et al. An epidemiological analysis of Stenotrophomonas maltophilia strains in a university hospital. Jpn J Infect Dis. 2004;57(2):37–40. [PubMed] [Google Scholar]
- 38.Spilker T, Coenye T, Vandamme P, LiPuma JJ. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J Clin Microbiol. 2004;42(5):2074–9. doi: 10.1128/JCM.42.5.2074-2079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulaboski R, Lovrić M, Mirceski V, Bogeski I, Hoth M. A new rapid and simple method to determine the kinetics of electrode reactions of biologically relevant compounds from the half-peak width of the square-wave voltammograms. Biophysical chemistry. 2008;138(3):130–137. doi: 10.1016/j.bpc.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Watson S, Cabrera-Aguas M, Khoo P. Common eye infections. Aust Prescr. 2018;41(3):67–72. doi: 10.18773/austprescr.2018.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–8. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 42.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22–7. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 43.Sheng XL, et al. Prevalence and associated factors of corneal blindness in Ningxia in northwest China. Int J Ophthalmol. 2014;7(3):557–62. doi: 10.3980/j.issn.2222-3959.2014.03.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta N, Tandon R, Gupta SK, Sreenivas V, Vashist P. Burden of corneal blindness in India. Indian J Community Med. 2013;38(4):198–206. doi: 10.4103/0970-0218.120153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eby A, Hazlett L. Pseudomonas keratitis, a review of where we’ve been and what lies ahead. Microb Biochem Technol. 2015;7:453–457. [Google Scholar]
- 46.Xu Z, Fang X, Wood TK, Huang ZJ, Systems-Level A. Approach for Investigating Pseudomonas aeruginosa Biofilm Formation. PloS one. 2013;8(2):e57050. doi: 10.1371/journal.pone.0057050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mesaros N, et al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clinical Microbiology and Infection. 2007;13(6):560–578. doi: 10.1111/j.1469-0691.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 48.Folkesson A, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10(12):841–51. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 49.Seviour T, et al. Voltammetric profiling of redox-active metabolites expressed by Pseudomonas aeruginosa for diagnostic purposes. Chemical Communications. 2015;51(18):3789–3792. doi: 10.1039/C4CC08590F. [DOI] [PubMed] [Google Scholar]
- 50.Coyle, S., Curto, V. F., Benito-Lopez, F., Florea, L. & Diamond, D., Wearable bio and chemical sensors. In Wearable sensors, Elsevier: pp 65–83 (2014).
- 51.Nayak S, Blumenfeld NR, Laksanasopin T, Sia SK. Point-of-care diagnostics: Recent developments in a connected age. Analytical chemistry. 2016;89(1):102–123. doi: 10.1021/acs.analchem.6b04630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carey T, et al. Fully inkjet-printed two-dimensional material field-effect heterojunctions for wearable and textile electronics. Nature communications. 2017;8(1):1202. doi: 10.1038/s41467-017-01210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amlil A, et al. Electrochemical Biosensor for the Immediate Detection of Bacteria. Biosens Bioelectron. 2016;7(197):2. [Google Scholar]
- 54.Liu C, et al. Electrochemical detection of Pseudomonas aeruginosa 16S rRNA using a biosensor based on immobilized stem-loop structured probe. Enzyme Microb Technol. 2011;49(3):266–71. doi: 10.1016/j.enzmictec.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Sismaet HJ, Pinto AJ, Goluch ED. Electrochemical sensors for identifying pyocyanin production in clinical Pseudomonas aeruginosa isolates. Biosens Bioelectron. 2017;97:65–69. doi: 10.1016/j.bios.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 56.Maugeri G, Lychko I, Sobral R, Roque ACA. Identification and Antibiotic-Susceptibility Profiling of Infectious Bacterial Agents: A Review of Current and Future Trends. Biotechnol J. 2019;14(1):e1700750–e1700750. doi: 10.1002/biot.201700750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Syal K, et al. Current and emerging techniques for antibiotic susceptibility tests. Theranostics. 2017;7(7):1795–1805. doi: 10.7150/thno.19217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sismaet HJ, et al. Electrochemical detection of Pseudomonas in wound exudate samples from patients with chronic wounds. Wound Repair Regen. 2016;24(2):366–72. doi: 10.1111/wrr.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.