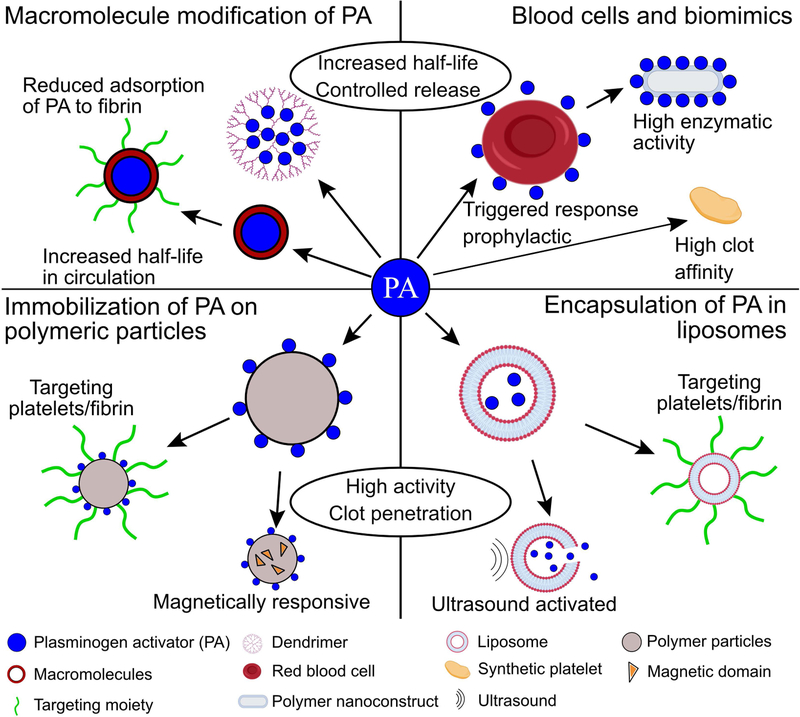
Summary
Fibrinolytic agents including plasmin and plasminogen activators improve outcomes in acute ischemic stroke and thrombosis by recanalizing occluded vessels. In the decades since their introduction into clinical practice, several limitations of have been identified both in terms of efficacy and bleeding risk associated with these agents. Engineered nano- and microparticles address some of these limitations by improving circulation time, reducing inhibition and degradation in circulation, accelerating recanalization, improving targeting to thrombotic occlusions, and reducing off-target effects; however, many particle-based approaches have only been used in pre-clinical studies to date. This review covers four advances in coupling fibrinolytic agents with engineered particles; (1) modifications of plasminogen activators with macromolecules, (2) encapsulation of plasminogen activators and plasmin in polymer and liposomal particles, (3) triggered release of encapsulated fibrinolytic agents and mechanical disruption of clots with ultrasound, and (4) enhancing targeting with magnetic particles and magnetic fields. Technical challenges for the translation of these approaches to the clinic are discussed.
Keywords: Drug delivery systems, fibrinolysis, magnetic fields, plasminogen activators, ultrasonic waves
Introduction
Plasminogen activators (PA) were introduced into clinical practice in the 1980s for indications of myocardial infarction and in the 1990’s for ischemic stroke [1]. Large clinical trials show the benefits of recombinant tissue plasminogen activator (tPA) in acute ischemic stroke [2,3]. During the last 30 years of clinical practice several limitations have arisen including a limited time window (4.5–6 hrs from onset of symptoms), neurotoxicity, and bleeding [4,5]. Over this same time, mechanical thrombectomy devices have emerged as an effective treatment for many types of occlusions in large arteries and veins [6–8]. In some cases, the combination of fibrinolytics and mechanical thrombectomy have been used successfully [9,10]. However, their use is limited to stroke centers that are not always available to individuals outside of major urban areas. Furthermore, mechanical thrombectomy devices cannot reach all types of clots. For example, in the brain, these devices cannot reach the M3/M4 branches where many strokes occur. Taken together, these considerations motivate the need for alternative drug delivery strategies for fibrinolytic agents.
The principle of fibrinolytic therapy is to deliver plasmin or PA to an occlusion to lyse fibrin, thus robbing the thrombus of its mechanical stability, and restoring blood flow in the vessel [11]. Plasmin binds directly to and lyses fibrin, however its efficacy is limited by rapid binding to its endogenous inhibitor α-antiplasmin. PA such as tPA and urokinase plasminogen activator (uPA) act indirectly by first cleaving plasminogen to plasmin, and SK forms a proactivator complex with plasmin(ogen) that converts other plasminogen molecules in the presence of fibrin [12]. Intravenous (IV) delivery provides an elevated systemic concentration of these fibrinolytic agents. PA are more effective in IV delivery than plasmin because their endogenous inhibitors, plasminogen activator inhibitor 1 (PAI-1) and 2 (PAI-2), are found at lower plasma concentrations compared to α-antiplasmin. Alternatively, local delivery to occluded vessels accessible to catheters can overcome some of the effects of these inhibitors with a high local concentration of plasmin or PA.
The most cited complication of fibrinolytic therapy is the risk for iatrogenic bleeding since these agents degrade hemostatic as well as thrombotic clots [13,14]. PA cause bleeding in a small fraction (1–6%) of patients when used to treat ischemic stroke [13], pulmonary embolism [15], or myocardial infarction [16]. Furthermore, it has been shown that tPA can increase the permeability of the blood brain barrier (BBB) while high concentrations of plasmin can damage the BBB, promoting bleeding [17]. Side effects are not the only limitation of PA; IV delivery of PA has a high incidence of failure for certain types of clots because it relies on blood flow, which is restricted or non-existent, to deliver them to the thrombus location [18]. Fibrinolytic therapy can also fail if a patient has high levels of PAI-1 [19] or SK antibody [20], or if the rate of deactivation exceeds the rate of delivery to the clot [21,22]. These limitations have prompted researchers to improve thrombolysis by developing artificial PA that are more enzymatically active [23], using adjuvants that assist PA [24], or inhibiting PAI-1 [25] or FXIIIa [26]. Another approach is to modify plasminogen activators (PA) with macromolecules to protect them from clearance and degradation in blood, thereby improving circulation half-life [27,28]. Further modifications of PA facilitate thrombus targeting via functionalization with moieties that bind to activated platelets or fibrin [29]. However, these approaches offer only marginal improvements over tPA, rely on blood flow for delivery, and still exhibit high risk of bleeding.
A more promising solution is through the use of nano-and microparticles. These can contain immobilized or encapsulated PA, preventing systemic interaction with the BBB, and can be actuated to improve at-site drug delivery. Nano- and microparticle carriers for PA have been developed to improve circulation time, reduce inhibition and degradation, and target occlusive thrombi [30,31]. Many of these are polymeric or liposomal spheres that encapsulate or embed PA. In addition to improving half-life and preventing loss of enzymatic activity in the circulation, these particles can be engineered to release PA at a controlled and prolonged rate [30]. Encapsulation allows delivery not only of PA, but also of plasmin itself by protecting it from inhibition by α2-antiplasmin [32]. Other novel fibrinolytic carriers include PA-functionalized red blood cells with prophylactic effects on thrombosis [33], nanoclusters that release PA in response to increased shear stress in stenosed vessels [34], and activated platelet-stimulated liposomes that release their PA cargo [35].
Though such approaches offer improvements, designing PA carriers that selectively target occlusive thrombi without causing bleeding or reaching small vessels remains a challenge and motivates the need for carriers that can be actuated using an external control mechanism. For example, echogenic liposomes only release a PA payload when exposed to ultrasound [36]. Similarly, magnetically responsive liposomes, microbubbles, and micro- and nanoparticles can be driven to accumulate at the thrombus site using external magnetic fields. This topic has been reviewed in the past with focus on targeting [37] and stage of development [38]. In this review, we highlight engineered micro- and nanoparticle PA carriers with special attention to particles externally actuated by ultrasound or magnetics fields.
Macromolecule modification of plasminogen activators
PA coupling to macromolecules can improve circulation time and protect against endogenous inhibitors (Fig. 1). One of the first examples of this approach was coupling streptokinase (SK) to polyethylene glycol (PEG), a process called PEGylation, which reduces α2-macroglobulin mediated catabolism of SK-plasmin complexes by 50% and extends its half-life from 5 min to 30 min [39]. Additionally, PEGylated SK binding to SK antibodies is reduced by 95%, indicating that this modification reduces the potential immunogenic response to SK [19]. Sakuragawa et al. PEGylated urokinase plasminogen activator (uPA) and showed extended activity over a 6 hr period, compared to a 1 hr period for native uPA, and 6-fold higher antithrombotic capabilities for PEGylated uPA compared to native uPA [40]. Berger and Pizzo showed that PEGylated tPA circulates for ten times longer in mice, rats, and beagles than tPA, though the active half-life is only increased threefold because of inhibition by PAI-1 and PAI-2 [41]. In all of these reports, the fibrinolytic rate of the PEGylated PA is slightly accelerated compared to the non-PEGylated control, likely due to reduced inhibition.
Figure 1.
Overview of engineered particles for the delivery of plasminogen activators (PA) covered in this review with features of different materials, targeting strategies, and actuation.
Molecules other than PEG have been used to increase the circulation time of PA. For instance, tPA has been coupled to albumin via a thrombin cleavable peptide [42]. The activity of the albumin coupled tPA is reduced by 25% but, upon exposure to thrombin, regains activity to 90% of the uncoupled tPA control. Additionally, circulation time is increased to 24 hr with a concentrated burst lasting 30 minutes after exposure to thrombin, and fibrinogenolysis is reduced by half. Another method of improving circulation time is embedding PA into branched synthetic polymers called dendrimers. Wang et al. embedded SK within a poly(amido amine) dendrimer and showed that it retained 80% of its activity and was stable in buffer for three-fold longer than free SK [43]. Fibrinolysis rates were nearly identical for the PA dendrimer and free SK. Other studies using SK in dendrimers report slightly enhanced lysis rates [44] and reduced fibrinogen degradation [45].
Other macromolecule modifications focus on coupling PA to macromolecules that target activated platelets, activated endothelial cells, or fibrin [28]. Bode et al. conjugated uPA to a monoclonal antibody to the integrin αIIbβ3 on platelets [46]. They found that anti-αIIbβ3 antibody modified uPA was drastically better at lysing platelet-rich clots in platelet density dependent manner. Similarly, targeting αIIbβ3 with a peptide attached to staphylokinase (SAK) improved fibrinolysis as well as decreased platelet aggregation relative to SAK without the peptide [47]. For a more detailed review of approaches for targeting platelets, endothelial cells, fibrin and erythrocytes, we refer the reader to the review by Absar et al. [29].
Encapsulation of plasminogen activators in liposomes and polymer particles
While PA modification increases circulation half-life, a primary disadvantage is that it offers moderate (if any) enhancements to fibrinolysis rates. An alternative way to prevent PA degradation and inhibition in circulation while increasing delivery and therefore lysis rates is to encapsulate them within lipid vesicles, liposomes, or polymer matrices (Fig. 1). SK encapsulated in unilamellar phosphatidylcholine liposomes retains 100% of its activity after 30 min incubation in plasma while unencapsulated SK loses more than 50% of its activity [48]. Stability tests indicate that liposomes do not leak SK over a period of 24 hours at body temperature. They release SK through membrane pores during interaction with a thrombus, resulting in similar lysis rates to unencapsulated SK. In another study, tPA encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles coated with the polysaccharide chitosan reduces tPA degradation while reducing lysis time of in vitro whole blood clots by 40% [49]. Both liposome and PEG encapsulated SK outperform free SK in thrombi formed in the carotid artery of rabbits; reperfusion was achieved in 75 min for free SK, 19 min for liposomal SK, and 7 min for PEG encapsulated SK [50]. Similarly, SK encapsulation in distearolphosphatidylcholine with PEG in the lipid bilayer increases half-life by 16-fold and activity by 6-fold in rats [51]. Such encapsulated PA are more effective, in part, because they display reduced adsorption to fibrin relative to non-encapsulated PA, and instead support penetration of PA into the thrombus [50].
To mitigate off-target fibrinolysis, micro- and nanoparticles carrying PA have also been decorated with targeting molecules. Huang et al. modified tPA carrying liposomes with PEG and cyclic arginine-glycine-aspartic acid (cRGD), which is found on the γ chains of fibrin and binds to activated αIIbβ3 [35]. The cRGD supports fusion between the liposomes and activated platelets, causing the liposomes to destabilize and release 90% of their tPA payload within 1 hr of interaction. Liposomes without cRGD motifs or those not exposed to activated platelets only release 10% of their tPA after 6 hr, indicating that tPA release is platelet-sensitive and targeted. Thrombolytic activity of the cRGD coated tPA-liposomes is equivalent to free tPA and three-fold faster than liposomes not triggered by platelets using cRGD. While there is no enhancement to lysis rate over the soluble drug, there are three advantages of cRGD PEGylated liposomes. First, liposomal encapsulation of tPA protects the drug from degradation in plasma. Second, PEGylation of tPA-liposomes make them less susceptible to unwanted destabilization. Finally, the cRGD motifs ensure that large payloads of tPA are only released in the presence of activated platelets, preventing systemic action of tPA. Nonetheless, liposomal delivery of PA has some disadvantages. Like free PA, liposomes rely on blood flow, which may be reduced or absent in occluded vessels, for delivery to a thrombus. However, because liposomes have larger size, their diffusion in occluded channels will be much slower, increasing the delay between injection and the initiation of fibrinolysis.
Another approach is to target the biophysical environment of thrombosis with pre-circulating nanoconstructs. While not useful for treating fully occluded vessels, this approach can be used prophylactically to prevent thrombus formation or to treat stenosed vessels. Korin et al. fabricated shear-activated nanotherapeutics (SA-NT) using nanoparticle aggregates formed from concentrated solutions of PLGA nanoparticles carrying immobilized tPA [34]. These 1–5 μm aggregates are stable up to shear stresses of ~100 dyn/cm2 and disassemble into their constituent nanoparticles at higher shear stresses, such as those experienced in stenotic or obstructed vessels. In a microfluidic model of stenosis with peak shear stress of 450 dyn/cm2, sixteen-fold more nanoparticles released from the SA-NT than in the straight part of the channel with shear stress of 30 dyn/cm2. Thrombi were lysed within 5 min of SA-NT administration in the ferric chloride thrombosis model in murine mesenteric arteries. This approach also shows promise as a prophylactic antithrombotic as pre-circulating SA-NT delayed time to full occlusion three-fold relative to control mice. In a murine pulmonary embolism model, the SA-NT conferred a survival advantage; 100% of control mice died while 80% of mice receiving SA-NT survived the embolism with >60% reperfusion after 45 min. Another advantage of SA-NT is that tPA-nanoparticles selectively concentrate near occlusions in regions of high shear stress, potentially reducing off-target side effects. However, as SA-NT are primarily useful as prophylactics, they would need to exist in the circulation prior to thrombus formation.
Coupling plasminogen activators to blood cells and blood cell mimics
Another targeting and triggered-release strategy relies on imitating or hijacking platelets or red blood cells (RBC) (Fig. 1). Pawlowski et al. designed platelet microparticle-inspired nanovesicles (PMIN) carrying SK that interact with platelets via ligands for αIIbβ3 and P-selectin [52]. Once the PMIN interacted with platelets, phospholipase-A2, an enzyme that is upregulated in sclerotic arteries [53], destabilizes the PMIN and triggers release of SK. In the ferric chloride thrombosis model in the murine carotid artery the SK-PMIN had antithrombotic properties indistinguishable from an equal dose of free SK. In a murine tail bleeding model the bleeding times for mice treated with SK-PMIN is equivalent to those for untreated controls, while bleeding times for mice receiving free SK was three-fold higher [52]. Unlike free SK, these SK-PMIN do not initiate systemic fibrinogenolysis.
PA can be bound directly to blood cells as a targeted therapeutic strategy. For example, tPA has been coupled to biotinylated red blood cells (RBC) to form functionalized RBC (tPA-RBC) [54]. In this case, tPA-RBC are an example of antithrombotic particles that are entrained in thrombi during their formation and either initiate or enhance fibrinolysis. Their mechanism is prophylactic rather than after a thrombus has formed. In the ferric chloride thrombosis model of the murine carotid artery, tPA-RBC restored blood flow in 20–30 min, where unbound tPA failed to reperfuse the vessel. tPA-RBC were also combined with traditional PA delivery to accelerate fibrinolysis by creating large (>20 µm) pores within the thrombus to promote tPA penetration [55]. Prophylaxis however requires particles to be present in the blood prior to an unpredictable event and therefore are likely most useful in individuals at high risk for thrombosis. See Greineder et al. for further discussion of antithrombotic drug delivery approaches [28].
Rather than using blood cells, Colasuonno et al. synthesized discoidal polymeric nanoconstructs (DPN) mimetics shaped like RBC and functionalized with tPA (tPA-DPN) [56]. DPN are 1 μm in diameter, 0.4 μm in height, biconcave, and can be synthesized from either PEG or PLGA. tPA-DPN have a fibrinolytic activity roughly 50% higher than free tPA at 10% the free tPA concentration. In thrombi formed in the the ferric chloride thrombosis model in murine mesentery venules, 0.1 mg/kg of tPA-DPN recanalizes 70% of thrombi in under 60 min, whereas 1 mg/kg of free tPA only recanalizes 40% thrombi after 90 min. Importantly, tPA associated to DPN is also protected from degradation by PAI-1 for longer than 3 hr in plasma, where free tPA is degraded to 30% activity.
Synthetic platelets have been developed as hemostats [57–60], but also for thrombolysis [61]. In this, polystyrene (PS) spheres are stretched into discoids and used as templates for the growth of cross-linked actin layers. The PS is solvated by a tetrahydrofuran and isopropanol mixture, and the remaining protein structure retains the size, shape and flexibility of platelets. Finally, the protein scaffolding is functionalized with the A1 domain of von Willebrand factor or the amino terminal domain of GPIbα to bind to platelet-rich thrombi. Thrombi formed in ex vivo whole blood perfusion over collagen at concentrations of 1.5–4×105 particles/µL comprised between 40–80% of the thrombus, while spheres functionalized with the same moieties only comprised between 5% and 10% of the volume. These data indicate that both physical and biochemical properties can be exploited to enhance thrombus targeting, and SP functionalized with PA could be a potent targeted thrombolytic.
Ultrasound mediated lysis and release
Echogenic Liposomes
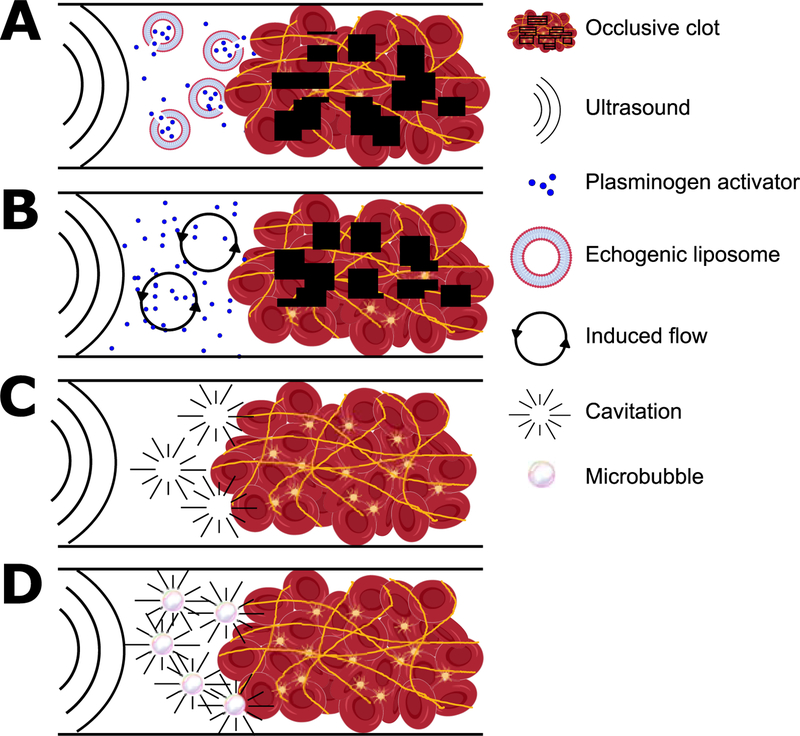
Instead of relying on systemic circulation, biochemical targeting, or blood flow induced mechanical forces to achieve local thrombolysis, external forces can trigger the release of encapsulated tPA from liposomes (Fig. 1, Fig. 2A). Echogenic liposomes (ELIP) release payloads on demand with application of sonic forces [62]. Shaw et al. used thrombi formed from whole blood ex vivo to test ELIP containing tPA (tPA-ELIP) with 120 kHz ultrasound. tPA-ELIP in the presence of ultrasound lysed thrombi four-fold faster than tPA alone [63]. The efficacy of tPA-ELIP was also shown in a murine model of thrombosis that uses denudation of the aorta followed by injection of 5% sodium ricinoleate and thrombin [64]. Blood flow was restored twice as fast by tPA-ELIP than empty ELIP in the presence of tPA and ultrasound at 5.7 MHz, and tPA-ELIP in the presence of ultrasound was twice as fast compared to no ultrasound. ELIP has also been used to deliver plasmin [32]. Kandadai et al. used plasmin-loaded ELIP to lyse blood clots formed from human whole blood in vitro, and reported that plasmin-ELIP exposed to 120 kHz ultrasound with 1.7 MHz pulses lysed clots at 15% faster rates than free tPA.
Figure 2.
Mechanisms of assisting thrombolysis using ultrasound. A) Ultrasound forces cause ELIP to deliver a payload of PA near a thrombus. B) Sonic forces cause acoustic streaming that induces flow and improves transport of PA to a thrombus. C) Sonic forces cause cavitation, which imparts mechanical energy into the thrombus and initiates degradation even in the absence of PA. D) Microbubbles increase the frequency of cavitation and accelerate lysis. See Table 1 for details and references.
Sonothrombolysis
Sonothrombolysis refers to methods using sonic energy to directly or indirectly degrade thrombi. For the interested reader, Bader et al. reviews the mechanisms of sonothrombolysis [36]. Here we focus on micro- and nanoparticles that enhance the these mechanisms: acoustic streaming, cavitation, and ultrasound-induced temperature rise. Acoustic streaming describes the use of acoustic radiation forces to create flow, which at the site of a thrombus helps overcome the slow, diffusion-mediated transport of PA in occluded vessels (Fig. 2B) [65]. Cavitation directly degrades thrombi with mechanical energy from bubble formation, oscillation, and collapse (Fig. 2C) [66]. Ultrasound can cause local temperature to increase by up to 5 °C and thereby accelerate fibrinolysis; however, when the contributions of each mechanism are decoupled, acoustic streaming and cavitation contribute more significantly to fibrinolytic enhancements than heating directly [67].
Sonothrombolysis is enhanced in the presence of microbubbles (Fig. 2D), gas-filled vesicles typically less than 8 µm in diameter [68]. Microbubbles are used as an ultrasound contrast agent because they scatter sonic waves more than blood, but they have recently garnered attention as agents for drug delivery and gene therapy [68]. Microbubbles can be stabilized with silanes, surfactants, protein shells, polymer coatings, or lipids. Bader et al. investigated the mechanism for microbubble-assisted sonothrombolysis using 50 µm octofluoropropane bubbles stabilized with a lipid monolayer [69]. They concluded that the oscillations and coalescence of microbubbles with a resonant frequency close to the frequency of ultrasound exposure were the primary contributors to lysis during exposure to sonic forces. They also observed more sustained cavitation over 50 s periods in the presence of microbubbles which further contributes to enhanced thrombolysis.
A series of clinical trials show the potential for sonothrombolysis in treatment of ischemic stroke of major cerebral arteries (Table 1). One trial found that ultrasound therapy in the presence microbubbles reduced reperfusion times to less than 20 min, while achieving full recanalization in 71% of patients within 2 hr [70]. By comparison, full recanalization was achieved in only 39% of patients receiving just tPA. There was no increased risk of intracranial hemorrhage due to the microbubbles compared to patients receiving only tPA. In another trial, sonothrombolysis accelerated reperfusion but increased risk of hemorrhage [71]. Out of 35 patients, risk of intracerebral hemorrhage was directly related to the dosage of microbubble infusion. Patients receiving a 1.4 mL dose of microspheres over a 90 min period experienced recanalization in half the time of control patients and did not experience an increase in hemorrhagic events; however, 27% of patients who received a double dose of microspheres experienced hemorrhage without a decrease in reperfusion time. A similar study validated these results [72]. The data from these studies indicate that the effects of microbubble size, composition, viscoelasticity and ultrasound frequency on microbubble-assisted sonothrombolysis merit further investigation.
Table 1.
Clinical studies using microbubble-assisted sonothrombolysis.
| Vessel | Number of patients | Initial NIHSS score (Range) | 3-month favorable outcome | Ultrasound frequency | Gas | Microbubble size | Microbubble stabilizing agent | Ref. |
|---|---|---|---|---|---|---|---|---|
| MCA | 15 | 17 (6–28) | 40% | 2 MHz | C3F8 | 1–2 µm | Lipids | [72] |
| MCA | 111 | 18 (15–19) | 56% | 2 MHz | Air | 2–8 µm | Galactose | [70] |
| MCA, ACA or PCA | 35 | 18 (9–21) | 72% | 2 MHz | C3F8 | 1–2 µm | Lipids | [71] |
| MCA | 138 | 17 (12–20) | 46% | 300 kHz | Air or SF6 | 2–8 µm (air) or 1.5–4.5 µm | Galactose (air) or Lipids | [99] |
National Institute of Health Stroke Scale (NIHSS) rates the severity of an occlusion. A higher score indicates a more severe clot with NIHSS > 4 requiring treatment. A 3-month favorable outcome is characterized by a decrease in the NIHSS score to 0–3. MCA, middle cerebral artery; ACA, anterior cerebral artery; PCA, posterior cerebral artery.
Magnetic particles and magnetic field control
Ultrasound waves attenuate in tissues, and thrombolytic methods that use ultrasound may be ineffective in vessels away from the body’s surface or not easily accessible by surgical intervention. Moreover, both ultrasound mediated drug release and microbubble enhanced sonothrombolysis rely on local catheter delivery or circulation to bring particles near a thrombus—a disadvantage shared by all approaches discussed heretofore. This could be challenging for occlusions in small vessels where catheters cannot reach. Magnetic fields overcome these limitations as they do not attenuate in tissue for frequencies <30 Mhz [73]. They can also be used to concentrate PA-laden magnetic particles at the thrombus periphery [74]. Magnetic nanoparticles (MNP) used for fibrinolysis are made from <30 nm superparamagnetic iron oxide crystals of magnetite (Fe3O4) or maghemite (γ-Fe2O3), FDA approved materials [75,76] with low toxicity in humans [77], embedded in a polymer matrix [75]. The small, randomly distributed domains allow the MNP to orient themselves in the direction of a magnetic field at all times with an induced dipole. Though magnetically guided particles are a promising direction for thrombolytic research, these approaches have only been used in preclinical studies to date.
Synthesis and functionalization of magnetic nanoparticles carriers
As in other fibrinolytic particles discussed above, encapsulating or immobilizing PA on MNP increases enzyme stability during storage [78] and improves half-life [79]. MNP carrying tPA, uPA, or SK have been decorated with coatings that further improve half-life and reduce immunogenicity including dextran [80,81], chitosan [82], silica [79,83], hydrosol [84], PLGA and PLA-PEG [85], polyacrylic acid (PAA) [86], polyethylene glycol (PEG) [87,88], poly[aniline-co-N-(1-one-butyric acid) aniline] [78], and heparin [89]. PA functionalization of MNP can rely on either physical adsorption to the MNP matrix or covalent attachment [90]. Adsorption yields high concentrations of tPA (> 20 μg/mL) near a thrombus [85]; however, PA desorbs from PLGA, PAA, and uncoated magnetite rods within 30 min of injection [85,90,91]. Premature release of PA before particles reach their target is problematic and compounded by the short half-life (5 min) of recombinant tPA in blood [92].
Limitations related to timing of PA release motivate the need for magnetic carriers with triggered release and alternative functionalization methods. Drozdov et al. developed a magnetite composite material that incorporates uPA that does not leach, enabling magnetically responsive drug carriers to have prolonged activity for several hours [84]. In this, negatively charged plasminogen interacts with a positively charged particle matrix containing uPA. Once converted, positively charged plasmin is repelled out of the MNP. A covalent bonding strategy is functionalization of PAA particle surfaces using N-hydroxysuccinimide followed by coupling with tPA [90]. Alternatively, streptavidin-functionalized particles can be conjugated to biotinylated tPA [74]. Such immobilization of PA protects the enzyme from inhibition by PAI-1 and degradation in the liver.
Magnetic field control of magnetic particles
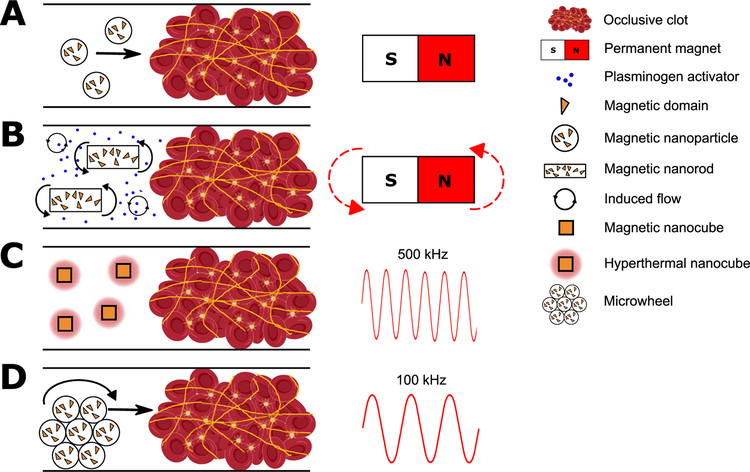
The simplest form of magnetic control is to use a permanent magnet to establish a magnetic field gradient to move MNP towards a target (Fig. 3A, Table 2). This is the most widely reported method for manipulating PA carriers [79,81,87,88,91]. Ma et al. used PAA particles conjugated with tPA to show recanalization of the iliac artery in rats and demonstrated reperfusion using only 20% of the therapeutic tPA concentration (1 mg/kg) [86]. Experiments in rat and mouse models also use a moving permanent magnet to drag MPN from the drug injection site to the thrombus [12,14,19]. Magnetic field gradients are difficult to scale to humans as the strength of a magnetic gradient force decays with the inverse square of the distance (1/r2) from the magnet. As an example, a 1.5 T magnet is capable of creating a 40 mT/m gradient in the carotid artery of pigs, which is sufficient to propel a 1.5 mm sphere at a velocity of 13 mm/s, but a 5 µm sphere only 1 µm/s [46]. The tortuosity of the human vasculature also renders a unidirectional field gradient potentially ineffective as MPN must navigate through vessels in varying orientations.
Figure 3.
Methods of magnetic control of magnetic nanoparticles (MNP). A) MNP move down a magnetic field gradient toward a clot using a permanent magnet. B) A rotating magnet is used to rotate rod-like MNP, inducing convection and improving transport of PA to a clot. C) A high frequency magnetic field induces local heating of cube-like MNP. D) A low frequency rotating magnetic field causes MNP to assemble into microwheels and roll into a thrombus. See Table 2 for details and references.
Table 2.
Summary of preclinical studies for MNP targeting and actuation.
| PA | Particle matrix | Magnetism | Iron oxide loading | Magnetic guidance | Type of experiment | Lysis rate relative to soluble PA | Ref. |
|---|---|---|---|---|---|---|---|
| tPA | PLGA | SP | 5 wt% | None | None | Not Compared | [85] |
| uPA | PEG | P | 0.54 wt% | PM | In vitro | 1.8 | [87] |
| uPA | PEG | P | 2 wt% | PM | In vitro | 3.7 | [88] |
| tPA | PLA-PEG | SP | Unmedicated | Translating PM | In vitro | 2.7 | [93] |
| tPA | Silica | SP | 9.4 wt% | PM | In vitro | 1.7 | [83] |
| tPA-SK | Silica | SP | 0.2 mg/mL | PM | In vitro | 2.2 | [79] |
| uPA | Hydrosol | SP | 1 wt% | PM | In vitro | 3.0 | [84] |
| tPA | None | SP | 6 wt% | Rotating PM | In vitro | 1.4 | [91] |
| tPA | Dextran | SP | 0.25 mg/mL | PM | In vitro | Not Compared | [81] |
| tPA | PAA | SP | 8 wt% | Translating PM | Rat, iliac artery embolism | >1.6 | [86] |
| uPA | Dextran | SP | 20000 IU/mL | PM | Rat, arteriovenous shunt | 5.0 | [80] |
| tPA | Chitosan | SP | 9.5 wt % | Translating PM | Rat, iliac artery embolism | 2.1 | [82] |
| tPA | Poly aniline | SP | 27.6 wt% | PM | Rat, iliac artery embolism | 6.5 | [78] |
| uPA | Heparin | SP | 8 wt% | PM | Rat and rabbit, carotid | 20.8 | [89] |
| tPA | Ferrolipids | SP | Unmedicated | PM | In vitro | ~20 | [97] |
| tPA | Aluminum | M | Unmedicated | Rotating PM | In vitro | 3.3 | [95] |
| tPA | Nickel | P | Unmedicated | Rotating PM | In vitro | 1.8 | [94] |
| tPA | BSA | SP | ~0.5 mg/mL | AC Field | Mouse, mesentery vasculature | ~1000 | [96] |
| tPA | Polystyrene | SP | 3.6 ug/ mL | Helmholtz coils | In vitro | 3.3 | [74] |
PA, plasminogen activator; PLGA, poly-(lactide-co-glycolic) acid; PEG, polyethylene glycol; PLA-PEG, poly(D,L-lactide)-co-poly(ethylene glycol); PAA, polyacrylic acid; BSA, bovine serum albumin; SP, superparamagnetic; P, paramagnetic; M, permanently magnetic; PM, permanent magnet; AC, alternating current.
Magnetic field induced mixing can enhance fibrinolysis by free PA (Fig. 3B, Table 2). Torno et al. used a permanent magnet to move magnetic microspheres back and forth, inducing a flow field near a bolus of tPA [93]. Mixing doubled the thrombolytic efficiency by reducing concentration gradients and, when combined with 20 kHz ultrasound, accelerated lysis three-fold over no mixing or ultrasound. A similar mixing strategy was reported by Huang et al. where rotating permanent magnets made rod-shaped particles spin [94]. In an embolic rat model, they reported that mixing doubled tPA-mediated lysis. Khalil showed thrombi removal using a ~1 mm helical robot made to abrade a thrombus via external magnetic control [95]. This purely mechanical mechanism removed a clot at three times the rate of the clinical dose of SK and may provide a less invasive alternative to catheters. MNP can also be heated with applied magnetic fields. For example, Voros et al. immobilized tPA on nanocubes, particles with cubic rather than spherical geometry, and subjected them to 500 kHz radio frequency (RF) fields to locally raise the temperature to 42 °C (Fig. 3C) [96]. Here, fibrin dissolution occurred an order-of-magnitude faster at 42 °C compared to 37 °C in vitro. In a ferric chloride thrombosis model in murine mesentery vessels, tPA nanocubes lysed clots in <1 min compared to 5–10 minutes in the absence of heating. This enhancement is greater than predicted based on the temperature-dependence of lysis kinetics and is also attributed to faster tPA release from the nanocubes [96].
Some of the most novel MNP strategies initiate thrombolysis through both chemical and mechanical mechanisms (Table 2). De Saint Victor et al. made 0.2–15 µm microbubbles containing air and 10 nm magnetite particles stabilized by phospholipids [97]. The mechanical energy from ultrasound cavitation combined with the biochemical action of free tPA degraded whole blood thrombi 2.5-fold faster than free tPA alone. Magnetic targeting provided further enhancement; retention of a high local concentration of magnetic microbubbles using a permanent magnet resulted in a continuous supply of high cavitation energy. The cavitation energy was itself enhanced by a large concentration of microbubbles resulting from magnetic focusing. This magnetically mediated sonothrombolysis degraded clots roughly twenty-fold faster than soluble tPA alone, ten-fold faster than ultrasound alone, and four-fold faster than tPA and ultrasound in the presence of microbubbles but in the absence of magnetic focusing.
Tasci et al. demonstrated enhanced fibrinolysis using a combination of biochemical and mechanical action using magnetically powered microwheels assembled in situ [74]. Biotinylated tPA was immobilized on 1 μm streptavidin-coated MNP. The microwheels rolled to the interface of a plasma clot (Fig. 3D) and accumulated tPA at this interface at a concentration 50 times higher than the injected concentration. Once at the interface, a corkscrew-like motion was used to drive the microwheels into the clot yielding a lysis rate six-fold faster than tPA alone. This approach relies on low strength (~10 mT), rotating magnetic fields rather than field gradients which is easier to scale-up to humans. The microwheels assemble to structures greater than 10 μm in diameter, which is within the detection limits of MRI [98], but disassemble into 1 μm spheres upon removal of the magnetic field, making them passable through capillaries.
Outlook
Table 3 provides a summary of the advantages and disadvantages of the different approaches to engineered fibrinolytic particles reviewed here. The external actuation of PA particles using ultrasound and magnetic fields is a promising direction, but several challenges must be overcome to translate these approaches into clinical practice. Ultrasound actuation has received positive results in small clinical trials but further characterization is needed on microbubble dosage [71], size, and composition, especially at lower frequencies [99]. Most studies of magnetic field actuation to date have been in preclinical models. A promising feature of magnetic approaches is the ability to both localize and concentrate fibrinolytic particles near a thrombus, reducing the effective circulating concentration of PA, helping to minimize side effects, and potentially broadening the indications for PA therapy. The scale-up of the hardware required for magnetic control in humans will have to overcome several technical obstacles: (i) the generation of sufficient magnetic forces in deep tissues, (ii) particle navigation in the complex, three-dimensional vasculature, (iii) combining actuation with imaging, and (iv) achieving translation across or against flowing blood. Nonetheless, the development of fibrinolytic particles has produced encouraging results. Some of the most recent work has made it clear that combining PA encapsulation with magnetic localization, ultrasound, and hyperthermia may produce lysis rates up to an order-of-magnitude faster than those achievable using free PA. Engineered particles also allow for the delivery of two or more agents, which could be exploited to couple PA with other emerging thrombolytics targeted to von Willebrand factor [100–102], neutrophil extracellular traps [103], and thrombin-activatable fibrinolysis inhibitor (TAFI) and PAI-1 [104]; alternatively, PA could be delivered alongside adjuvants that enhance PA activity [24], PAI-1 inhibitors [25], or FXIIIa inhibitors to accelerate thrombolysis [26]
Table 3.
Summary of approaches to engineered fibrinolytic macromolecules and particles
| Method | Advantages | Disadvantages |
|---|---|---|
| Macromolecules | ||
| Polymer coatings | Increased half-life | Reduced activity |
| FDA approved materials available | Low specificity | |
| Low mobility in occluded vessels | ||
| Dendrimers | Increased half-life | Low specificity |
| Reduced adsorption | Low mobility in occluded vessels | |
| Variable loading | ||
| Modified blood cells and cell mimics | ||
| Targeting | Targets components of thrombi | Low mobility in occluded vessels |
| Reduced bleeding risk | ||
| Prophylaxis | Preventive Triggered release |
Requires presence in blood before vessel occlusion |
| Degrades clot from inside out | Complicated preparation/synthesis | |
| Shear-activated release | Reduced bleeding risk | No shear in fully occluded vessel |
| Triggered release | Requires presence in blood before vessel occlusion | |
| Liposomes and polymer particles | ||
| PA Encapsulation | Increased half-life | Low specificity |
| Controlled release | Difficult to control stability | |
| Reduces adsorption to fibrin | Low mobility in occluded vessels | |
| PA Immobilization | Increased half-life | Reduced activity |
| Decreased PA inhibition | Low mobility in occluded vessels | |
| Increased clot penetration | ||
| Used in other clinical applications | ||
| Actuation | ||
| Sonic actuation | Enhanced lysis | Limited mobility in occluded vessels |
| Locally induced flows | Limited targeting/specificity | |
| Successful phase II clinical trials | ||
| Magnetic actuation | Compatible with most engineered particles | Early stages of development (preclinical) |
| High mobility, targeting | Challenging scale-up | |
| Local hyperthermia | ||
| High local concentration | ||
Acknowledgments
The authors acknowledge support from the National Institutes of Health under grants R21AI138214 and R01NS102465. D.D. was supported by an American Heart Association Predoctoral Fellowship Award 18PRE34070076. Figure icons created with Biorender.com.
Footnotes
Disclosure of Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Marder VJ. Historical perspective and future direction of thrombolysis research: The re-discovery of plasmin. J Thromb Haemost 2011; 9: 364–73. [DOI] [PubMed] [Google Scholar]
- 2.Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, Innes K, Venables G, Czlonkowska A, Kobayashi A, Ricci S, Murray V, Berge E, Slot KB, Hankey GJ, Correia M, Peeters A, Matz K, Lyrer P, Gubitz G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet Elsevier Ltd; 2012; 379: 2352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, Von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy DE, Brott TG, Haley EC, Marler JR, Sheppard GL, Barsan W, Broderick JP. Factors Related to Intracranial Hematoma Formation in Patients Receiving Tissue-Type Plasminogen Activator for Acute Ischemic Stroke. Stroke 1994; 25: 291–7. [DOI] [PubMed] [Google Scholar]
- 5.Abu R, Nassar T, Yarovoi S, Rayan A, Lamensdorf I, Karakoveski M, Vadim P, Jammal M, Cines DB, Higazi AA. Neuropharmacology Blood e brain barrier permeability and tPA – mediated neurotoxicity. Neuropharmacology Elsevier Ltd; 2010; 58: 972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal M, Menon BK, Eesa M, Barber PA, Morrish WF, Demchuk AM, Kamal NR, Ryckborst KJ, Coutts SB, Smith EE, Subramaniam S, Mitha AP, Wong JH, Sajobi TT, Hill MD, Lowerison MW, Rempel JL, Shuaib A, Roy D, Weill A, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–30. [DOI] [PubMed] [Google Scholar]
- 7.Campbell BCV, Yassi N, Yan B, Oxley TJ, Wu TY, Davis SM, Mitchell PJ, Dowling RJ, Churilov L, Donnan GA, Kleinig TJ, Scroop R, Dewey HM, Brooks M, Simpson MA, Parsons MW, Miteff F, Levi CR, Ang T, Krause M, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–18. [DOI] [PubMed] [Google Scholar]
- 8.Tomasello A, Castaño C, Blasco J, Aja L, Dorado L. Thrombectomy within eight hours after symptom onset in ischemic stroke. Turk Noroloji Derg 2016; 22: 36. [Google Scholar]
- 9.Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N Engl J Med 2015; 372: 2285–95. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Dewey HM, Thijs V, Scroop R, Simpson M, Brooks M, Asadi H, Wu TY, Shah DG, Wijeratne T, Ang T, Miteff F, Levi CR, et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N Engl J Med 2018; 378: 1573–82. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol 2005; 129: 307–21. [DOI] [PubMed] [Google Scholar]
- 12.Nagendra K, Memorial RP, Depart- NYS. of Activation of Human Plasminogen by Streptokinase 1972;.
- 13.Miller DJ, Simpson JR, Silver B. Safety of Thrombolysis in Acute Ischemic Stroke: A Review of Complications, Risk Factors, and Newer Technologies 2011; 1: 138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van De Werf F, Barron HV., Armstrong PW, Granger CB, Berioli S, Barbash G, Pehrsson K, Verheugt FWA, Meyer J, Betriu A, Califf RM, Li X, Fox NL. Incidence and predictors of bleeding events after fibrinolytic therapy with fibrin-specific agents: A comparison of TNK-tPA and rt-PA. Eur Heart J 2001; 22: 2253–61. [DOI] [PubMed] [Google Scholar]
- 15.Daley MJ, Murthy MS, Peterson EJ. Bleeding risk with systemic thrombolytic therapy for pulmonary embolism: Scope of the problem. Ther Adv Drug Saf 2015; 6: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bundhun PK, Janoo G, Chen M-H. Bleeding events associated with fibrinolytic therapy and primary percutaneous coronary intervention in patients with STEMI. Medicine (Baltimore) 2016; 95: e3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niego B, Medcalf RL, Barrier BB. Plasmin-dependent modulation of the blood – brain barrier: a major consideration during tPA-induced thrombolysis? 2014;: 1283–96. [DOI] [PMC free article] [PubMed]
- 18.von Kummer R Early Major Ischemic Changes on Computed Tomography Should Preclude Use of Tissue Plasminogen Activator. Stroke 2003; 34: 820–1. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Han SW, Kim EH, Kim DJ, Lee KY, Kim DI, Heo JH. Plasma Fibrinolysis Inhibitor Levels in Acute Stroke Patients with Thrombolysis Failure. J Clin Neurol 2009; 1: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lew AS, Neer T, Rodriguez L, Geft IL, Shah PK, Ganz W. Clinical failure of streptokinase due to an unsuspected high titer of antistreptokinase antibody. J Am Coll Cardiol 1984; 4: 183–5. [DOI] [PubMed] [Google Scholar]
- 21.Rijken DC, Sakharov D V. Molecular transport during fibrin clot lysis. Fibrinolysis and Proteolysis 2000; 14: 98–113. [Google Scholar]
- 22.Blinc A, Francis CW. Transport Processes in Fibrinolysis and Fibrinolytic Therapy. Thromb Haemost 1996; 76: 481–91. [PubMed] [Google Scholar]
- 23.Keyt BA, Paoni NF, Refino CJ, Berleau L, Nguyen H, Chow A, Lai J, Pena L, Pater C, Ogezt J, Etcheverryt T, Botstein D, Bennetrt WF. A faster-acting and more potent form of tissue plasminogen activator 1994; 91: 3670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frostfeldt G, Ahlberg G, Gustafsson G, Helmius G, Swahn E, Venge P, Wallentin L. Low Molecular Weight Heparin ( Dalteparin ) as Adjuvant Treatment to Thrombolysis in Acute Myocardial Infarction — A Pilot Study: Biochemical Markers in Acute Coronary Syndromes ( BIOMACS II ). J Am Coll Cardiol Elsevier Masson SAS; 1999; 33: 627–33. [DOI] [PubMed] [Google Scholar]
- 25.Levi M, Biemond BJ, Zonneveld A Van. Inhibition of Plasminogen Activator Inhibitor-1 Activity Results in Promotion of Endogenous Thrombolysis and Inhibition of Thrombus Extension in Models of Experimental Thrombosis 1: 305–12. [DOI] [PubMed] [Google Scholar]
- 26.Leidy EM, Stern AM, Friedman PA, Bush LR. Enhanced Thrombolysis by a Factor XIIIa Inhibitor in a Rabbit Model of Femoral Artery Thrombosis. Thromb Res 1990; 59: 15–26. [DOI] [PubMed] [Google Scholar]
- 27.El-Sherbiny IM, Elkholi IE, Yacoub MH. Tissue plasminogen activator-based clot busting: Controlled delivery approaches. Glob Cardiol Sci Pract 2014; 2014: 336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greineder CF, Howard MD, Carnemolla R, Cines DB, Muzykantov VR. Review Article Advanced drug delivery systems for antithrombotic agents. Blood J 2016; 122: 1565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Absar S, Gupta N, Nahar K, Ahsan F. Engineering of plasminogen activators for targeting to thrombus and heightening thrombolytic efficacy. J Thromb Haemost 2015; 13: 1545–56. [DOI] [PubMed] [Google Scholar]
- 30.Vaidya B, Agrawal GP, Vyas SP. Functionalized carriers for the improved delivery of plasminogen activators. Int J Pharm Elsevier B.V; 2012; 424: 1–11. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Feng X, Jin R, Li G. HHS Public Access 2018; 15: 173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kandadai MA, Meunier JM, Hart K, Holland CK, Shaw GJ. Plasmin-Loaded Echogenic Liposomes for Ultrasound-Mediated Thrombolysis. Transl Stroke Res 2014; 6: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganguly K, Krasik T, Medinilla S, Bdeir K, Cines DB, Muzykantov VR, Murciano JC. Blood clearance and activity of erythrocyte-coupled fibrinolytics. The Journal of pharmacology and experimental therapeutics 2005. [DOI] [PubMed]
- 34.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, Feldman CL, Wagner DD, Ingber DE. Shear-Activated Nanotherapeutics for Drug Targeting to Obstructed Blood Vessels. Science (80- ) 2012; 337: 738–42. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Yu L, Ren J, Gu B, Longstaff C, Hughes AD, Thom SA, Xu XY, Chen R. An activated-platelet-sensitive nanocarrier enables targeted delivery of tissue plasminogen activator for effective thrombolytic therapy. J Control Release 2019; 300: 1–12. [DOI] [PubMed] [Google Scholar]
- 36.Bader KB, Bouchoux G, Holland CK. Sonothrombolysis. Adv Exp Med Biol 2016. [DOI] [PMC free article] [PubMed]
- 37.Huang T, Li N, Gao J. Recent strategies on targeted delivery of thrombolytics 2019; 14: 233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamanlu M, Farhoudi M, Eskandani M, Mahmoudi J, Barar J, Rafi M, Omidi Y. Recent advances in targeted delivery of tissue plasminogen activator for enhanced thrombolysis in ischaemic stroke. J Drug Target Informa UK Ltd.; 2018; 0: 95–109. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopalan S, Gonias SL, Pizzo S V. A nonantigenic covalent streptokinase-polyethylene glycol complex with plasminogen activator function. J Clin Invest 1985; 75: 413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakuragawa N, Shimizu K, Kondo K, Kondo S, Niwa M. Studies on the effect of PEG-modified urokinase on coagulation-fibrinolysis using beagles. Thromb Haemost 1986; 41: 627–35. [DOI] [PubMed] [Google Scholar]
- 41.Berger H Jr, Pizzo S V. Preparation of Polyethylene Glycol-Tissue Plasminogen Activator Adducts That Retain Functional Activity: Characteristics and Behavior in Three Animal Species. Blood 1988; 71: 1641–7. [PubMed] [Google Scholar]
- 42.Absar S, Kwon YM, Ahsan F. Bio-responsive delivery of tissue plasminogen activator for localized thrombolysis. J Control Release Elsevier B.V; 2014; 177: 42–50. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Inapagolla R, Kannan S, Lieh-Lai M, Kannan RM. Synthesis, characterization, and in vitro activity of dendrimer- streptokinase conjugates. Bioconjug Chem 2007; 18: 791–9. [DOI] [PubMed] [Google Scholar]
- 44.Ramos Fernandes EG, Alencar De Queiroz AA, Abraham GA, San Román J. Antithrombogenic properties of bioconjugate streptokinase-polyglycerol dendrimers. J Mater Sci Mater Med 2006; 17: 105–11. [DOI] [PubMed] [Google Scholar]
- 45.Mukhametova LI, Aisina RB, Zakharyan EM, Karakhanov EA, Gershkovich KB, Varfolomeyev SD. Thrombolytic and fibrinogenolytic properties of bioconjugate streptokinase-polyamidoamine dendrimers in vitro. Thromb Res 2017; 154: 50–2. [DOI] [PubMed] [Google Scholar]
- 46.Bode C, Meinhardt G, Runge MS, Freitag M, Nordt T, Arens M, Newell JB, Kübler W, Haber E. Platelet-targeted fibrinolysis enhances clot lysis and inhibits platelet aggregation. Circulation 1991; 84: 805–13. [DOI] [PubMed] [Google Scholar]
- 47.Chen H, Mo W, Zhang Y, Su H, Ma J, Yao R, Zhang S, Ge J, Song H. Functional properties of a novel mutant of staphylokinase with platelet-targeted fibrinolysis and antiplatelet aggregation activities. Eur J Pharmacol 2007; 566: 137–44. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen PD, O’Rear EA, Johnson AE, Lu R, Fung BM. Thrombolysis Using Liposomal-Encapsulated Streptokinase: An In Vitro Study. Exp Biol Med 1989; 192: 261–9. [DOI] [PubMed] [Google Scholar]
- 49.Chung TW, Wang SS, Tsai WJ. Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials 2008; 29: 228–37. [DOI] [PubMed] [Google Scholar]
- 50.Leach JK, Patterson E, O’Rear EA. Improving thrombolysis with encapsulated plasminogen activators and clinical relevance to myocardial infarction and stroke. Clin Hemorheol Microcirc 2004; 30: 225–8. [PubMed] [Google Scholar]
- 51.Kim IS, Choi HG, Choi HS, Kim BK, Kim CK. Prolonged systemic delivery of streptokinase using liposome. Arch Pharm Res 1998; 21: 248–52. [DOI] [PubMed] [Google Scholar]
- 52.Pawlowski CL, Li W, Sun M, Ravichandran K, Hickman DS, Kos C, Kaur G, Sen Gupta A. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials Elsevier Ltd; 2017; 128: 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: Biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol 2005; 25: 923–31. [DOI] [PubMed] [Google Scholar]
- 54.Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol 2003; 21: 891–6. [DOI] [PubMed] [Google Scholar]
- 55.Gersh KC, Zaitsev S, Cines DB, Muzykantov V, Weisel JW. Brief report Flow-dependent channel formation in clots by an erythrocyte-bound fibrinolytic agent 2015; 117: 4964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colasuonno M, Palange AL, Aid R, Ferreira M, Mollica H, Palomba R, Emdin M, Del Sette M, Chauvierre C, Letourneur D, Decuzzi P. Erythrocyte-Inspired Discoidal Polymeric Nanoconstructs Carrying Tissue Plasminogen Activator for the Enhanced Lysis of Blood Clots. ACS Nano 2018; 12: 12224–37. [DOI] [PubMed] [Google Scholar]
- 57.Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL, Gupta A Sen. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials Elsevier Ltd; 2013; 34: 3031–41. [DOI] [PubMed] [Google Scholar]
- 58.Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Synthetic Platelets: Nanotechnology to Halt Bleeding. Sci Transl Med 2009; 1: 11ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nandi S, Brown AC. Platelet-mimetic strategies for modulating the wound environment and inflammatory responses. Exp Biol Med 2016; 241: 1138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown AC, Stabenfeldt SE, Ahn B, Hannan RT, Dhada KS, Herman ES, Stefanelli V, Guzzetta N, Alexeev A, Lam WA, Lyon LA, Barker TH. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat Mater 2014; 13: 1108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doshi N, Orje JN, Molins B, Smith JW, Mitragorti S, Ruggeri ZM. Platelet Mimetic Particles for Targeting Thrombi in Flowing Blood 2013; 24: 3864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res 2007; 119: 777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res Elsevier Ltd; 2009; 124: 306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laing ST, Moody MR, Kim H, Smulevitz B, Huang SL, Holland CK, McPherson DD, Klegerman ME. Thrombolytic efficacy of tissue plasminogen activator-loaded echogenic liposomes in a rabbit thrombus model. Thromb Res Elsevier Ltd; 2012; 130: 629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol 1995; 21: 419–24. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Leeman JE, Wang J, Pacella JJ, Villanueva FS. New insights into mechanisms of sonothrombolysis using ultra-high-speed imaging. Ultrasound Med Biol 2014; 40: 258–62. [DOI] [PubMed] [Google Scholar]
- 67.Sakharov D V, Hekkenberg RT, Rijken DC. Acceleration of Fibrinolysis by High-frequency Ultrasound: The Contribution of Acoustic Streaming and Temperature Rise. Thromb Res 2000; 100: 333–40. [DOI] [PubMed] [Google Scholar]
- 68.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev 2008; 60: 1153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bader KB, Gruber MJ, Holland CK. Shaken and Stirred: Mechanisms of Ultrasound-Enhanced Thrombolysis. Ultrasound Med Biol 2014; 41: 187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabín J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke 2006; 37: 425–9. [DOI] [PubMed] [Google Scholar]
- 71.Molina CA, Barreto AD, Tsivgoulis G, Sierzenski P, Malkoff MD, Rubiera M, Gonzales N, Mikulik R, Pate G, Ostrem J, Singleton W, Manvelian G, Unger EC, Grotta JC, Schellinger PD, Alexandrov A V. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Ann Neurol 2009; 66: 28–38. [DOI] [PubMed] [Google Scholar]
- 72.Alexandrov AV, Mikulik R, Ribo M, Sharma VK, Lao AY, Tsivgoulis G, Sugg RM, Barreto A, Sierzenski P, Malkoff MD, Grotta JC. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke 2008; 39: 1464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma PK, Guha SK. Transmission of time varying magnetic field through body tissue. J Biol Phys 1975; 3: 95–102. [Google Scholar]
- 74.Tasci TO, Disharoon D, Schoeman RM, Rana K, Herson PS, Marr DWM, Neeves KB. Enhanced Fibrinolysis with Magnetically Powered Colloidal Microwheels. Small 2017; 1700954: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeong U, Teng X, Wang Y, Yang H, Xia Y. Superparamagnetic colloids: Controlled synthesis and niche applications. Adv Mater 2007; 19: 33–60. [Google Scholar]
- 76.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res Pharmaceutical Research; 2016; 33: 2373–87. [DOI] [PubMed] [Google Scholar]
- 77.Arami H, Khandhar A, Liggitt D, Krishnan KM. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles 2016; 25: 289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang HW, Hua MY, Lin KJ, Wey SP, Tsai RY, Wu SY, Lu YC, Liu HL, Wu T, Ma YH. Bioconjugation of recombinant tissue plasminogen activator to magnetic nanocarriers for targeted thrombolysis. Int J Nanomedicine 2012; 7: 5159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tadayon A, Jamshidi R, Esmaeili A. Delivery of tissue plasminogen activator and streptokinase magnetic nanoparticles to target vascular diseases. Int J Pharm Elsevier B.V.; 2015; 495: 428–38. [DOI] [PubMed] [Google Scholar]
- 80.Bi F, Zhang J, Su Y, Tang YC, Liu JN. Chemical conjugation of urokinase to magnetic nanoparticles for targeted thrombolysis. Biomaterials Elsevier Ltd; 2009; 30: 5125–30. [DOI] [PubMed] [Google Scholar]
- 81.Heid S, Unterweger H, Tietze R, Friedrich RP, Weigel B, Cicha I, Eberbeck D, Boccaccini AR, Alexiou C, Lyer S. Synthesis and Characterization of Tissue Plasminogen Activator — Functionalized Superparamagnetic Iron Oxide Nanoparticles for Targeted Fibrin Clot Dissolution. Int J Mol Sci 2017; 18: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen JP, Yang PC, Ma YH, Wu T. Characterization of chitosan magnetic nanoparticles for in situ delivery of tissue plasminogen activator. Carbohydr Polym Elsevier Ltd.; 2011; 84: 364–72. [Google Scholar]
- 83.Chen JP, Yang PC, Ma YH, Tu SJ, Lu YJ. Targeted delivery of tissue plasminogen activator by binding to silica-coated magnetic nanoparticle. Int J Nanomedicine 2012; 7: 5137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drozdov AS, Vinogradov VV, Dudanov IP, Vinogradov VV. Leach-proof magnetic thrombolytic nanoparticles and coatings of enhanced activity. Sci Rep Nature Publishing Group; 2016; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie Y, Kaminski MD, Torno MD, Finck MR, Liu X, Rosengart AJ. Physicochemical characteristics of magnetic microspheres containing tissue plasminogen activator. J Magn Magn Mater 2007; 311: 376–8. [Google Scholar]
- 86.Ma YH, Wu SY, Wu T, Chang YJ, Hua MY, Chen JP. Magnetically targeted thrombolysis with recombinant tissue plasminogen activator bound to polyacrylic acid-coated nanoparticles. Biomaterials Elsevier Ltd; 2009; 30: 3343–51. [DOI] [PubMed] [Google Scholar]
- 87.Inada Y, Ohwada K, Yoshimoto T, Kojima S, Takahashi K, Kodera Y, Matsushima A, Saito Y. Fibrinolysis by Urokinase Endowed with Magnetic Property. Biochem Biophys Res Commun 1987; 148: 392–6. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimoto T, Ohwada K, Takahashi K, Matsushima A, Saito Y, Inada Y. Magnetic Urokinase: Targeting of Urokinase to Fibrin Clot. Biochem Biophys Res Commun 1988; 152: 739–43. [DOI] [PubMed] [Google Scholar]
- 89.Prilepskii AY, Fakhardo AF, Drozdov AS, Vinogradov VV, Dudanov IP, Shtil AA, Bel’Tyukov PP, Shibeko AM, Koltsova EM, Nechipurenko DY, Vinogradov VV Urokinase-Conjugated Magnetite Nanoparticles as a Promising Drug Delivery System for Targeted Thrombolysis: Synthesis and Preclinical Evaluation. ACS Appl Mater Interfaces American Chemical Society; 2018; 10: 36764–75. [DOI] [PubMed] [Google Scholar]
- 90.Friedrich RP, Zaloga J, Schreiber E, Tóth IY, Tombácz E, Lyer S, Alexiou C. Tissue Plasminogen Activator Binding to Superparamagnetic Iron Oxide Nanoparticle—Covalent Versus Adsorptive Approach. Nanoscale Res Lett Nanoscale Research Letters; 2016; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu J, Huang W, Huang S, ZhuGe Q, Jin K, Zhao Y. Magnetically active Fe3O4 nanorods loaded with tissue plasminogen activator for enhanced thrombolysis. Nano Res 2016; 9: 2652–61. [Google Scholar]
- 92.Gravanis I, Tsirka SE. tPA as a therapeutic target in stroke. Expert Opin Ther Targets 2008; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Torno MD, Kaminski MD, Xie Y, Meyers RE, Mertz CJ, Liu X, O’Brien WD, Rosengart AJ. Improvement of in vitro thrombolysis employing magnetically-guided microspheres. Thromb Res 2008; 121: 799–811. [DOI] [PubMed] [Google Scholar]
- 94.Huang L, ZhuGe Q, Cheng R, Zhao Y, Mao L, Yang B, Jin K, Huang W. Acceleration of Tissue Plasminogen Activator-Mediated Thrombolysis by Magnetically Powered Nanomotors. ACS Nano 2014; 8: 7746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sitti M, Khalil ISM, Mahdy D, Sadek K, Tabak AF, Hamdi N. Rubbing Against Blood Clots Using Helical Robots: Modeling and In Vitro Experimental Validation. IEEE Robot Autom Lett IEEE; 2017; 2: 927–34. [Google Scholar]
- 96.Voros E, Cho M, Ramirez M, Palange AL, De Rosa E, Key J, Garami Z, Lumsden AB, Decuzzi P. TPA Immobilization on Iron Oxide Nanocubes and Localized Magnetic Hyperthermia Accelerate Blood Clot Lysis. Adv Funct Mater 2015; 25: 1709–18. [Google Scholar]
- 97.de Saint Victor M, Barnsley LC, Carugo D, Owen J, Coussios CC, Stride E. Sonothrombolysis with Magnetically Targeted Microbubbles. Ultrasound Med Biol 2019; 45: 1151–63. [DOI] [PubMed] [Google Scholar]
- 98.Nilsson M, Lasič S, Drobnjak I, Topgaard D, Westin CF. Resolution limit of cylinder diameter estimation by diffusion MRI: The impact of gradient waveform and orientation dispersion. NMR Biomed 2017; 30: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rubiera M, Ribo M, Delgado-Mederos R, Santamarina E, Maisterra O, Delgado P, Montaner J, Alvarez-Sabín J, Molina CA. Do Bubble Characteristics Affect Recanalization in Stroke Patients Treated with Microbubble-Enhanced Sonothrombolysis? Ultrasound Med Biol 2008; 34: 1573–7. [DOI] [PubMed] [Google Scholar]
- 100.De Lizarrondo SM, Gakuba C, Herbig BA, Repessé Y, Ali C, Denis CV., Lenting PJ, Touzé E, Diamond SL, Vivien D, Gauberti M. Potent thrombolytic effect of N-acetylcysteine on arterial thrombi. Circulation 2017; 136: 646–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Denorme F, Desender L, Vandenbulcke A, Deckmyn H, Vanhoorelbeke K, De Meyer SF, Langhauser F, Kleinschnitz C, Rottensteiner H, Plaimauer B, Scheiflinger F, François O, Andersson T. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood 2016; 127: 2337–45. [DOI] [PubMed] [Google Scholar]
- 102.Bustamante A, Ning MM, García-Berrocoso T, Penalba A, Boada C, Simats A, Pagola J, Ribó M, Molina C, Lo E, Montaner J. Usefulness of ADAMTS13 to predict response to recanalization therapies in acute ischemic stroke. Neurology 2018; 90: e995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ducroux C, Di Meglio L, Loyau S, Delbosc S, Boisseau W, Deschildre C, Maacha M Ben, Blanc R, Redjem H, Ciccio G, Smajda S, Fahed R, Michel JB, Piotin M, Salomon L, Mazighi M, Ho-Tin-Noe B, Desilles JP. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 2018; 49: 754–7. [DOI] [PubMed] [Google Scholar]
- 104.Wyseure T, Peeters M, Gils A, Declerck PJ, Rubio M, De Lizarrondo SM, Vivien D, Denorme F, De Meyer SF. Innovative thrombolytic strategy using a heterodimer diabody against TAFI and PAI-1 in mouse models of thrombosis and stroke. Blood 2015; 125: 1325–32. [DOI] [PubMed] [Google Scholar]