Abstract
Behavioral traits associated with various forms of psychopathology are conceptualized as dimensional, varying from those present in a frank disorder to subclinical expression. Demonstrating links between these behavioral traits and neurobiological indicators, such as brain structure, provides one form of validation for this view. However, unlike behavioral dimensions associated with other forms of psychopathology (e.g., autism spectrum disorder, attention deficit hyperactivity disorder, antisocial disorders), eating disorder traits have not been investigated in this manner in spite of the potential that such an approach has to elucidate etiological mechanisms. Therefore, we examined for the first time neural endophenotypes of Anorexia Nervosa and Bulimia via dimensional traits (measured using the Eating Disorders Inventory-3) in a large subclinical sample of young adults (n=456 and n=247, respectively; ages=18-22 years) who each provided a structural magnetic resonance imaging scan. Cortical thickness was quantified at 81,924 vertices across the cortical surface. We found: 1) increasing eating disorder traits correlated with thinner cortex in the insula and orbitofrontal cortex, among other regions, and 2) using these regions as seeds, increasing eating disorder trait scores negatively modulated structural covariance between these seed regions and other cortical regions linked to regulatory and sensorimotor functions (e.g., frontal and temporal cortices). These findings parallel those found in the clinical literature (i.e., thinner cortex in these food-related regions in individuals with eating disorders) and therefore provide evidence supporting the dimensional view of behavioral traits associated with eating disorders. Extending this approach to genetic and neuroimaging genetics studies holds promise to inform etiology.
Keywords: eating disorder, anorexia, bulimia, behavioral traits, brain, cortical thickness
Introduction
Historically, psychopathology was viewed as a qualitative or categorical difference in the expression of behavioral symptoms as compared to behaviors found in the general population. Increasingly however, behavioral traits associated with various forms of psychopathology are conceptualized to exist along a continuum that varies from clinical (i.e., the disorder) to subclinical (i.e., normal variation) expression; in other words, a quantitative difference in behavioral expression (Plomin, Haworth, & Davis, 2009). One form of validation for this dimensional view of psychopathology is derived from linking these behaviors with neurobiology, such as brain structure and function. Indeed, several studies demonstrate parallels between structural brain differences in frank disorders and neural correlates of subclinical behavioral traits associated with various forms of psychopathology. For example, neuroanatomical regions associated with social-communication, such as the posterior superior temporal sulcus, not only differ in their structural features among individuals with an autism spectrum disorder diagnosis (pathognomonic with social-communication deficits; American Psychiatric Association, 2013) as compared to typically developing controls (e.g., thinner cortex: Hadjikhani et al., 2006; Scheel et al., 2011; Wallace et al., 2010), but also these structural features vary in these same regions as a function of subclinical autistic traits in typically developing individuals (e.g., increasing autistic traits associated with thinner cortex: Wallace et al., 2012). Similar patterns have been observed in relation to both clinical and subclinical expressions of attention-deficit/hyperactivity disorder-related (e.g., Ducharme et al., 2012; Mous et al., 2017; Narr et al., 2009; Shaw et al., 2007, 2011) and antisocial behaviors (e.g., Smaragdi et al., 2017; Wallace et al., 2012, 2014; Yang et al., 2015), for example. However, these neuroanatomical parallels have been largely ignored for various forms of eating-related pathology. Anorexia Nervosa and Bulimia are eating disorders with distinct behavioral expressions (i.e., restricted energy intake in the former vs. binge eating with compensatory actions such as vomiting or excessive exercise in the latter), but they share a hyperfocus on body weight and shape that detrimentally impacts self-evaluations (American Psychiatric Association, 2013). An increasing number of neuroimaging studies have documented brain structural atypicalities in patients with these eating disorders, particularly anorexia nervosa. Recurring brain regions implicated in these studies thus far include those associated with food reward and perception, such as the somatosensory and orbitofrontal cortices, as well as interoception, such as the insula (for review, see Frank, 2015). Nevertheless, there are significant impediments to conducting this research with these clinical groups including the potential influence of common comorbidities (e.g., anxiety disorders) and state-based confounds (e.g., malnutrition) that interfere with the ability to attribute observed differences to the presence of an eating disorder. More specifically, state-based differences in the course of Anorexia Nervosa have been shown to dramatically affect structural brain metrics, including cortical thickness, with, for example, larger group differences occurring during the height of the illness and diminished differences occurring after recovery (Bernardoni et al., 2016; King et al., 2015; Lovagnino et al., 2018). Therefore, utilizing a trait-based approach to examine the dimensionality of eating disorder behaviors in a sample free from various forms of psychopathology and the association of these eating behaviors with brain structure could prove insightful and critically important to elucidating etiological mechanisms. Thus, we explore for the first time neural endophenotypes of Anorexia Nervosa and Bulimia traits, examined continuously, in a large subclinical sample of young adult volunteers.
Methods
885 young adult participants (age range=18-22 years) completed two of the Eating Disorder Inventory-3 (EDI-3; Garner, 2004) subtests (Drive for Thinness [EDIThn] and Bulimia [EDIBul]) and provided one anatomic magnetic resonance imaging scan. Scores on EDIThn ranged from 0 to 21 and scores on EDIBul ranged from 0 to 24; however, due to skewness in data, self-ratings of zero were discarded. Furthermore, participants with past or present DSM-IV psychiatric disorders were excluded (EDIThn group n=92; EDIBul group n=63) from analyses resulting in 456 psychiatrically healthy participants providing non-zero EDIThn scores and 247 psychiatrically healthy participants providing non-zero EDIBul scores. See Table 1 for demographic details on the final samples utilized in analyses.
Table 1.
Demographic characteristics of study participants.
| Final EDIThn Sample (n=456) |
Final EDIBul Sample (n=247) |
|
|---|---|---|
| Mean Age (SD) | 19.58 (1.21) | 19.43 (1.23) |
| Sex Ratio (M:F) | 143:313 | 78:169 |
| Race (Wh:AfAm:As:Oth) | 206:57:138:55 | 117:25:84:21 |
Note: EDIThn=Eating Disorder Inventory-3 Drive for Thinness; EDIBul= Eating Disorder Inventory-3 Bulimia; SD=standard deviation; M=Male; F=Female; Wh=White; AfAm=African-American; As=Asian; Oth=Other
Magnetic resonance images were acquired on one of two identical 3T GE MR750 Scanners equipped with an 8-channel head-coil. All T-1 weighted MPRAGE scans passed quality control standards implemented via blind ratings both pre- and post-processing. The Civet pipeline from the Montreal Neurological Institute was used to quantify cortical thickness across 81,924 vertices on the cortical surface. In brief, the Constrained Laplacian Anatomic Segmentation using Proximity (CLASP) method was utilized to extract gray and white matter surface meshes (Kim et al., 2005) from which thickness could be calculated in native space across maximally aligned nodes using surface registration (Lyttelton et al., 2007; MacDonald et al., 2000). A blurring kernel of 30mm was used to reduce noise in cortical thickness quantification (Lerch & Evans, 2005).
Statistical analyses were performed using SurfStat (e.g., Taylor & Worsley, 2007) in the Matlab environment. First, linear associations between thickness across the cortical surface and both EDIThn and EDIBul, applying family-wise error (FWE) correction procedures to account for multiple comparisons (FWE-corrected p<.05), were examined using regression procedures. Second, neuroanatomic regions where significant associations between cortical thickness and EDI-3 subscores were noted were utilized as seeds. Then, structural covariance between cortical thickness in each seed region and thickness in all other vertices across the cortical surface was examined as a function of EDIThn or EDIBul scores using linear regression. In other words, we examined whether EDI-3 subscores significantly modulated intra-cortical correlations using the Mapping Anatomical Correlations Across Cerebral Cortex (MACACC) procedure (Lerch et al., 2006). Once again, FWE correction procedures were implemented to account for multiple comparisons (FWE-corrected p<.05).
All participants provided informed consent. Furthermore, this study was approved by the local institutional research ethics committee and was completed in accordance with the ethical standards delineated in the 1964 Declaration of Helsinki.
Results
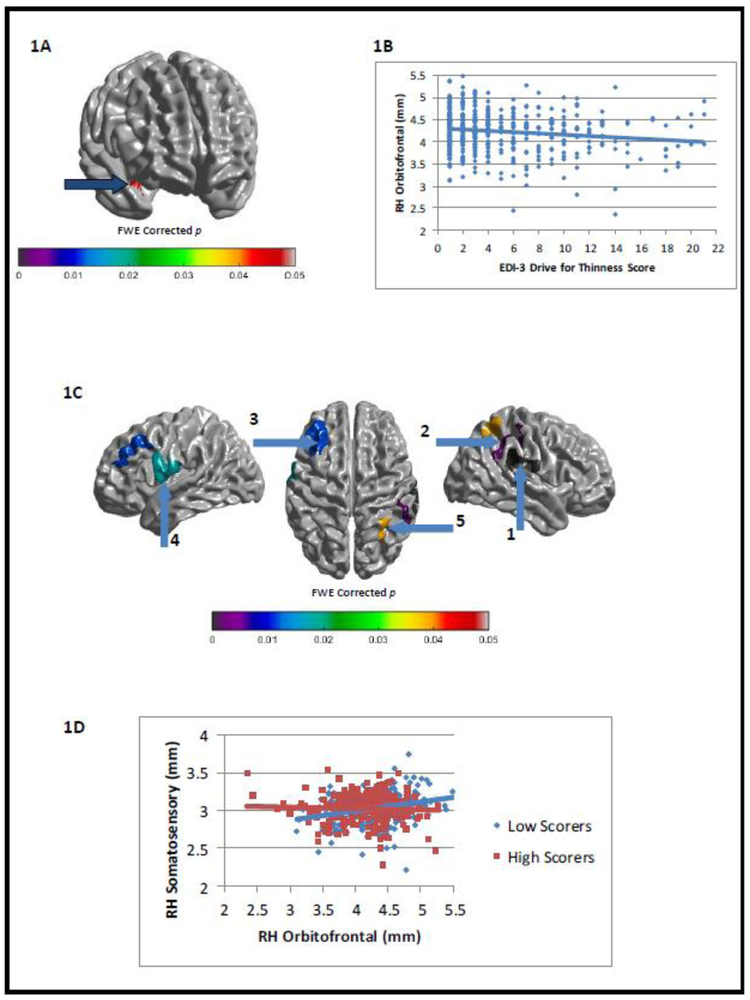
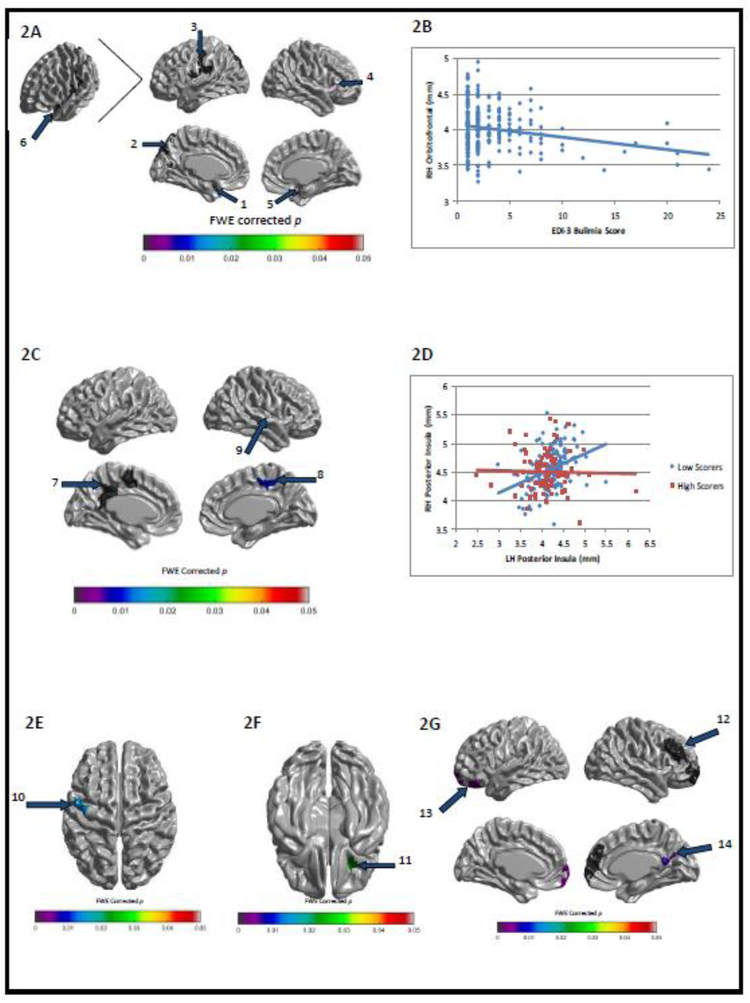
Self-ratings of both EDIThn and EDIBul were negatively correlated with thickness in orbitofrontal and insular cortices, while EDIBul scores were further negatively correlated with somatosensory cortical thickness (FWE-corrected ps<.05). Utilizing these regions as seeds, we also found that both EDIThn and EDIBul ratings negatively modulated (i.e., weakened) the positive relationship between thickness in these regions and thickness in other cortical regions (e.g., left prefrontal and right temporoparietal regions for EDIThn and bilateral cingulate and sensorimotor cortices for EDIBul; FWE-corrected ps<.05). See Figures 1 and 2 for correlations between EDI-3 subscores and cortical thickness, and for modulations of intra-cortical correlations by EDI-3 subscores. Notably, significant interactions with sex were not observed for EDIThn and were minimal for EDIBul, occurring in two very small brain regions that did not overlap with the regions implicated above.
Figure 1.
Family wise error-corrected associations between EDI-3 Drive for Thinness (EDIThn) scores and cortical thickness/intracortical correlations: (A) negative correlation between EDIThn and cortical thickness in right orbitofrontal cortex (x=33, y=10, z=−15) and (B) its associated scatterplot; (C) group differences between low- and high-EDIThn scorers in intracortical correlations between right orbitofrontal cortex (see Figure 1A) and the rest of cortex and (D) a sample scatterplot of the relationship between right orbitofrontal cortex thickness (see Figure 1A) and right somatosensory cortex thickness (Figure 1C, seed 2; x=45, y=−24, z=44) as a function of low- and high-EDIThn scorers.
Figure 2.
Family wise error-corrected associations between EDI-3 Bulimia (EDIBul) scores and cortical thickness/intracortical correlations: (A) negative correlation between EDIBul and cortical thickness in bilateral insula and orbitofrontal cortices as well as left somatosensory and inferior parietal cortices and (B) a sample scatterplot of the relationship between EDIBul scores and cortical thickness from Figure 2A, seed 5 (x=29, y=22, z=−24); (C) group differences between low- and high-EDIBul scorers in intracortical correlations between left posterior insula (see Figure 2A, seed 1; x=−42, y=−6, z=−14) and the rest of cortex and (D) a sample scatterplot of the relationship between cortical thickness in Figure 2A, seed 1 and Figure 2C, seed 9 (x=38, y=−8, z=−9) as a function of low- and high-EDIBul scorers; (E) group differences between low- and high-EDIBul scorers in intracortical correlations between right anterior insula (see Figure 2A, seed 4; x=30, y=22, z=9) and left sensorimotor cortex thickness (x=−45, y=−7, z=58); (F) group differences between low- and high-EDIBul scorers in intracortical correlations between right lateral orbitofrontal (see Figure 2A, seed 5) and right inferior orbitofrontal cortex thickness (x=28, y=23, z=−24); (G) group differences between low- and high-EDIBul scorers in intracortical correlations between left orbitofrontal cortex (see Figure 2A, seed 6; x=−26, y=23, z=−23) and each of the following: right anterior prefrontal cortex (seed 12; x=14, y=63, z=−9), left orbitofrontal cortex (seed 13; x=−18, y=38, z=−22), left precuneus (seed 14; x=3, y=−64, z=27).
Discussion
The current study provides neurobiological evidence for the dimensional view of behaviors associated with eating disorders by linking variation in cortical structure with subclinical eating disorder traits for the first time. More specifically, self-ratings of Anorexia Nervosa and Bulimia traits were negatively correlated with thickness in distinct cortical regions (e.g., orbitofrontal cortex and insula) that are crucial to food perception, reward, and interoception (Frank, 2015; Rolls, 2015). Furthermore, greater endorsement of these traits negatively modulated anatomical covariance between these regions and other cortical areas, including those serving regulatory and sensorimotor functions, paralleling studies documenting atypical functional connectivity of these regions in eating disorders (Gaudio, Wiemerslage, Brooks, & Schiöth, 2016). Therefore, not only does cortical thickness in select regions reflect ratings of behaviors related to eating disorders, but also the relationships among these and other functionally integrated brain regions are modulated by the level of these eating disorder-related behaviors. These findings complement the clinical literature reporting group differences in brain structure among individuals with eating disorders (Frank, 2015) by implicating an overlapping set of regions (e.g., orbitofrontal, insula, and somatosensory cortices) as associated with subclinical eating disorder traits. However, it does so in the absence of at least one potentially significant confound (i.e., psychiatric comorbidity). Thus, not only does linking regionally-specific brain structure to eating disorder-related behavioral traits validate viewing these behaviors dimensionally, but also it joins similar approaches to other forms of psychopathology (e.g., autism spectrum disorder: Wallace et al., 2012; attention deficit/hyperactivity disorder: Ducharme et al., 2012; Mous et al., 2017; Shaw et al., 2011; and antisocial behavior: Yang et al., 2015) consistent with broader conceptualizations linking behavior and biology, such as the Research Domain Criteria (Insel et al., 2010). More to the point, parallels drawn between our subclinical behavior-structural brain associations and the previously established clinical group differences (Frank, 2015) provide additional evidence that these neural signatures have the potential to serve as informative endophenotypes for future genetic studies that require large samples. Nevertheless, although these findings are novel, limitations to the current study should be considered. For example, the present study included a relatively large sample size for a neuroimaging study; however, replication is required, particularly across a broader age range that extends into adolescence given the prototypical emergence of Anorexia Nervosa and Bulimia during this developmental window. Similarly, longitudinal studies are needed given the well-established dynamic nature of brain development across the lifespan (e.g., Fjell et al., 2014; Raznahan et al., 2011) and an emerging trend of developmentally dynamic findings within the brain imaging literature on eating disorders (e.g., Cyr et al., 2017). Finally, it is important to point out that although the EDI-3 is touted as a trait-based measure, state-based influences on ratings cannot be ruled out (Cyr et al., 2017; Treadway & Leonard, 2016). Only through longitudinal designs can the potential for confounding state-based impacts be rigorously evaluated and potentially teased apart from stable, behavioral trait-based influences.
Acknowledgements:
We thank all members of the Laboratory of NeuroGenetics for their assistance in conducting the Duke Neurogenetics Study, which was supported by Duke University and NIH grant R01DA033369. A.R.H. is further supported by NIH grant R01AG049789. We also thank Esha Mehta and Caylynn Yao for their assistance in completing this research.
Funding: This study was supported by Duke University and NIH grant R01DA033369. A.R.H. is further supported by NIH grant R01AG049789.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose.
Ethical approval: This study was approved by the Duke University Institutional Review Board.
Informed consent: All participants provided informed consent.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Bernardoni F, King JA, Geisler D, Stein E, Jaite C, Nätsch D, Tam FI, Boehm I, Seidel M, Roessner V, & Ehrlich S (2016). Weight restoration therapy rapidly reverses cortical thinning in anorexia nervosa: A longitudinal study. NeuroImage, 130, 214–222. [DOI] [PubMed] [Google Scholar]
- Cyr M, Kopala-Sibley DC, Lee S, Chen C, Stefan M, Fontaine M, Terranova K, Berner LA, & Marsh R (2017). Reduced inferior and orbital frontal thickness in adolescent bulimia nervosa persists over two-year follow-up. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Hudziak JJ, Botteron KN, Albaugh MD, Nguyen TV, Karama S, & Evans AC (2012). Brain Development Cooperative Group. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Dale AM, Walhovd KB, & Alzheimer Disease Neuroimaging Initiative. (2014). Accelerating cortical thinning: unique to dementia or universal in aging? Cerebral Cortex, 24, 919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK (2015). Advances from neuroimaging studies in eating disorders. CNS Spectrums, 20, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DM (2004). Eating Disorder Inventory-3 Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Gaudio S, Wiemerslage L, Brooks SJ, & Schiöth HB (2016). A systematic review of resting-state functional-MRI studies in anorexia nervosa: Evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neuroscience and Biobehavioral Reviews, 71, 578–589. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, & Tager-Flusberg H (2006). Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex, 16, 1276–1282. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, & Wang P (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167, 748–751. [DOI] [PubMed] [Google Scholar]
- King JA, Geisler D, Ritschel F, Boehm I, Seidel M, Roschinski B, Soltwedel L, Zwipp J, Pfuhl G, Marxen M, Roessner V, & Ehrlich S (2015). Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biological Psychiatry, 77, 624–632. [DOI] [PubMed] [Google Scholar]
- Lavagnino L, Mwangi B, Cao B, Shott ME, Soares JC, & Frank GKW (2018). Cortical thickness patterns as state biomarker of anorexia nervosa. International Journal of Eating Disorders, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, & Evans AC (2005). Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage, 24, 163–173 [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, & Evans AC (2006). Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage, 31, 993–1003. [DOI] [PubMed] [Google Scholar]
- Lyttelton O, Boucher M, Robbins S, & Evans A (2007). An unbiased iterative group registration template for cortical surface analysis. NeuroImage, 34, 1535–1544. [DOI] [PubMed] [Google Scholar]
- Mous SE, White T, Muetzel RL, El Marroun H, Rijlaarsdam J, Polderman TJ, Jaddoe VW, Verhulst FC, Posthuma D, & Tiemeier H (2017). Cortical morphology as a shared neurobiological substrate of attention-deficit/hyperactivity symptoms and executive functioning: a population-based pediatric neuroimaging study. Journal of Psychiatry & Neuroscience, 42, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del'Homme M, Caplan R, Toga AW, McCracken JT, & Levitt JG (2009). Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Haworth CM, & Davis OS (2009). Common disorders are quantitative traits. Nature Reviews Genetics, 10, 872–878. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, & Giedd JN (2011). How does your cortex grow? Journal of Neuroscience, 31, 7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2015). Taste, olfactory, and food reward value processing in the brain. Progress in Neurobiology, 127–128, 64-90. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, & Rapoport JL (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences, 104, 19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, & Giedd J (2011). Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. American Journal of Psychiatry, 168, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaragdi A, Cornwell H, Toschi N, Riccelli R, Gonzalez-Madruga K, Wells A, Clanton R, Baker R, Rogers J, Martin-Key N, Puzzo I, Batchelor M, Sidlauskaite J, Bernhard A, Martinelli A, Kohls G, Konrad K, Baumann S, Raschle N, Stadler C, Freitag C, Sonuga-Barke EJS, De Brito S, & Fairchild G (2017). Sex differences in the relationship between conduct disorder and cortical structure in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 703–712. [DOI] [PubMed] [Google Scholar]
- Taylor JE & Worsley KJ (2007). Detecting sparse signal in random fields, with an application to brain mapping. Journal of the American Statistical Association, 102, 913–928. [Google Scholar]
- Treadway MT, & Leonard CV (2016). Isolating biomarkers for symptomatic states: considering symptom–substrate chronometry. Molecular Psychiatry, 21, 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, & Martin A (2010). Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain, 133, 3745–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Shaw P, Lee NR, Clasen LS, Raznahan A, Lenroot RK, Martin A, & Giedd JN (2012). Distinct cortical correlates of autistic versus antisocial traits in a longitudinal sample of typically developing youth. Journal of Neuroscience, 32, 4856–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, White S, Robustelli B, Sinclair S, Hwang S, Martin A, & Blair RJ (2014). Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous-unemotional traits. Journal of the American Academy of Child and Adolescent Psychiatry, 53, 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang P, Baker LA, Narr KL, Joshi SH, Hafzalla G, Raine A, & Thompson PM (2015). Thicker temporal cortex associates with a developmental trajectory for psychopathic traits in adolescents. PLoS One, 10, e0127025. [DOI] [PMC free article] [PubMed] [Google Scholar]