Abstract
Background:
The “double bubble” sign is an ultrasonographic finding that commonly represents duodenal atresia and is associated with trisomy 21.
Objectives:
We sought to evaluate the positive predictive value of a prenatally identified double bubble sign for duodenal atresia and the genetic etiologies associated with it.
Methods:
We examined a retrospective cohort with prenatal double bubble sign between January 1, 2008, and June 30, 2017. Postnatal diagnoses were determined by review of operative reports and additional postnatal evaluation including cytogenetic analysis, molecular analysis, and/or clinical genetic evaluation.
Results:
All live births at our institution with a prenatal double bubble sign had confirmed duodenal atresia. Additional anatomic anomalies and/or genetic abnormalities were identified in 62% of cases. Out of 21 cases, 6 had trisomy 21. Of the remaining 15 cases, 8 were nonisolated duodenal atresia, 3 of which had a heterotaxy syndrome. In the 7 isolated cases, 1 likely pathogenic chromosomal microdeletion was identified.
Conclusions:
Prenatal double bubble sign is a reliable predictor of duodenal atresia. In addition to trisomy 21, heterotaxy may be encountered. ZIC3 mutations as well as microdeletion of 4q22.3 may be underlying genetic etiologies to be considered in the diagnostic evaluation of a prenatal double bubble sign.
Keywords: Duodenal atresia, Double bubble sign, Prenatal diagnosis
Introduction
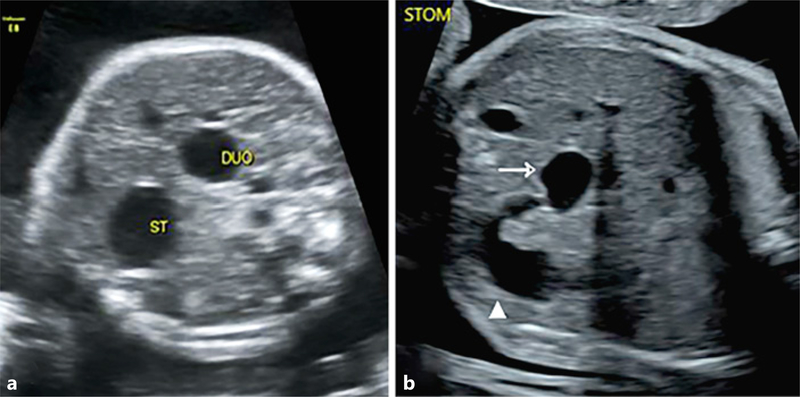
The “double bubble” sign is an ultrasonographic finding that signifies the presence of two adjacent fluid-filled echolucent structures within the abdomen of a fetus (Fig. 1). This can result when, in the presence of obstruction within the proximal small bowel (most often duodenal atresia), there is dilatation of both the stomach and the duodenal bulb. While the association between a double bubble sign and duodenal atresia is well established, the positive predictive value of the prenatal ultrasonographic sign is not known, with some studies suggesting that false positives or other intestinal obstructions may underlie the finding [1–3]. Other gastrointestinal obstructions, such as fetal volvulus, jejunal atresia, or ileal atresia have all been reported to be present in some cases after an ultrasound presentation of a double bubble sign [4, 5]. Conditions such as fetal volvulus can be life threatening, particularly after late diagnosis [6]; thus, analysis of the positive predictive value of the double bubble sign is of importance for ensuring proper prenatal diagnosis counseling and postnatal medical treatment.
Fig. 1.
Double bubble sign. a Stomach and duodenum. b Stomach (arrow) with dilatation of proximal bowel (white triangle). Representative prenatal ultrasound images from cases in the study.
Duodenal atresia occurs with an incidence of 1 in 5,000 to 1 in 10,000 live births [6]. It can present in both an isolated form, in combination with other congenital anomalies, or in association with a known or suspected chromosomal abnormality, particularly trisomy 21. Studies suggest that about one-third of those diagnosed prenatally with duodenal atresia have Down syndrome, and that overall 3–5% of those with trisomy 21 have duodenal atresia [6, 7]. Few studies to date have explored other potential genetic etiologies of duodenal atresia, with most genetic investigations being limited to aneuploidy testing [8–10]. Identification of other genetic etiologies of duodenal atresia would be beneficial for purposes of disease characterization, screening and detection, and prenatal diagnosis counseling.
We have reviewed a cohort of cases to evaluate the positive predictive value of the prenatal double bubble sign for duodenal atresia. Within this cohort, we have further evaluated for associated anomalies and genetic etiologies for duodenal atresia.
Materials and Methods
We performed a retrospective cohort analysis of all cases of double bubble visualized on prenatal ultrasound at a single center between January 1, 2008, and June 30, 2017. Our institution serves both the general population for routine anomaly screening, but also serves as a referral center for evaluation of fetal anomalies. For cases born at our institution, we analyzed pregnancy outcome, postnatal findings, and postnatal diagnosis. Postnatal diagnosis was determined by review of operative reports. Cases were further investigated for postnatal genetic evaluation via cytogenetic analysis, molecular analysis, and/or via clinical diagnosis. Neonatal outcomes were also investigated.
All prenatal ultrasound examinations were performed by certified ultrasonographers with Maternal Fetal Medicine specialists using Voluson E7, E8, or E10 GE Healthcare ultrasounds. In all cases, a detailed ultrasound anatomic evaluation was performed, after 18 weeks estimated gestational age, regardless of gestational age at presentation to our institution. Data on ultrasound reports were extracted from our computer database. All cases had prenatal genetic counseling and were offered prenatal genetic testing. All cases born at our institution had postnatal genetic evaluation by Medical Genetics specialists and were offered postnatal genetic testing as indicated by postnatal evaluation findings. Postnatal examination findings and clinical diagnoses were extracted from operative reports and genetic consultative reports. Genetic testing results were obtained from our Department of Pathology, Cytogenetics Laboratory and/or commercial testing labs, when indicated. Prenatal cytogenetic testing results were analyzed for cases of termination of pregnancy or intrauterine demise. Descriptive analysis of the collected data was presented.
Results
Between January 1, 2008, and June 30, 2017, 21 fetuses evaluated by prenatal ultrasound were found to have a double bubble sign. During the timeframe of this study, our institution performed a total of 29,792 anatomy ultrasounds, of which 7,580 (25%) had been referred from outside institutions for any anomaly finding, resulting in a prenatal double bubble incidence of 1: 1,420 at our center. With regard to double bubble sign, 2 cases were found on routine anomaly screen from within our institution, and 19 cases were referred from outside institutions and had a repeat anomaly screen upon presentation to our center. Gestational ages of initial sonographic evaluation at our center were between 19 + 6/7 and 38 + 4/7 weeks. Patients from outside centers after a second trimester ultrasound was positive for an anomaly presented at a mean gestational age of 29 + 5/7 weeks (range 19 + 6/7 to 38 + 4/7 weeks).-
Maternal and gestational demographics were as listed in Table 1. The mean maternal age was 30.5 years. A majority of the women in the cohort were Caucasian (57.1%) or African-American (28.6%), while only 9.5% were of Hispanic descent, and 4.8% were of Asian descent. This very closely resembles the race and ethnicity of the local population for the state of Maryland (50.9% Caucasian, 30.8% African-American, 10.1% Hispanic, 6.7% Asian) [11]. The majority of the cohort was married (76%) and denied drug, alcohol, or tobacco use during pregnancy (95%). Approximately half of the cohort was relatively healthy without notable maternal comorbidity. Maternal comorbidities were various, with no one disease being exceedingly present amongst the cohort as a whole. Approximately half of the cohort (10 of 21) were multiparous, with 11 being nulliparous, 5 of whom had had prior miscarriage or terminations, and 6 of whom were primigravida. The majority of cases were singleton pregnancies (95%) with one case in which only one twin of a dichorionic diamniotic twin pregnancy had prenatal double bubble sign.
Table 1.
Maternal and gestational demographics (n = 21)
| Age, years | 30.5 (16–40) |
|---|---|
| Maternal age groups | |
| <20 | 2 (9.5) |
| 20–24 | 2 (9.5) |
| 25–29 | 6 (28.6) |
| 30–34 | 3 (14.3) |
| >34 | 8 (38.1) |
| Maternal race | |
| African-American | 6 (28.6) |
| Asian | 1 (4.8) |
| Caucasian | 12 (57.1) |
| Hispanic | 2 (9.5) |
| Marital status | |
| Single | 5 (23.8) |
| Married | 16 (76.2) |
| Drug/smoking/alcohol use in pregnancy | |
| Yesa | 1 (4.8) |
| No | 20 (95.2) |
| Maternal comorbidities | |
| Hypertension | 2 (9.5) |
| Gestational diabetes | 3 (14.3) |
| Asthma | 1 (4.8) |
| Depression | 2 (9.5) |
| Otherb | 3 (14.3) |
| None | 10 (47.6) |
| Parity | |
| Nulliparous | 11 (52.4) |
| Multiparous | 10 (47.6) |
| Gestational count | |
| Singleton | 20 (95.2) |
| Multifetal gestation | 1 (4.8) |
| Gestational age at first ultrasound | |
| <10 weeks | 0 (0) |
| 10–20 weeks | 2 (9.5) |
| 20–30 weeks | 7 (33.3) |
| >30 weeks | 12 (76.2) |
| Gestational age at delivery, weeks | 35.6 (26.1–41.2) |
Values are presented as n (%) or mean (range).
Tobacco, marijuana, and opiates.
Chronic anemia, uterine fibroids, sexually transmitted infection.
Three of the 21 cases of prenatal double bubble sign did not have postnatal diagnostic evaluation from which duodenal atresia could be confirmed. One patient opted for termination for prenatally confirmed fetal trisomy 21. Another pregnancy with prenatally confirmed fetal trisomy 21 ended in intrauterine fetal demise for which no autopsy or further evaluation was pursued. One case of prenatal double bubble sign, with an additional anomaly of bilateral renal agenesis, delivered at an outside hospital with neonatal demise within hours of delivery; this patient was lost to postnatal follow-up. While postnatal evaluation was not available for the 3 aforementioned cases, prenatal examination and/or genetic diagnosis was available; thus, all 21 cases were included in further analysis of associated anomalies and genetic etiologies. Out of 21 cases of prenatal double bubble sign, there were 6 cases with trisomy 21 and 8 cases with other anomalies, 3 of which had heterotaxy syndrome. Seven cases had isolated duodenal atresia.
With the exception of the case with bilateral renal agenesis, all live births (n = 18) and delivery of the case of intrauterine fetal demise (n = 1) occurred at our institution. In these 18 live births, duodenal atresia was confirmed by direct examination during surgical corrective procedures. Additional intraoperative findings included gastric volvulus in 1 case and causative annular pancreas in 4 other cases.
Genetic testing was pursued in 18 cases, declined in 2 cases, and unknown in the one case that delivered at an outside hospital and was lost to postnatal follow-up. All 18 cases for which genetic testing was pursued had chromosomal analysis by: karyotype (n = 6), microarray (n = 8), or both (n = 4). Additionally, 2 of the 3 cases of clinical heterotaxy had ZIC3/CFC1 heterotaxy gene testing (the 3rd case declined to have this testing performed). One case within the cohort had continued follow-up with the Medical Genetics service for multiple anomalies (duodenal atresia + butterfly vertebrae) leading to additional genetic testing via whole exome sequencing with unrevealing results.
A syndromic etiology, either Down syndrome or heterotaxy syndrome, was present in 9 of our 21 cases (43%). The genetic etiology of duodenal atresia was identified in 8 of the 21 cases (38.1%): 6 cases of trisomy 21, 1 case with a novel variant in heterotaxy-associated ZIC3 gene, and 1 case with microdeletion (arr 4q22.3(95,859,913–95,941,464)×1) which was classified as likely pathogenic by the resulting laboratory per American College of Medical Genetics (ACMG) standards.
In the 6 cases of trisomy 21, other ultrasonographic findings included absent nasal bone (n = 4), echogenic bowel (n = 3), echogenic intracardiac foci (n = 3), and atrioventricular canal defect (n = 2), but no other major structural anomalies were visualized. Four of the trisomy 21 cases were live births and had postnatal surgical evaluation, 1 pregnancy was terminated, and 1 suffered fetal demise. Of the 15 non-Down syndrome fetuses, 8 (38%) had additional findings or major structural anomalies visualized on prenatal ultrasound, including the 3 cases of heterotaxy, 2 others with cardiac defects, 1 with bilateral renal agenesis, 1 with butterfly vertebrae, and 1 with echogenic bowel apart from the duodenum. Seven of the 21 cases (33%) were isolated cases of duodenal atresia and had no other anatomic findings. All 7 of those cases had cytogenetic analysis by SNP microarray, only 1 of which was abnormal: the aforementioned 4q22.3 microdeletion. Polyhydramnios was present in 16 of the 21 cases (76%) of prenatal double bubble but showed no association with genetic etiologies. Of the 4 cases having normal amniotic fluid indices, 2 were trisomy 21, 1 was a heterotaxy, and 1 was isolated duodenal atresia. Severe oligohydramnios was noted in the 1 case with bilateral renal agenesis.
Discussion
There is a paucity of literature available on prenatal double bubble sign beyond its association with trisomy 21, its positive predictive value for duodenal atresia, and its potential diversity of genetic etiologies. Earlier case reports or case series aided to establish the association of prenatal double bubble sign to duodenal atresia and its association to aneuploidy, mostly trisomy 21 [4–8]. More recent studies have presented additional anatomical and chromosomal defects, as well as outcome data, including postnatal findings, though none were within the United States [9, 10].
Duodenal atresia is the most common congenital gastrointestinal obstruction. However, when a double bubble sign is present on prenatal ultrasound, other gastrointestinal anomalies, such as jejunal atresia, ileal atresia, intestinal volvulus, and abdominal duplication cysts, have been found postnatally [10]. As a referral center, the incidence of duodenal atresia at our institution was higher than that of the general population [6]. Within our cohort, all 18 of the pregnancies with prenatal double bubble sign and live birth at our institution had postnatal confirmation of duodenal atresia during surgical exploration and corrective procedures. This suggests that the prenatal double bubble sign is a reliable predictor of duodenal atresia and other etiologies are likely rare. With this knowledge, pediatric surgeons may provide more informed counseling regarding the likely management and postnatal course for the fetus found to have a prenatal double bubble sign.
Although 3 cases within our cohort did not have postnatal evaluation for confirmation of duodenal atresia (due to termination or demise), those cases had prenatally identified additional anomalies or a genetic diagnosis and were included in our outcomes analysis. Our cohort of 21 cases of fetal double bubble sign appeared to segregate into three groups: cases of confirmed trisomy 21, cases with an isolated finding of prenatal double bubble, and cases with additional structural anomalies (Table 2). Nearly 30% of our cases had trisomy 21 which is similar to the 30–40% of duodenal atresia associated with trisomy 21 found in previous studies [12–14]. A third of our cases had duodenal atresia as an isolated structural finding, and the remaining 38% of cases had additional structural anomalies identified on prenatal ultrasound. Previous studies have reported between 38 and 72% of prenatally diagnosed duodenal atresia had associated anomalies, including both chromosomal and structural anomalies within that estimate. This aligns with our findings of 62% (13 of 21) of our cohort having chromosomal or additional major structural anomalies, suggesting that finding a double bubble sign mandates careful evaluation for other anomalies.
Table 2.
Outcomes of fetuses with prenatal double bubble sign
| Trisomy 21 (n = 6) |
Additional anomalies (n = 8) |
Isolated (n = 7) | |
|---|---|---|---|
| Termination | 1 (17) | 0 | 0 |
| Fetal demise | 1 (17) | 0 | 0 |
| Neonatal demise | 0 | 3 (33) | 0 |
| Living | 4 (67) | 5 (67) | 7 (100) |
| Preterm delivery | |||
| <37 weeks | 3 (50) | 4 (50) | 2 (29) |
| <32 weeks | 2 (33) | 2 (25) | 1 (14) |
| Cesarean delivery | 1 (17) | 4 (50) | 3 (43) |
| Birthweight, g | 2,588 (2,240–2,940) | 2,440 (1,970–2,590) | 2,940 (1,700–3,980) |
| NICU stay, days | 17 (12–22) | 42 (1–139) | 27 (10–67) |
Data are presented as n (%) or mean of live births (range).
Genetic technologies, which were previously limited to karyotyping for aneuploidy, have evolved over the past decade, allowing us to explore additional potential genetic etiologies for duodenal atresia. Beyond evaluating for trisomy 21 or other aneuploidy, the patients within our cohort had additional genetic evaluation available to them via a clinical genetics consultation, microarray testing for chromosomal copy number variation, and/or molecular testing for single genes (Table 3). Additional associations with double bubble in previous studies were mostly Down syndrome and cardiac defects. Whereas these findings were also present in our cohort, heterotaxy was an additional notable condition comprising 20% (3 of 8 cases) of those with additional anomalies without trisomy 21. In 1 of our 3 heterotaxy cases, a variation in ZIC3 gene (a heterotaxy-associated gene) was found. This adds heterotaxy as both a clinical syndrome and as a monogenic disorder to the potential genetic etiologies associated with duodenal atresia.
Table 3.
Genetic etiologies and additional associated findings in fetuses with double bubble sign
| Prenatal finding / suspected diagnosis |
Cases, n | Prenatal polyhydramnios, n |
Postnatal surgical findings | Genetic testing results |
|---|---|---|---|---|
| Trisomy 21a | 6 | 4 | 3 duodenal atresia | 6 trisomy 21 karyotype |
| 1 duodenal atresia/duodenal web | ||||
| Isolated | 7 | 6 | 5 duodenal atresia | 6 normal microarray |
| 2 duodenal atresia/annular pancreas | 1 microdeletionb | |||
| Heterotaxyc | 3 | 2 | 2 duodenal atresia/annular pancreas | 1 heterotaxy-associated ZIC3 gene variant |
| 1 duodenal atresia/duodenal web + gastric volvulus | 1 normal microarray with negative ZIC3/CFC1 gene testing |
|||
| 1 normal karyotype and microarray | ||||
| Cardiac defect | 2 | 2 | 2 duodenal atresia | 1 normal microarray and karyotype |
| 1 normal cfDNA screen with no further genetic testing | ||||
| Bilateral renal agenesisd | 1 | 0 | N/A | N/A |
| Vertebral anomaly | 1 | 1 | duodenal atresia | normal karyotype, microarray, and whole-exome sequencing |
| Echogenic bowel | 1 | 1 | duodenal atresia | normal microarray |
Trisomy 21: 6 total cases – 4 diagnosed by prenatal chromosomal analysis, 2 suspected prenatally by cfDNA screen result and ultrasound findings, both confirmed by postnatal karyotype.
Interstitial microdeletion of chromosome band 4q22.3.
Heterotaxy cases included multiple anomalies including complex cardiac defects and right-sided stomachs.
Delivered at outside hospital with neonatal demise, lost to follow-up postnatally.
Of the 7 isolated cases of duodenal atresia, 6 had normal SNP microarray and 1 had likely pathogenic interstitial microdeletion of chromosome 4q22.3. Hou and Wang [15] reported a case of a neonate with 4q22.3 deletion as a result of a ring chromosome 4 mosaicism. In addition to other anomalies, the neonate in this case report had congenital short bowel with midgut malrotation and pseudo-obstruction. This, along with our case, suggests that the 4q22.3 microdeletion may be an additional genetic etiology of duodenal atresia.
There were no fetal or neonatal demises within our cases of isolated duodenal atresia. Furthermore, only 1 of 7 isolated cases (14%) of duodenal atresia delivered at less than 32 weeks gestation. Thus, our data support that fetuses with isolated duodenal atresia on prenatal ultrasound generally have favorable outcomes. As would be expected, cases with additional anomalies appear to have less favorable outcomes, as the only fetal or neonatal demises seen in our cohort were in those cases of duodenal atresia with trisomy 21 and/or additional associated structural anomalies.
Conclusion
Prenatal double bubble sign is a reliable positive predictor of duodenal atresia, and a significant proportion of cases have associated anatomic anomalies and/or genetic abnormalities. While duodenal atresia is mostly associated with Down syndrome, there are additional genetic etiologies one should be cognizant of in the diagnostic evaluation, including 4q22.3 microdeletion and heterotaxy syndrome. Outcomes appear to be favorable when duodenal atresia is found in isolation and appear to be less favorable when additional anatomic anomalies are found. These findings underscore the need for skilled sonographic examination and advanced genetic investigation for prenatal diagnosis and informed, appropriate perinatal care of a fetus with duodenal atresia.
Footnotes
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Morris G, Kennedy A Jr, Cochran W. Small Bowel Congenital Anomalies: a Review and Update. Curr Gastroenterol Rep. 2016. April; 18(4): 16. [DOI] [PubMed] [Google Scholar]
- 2.Koberlein G, DiSantis D. The “double bubble” sign. Abdom Radiol (NY). 2016. February; 41(2): 334–5. [DOI] [PubMed] [Google Scholar]
- 3.Traubici J The double bubble sign. Radiology. 2001. August; 220(2): 463–4. [DOI] [PubMed] [Google Scholar]
- 4.Gilbertson-Dahdal DL, Dutta S, Varich LJ, Barth RA. Neonatal malrotation with midgut volvulus mimicking duodenal atresia. AJR Am J Roentgenol. 2009. May; 192(5): 1269–71. [DOI] [PubMed] [Google Scholar]
- 5.Ohuoba E, Fruhman G, Olutoye O, Zacharias N. Perinatal survival of a fetus with intestinal volvulus and intussusception: a case report and review of the literature. AJP Rep. 2013. October; 3(2): 107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eovaldi BJ, Cohen H. Duodenal Atresia And Stenosis [Updated 2018 Nov 13] StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018. January Available from https://www.ncbi.nlm.nih.gov/pubmed/29261981 [PubMed] [Google Scholar]
- 7.Choudhry MS, Rahman N, Boyd P, Lakhoo K. Duodenal atresia: associated anomalies, prenatal diagnosis and outcome. Pediatr Surg Int. 2009. August; 25(8): 727–30. [DOI] [PubMed] [Google Scholar]
- 8.Sonek J, Croom C. Second trimester ultrasound markers of fetal aneuploidy. Clin Obstet Gynecol. 2014. March; 57(1): 159–81. [DOI] [PubMed] [Google Scholar]
- 9.Kucińska-Chahwan A, Posiewka A, Bijok J, Jakiel G, Roszkowski T. Clinical significance of the prenatal double bubble sign - single institution experience. Prenat Diagn. 2015. November; 35(11): 1093–6. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, He P, Li DZ. Clinical outcome of pregnancies with the prenatal double bubble sign - a five-year experience from one single centre in mainland China. J Obstet Gynaecol. 2018. February; 38(2): 206–9. [DOI] [PubMed] [Google Scholar]
- 11.United States Census Bureau. Population estimates, July 1, 2018 [cited 2018 Jan 28]. Available from https://www.census.gov/quickfacts/md
- 12.Freeman SB, Torfs CP, Romitti PA, Royle MH, Druschel C, Hobbs CA, et al. Congenital gastrointestinal defects in Down syndrome: a report from the Atlanta and National Down Syndrome Projects. Clin Genet. 2009. February; 75(2): 180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jassani MN, Gauderer MW, Fanaroff AA, Fletcher B, Merkatz IR. A perinatal approach to the diagnosis and management of gastrointestinal malformations. Obstet Gynecol. 1982. January; 59(1): 33–9. [PubMed] [Google Scholar]
- 14.Nicolaides KH, Snijders RJ, Cheng HH, Gosden C. Fetal gastro-intestinal and abdominal wall defects: associated malformations and chromosomal abnormalities. Fetal Diagn Ther. 1992; 7(2): 102–15. [DOI] [PubMed] [Google Scholar]
- 15.Hou JW, Wang TR. Amelia, dextrocardia, asplenia, and congenital short bowel in deleted ring chromosome 4. J Med Genet. 1996. October; 33(10): 879–81. [DOI] [PMC free article] [PubMed] [Google Scholar]