Abstract
Early studies following perinatal hypoxic-ischemic encephalopathy (HIE) suggested expressive language deficits and academic difficulties, but detailed study of language development in this population since widespread adoption of therapeutic hypothermia has been limited. Expressive and receptive language testing was performed as part of a larger battery with 45 children with a mean age of 26 months following perinatal HIE treated with therapeutic hypothermia. Overall cohort outcomes as well as the effects of gender, estimated household income, initial pH and base excess, and pattern of injury on neonatal brain MRI were assessed. The cohort overall demonstrated expressive language subscore, visual-reception subscore, and early learning composite scores significantly below test norms with relative sparing of receptive language subscores. Poorer expressive language manifested as decreased vocabulary size and shorter utterances. Expressive language subscores showed a significant gender effect, and estimated socioeconomic status showed a significant effect on both receptive and expressive language subscores. Initial blood gas markers and modified Sarnat scoring did not show a significant effect on language subscores. Binarized MRI abnormality predicted a significant effect on both receptive and expressive language subscores; presence of specific cortical/subcortical abnormalities predicted receptive language deficits. Overall, the language development profile of children following HIE in the era of hypothermia shows a relative strength in receptive language. Gender and socioeconomic status predominantly predict expressive language deficits; abnormalities detectable on MRI predominantly predict receptive language deficits.
Keywords: Language development, hypoxic-ischemic encephalopathy, therapeutic hypothermia
2. Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) affects approximately 1.5 in 1000 births and is the most common cause of perinatal brain injury in full-term neonates (1). Even with therapeutic hypothermia, now standard of care when available for neonates with HIE, there is incomplete neuroprotection-- on long-term follow-up, 17% of these children develop cerebral palsy, and 27% demonstrate IQ<70 (2). Prior to therapeutic hypothermia, a number of studies demonstrated poorer speech and language skills including selective reading and spelling difficulties in school age in children with HIE even in the absence of more serious cognitive or motor difficulties (3–5); therefore, it is critical to evaluate speech and language skills in the era of therapeutic hypothermia.
There have been few evaluations of language development in children with HIE post therapeutic hypothermia. Both the NICHD and TOBY trial of hypothermia for perinatal HIE performed neurocognitive follow-up studies at age 6–7 (using either the Wechsler Preschool and Primary Scale of Intelligence, Third Edition (6) or Wechsler Intelligence Scale for Children, Fourth Edition (7). The NICHD follow-up study (2) noted low-normal verbal IQ in both hypothermia (mean standard score 85.9) and normothermia (mean standard score 86.4) groups, though a fraction of the sample population was deemed too low-functioning to assess verbal IQ. The TOBY trial (8) reported average verbal IQ in both hypothermia (mean standard score 105) and normothermia (mean standard score 101) groups. While correlations between Verbal IQ and scores on language assessments are generally moderate to high (9), they measure different skills and differently predict academic function (10).
In attempts to understand later neurodevelopmental outcomes following neonatal HIE, degree of injury as measured by modified Sarnat score, blood biomarkers, and patterns of brain injury seen on MRI have been used to predict outcome (11,12). There is evidence that such measures predict broad outcomes such as mortality and eventual diagnoses of cerebral palsy and intellectual disability (12,13). Little is known about value of these markers in predicting higher frequency, lower severity disorders such as language impairment. To date, the most promising predictive markers have been MRI-- findings of white matter or basal ganglia injury have been shown to correlate with lower language scores at age 30 months (12,14) and with both lower performance and verbal scores at age 4 years (14).
In our cohort of children with moderate to severe HIE treated with therapeutic hypothermia, we hypothesized that while gender and socio-economic status would influence language outcomes (15–17), some early markers of hypoxic-ischemic injury severity (initial pH/base deficit, severity of HIE on post-cooling MRI) would also correlate with language outcomes. Further, we hypothesized that specific injury patterns seen on MRI may predict specific types of language skill deficits. Specifically, basal ganglia injury has a strong relationship with motor impairment, which is thought to affect speech and would therefore be expected to predominantly affect expressive language measures (18) while cortical injury might be expected to more likely affect language learning resulting in receptive language deficits (13,14,19).
3. Materials and Methods
Study Design
This is a convenience sample of infants with hypoxic-ischemic encephalopathy who were treated with therapeutic hypothermia and participated in a comprehensive follow-up evaluation at age 2 years.
Neonatal Timepoint
All neonates who underwent whole-body cooling for moderate-to-severe encephalopathy between 2010 and 2014 at Johns Hopkins Children’s Hospital neonatal intensive care unit (NICU) and participated in a comprehensive neurodevelopmental battery at age 2 were included in the study. Infants eligible for cooling were diagnosed with moderate to severe HIE on clinical exam based on modified Sarnat criteria (20,21) and blood gas from the umbilical cord or first hour of life with pH <7.15 or a base deficit >10 mmol/L. If a blood gas measurement was not available, 10-minute Apgar score <5 or assisted ventilation for ≥10 minutes after birth, an acute perinatal event, and moderate to severe encephalopathy were used to diagnose HIE. Additional eligibility criteria for this study included gestational age ≥35 weeks, birth weight ≥1800 g, initiation of whole-body cooling within 6 hours of birth, and a parent who spoke English as the primary language. Neonates with a contraindication to hypothermia therapy (e.g. coagulopathy) with active bleeding or congenital anomalies that could make cooling unsafe were not eligible for the study.
Variables gathered from the NICU period used for analysis in this study included initial blood gas (either cord or within the first hour of life as available) pH and base deficit. Lactate was not consistently obtained in this cohort (Table 1). Household income was not directly assessed on admission, but socioeconomic status was estimated using well-established (22,23) proxies: 1) 2015 median income of the census tract containing the family’s home address at the time of delivery and 2) family insurance status (private vs. medical assistance/public).
Table 1 -. Participant characteristics.
Measures obtained from NICU admission include proxies of socioeconomic status (parental public vs. private insurance and home census tract-based estimate of household income) and injury markers. N reflects number of subjects for which the measure was obtained and (for categorical variables) the number of subjects falling into each category. Modified Sarnat Score reflects documented degree of encephalopathy on exam within the first six hours of life (moderate (2) vs. severe (3)). NICHD MRI grade reflects both the degree and gross distribution of brain injury on conventional MRI. Grades include categories for normal MRI (0), cortical/subcortical involvement only (1A (minimal cerebral lesions) and 1B (more extensive cerebral lesions), basal ganglia/thalamic/deep white matter involvement only (2A (basal ganglia, thalamic, internal capsule lesions only), or involvement of both areas (2B (basal ganglia, thalamic, internal capsule, and cerebral lesions) and 3 (cerebral devastation)). Parent-reported household tax bracket and clinical diagnosis of cerebral palsy were obtained from the 2-year research visit.
| Demographics | N | % | Mean +/− SD | Range |
|---|---|---|---|---|
| Female sex | 21/45 | 47% | ||
| Private insurance | 34/45 | 76% | ||
| Maternal education (years, 12 = completed high school) | 18/45 | 15.1+/−2.1 | 11–17 | |
| Annual household income (parent-reported) | 18/45 | $126.1k+/−83.7k | $31.2k-305.9k | |
| Annual household income (estimated from median by census tract) | 45/45 | $75.5k+/−33.4k | $16.9k-167.6k | |
| NICU biomarkers | ||||
| Modified Sarnat Score | ||||
| 2 (moderate encephalopathy) | 32/45 | 71% | ||
| 3 (severe encephalopathy) | 13/45 | 29% | ||
| NICHD MRI classification | ||||
| 0 (normal) | 25/44 | 57% | ||
| 1A (minimal cerebral lesions) | 11/44 | 25% | ||
| 1B (more extensive cerebral lesions without other involvement) | 2/44 | 4.5% | ||
| 2A (basal ganglia, thalamic, internal capsule lesions only) | 3/44 | 6.8% | ||
| 2B (basal ganglia, thalamic, internal capsule, and cerebral lesions) | 3/44 | 6.8% | ||
| 3 (cerebral devastation) | 0/44 | 0% | ||
| pH | 45/45 | 6.96+/−0.13 | 6.62–7.26 | |
| Base excess/deficit | 43/45 | −15.9+/−6.2 | −33.8−−7.0 | |
| Serum lactate (mmol/L) | 22/45 | 3.9+/−4.1 | 0.7–16.7 | |
| Other diagnoses at age 2 | ||||
| Cerebral palsy | 3/45 | 6.7% | ||
Categorization of encephalopathy was determined by review of neurologic status as described in the admission history and physical. A neonatologist and a pediatric neurologist both with additional training in developmental disabilities independently reviewed the admission records to determine moderate or severe encephalopathy. Level of encephalopathy was determined using modified Sarnat criteria (20,21). If infants had features from both categories, the level of encephalopathy was determined with the most criteria present. Agreement between the record reviewers was a kappa of 0.78. Disagreements were reviewed and determined by consensus.
MRI was obtained as a part of usual NICU care in the first two weeks of life in 44/45 subjects (average age 8.7±3.0 days). NICHD MRI grade (a grading scheme ranging between 0, or no detectable HIE-related injury to 3, or “global devastation” with distinction between cortical/subcortical and basal ganglia/thalamic injury based on T1- and T2-weighted sequences; see Table 1 for brief description of NICHD MRI grades; described in detail in (24) was assessed by a board-certified pediatric neuroradiologist blinded to outcomes.
Preschool-age Timepoint
As standard of care, starting in 2010 all children treated for neonatal HIE in our NICU are referred for neurodevelopmental follow-up on discharge. In addition, families were eligible to participate in a research evaluation between 18–30 months. Children who were involved in the foster care system at the time of neurodevelopmental follow-up were not included in the research study.
As part of a larger battery, the research evaluation included the Mullen Scales of Early Learning (MSEL; Pearson Education, Inc., London, UK) and the MacArthur-Bates Communicative Development Inventories (MB-CDI; Brookes Publishing; Baltimore, MD). MSEL is a developmental battery assessing gross motor (GM), fine motor (FM), visual reception (VR), expressive language (EL), and receptive language (RL) which also provides a summary early learning composite (ELC) incorporating information from VR, EL, RL, and FM subscores. Testing norms are established for children from birth to 68 months of age. Subscores are reported as T-scores (population mean of 50; standard deviation 10; floor of instrument 20), and the ELC is reported as a standard score (population mean of 100; standard deviation 15). The MB-CDI is a detailed, standardized parent report instrument including subscores describing overall volume of word production (MB-PROD), and average length of longest 3 sentences (MB-M3L). Other MB-CDI subscores were not included in this analysis as they reflect skills (e.g. correct contextual matching of later developed word endings) not expected to be reliably present at this age. Norms for age and gender are reported as percentiles, and are available between ages 8–30 months. In normative samples, subscore performance below 10th percentile is considered concerning for language disability.
Forty-five children in this convenience sample had completed at least MSEL-RL, MSEL-EL, and MSEL-VR and were included in this analysis. Of these, 44/45 completed all MSEL subscores, and completed MB-CDI reports were obtained for 33/45 within the normed window. In this study, we were particularly interested in MB-PROD and MB-M3L as measures of speech output and complexity, respectively; for these measures, normed percentile values were available for 33/45 and 32/45 subjects, respectively.
In addition to primary study outcome measurements, other information collected at the preschool age timepoint included whether the individual had been clinically diagnosed with cerebral palsy. Additional demographic information (e.g. household tax bracket) was also elicited but with incomplete response (Table 1).
Statistical Analysis
Overall cohort outcomes were assessed; statistics were performed using R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), and figures were constructed using MATLAB R2018b (MathWorks, Natick, MA). Distributions of scores for each subtest were described and compared to typical average scores for age using a non-parametric Wilcoxon signed-rank test so as not to assume normally-distributed data. Developmental dissociation was assessed between subscores within the tested cohort using the Wilcoxon rank-sum test.
In order to better define motor contributions to language scores, post-hoc analysis was performed 1) on subjects with a clinical diagnosis of cerebral palsy, and 2) on all subjects. As only three subjects in this convenience sample were diagnosed with cerebral palsy, language scores were described narratively. To identify motor and cognitive contributions more quantitatively, a 2-way Type II ANOVA model was constructed using MSEL-VR (as a proxy for non-verbal intelligence) and MSEL-FM (a measure of fine motor performance) as predictors of MSEL-EL and MSEL-RL.
To investigate the value of potential prognostic factors available in the NICU setting to later language ability, we examined covariance of selected expressive and receptive language subscores (MSEL-EL, MSEL-RL, and MB-PROD) with gender, geocode-based estimated income, initial pH and base excess, modified Sarnat scores and binarized normal/abnormal MRI.
Data from additional variables of interest (reported household income, level of maternal education, and lactate) were only available from a minority of subjects. Cohort-wide values were summarized (Table 1), but these variables were not included in further analysis.
We examined covariance of selected subscores (MSEL-EL, MSEL-RL, MSEL-VR, MB-PROD, MB-M3L) with respect to 2 × 2 covariates of the presence vs. absence of cortical/subcortical lesions and basal ganglia/thalamic lesions, respectively. Determination of anatomical location was based on NICHD MRI criteria-- grades 1A and 1B were considered to have cortical/subcortical involvement alone; grade 2A was considered to have basal ganglia/thalamic involvement alone; grades 2B and 3 were considered to have both types of involvement.
Given the sample size, analysis was first performed in a univariate manner on subjects with data including the variable of interest. Again, non-parametric statistics were used; a Wilcoxon rank-sum test was used for binary covariates, and a significance test on Kendall’s tau correlation vs. a null hypothesis of no effect was used for continuous covariates. The 2 × 2 model based on factors of presence/absence of 1) cortical/subcortical (MRI-CORT) and 2) basal ganglia/thalamic lesions (MRI-BGT) was analyzed using a two-way Type II ANOVA structure; significance was determined based on an F test. To better understand the relative contributions of variables studied, we also constructed a 4-way Type II ANOVA model including all variables that showed significant associations on univariate analyses (MRI-CORT, MRI-BGT, gender, insurance status, initial pH, and initial base excess).
4. Results
Sample Characteristics
127 infants were treated with therapeutic hypothermia during the study time period. 14 of these infants were deceased at the time of follow-up. A subset of 45 of these children participated in a comprehensive research evaluation near two years of age. Participant characteristics are summarized in Table 1.
Whole-Cohort Language and Cognitive Outcomes
Whole-cohort testing characteristics are summarized in Table 2. MSEL-RL scores were not significantly different from those of the test norms (T-score estimate 49.0, CI [45, 52.5], p=0.57), but MSEL-EL (44.5, CI [41,48], p=0.0023), MSEL-VR (42.5, CI [38.5,46.5], p=0.0015), and MSEL-ELC (SS=90.5, CI [85,96], p=0.0016) were all significantly lower than test norms (Figure 1A, indicated by *). 4/44 subjects (9.1%) had MSEL-ELC standard scores < 70; 3/45 (6.7%) and 4/45 (8.9%) had MSEL-RL and MSEL-EL T-scores < 30, respectively (2 standard deviations below the population mean in all cases). MB-PROD and MB-M3L measures were both significantly lower than test norms (Figure 1B; estimate 27.5 percentile, CI [20–40 percentiles], p=0.00087; estimate 35 percentile, CI [25–47.5 percentiles], p=0.016, respectively). 24% of subjects (MB-PROD) and 16% of subjects (MB-M3L), respectively had scores below 10th percentile for age and gender, representing classification as “high-risk”. MSEL-RL scores were significantly higher than MSEL-VR scores (p=0.018) and the early learning composite (p=0.037) but non-significantly higher than MSEL-EL scores (p=0.083; Figure 1A).
Table 2: Whole-cohort testing characteristics.
Bolded entries indicate p<0.05 for significant group difference below testing norms. T-scores are normed against typical validation groups with mean scores of 50 and a standard deviation of 10. Standard scores are normed against typical validation groups with mean scores of 100 and a standard deviation of 15.
| Estimate | 95% Confidence interval |
p-value | |
|---|---|---|---|
| Mullens RL T-score | 49.0 | [45.0,52.5] | 0.57 |
| Mullens EL T-score | 44.5 | [41.0,48.0] | 0.0023 |
| Mullens VR T-score | 42.5 | [38.5,46.5] | 0.0015 |
| Mullens FM T-score | 42.0 | [38.5, 45.0] | 0.000062 |
| Mullens ELC standard score | 90.5 | [85.0,96.0] | 0.0016 |
| M-B Production percentile | 27.5 | [20.0,40.0] | 0.00087 |
| M-B M3L percentile | 35.0 | [25.0,47.5] | 0.016 |
| Estimated group difference | 95% Confidence interval | p-value | |
| Mullens RLt-VRt | +6.0 | [1.0,11.0] | 0.018 |
| Mullens RLt-ELCt | +5.0 | [0.67,10.0] | 0.037 |
| Mullens RLt-ELt | +4.0 | [0,9.0] | 0.083 |
Abbreviations: ELt: Mullen expressive language T-score; RLt: Mullen receptive language T-score; ELC: Mullen Early Learning Composite; M-B: MacArthur-Bates Communicative Development Inventories; M3L: MacArthur-Bates mean of 3 longest utterance percentile
Figure 1: Whole-cohort testing characteristics.
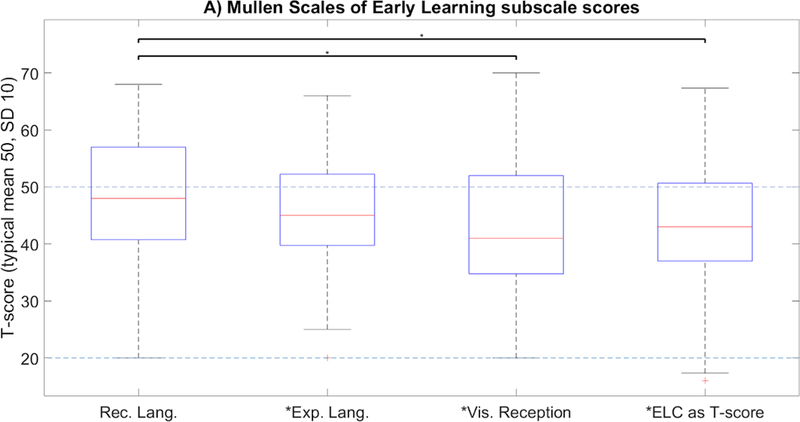
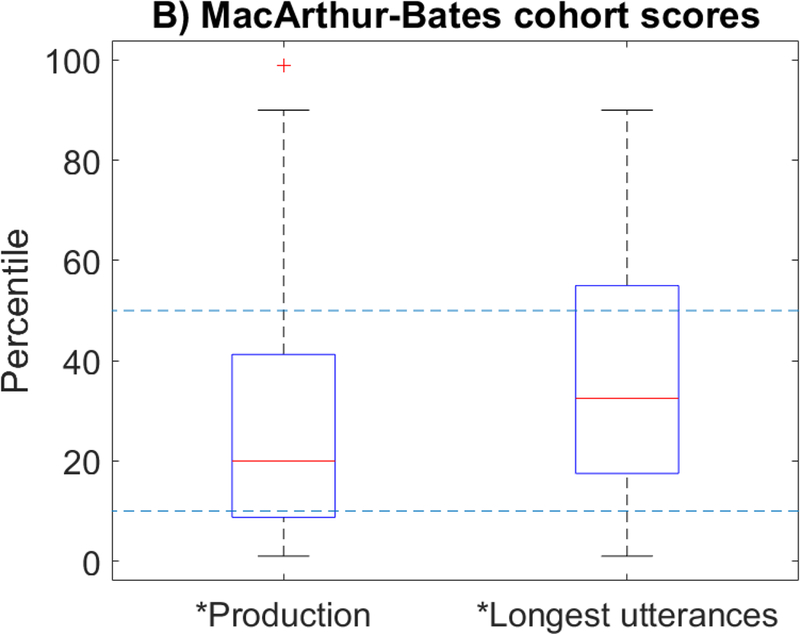
Asterisk on x-axis label indicates significant group difference below testing norms (p<0.05); starred bracket indicates significant discrepancy in performance between domains (p<0.05). T-scores are normed against typical validation groups with mean scores of 50 and a standard deviation of 10.
Covariate Analysis
All 3 children with CP had basal ganglia/thalamic involvement (one individual with MRI grade 2A; two individuals with MRI grade 2B). Those with grade 2B MRI scores had variable motor functioning (one with Gross Motor Functional Classification System (GMFCS) I, one with GMFCS V) and very poor language outcomes (MSEL-RL and MSEL-EL T-scores <25); the individual with a grade 2A MRI had GMFCS II and borderline language scores (MSEL-RL T=46; MSEL-EL T=37).
Variance in MSEL-VR (a proxy for non-verbal cognitive skills) predicted a significant minority of variance in both MSEL-EL (27.2% of variance; p=0.00014) and MSEL-RL (23.1% of variance; p=0.00074). Variance in MSEL-FM contributed significantly only to variance in MSEL-EL (10.0% of variance; p=0.014; Table 3).
Table 3: Cognitive and motor determinants of language function.
Table entries indicate percent of variance of each outcome measure (columns) by each predictor (row) explained within a 2-factor ANOVA based on proxies of non-verbal cognitive (visual reception scores) and motor (fine motor) functioning. The third row represents the interaction term between the two factors as a predictor.
| Predictor | MSEL Expressive language T- score |
MSEL Receptive language T-score |
|---|---|---|
| MSEL Visual reception T-score | 27.2%*** | 23.1%*** |
| MSEL Fine motor T-score | 10.0%* | 2.5% |
| MSEL-VR × MSEL-FM interaction effect | 1.7% | 5.4% |
| Total explained variance | 38.9% | 31.0% |
Bolded entries indicate p<0.05 by F-test (* p<0.05; *** p<0.001).
Abbreviations: MSEL: Mullen Scales of Early Learning; MSEL-VR: Mullen Scales Visual Reception T-score; MSEL-FM: Mullen Scales Fine Motor T-score
Univariate evaluation of effects of covariates is summarized in Table 4. There were gender effects in MSEL-EL subscores (estimated T-score 7.0 points greater in females than males; p=0.024) but not MSEL-RL subscores. Estimated household income predicted MSEL-EL and MSEL-RL deficits. pH and base excess did not predict MSEL-EL, MSEL-RL, or MB-PROD. Modified Sarnat scores did not predict deficits. Binarized normal vs. abnormal MRI (NICHD score > 0) showed significant effects on both expressive language (estimated difference in T-scores 7.0 points; p=0.038) and receptive language (estimated difference in T-scores 10.1 points; p=0.0061) subscores.
Table 4: Effects of NICU covariates.
Bolded entries indicate p<0.05 for significant between-group differences. T-scores are normed against typical validation groups with mean scores of 50 and a standard deviation of 10.
| Estimated group difference |
95% Confidence interval |
p-value | |
|---|---|---|---|
| Mullens ELt F-M | +7.0 | [1.0,14.0] | 0.024 |
| M-B Production Percentile F-M | +15.0 | [−9.5,35.0] | 0.063 |
| Mullens RLt F-M | +4.0 | [−3.0,11.0] | 0.32 |
| Mullens ELt ModSarnat 2- ModSarnat 3 | +4.0 | [−3.0,12.0] | 0.23 |
| M-B Production Percentile ModSarnat 2- ModSarnat 3 | +0.0 | [−19.0,25.0] | 0.85 |
| Mullens RLt ModSarnat 2- ModSarnat 3 | +3.0 | [−5.0,11.0] | 0.42 |
| Mullens ELt Nl-Abnl MRI | +7.0 | [0.0,14.0] | 0.038 |
| Mullens RLt Nl-Abnl MRI | +10.1 | [3.0,19.0] | 0.0061 |
| Mullens VRt Nl-Abnl MRI | +7.0 | [−2.0,15.0] | 0.10 |
| Mullens FMt Nl-Abnl MRI | +1.0 | [−6.0,9.0] | 0.89 |
| MB ProdPerc Nl-Abnl MRI | +15.0 | [0.0,34.0] | 0.070 |
| MB M3LPerc Nl-Abnl MRI | +11.6 | [−10.0,30.0] | 0.28 |
| Estimated Kendall’s rank correlation coefficient (τ) | p-value | ||
| Mullens ELt vs. Estimated Income | 0.305 | 0.0035 | |
| M-B Production Percentile vs. Estimated Income | 0.180 | 0.15 | |
| Mullens RLt vs. Estimated Income | 0.250 | 0.017 | |
| Mullens ELt vs. pH | −0.045 | 0.67 | |
| M-B Production Percentile vs. pH | 0.10 | 0.43 | |
| Mullens RLt vs. pH | −0.0010 | 0.99 | |
| Mullens ELt vs. BE | 0.11 | 0.33 | |
| M-B Production Percentile vs. BE | −0.027 | 0.84 | |
| Mullens RLt vs. BE | 0.091 | 0.40 | |
Abbreviations: ELt: Mullen expressive language T-score; RLt: Mullen receptive language T-score; M-B: MacArthur-Bates Communicative Development Inventories; M3L: MacArthur-Bates mean of 3 longest utterance percentile; ModSarnat: Modified Sarnat score; BE: Base excess
In the 2-factor anatomical injury model (Figure 2, Table 5), only presence of cortical/subcortical lesions showed significant effects on receptive language subscores (p=0.0023), but neither showed significant effects on expressive language or visual reception subscores. There was not a significant interaction effect between the two factors. This model explained a total of 28.4% of variance in MSEL-RL as compared to 17.8% of variance in MSEL-EL and 10.6% of variance in MSEL-VR. The same model also explained a total of 10.9% of variance in MB-PROD and 18.9% of the variance in MB-M3L -- in the case of MB-M3L, it was predominantly the interaction term (presence of both cortical/subcortical and basal ganglia/thalamic lesions) that predicted deficits (p=0.036).
Figure 2: Assessment outcomes by modified NICHD MRI grade.
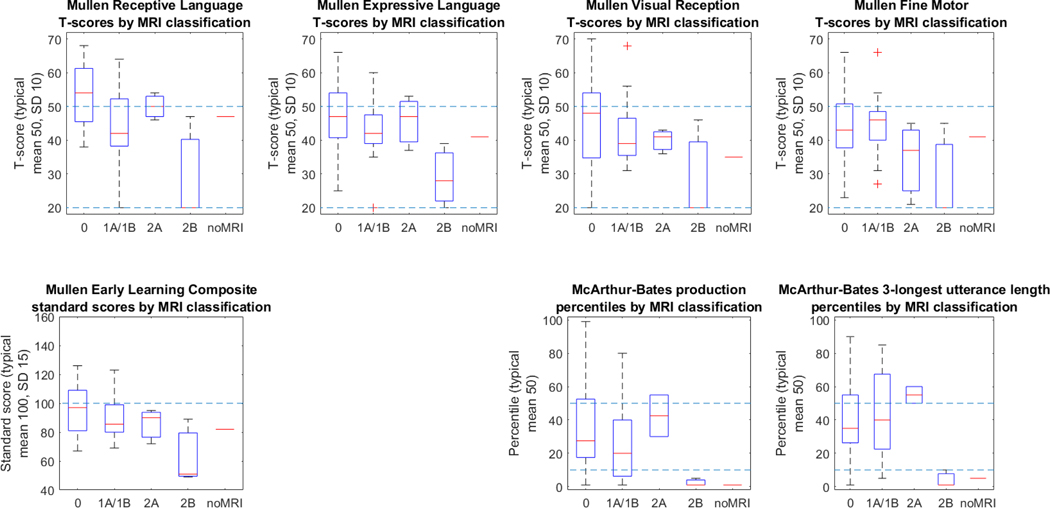
NICHD MRI grade reflects both the degree and gross distribution of brain injury on conventional MRI. Grades include categories for normal MRI (0), cortical/subcortical involvement only (1A (minimal cerebral lesions) and 1B (more extensive cerebral lesions), basal ganglia/thalamic/deep white matter involvement only (2A (basal ganglia, thalamic, internal capsule lesions only), or involvement of both areas (2B (basal ganglia, thalamic, internal capsule, and cerebral lesions) and 3 (cerebral devastation)). T-scores are normed against typical validation groups with mean scores of 50 (higher dotted line) and a standard deviation of 10; a T-score of 20 represents the floor of the instrument for individual subscores (lower dotted line).
Table 5: Outcomes by pattern of injury on MRI.
Table entries indicate percent of variance of each outcome measure (columns) by each predictor (row) explained within a 2-factor ANOVA based on NICHD MRI scoring. Grades 1A/1B represent presence of cortical/subcortical injury only; grade 2A represents presence of basal ganglia/thalamic injury only, and grades 2B/3 represent presence of both types of injury. The last row represents the interaction term between the two factors as a predictor.
| Predictor | MSEL Expressive language t- score |
MSEL Receptive language t- score |
MSEL Visual reception t-score |
MB Production Percentile |
MB 3- longest utterance length |
|---|---|---|---|---|---|
| Presence of cortical/subcortical injury | 8.1% | 18.5%** | 3.3% | 4.3% | 1.3% |
| Presence of basal ganglia/ thalamic injury | 5.7% | 6.5% | 6.3% | 1.6% | 3.4% |
| (Cortical/ subcortical injury) × (basal ganglia/ thalamic injury) interaction effect | 4.0% | 3.5% | 1.1% | 4.9% | 14.1%* |
| Total explained variance | 17.8% | 28.4% | 10.6% | 10.9% | 18.9% |
Bolded entries indicate p<0.05 by F-test (* p<0.05; ** p<0.01).
Abbreviations: MSEL: Mullen Scales of Early Learning; MB: MacArthur-Bates Communicative Development Inventories
In the 4-factor ANOVA model (Table 6), total explained variance ranged between 26.3% (MB-PROD) to 41.4% (MSEL-RL). Estimated household income was the most consistent predictor with significant impacts on MSEL-EL (p=0.0068), MSEL-RL (p=0.014), and MSEL-VR (p=0.0047). Gender also contributed significantly to MSEL-EL (p=0.027), and MRI-CORT also contributed significantly to MSEL-RL (p=0.010).
Table 6: 4-factor ANOVA including all significant covariates.
Table entries indicate percent of variance of each outcome measure (columns) by each predictor (row) explained within the 4-factor model. Rows with multiple predictors listed separated by colons represent interaction term predictors.
| MSEL-EL | MSEL-RL | MSEL-VR | MB-PROD | MB-M3L | |
|---|---|---|---|---|---|
| MRIcort | 4.0% | 13.7%** | 0.9% | 0.4% | 0.1% |
| MRIbgt | 1.0% | 2.1% | 2.6% | 0.0% | 0.1% |
| Gender | 10.5%* | 1.1% | 4.4% | 9.6% | 0.7% |
| TractEstIncome | 16.4%** | 12.5%* | 19.1%* | 1.0% | 5.4% |
| MRIbgt:Gender | 1.1% | 0.9% | 0.0% | 0.0% | 0.0% |
| MRIbgt:MRIcort | 0.3% | 0.0% | 0.1% | 2.4% | 9.9% |
| Gender:MRIcort | 0.6% | 2.3% | 0.6% | 8.5% | 0.5% |
| MRIbgt:TractEstIncome | 0.7% | 0.9% | 0.6% | 0.2% | 3.4% |
| Gender:TractEstIncome | 0.0% | 0.0% | 4.0% | 0.5% | 0.5% |
| MRIcort:TractEstIncome | 1.1% | 2.4% | 0.0% | 3.0% | 8.0% |
| MRIbgt:Gender:MRIcort | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| MRIbgt:Gender:TractEstIn come | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| MRIbgt:MRIcort:TractEstI ncome | 1.0% | 0.5% | 1.2% | 0.0% | 0.0% |
| Gender:MRIcort:TractEstI ncome | 0.7% | 4.9% | 0.2% | 0.6% | 2.9% |
| MRIbgt:Gender:MRIcort:T ractEstIncome | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Total Explained Variance | 37.4% | 41.4% | 33.8% | 26.3% | 31.5% |
Bolded entries indicate p<0.05 by F-test (* p<0.05; ** p<0.01).
Abbreviations: MSEL-EL: Mullens expressive language T-score; MSEL-RL: Mullens receptive language T-score; MSEL-VR: Mullens visual reception T-score; MB-PROD: MacArthur-Bates production percentile; MB-M3L: MacArthur-Bates mean of 3 longest utterance percentile; MRIbgt: Presence of basal ganglia/thalamic injury on MRI; MRIcort: presence of cortical/subcortical injury on MRI; TractEstIncome: household income as estimated based on home census tract.
5. Discussion/Conclusion
Mean performance on language measures fell within the normal range for all measures, and most individuals performed within the normal range for cognitive and language measures. While receptive language scores on MSEL were not significantly different from existing normative samples, expressive language and visual reception subscales were significantly lower as were composite scores. These findings are consistent with previous studies that as a group, children with perinatal HIE treated with therapeutic hypothermia perform in the average to low-average range on some language measures. This study demonstrates that patterns of strengths and weakness previously demonstrated at age 4–7 years are evident by age two (2,13). Receptive language at age two was most preserved when compared to expressive language and a non-verbal visual reception task. While expressive language measures fell in the average range for the group, mean performance was significantly lower than published normative samples on a performance-based measure (MSEL) indicating a larger number of children than would be predicted with low-average or below average expressive and non-verbal skills. Additionally, parent report of expressive one-word vocabulary on the MacArthur-Bates was consistent with the children’s performance on MSEL with 24% of the study group with scores classified as highrisk. While language performance at age 2 is variable and children with isolated expressive language delays have a good prognosis, early language disability can be an indicator for neurodevelopmental problems and should be monitored in a high risk population such as children with a history of perinatal HIE. There is evidence of good concurrent and predictive validity of parent report measures such as the MacArthur Bates in this age range (25). Further, there is growing evidence that while ‘late talkers’, defined as children between the ages of 18–35 months who acquire language at a slower rate than their typically developing peers, may catch-up in terms of vocabulary, language-based learning difficulties re-emerge as reading and writing difficulties in school age (26–29). Pre-hypothermia outcomes have found these very areas of difficulty in children with perinatal HIE (2,8).
Variation in general cognitive factors and, in the case of expressive language, in motor skills appear to explain a portion of the variation in preschool language skills. However, large portions of variation in language functioning also remain independent of these factors, which highlights the need for serial developmental monitoring across domains in this high-risk population.
As expected, there were intervening gender and environmental effects on language outcomes following perinatal HIE. Girls had better performance than boys on the expressive portions of MSEL, but there were not receptive differences. As the MacArthur-Bates normative percentiles are based on gender and age, gender was already accounted for on these measures. Proxies of socioeconomic status predicted differences in both expressive and receptive portions of the Mullen. This finding is not unexpected given the large body of literature demonstrating socio-economic effects on language production; however it reinforces the idea that additional exposures, especially in low-income households, are important for language learning. This is particularly important in a vulnerable population such as children with a history of HIE and provides some evidence for early intervention.
Consistent with previous studies, while neither modified Sarnat score nor pH/BE independently correlated with 2 year language outcomes, injury as demonstrated on early MRI did relate to outcome. Specifically, children with normal early MRI had significantly higher expressive and receptive language scores than those with any MRI abnormalities. As expected, children with the most extensive injury patterns had lower scores in all measures. Presence of cortical/subcortical lesions showed effects on receptive language subscores, but neither cortical/subcortical nor basal ganglia/thalamic types of injury showed significant effects on expressive language or visual reception subscores.
Limitations
This study was a convenience sample and was not fully powered for multivariate analysis. We did not intentionally select for participants based on injury characteristics or socioeconomic factors, but inclusion only of individuals maintaining research follow-up may have biased our sample.
As with any preschool outcome, caution should be used when interpreting the relationship between performance in any specific domain and later functional outcomes. This is especially true for language, which is rapidly developing at this age.
High-grade injury (by encephalopathy score or MRI) was not equally represented in this sample, and our findings apply most directly to individuals without devastating injury on MRI (NICHD grade 3). That said, the percentage of normal (NICHD grade 0) MRI scores in our cohort was similar to that of prior studies (e.g. 57% vs. 52% in (21)), and data collected reflect on the developmental status of individuals who have sustained a broad spectrum of hypoxic-ischemic brain injury patterns.
Implications
This cohort of children with perinatal HIE treated with therapeutic hypothermia had relatively preserved receptive language skills at age 2. Expressive language and visual reception were in the normal range but significantly lower than normative samples. The MacArthur-Bates has been validated in many populations, and it similarly appears to be appropriate to identify expressive language deficits in preschool children with a history of HIE. While blood gas variables and modified Sarnat score did not predict outcome, only a single subject in our study with a normal MRI demonstrated below-normal expressive language functioning (and none demonstrated below-normal receptive language functioning), allowing for some reassurance about language outcomes at the age of 2 for children with a normal MRI. Further, one of the major determinants of expressive and receptive language was estimated socioeconomic status, suggesting that much remaining risk following HIE treated with therapeutic hypothermia may be modifiable through environmental enrichment and early intervention. While portions of variance in language outcomes appear to be driven by variance in motor and cognitive domains, significant unexplained variance remains. This highlights the need for close monitoring of all developmental domains in this high-risk population.
8.1. Acknowledgement
We acknowledge the members of the Johns Hopkins Neurosciences Intensive Care Nursery whose collaboration has provided a platform for this research. We thank the participants of the 11th Hershey Conference on Developmental Brain Injury for their early feedback on this data. We are particularly grateful to the participants and their families without whom this research would not be possible.
8.4. Funding Sources
Vera Joanna Burton was supported by the NINDS/NIH K12-NS001696 during data collection for this project. Vera Joanna Burton, Gwyn Gerner, Frances Northington were all supported in part by NIH/NICHD 1 R01 HD086058-01A1 during the preparation of this manuscript.
Footnotes
Statement of Ethics
This study was performed following IRB approval. Parents/guardians of subjects provided written informed consent for all study procedures
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329–38. [DOI] [PubMed] [Google Scholar]
- 2.Natarajan G, Pappas A, Shankaran S. Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE). Semin Perinatol. 2016. December;40(8):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza SW, McCartney E, Nolan M, Taylor IG. Hearing, speech, and language in survivors of severe perinatal asphyxia. Arch Dis Child. 1981;56(4):245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson C, Finer N. Term infants with hypoxic-ischemic encephalopathy: outcome at 3.5 years. Dev Med Child Neurol. 1985. August;27(4):473–84. [DOI] [PubMed] [Google Scholar]
- 5.Marlow N, Rose AS, Rands CE, Draper ES. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2005. September;90(5):F380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wechsler D, Tideman E. WPPSI-III: Wechsler Preschool and Primary Scale of Intelligence-third edition : manual. 2005. 164p. [Google Scholar]
- 7.Wechsler D WISC-IV: Wechsler Intelligence Scale for Children, 4th Edition Integrated: Technical and Interpretive Manual. 2004. [Google Scholar]
- 8.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014. July 10;371(2):140–9. [DOI] [PubMed] [Google Scholar]
- 9.Pearson Education. Clinical Evaluation of Language Fundamentals: Technical report: Correlations between the CELF-4 and WISC-4 Integrated. Hämtad från https://imagespearsonclinicalcom/images/resource/techrpts/CELF4_WISC4_TechReport.pdf.2008.
- 10.Stage SA, Abbott RD, Jenkins JR, Berninger VW. Predicting response to early reading intervention from verbal IQ, reading-related language abilities, attention ratings, and verbal IQ—word reading discrepancy: Failure to validate discrepancy method. J Learn Disabil.2003;36(1):24–33. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics. Neonatal Encephalopathy and Neurologic Outcome, Second Edition Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Pediatrics. 2014. May 1;133(5):e1482–8. [DOI] [PubMed] [Google Scholar]
- 12.Allanson ER, Waqar T, White C, Tunçalp Ö, Dickinson JE. Umbilical lactate as a measure of acidosis and predictor of neonatal risk: a systematic review. BJOG. 2017. March;124(4):584–94. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro KA, Kim H, Mandelli ML, Rogers EE, Gano D, Ferriero DM, et al. Early changes in brain structure correlate with language outcomes in children with neonatal encephalopathy. Neuroimage Clin. 2017. June 10;15:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman KJ, Gorno-Tempini ML, Glidden DV, Kramer JH, Miller SP, Barkovich AJ, et al. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009. March;123(3):1025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Washington JA, Craig HK. Performances of At-Risk, African American Preschoolers on the Peabody Picture Vocabulary Test-III. Lang Speech Hear Serv Sch. 1999. January 1;30(1):75–82. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez-Clellen VF (1996). Language diversity: Implications for assessment. In Cole K, Dale P, & Thal D (Eds.), Advances in assessment of communication and language; (pp. 29–56). [Google Scholar]
- 17.Whitehurst GJ (1997). Language processes in context: Language learning in children reared in poverty. In Adamson LB, & Romski MA (Eds.), Communication and language acquisition: Discoveries from atypical development; (pp. 233–265). [Google Scholar]
- 18.Martinez-Biarge M, Diez-Sebastian J, Rutherford MA, Cowan FM. Outcomes after central grey matter injury in term perinatal hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010. November;86( 11):675–82. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew ATM, Diez-Sebastian J, Rutherford MA, et al. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr. 2012. November;161(5):799–807. [DOI] [PubMed] [Google Scholar]
- 20.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976. Oct;33(10):696–705. [DOI] [PubMed] [Google Scholar]
- 21.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005. October 13;353(15):1574–84. [DOI] [PubMed] [Google Scholar]
- 22.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Multiple Risk Factor Intervention Trial (MRFIT) Research Group. ZIP-code-based versus tract-based income measures as long-term risk-adjusted mortality predictors. Am J Epidemiol. 2006. September 15;164(6):586–90. [DOI] [PubMed] [Google Scholar]
- 23.Diez-Roux AV, Kiefe CI, Jacobs DR Jr, Haan M, Jackson SA, Nieto FJ, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001. August;11(6):395–405. [DOI] [PubMed] [Google Scholar]
- 24.Shankaran S, McDonald SA, Laptook AR, Hintz SR, Barnes PD, Das A, et al. Neonatal Magnetic Resonance Imaging Pattern of Brain Injury as a Biomarker of Childhood Outcomes following a Trial of Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy. J Pediatr. 2015. November;167(5):987–93.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman HM, Dale PS, Campbell TF, Colborn DK, Kurs-Lasky M, Rockette HE, et al. Concurrent and predictive validity of parent reports of child language at ages 2 and 3 years. Child Dev. 2005. July;76(4):856–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snowling MJ, Adams JW, Bishop DV, Stothard SE. Educational attainments of school leavers with a preschool history of speech-language impairments. Int J Lang Commun Disord. 2001. April;36(2):173–83. [DOI] [PubMed] [Google Scholar]
- 27.Rescorla L. Language and reading outcomes to age 9 in late-talking toddlers. J Speech Lang Hear Res. 2002. April;45(2):360–71. [DOI] [PubMed] [Google Scholar]
- 28.Rice ML, Taylor CL, Zubrick SR. Language outcomes of 7-year-old children with or without a history of late language emergence at 24 months. J Speech Lang Hear Res. 2008. April;51(2):394–407. [DOI] [PubMed] [Google Scholar]
- 29.Preston JL, Frost SJ, Mencl WE, Fulbright RK, Landi N, Grigorenko E, et al. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain. 2010. August;133(Pt 8):2185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


