Abstract
In the present review, we have been able to describe the different families of dyes and pigments used in textile finishing processes (Yarns, fabrics, nonwovens, knits and rugs) such as dyeing and printing. These dyes are reactive, direct, dispersed, indigo, sulphur and vats. Such that their presence in the liquid effluents resulting from the textile washing constitutes a serious risk, in the absence of their purification, for the quality of receiving aquatic environments. Indeed, the presence of these dyes and pigments can cause a significant alteration in the ecological conditions of the aquatic fauna and flora, because of the lack of their biodegradability. This has a negative impact on the equilibrium of the aquatic environment by causing serious dangers, namely the obvious dangers (Eutrophication, under-oxygenation, color, turbidity and odor), the long-term dangers (Persistence, bioaccumulation of carcinogenic aromatic products and formation of by-products of chlorination), mutagenicity and carcinogenicity.
Keywords: Organic chemistry, Environmental science, Textile finishing processes, Liquid effluents, Aquatic environment, Serious dangers
Organic chemistry; Environmental science; Textile finishing processes; Liquid effluents; Aquatic environment; Serious dangers
1. Introduction
The textile finishing industries are chemical industries in which dyes and pigments are used in very large quantities with such a large volume of water [1, 2, 3, 4]. The dyes and pigments of textile finishing used in the dyeing and printing of natural, synthetic, man-made and mixed textile materials such as wool, silk, nylon, polyester, acrylic, polyacetate and polyurethane are numerous and can reach 100,000 types on the market of which world annual production is about 700, 000 tonnes [5, 6, 7]. The washing of tinted or printed textile articles/fabrics produces a large volume of liquid effluents laden with these dyes and pigments with a variable proportion (10–60 %) cause a waste of 280,000 tons of dyes per year, and chemical products and auxiliaries such as phosphates, nitrates who are direct effects not inconsiderable on aquatic fauna, flora and human health [8, 9, 10, 11, 12, 13]. Because, these textile effluents are characterized by high values in terms of several physicochemical and biological parameters, indicating the degree of pollution, including coloring, temperature, salinity, pH, biological oxygen demand (BOD), chemical oxygen demand (COD), total dissolved solids (TDS), total nitrogen (TN), total phosphorus (TP) and non-biodegradable organic compounds, on the other hand, these effluents also contain heavy metals, metalliferous and phthalocyanine dyes, such as chromium (Cr), arsenic (Ar), copper (Cu) and zinc (Zn) [14, 15, 16]. The objective of this review is to describe the different families of textile dyes (Chemical and dye classification) and pigments, and their impact on the aquatic environment.
2. Main text
2.1. Textile finishing dyes
The textile finishing dyes are organic compounds capable of absorbing the light radiation in the visible range of the spectrum and of reflecting or diffusing complementary radiation on the one hand, and dyeing a substance in a sustainable manner on the other hand [17]. They consist of chromophoric groups, auxochromes and conjugated aromatic structures. These groups have specific independent properties, i.e., the color and the ability to be fixed on such a textile support, during dyeing and/or printing [18]. Chromophoric groups are unsaturated groups consisting of atoms or groups of atoms, in which the arrangement of successive single and double bonds resonates with the unstable mesomeric form thus allowing the absorption of light rays [17]. Table 1 gathers the mainchromophoric and auxochromic groups classified by the increasing intensity [17, 18, 19].
Table 1.
Chromophoric and auxochromic groups of textile dyes.
| Groups chromophoric | Groups auxochromic | ||
|---|---|---|---|
| Azo | (-N=N-) | amino | (-NH2) |
| Nitroso | (-NO or –N-OH) | methylamino | (-NHCH3) |
| Carbonyl | (>C=O) | dimethylamino | (-N(CH3)2) |
| Ethylenic | (>C=C<) | hydroxyl | (-OH) |
| Nitro | (-NO2 or = NO–OH) | alkoxyl | (-OR) |
| Sulphide | (>C=S) | electron donor groups | (-NO2) |
| Ketone-imine | (>C=NH) | (-CO2H) | |
| Polymethine | (=HC-HC = CH–CH = ) | (-SO3H) | |
| Anthraquinone | 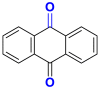 |
(-OCH3) Cl, Br, I, At | |
| Phtalocyanine | 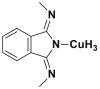 |
||
| Triphenylmethane | 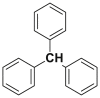 |
||
The classification of the dyes of textile finishing is based either on their chemical structure or on their method of application to different substrates such as textile fibers, paper, leathers and plastics [17, 19, 20]. Thus, there are two types of classifications such as chemical and dye classification's, in the following.
2.2. Chemical classification
It is based on the chemical structure of the dyes and more particularly on the nature of their chromophore grouping, such that dyeing plants will be able to predict the chemical reactions between the dye and the reducing agents, the oxidants and the other compounds [19].
2.2.1. Azo dyes
They constitute the largest family, including 70% of the world's annual production of synthetic dyes [17, 18, 19]. They are characterized by the presence of one or more azo groups (-N=N-) linked to the –OH or –NH2 type auxochrome groups, namely the monoazo, the diazo and the triazo dyes whose grouped examples are in Table 2 [21,22].
Table 2.
Chemical structures and maximum wave lengths (λmax) of some azo dyes.
| Dyes | Chemical structures | λmax (nm) | Groupings |
|---|---|---|---|
| Yellow reactive 4 (YR4) | 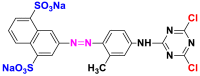 |
385 | monoazo |
| Black reactive 5 (BR5) | 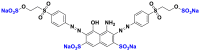 |
590 | disazo |
| Direct blue 71 (DB71) | 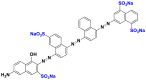 |
575 | triazo |
2.2.2. Anthraquinone dyes
They are the most important class after azo dyes. Their general formulas derived from anthracene show that the chromophore group is a quinone nucleus to which hydroxyl or amino groups can be attached [23]. This group gives the dye molecule good light resistance. Table 3 lists some examples of anthraquinone dyes [24, 25].
Table 3.
Chemical structures and λmax of some anthraquinone dyes.
| Dyes | Reactive blue 19 (RB19) | Acid blue 62 (AB62) |
|---|---|---|
| Chemical structures | 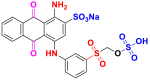 |
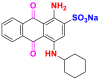 |
| λmax (nm) | 592 | 635 |
2.2.3. Indigo dyes
Indigo dyes are derivatives of indigo. Thus, the selenium, sulfur and oxygen homologues of indigo blue cause significant hypochromic effects with colors ranging from orange to turquoise, some examples of which are summarized in Table 4 [26,27].
Table 4.
Chemical structures and λmax of some indigo dyes.
| Dyes | Indigo blue | Blue acid 74 (BA74) |
|---|---|---|
| Chemical structures | 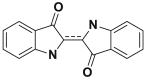 |
 |
| λmax (nm) | 665 | 612 |
2.2.4. Xanthene dyes
They are endowed with an intense fluorescence of which the best known compound is fluorescein. These dyes are little used in dyeing but they are used in the marking technique as markers in maritime accidents or tracers in underground rivers [28]. Table 5 groups together chemical structures of some xanthene dyes [29, 30].
Table 5.
Chemical structure and λmax of some xanthene dyes.
| Dyes | Chemical structure | λmax (nm) |
|---|---|---|
| Fluorescein: R1 = R2 = H | 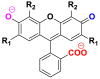 |
480–500 |
| Dibromofluorescein: R1 = H, R2 = Br | 490–510 | |
| Eosin Y: R1 = R2 = Br | 510–530 | |
| Erythrosine B: R1 = R2 = I | 520–540 |
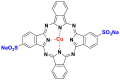
2.2.5. Phthalocyanine dyes
The phthalocyanine dyes are a family of dyes that are obtained by the reaction of dicyanobenzene in the presence of a metal type of Cu, Ni, Co, Pt. The phthalocyanine nucleus gives the molecule a good fastness to light. The most widely used dye in this family is copper phthalocyanine, because of its high chemical stability [31]. Table 6 summarizes the chemical structures of some phthalocyanine dyes molecules [32, 33, 34].
Table 6.
Chemical structures and λmax of some phthalocyanine dyes.
| Dyes | Chemical structures | λmax (nm) |
|---|---|---|
| Pigment blue 15/3 | 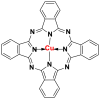 |
785–793 |
| Nickel (II) tetrasulfonic acid | 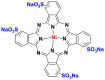 |
620–626 |
| Iron (III) phthalocyanine chloride | 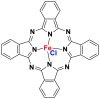 |
650–658 |
2.2.6. Nitrated and nitrosated dyes
Nitrated and nitrosated are class of dyes very limited in number and relatively old. They are still in use because of their very moderate price linked to the simplicity of their molecular structure characterized by the presence of a nitro group (-NO2) at ortho position relative to an electron-donor group (hydroxyl or amino groups) [17]. Table 7 groups the chemical structures of some nitro and nitrosated coloring molecules [35, 36].
Table 7.
Chemical structures and λmax of some nitro and nitrosated dyes.
| Dyes | Picric acid (2, 4, 6-trinitrophenol) | 2-nitrophenol | 2-amino-4-nitrophenol |
|---|---|---|---|
| Chemical structures | 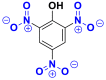 |
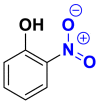 |
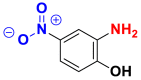 |
2.2.7. Diphenylmethane and triphenylmethane dyes
Diphenylmethane and triphenylmethane dyes represent the least important category, diphenyl methane is derivatives of auramine and triphenylmethanes are the origin of the oldest synthetic dyes such as fuchsin and malachite green [37]. The sulfonation of triphenylmethane gives acid dyes. While OH-auxochromes, neighboring carboxyl groups, form metallizable dyes. Table 8 below lists some chemical structures of diphenylmethane and triphenylmethane dyes [38, 39].
Table 8.
Chemical structures and λmax of diphenylmethane and triphenylmethane dyes.
| Dyes | p-dimethylamino-phenylethynyl | Light green |
|---|---|---|
| Chemical structures | 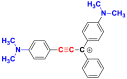 |
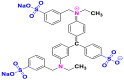 |
| λmax (nm) | 727 | 660 |
| Type of dyes | diphenylmethane | triphenylmethane |
2.2.8. Polymethinic dyes
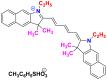
Also, called cyanines, they are constituted by a polymethine chain carrying at the ends of various heterocyclic whose examples are grouped in Table 9 [40,41].
Table 9.
Chemical structures and λmax of some polymethine dyes.
| Dyes | Basic yellow 28 | Polymethine dye 2630 | |
|---|---|---|---|
| Chemical structures | 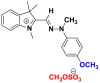 |
 |
|
| λmax (nm) | 439 | 700 | |
2.3. Dyes classification
Dyes classification is defined by the grouping auxochromes which is of interest for the dyer who prefers a classification by fields of application. This classification is informed about the dye solubility in the dye bath, its affinity for the various fibers and the nature of the fixation [42, 43]. In this classification, there are two families of dyes, soluble and water-insoluble dyes [44, 45].
2.3.1. Water-soluble dyes
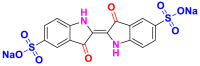
2.3.1.1. Acid or anionic dyes
They are also called anionic dyes used for the dyeing of fibers which contain an amino group (NH2) such as wool, polyamide, silk and mod acrylic which should be fixed on the NH4+ cations of these fibers in acid medium. They consist of a chromophore group and one or more sulphonates groups allowing their solubilization in water [46]. Most of these dyes are azo, anthraquinone or triarylmethanes [26, 47]. Table 10 lists the chemical structures and maximum wave lengths λmax of some examples of acid dyes [48, 49, 50].
Table 10.
Chemical structures and λmax of some acid dyes.
| Chemical structures | Dyes | λmax (nm) | Groupings |
|---|---|---|---|
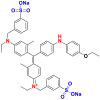 |
Acid Blue 74 | 612 | indigoid |
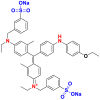 |
Acid Blue 90 | 580 | triphenylmethane |
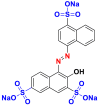 |
Red acid 27 (AR27) | 521 | azo |
2.3.1.2. Basic dyes or cationic dyes
They are also called cationic dyes which are salts of organic bases capable of directly dyeing the fibers bearing anionic sites and the cost only after treatment with metal salts. They are composed of diarylmethane, triarylmethane, anthraquinone and/or azo structures [51, 52]. Table 11 groups together some chemical structures and maximum wave lengths λmax of basic dyes [53, 54].
Table 11.
Chemical structures and λmax of some basic dyes.
| Dyes | Basic blue 9 (BB9) | Basic yellow 37 (BY37) | Blue nile (BN) |
|---|---|---|---|
| Chemical structures | 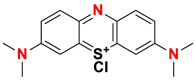 |
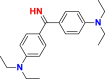 |
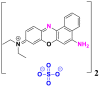 |
| λmax (nm) | 665 | 440 | 638 |
| Groupings | phthalocyanine | ketone imine | sulphonates |
2.3.1.3. Metalliferous dyes
In order to facilitate the dyer work by avoiding the etching operation, the idea has been to incorporate the metal into the dye itself by forming the metalliferous complex beforehand instead of precipitating it into the fiber in a subsequent manner [55]. Thus, metalliferous dyes are molecules derived from acid dyes with a metal atom (Cr, Cu, Ni and Co) incorporated into their chemical structures [55]. These dyes can be categorized in two complex forms: A metalliferous complex 1/1, in which the metal atom can be associated with a single molecule of the dye and a metalliferous complex 1/2, in which the metal atom can be associated with two molecules of dyes. Metalliferous dyes are often azo and phthalocyanine structures of which some examples are given in previous Table 6 [32, 33, 34].
2.3.1.4. Reactive dyes
Reactive dyes contain chromophore groups mainly derived from azo, anthraquinone and phthalocyanine families [43]. Their name is linked to the presence of a reactive chemical function, of the triazine or vinylsulfone type, ensuring the formation of a strong covalent bond with the fibers [17]. They are more soluble in water and in the dyeing of cotton and possibly in that of wool and polyamides. Table 12 lists some examples of reactive dyes [56, 57].
Table 12.
Chemical structures and λmax of some reactive dyes.
| Chemical structures | Dyes | λmax (nm) | Groupings |
|---|---|---|---|
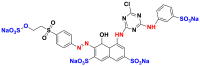 |
Reactive red 198 (RR 198) | 515 | carbonyl |
 |
Red cibacrom 3 (RC3) | 540 | azo |
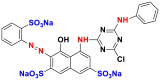
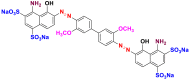
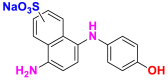
2.3.1.5. Direct or substantive dyes
Direct or substantive dyes are large molecules capable of forming positive or negative charges electrostatically attracted by fiber charges. They are distinguished by their affinity for cellulosic fibers without mordant application that is related to the plane structure of their molecule [17]. They are used for dyeing cellulosic fibers. These dyes are applied directly to the bath containing the salt (sodium chloride or sodium sulfate) and auxiliary products that facilitate the wettability of the fiber and the dispersion effect. The mixtures of nonionic and anionic surfactants are used for this purpose. The main advantages of these dyes are the wide variety of colors, their ease of application and their low price. On the other hand, their main disadvantage is their low wet strength. Table 13 below gives some examples of direct dyes [58, 59].
Table 13.
Chemical structures and λmax of some direct dyes.
| Chemical structures | Dyes | λmax (nm) | Groupings |
|---|---|---|---|
 |
Direct blue 1 (BD-1) | 594 | azo |
 |
Direct blue 86 (BD-86) | 594 | phthalocyanine |
2.3.2. Insoluble dyes in water
2.3.2.1. Vat dyes
The vat dyes are water-insoluble dyes when they are fixed on the fibers, but they become soluble and substantive by the reduction in very alkaline medium (in vat) in leuco-derivatives soluble in water [44, 60, 61]. They are then re-solubilized by oxidation in air or with the aid of an oxidizing agent and are generated within the fiber, on the other hand [62]. They are known for their good resistance to degradation agents (washing and solar rays) and their affinity for certain fibers such as cotton, linen, wool, silk, rayon and other cellulosic fibers such as indigo for dyeing jeans (or denim). Among the vat dyes used, there is indigo (vat blue 1) which is already mentioned in Table 4 [27]. Also, there is the blue indanthrene RS (vat blue 4) and vat green 1 which are collated in Table 14 [61, 62, 63, 64].
Table 14.
Chemical structures and λmax of some vat dyes.
| Dyes | Blue indanthrene RS | Vat green 1 |
|---|---|---|
| Chemical structures | 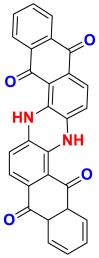 |
 |
| λmax (nm) | 631 | 630 |
| Groupings | anthraquinonique | anthraquinonique |
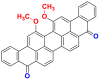
2.3.2.2. Sulfur dyes
Sulfur dyes are quite similar to vat dyes by the method of use, but they represent sulfur-containing molecules and have different chemical structures with high molecular weight [65]. They are insoluble in water but applied as a soluble derivative after reduction in an alkaline medium by sodium sulphide. They are then reoxidized to their insoluble state in the fiber. Sulfur dyes are typically applied to cotton to produce cost-effective dark shades with a medium to good wash and light fastness [17]. Table 15 lists some examples of sulfur dyes [26, 27, 65].
Table 15.
Chemical structures and λmax of some sulfur dyes.
| Dyes | Sulfur black (SB) | Sulfur blue 15 (SB15) |
|---|---|---|
| Chemical structures | 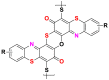 |
 |
| λmax (nm) | 597 | 593 |
2.3.2.3. Disperse or dispersible dyes
They are also called plastosolubles, examples of which are shown in Table 16, which are very insoluble in water and applied as a fine powder dispersed in the dye bath [57, 66]. They are stable during at high temperature dyeing, to diffuse in the synthetic fibers and then to fix it. Disperse dyes are widely used in the dyeing of most manufactured fibers, especially polyester and polyamide in the presence of a dispersing agent that is always added to the solution [17].
Table 16.
Chemical structures and λmax of some disperses dyes.
| Dyes | Red disperse60 (RD60) | Blue disperse 7 (BD7) | |
|---|---|---|---|
| Chemical structures | 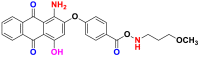 |
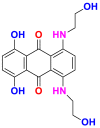 |
|
| λmax (nm) | 536 | 570 | |
| Groupings | anthraquinonique | anthraquinonique | |
2.3.2.4. Pigments
The pigments are colored compounds that are insoluble in water, in the solvent (basic and acidic) and do not contain any group that can react with the textile fiber by means of a binder. They are commonly used in printing processes (pigment printing) [67, 68]. Organic pigments are largely benzoic derivatives and inorganic pigments (minerals) are derivatives of metals such as titanium, zinc, barium, lead, iron, molybdenum, antimony, zirconium, calcium, aluminum, magnesium, cadmium and chromium [69]. They must be very finely divided so that they can be kept in suspension thanks to dispersants, such as the film formed by the heat treatment (drying), is flexible and transparent and leaves the pigments all their vivacity and their solidarity to the light with good resistance to mechanical tests.
2.3.2.5. Fixing different dye classes
During the various stages of dyeing and printing process, a greater or lesser quantity of dyes and pigments is lost, despite the use of fixing agents, due to affinity for the surfaces [70, 71]. Indeed, the dyes are fixed on the textile fibers by Van der Waals type interaction, hydrogen and hydrophobic interaction, the fixing of the dye depends on its nature, its chemical composition and the type of fiber on which it is applied [17]. When the dye and the fiber have opposite charges, a very strong bond between the dye and the fiber within the complex is enhanced by additional electrostatic interaction [18, 43]. Table 17 presents a relative estimate of the rates of fixation and rejection of different dyes tuffs according to the nature of the textile fibers to be dyed [17, 72]. Besides, it appears that a fairly large percentage of the dyes are discharged with the effluents which are, in most of the time, discharged directly into the streams without any prior treatment. This has a negative influence on the aquatic environment.
Table 17.
Rate of fixation and rejection of dyes in relation to the textile fibers used.
| Dyes | Textile fibers | Fixation rate (%) | Discharge rate (%) |
|---|---|---|---|
| Acid | wool and nylon | 80–93 | 7–20 |
| Azo | cellulose | 90–95 | 5–10 |
| Basic | acrylic | 97–98 | 2–3 |
| Vat | cellulose | 80–95 | 5–20 |
| Direct | cellulose | 70–95 | 5–30 |
| Dispersed | synthetic | 80–92 | 2–20 |
| Reagents | cellulose | 50–80 | 20–50 |
| Sulfur | cellulose | 60–70 | 30–40 |
According to this table, it turns out that larger amounts of dyes are lost due to the lack of affinity for the textile surfaces to be dyed or colored. This requires a more appropriate regeneration technique (or treatment).
2.4. Impact of dyes textile finishing on aquatic environment
The presence of textile dyes in wastewater discharged by textile industries in aquatic environments such as wadis, rivers, seas, oceans... and the lack of their biodegradability, under normal ecological conditions can destroy the vital conditions of these different environments, while preventing the penetration of light to the depths of aquatic environments [73, 74, 75, 76]. As a result, there are serious ecological consequences such as changing the nature of aquatic environments and reducing photosynthesis compared to aquatic flora [8, 77, 78, 79].
In addition, these waste waters can produce several dangerous problems, namely aesthetic and health problems such as changes in the quality (color and odor) of water and make it toxic, as they can cause allergies, dermatitis, skin irritations, cancers and mutations in humans [77, 80, 81, 82, 83]. Furthermore, between 60 and 70% of azo dyes are toxic, carcinogenic and are refractory to conventional treatment processes because of their resistance to conventional physicochemical destruction and the absence of their biodegradability [84]. This interrupted the phenomenon of photosynthesis in marine plants by triggering the phenomenon of eutrophication, under the effect of the release of mineral elements such as nitrates, nitrites and phosphates in an uncontrolled manner, and produced the long-term hazards of persistence, bioaccumulation, by products of chlorination, mutagenicity and carcinogenicity [84, 85, 86].
In addition, the existence of dyes and textile pigments in wastewaters makes them very characterized by a high degree of coloration, a very fluctuating pH and high levels in terms of biochemical oxygen demand (BOD), chemical oxygen demand (COD), total organic carbon (TOC) and suspended solids (TSS) [3, 87, 88, 89, 90]. The uncontrolled increase in these pollution indicators can cause an imbalance in biological treatment plants because of the high toxicity of the metal elements contained in these dyes (metalliferous and pigments) [91]. As well as, the presence of mineral elements with very high levels in water and lack of their consumption by aquatic plants (algae, bryophytes, pteridophytes, spermatophytes and vascular plants) accelerates their uncontrolled proliferation in an uncontrolled manner and consequently leads to the oxygen depletion by inhibition of photosynthesis in the strata, deeper watercourses and stagnant waters [17, 77, 78, 79, 80]. This can affect the life of aquatic fauna and flora, modify the quality of drinking water production [81].
In case of disposal of organic matter including dyes and pigments to the receiving media via textile finishing effluents, the natural processes of regulation can no longer compensate the bacterial oxygen consumption [47, 92]. This may lead to under-oxygenation of stagnant aquatic environments, such that the degradation of 7–8 mg of organic matter by microorganisms is sufficient to consume the amount of oxygen in one liter of water [17, 93].
The agglomeration of organic matter (dyes, pigments, etc.) in watercourses induces the appearance of bad taste, bacterial proliferation, pestilential odors and abnormal colorations [68, 71, 85, 86]. In such a way that coloration could be perceived with the naked eye from a mass concentration of dyes equal to 5 μg/L [17, 94]. Apart from the unsightly appearance, the coloring agents have the ability to interfere with the transmission of light in the water, thus blocking the photosynthesis of aquatic plants.
Synthetic organic dyes are compounds that cannot be purified (persistent) by the processes of natural biological degradation [95]. This persistence is closely related to their chemical reactivity, so that the unsaturated compounds are less persistent than the saturated compounds. The persistence of aromatic compounds increases with the number of substituents and the halogen substituents increase the persistence of the dyes having the alkyl groups [17].
Given the widespread use of dyes, these contaminants can be found in the environment and accumulate biologically throughout the food chain in organisms (fish, algae...) of freshwater [70].
Species at the high end of the food web, including humans, are exposed to toxic concentrations up to a thousand times higher than initial concentrations in water [17, 64]. Such as the analysis of the data recorded by the Ecological and Toxicological Association of Dyes and synthetic organic pigments (ETAD) concerning their toxicity towards fish, showed that 98% of these dyes have a limited concentration CL50 above 1 mg/L, and 59% have a CL50 greater than 100 mg/L [96].
During the elimination of pathogenic micro-organisms by chlorine, the latter reacts with the organic matter to form trihalomethanes (THM) chlorination by-products [17, 41]. The prolonged exposure to these substances causes’ health problems in humans, including carcinogenic effects (bladder, colon, colorectal etc.), health effects, including the immune and reproductive system with genotoxic and carcinogenic properties [70, 97, 98].
In terms of toxicity, the most toxic synthetic organic dyes are azo dyes and more particularly diazo and cationic dyes [81, 99, 100]. These types of dyes have carcinogenic effects on humans and animals primarily, as the electron-withdrawing character of azo groups generates electronic deficiencies. This makes azo compounds uncomfortable with oxidative catabolism under aerobic environmental conditions [101, 102, 103].
3. Conclusion
In the light of this review, we can conclude that synthetic dyes and pigments applied to textile finishing processes are numerous and diverse in terms of chromophore groups (Azo groups, anthraquinone groups, indigoids, xanthenes, phthalocyanines, nitroseds, triphenylmethanes and polymethines) and application techniques (Water-soluble dyes (Acidic, basic, metalliferous, reactive and direct dyes) and water-insoluble dyes (Vat dyes, sulfur dyes and dispersible)). The majority of these dyes are azo (60–70 %) characterized by high chemical stability with respect to chemicals and products due to the combination of azo groups with aromatic rings. The presence of these dyes and its residues in the effluents discharged into aquatic environments, as well as their relative biodegradability, have a negative impact on the environment and more particularly on aquatic ecosystems because of their toxic and carcinogenic effects as they occur. Accumulate throughout the food chain of aquatic fauna, on the one hand and dysfunctional physiological processes of aquatic flora (Plants, diatoms and algae) by disrupting their photosynthesis mechanisms by the lack of circulation of the oxygen and absorption of light in aquatic environments, on the other hand. Besides, these textile dyes must eliminate according to processes, which will be the interest of a future review, of biological, chemical, physical and physicochemical treatment before their evacuation in aquatic environments.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to express their sincere thanks to the reading committee for its meticulous reading of our work as well as the questions and remarks made. Our recognition is also addressed to Dr. Rachid Hsissou and Mr. Mohamed El Hamzaoui for the linguistic assistance and encouragement, to Mr. Jamal Abdennasser Berradi for his help and supports and to researchers of LAPGP Laboratory, Department of Chemistry, Faculty of Sciences-Ibn Tofail University-Kenitra, for their support to conduct this review.
Contributor Information
Mohamed Berradi, Email: mberradi24@gmail.com.
Rachid Hsissou, Email: r.hsissou@gmail.com.
References
- 1.Akbari A., Desclaux S., Rouch J.C., Remigy J.C. Application of nanofiltration hollow fiber membranes developed by photografting, to treatment of anionic dye solutions. J. Membr. Sci. 2007;297:243–252. [Google Scholar]
- 2.Verma A.K., Dash R.R., Bhunia P. A review on chemical coagulation/flocculation technologies for removal of color from textile wastewaters. J. Environ. Manag. 2012;93:154–168. doi: 10.1016/j.jenvman.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Poon C.S., Huang Q., Fung P.C. Degradation kinetics of cuprophenyl yellow RL by UV/H2O2/ultrasonication (US) process in aqueous solution. Chemosphere. 1999;38(5):1005–1014. [Google Scholar]
- 4.Joshi M., Bansal R., Purwar R. Colour removal from textile effluents: a review. Indian J. Fibre Text. Res. 2004;29:239–259. [Google Scholar]
- 5.Sahoo P.R., Prakash K., Kuma S. Light controlled receptors for heavy metal ions. Coord. Chem. Rev. 2018;357:18–49. [Google Scholar]
- 6.Xu X.R., Li H.B., Wang W.H., Gu J.D. Decolorization of dyes and textile wastewater by potassium permanganate. Chemosphere. 2005;59:893–898. doi: 10.1016/j.chemosphere.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Rajamohan N., Rajasimman M. Kinetic modeling of dye effluent biodegradation by pseudomonas stutzeri. Eng. Technol. Appl. Sci. Res. 2013;3(2):387–390. [Google Scholar]
- 8.Royer B., Cardoso N.F., Lima E.C., Ruiz V.S.O., Macedo T.R., Airoldi C. Organo functionalized kenyaite for dye removal from aqueous solution. J. Colloid Interface Sci. 2009;336:398–405. doi: 10.1016/j.jcis.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Royer B., Cardoso N.F., Lima E.C., Macedo T.R., Airoldi C. A useful organofunctionalized layered silicate for textile dye removal. J. Hazard Mater. 2010;181:366–374. doi: 10.1016/j.jhazmat.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Fazal T., Mushtaq A., Rehman F., Khan A.U., Rashid N., Farooq Ur W., Rehman M.S., Xu J. Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renew. Sustain. Energy Rev. 2018;82(3):3107–3126. [Google Scholar]
- 11.Rahman Bhuiyan M.A., Mizanur Rahman M., Shaid A., Bashar M.M., Khan M.A. Scope of reusing and recycling the textile wastewater after treatment with gamma radiation. J. Clean. Prod. 2016;112:3063–3071. [Google Scholar]
- 12.Sahoo P., Prakash K., Kumar S. Synthesis of an oxadiazole through an indole mediated single step procedure for selective optical recognition of Cu2+ ions. Sens. Actuat. Chem. 2017;242:299–304. [Google Scholar]
- 13.Berradi M., Hsissou R., Khudhair M., Lakdioui T., Bekhta A., El Gouri M., Rafik M., El Bachiri A., El Harfi A. Ultrafiltration of wastewater-models loaded with indigo blue by membranes composed of organic polymers at different percentages: comparative study. Moroc. J. Chem. 2019;7(2):230–235. [Google Scholar]
- 14.Oh J.M., Biswick T.T., Choy J.H. Layered nanomaterials for green materials. J. Math. Chem. 2009;19:2553–2563. [Google Scholar]
- 15.Es-sahbany H., Berradi M., Nkhili S., Hsissou R., Allaoui M., Loutfi M., Bassir D., Belfaquir M., El Youbi M.S. Removal of heavy metals (nickel) contained in wastewater models by the adsorption technique on natural clay. Mat. Tod. Proc. 2019;13:866–875. [Google Scholar]
- 16.Mantzavinos D., Psillakis E. Enhancement of biodegradability of industrial wastewaters by chemical oxidation pre-treatment. J. Chem. Technol. Biotechnol. 2004;79:431–454. [Google Scholar]
- 17.Lim S.L., Chu W.L., Phang S.M. Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour. Technol. 2010;1:7314–7322. doi: 10.1016/j.biortech.2010.04.092. [DOI] [PubMed] [Google Scholar]
- 18.Rajasimman M., Babu S.V., Rajamohan N. Biodegradation of textile dyeing industry wastewater using modified anaerobic sequential batch reactor–Start-up, parameter optimization and performance analysis. J. Taiw. Inst. Chem. Eng. 2017;72:171–181. [Google Scholar]
- 19.Ben Mansour H., Boughzalaa O., Dridi D., Barillier D., Chekir-Ghedira L., Mosrati R. Textiles dyes as a source of wastewater contamination: screening of the toxicity and treatment methods. J. Water Sci. 2011;24(3):209–238. [Google Scholar]
- 20.Sahoo P.R., Prakash K., Kumar A., Kumar S. Efficient reversible optical sensing of water achieved through the conversion of H-aggregates of a merocyanine salt to J-aggregates. Chem. Sel. 2017;2(21):5924–5932. [Google Scholar]
- 21.Devi Saini R. Textile organic dyes: polluting effects and elimination methods from textile waste water. Int. J. Chem. Eng. Res. 2017;9(1):121–136. [Google Scholar]
- 22.Holkar C.R., Jadhav A.J., Pinjari D.V., Mahamuni N.M., Pandit A.B. A critical review on textile wastewater treatments: possible approaches. J. Environ. Manag. 2016;182:351–366. doi: 10.1016/j.jenvman.2016.07.090. [DOI] [PubMed] [Google Scholar]
- 23.Franciscon E., Zille A., Fantinatti-Garboggini F., Silva I.S., Cavaco-Paulo A., Durrant L.R. Microaerophilic–aerobic sequential decolourization/biodegradation of textile azo dyes by a facultative Klebsiella sp. Strain VN-31, Process. Biochemistry. 2009;44:446–452. [Google Scholar]
- 24.El Boujaady H., Mourabet M., El Rhilassi A., Bennani-Ziatni M., El Hamri R., Taitai A. Adsorption of a textile dye on synthesized calcium deficient hydroxyapatite (CDHAp): kinetic and thermodynamic studies. J. Mater. Environ. Sci. 2016;7(11):4049–4063. [Google Scholar]
- 25.Robinson T., McMullan G., Marchant R., Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001;77:247–255. doi: 10.1016/s0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- 26.Alkan M., Celikcapa S., Demirbasx O.Z., Dogan M. Removal of reactive blue 221 and acid blue 62 anionic dyes from aqueous solutions by sepiolite. Dyes Pigments. 2005;65:251–259. [Google Scholar]
- 27.Bilal M., Rasheed T., Iqbal H.M.N., Chuanlong L., Hang W., Hongbo H., Wei W., Xuehong Z. Photocatalytic degradation, toxicological assessment and degradation pathway of C.I. Reactive blue 19 dye. Chem. Eng. Res. Des. 2018;129:384–390. [Google Scholar]
- 28.Berradi M., Essamri A., El Harfi A. Discoloration of water loaded with vat dyes by the membrane process of ultrafiltration. J. Mater. Environ. Sci. 2016;7(4):1098–1106. [Google Scholar]
- 29.Paz A., Carballo J., Pérez M.J., Domínguez J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere. 2017;181:168–177. doi: 10.1016/j.chemosphere.2017.04.046. [DOI] [PubMed] [Google Scholar]
- 30.Subodh, Mogha N.K., Chaudhary K., Kumar G., Masram D.T. Fur-imine-functionalized graphene oxide-immobilized copper oxide nanoparticle catalyst for the synthesis of xanthene derivatives. ACS Omega. 2018;3(11):16377–16385. doi: 10.1021/acsomega.8b01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamagami A., Kawano K., Futaki S., Kuramochi K., Tsubaki K. Syntheses and properties of second-generation V-shaped xanthene dyes with piperidino groups. Tetrahedron. 2017;73:7061–7066. [Google Scholar]
- 32.Li S., Zhang H., Lu R., Yu A. Interaction between triethanolamine and singlet or triplet excited state of xanthene dyes in aqueous solution. Spect. Act. Mol. Biomol. Spect. 2017;184:204–210. doi: 10.1016/j.saa.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Namgoong J.W., Kim S.H., Chung S.W., Kim Y.H., Kwak M.S., Kim J.P. Aryloxy and chloro-substituted zinc (II) phthalocyanine dyes: synthesis, characterization and application for reducing the thickness of color filters. Dyes Pigments. 2018;154:128–136. [Google Scholar]
- 34.Fu S., Xu C., Du C., Tian A., Zhang M. Encapsulation of C.I. Pigment blue 15:3 using a polymerizable dispersant via emulsion polymerization. Col. Surf. Physicochem. Eng. Asp. 2011;384:68–74. [Google Scholar]
- 35.Ahmad Z., Abdullah S.M., Sulaiman K. Temperature-sensitive chemical cell based on nickel (II) phthalocyanine-tetrasulfonic acid tetra sodium salt. Sens. Actuators. 2012;179(A):146–150. [Google Scholar]
- 36.Arıcı M., Arıcan D., Lutfi Ugur A., Erdogmus A., Koca A. Electrochemical and spectroelectrochemical characterization of newly synthesized manganese, cobalt, iron and copper phthalocyanines. Electrochim. Acta. 2013;87:554–566. [Google Scholar]
- 37.Wang X., Guan W., Pan J. Preparation of magnetic imprinted polymer particles via microwave heating initiated polymerization for selective enrichment of 2-amino-4-nitrophenol from aqueous solution. Chem. Eng. J. 2011;178:85–92. [Google Scholar]
- 38.Hebert R.M., Jack Ovitz A.M. Wildlife toxicity assessment for picric acid (2, 4, 6-trinitrophenol) Wild. Tox. Assess. Chemical. Milit. Concer. 2015:271–277. [Google Scholar]
- 39.Wei S.C., Shen F., Lien C.W., Unnikrishnan B., Wang Y.S., Chu H.W., Huang C.C., Hsu P.H., Chang H.T. Graphene oxide membrane as an efficient extraction and ionization substrate for spray-mass spectrometric analysis of malachite green and its metabolite in fish samples. Anal. Chem. Act. 2018;1003:42–48. doi: 10.1016/j.aca.2017.11.076. [DOI] [PubMed] [Google Scholar]
- 40.Akiyama S., Nakatsuji S., Nakashima K., Yamasaki S. Diphenylmethane and triphenylmethane dye ethynovinylogues with absorption bands in the near-infrared. Dyes Pigments. 1988;9:459–466. [Google Scholar]
- 41.Zhao B., Xiao W., Shang Y., Zhu H., Han R. Adsorption of light green anionic dye using cationic surfactant-modified peanut husk in batch mode. Arab. J. Chem. 2017;10:S3595–S3602. [Google Scholar]
- 42.Webster S., Fu J., Padilha L.A., Przhonska O.A., Hagan D.J., Van Stryland E.W., Bondar M.V., Slominsky Y.L., Kachkovski A.D. Comparison of nonlinear absorption in three similar dyes: polymethine, squaraine and tetra one. Chem. Phys. 2008;348:143–151. [Google Scholar]
- 43.Mijin D.Z., Avramov M.L., Antonije I., Onjia E., Grgur B.N. Decolorization of textile dye CI basic yellow 28 with electrochemically generated active chlorine. Chem. Eng. J. 2012;204–206:151–157. [Google Scholar]
- 44.Chakraborty J.N. Dye-Fibre interaction. Fund. Pract. Col. Text. 2010;2010:20–28. [Google Scholar]
- 45.Siddiqua U.H., Shaukat Z., Munawar I., Tanveer H. Relationship between structures and dyeing properties of reactive dyes for cotton dyeing. J. Mol. Liq. 2017;241:839–844. [Google Scholar]
- 46.Santos Pisoni D.D., Marluza P.D.A., Cesar L.P., Severo Rodembusch F., Campo L.F. Synthesis, photo physical study and BSA association of water-insoluble squaraine dyes. J. Photochem. Photob. Chem. 2013;252:77–83. [Google Scholar]
- 47.Wan T., Li D., Jiao Y., Ouyang X., Chang L., Wang X. Bifunctional MoS2 coated melamine-formaldehyde sponges for efficient oil-water separation and water-soluble dye removal. Appl. Mat. Tod. 2017;9:551–559. [Google Scholar]
- 48.Jarmany A., Kheribech A., Mountadar M. La décoloration des rejets liquides de textile par électrocoagulation. Phys. Chem. New. 2002;6:101–109. [Google Scholar]
- 49.Moussavi G., Mahmoudi M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard Mater. 2009;168:806–812. doi: 10.1016/j.jhazmat.2009.02.097. [DOI] [PubMed] [Google Scholar]
- 50.Daneshvar N., Rabbani M., Modirshahla N., Behnajady M.A. Kinetic modeling of photocatalytic degradation of acid red 27 in UV/TiO2 process. Photochemistry J. 2004;168(1-2):39–45. [Google Scholar]
- 51.Puttaswamy Vinod K.N., Ninge Gowda K.N. Oxidation of C.I. Acid red 27 by chloramine-T in perchloric acid medium: spectrophotometric, kinetic and mechanistic approaches. Dyes Pigments. 2008;78:131–138. [Google Scholar]
- 52.Umukoro E.H., Peleyeju M.G., Ngila J.C., Arotiba O.A. Photocatalytic degradation of acid blue 74 in water using Ag-Ag2O-ZnO nanostructures anchored on graphene oxide. Solid State Sci. 2016;51:66–73. [Google Scholar]
- 53.Aly R.O. Implementation of chitosan inductively modified by c-rays copolymerization with acrylamide in the decontamination of aqueous basic dye solution. Arab. J. Chem. 2017;10:S121–S126. [Google Scholar]
- 54.Bentahar S., Dbik A., El Khomri M., El Messaoudi N., Lacherai A. Removal of a cationic dye from aqueous solution by natural clay. J. Ground. Sus. Develop. 2018;6:255–262. [Google Scholar]
- 55.İyim T.B., Güçlü G. Removal of basic dyes from aqueous solutions using natural clay. Desalination. 2009;249:1377–1379. [Google Scholar]
- 56.Rani S.S., Mahajan R.K., Equilibrium R.K. Kinetics and thermodynamic parameters for adsorptive removal of dye basic blue 9 by ground nut shells and Eichhornia. Arab. J. Chem. 2016;9(2):1464–1477. [Google Scholar]
- 57.Chakraborty J.N. Dyeing with metal-complex dye. Fund. Pract. Col. Text. 2014:187–199. [Google Scholar]
- 58.Aghajani K., Tayebi H.A. Adaptive neuro-fuzzy inference system analysis on adsorption studies of reactive red 198 from aqueous solution by SBA-15/CTAB composite. Spectro. Act. Mol. Biomol. Spect. 2017;171:439–448. doi: 10.1016/j.saa.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 59.Berradi M., El Harfi A. Discoloration of charged models wastewater with reactive and dispersed dyes by the combined process of coagulation-ultrafiltration. J. Mater. Environ. Sci. 2017;8(5):1762–1769. [Google Scholar]
- 60.Arun A.S., Prasad, Satyanarayana S.V., Bhaskara Rao K.V. Biotransformation of direct blue 1 by a moderately halophilicbacterium marinobacter sp. strain HBRA and toxicity assessment of degraded metabolites. J. Hazard Mater. 2013;262:674–684. doi: 10.1016/j.jhazmat.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Hassaan M.A., El Nemr A., Madkour F.F. Testing the advanced oxidation processes on the degradation of direct blue 86 dye in wastewater, Egypt. J. Aqua. Res. 2017;43(1):11–19. [Google Scholar]
- 62.Burkinshaw S.M., Son Y.A. The dyeing of supermicrofibre nylon with acid and vat dyes. Dyes Pigments. 2010;87:132–138. [Google Scholar]
- 63.Santhi P., Jeyakodi Moses J. Reduction process of vat dye on cotton fabric assisted by ferrous sulphate. As. J. Chem. 2011;23(1):169–172. [Google Scholar]
- 64.Ji Q., Liu G., Jiti Z., Jing W., Jin R., Lv H. Removal of water-insoluble Sudan dyes by Shewanella oneidensis MR-1. Bioresour. Technol. 2012;114:144–148. doi: 10.1016/j.biortech.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Chaari I., Feki M., Medhioub M., Bouzid J., Fakhfakh E., Jamoussi F. Adsorption of a textile dye “indanthrene blue RS (C.I. vat blue 4)” from aqueous solutions onto smectite-rich clayey rock. J. Hazard Mater. 2009;172:1623–1628. doi: 10.1016/j.jhazmat.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 66.Muzamil K., Farooq A., Irfan S., Duy-Nam P., Qamar K., Zeeshan K., Hoik L., Ick Soo K. Dyeing and characterization of regenerated cellulose nanofibers with vat dyes. Carbohydr. Polym. 2017;174:443–449. doi: 10.1016/j.carbpol.2017.06.125. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen T.A., Fu C.-C., Juang R.-S. Effective removal of sulfur dyes from water by biosorption and subsequent immobilized laccase degradation on crosslinked chitosan beads. Chem. Eng. J. 2016;304:313–324. [Google Scholar]
- 68.Zhang B., Dong X., Yu D., He J. Stabilization mechanisms of C.I. disperses red 60 dispersions in the presence of its dye-polyether derivatives. Coll. Surf. Physicochem. Eng. Asp. 2012;405:65–72. [Google Scholar]
- 69.Es-sahbany H., Berradi M., Nkhili S., Bassir D., Belfaquir M., El Youbi M.S. Valorization of Moroccan clay: application to the adsorption of cobalt ions contained in wastewater synthesized. Moroc. J. Chem. 2018;6(1):173–179. [Google Scholar]
- 70.Hossain L., Kanti Sarker S., Samad Khan M. Evaluation of present and future wastewater impacts of textile dyeing industries in Bangladesh. Envir. Develop. 2018;26:23–33. [Google Scholar]
- 71.Khattab T.A., Rehan M., Hamouda T. Smart textile framework: photochromic and fluorescent cellulosic fabric printed by strontium aluminate pigment. Carbohydr. Polym. 2018;195:143–152. doi: 10.1016/j.carbpol.2018.04.084. [DOI] [PubMed] [Google Scholar]
- 72.Belpaire C., Reyns T., Geeraerts C., Van Loco J. Toxic textile dyes accumulate in wild European eel Anguilla. Chemosphere. 2015;138:784–791. doi: 10.1016/j.chemosphere.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 73.Liang J., Ning X., Kong M., Liu D., Wang G., Cai H., Sun J., Zhang Y., Lu X., Yuan Y. Elimination and ecotoxicity evaluation of phthalic acid esters from textile-dyeing wastewater. Environ. Pollut. 2017;231:115–122. doi: 10.1016/j.envpol.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 74.Azbar N., Yonar T., Kestioglu K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere. 2004;55:35–43. doi: 10.1016/j.chemosphere.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Srinivasan A., Viraraghavan T. Decolorization of dye wastewaters by biosorbents: a review. J. Environ. Manag. 2010;91(10):1915–1929. doi: 10.1016/j.jenvman.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Eren Z. Ultrasound as a basic and auxiliary process for dye remediation: a review. J. Environ. Manag. 2012;104:127–141. doi: 10.1016/j.jenvman.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 77.Hosseini Koupaie E., Alavi Moghaddam M.R., Hashemi S.H. Bioelectricity generation and bioremediation of an azo-dye in a microbial fuel cell coupled activated sludge process. Int. Biodeterior. Biodegrad. 2012;71:43–49. [Google Scholar]
- 78.Solpan D., Guven O. Discoloration and degradation of some textile dyes by gamma irradiation. Radiat. Phys. Chem. 2002;65:549–558. [Google Scholar]
- 79.Pearce C.I., Lioyd J.R., Guthrie J.T. The removal of color from textile wastewater using whole bacterial cells: a review. Dyes Pigments. 2003;58:179–196. [Google Scholar]
- 80.Cardoso N.F., Lima E.C., Royer B., Bach M.V., Dotto G.L., Pinto L.A.A. Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of reactive red 120 dye from aqueous effluents. J. Hazard Mater. 2012;241–242:146–153. doi: 10.1016/j.jhazmat.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 81.Cardoso N.F., Lima E.C., Calvete T., Pinto I.S., Amavisca C.V., Fernandes T.H.M., Pinto R.B., Alencar W.S. Application of aqai atalks as biosorbents for the removal of the dyes reactive black 5 and reactive orange 16 from aqueous solution. J. Chem. Eng. Data. 2011;56(5):1857–1868. [Google Scholar]
- 82.Cardoso N.F., Lima E.C., Pinto I.S., Amavisca C.V., Royer B., Pinto R.B., Alencar W.S., Pereira S.F.P. Application of cupuassu shell as biosorbents for the removal of textile dyes from aqueous solution. J. Environ. Manag. 2011;92(4):1237–1247. doi: 10.1016/j.jenvman.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 83.Sarvajith M., Kiran Kumar Reddy G., Nancharaiah Y.V. Textile dye biodecolourization and ammonium removal over nitrite in aerobic granular sludge sequencing batch reactors. J. Hazard Mater. 2018;342:536–543. doi: 10.1016/j.jhazmat.2017.08.064. [DOI] [PubMed] [Google Scholar]
- 84.Rawat D., Shyam Sharma R., Karmakar S., Singh Arora L., Mishra V. Ecotoxic potential of a presumably non-toxic azo dye. Ecotoxicol. Environ. Saf. 2018;148:528–537. doi: 10.1016/j.ecoenv.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 85.De Lima R.O.A., Bazo A.P., Salvadori D.M.F., Rech C.M., Oliveira D.P., Umbuzeiro G.A. Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat. Res. 2007;626(1-2):53–60. doi: 10.1016/j.mrgentox.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Carneiro P.A., Umbuzeiro G.A., Oliveira D.P., Zanoni M.V.B. Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperses dyes. J. Hazard Mater. 2010;174(1-3):694–699. doi: 10.1016/j.jhazmat.2009.09.106. [DOI] [PubMed] [Google Scholar]
- 87.Croce R., Cinà F., Lombardo A., Crispeyn G., Cappelli C.I., Vian M., Maiorana S., Benfenati E., Baderna D. Aquatic toxicity of several textile dye formulations: acute and chronic assays with Daphnia magna and Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2017;144:79–87. doi: 10.1016/j.ecoenv.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 88.Berradi M., El Harfi A. Review of the water resources, pollution sources and wastewaters of the Moroccan textile industry. J. Wat. Sci. Envir. Tech. 2016;1(2):80–86. [Google Scholar]
- 89.Berradi M., Cherkaoui O., El Harfi A. Comparative study the performance ofultrafiltration asymmetric membranes. Application to the bleaching of colored water with vat dyes. Appl. J. Envir. Eng. Sci. 2017;3(2):114–120. [Google Scholar]
- 90.Yuseuf R.O., Sonibare J. Caracterization of textile industries. Effluents in Kaduna, Nigeria and pollution implications. J.A. Glob. Nes. Inter. J. 2004;6(1):212–221. [Google Scholar]
- 91.Zaroual Z., Azzi M., Saib N., Chainet E. Contribution to the study of electrocoagulation mechanism in basic textile effluent. J. Hazard Mater. 2006;131(B):73–78. doi: 10.1016/j.jhazmat.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 92.Riera-Torres M., Gutiérrez-Bouzán C., Crespi M. Combination of coagulation-flocculation and nanofiltration techniques for dye removal and water reuse in textile effluents. Desalination. 2010;252(1-3):53–59. [Google Scholar]
- 93.Khandegar V., Saroha A.K. Electrocoagulation for the treatment of textile industry effluent- A review. J. Environ. Manag. 2013;128:949–963. doi: 10.1016/j.jenvman.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 94.Berradi M., Chabab Z., Arroub H., Nounah H., El Harfi A. Optimization of the coagulation/flocculation process for the treatment of industrial wastewater from the hot dip galvanizing of steel. J. Mater. Environ. Sci. 2014;5(2):360–365. [Google Scholar]
- 95.Allaoui M., Berradi M., El Hassan M., Saadallah M., El Harfi A. Synthesis of a new asymmetric semi permeable membrane and based alloy of two polymers PVC and PSU. Application in the treatments of a colored solution. Moroc. J. Chem. 2016;1:251–258. [Google Scholar]
- 96.Willmott N.J., Gutherie J.T., Nelson G. The biotechnology approach to color removal from textile effluent. J. Soc. Dye. Colour. 1998;6:212–221. [Google Scholar]
- 97.Prasad M.P., Bhakat P., Chatterjee S. Optımızatıon of textıle dye degradatıon by bacterıal specıes ısolated from natural sources. J. Ecol. Environ. Sci. 2013;4(1):97–99. [Google Scholar]
- 98.Srivastava S., Sinha R., Roya D. Toxicological effects of malachite green. Aquat. Toxicol. (N. Y.) 2004;66:319–329. doi: 10.1016/j.aquatox.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 99.Salazar R., Brillas E., Sirés I. Finding the best Fe2+/Cu2+ combination for the solar photoelectro-Fenton treatment of simulated wastewater containing the industrial textile dye disperse blue 3. Appl. Catal. Environ. 2012;115–116:107–116. [Google Scholar]
- 100.Suryavathi V., Sharma S., Sharma S., Saxena P., Pandey S., Grover R., Kumar S., Sharma K.P. Acute toxicity of textile dye wastewaters (untreated and treated) of Sanganer on male reproductive systems of albino rats and mice. Reprod. Toxicol. 2005;19:547–556. doi: 10.1016/j.reprotox.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 101.Hsissou R., Abbout S., Berisha A., Berradi M., Assouag M., Hajjaji N., El Harfi A. Experimental, DFT and molecular dynamics simulation on the inhibition performance of the DGDCBA epoxy polymer against the corrosion of the E24 carbon steel in 1.0 M HCl solution. J. Mol. Struct. 2019;1182:340–351. [Google Scholar]
- 102.Srivastava S.J., Singh N.D., Srivastava A.K., Sinha R. Acute toxicity of malachite green and its effects on certain blood parameters of a catfish, Heteropneustes fossilis. Aquat. Toxicol. (N. Y.) 1995;31:241–247. [Google Scholar]
- 103.Hsissou R., Dagdag O., Abbout S., Benhiba F., Berradi M., El Bouchti M., Berisha A., Hajjaji N., El Harfi A. Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. J. Mol. Liq. 2019;284:182–192. [Google Scholar]


