Abstract
In this study, a full-length cDNA sequence of SoxE (subgroup E within the Sox family of transcription factors) was cloned from Macrobrachium nipponense and named MnSoxE1. The full-length cDNA of MnSoxE1 is 1748 bp, consisting of a 110 bp 5′ UTR, a 105 bp 3′ UTR, and a 1533 bp ORF that encodes 510 amino acids. Conserved domains showed that MnSoxE1 has a high similarity to the SoxE gene of Penaeus vannamei. Phylogenetic tree analysis classified that MnSoxE1 with the SoxE gene of other arthropods into one clade. These results suggested that MnSoxE1 belongs to the SoxE subgroup. During embryonic development, MnSoxE1 was mainly expressed in the gastrula stage, implicating its involvement in tissue cell differentiation and formation. In the post-larval stages, the expression of MnSoxE1 continued to increase on days 1–10. The expression level in males was significantly higher than that in females. Males are clearly distinguishable from females on post-larval day 25, showing that MnSoxE1 may play a role in promoting early development and germ cell and gonadal differentiation, especially for males. qPCR analysis showed that MnSoxE1 may also be involved in oogonium proliferation during ovary development. Further in situ hybridization analysis revealed that MnSoxE1 was mainly located in oocytes and spermatocytes, especially in sertoli cells, and implies that it may be involved in the development of oocytes and spermatocytes, as well as the maintenance of testes in mature prawns. These results indicate that MnSoxE1 is involved in gonadal differentiation and development in M. nipponense, especially testis development.
Keywords: Macrobrachium nipponense, SoxE, Temporal and spatial expression, In situ hybridization
Introduction
The oriental river prawn Macrobrachium nipponense is one of the most important economic aquaculture species in China. Male prawns grow faster reaching a larger size than females at harvest time, which means that males have more economic value (Li et al. 2018; Zhang et al. 2013). Therefore, we are interested in researching the sex-determination mechanism of M. nipponense.
In crustaceans, the androgenic gland plays a key role in sex determination and differentiation. In Macrobrachium rosenbergii, mass production of all-male populations can be achieved by ablating the androgenic gland. Insulin-like androgenic gland factor secreted by the androgenic gland was thought to play a key role as a sex-determining gene in male crustaceans (Sagi et al. 1990; Aflalo et al. 2006). Although a lot of work has been focused on the study of sex differentiation in crustaceans, its molecular mechanisms remain unclear (Yu et al. 2014; Guo et al. 2018). Therefore, the identification of more gender-related genes is necessary.
A sequence encoding the SoxE protein was identified from the early transcriptome of M. nipponense (Jin et al. 2013). The Sox gene family is a group of genes that encode the mammalian male determining gene, Sry-like HMG (High Mobility Group) box. Researchers used the similarity of the HMG-box motif in the Sox gene to divide Sox genes into ten subfamilies, revealing that the HMG box contains a specific 9-amino acid sequence (RPMNAFMVW) (Bowles et al. 2000). In 1990, the discovery of SRY, the sex-determining region of Y chromosome genes, was an important breakthrough in mammalian gender determination research (Gubbay et al. 1990; Sinclair et al. 1990). Many transcription factors belonging to the Sox gene family are involved in diverse developmental processes, including sex determination, neural crest development, and neurogenesis (Laudet et al. 1993; Hong and Saint-Jeannet 2005; Paola et al. 2010). Previous studies proved that SRY is conserved and specific to the Y chromosome in mammals. Deletions or point mutations in the HMG domain of the Sry/SRY gene may cause an autosomal XY female sex-reversed phenotype in mice or humans (Jäger et al. 1990; Gubbay et al. 1990; Foster et al. 1994; Barrionuevo et al. 2006). SoxE subgroup transcription factors consist of SOX8, SOX9, and SOX10 proteins, which are thought to play essential roles in mammalian sex determination and gonadal development (Silva et al. 1996; Koopman 1999; Chaboissier et al. 2004; She and Yang 2017). Many Sox genes have also been identified in invertebrates, in addition to widely studied model animals such as Drosophila, sea urchin, and nematode, but the function of the Sox gene is poorly understood, especially in crustaceans (Phochanukul and Russell 2010).
In this study, the full-length cDNA of MnSoxE1 was cloned from M. nipponense and phylogenic analysis was employed to clarify its evolutionary progression. The expression profiles of MnSoxE1 gene in ovarian maturation, and embryonic, larval, and mature tissues were quantified by qPCR. Localization of MnSoxE1 mRNA in testis and ovary tissue was investigated using in situ hybridization (ISH). We aimed to provide an in-depth understanding of the MnSoxE1 gene in gender-related development of M. nipponense, and lay a foundation for future functional studies.
Materials and methods
Experimental material
Wild healthy prawns approximately 1.20–3.85 g (males 3–3.85 g; females 1.20–2 g) were obtained from Tai lake in Wuxi, China (120° 13′ 44″ E, 31° 28′ 22″ N). Recirculating water aquarium system was used for culturing at 24–26 °C, and prawns were fed paludina twice per day. After 1 week culture, embryo, larva, muscle, testis, ovary, hepatopancreas, brain, eyestalk, and gill were dissected out from mature prawns. Different developmental stages of prawns were collected from the breeding process. Developmental stages of larvae were determined by the criteria of previous research (Li et al. 2018). Liquid nitrogen was used to freeze the sample and then stored them at − 80 °C.
Total RNA isolation
A variety of tissues including embryo, larva, muscle, testis, ovary, hepatopancreas, brain, eyestalk, and gill, were dissected out from mature prawns. Total RNA was isolated with RNAiso Plus Reagent (TaKaRa, Japan). At least five prawns were analyzed for each type of tissue and 1.2% agarose gel was used to test all of the RNA samples.
Cloning and sequence analysis
The partial fragment of SoxE cDNA was obtained from normalized cDNA library in our lab (Jin et al. 2013). The RACE technique was utilized to clone the full-length cDNA sequence of SoxE from hepatopancreas, based on the known fragment. First-strand cDNA was synthesized from total RNA of hepatopancreas using a Reverse Transcriptase M-MLV Kit (TaKaRa, Japan). The cDNA was kept at − 20 °C, used for terminal fragments amplification with the 3′/5′-RACE. The 3′-RACE and 5′-RACE were performed using 3′-full RACE Core Set Ver.2.0 Kit and 5′-full RACE Kit (TaKaRa, Japan) to get cDNA 3′ and 5′ ends. The primers used in the clone are listed in Table 1. The PCR products were purified with Gel Extraction kit (Sangon, China), and sequenced by ABI3730 DNA Analyzer after inserting into PMD-18T vector (TaKaRa, Japan).
Table 1.
Primers of sequence used in this research
| Sequence (5′ → 3′) | Description | |
|---|---|---|
| 5′-RACE outer | CATGGCTACATGCTGACAGCCTA | Primer for 5′-RACE |
| 5′-RACE inner | CGCGGATCCACAGCCTACTGATGATCAGTCGATG | Primer for 5′-RACE |
| MnSoxE1 -5′R | GAATCTTGCTCCAGTCGTAG | Primer for 5′-RACE |
| 3′-RACE outer | TACCGTCGTTCCACTAGTGATTT | Primer for 3′-RACE |
| 3′-RACE inner | CGCGGATCCTCCACTAGTGATTTCACTATAGG | Primer for 3′-RACE |
| MnSoxE1-3′F | CCGTCGGAAACACAAGCAGGA | Primer for 3′-RACE |
| MnSoxE1 -qF | ACATAGATCGCGCAGAAATGAAC | primer for qPCR |
| MnSoxE1 -qR | CCAAGGAAGGAAGACTTGTGAGT | primer for qPCR |
| MnEIF-F | CATGGATGTACCTGTGGTGAAAC | Reference gene primer |
| MnEIF-R | CTGTCAGCAGAAGGTCCTCATTA | Reference gene primer |
BLASTX and BLASTN programs (http://www.ncbi.nlm.nih.gov/BLAST/), open-reading frame (ORF) finder (http://www.ncbi.nlm.nih.gov/gorf/), and SMART modular architectural analysis programs (http://smart.embl-heidelberg.de/) were used for sequences analysis, and protein and conserved domains’ prediction. Phylogenetic trees were generated by the neighbor-joining method by Molecular Evolutionary Genetics Analysis (MEGA 5.0) (Bairoch et al. 1997; Combet et al. 2000). And multiple alignments of amino acid sequences encoding MnSoxE1 were created using DNAMAN 6.0.
Tissue expression by real-time quantitative PCR
Approximately 1 μg of total RNA from each tissue (embryo, larva, muscle, testis, ovary, hepatopancreas, brain, eyestalk, and gill) was reverse-transcribed by iscript™ cDNA Synthesis Kit Perfect Real Time (BIO-RAD). Expression level of MnSoxE1 in various tissue was tested using a quantitative real-time PCR assay on Bio-Rad iCycler iQ5 Real-Time PCR System (Bio-Rad, USA).
The primer pair MnSoxE1-qF and MnSoxE1-qR of qPCR was designed using Primer-Blast tools in NCBI (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) based on the open-reading frame. MnEIF was used as a reference gene (Hu et al. 2018). All primers were synthesized by Shanghai Exsyn-bio Technology Company and are presented in Table 1. Per sample was performed by four replicate qPCR reaction, and for each sample, five prawns were analyzed (Zhang et al. 2013). Differences of expressions were considered to be significant if P < 0.05. Relative copy number of MnSoxE1 mRNA was calculated with the 2−ΔΔCT comparative CT method (Livak and Schmittgen 2001).
Expression profiles of MnSoxE1 in different stages of embryos and ovarian development
According to previous research, the developmental stages of embryo were classified into several stages (Qiao et al. 2015): Cleavage stage (CS), Blastula stage (BS), Gastrul stage (GS), Nauplius stage (NS), and Zoea stage (ZS). The selection of larvae is based on previous research in the laboratory(Jin et al. 2016): L1: the first day larvae after hatching; L5: the 5 day larvae after hatching; L10: the 10 day larvae after hatching; L15: the 15 day larvae after hatching; PL1: post-larval stage of the first day; PL5: post-larval stage of 5 days; PL10: post-larval stage of 10 days; PL15: post-larval stage of 15 days; Fe-PL25: post-larval stage of 25 days of females; M-PL25: post-larval stage of 25 days of males. The ovarian cycle of M. nipponense was classified into five stages based on the previous results (Qiao et al. 2015).
In situ hybridization (ISH)
The ovaries, testis, and vas deferens were fixed by 4% paraformaldehyde in phosphate-buffered saline (PBS) and performed with Zytofast PLUS CISH implementation kit (Zyto Vision GmBH, Germany). Experimental steps are in accordance with previous criteria, then counterstaining with hematoxylin and dehydrated in a gradient alcohol solution, dried in air, and fixed with DPX (Li et al. 2018). The sequence of 5′-GTGAGGAGCGGAAGTGGCATACTGGGGTCCTG-3′ was used as probe. The probe sequence in the control was 5′- CAGGACCCCAGTATGCCACTTCCGCTCCTCAC-3′.
Data analysis
Quantitative data were displayed as mean ± SD. Statistical difference analysis was tested using one-way ANOVA and Duncan’s multiple range tests. A probability level of 0.05 was used to indicate significance (P < 0.05). All statistical analyses were performed using SPSS 20.0.
Results
Cloning and characterization of MnSoxE1
The MnSoxE1 gene was cloned using 3′ and 5′ RACE technology and resulted in a full-length 1748 bp long MnSoxE1 cDNA, which included a 110 bp 5′ UTR, and a 105 bp 3′ UTR. The deduced open-reading frame was 1533 bp, encoding 510 amino acids (Fig. 1) with a predicted molecular mass of 53.8 kDa and calculated isoelectric point of 7.69. SMART software analysis revealed that the deduced amino acid sequence contained an HMG domain (residues 164–234). The specific sequence information for MnSoxE1 is presented in Fig. 1.
Fig. 1.
Nucleotide and deduced amino acid sequence of the M. nipponense SoxE1 gene (MnSoxE1). The HMG-box domain is marked with gray and specific amino acid sequence is in the red box
Sequence alignment of MnSoxE1 and phylogenic tree
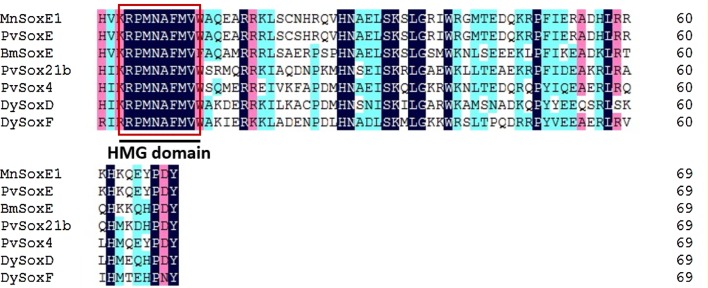
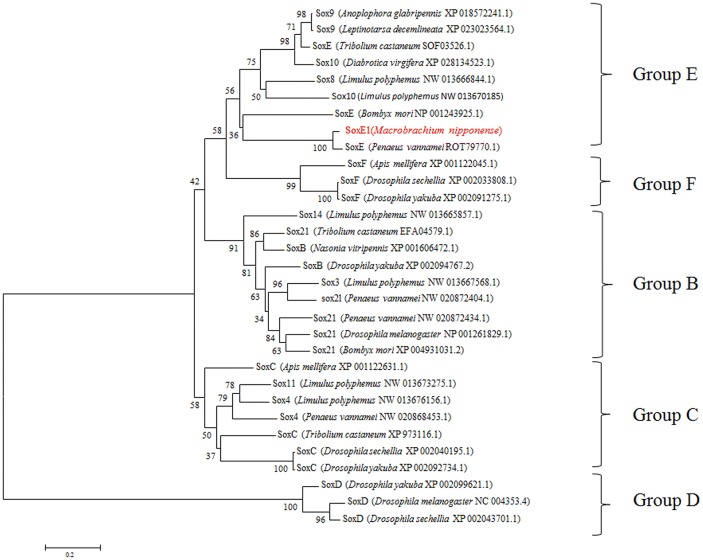
In this study, MEGA5.1 and DNAMAN 6.0 softwares were used to create multiple alignments of MnSoxE1 and SoxE homologs from several arthropods. MnSoxE1 has a high similarity to the HMG region of the SoxE gene of other species; the similarity to SoxE of Penaeus vannamei reached 97.14%, but the similarity with the Sox gene of other subgroups of other species was less than 60% (Fig. 2). A phylogenetic tree was constructed including arthropod and some non-arthropod Sox genes. The species used for the construction of the phylogenetic tree are listed in Figure 3. All Sox genes selected were classified into five groups (B–F) (Fig. 3). The MnSoxE1 gene was clustered within Group E.
Fig. 2.
The alignment of amino acid sequences of the conserved HMG-box domain of MnSoxE1 and that in other homologs. The location of the HMG box is marked in the figure
Fig. 3.
Phylogenetic analysis of different Sox family members using the MEGA 5.1 program. GenBank accession numbers were indicated after species name
Transcriptional profile of MnSoxE1 during embryonic and larval development stages
qPCR was used to analyze the expression of MnSoxE1 in six different stages of embryonic development and found to be expressed in all tested samples. The expression was lowest at the unfertilized stage (US), and showed continuous low expression in the cleavage (CS) and blastula stages (BS). Next, a sharp rise occurred in expression, reaching the highest level at the gastrula stage (GS), about 10–20 times higher than the earlier stages. Then, the expression significantly decreased in the next stages: nauplius (NS) and zoea stages (ZS) (Fig. 4).
Fig. 4.
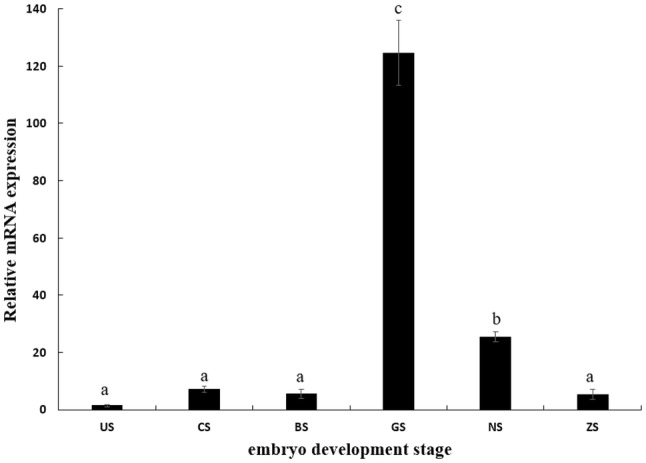
Transcriptional profile of MnSoxE1 during embryonic development. US unfertilized stage, CS cleavage stage, BS blastula stage, GS gastrula stage, NS nauplius stage, ZS zoea stage. Different letters denote significant differences (P < 0.05). Error bars represent the mean ± standard error
Expression pattern of MnSoxE1 in different larval stages was also examined by qPCR. The expression of MnSoxE1 was lowest in larvae on the 5th day of after hatching, and then continued to rise for 1–10 days post-larval stage. At day 25 post-larval, the sex of M. nipponense was visually distinguished. At this time, the expression level of MnSoxE1 in the male prawns reached its highest point and was significantly higher by twofold than that of the females (Fig. 5).
Fig. 5.
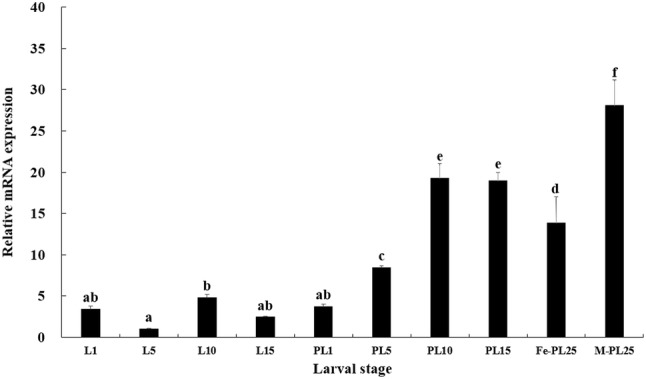
Transcriptional profile of MnSoxE1 during larval stages. L1: the first day larvae after hatching; L5: the 5 day larvae after hatching; L10: the 10 day larvae after hatching; L5: the 15 day larvae after hatching; PL1: post-larval stage of 1 day; PL5: post-larval stage of 5 days; PL10: post-larval stage of 10 days; PL15: post-larval stage of 15 days; Fe-PL25: post-larval stage of 25 days of females; M-PL25: post-larval stage of 25 days of males. Different letters denote significant differences (P < 0.05). Error bars represent the mean ± standard error
Transcriptional profile of MnSoxE1 during different ovary stages
The expression pattern of MnSoxE1 in the ovary was examined during the various reproductive cycles. The qPCR results showed that the expression level of MnSoxE1 transcripts in ovary presented a peak in stage I, and then dropped until the stage III, reaching its lowest point. After that, the expression rose sharply until stage V (Fig. 6).
Fig. 6.
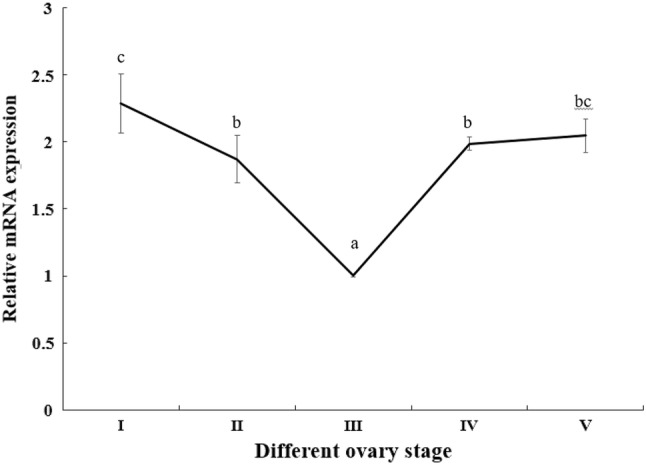
Transcriptional profile of MnSoxE1 in ovaries during ovarian reproductive stages. Stage I: oogonium proliferation; Stage II: primary vitellogenesis; Stage III: secondary vitellogenesis; Stage IV: vitellogenesis termination; and Stage V: spent stage. Different letters denote significant differences (P < 0.05). Error bars represent the mean ± standard error
Expression of MnSoxE1 in mature different tissues
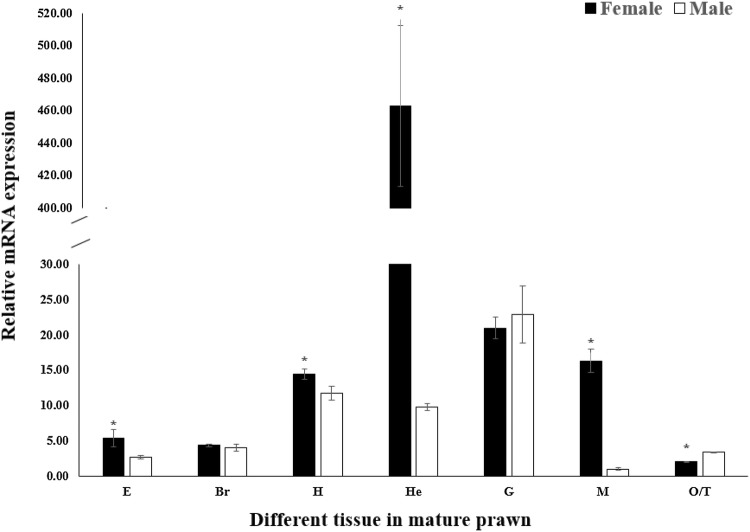
The expression distribution of MnSoxE1 was analyzed by qPCR in all tissues in adult male and female prawns. The results showed that MnSoxE1 mRNA was distributed in all tissues tested (Fig. 7). In males, MnSoxE1 displayed the highest expression level in the gill and with the lowest in muscle. In females, the lowest expression of MnSoxE1 was in ovary, and the highest was in the hepatopancreas. When comparing expression between males and females, expression in the hepatopancreas was significantly higher in females than in males, by roughly 47-fold (Fig. 4) (P < 0.05).
Fig. 7.
The expression profile of MnSoxE1 in different tissues was revealed by real-time quantitative PCR. The amount of MnSoxE1 mRNA was normalized to the MnEIF transcript level. Data are shown as mean ± SD of three replicates in various tissues. E eyestalk, Br brain; H heart, He hepatopancreas, G gill, M muscle, O ovary, T testis. Data indicated with asterisks are significantly different between different groups (P < 0.05). Error bars represent the mean ± standard error
Localization of MnSoxE1 mRNA
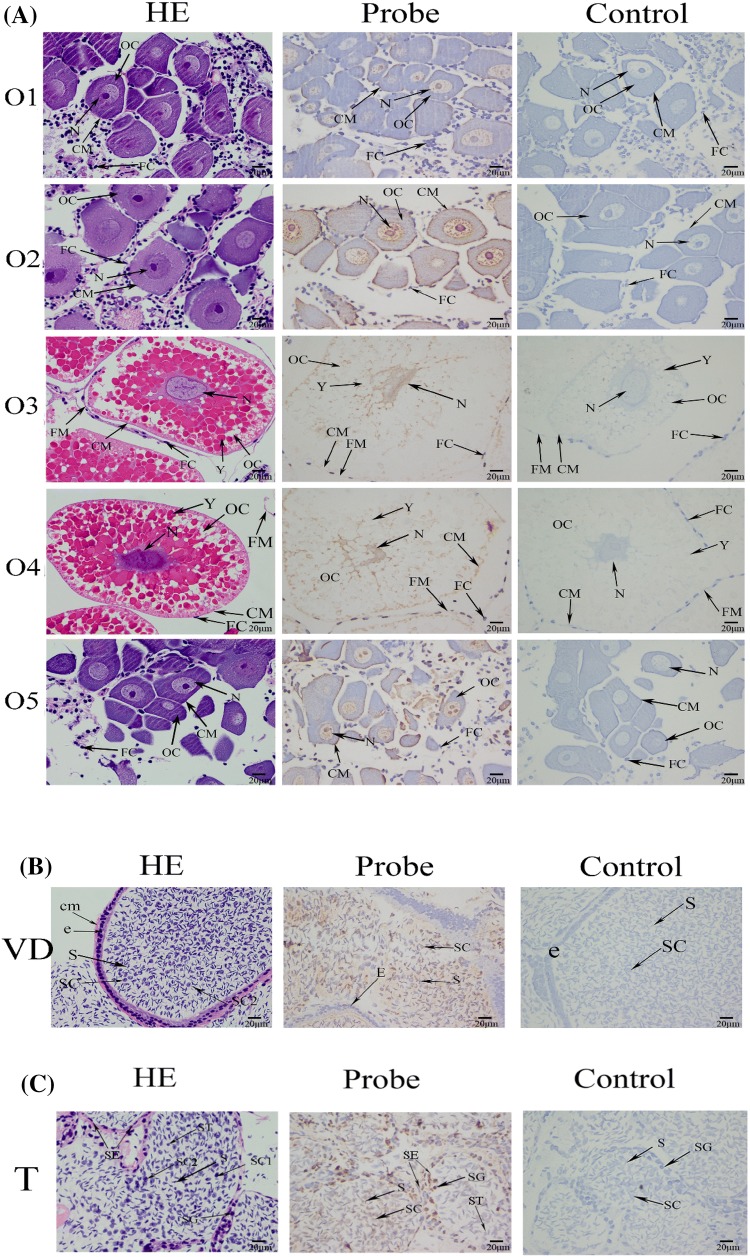
Localization of MnSoxE1 mRNA in sex-related organs (ovaries, vas deferens and testes) was studied by ISH. Ovarian development is accompanied by the formation of eggs and the accumulation of vitellogenin. Ovarian reproductive epithelium differentiates into oogonium and follicular cells. With the development of the ovary, the oogonium gradually differentiates and the volume and color of ovarian tissue also changes accordingly (Stage I, oocyte proliferation, transparent; stage II, yolk formation, khaki; stage III, secondary yolk stage, light green; stage IV, yolk termination, dark green; stage V, regression period, dark gray) (Li et al. 2018). The result of ISH showed that an MnSoxE1 signal was present in oocytes (Fig. 8a). Testes and vas deferens are important components of the male prawn reproductive system. The anterior segments of two vas deferens are connected to the testis, and the vas deferens are full of mature sperm and some spermatocytes. ISH revealed a MnSoxE1 signal in the vas deferens spermatocytes (Fig. 8b). In testis, there are many seminiferous tubules with spermatogonium, spermatocytes, and spermatozoa cells. MnSoxE1 transcripts were localized in sertoli cells, spermatogonia, and testis spermatocytes, and no signal was observed in the negative control using a sense-strand probe (Fig. 8c).
Fig. 8.
Location of MnSoxE1 gene detected by in situ hybridization. a Histological section of ovary at different ovary stages of M. nipponense. OC oocyte, N nucleus, CM cytoplasmic membrane, Y yolk granule, FC follicle cell, FM follicle membrane. b Histological section of vas deferens of M. nipponense. SC spermatocyte, E epithelial cell. CM cytoplasmic membrane, S sperm, c histological section of testis of M. nipponense. CT collecting tissue, SG spermatogonium, SE sertoli cells, SC spermatocyte, SC1 primary spermatocyte, SC2 second spermatocyte, ST spermatid, S sperm
Discussion
In the present study, the MnSoxE1 gene was cloned, and sequence analysis revealed an HMG-box domain, indicating that the gene belongs to the HMG-box gene superfamily. In addition, the MnSoxE1 protein contains the motif “RPMNAFMVW” located within the HMG box which is conserved for all Sox sequences (Bowles et al. 2000; Wilson and Dearden 2008). The HMG-box domain of the Sox gene is highly conserved, and is attributed to its ability to specifically bind to its DNA recognition sequence. Previous studies have shown that structural, functional, and evolutionary relationships of Sox genes have relied largely on the HMG-box sequence (Bowles et al. 2000). The Sox protein could specifically bind to the DNA sequence of 5′(A/T) (A/T)CAA(A/T)G3′, and after binding to the DNA sequence, the overall configuration of the HMG cassette was unchanged, inducing its target DNA to bend to varying degrees (Sugimoto et al. 1991; Seeliger et al. 1992; Bowles et al. 2000; Nagai 2001).
Previous analysis has shown that the Sox family is classified using phylogenetic comparisons into ten groups (A–J) (Bowles et al. 2000). In Oryzias latipes, researchers found that Sox32 was assigned to a new group K. In the arthropod, there are five groups (B–F) that have been identified (Crémazy et al. 2001; Wilson and Dearden 2008). The gene in this study belongs to the SoxE family based on sequence alignment and phylogenetic tree analysis (Figs. 2, 3). Previous studies have also shown that the HGM of the Sox gene maintains a high similarity in the same subfamily (Shinzato 2007; Focareta and Cole 2016). In this study, MnSoxE1 was clustered into the SoxE subgroup, and has the closest evolutionary relationship with P. vannamei. Moreover, phylogenetic analysis showed that the Sox genes of different subgroups from the same species did not cluster into the same class, while Sox genes of the same subgroup from different species clustered together (Fig. 3), which is consistent with previous studies. SRY was thought to be involved in gonadal differentiation (Koopman 1999), and in mammals, the SoxE gene was found to be involved in male sex determination and gonadal development. These and related studies of fishes demonstrated that SoxE may regulate testicular differentiation (Kobayashi and Nagahama 2009). However, there is no direct evidence for a similar function of SoxE in crustaceans.
The expression pattern of MnSoxE1 in different embryonic stages of M. nipponense was analyzed by qPCR. Results showed that the expression of MnSoxE1 was rapidly increased in the gastrula stage, and then decreased until to zoea stage (Fig. 4). The gastrula stage is a critical period of cell differentiation and is related to the generation of mesoderm. In previous studies, SoxE was found to be expressed throughout the mesodermal regions in human, sea urchin, and Sepia officinalis (De et al. 2000; Juliano et al. 2006; Focareta and Cole 2016), this suggested that MnSoxE1 is involved in the formation of mesoderm of M. nipponense. Moreover, researchers found that the SoxE gene was not expressed during the early stages of embryonic development in panarthropod species, but expression occurred in mesodermal tissue approximately 8.5 h after formation of the gastrula. Since SoxE is expressed in the posterior abdominal segments,similar to other arthropods and onychophora, it was thought that SoxE could be associated with gonad formation (Janssen et al. 2018). This supports our finding that MnSoxE1 may play the same role in the embryonic development of M. nipponense.
Further study was conducted on the expression of larvae after embryonic development was completed, especially during the post-larval phase. It is worth noting that in the period of a large number of moltings (L1–L15) and dramatic changes (L15–PL1, metamorphosis process), common organs are drastically differentiated, but the expression of MnSoxE1 did not change much. Interestingly, a significant increase occurred during days 1–10 post-larval (Fig. 5). Previous studies have shown that the germ gland anlage appears around post-larval day 9 (Zhang et al. 2015; Jin et al. 2016). Combined with the above evidence, MnSoxE1 is involved in early gonadal differentiation but not in other tissues of M. nipponense. On post-larval day 25, the male and female larvae can be visually distinguished. At this time, the expression of MnSoxE1 in males is about twofold higher than that of females, which implies that MnSoxE1 is more involved in the early differentiation of males.
The expression pattern of MnSoxE1 was, therefore, detected in various ovarian reproductive cycles. In different stages of ovarian development of M. nipponense, the expression of the MnSoxE1 gene was high in the first phase of oogonium proliferation, and the overall trend was a fold line, showing that third-stage expression was significantly lower (Fig. 6). At this time, the secondary vitellogenesis of the ovary of M. nipponense sinensis was the accumulation period of yolk and less active cell differentiation. MnSoxE1 showed high expression level in OI and OV during the ovarian reproductive cycle. OI and OV play essential roles in oogonium proliferation and cell differentiation, suggesting that MnSoxE1 may be involved in these processes during ovarian development in M. nipponense. The expression of the MnSoxE1 gene was also analyzed in different mature tissues. In adult tissues, qPCR analysis showed that the MnSoxE1 gene did not show significant differences between males and females in the same tissue, except in hepatopancreas and muscle. However, MnSoxE1 was expressed at a relatively low level in adult gonads, whether in testis or ovary (Fig. 7). Thus, MnSoxE1 was predicted to perform different functions in adult prawns relative to embryonic and larval prawns, and needs further research.
ISH was used to locate MnSoxE1 in different reproductive cycles of the ovary, testis, and vas deferens. Results showed that MnSoxE1 was expressed on the nucleus, cytoplasm, and cell membrane of the oocyte. In the in situ hybridization of testis and vas deferens, strong signals were observed in sertoli cells and spermatocytes, but not in mature sperm (Fig. 8b, c). Combined with these results, we can speculate that the MnSoxE1 gene may be involved in cell differentiation, especially in sertoli cells of the gonads in M. nipponense. Sertoli cells are the somatic cells of the testes, essential for testis formation and spermatogenesis, which can facilitate the progression of germ cells to spermatozoa by controlling the environment in seminiferous tubules (Griswold 1998). Previous studies showed that Sox8 was observed in the perinuclear area of sertoli cells and the interstitium in adult mouse, consistent with our results (O’Bryan et al. 2008; Roumaud et al. 2018). Sox9 is also a key gene involved in sertoli cell and testis differentiation (Barrionuevo et al. 2009; Ting et al. 2013). Strong signals of Sox9 were localized in oocyte and sertoli cells in amphibian, including xenopus tropicalis and Bufo marinus (El Jamil et al. 2008; Abramyan et al. 2009), and only localized in sertoli cells in mouse (Kent et al. 1996). These results indicate that the functions of the SoxE subgroup have slight differences in different species (Silva et al. 1996; Western et al. 1999). However, a few previous studies reported that the SoxE subgroup members affected sex differentiation or resulted in sexual reversal in crustacean species. This may be due to functional bias caused by differences in species, but based on gene conservation and related tissue expression and localization, MnSoxE1 may still be an important sex-related gene in M. nipponense.
In summary, in this study, MnSoxE1 was analyzed in M. nipponense by RACE cloning, qPCR, and in situ hybridization analysis. Based on the results of qPCR and localization of in situ hybridization, we hypothesize that MnSoxE1 may play a role in cell differentiation during embryonic development and the formation of germ gland anlage. MnSoxE1 is also involved in early gonad differentiation, especially in males and the maintenance of adult testes in M. nipponense. As far as we know, this is the first report of the SoxE gene in M. nipponense. Further study of SoxE genes is of great significance for revealing their function in the molecular mechanisms of sex determination in M. nipponense.
Acknowledgements
This research was supported by National Key R&D Program of China (2018YFD0900201); Central Public-Interest Scientific Institution Basal Research Fund CAFS (2019JBFM02); Jiangsu Agricultural Industry Technology System (JFRS-02); National Natural Science Foundation of China (31572617); China Agriculture Research System-48 (CARS-48); New cultivar breeding Major Project of Jiangsu province (PZCZ201745).
Author contributions
Conceived and designed the experiments: YH, HF, SJ, and HQ. Performed the experiments: YH, SJ, and WZ. The specimens were maintained by YH and YX. Analyzed the data: YH. Contributed reagents/materials/analysis tools: YG and YW.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abramyan J, Feng CW, Koopman P. Cloning and expression of candidate sexual development genes in the cane toad (Bufo marinus) Dev Dyn. 2009;238(9):2430–2441. doi: 10.1002/dvdy.22055. [DOI] [PubMed] [Google Scholar]
- Aflalo ED, Hoang TTT, Nguyen VH, Lam Q, Nguyen DM, Trinh QS, Raviv S, Sagi A. A novel two-step procedure for mass production of all-male populations of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture. 2006;256(1):468–478. [Google Scholar]
- Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1997. Nucleic Acids Res. 1997;25(1):217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Jr Klattig, Kist R, Taketo MM, Englert C, Scherer G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74(1):195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Georg I, Scherthan H, Lécureuil C, Guillou F, Wegner M, Scherer G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol. 2009;327(2):301–312. doi: 10.1016/j.ydbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227(2):239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Chaboissier M-C, Kobayashi A, Vidal VI, Lützkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131(9):1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Combet C, Blanchet C, Geourjon C, Deleage G. NPS@: network protein sequence analysis. Trends Biochem Sci. 2000;25(3):147–150. doi: 10.1016/s0968-0004(99)01540-6. [DOI] [PubMed] [Google Scholar]
- Crémazy F, Berta P, Girard F. Genome-wide analysis of Sox genes in Drosophila melanogaster. Mech Dev. 2001;109(2):371–375. doi: 10.1016/s0925-4773(01)00529-9. [DOI] [PubMed] [Google Scholar]
- De SBP, Moniot B, Poulat F, Berta P. Expression and subcellular localization of SF-1, SOX9, WT1, and AMH proteins during early human testicular development. Dev Dyn. 2000;217(3):293–298. doi: 10.1002/(SICI)1097-0177(200003)217:3<293::AID-DVDY7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- El Jamil A, Kanhoush R, Magre S, Boizet-Bonhoure B, Penrad-Mobayed M. Sex-specific expression of SOX9 during gonadogenesis in the amphibian Xenopus tropicalis. Dev Dyn. 2008;237(10):2996–3005. doi: 10.1002/dvdy.21692. [DOI] [PubMed] [Google Scholar]
- Focareta L, Cole AG. Analyses of Sox-B and Sox-E Family Genes in the Cephalopod Sepia officinalis: revealing the conserved and the unusual. PLoS ONE. 2016;11(6):e0157821. doi: 10.1371/journal.pone.0157821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanović M, Weissenbach J, Mansour S, Young ID, Goodfellow PN. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372(6506):525. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9(4):411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovellbadge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346(6281):245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Guo Q, Li S, Lv X, Xiang J, Sagi A, Manor R, Li F. A putative insulin-like androgenic gland hormone receptor gene specifically expressed in male Chinese shrimp. Endocrinology. 2018;159(5):2173–2185. doi: 10.1210/en.2017-03253. [DOI] [PubMed] [Google Scholar]
- Hong CS, Saint-Jeannet JP. SOX proteins and neural crest development. Semin Cell Dev Biol. 2005;16(6):694–703. doi: 10.1016/j.semcdb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Hu Y, Fu H, Qiao H, Sun S, Zhang W, Jin S, Jiang S, Gong Y, Xiong Y, Wu Y. Validation and evaluation of reference genes for quantitative real-time PCR in Macrobrachium Nipponense. Int J Mol Sci. 2018;19(8):2258. doi: 10.3390/ijms19082258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger RJ, Anvret M, Hall K, Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990;348(6300):452. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Janssen R, Andersson E, Betnér E, Bijl S, Fowler W, Höök L, Leyhr J, Mannelqvist A, Panara V, Smith K. Embryonic expression patterns and phylogenetic analysis of panarthropod sox genes: insight into nervous system development, segmentation and gonadogenesis. BMC Evol Biol. 2018;18(1):88. doi: 10.1186/s12862-018-1196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Fu H, Zhou Q, Sun S, Jiang S, Xiong Y, Gong Y, Qiao H, Zhang W. Transcriptome analysis of androgenic gland for discovery of novel genes from the oriental river prawn, Macrobrachium nipponense, using Illumina Hiseq 2000. PLoS ONE. 2013;8(10):e76840. doi: 10.1371/journal.pone.0076840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Zhang Y, Guan H, Fu H, Jiang S, Xiong Y, Qiao H, Zhang W, Gong Y, Yan W. Histological observation of gonadal development during post-larva in oriental river prawn, Macrobrachium nipponense. Chin J Fish. 2016;29(4):11–16. [Google Scholar]
- Juliano CE, Voronina E, Stack C, Aldrich M, Cameron AR, Wessel GM. Germ line determinants are not localized early in sea urchin development, but do accumulate in the small micromere lineage. Dev Biol. 2006;300(1):406–415. doi: 10.1016/j.ydbio.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122(9):2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nagahama Y. Molecular aspects of gonadal differentiation in a teleost fish, the Nile tilapia. Sex Dev. 2009;3(2–3):108–117. doi: 10.1159/000223076. [DOI] [PubMed] [Google Scholar]
- Koopman P. Sry and Sox9: mammalian testis-determining genes. Cell Mol Life Sci CMLS. 1999;55(6–7):839–856. doi: 10.1007/PL00013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V, Stehelin D, Clevers H. Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res. 1993;21(10):2493–2501. doi: 10.1093/nar/21.10.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Qiao H, Fu H, Sun S, Zhang W, Jin S, Jiang S, Gong Y, Xiong Y, Wu Y. Identification and characterization of opsin gene and its role in ovarian maturation in the oriental river prawn Macrobrachium nipponense. Comp Biochem Phys B. 2018;218:1–12. doi: 10.1016/j.cbpb.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nagai K. Molecular evolution of Sry and Sox gene. Gene. 2001;270(1):161–169. doi: 10.1016/s0378-1119(01)00479-6. [DOI] [PubMed] [Google Scholar]
- O’Bryan MK, Shuji T, Kennedy CL, Greg S, Shun-Ichi H, Ray MK, Qunsheng D, Dagmar W, Kretser DM, De Mitch E. Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev Biol. 2008;316(2):359–370. doi: 10.1016/j.ydbio.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paola B, Marianne BF, Tatjana SS. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc Natl Acad Sci U S A. 2010;107(8):3570–3575. doi: 10.1073/pnas.0906596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phochanukul N, Russell S. No backbone but lots of Sox: invertebrate Sox genes. Int J Biochem Cell Biol. 2010;42(3):453–464. doi: 10.1016/j.biocel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Qiao H, Xiong Y, Zhang W, Fu H, Jiang S, Sun S, Bai H, Jin S, Gong Y. Characterization, expression, and function analysis of gonad-inhibiting hormone in Oriental River prawn, Macrobrachium nipponense and its induced expression by temperature. Comp Biochem Physiol A Mol Integr Physiol. 2015;185:1–8. doi: 10.1016/j.cbpa.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Roumaud P, Haché J, Martin LJ. Expression profiles of Sox transcription factors within the postnatal rodent testes. Mol Cell Biochem. 2018;447(1–2):1–13. doi: 10.1007/s11010-018-3302-3. [DOI] [PubMed] [Google Scholar]
- Sagi A, Dan C, Milner Y. Effect of androgenic gland ablation on morphotypic differentiation and sexual characteristics of male freshwater prawns, Macrobrachium rosenbergii. Gen Comp Endocrinol. 1990;77(1):15–22. doi: 10.1016/0016-6480(90)90201-v. [DOI] [PubMed] [Google Scholar]
- Seeliger S, Derian CK, Vergnolle N, Bunnett NW, Nawroth R, Schmelz M, Von Der Weid PY, Buddenkotte J, Sunderkötter C, Metze D, Andrade-Gordon P, Harms E, Vestweber D, Luger TA, Steinhoff M. An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein. EMBO J. 1992;11(10):3705–3712. doi: 10.1002/j.1460-2075.1992.tb05455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She ZY, Yang WX. Sry and SoxE genes: How they participate in mammalian sex determination and gonadal development? Semin Cell Dev Biol. 2017;63:13–22. doi: 10.1016/j.semcdb.2016.07.032. [DOI] [PubMed] [Google Scholar]
- Shinzato C (2007) Cnidarian Sox genes and the evolution of function in the Sox gene family. PhD thesis, James Cook University
- Silva S, Da Morais, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14(1):62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovellbadge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346(6281):240. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Sugimoto A, Iino Y, Maeda T, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe ste11 + encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 1991;5(11):1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- Ting J, Cong-Cong H, Zhen-Yu S, Wan-Xi Y. The SOX gene family: function and regulation in testis determination and male fertility maintenance. Mol Biol Rep. 2013;40(3):2187–2194. doi: 10.1007/s11033-012-2279-3. [DOI] [PubMed] [Google Scholar]
- Western PS, Harry JL, Graves JA, Sinclair AH. Temperature-dependent sex determination: upregulation of SOX9 expression after commitment to male development. Dev Dyn. 1999;214(3):171–177. doi: 10.1002/(SICI)1097-0177(199903)214:3<171::AID-AJA1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Dearden PK. Evolution of the insect Sox genes. BMC Evol Biol. 2008;8(1):120. doi: 10.1186/1471-2148-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y-Q, Ma W-M, Zeng Q-G, Qian Y-Q, Yang J-S, Yang W-J. Molecular cloning and sexually dimorphic expression of two Dmrt genes in the giant freshwater prawn, Macrobrachium rosenbergii. Agric Res. 2014;3(2):181–191. [Google Scholar]
- Zhang Y, Fu H, Qiao H, Jin S, Jiang S, Xiong Y, Gong Y, Zhang X. Molecular cloning and expression analysis of transformer-2 gene during development in Macrobrachium nipponense (de Haan 1849) J World Aquacult Soc. 2013;13(2):331–340. [Google Scholar]
- Zhang Y, Sun S, Fu H, Ge X, Qiao H, Zhang W, Xiong Y, Jiang S, Gong Y, Jin S. Characterization of the male-specific lethal 3 gene in the oriental river prawn, Macrobrachium nipponense. Genet Mol Res. 2015;14(2):3106–3120. doi: 10.4238/2015.April.10.21. [DOI] [PubMed] [Google Scholar]