Abstract
Asymmetric patterns of frontal brain electrical activity reflect approach and avoidance tendencies, with stability of relative right activation associated with withdrawal emotions/motivation and left hemisphere activation linked with approach and positive affect. However, considerable shifts in approach/avoidance-related lateralization have been reported for children not targeted because of extreme temperament. In this study, dynamic effects of frontal electroencephalogram (EEG) power within and across hemispheres were examined throughout early childhood. Specifically, EEG indicators at 5, 10, 24, 36, 48, and 72 months-of-age (n=410) were analyzed via a hybrid of difference score and panel design models, with baseline measures and subsequent time-to-time differences modeled as potentially influencing all subsequent amounts of time-to-time change (i.e., predictively saturated). Infant sex was considered as a moderator of dynamic developmental effects, with temperament attributes measured at 5 months examined as predictors of EEG hemisphere development. Overall, change in left and right frontal EEG power predicted declining subsequent change in the same hemisphere, with effects on the opposing neurobehavioral system enhancing later growth. Infant sex moderated the pattern of within and across-hemisphere effects, wherein for girls more prominent left hemisphere influences on the right hemisphere EEG changes were noted and right hemisphere effects were more salient for boys. Largely similar patterns of temperament prediction were observed for the left and the right EEG power changes, with limited sex differences in links between temperament and growth parameters. Results were interpreted in the context of comparable analyses using parietal power values, which provided evidence for unique frontal effects.
Keywords: developmental processes, EEG asymmetry, temperament, sex differences, latent change scores, panel models
Developmental researchers have long aspired to capture the process of development, rather than individual static indicators that are, in effect, “snapshots” in time. Key developmental pathways emerge during infancy either reducing or increasing the probability of later behavioral/emotional dysfunction (Bornstein, 2014; Crockenberg & Leerkes, 2000). Studies have only recently begun to address such pathways and outcomes associated with individual differences in trajectories (Bridgett et al., 2009; Lengua, 2006) to understand emotion-related processes that result in either risk or protection. Although important, these existing longitudinal investigations relied on panel and growth models (Gartstein, Hancock, & Iverson, 2018; Howarth, Fettig, Curby, & Bell, 2016) with the former not assessing temporal change per se, and the latter focused on functional forms of growth. Neither strategy embodies the sensitivity, responsiveness, and cross-domain interactivity of the recently developed family of latent change score models ideally suited for the study of developmental processes in early childhood, during a period of markedly rapid transitions (Grimm, Mazza, & Mazzocco, 2016). Latent change score techniques allow us to consider the interplay between left and right frontal hemisphere activity that shapes the negotiation of approach/avoidance motives, a process open to influences of child sex and temperament. This novel analytic approach was used to do just this, as we modeled the developmental progression of frontal electroencephalogram (EEG) activity effects within and across hemispheres, advancing our conceptual understanding of emotion/motivation and underlying developmental processes by providing a dynamic representation of frontal EEG asymmetry based on multiple longitudinal evaluations across early childhood. The purpose of our study was twofold. First, we aimed primarily to address an important gap in research concerning developmental changes in neurophysiology, focusing on sex differences and the impact of infant temperament on the cascade of developmental effects. The second goal was to provide an illustration of an emerging statistical technique that can prove beneficial in answering a host of research questions in developmental science.
Differential asymmetric patterns of frontal brain electrical activity have been shown to reflect approach/avoidance tendencies, insofar as relative right hemisphere activation is linked with withdrawal behaviors and emotions (e.g., behavioral inhibition, fear), and left hemisphere activation associated with approach and positive affect (Calkins, Fox, & Marshall, 1996; Hane, Fox, Henderson, & Marshall, 2008). For example, infants demonstrating resting right frontal EEG asymmetry (i.e., greater relative right frontal activation) during a baseline condition were more likely to cry upon separating from mothers, relative to babies exhibiting left frontal EEG asymmetry (Bell & Fox, 1994; Davidson & Fox, 1989; Fox, Calkins, & Bell, 1994). Conversely, infants exhibiting relative left frontal activation expressed more positive affect and more readily approached an unfamiliar experimenter during a playful episode (Hane et al., 2008). Thus, resting frontal EEG asymmetry reflects individual differences in approach/avoidance related emotions and motivation, central to a number of temperament and personality theories (e.g., Rothbart, Ahadi, & Evans, 2000). Further, there is some indication of sex differences in the context of asymmetry-behavior connections (e.g., Gartstein, Bell, & Calkins, 2014; Theall-Honey & Schmidt, 2006), although the latter has not been sufficiently investigated.
EEG yields a continuous measure of synchronized firing of neuronal ensembles in a given frequency, referred to as power (μV2). Studies of frontal EEG asymmetry focused on emotion have typically considered EEG power values in the alpha frequency band: 8–13 Hz for adults; 6–9 Hz for infants and young children (e.g., Bell & Fox, 1992; Marshall, Bar-Haim, & Fox, 2002; Pizzagalli, 2007). In adults, EEG alpha activity was correlated with lower blood oxygen level-dependent (BOLD) signals in cortical areas underneath specific scalp electrodes (Goldman, Stern, Engel, & Cohen, 2002). Recent functional magnetic resonance (fMRI) research with adults using simultaneous EEG/fMRI recordings confirmed that the dorsolateral prefrontal cortex and the amygdala are involved in approach and withdrawal motivation, with temporal correlations between frontal EEG asymmetry and amygdala BOLD activity (Zotev et al., 2016). The standard in the field is to compute frontal EEG asymmetry as a difference in EEG alpha power between homologous electrodes: right alpha power minus left alpha power (Coan & Allen, 2004; Davidson, 1998; Fox & Davidson, 1987; 1988; Reznik & Allen, 2018). Alpha is inhibitory with respect to cortical network activity; thus, activation is inversely related to power. As a result, lower/negative frontal asymmetry scores reflect relatively greater right frontal activation and higher/positive values are interpreted as relative left activation (Allen, Coan, & Nazarian, 2004). Similar to adults, frontal EEG asymmetry scores in childhood are thought to measure differences in hemispheric activity of the dorsolateral prefrontal cortex underlying electrodes F3 and F4 (Meyer et al., 2015; Pizzagalli, Sherwood, Henriques, & Davidson, 2005).
Although asymmetrical frontal activation patterns and emotion lateralization are widely investigated, a number of questions remain unanswered. Importantly, frontal EEG asymmetry effects can be a function of significant differences at either, neither, or both of the constituent recording electrodes. Thus, activity at left and right sites must be investigated independently in order to determine which hemisphere contributes to the asymmetry related patterns of results (Allen, Coan, & Nazarian, 2004; Harmon-Jones & Allen, 1998; Reznik & Allen, 2018). Approach and avoidance emotions and motivation are thought to regulate each other, with reciprocal effects on the biological level reflected in frontal EEG activation/inhibition patterns (Fox, 1994). This dynamic interplay between the two specialized hemispheres over time results in either regulated or dysregulated approach and avoidance tendencies, especially critical in early childhood, during rapid developmental shifts in physiology and behavior. In fact, this reciprocal exchange between effects exerted by the left hemisphere on the activity of the right and vice versa likely contributes to the “tuning” of the central nervous system (CNS) to more effectively negotiate the tension between approach and avoidance motives. Quantifying dynamic interactions between left and right frontal EEG activity over time provides an opportunity to observe the regulatory nature of cross-hemisphere interplay via their electrophysiological signatures. The latent change score analytical approach applied here, in the context of a hybrid panel design, offers mathematical advantages (e.g., more robust estimation of effects), and can significantly advance our conceptual understanding of EEG growth and brain development processes more broadly. Specifically, it can provide a window into change driving change within and across hemispheres, while taking sex differences and contributions of temperament into account.
Stability of EEG asymmetry is often emphasized, yet considerable shifts in neuro-electrophysiology in community samples of children have been noted. In an unselected (i.e., not targeting temperament extremes) group of children representing a portion of the dataset used in the current study, frontal EEG asymmetry was only moderately stable from 10 to 24 months, but not between 24 and 36 or between 36 and 48 months (Howarth, Fettig, Curby, & Bell, 2015). In other studies, frontal EEG power demonstrated significant month-to-month correlations during the first year (Bell & Fox, 1994) and at the same time month-to-month recordings with infants in three independent samples indicated EEG power increased, on average, across the second half of the first year (Bell & Fox, 1992; Cuevas & Bell, 2011; MacNeill, Ram, Bell, Fox, & Perez-Edgar, 2018). An increase in EEG power for most sites was also observed in more widespread recordings across the first 4 years (5, 10, 14, 24, 51 months; Marshall, Bar-Haim, & Fox, 2002). In older children (8 to 12 years) relative alpha increased with age, indicating a shift to faster wave activity (Clarke, Barry, McCarthy, & Selikowitz, 2001), with hemispheric differences: there was greater relative power on the left compared to the right frontal region. Importantly, shifts in alpha power have been linked with brain development, including neuronal maturation and myelination across the cortex. Neural processes related to changes in network size and density (e.g., increase in the interconnected cortical cell assemblies) are likely responsible for the developmental trajectories of EEG power, although physical growth of the skull and supportive tissue may play a part a well (Anokhin, Lutzenberger, Nikolaev, & Birbaumer, 2000; Marshall et al., 2002; Martincović, Jovanović, & Ristanović, 1998).
Sex differences in EEG development and related temperament traits have been reported. Greater increases in EEG power trajectories were reported for girls, suggesting faster maturation (Anokhin, 2000; Martincović et al., 1998). In terms of temperament, girls demonstrated greater fear/behavioral inhibition in infancy across multiple studies, with higher levels of approach-related traits (e.g., activity, high intensity pleasure) noted for boys (Campbell & Eaton, 1999; Carey & McDevitt, 1978; Gartstein & Rothbart, 2003; Kivijärvi et al., 2005; Martin, Wisenbaker, Baker, & Huttunen, 1997; Maziade, 1984; Rothbart, 1988). Significant sex-related variability are also reported based on a meta-analysis of the temperament literature. Specifically, a large difference on effortful control favored girls, with inhibitory control and perceptual sensitivity sub-dimensions shown as main contributors. For surgency, boys demonstrated higher levels, especially on activity and high-intensity pleasure components (Else-Quest, Hyde, Goldsmith, & Van Hulle, 2006). Although not entirely consistent, early sex differences in temperament have emerged across different methods of assessment, and parallel those documented in personality studies with adults (Olino, Durbin, Klein, Hayden, & Dyson, 2013). For example, parent-report and structured laboratory observations indicated that boys demonstrate higher levels of activity level, less shyness and inhibitory control, compared to girls (Gagne, Miller, & Goldsmith, 2013).
Child sex has not been sufficiently investigated in the context of emotion-related brain lateralization literature; however, recent studies suggest potential moderation of EEG asymmetry effects. Shy preschool-age girls displayed greater relative right EEG activation during emotion-eliciting video-clips relative to shy boys, who displayed greater relative left EEG activation, leading authors to suggest that frontal EEG activation/emotion models could be gender-specific (Theall-Honey & Schmidt, 2006). In addition, the association between exposure to psychosocial risk and greater relative right frontal asymmetry was stronger for girls starting in infancy, and stronger associations between right frontal asymmetry and internalizing symptoms emerged for samples with a larger proportion of girls (Peltola et al., 2014). Thus, we considered whether or not infant sex altered the nature of emotion lateralization, moderating within and between hemisphere relations over time, as well as links between temperament and neurophysiological changes.
Temperament is relevant to the study of emotion-related frontal EEG activity because of connections between asymmetry indicators and individual differences in reactivity and regulation, especially approach/positive affectivity and fear/negative emotionality (e.g., Bell & Fox, 1994; Buss et al., 2003; Davidson & Fox, 1989; Fox et al., 1994; Goldstein et al., 2019; Lusby et al., 2016). Additional temperament domains have also been implicated. Gartstein et al. (2014) demonstrated that 5-month regulatory capacity/orienting was associated with greater right frontal activation in the context of an arm restraint task at 10 months for girls, using a portion of the same dataset as the present study. As just two time-points were considered, this study did not permit an examination of developmental changes or growth in the emotion-related EEG activity lateralization. Nonetheless, existing work suggests that temperament dimensions beyond fear and approach related attributes, although not typically examined, could provide critical inputs driving developmental changes in neurophysiological profiles.
Current Study
Our study was aimed at addressing important gaps in existing research, which has not sufficiently addressed questions related to developmental changes in the EEG effects contributing to frontal asymmetry. In addition, sex differences in EEG changes across early childhood have been largely neglected, especially with respect to potential contributions of temperament. Although fear/avoidance and approach/positive affectivity aspects of temperament have been frequently studies in the context of EEG asymmetry investigations, the role of broader factors, including Positive Affectivity/Surgency, Negative Emotionality, and Regulatory Capacity/Orienting has not been sufficiently considered, despite the emphasis on these over-arching dimensions in the general temperament literature (Gartstein et al., 2016). Moreover, temperament attributes are most widely studied as outcomes of neurophysiological profiles, with some notable exceptions (e.g., Goldstein et al., 2019); yet individual differences in reactivity and regulation in infancy can be expected to set the stage for developmental cascades of EEG asymmetry-related effects (Gartstein et al., 2014). Finally, our work illustrates the use of a versatile statistical technique capable of illuminating developmental processes, which can be widely applied across different areas of growth-related scientific inquiry.
Our first goal was to examine the cascade of dynamic effects among frontal EEG power indicators within and across hemispheres. Next, infant sex was considered as a moderator with respect to within and cross-hemisphere latent change score effects. We modeled predictive links between early infant temperament factors and changes in EEG power across six time points from infancy until 6 years of age, and sex differences in the context of temperament predictor analyses. Thus, our study provides insight into developmental processes involved in the balance between left and right hemisphere activation underlying approach and avoidance motivation, and their mutual regulation effects across early childhood.
We hypothesized a pattern of change within left and right frontal activation wherein initial (or intercept) values predict rate of change across different developmental segments considered, with earlier indices of change expected to influence subsequent growth occurring downstream in the system. Although prior research suggests an overall increase in power values across childhood (e.g., Marshall et al., 2002), change-related effects have not been examined. Our study is the first to consider whether more rapid shifts in EEG frontal power potentiate further acceleration of growth. We hypothesized cross-domain relations based on the tandem action of the associated neurobehavioral systems (Fox, 1994). Initial levels and changes in the left frontal activity were expected to drive growth of the right regional activity, and initial levels and changes in the right frontal activity were hypothesized to shape changes on the left, thereby regulating each other. Cross-hemisphere effects were anticipated consistent with the principle of the left hemisphere activation having an inhibitory effect on the right and vice versa. Thus, more rapid changes in power on the left were hypothesized to downregulate changes in the right hemisphere power values, slowing growth.
We expected that the resulting patterns of emotion-related EEG activity effects would differ by infant sex and be associated with infant temperament in a manner that varies for boys and girls. As greater developmental increases in EEG power have been reported for girls (Anokhin, 2000; Martincović et al., 1998), more prominent change-related effects were hypothesized for girls in our analyses as well. That is, we anticipated that significantly greater effects of initial level and earlier shifts in EEG power would be observed for girls compared to boys; however, existing studies did not provide the basis for a-priori expectations concerning cross-domain relations. Connections between fear/negative emotionality and increased changes in right frontal power, as well as between approach/positive affect and accelerated shifts in power for the frontal region of the left hemisphere, are indicated by prior research (e.g., Bell & Fox, 1994; Hane et al., 2008). We hypothesized that Regulatory Capacity/Orienting would predict greater changes in right frontal power, especially between 5 and 10 months, consistent with Gartstein et al. (2014). Moreover, it was anticipated that Positive Affectivity/Surgency would contribute to accelerated changes in right frontal EEG power for boys, and that the hypothesized effect associated with Regulatory Capacity/Orienting would be observed primarily for girls (Gartstein et al., 2014).
Method
Participants
As part of a longitudinal study examining individual differences in cognition-emotion integration across early development, 410 healthy children (i.e., generally born full-term, typical birthweight, and not suffering from birth/medical complications or congenital/developmental conditions) were recruited (209 girls). Laboratory visits were conducted when these children were 5, 10, 24, 36, 48, and 72 months of age. Ethnically, this sample was primarily non-Hispanic (n=383), with 27 Hispanic participants, and included the following racial categories: 318 Caucasian, 56 African American, 34 Multi-Racial/Other, and 2 Asian. Participants were recruited at two research locations (Blacksburg, VA; Greensboro, NC), with each collecting data from approximately half of the total sample. For parents who reported educational information (404 mothers, 392 fathers), 99% of mothers and 97% of fathers graduated from high school (6% and 7% technical degree, 42% and 31% bachelor’s degree, and 22% and 24% graduate degree, respectively). Mothers and fathers were approximately 29 and 32 years old (SD = 6 and 7), respectively, when the infants were born. With respect to infants, they were primarily second born (35%), although first-born (20%), only children (18%), and third-born (12%) or later (15%) were also relatively common in this sample (mean number of siblings = 1.42; SD = 1.07). Information concerning gestational age (mean = 38.89 weeks; SD = 1.48) and birth weight (mean = 7.49 lbs.; SD = 1.15) indicated term and typical weight infants. Recruitment was accomplished via commercial mailing lists, newspaper birth announcements, and word of mouth.
Data were collected in both research locations using identical protocols. Research teams were trained together by the last author on protocol administration, including psychophysiological coding. To ensure that identical protocol administration was maintained between the labs, the (Blacksburg, VA) site team periodically viewed video recordings and EEG files collected by the (Greensboro, NC) lab and provided verification of artifact screening for EEG data. The Institutional Review Boards at both universities approved this work.
EEG Recording and Analyses
Infants and their mothers visited the research lab on or within two weeks of turning 5- and 10-months of age. Baseline EEG was recorded while infants sat in an infant seat (5 months) or high chair (10 months) and watched a 45-second video clip depicting a visually-dynamic musical segment from Sesame Street (Richards, 1997). This procedure quieted the infants yielding minimal eye and gross motor movements, thus helping infants tolerate the EEG cap for the recording. Mothers sat beside their infants and were instructed not to talk with them during the EEG recording. Mothers were reimbursed for participation.
For the 24-, 36-, and 48-month assessments, children visited the lab on or within two months after their respective birthdays. Baseline EEG was recorded for 1 minute while children sat in a highchair (24 months) or chair (36 and 48 months) and watched a clip of the film Finding Nemo (sea turtles riding the East Australian Current). Mothers sat in a chair to the right of their children. During the baseline recording, the TV monitor was 1.8 meters from the children, and mothers were instructed not to talk. At the end of each yearly visit, mothers were paid for participating and children were given a small toy.
The 72-month lab visit occurred on or within six months after the children’s birthday. Baseline EEG was recorded for 2 minutes while the children sat in a chair and watched a clip of the film Lion King (opening scene). The TV monitor was 1.8 meters from the children and mothers were in an adjacent room. Mothers were reimbursed for participation; in addition, children were given a small toy.
Recordings were made from 16 left and right scalp sites: frontal pole (Fp1, Fp2), medial frontal (F3, F4), lateral frontal (F7, F8), central (C3, C4), temporal (T7, T8), medial parietal (P3, P4), lateral parietal (P7, P8), and occipital (O1, O2). All electrode sites were referenced to Cz during recording. Based on publication guidelines for studies using EEG methodology (Keil et al., 2014), we report the following details. The recordings were obtained using a stretch cap (Electro-Cap, Inc.; Eaton, OH; E1-series cap) with 16 electrodes in the 10/20 system pattern (Jasper, 1958). After the cap was placed on the head, a small amount of abrasive gel was placed into each recording site and the scalp was gently rubbed. Conductive gel was then added to the recording sites. Electrode impedances were measured and accepted if they were below 20 kΩ. The electrical activity from each lead was amplified using separate James Long Company Bioamps (James Long Company; Caroga Lake, NY). During data collection, the high pass filter was a single pole RC filer with a 0.1 Hz cut-off (3 dB or half-power point) and 6 dB per octave roll-off. The low pass filter was a two-pole Butterworth type with a 100 Hz cut-off (3 dB or half-power point) and 12 dB octave roll-off. Activity for each scalp lead was displayed on the monitor of the acquisition computer. The EEG was digitized on-line at 512 samples per second for each channel to eliminate the effects of aliasing. Snapshot-Snapstream (HEM Data Corp., Southfield, MI) acquisition software was used with raw data stored for later analyses. Prior to the recording of each subject a 10 Hz, 50 uV peak-to-peak sine wave was input through each amplifier. This calibration signal was digitized for 30 seconds and stored for subsequent analysis. Spectral analysis of the calibration signal and computation of power at 9–11 Hz was performed. Then, EEG data were examined and analyzed using EEG Analysis software developed by the James Long Company. Data were re-referenced via software to an average reference configuration and then artifact scored for eye movements using a peak-to-peak criterion of 100μV or greater. Gross motor movements over 200μV peak to peak were also scored. These artifact-scored epochs were eliminated from all subsequent analyses. No artifact correction procedures were used. The data were then analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1 second width and 50% overlap.
Power was computed for the 6 to 9 Hz frequency band (i.e., “infant and child alpha”) for all EEG records/visits. Infants and children have a dominant frequency between 6 to 9 Hz (Bell & Fox, 1994; Marshall, Bar-Haim, & Fox, 2002). This band has been correlated with patterns of emotion reactivity and regulation during infancy and early childhood (e.g., Bell & Fox, 1994; Buss et al., 2003; Dawson, 1994; Diaz & Bell, 2012; Hannesdottir, Doxie, Bell, Ollendick, & Wolfe, 2010). EEG power was expressed as mean square microvolts and the data transformed using the natural log (ln) to normalize the distribution. Ln power at left frontal (F3) and right frontal (F4) values were utilized because these regions are typically evaluated in the studies of frontal asymmetry (e.g., Coan & Allen, 2004; Reznik & Allen, 2018). Higher power values reflect lower activation at a particular electrode site, whereas lower power is indicative of greater activation (Lindsley & Wicke, 1974). For the data associated with this report, all participants deemed as outliers with respect to EEG power values (+/− 3 SDs) were eliminated, consistent with prior studies (e.g., Miskovic, Schmidt, Boyle, & Saigal, 2009), resulting in a sample size of 387 (198 girls). Simulation-based power analysis methods (Hancock & French, 2013; Muthén & Muthén, 2002) using Mplus 7.4 (Muthén, & Muthén, 2012) indicated this sample size enables detection of small to medium effects with .80 power.
Infant Temperament
The Infant Behavior Questionnaire-Revised (IBQ-R; Gartstein & Rothbart 2003), a 191-item parent-report instrument that yields 14 scales comprising three over-arching factors: Positive Affectivity/Surgency (Activity Level, Approach, High Intensity Pleasure, Perceptual Sensitivity, Smiling and Laughter, and Vocal Reactivity; Negative Emotionality (Distress to Limitations, Fear, Sadness, and Falling Reactivity); and Regulatory Capacity/Orienting (Cuddliness/Affiliation, Duration of Orienting, Low Intensity Pleasure, and Soothability). Reliability and validity of the IBQ-R have been consistently demonstrated, with Cronbach’s α’s ranging from .70 to .96 (Gartstein & Rothbart, 2003; Gartstein, Slobodskaya, & Kinsht, 2003). Satisfactory inter-rater agreement was also reported (Gartstein & Rothbart, 2003; Parade & Leerkes, 2008), with validity supported by studies incorporating the IBQ-R and laboratory indicators of temperament (Gartstein et al., 2010; Gartstein & Marmion, 2008; Parade & Leerkes, 2008). For the present sample, internal consistency was good, with Cronbach’s α’s ranging from .86 to .96 (Mean = .91) for the three factors. The IBQ-R was administered at 5 months, as this age marks an important transition in the first year of life, in terms of motor, cognitive, and social-emotional milestones (Bornstein, Arterberry, & Lamb, 2014; Shonkoff & Phillips, 2000). Improvements in memory likely contribute to increased displays of negative affect, for example those related to fear, as infants are able to discern the difference between previously encountered and unfamiliar stimuli with greater efficiency, responding to the latter in a more salient manner. Motor milestones related to reaching and grasping can be expected to facilitate expression of approach-based tendencies and positive affectivity, as infants are able to manipulate desirable objects in a more effective manner (Gartstein & Rothbart, 2003).
Missing Data
Participants providing data (“with”) and those not providing data (“without”) at each of the assessment points were compared on all of the EEG and temperament variables considered in this study. Infants who did not provide EEG data at 5 months (n=36) had lower EEG power relative to those who provided EEG data on F3 (left) power at 10 months (t = 2.70, p < .05; with M = 0.92, SD = 0.22; without M = 0.80, SD = 0.31). Infants who did not provide EEG data at 10 months (n=58) did not differ from those infants who provided EEG data at 10 months on subsequent EEG power measurements. Infants not providing EEG data at 24 months (n=162) were different from those who did on several subsequent frontal EEG power values: (1) at 48 months on F3 (t = 3.18, p < .01; with M = 1.11, SD = 0.14; without M = 1.04, SD = 0.15) and F4 (t = 3.93, p < .01; with M = 1.12, SD = 0.14; without M = 1.04, SD = 0.13); (2) at 72 months on F3 (t = 2.32, p < .05; with M = 1.11, SD = 0.15; without M = 1.04, SD = 0.21) and F4 (t = 2.19, p < .05; with M = 1.11, SD = 0.15; without M = 1.03, SD = 0.22). Infants who did and did not provide EEG data at 36 (n=147) and 48 months (n=161) did not differ statistically from infants who provided data on any subsequent EEG measures.
Infants providing (“with”) EEG data at 5 months were rated lower on Negative Emotionality relative to infants not providing (“without”) EEG data (t = −2.50, p < .05; with M = 2.98, SD = 0.64; without M = 3.28, SD = 0.72). The same pattern occurred for infants with and without EEG data at 10 months (t = −3.23, p < .01; with M = 2.96, SD = 0.64; without M = 3.26, SD = 0.65). No other temperament differences emerged for infants with and without EEG data.
Analytic Strategy
Descriptive statistics were computed (Table 1). Then, data from the entire sample were analyzed using a hybrid of a difference (or latent change) score model and a panel design, in which the baseline measures and subsequent time-to-time differences (i.e., amounts of change obtained by subtraction) were modeled as potentially influencing all subsequent amounts of time-to-time change within and across hemispheres (i.e., predictively saturated). This approach was selected due to varying durations between times of measurement as well as the expected differences in growth during these intervals. The first such completely lagged model was computed for the entire sample. Second, relations for boys and girls were examined separately in a multisample model, wherein parameters were unconstrained but tested for equivalence across the sexes. Third, infant temperament factors (Positive Affectivity/Surgency, Negative Emotionality, and Regulatory Capacity/Orienting) were considered as predictors of the time-to-time differences for the entire sample, and the final set of models introduced three IBQ-R factors as predictors of baseline and latent change in frontal EEG power for girls and boys, with parameters tested for equivalence between sexes. A number of covariates were included in all models: infant race (Caucasian, African-American, Asian, Multi-Racial), ethnicity (Hispanic, non-Hispanic), and dft windows – amount of usable (i.e., artifact free) data across all assessment points. Analyses were conducted in Mplus 7.4 (Muthén, & Muthén, 2012), using full information maximum likelihood (FIML) to accommodate missing data and Satorra-Bentler corrections to fit indices and standard errors to adjust for potential nonnormality. Parallel models were considered for parietal sites (P3/P4), to determine if the anticipated results were specific to the frontal region (Poole, Santesso, Van Lieshout, & Schmidt, 2018).
Table 1.
Descriptive statistics: Electrophysiological and Temperament Indicators
| Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|
| F3 Baseline 5 monthsa | −.55 | 1.35 | .65 | .28 |
| F4 Baseline 5 months | −.62 | 1.42 | .69 | .26 |
| F3 Baseline 10 months | −.39 | 1.60 | .91 | .23 |
| F4 Baseline 10 months | −1.16 | 1.58 | .93 | .25 |
| F3 Baseline 24 months | −.22 | 1.55 | 1.05 | .22 |
| F4 Baseline 24 months | −.21 | 1.50 | 1.07 | .19 |
| F3 Baseline 36 months | .72 | 1.74 | 1.14 | .19 |
| F4 Baseline 36 months | .65 | 1.72 | 1.15 | .20 |
| F3 Baseline 48 months | .62 | 1.43 | 1.09 | .14 |
| F4 Baseline 48 months | .64 | 1.46 | 1.10 | .13 |
| F3 Baseline 72 months | .48 | 1.62 | 1.09 | .18 |
| F4 Baseline 72 months | .42 | 1.58 | 1.09 | .18 |
| P3 Baseline 5 monthsa | −.48 | 1.41 | .80 | .22 |
| P4 Baseline 5 months | .06 | 1.39 | .83 | .21 |
| P3 Baseline 10 months | −1.23 | 1.61 | 1.02 | .23 |
| P4 Baseline 10 months | .25 | 1.53 | 1.05 | .19 |
| P3 Baseline 24 months | −.11 | 1.51 | 1.10 | .27 |
| P4 Baseline 24 months | −.47 | 1.60 | 1.14 | .26 |
| P3 Baseline 36 months | .66 | 1.81 | 1.20 | .21 |
| P4 Baseline 36 months | .71 | 1.82 | 1.20 | .21 |
| P3 Baseline 48 months | .65 | 1.53 | 1.16 | .17 |
| P4 Baseline 48 months | .66 | 1.56 | 1.18 | .17 |
| P3 Baseline 72 months | .13 | 1.66 | 1.12 | .23 |
| P4 Baseline 72 months | .27 | 1.68 | 1.12 | .22 |
| Positive Affectivity/Surgencyb | 2.77 | 6.68 | 4.73 | .70 |
| Negative Emotionality | 1.54 | 5.30 | 3.00 | .65 |
| Regulatory Capacity/Orienting | 3.57 | 6.60 | 5.04 | .55 |
| dftc 5 months | 0 | 286 | 52.79 | 22.87 |
| dft 10 months | 0 | 99 | 51.02 | 18.02 |
| dft 24 months | 0 | 152 | 64.85 | 29.81 |
| dft 36 months | 24 | 264 | 143.78 | 53.67 |
| dft 48 months | 21 | 300 | 149.07 | 60.58 |
| dft 72 months | 18 | 294 | 191.98 | 37.71 |
Note.
Power expressed in terms of square microvolts, transformed using the natural log (ln).
Temperament scores reflect a 7-point Likert scale (1–7); summed and averaged items/scales yielding factors.
dft windows - artifact free usable EEG data, 2 windows per second.
Results
Fit indicators for the initial frontal EEG (F3, F4) hybrid difference score/panel model examined with the total sample were satisfactory: Root Mean-Square Error of Approximation (RMSEA) = 0.00; Standardized Root Mean-Square Residual (SRMR) = 0.03; Akaike Information Criterion (AIC) = −654.94; Bayesian Information Criterion (BIC) = 207.99. A number of within and several cross-domain effects were observed for the entire sample, as can be seen in Supporting Materials Figure 1. Of interest, within domain paths (i.e., within F3 left hemisphere or within F4 right hemisphere) were in the negative direction (see standardized paths in Supporting Materials, Table 1), indicating a declining rate of change or effects consistent with deceleration. These within-hemisphere influences were coupled with a counterbalancing between-hemisphere action in the positive direction, so that changes in the left hemisphere (F3) were associated with accelerated growth in subsequent power values in the right hemisphere (F4), and vice versa (Supporting Materials, Table 1). Within-hemisphere influences were more prominent on the right (F4), with cross-hemisphere effects focused on the final segment of growth, from 48 to 72 months of age. The overall developmental patterns of the power signature at left and right hemispheres were very similar (Supporting Materials, Figure 2), beginning with relatively rapid increases and plateauing around 36 months.
A parallel solution based on parietal (P3/P4) power values was considered and demonstrated satisfactory fit (RMSEA = 0.04; SRMR = 0.03; AIC = −518.42; BIC = −347.17), indicating that a number of developmental effects are not frontal region-specific (Supporting Materials, Table 2). The primary difference between frontal and parietal models involved: (1) more prominent left within-hemisphere effects; (2) a more uniform pattern of left-to-right cross-hemisphere effects for the final lag (48 to 72 months of age) based on the parietal values.
Infant Sex as Moderator
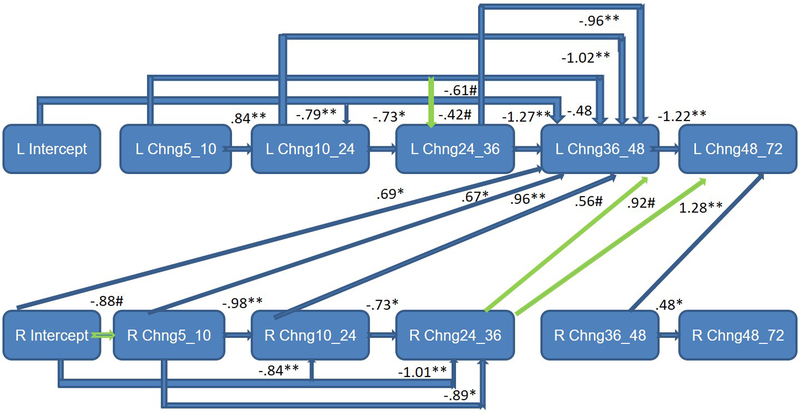
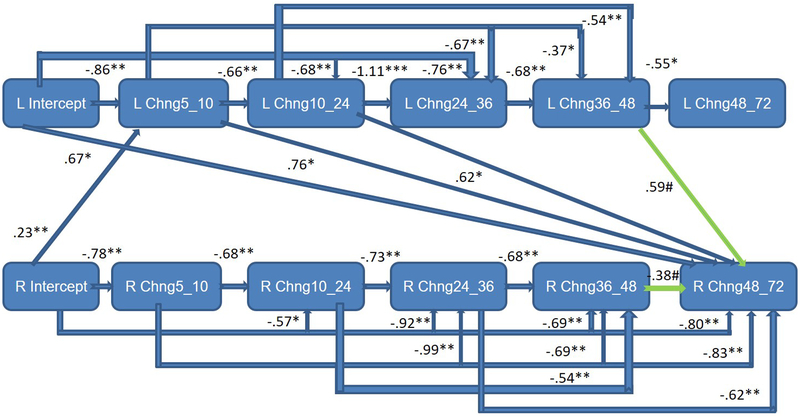
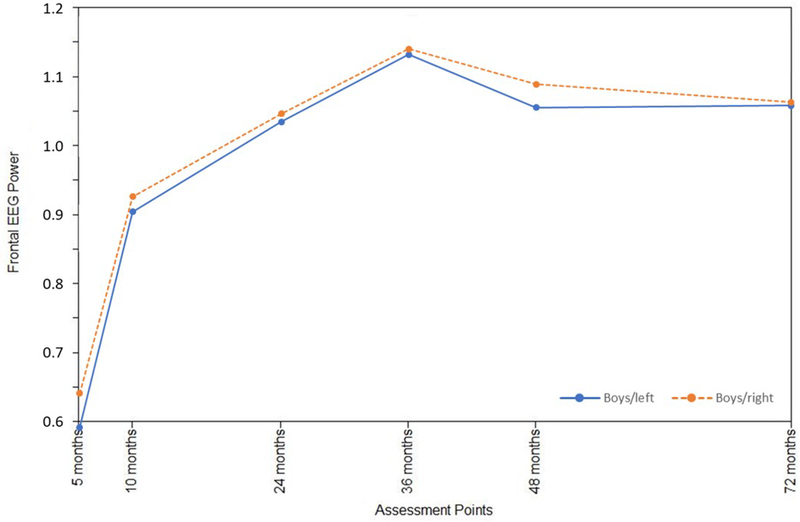
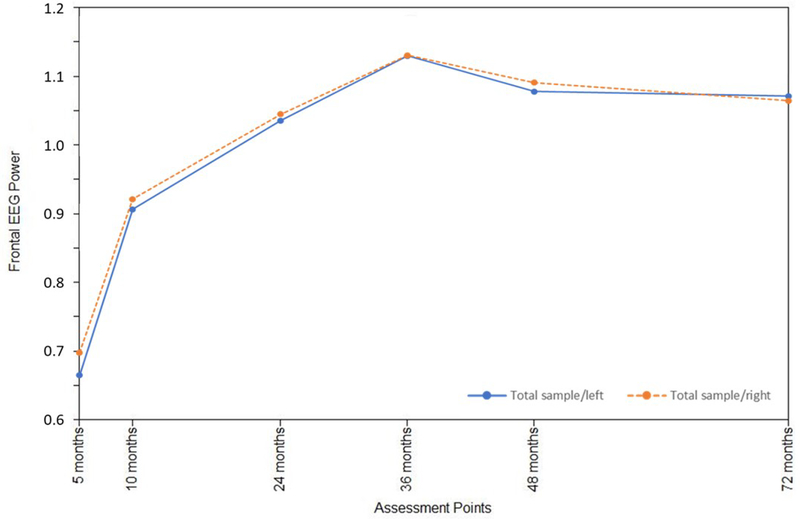
Infant sex was examined as a moderator of the frontal EEG hybrid difference score/panel model, with multisample model fit indicators deemed satisfactory: RMSEA = 0.06; SRMR = 0.06; AIC = −2048.48; BIC = −417.61. These analyses provided a picture of sex differences in the interplay between left and right frontal activation across early childhood (Table 2). Standardized path coefficients indicated that right frontal power was associated with fewer within-domain effects for boys (Figure 1a). For girls, primary cross-domain paths were directed from left to right (Figure 1b), whereas for boys the pattern was the opposite, with the right frontal power changes primarily affecting growth on the left (Figure 1a). Statistically significant differences (p<.05) between boys and girls were observed for a number of paths (Table 2), particularly notable from 36 to 48 months for both left and right hemisphere effects. Overall development of the frontal EEG power signature at left and right hemispheres was nonetheless rather similar for boys and girls (Figures 2a and 2b), beginning with relatively rapid increases and plateauing around 36 months, similar to the growth pattern that emerged for the total sample. Moreover, consistent variability was observed around boy and girl means (Figures 2a and 2b), as well as for the total sample (Supporting Materials/Figure 2).
Table 2.
Standardized Path Coefficients: Frontal Mutisample Hybrid Difference Score/Panel Model
| Boys: Left (F3 above diagonal) and Right (F4 below diagonal) Within-Domain Coefficients | ||||||
| Growth Parameter |
Intercept | Chng5_10 | Chng10_24 | Chng24_36 | Chng36_48 | Chng48_72 |
| Intercept | ------------ | −.84(.61) | −.79**(.20) | −.42#(.24) | −1.01**(.24) | −.48(.44) |
| Chng5_10 | −.88#(.49) | -------------- | −.84**(.15) | −.61#(.31) | −1.02**(.32) | −.50(.60) |
| Chng10_24 | −.84**(.21) | −.98**(.17) | -------------- | −.73*(.30) | −.96**(.32) | −.63(.60) |
| Chng24_36 | −1.01**(.37) | −.89*(.36) | −.73*(.30) | -------------- | −1.27**(.34) | −.94(.61) |
| Chng36_48 | .00(.27) | −.08(.28) | −.26(.31) | −.38(.31) | -------------- | −1.22**(.24) |
| Chng48_72 | −.32(.48) | −.26(.52) | −.09(.54) | .13(.52) | .48*(.22) | ------------- |
| Girls: Left (F3 above diagonal) and Right (F4 below diagonal) Within-Domain Coefficients | ||||||
| Growth Parameter |
Intercept | Chng5_10 | Chng10_24 | Chng24_36 | Chng36_48 | Chng48_72 |
| Intercept | ------------ | −.86**(.09) | −.68**(.22) | −.76**(.24) | −.31(.19) | −.13(.27) |
| Chng5_10 | −.78**(.10) | -------------- | −.66**(.19) | −.67**(.21) | −.37*(.18) | −.03(.29) |
| Chng10_24 | −.57*(.26) | −.68**(.23) | -------------- | −1.11**(.15) | −.54**(.15) | −.00(.26) |
| Chng24_36 | −.92**(.22) | −.99**(.20) | −.73**(.15) | -------------- | −.68**(.14) | −.26(.28) |
| Chng36_48 | −.69**(.20) | −.69**(.17) | −.54**(.15) | −.68**(.14) | -------------- | −.55*(.26) |
| Chng48_72 | −.80**(.29) | −.83**(.30) | −.22(.25) | −.62*(.28) | −.38#(.23) | ------------- |
| Boys: Left (F3) to Right (F4) and Right (F3) to Left (F4) Cross-Domain Coefficients | ||||||
| Growth Parameter |
Intercept | Chng5_10 | Chng10_24 | Chng24_36 | Chng36_48 | Chng48_72 |
| Intercept | ------------ | .20(.60) | .16(.15) | .44(.30) | −.31(.23) | .29(.47) |
| Chng5_10 | .15(.50) | -------------- | .14(.14) | .28(.28) | −.29(.31) | .28(.59) |
| Chng10_24 | .17(.24) | −. 01(.16) | -------------- | .01(.19) | −.18(.33) | .11(.60) |
| Chng24_36 | −.13(.31) | −.02(.33) | .16(.29) | ------------ | −.33(.37) | −.10(.63) |
| Chng36_48 | .69*(.28) | .67*(.29) | .96**(.32) | .56#(.29) | -------------- | −.34(.24) |
| Chng48_72 | .52(.45) | .56(.48) | .72(.53) | .92#(.49) | 1.28**(.21) | ------------- |
| Girls: Left (F3) to Right (F4) and Right (F3) to Left (F4) Cross-Domain Coefficients | ||||||
| Growth Parameter |
Intercept | Chng5_10 | Chng10_24 | Chng24_36 | Chng36_48 | Chng48_72 |
| Intercept | ------------ | .09(.09) | .19(.16) | .20(23) | .25(.22) | .67*(.30) |
| Chng5_10 | .23**(.09) | ------------ | .23(.14) | .33(.21) | .21(.20) | .76*(.31) |
| Chng10_24 | .33(.27) | . 22(.25) | -------------- | −.17(.16) | .10(.17) | .62*(.28) |
| Chng24_36 | .04(.22) | −.05(.19) | .19(.15) | -------------- | .05(.16) | .59#(.30) |
| Chng36_48 | −.18(.17) | −.06(.16) | −.06(.14) | −.06(.15) | -------------- | .31(.26) |
| Chng48_72 | −.01(.27) | −.06(.28) | .00(.26) | .11(.26) | .30(.23) | ------------- |
Note. Chng = Change. Boy and girl cross-domain coefficients: Left (F3) to Right (F4) path coefficients above the diagonal and Right (F4) to Left (F3) below; standard errors in parentheses. Dark-shaded values represent paths significantly different by infant sex (p<.05); light-shade (p<.10).
p<.01,
p<.05,
p<.10.
Figure 1a.
Completely lagged frontal hybrid difference score/panel design model: F3 (left)/F4 (right) mutual regulation from 5 to 72 months of age for boys only - significant sex differences were particularly notable from 36 to 48 months; earlier right frontal EEG power predicted change on the left.
Green paths represent trend level effects (p<.10).
Figure 1b.
Completely lagged frontal hybrid difference score/panel design model: F3 (left)/F4 (right) mutual regulation from 5 to 72 months of age for girls only - significant sex differences were particularly notable from 36 to 48 months; earlier left frontal EEG power generally predicted change on the left.
Green paths represent trend level effects (p<.10).
Figure 2a.
Growth trajectories for left and right frontal EEG power from 5 to 72 months of age: Boys only.
Figure 2b.
Growth trajectories for left and right frontal EEG power from 5 to 72 months of age: Girls only
The multisample model based on parietal power values (P3/P4) demonstrated satisfactory fit (RMSEA = 0.06; SRMR = 0.05; AIC = −1956.39; BIC = −326.58), producing a single statistically significant difference between boys and girls. The path from the right hemisphere intercept to the 5-to-10-month latent change score emerged as significantly different (Table 3), although coefficients obtained for boys and girls reflected the same direction of effect. This pattern of results contrasts a number of significant sex differences obtained with the frontal power values.
Table 3.
Standardized Path Coefficients: Mutisample Parietal Hybrid Difference Score/Panel Model
| Boys: Left (P3 above diagonal) and Right (P4 below diagonal) Within-Domain Coefficients | ||||||
| Growth Parameter |
Intercept | Chng5_10 | Chng10_24 | Chng24_36 | Chng36_48 | Chng48_72 |
| Intercept | ------------ | −.71**(.31) | −1.15**(.31) | −.52#(.29) | −.91**(.22) | −.09(.92) |
| Chng5_10 | −.89**(.13) | -------------- | −1.06**(.18) | −.59*(.23) | −.92**(.21) | −.26(.54) |
| Chng10_24 | −.33(.38) | −.70**(.20) | -------------- | −.64**(.22) | −.98**(.20) | −.18(.56) |
| Chng24_36 | −.98**(.34) | −.99**(.30) | −1.10**(.27) | -------------- | −.74**(.21) | −.07(.41) |
| Chng36_48 | .30(.25) | −.29(.23) | −.31(.22) | −.90**(.21) | -------------- | −.47#(.26) |
| Chng48_72 | −.63(.78) | −.56(.49) | −.65(.46) | −.94#(.53) | −.70#(.38) | ------------- |
| Girls: Left (P3 above diagonal) and Right (P4 below diagonal) Within-Domain Coefficients | ||||||
| Growth Parameter |
Intercept | Chng5_10 | Chng10_24 | Chng24_36 | Chng36_48 | Chng48_72 |
| Intercept | ------------ | −1.03**(.29) | −.71*(.34) | −.69*(.28) | −.73**(.23) | −.16(.28) |
| Chng5_10 | −.61**(.07) | -------------- | −.81**(.25) | −.65**(.25) | −.76**(.22) | −.18(.27) |
| Chng10_24 | −.97*(.38) | −.98**(.24) | -------------- | −.92**(.18) | −.60**(.22) | −.24(.22) |
| Chng24_36 | −.52*(.30) | −.63*(.27) | −.92**(.17) | -------------- | −.76**(.18) | −.32(.21) |
| Chng36_48 | −.18(.23) | −.19(.23) | −.39*(.19) | −.53**(.16) | -------------- | −.16(.24) |
| Chng48_72 | −.80**(.24) | −.74**(.24) | −.64**(.18) | −.52**(.20) | .02(.25) | ------------- |
| Boys: Left (P3) to Right (P4) and Right (P3) to Left (P4) Cross-Domain Coefficients | ||||||
| Growth Parameter |
Intercept | Chng5_10 | Chng10_24 | Chng24_36 | Chng36_48 | Chng48_72 |
| Intercept | ------------ | .26(.17) | −.07(.33) | .43(.31) | −.09(.23) | .62(.75) |
| Chng5_10 | .24(.28) | -------------- | −.05(.21) | .30(.24) | −.11(.22) | .52(.44) |
| Chng10_24 | .70#(.39) | −. 34(.18) | -------------- | .27(.23) | −.16(.22) | .56(.46) |
| Chng24_36 | −.06(.31) | −.09(.28) | −.18(.25) | ------------ | −.17(.23) | .66#(.37) |
| Chng36_48 | .50*(.23) | .44*(.21) | .41*(.20) | −.08(.20) | -------------- | .38(.22) |
| Chng48_72 | .07(.94) | .22(.61) | .16(.57) | −.23(.59) | .15(.42) | ------------- |
| Girls: Left (P3) to Right (P4) and Right (P3) to Left (P4) Cross-Domain Coefficients | ||||||
| Growth Parameter |
Intercept | Chng5_10 | Chng10_24 | Chng24_36 | Chng36_48 | Chng48_72 |
| Intercept | ------------ | −.02(.02) | .41(.37) | .16(29) | −.14(.22) | .75**(.23) |
| Chng5_10 | .43**(.08) | ------------ | .31(.25) | .19(.25) | −.17(.20) | .72**(.22) |
| Chng10_24 | .29(.35) | . 22(.25) | -------------- | .07(.21) | −.02(.19) | .63**(.19) |
| Chng24_36 | .31(.31) | .19(.28) | .05(.17) | -------------- | −.04(.15) | .62**(.19) |
| Chng36_48 | .45(.23) | .53*(.24) | .20(.21) | .23(.19) | -------------- | .78**(.22) |
| Chng48_72 | −.01(.27) | .03(.28) | .07(.22) | .18(.23) | .02(.25) | ------------- |
Note. Chng = Change. Boy and girls cross-domain coefficients: Left (P3) to Right (P4) path coefficients above the diagonal and Right (P4) to Left (P3) below. Dark-shaded values represent paths significantly different by infant sex (p<.05).
p<.01,
p<.05,
p<.10.
Temperament Predictors
All three temperament factors were considered simultaneously as predictors of frontal EEG power latent change scores with the resulting model demonstrating satisfactory fit (RMSEA = 0.00; SRMR = 0.03; AIC = 1382.96; BIC = 2530.90). For simplicity, each factor and its relations to EEG power changes is presented separately (Figure 3). Positive Affectivity/Surgency, Negative Emotionality, and Regulatory Capacity/Orienting made multiple significant contributions to latent change scores for the entire sample, which were often similar across the left (F3) and right (F4) hemispheres. No significant (or trend-level) temperament-related effects emerged for the parietal power (P3/P4) model, which had satisfactory fit: RMSEA = 0.04; SRMR = 0.03; AIC = 1547.28; BIC = 2695.22.
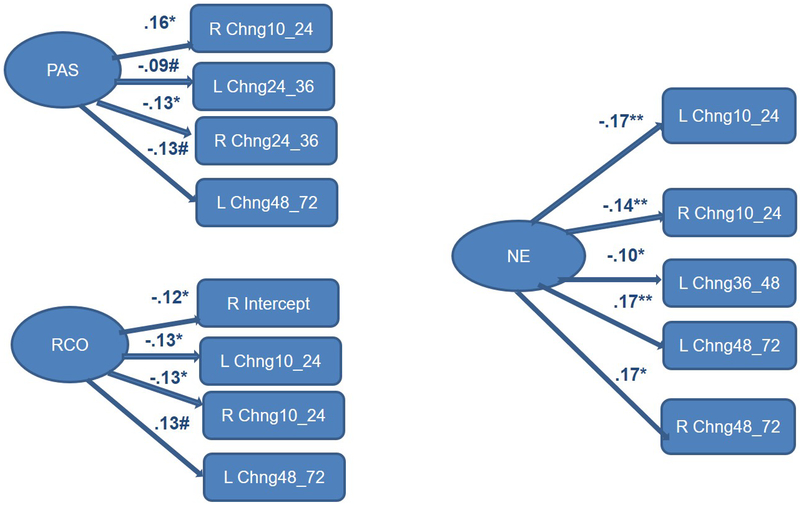
Figure 3.
Completely lagged frontal hybrid difference score/panel design model: Total sample F3 (left)/F4 (right) from 5 to 72 months of age with temperament predictors - Positive affectivity/surgency, negative emotionality, and regulatory capacity/orienting made multiple significant contributions to latent change scores for the entire sample, which were often similar across the left (F3) and right (F4) hemispheres.
Standardized paths for Positive Affectivity/Surgency (PAS), Negative Emotionality (NE), Regulatory Capacity/Orienting (RCO); *+p<.01, *p<.05, #p<.10.
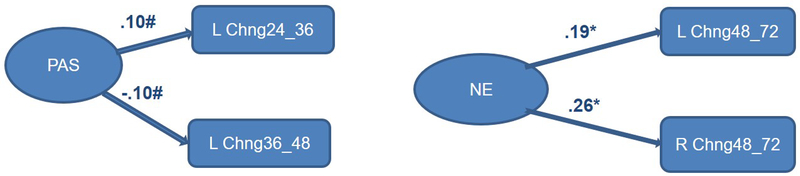
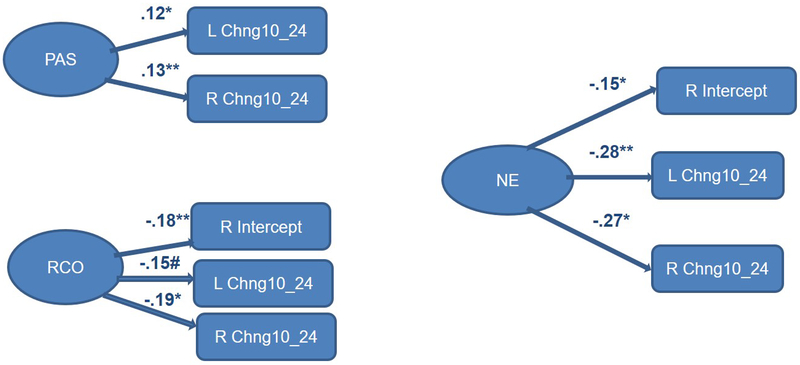
Finally, we considered infant sex as a moderator of links between temperament predictors and frontal EEG growth parameters. The multisample model with the three IBQ-R factors (Positive Affectivity/Surgency, Negative Emotionality, and Regulatory Capacity/Orienting) as predictors yielded satisfactory fit: RMSEA = 0.03; SRMR = 0.07; AIC = 289.20; BIC = 2197.16. For boys, only Positive Affectivity/Surgency and Negative Emotionality made significant contributions to explaining latent changes in EEG power/activation from 24 to 72 months of age (Figure 4a). For Negative Emotionality, its effect was limited to the change between 48 and 72 months, our final assessment interval. For girls, overall there were more significant paths associated with temperament variables, and some involving Regulatory Capacity/Orienting (Figure 4b). Moreover, for girls, significant paths from temperament factors to latent change scores were more concentrated earlier in development, from 5 to 24 months of age.
Figure 4a.
Completely lagged frontal hybrid difference score/panel design model: F3 (left)/F4 (right) from 5 to 72 months of age with temperament predictors for boys only - Positive affectivity/surgency and negative emotionality made several significant contributions to latent change scores, similar across the left (F3) and right (F4) hemispheres, but variable in direction across developmental periods.
Standardized paths for Positive Affectivity/Surgency (PAS), Negative Emotionality (NE); **p<.01, *p<.05, #p<.l0.
Figure 4b.
Completely lagged frontal hybrid difference score/panel design model: F3 left)/F4 (right) from 5 to 72 months of age with temperament predictors for girls only - Positive affectivity/surgency, negative emotionality, and regulatory capacity/orienting made a number significant contributions to latent change scores, often similar across the left (F3) and right (F4) hemispheres.
Standardized paths for Positive Affectivity/Surgency (PAS), Negative Emotionality (NE), Regulatory Capacity/Orienting (RCO); **p<.01, *p<.05, #p<.10.
Several significant differences among temperament-related standardized path coefficients were observed, all involving Negative Emotionality. For girls, Negative Emotionality was negatively associated with a right hemisphere latent change score, thus decrease in change from 10 to 24 months of age (boys: β = −0.05, NS; girls: β = −0.27, p<.01). In boys, positive relations with Negative Emotionality were observed, wherein higher Negative Emotionality translated into left hemisphere (boys: β = 0.19, p<.05; girls: β = −0.03, NS) and right hemisphere (boys: β = 0.26, p<.05; girls: β = −0.01, NS) latent change increases from 48 to 72 months of age. Overall, Negative Emotionality contributed to shaping frontal EEG power changes later in childhood in the boy relative to the girl subsample.
Minimal significant sex differences were noted for the parietal power temperament prediction model demonstrating satisfactory fit: RMSEA = 0.05; SRMR = 0.05; AIC = 430.63; BIC = 2338.56. One comparison reached statistical significance, wherein the association between Negative Emotionality and left hemisphere power latent change from 5 to 10 months of age differed for boys and girls (boys: β = −0.08, p<.10; girls: β = 0.08, NS). That is, Negative Emotionality was associated with decreased changes in left hemisphere power for boys and accelerated growth for girls during the same developmental period (i.e., 5 to 10 months of age).
Discussion
Results for frontal EEG power hybrid difference score/panel models across early childhood provide support for meaningful shifts in electrophysiology reflecting activity of neurobehavioral systems previously linked with approach/avoidance tendencies (e.g., Calkins et al., 1996; Fox, 1994; Hane et al., 2008), and associated with additional temperament attributes in the present investigation. Our findings suggest a complex pattern, likely serving a self-organizing function, wherein within-domain (i.e., within hemisphere) action downregulates changes in power, decreasing growth. Between-domain (i.e., between hemisphere) associations for frontal EEG power latent change scores were generally positive, indicating acceleration of growth effects with respect to the opposite hemisphere. This pattern of results can be viewed as consistent with the Fox (1994) conceptual model and emotion theories emphasizing mutual regulation more broadly (Cole, Martin, & Dennis, 2004), but not with the principle of each hemisphere inhibiting growth on the opposing side. Overall developmental patterns for EEG power values reflected initially rapid increases in alpha power for left and right frontal regions, with growth plateauing around 36 months, possibly due to the within and cross-domain effects on change in power over time. The observed change-related effects likely reflect systemic regulatory efforts within and across hemispheres, and our findings suggest that infant sex and temperament play a role in shaping the development of this frontal brain activity.
To some extent, results obtained for parietal values mirror the developmental progression that emerged for the frontal sites, yet there are notable distinctions. With respect to the latent change model for the entire sample, parietal power values were associated with a more consistent pattern of within-hemisphere effects on the left, as well as left-to-right cross-hemisphere predictions for the final latent change score (48 to 72 months of age), with all prior lags making significant contributions. There were also notable differences in the patterns of infant sex and temperament-related effects across frontal and parietal EEG power hybrid difference score/panel model solutions. Importantly, modeling the interplay between lateralized brain activity as a cascade of developmental changes provides a viable lens for examining the dynamic nature of neurodevelopment, indicating this approach would be useful in the study of other developmental processes.
Significant sex differences in the pattern of developmental EEG effects speak to the importance of examining infant sex as a moderator in the study of neurophysiology in childhood. The primary direction of cross-domain effects differed for boys and girls, with earlier left frontal EEG power generally predicting change on the right for girls, and right hemisphere effects more dominant in predicting change in left hemisphere power for boys. This pattern of results could account for previously reported sex differences in temperament and behavior problems, especially under-controlled behavior more frequently reported for boys (e.g., Chen, 2010; Else-Quest et al., 2006). That is, the direction of predominant effects for girls, with the left frontal EEG change parameters driving change on the right across early childhood and serving to increase growth, could be associated with behavioral and emotional consequences. Relative left frontal activation has been associated with self-regulation as well as approach (Goodman, Rietschel, Lo, Costanzo, & Hatfield, 2013; Jackson et al., 2003; Papousek, Freudenthaler, & Schulter, 2011). Thus, the tendency for left frontal activity to stimulate changes on the right could in part be responsible for the frequent early childhood finding of greater self-regulation abilities in girls (Else-Quest et al., 2006; Gagne et al., 2013; Matthews, Ponitz, & Morrison, 2009). Considerable sex differences in the patterns of effects among frontal EEG changes across early childhood likely parallel additional behavioral manifestations, such as expression of fear/avoidance and approach, wherein consistent differences between boys and girls have been demonstrated (e.g., Campbell & Eaton, 1999; Kivijärvi et al., 2005; Martin et al., 1997; Olino et al., 2013). Sex differences in the pattern of predictive links between temperament at 5 months of age and subsequent changes in frontal EEG power were also observed, and will be discussed after considering overall contributions of Positive Affectivity/Surgency, Negative Emotionality, and Regulatory Capacity/Orienting.
All three temperament factors (e.g., Positive Affectivity/Surgency, Negative Emotionality, and Regulatory Capacity/Orienting) were associated with changes in frontal EEG power across early childhood. Although we anticipated a differential pattern in prediction of changes in power for the two hemispheres, our results suggest these shifts are largely consistent. That is, Positive Affectivity/Surgency was associated with a declining change from 24 to 36 months of age in left and right hemisphere power (although the left hemisphere effect only approached significance). Regulatory Capacity/Orienting also resulted in decelerated growth for the left and the right between 10 and 24 months of age, and Negative Emotionality was associated with similarly decreasing changes from 10 to 24 months, along with increased changes between 48 and 72 months, also for the left and right hemispheres. The direction of several paths between temperament and change in frontal activity reversed direction across assessment waves considered in this study. These results require replication, and the noted lack of consistency could be viewed as limiting confidence in the findings, yet the directional shifts may reflect developmental processes that depend on what particular time period is being examined with respect to changes in frontal EEG power. The latter interpretation is likely tenable in early childhood because of particularly rapid development across different areas of functioning (e.g., cognitive, motor).
A sizable literature supports variability in links between temperament and frontal EEG asymmetry scores as favoring the left or the right, capturing differences in absolute power (Bell & Fox, 1994; Degnan et al., 2011; Hane et al., 2008). However, our results suggest that individual differences in reactivity and regulation play a largely equivalent role in shaping developmental patterns for frontal EEG power in the left and right hemispheres across early childhood. The finding of higher Regulatory Capacity/Orienting predicting lower initial power levels in the right hemisphere (thus, greater activation) is consistent with Gartstein et al. (2014) results, indicating greater Regulatory Capacity/Orienting was associated with relative right frontal activation in the context of an arm restraint task at 10 months. This pattern of results is also in line with a recently offered revision of the Behavioral Inhibition System definition (Gable, Neal, & Threadgill, 2018), which describes this system and the associated right frontal EEG asymmetry as linked with self-regulation. It should be noted that none of the temperament/latent change score associations emerged as statistically significant, or approaching significance, in the parietal power model. The latter pattern of results indicates that temperament predictive relations are indeed unique to the developmental progression observed for the frontal power values.
These temperament prediction results for the overall sample are informed by several sex differences. Only Positive Affectivity/Surgency and Negative Emotionality made significant contributions to explaining latent changes in EEG power from 24 to 72 months of age for boys. More numerous significant temperament effects were observed for girls, including Regulatory Capacity/Orienting along with the reactivity-related factors. Significant paths in the model for girls were more concentrated during earlier developmental periods between 5 to 24 months of age. The significant contribution of regulatory capacity/orienting to EEG power changes for girls, but not boys is consistent with prior research (Gartstein et al., 2014), and could also be associated with previously observed differences in early self-regulation generally favoring girls (e.g., Else-Quest et al., 2006). Statistically significant differences among paths were noted for Negative Emotionality in the frontal EEG power model. Specifically, higher Negative Emotionality translated into latent change increases in EEG power from 48 to 72 months of age on the left and the right for boys, but not girls. On the other hand, for girls, Negative Emotionality effects were noted earlier in childhood, wherein right hemisphere latent change scores decreased from 10 to 24 months of age with higher levels of distress proneness. This pattern of results requires further study, yet our findings suggest that for girls, higher levels of Negative Emotionality are associated with stabilization (i.e., decreased growth) in EEG power for the right hemisphere in late infancy/toddler period, whereas for boys, greater distress proneness contributes to more dynamic shifts in EEG power for both hemispheres later in childhood. Importantly, this pattern of sex differences also appears to be frontal-site specific. A single statistically significant effect involving Negative Emotionality emerged in the analyses of parietal values.
Contextual influences are generally invoked in explaining sex differences in early childhood similar to the ones noted in our study (e.g., Gartstein et al., 2014). Yet the sex-dependent pattern of results is also consistent with extensive evidence from animal studies indicating that dimorphic behaviors are common in sexually reproducing species and result from neural circuits developmentally programmed to be different in males and females (Morris, Jordan, & Breedlove, 2004; Wu & Shah, 2011). Some of this programming in humans appears to be a function of prenatal influences that shape sex differences in temperament development, likely via epigenetic processes (Gartstein & Skinner, 2018). For example, in utero exposure to high levels of androgens was linked to later externalizing difficulties (e.g., ADHD; Martel, Klump, Nigg, Breedlove, & Sisk, 2009) more common in boys, and could contribute to sex differences in approach/avoidance related frontal EEG developmental patterns. Additional studies are required to explore pathways driving gene expression in alternate direction in boys and girls, and initial research suggests sex differences in stress-related gene expression shifts, wherein increased NR3C1 methylation was positively associated with fearfulness for girls only (Ostlund et al., 2016). Postpartum biological effects are also possible, for example via testosterone increases for boys in infancy, referred to as “mini-puberty”, peaking by the second month and returning to baseline at about 6 months (Hines, Constantinescu, & Spencer, 2015).
Results of our study suggest that multiple longitudinal measures of left and right resting baseline frontal EEG activity can be effectively framed as a part of a self-regulating system, open to infant sex and temperament influences. A recent study demonstrated that both positive and negative emotionality contributed to frontal EEG asymmetry changes later in childhood (i.e., from 3 to 6 years of age; Goldstein et al., 2019), and our investigation extends this work showing such effects can be observed earlier in infancy, and involve Regulatory Capacity/Orienting as well. Dynamic shift in frontal EEG power have been demonstrated in prior research, suggesting influences of additional contextual factors, such as foster care placement, with the latter effects particularly salient prior to 24 months of age (McLaughlin, Fox, Zeanah, & Nelson, 2011). Together this evidence points to the malleability of early neurophysiology, wherein the development of emotion/motivation related EEG lateralization appears to be open to multiple inputs. Although some parallels in developmental effects emerged in comparable models examined for parietal sites (P3/P4), the latter analyses failed to yield a pattern that differed significantly as a function of infant sex, or notable relations with temperament. Importantly, our results speak to dynamic effects, with change influencing subsequent change, within and across hemispheres. Direction of associations is also of interest, as within-domain relations resulted in slowed developmental shifts, whereas cross-domain paths generally led to increases in growth. Additional research is required to clarify the meaning and significance of these dynamics, for example, making connections with brain development (e.g., myelination), and our results suggest such effects are not limited to the frontal region.
As noted, results of this study also speak to the viability of latent-change score models in elucidating the process of development, considered at the neurophysiological level in the context of this study. These computational techniques are capable of capturing dynamic effects, a subject of considerable interest across different areas of developmental science. Thus, latent-change score models allow researchers to go beyond putting together “snap-shots” or describing the functional form of a growth trajectory, in effect capturing the process of development. As such, in the present study these models provide evidence of some dynamic developmental effects ubiquitous across frontal and parietal regions, and others limited to the frontal sites, demonstrating a complex pattern of growth inhibition (within hemisphere) and potentiation (across hemispheres) which differed by child sex almost exclusively in the frontal region of the brain, and was only linked to temperament at the frontal sites.
Our study is subject to several limitations, including a relatively homogeneous sample and a mono-method/single time point assessment of temperament, which should be addressed in future research, examining behavioral manifestations of temperament and ensuring greater demographic variability. Moreover, future research should consider latent changes in temperament, not addressed in the present investigation. Although typical for EEG work in early childhood, missing data represents a limitation as well. Links between EEG latent change scores and distal outcomes (e.g., anxiety symptoms, self-regulation), were not addressed, and are essential to consider in the future. Elucidating such links will be critical to clarifying the interpretation of sex differences and to determining functional consequences of the observed developmental patterns (e.g., shifts in directionality of associations with temperament). Analyses focused on the developmental progression for absolute power and future work could consider relative power and ratio models (i.e., theta/alpha or theta/beta), beyond the scope herein. It should be noted that the baseline recording was conducted using different visual stimuli, in order to provide a developmentally-appropriate context for eliciting a calm/alert state. Nonetheless, reliance on different video content could be viewed as a limitation to be addressed in future research. Moreover, although commonly used with young children, videos represent a different condition for EEG recording than a resting state measured with older participants, although questions have been raised about the latter as well (e.g., eyes open vs. closed; for a discussion, see Anderson & Perone, 2018). Finally, future studies could consider recruitment-related variability, as multiple sources are typically utilized, and we were not able to consider the impact of such differences in this study.
In conclusion, our study provides a developmental/quantitative platform for conceptualizing the interplay between left and right frontal EEG activity demonstrating moderation by infant sex and contributions of temperamental reactivity and regulation. Although demanding in terms of acquisition and analysis, longitudinal data provide a valuable window into dynamic neurodevelopmental processes, especially during rapid transitions in early childhood. The quantitative approach described in this study could be readily applied in other areas of developmental science to elucidate dynamic/process-related effects.
Supplementary Material
Research Highlights.
Within hemisphere developmental changes in EEG became less pronounced with age, whereas development in one hemisphere influenced accelerated growth in the opposite hemisphere.
For girls, stronger left hemisphere influences on the right hemisphere changes were noted; whereas for boys, right hemisphere effects emerged as more prominent.
Three temperament factors of Negative Emotionality, Positive Affectivity/Surgency, and Regulatory Capacity/Orienting were associated with similar latent change score effects across the two hemispheres.
Findings demonstrate the importance of considering dynamic developmental effects in the study of neurophysiological underpinnings for emotional processing and more broadly.
Acknowledgements
We gratefully acknowledge participating families who made our publication possible. This research was supported by grants HD049878 and HD043057 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell.
Footnotes
Conflict of Interest Statement
None of the contributing authors have any conflicts of interest to report.
Data Availability Statement
Data included in this manuscript will be made available upon request by the corresponding author (Maria A. Gartstein).
Contributor Information
Maria A. Gartstein, Washington State University
Gregory R. Hancock, University of Maryland
Natalia V. Potapova, Washington State University
Susan D. Calkins, University of North Carolina at Greensboro
Martha Ann Bell, Virginia Tech.
References
- Allen JJB, Coan JA, & Nazarian M (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology, 67, 183–218. [DOI] [PubMed] [Google Scholar]
- Anderson AJ, & Perone S (2018). Developmental change in the resting state electroencepha-logram: Insights into cognition and the brain. Brain and Cognition, 126, 40–52. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Lutzenberger W, Nikolaev A, & Birbaumer N (2000). Complexity of electrocortical dynamics in children: Developmental aspects. Developmental Psychobiology, 36, 9–22. [PubMed] [Google Scholar]
- Bell MA, & Fox NA (1992). The relations between frontal brain electrical activity and cognitive development during infancy. Child Development, 63, 1142–1163. doi: 10.1111/j.1467-8624.1992.tb01685.x [DOI] [PubMed] [Google Scholar]
- Bell MA, & Fox NA (1994). Brain development over the first year of life: Relations between electroencephalographic frequency and coherence and cognitive and affective behaviors In Dawson G, & Fischer KW (Eds.), Human behavior and the developing brain (pp. 314–345). New York, NY: Guilford Press. [Google Scholar]
- Bornstein MH (2014). Human infancy…And the rest of the lifespan. Annual Review of Psychology, 65, 121–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Arterberry ME, & Lamb ME (2014). Development in infancy: A contemporary introduction (5th ed.). New York, NY: Psychology Press. [Google Scholar]
- Bridgett DJ, Gartstein MA, Putnam SP, McKay T, Iddins E, Robertson C, Ramsay K, & Rittmueller A (2009). Maternal and contextual influences and the effect of temperament development during infancy on parenting in toddlerhood. Infant Behavior & Development, 32, 103–116. [DOI] [PubMed] [Google Scholar]
- Buss KA, Malmstadt J, Dolski I, Kalin N, Goldsmith H, & Davidson R (2003). Right frontal brain activity, cortisol, and withdrawal behavior in 6 months old infants. Behavioral Neuroscience, 117, 11–20. doi: 10.1037/0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, & Marshall TR (1996). Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development, 67, 523–540. doi: 10.2307/1131830 [DOI] [PubMed] [Google Scholar]
- Campbell DW, & Eaton WO (1999). Sex differences in the activity level of infants. Infant and Child Development, 8, 1–17. [Google Scholar]
- Carey WB, & McDevitt SC (1978). Revisions of the infant temperament questionnaire. Pediatrics, 61, 735–739. [PubMed] [Google Scholar]
- Chen JJ (2010). Gender differences in externalizing problems among preschool children: Implications for early childhood educators. Early Child Development and Care, 180, 463–474. [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, & Selikowitz M (2001). Electroencephalogram differences in two subtypes of Attention-Deficit/Hyperactivity Disorder. Psychophysiology, 38, 212–221. [PubMed] [Google Scholar]
- Coan JA, & Allen JJ (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67, 7–50. doi: 10.1016/j.biopsycho.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, & Dennis TA (2004). Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Development, 75, 317–333. [DOI] [PubMed] [Google Scholar]
- Crockenberg S, & Leerkes E (2000). Infant social and emotional development in family context In Zeanah C (Ed.), Handbook of Infant Mental Health (et al. ) (pp. 60–90). New York: Guilford Press. [Google Scholar]
- Cuevas K, & Bell MA (2011). EEG and ECG from 5 to 10 months of age: Developmental changes in baseline activation and cognitive processing during a working memory task. International Journal of Psychophysiology, 80, 119–128. doi: 10.1016/j.ijpsycho.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, & Fox NA (1989). Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of Abnormal Psychology, 98, 127–131. [DOI] [PubMed] [Google Scholar]
- Dawson G (1994). Frontal electroencephalographic correlates of individual differences in emotion expression in infants: A brain systems perspective on emotion In Fox NA (Ed.), The development of emotion regulation: Biological and behavioral considerations. Monographs of the Society for Research in Child Development, 59, 135–151. Chicago: University of Chicago Press. [PubMed] [Google Scholar]
- Degnan KA, Hane AA, Henderson HA, Moas OL, Reeb-Sutherland BC, & Fox NA (2011). Longitudinal stability of temperamental exuberance and social-emotional outcomes in early childhood. Developmental Psychology, 47, 765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, & Bell MA (2012). Frontal EEG asymmetry and fear reactivity in different contexts at 10 months. Developmental Psychobiology, 54, 536–545. doi: 10.1002/dev.20612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else-Quest NM, Hyde JS, Goldsmith HH, & Van Hulle CA (2006). Gender differences in temperament: A meta-analysis. Psychological Bulletin, 132, 33–72. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- Fortier P, Van Lieshout RJ, Waxman JA, Boyle MH, Saigal S, & Schmidt LA (2014). Are orchids left and dandelions right? Frontal brain activation asymmetry and its sensitivity to developmental context. Psychological Science, 25, 1526–1533. doi: 10.1177/0956797614534267 [DOI] [PubMed] [Google Scholar]
- Fox N (1994). Dynamic cerebral processes underlying emotion regulation. Monographs of the Society for Research in Child Development, 59, 152–166. doi: 10.2307/1166143. [DOI] [PubMed] [Google Scholar]
- Fox NA, Calkins SD, & Bell MA (1994). Neural plasticity and development in the first two years of life: Evidence from cognitive and socio-emotional domains of research. Development and Psychopathology, 6, 677–698. doi: 10.1017/S0954579400004739 [DOI] [Google Scholar]
- Fox NA, & Davidson RJ (1987). EEG asymmetry in ten month-old infants in response to approach of a stranger and maternal separation. Developmental Psychology, 23, 233–240. [Google Scholar]
- Fox NA, & Davidson RJ (1988). Patterns of brain electrical activity during facial signs of emotion in ten-month-old infants. Developmental Psychology, 24, 230–236. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, & Schmidt LA (2001). Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development, 72, 1–21. doi: 10.1111/1467-8624.00262 [DOI] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Henderson HA, & Marshall P (2007). Developmental psychophysiology: Conceptual and methodological issues In Cacioppo JT, Tassinary LG, & Berntson GG (Eds.), Handbook of psychophysiology, (3rd ed., pp. 453–481). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Gable PA, Neal LB, & Threadgill AH (2018). Regulatory behavior and frontal activity: Considering the role of revised-BIS in relative right frontal asymmetry. Psychophysiology, 55, e12910 10.1111/psyp.12910 [DOI] [PubMed] [Google Scholar]
- Gagne JR, Miller MM, & Goldsmith HH (2013). Early-but modest-gender differences in focal aspects of childhood temperament. Personality and Individual Differences, 55, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Bell MA, & Calkins SD (2014). EEG asymmetry at 10 months of age: Are temperament trait predictors different for boys and girls? Developmental Psychobiology, 56, 1327–1340. doi: 10.1002/dev.21212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Bridgett DJ, Rothbart MK, Robertson C, Iddins E, Ramsay K, & Schlect S (2010). A latent growth examination of fear development in infancy: Contributions of maternal depression and the risk for toddler anxiety. Developmental Psychology, 46, 651–668. doi: 10.1037/a0018898 [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Hancock GR, & Iverson SL (2018). Positive affectivity and fear trajectories in infancy: Contributions of mother-child interaction factors. Child Development, 89, 1519–1534. doi: 10.1111/cdev.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, & Marmion J (2008). Fear and positive affectivity in infancy: Convergence/discrepancy between parent-report and laboratory-based indicators. Infant Behavior and Development, 31, 227–238. doi: 10.1016/j.infbeh.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Prokasky A, Bell MA, Calkins S, Bridgett DJ, Braungart-Rieker J, Leerkes E, Cheatham C, Eiden RD, Mize KD, Jones N, Mireault G, & Seamon E (2017). Latent profile and cluster analysis of infant temperament: Comparisons across person-centered approaches. Developmental Psychology, 53, 1811–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the revised infant behavior questionnaire. Infant Behavior and Development, 26, 64–86. doi: 10.1016/j.infbeh.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, & Skinner MK (2018). Prenatal influences on temperament development: The role of environmental epigenetics. Development and Psychopathology, 30, 1269–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Slobodskaya HR, & Kinsht IA (2003). Cross-cultural differences in the first year of life: United States of America (U.S.) and Russia. International Journal of Behavioral Development, 27, 316–328. doi: 10.1080/01650250244000344 [DOI] [Google Scholar]
- Goldman RI, Stern JM, Engel J, & Cohen MS (2002). Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport, 13, 2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Shankman SA, Kujawa A, Torpey-Newman DC, Dyson MW, Olino TM, & Klein DN (2019). Positive and negative emotionality at age 3 predicts change in frontal EEG asymmetry across early childhood. Journal of Abnormal Child Psychology, 47, 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RN, Rietschel JC, Lo L, Costanzo ME, & Hatfield BD (2013). Stress, emotion regulation and cognitive performance: The predictive contributions of trait and state relative frontal EEG alpha asymmetry. International Journal of Psychophysiology, 87, 115–123. doi: 10.1016/j.ijpsycho.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Grimm KJ, Mazza GL, & Mazzocco MM (2016). Advances in methods for assessing longitudinal change. Educational Psychologist, 51, 342–353. doi: 10.1080/00461520.2016.1208569 [DOI] [Google Scholar]
- Hancock GR, & French BF (2013). Power analysis in covariance structure models In Hancock GR & Mueller RO (Eds.), Structural equation modeling: A second course (2nd ed., pp. 117–159). Charlotte, NC: Information Age Publishing. [Google Scholar]
- Hane AA, Fox NA, Henderson HA, & Marshall PJ (2008). Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology, 44, 1491–1496. doi: 10.1037/a0012855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesdottir DK, Doxie J, Bell MA, Ollendick TH, & Wolfe CD (2010). A longitudinal study of emotion regulation and anxiety in middle childhood: Associations with frontal EEG asymmetry in early childhood. Developmental Psychobiology, 52, 197–204. doi: 10.1002/dev.20425 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Allen JJB (1998). Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology, 74, 1310–1316. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Gable PA (2018). On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology, 55, e12879. doi: 10.1111/psyp.12879 [DOI] [PubMed] [Google Scholar]
- Hines M, Constantinescu M, & Spencer D Early androgen exposure and human gender development. Biology of Sex Differences, 26, 6:3. doi: 10.1186/s13293-015-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth GZ, Fettig NB, Curby TW, & Bell MA (2016). Frontal electroencephalogram asymmetry and temperament across infancy and early childhood: An exploration of stability and bidirectional relations. Child Development, 87, 465–476. doi: 10.1111/cdev.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, et al. (2003). Now you feel it, now you don’t: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science, 14, 612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x [DOI] [PubMed] [Google Scholar]
- Jasper HH (1958). The ten twenty-electrode system of the international federation. Electroencephalography Clinical Neurophysiology, 10, 371–375. [PubMed] [Google Scholar]
- Keil A, et al. (2014). Committee report: Publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology, 51, 1–21. doi: 10.1111/psyp.12147 [DOI] [PubMed] [Google Scholar]
- Kivijärvi M, Räihä H, Kaljonen A,Tamminen T, & Piha J (2005). Infant temperament and maternal sensitivity behavior in the first year of life. Scandinavian Journal of Psychology, 46, 421–428. [DOI] [PubMed] [Google Scholar]
- Lengua LJ (2006). Growth in temperament and parenting as predictors of adjustment during children’s transition to adolescence. Developmental Psychology, 42, 819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DB, & Wicke JD (1974). The EEG: Autonomous electrical activity in man and animals In Thompson R & Patterson MN (Eds.), Bioelectrical recording techniques (pp. 3–83). New York: Academic Press. [Google Scholar]
- Lopez-Duran NL, Nusslock R, George C, & Kovacs M (2012). Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology, 49, 510–521. doi: 10.1111/j.1469-8986.2011.01332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusby CM, Goodman SH, Yeung EW, Bell MA, & Stowe ZN (2016). Infant EEG and temperament negative affectivity: Coherence of vulnerabilities to mothers’ perinatal depression. Development and Psychopathology, 28, 895–911. [DOI] [PubMed] [Google Scholar]
- MacNeill LA, Ram N, Bell MA, Fox NA & Perez-Edgar K (2018). Trajectories of infants’ biobehavioral development: Timing and rate of A-Not-B performance gains and EEG maturation. Child Development, 89, 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113, 1199–1208. doi: 10.1016/S1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- Martel MM, Klump K, Nigg JT, Breedlove SM, & Sisk CL (2009). Potential hormonal mechanisms of Attention-Deficit/Hyperactivity Disorder and Major Depressive Disorder: A new perspective. Hormones and Behavior, 55, 465–479. doi: 10.1016/j.yhbeh.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RP, Wisenbaker J, Baker J, & Huttunen MO (1997). Gender differences in temperament at six months and five years. Infant Behavior and Development, 20, 339–347. [Google Scholar]
- Martincović Z, Jovanović V, & Ristanović D (1998). EEG power sprectra of normal preadolescent twins. Gender differences of quantitative EEG maturation. Clinical Neurophysiology, 28, 231–248. [DOI] [PubMed] [Google Scholar]
- Matthews JS, Ponitz CC, & Morrison FJ (2009). Early gender differences in self-regulation and academic achievement. Journal of Educational Psychology, 101, 689–704. [Google Scholar]
- Maziade M, Boudreault M, Thivierge J, Caperaa P, & Cote R (1984). Infant temperament: SES and gender differences and reliability of measurement in a large Quebec sample. Merrill-Palmer Quarterly, 30, 213–226. [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, & Nelson CA (2011). Adverse rearing environments and neural development in children: The development of frontal electroencephalogram asymmetry. Biological Psychiatry, 70, 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Smeets T, Giesbrecht T, Quaedflieg CWEM, Smulders FTY, Meijer EH, & Merckelbach HLGJ (2015). The role of frontal EEG asymmetry in post-traumatic stress disorder. Biological Psychology, 108, 62–77. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA, Boyle M, & Saigal S (2009). Regional electroencephalogram (EEG) spectral power and hemispheric coherence in young adults born at extremely low birth weight. Clinical Neurophysiology, 120, 231–238. doi: 10.1016/j.clinph.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, & Breedlove SM (2004). Sexual differentiation of the vertebrate nervous system. Nature Neuroscience, 7, 1034–1039. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2002). How to use a Monte Carlo study to decide on sample size and determine power. Structural Equation Modeling: A Multidisciplinary Journal, 9, 599–620. [Google Scholar]
- Muthén LK, & Muthén BO (2012). Mplus 7.4 [Computer software]. Retrieved from http://statmodel.com.
- Olino TM, Durbin CE, Klein DN, Hayden EP, & Dyson MW (2013). Gender differences in young children’s temperament traits: Comparisons across observational and parent‐report methods. Journal of Personality, 81, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund BD, Conradt E, Crowell SE, Tyrka AR, Marsit CJ, & Lester BM (2016). Prenatal stress, fearfulness, and the epigenome: Exploratory analysis of sex differences in DNA methylation of the glucocorticoid receptor gene. Frontiers in Behavioral Neuroscience, 10: 147. doi: 10.3389/fnbeh.2016.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papousek I, Freudenthaler H, & Schulter G (2011). Typical performance measures of emotion regulation and emotion perception and frontal EEG asymmetry in an emotional contagion paradigm. Personality and Individual Differences, 51, 1018–1022. doi: 10.1016/j.paid.2011.08.013 [DOI] [Google Scholar]
- Parade S, & Leerkes E (2008). The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behavior and Development, 31, 637–646. doi: 10.1016/j.infbeh.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola MJ, Bakermans-Kranenburg MJ, Alink LRA, Huffmeijer R, Biro S, & van IJzendoorn MH (2014). Resting frontal EEG asymmetry in children: Meta-analyses of the effects of psychosocial risk factors and associations with internalizing and externalizing behavior. Developmental Psychobiology, 56, 1377–1389. doi: 10.1002/dev.21223 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA (2007). Electroencephalography and high-density electrophysiological source localization In Cacioppo JT, Tassinary LG, & Berntson GG (Eds.), Handbook of psychophysiology (3rd ed.) (pp. 56–84). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, & Davidson RJ (2005). Frontal brain asymmetry and reward responsiveness—A source-localization study. Psychological Science, 16, 805–813. [DOI] [PubMed] [Google Scholar]
- Poole KL, Santesso DL, Van Lieshout RJ, & Schmidt LA (2018). Trajectories of frontal brain activity and socio-emotional development in children. Developmental Psychobiology, 60, 353–363. doi: 10.1002/dev.21620 [DOI] [PubMed] [Google Scholar]
- Reznik SJ, & Allen JJB (2018). Frontal asymmetry as a mediator and moderator of emotion: An updated review. Psychophysiology, 5, e1296. doi: 10.1111/psyp.12965. [DOI] [PubMed] [Google Scholar]
- Richards JE (1997). Effects of attention in infants’ preference for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Developmental Psychology, 33, 22–31. doi: 10.1037/0012-1649.33.1.22 [DOI] [PubMed] [Google Scholar]
- Rothbart MK (1988). Temperament and the development of the inhibited approach. Child Development, 59, 1241–1250. [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, & Evans DE (2000). Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology, 78, 122–135. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, & Phillips DA (Eds.). (2000). From neurons to neighborhoods: The science of early child development. Washington, D.C: National Academy Press. [PubMed] [Google Scholar]
- Theall-Honey LA, & Schmidt LA (2006). Do temperamentally shy children process emotion differently than non-shy children? Behavioral, psychophysiological, and gender differences in reticent preschoolers. Developmental Psychobiology, 48, 187–196. [DOI] [PubMed] [Google Scholar]
- Wu MV, & Shah NM (2011). Control of masculinization of the brain and behavior. Current Opinions in Neurobiology, 21, 116–123. doi: 10.1016/j.conb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V, Yuan H, Misaki M, Phillips R, Young KD, Feldner MT, & Bodurka J (2016). Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. NeuroImage: Clinical, 11, 224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.