Abstract
We report the first documented clinical case of the use of magnetic seeds to mark axillary lymph node metastasis in breast cancer before neoadjuvant chemotherapy. After chemotherapy, the patient showed a complete radiological response. One single sentinel lymph node was detected using a radiotracer, while the marked node was intraoperative magnetometer-guided identified. The analysis of the nodes showed negative sentinel lymph node and positive marked node, and the subsequent targeted axillary dissection was performed. Marking axillary positive lymph nodes with a magnetic seed is a simple and effective procedure for the intraoperative localisation of the node after neoadjuvant treatment.
INTRODUCTION
We present the first documented case of the use of magnetic seeds to mark axillary lymph node metastasis in breast cancer prior to neoadjuvant chemotherapy with magnetometer-guided intraoperative identification of the marked lymph node.
CASE REPORT
A 73-year-old woman diagnosed with multifocal triple-negative invasive ductal carcinoma in her left breast, with a Ki-67 index of 80% and axillary involvement evidenced by ultrasound and confirmed by core needle biopsy.
Magnetic resonance imaging showed two adjacent lesions, measuring 29 and 12 mm, where the upper quadrants meet.
Before neoadjuvant treatment, the metastatic lymph node was marked with a magnetic seed (Magseed, Endomag, Cambridge, UK), so that it would subsequently be easier to localise during the operation. The patient received 6 cycles of FEC (fluorouracil, epirubicin, cyclophosphamide) chemotherapy, after which MRI revealed a 40% decrease in the breast tumour volume and ultrasound showed the axillary lymphadenopathy had developed a complete radiological response. After obtaining the patient’s informed consent, we performed a hookwire-guided lumpectomy of the breast lesions, a targeted axillary dissection through the localisation of the marked lymph node (Fig. 1), and sentinel node biopsy using a radiotracer, which revealed one sentinel node.
Figure 1.

Intraoperative magnetometric identification of marked lymph node.
The lymphadenopathies were assessed intraoperatively using the OSNA (one-step nucleic acid amplification) method, leading to the diagnosis of macrometastasis in the lymph node marked with the magnetic seed (45 000 copies/μl) (Fig. 2), while the sentinel node was negative for metastasis (less than 250 copies/μl). Following the intraoperative identification of the lymphadenopathies, we performed axillary lymph node dissection without observing any metastatic involvement in the rest of the lymph nodes studied.
Figure 2.
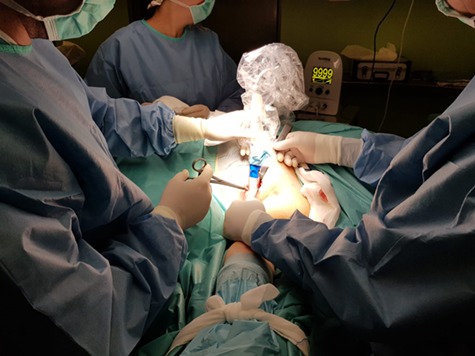
Marked lymph node opened showing magnetic seed placement.
DISCUSSION
Lymph node involvement in breast cancer is an important prognostic factor used to guide local and systemic therapies. Neoadjuvant chemotherapy can achieve an axillary pathological complete response in 35–49% of patients with previously positive axillary lymph nodes [1–3].
There is now evidence that axillary lymph node dissection can be omitted in patients with a complete response [4, 5].
However, early prospective clinical trials found that sentinel node biopsy, which is currently considered the standard for primary surgery in clinically negative axillary lymph nodes, has a high false-negative rate in the context of axillary clinical complete response following neoadjuvant treatment [6].
The SENTINA study revealed a false-negative rate of 14.2%, which fell to 9.6% if more than one node was biopsied [7]. Clinical trial ACOSOGZ1071 revealed a false-negative rate of 12.6%. This rate decreased to 10.8% if a dual tracer was used and to 8.7% if the lymph nodes were studied using immunohistochemistry. The same study showed that when the affected lymph nodes were marked with a clip prior to the neoadjuvant treatment, then post-treatment removal of these clipped nodes reduced the false-negative rate to 6.8% [1].
Donker et al. described the MARI (marking the axillary lymph node with radioactive iodine seeds) technique (marking the axillary lymph node with radioactive iodine seeds), in which the affected lymph node was marked before starting chemotherapy and subsequently identified by locating the seed with a gamma detection probe. This method correctly identified the marked lymph node in 97% of cases, with a false-negative rate of 7% [8].
Caudle et al. described the targeted axillary dissection technique, in which biopsied suspected lymphadenopathies were marked with a clip before starting the neoadjuvant therapy. After treatment, in the event of a clinical complete response, a second technique was used to facilitate intraoperative localisation and biopsy of the marked node. The sentinel lymph node was subsequently identified and the marked node removed. False-negative rates of 10.1% were reported for the isolated identification of just the sentinel lymph node alone, 4.2% in the case of just the marked node, and 1.4% for the identification of both the marked and sentinel lymph nodes. There is also evidence that in up to 23% of the cases the clipped node was not a sentinel node [9].
The reduction of false-negative rates using the targeted axillary dissection technique, and the fact that the clipped node did not correspond to the sentinel lymph node in up to 23% of cases, suggests that the presence of metastasis in the axillary nodes and the changes caused by the response to chemotherapy can alter axillary lymphatic drainage pathways, which could explain the high rate of false negatives.
The integration of targeted axillary dissection into clinical practice presents a series of difficulties with respect to the method chosen to guide the surgical removal of the marked node: the use of an iodine-125 seed entails the handling of radioactive material, which could lead to regulatory problems in some countries, and the methods used after neoadjuvant treatment have the disadvantage of routinely requiring a second procedure in which a normalised lymph node has to be marked following a radiological complete response.
The magnetic seed is a new technology that uses a 1 × 5 mm surgical stainless steel marker. It can be detected using a magnetometer (Sentimag, Endomag, Cambridge, UK), which provides information on the direction to the marker and how far away it is. This means the marked node can be located in a similar fashion as the way in which a radioactive iodine seed would be detected, but without the regulatory problems associated with the use of radioactive materials.
The magnetic seed has been approved by the FDA since 2016 and has had CE marking since 2017 for the localisation of breast lesions. In 2018, it was approved for marking soft tissues. The product’s technical characteristics ensure that an MRI can be performed without the risk of the seed moving. However, the seed generates an MRI artefact that could affect the visualisation of the tumour within 3 cm of the seed. Therefore, the radiological response in axillary lymph nodes following neoadjuvant treatment should be assessed with a different imaging technique; in our case, we used axillary ultrasound.
In our opinion, this MRI artefact can complicate assessment of treatment response in tumours located within 3 cm of the axillary cavity. As such, we do not recommend implanting the magnetic seed prior to neoadjuvant treatment on any tumours in this area.
We believe marking positive lymph nodes before commencing neoadjuvant treatment with a magnetic seed could provide a simple and effective means of intraoperative localisation of the marked node. Future studies will provide more information on the usefulness of magnetic seeds in this clinical context.
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interests.
REFERENCES
- 1. Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (alliance). Ann Surg 2016;263:802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015;33:258–64. [DOI] [PubMed] [Google Scholar]
- 3. Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol 2016;23:3467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nguyen TT, Hoskin TL, Day CN, Degnim AC, Jakub JW, Hieken TJ, et al. Decreasing use of axillary dissection in node-positive breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 2018;25:2596–602. [DOI] [PubMed] [Google Scholar]
- 5. Galimberti V, Ribeiro Fontana SK, Maisonneuve P, Steccanella F, Vento AR, et al. Sentinel node biopsy after neoadjuvant treatment in breast cancer: Five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur J Surg Oncol 2016;42:361–8. [DOI] [PubMed] [Google Scholar]
- 6. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Alliance for clinical trials in oncology. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (alliance) clinical trial. JAMA 2013;310:1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14:609–18. [DOI] [PubMed] [Google Scholar]
- 8. Donker M, Straver ME, Wesseling J, Loo CE, Schot M, Drukker CA, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 2015;261:378–82. [DOI] [PubMed] [Google Scholar]
- 9. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 2016;34:1072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


