Abstract
Background:
Previous imaging studies using Positron Emission Tomography (PET) have shown that alcohol use disorder (AUD) is associated with a decrease in dopamine type 2/3 receptor (D2/3) binding and dopamine transmission. Although binge drinking is a risk factor for future AUD, little is known about the neurobiology of binge drinking in young adults. This study measured D2/3 receptor binding and stimulant-induced dopamine release using PET and [11C]raclopride in binge drinkers without an AUD.
Methods:
This study included 14 healthy controls (HC) and 14 young adult binge drinkers (BD), aged 18–25. The BD met National Institute on Alcohol Abuse and Alcoholism (NIAAA) criteria for binge drinking and the HC subjects were social drinkers. The subjects were scanned with [11C]raclopride before and after the administration of oral methylphenidate (60 mg) to measure D2/3 binding and dopamine release.
Results:
There was no significant difference in the PET measures of D2/3 binding or methylphenidate-induced dopamine release between the two groups. There was no significant association between Alcohol Use Disorders Identification Test (AUDIT) scores or 30-day drinking history and the imaging data.
Conclusion:
In this sample of 18–25-year-old binge drinkers without a diagnosis of a substance use disorder, there were no significant differences in D2/3 receptor binding potential or methylphenidate-induced dopamine release relative to healthy controls.
Keywords: Alcohol, Binge drinking, Dopamine, PET, Imaging, Raclopride
1. Introduction
Excessive alcohol drinking in young adulthood is a persistent public health problem (Hasin et al., 2007; Mokdad et al., 2004). The National Survey on Drug Use and Health indicates that 37.8% of college students and 32.6% of non-college young adults (ages 18–22) binge drank in the last month (SAMHSA, 2016). Even though binge drinking is associated with an increased risk of future alcohol use disorder (AUD) (Bonomo et al., 2004; Hasin et al., 2001), little is known about the neurobiology of binge drinking in this age group and how it compares to the known alterations seen in AUD.
Previous imaging studies have shown that AUD is associated with a disruption in striatal dopamine neurotransmission. Perhaps the most recognized neurobiological marker of addiction is low dopamine type 2/3 receptor (D2/3) binding measured with Positron Emission Tomography (PET) (for review see (Trifilieff and Martinez, 2014)). Low D2/3 receptor binding has been shown in at least eight human studies of AUD (Heinz et al., 2005, 2004; Hietala et al., 1994; Martinez et al., 2005; Rominger et al., 2012; Volkow et al., 1996, 2002; Volkow et al., 2007), and in laboratory animals, low D2/3 receptor binding has been shown to predict alcohol self-administration (for review see (Trifilieff and Martinez, 2014)).
In addition to low D2/3 receptor availability, low dopamine transmission has been demonstrated in PET research of AUD. Dopamine transmission can be measured by obtaining scans with [11C]raclopride before and after the administration of a psychostimulant, such as amphetamine or methylphenidate. Two studies have used these methods in AUD and both showed that dopamine transmission was blunted compared to matched controls (Martinez et al., 2005; Volkow et al., 2007). Another PET study used [18F]DOPA to measure pre-synaptic dopamine synthesis capacity in AUD, and showed that low radiotracer uptake correlated with greater craving for alcohol (Heinz et al., 2005). Taken together, these PET studies indicate that AUD is associated with a decrease in striatal dopamine signaling which correlates with a greater severity of disease.
In this study, PET was used to investigate this neurobiology in binge drinking. A group of young adult binge drinkers and matched control subjects underwent two scans with [11C]raclopride before and after a psychostimulant challenge (methylphenidate 60 mg PO) in order to obain baseline measures of D2/3 receptor binding and methylphenidate-induced dopamine release. Based on previous research, we hypothesized that binge drinking subjects would show a decrease in baseline D2/3 receptor binding and decreased stimulant-induced dopamine increase.
2. Methods and materials
2.1. Subjects
The study was approved by the Institutional Review Board of the New York State Psychiatric Institute and all subjects provided written informed consent. Inclusion criteria for the binge drinking (BD) subjects were as follows: 1) ages 18–25 years old; 2) absence of significant medical illness; 3) absence of past or current substance use disorder, with a negative urine toxicology, but meet the National Institute on Alcohol Abuse and Alcoholism (NIAAA) definition of binge drinking (NIAAA, 2015). The NIAAA criteria consists of a pattern of drinking alcohol that brings blood alcohol concentration (BAC) to 0.08 g/dl or above. For the typical adult, this corresponds to consuming 5 or more drinks in males and 4 or more drinks in females within two hours. The BD subjects were required to have at least 4 binge drinking episodes resulting in acute intoxication in the month prior to study entry.
The criteria for healthy controls (HC) were: 1) ages 18–25 years old; 2) absence of psychiatric or medical illness; 3) absence of past or current substance use disorder, with a negative urine toxicology; 4) meet criteria for social drinking defined as no more than one drink a day for women, and no more than two drinks a day for men over the past month (NIAAA, 2016). The control subjects could not have consumed more than 7 (women) or 14 (men) drinks per week in the last month to meet NIAAA criteria for low-risk drinking, and could not have had more than two episodes of binge drinking in the past year.
Each subject underwent a psychiatric and medical evaluation to determine that these criteria were met. The Structured Clinical Interview for DSM-IV (SCID-IV) and a psychiatric interview by a psychiatrist was used to verify lack of psychiatric disorders. The time-line follow-back interview (Maisto et al., 1982) was used to estimate daily drinking over the 30 days prior to study entry. Socioeconomic position was assessed using years of education as a proxy (Sirin, 2005). The Alcohol Use Disorder Identification Test (AUDIT) was used to assess harmful alcohol consumption (Saunders et al., 1993). Participants were requested to be abstinent from alcohol two days prior to scanning and were breathalyzed on the day of scan to ensure that they were not intoxicated (breath alcohol content = 0.0%).
2.2. Imaging methods
The PET scans were obtained at the Yale University Positron Emission Tomography Center using the High Resolution Research Tomograph (HRRT, Siemens/CTI, Knoxville, TN) in list mode and reconstructed using the MOLAR algorithm. Subjects underwent two scans with [11C]raclopride, administered as a bolus and acquired over 60 min. Following a baseline scan, subjects were administered oral methylphenidate (60 mg) in an open-labelled fashion before undergoing a second scan using methods previously described (Martinez et al., 2011; Volkow et al., 2001). The PET data were analyzed using the Simplified Reference Tissue Model (Lammertsma and Hume, 1996) with the cerebellum as a reference region. The PET outcome measure was binding potential BPND= fND *BAVAIL/KD, where ND refers to the non-displaceable compartment, fND is the free fraction of the free plus non-specifically bound tracer in brain, BAVAIL is the concentration of D2/3 receptors available to bind to the tracer (nmol *cm-3 of tissue), and KD is the equilibrium dissociation constant (Slifstein and Laruelle, 2001). The percent change in [11C]raclopride BPND following methylphenidate administration was calculated as ΔBPND = (BPND methylphenidate – BPND baseline)/BPND baseline*100 (Innis et al., 2007).
Each subject had a T1-weighted structural MRI scan for identification of the regions of interest (ROIs). The analysis of the [11C]raclopride scans was limited to the striatum which was divided into 5 subdivisions: 1) the caudate (anterior and posterior); 2) the putamen (anterior and posterior); and 3) the ventral striatum, which includes the nucleus accumbens, ventral caudate, and ventral putamen, as described previously (Martinez et al., 2003).
2.3. Statistical analysis
Demographic variables were compared with two-group t-tests or Chi-Squared statistics. PET measures were tested in a mixed model framework with ROIs as repeated measures and ROI and group as fixed regressors. Relationships between PET data and clinical characteristics were analyzed with Pearson product moments. A two-tailed probability value of p ≤ 0.05 was chosen as the level of significance.
3. Results
3.1. Group composition
14 HC and 14 BD subjects completed the PET scans with [11C]raclopride (see Table 1). The subjects were matched for cigarette smoking (only 2 smokers in each group, who did not meet criteria for tobacco use disorder) and ethnicity (HC: 7 African American, 5 Hispanic, 2 Caucasian; BD: 9 African American, 3 Hispanic, 1 Caucasian, 1 Asian). The BD group reported more recent cannabis use than the HC (5 of the BD reported weekly use, with no weekly use reported in HC). No subjects met criteria for cannabis dependence per DSM-IV. The BD group also reported higher AUDIT scores, more drinks in the past month, and began drinking at an earlier age compared to the HC group (reported in Table 1).
Table 1.
Demographic data.
| HC | BD | p | |
|---|---|---|---|
| Sex | 6M/8F | 10M/4F | 0.14 |
| Age (yrs) | 22.9 ± 1.3 | 22.9 ± 1.8 | 1 |
| Age started drinking (yrs) | 20.2 ± 1.3 | 17.4 ± 1.8 | < 0.01 |
| Years education | 15.2 ± 2.3 | 13.7 ± 1.9 | 0.07 |
| Family history of addiction (n) | 6 | 8 | 0.47 |
| AUDIT | 3.7 ± 2.3 | 12.6 ± 7.0 | < 0.01 |
| Drinks/30 days | 7.4 ± 7.0 | 84.9 ± 93.3 | <0.01 |
Abbreviations: HC = healthy control and BD = binge drinker.
3.2. Imaging results
3.2.1. Scan Parameters
There was no significant difference in the [11C]raclopride injected dose between the HC and BD subjects both at baseline (HC: 13.8 ± 3.9 mCi; BD: 14.7 ± 2.4 mCi, p = 0.5) and post-methylphenidate (HC: 13.5 ± 4.3 mCi; BD: 13.7 ± 2.7 mCi, p = 0.9). There were also no significant differences in injected mass between the groups at baseline (HC: 1.26 ± 1.11 μg; BD: 0.87 ± 0.98 μg, p = 0.3) and post-methylphenidate (HC:1.49 ± 1.25 μg; BD: 0.81 ± 0.68 μg, p = 0.08). The average specific activity was 1932 ± 967 Ci/mmoles for HC and 1787 ± 955 Ci/mmoles for the BD (p = 0.7) at baseline and 1881 ± 731 Ci/mmoles for HC and 1570 ± 753 Ci/mmoles for the BD (p = 0.3) post-methylphenidate.
3.2.2. Baseline D2 receptor availability and methylphenidate-induced dopamine release
There were no significant group differences in receptor availability (BPND, F(1,26) = 0.802, p = 0.379) or dopamine increase (ΔBPND, F(1,26) = 0.432, p = 0.517) in the mixed models (Fig. 1). Methylphenidate significantly decreased striatal [11C]raclopride binding in the HC (p < 0.001) and BD (p < 0.001) groups, as well as in the entire sample (p < 0.001). Table 2 shows group means ± SD for BPND and ΔBPND with p values from two-group t-tests for illustration.
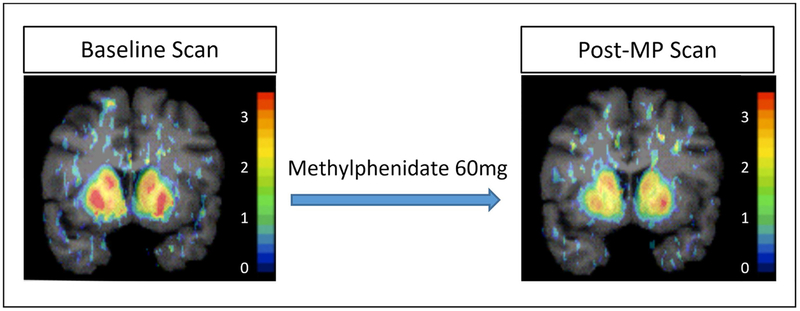
Fig. 1. Representative PET scans from binge drinking participants and healthy controls.
The scan on the left is at baseline, while the scan on the right is after the oral methylphenidate 60 mg challenge. Post-challenge, synaptic dopamine is increased so that there are fewer available D2/3 receptors to bind to [11C]raclopride. Groups did not differ in their baseline scans, or in percent difference after the methylphenidate challenge. The color bar shows values for BPND. Abbreviations: MP = methylphenidate.
Table 2.
Comparison of [11C]raclopride BPND and ΔBPND between groups.
| ROI | HC BPND | BD BPND | p | HC ΔBPND | BD ΔBPND | p |
|---|---|---|---|---|---|---|
| prePU | 3.15 ± 0.29 | 3.30 ± 0.30 | 0.20 | 13 ± 7% | 14 ± 5% | 0.65 |
| preCA | 2.43 ± 0.26 | 2.63 ± 0.34 | 0.09 | 10 ± 8% | 14 ± 8% | 0.18 |
| postPU | 3.28 ± 0.33 | 3.36 ± 0.40 | 0.55 | 22 ± 10% | 19 ± 10% | 0.50 |
| postCA | 1.75 ± 0.38 | 1.84 ± 0.35 | 0.51 | 12 ± 14% | 16 ± 11% | 0.40 |
| VST | 2.52 ± 0.20 | 2.60 ± 0.25 | 0.36 | 12 ± 5% | 14 ± 6% | 0.56 |
| STR | 2.79 ± 0.23 | 2.92 ± 0.33 | 0.24 | 15 ± 7% | 15 ± 6% | 0.75 |
All values are mean ± standard deviation (SD). Abbreviations: pre/post = anterior (pre-) or posterior (post-) to the anterior commissure. ROI = region of interest, PU = putamen, CA = caudate, VST = ventral striatum, STR = whole striatum, HC = healthy control, and BD = binge drinker.
3.2.3. Relationships between scan data and clinical measures
There were no significant associations between AUDIT score and [11C]raclopride BPND or ΔBPND in BD or HC subjects (p > 0.3 in all cases). There were no significant associations between 30-day drinking history and [11C]raclopride BPND or ΔBPND.
4. Discussion
This study did not find any difference in D2/3 receptor availability or dopamine release in binge drinkers when compared to healthy controls. Additionally, AUDIT and 30-day drinking scores were not significantly associated with the PET outcome measures.
Our findings did not support the hypothesis that binge drinking is associated with decreased striatal D2/3 binding potential or blunted dopamine transmission. We had expected to find measures of reduced striatal dopamine signaling, as has been reported previously for AUD (for review see (Ravan et al., 2014)). The mostly likely reason for our findings is that our study sample included binge drinking young adults who did not meet criteria for AUD. Thus, while this sample is at risk for developing AUD (Bonomo et al., 2004; Hasin et al., 2001), they had not met this milestone.
Casey et al. (Casey et al., 2014) previously performed a PET study imaging striatal dopamine signaling in a similar population of young adults. They used [11C]raclopride and an amphetamine challenge to measure BPND and ΔBPND in volunteers with a high risk of developing a substance use disorder, but who did not meet this criteria. They scanned three groups of subjects: 1) drug users (cocaine or amphetamines) with a multigenerational family history of a substance use disorder; 2) drug using subjects without a family history; and 3) drug naive healthy controls, also without a family history. Their results showed that subjects with cocaine/amphetamine use and a family history of a substance use disorder had blunted measures of ΔBPND compared to the other two groups. Subjects with risky stimulant use, but without a family history, did not differ from the drug naïve controls. Our study shows a similar finding, although without the group of high-risk family history positive subjects. We saw no difference in BPND and ΔBPND when comparing risky alcohol users to controls. However, our study sample did not include enough subjects with a strong family history of addiction to allow for an investigation of this factor. Thus, a future study of binge drinkers with a prominent family history of addiction would be needed to address this relationship.
4.1. Limitations
This study has several limitations that should be recognized. We had a relatively small number of subjects per group with a wide range of 30-day drinking in the BD group. Also, since participants were not detoxified or hospitalized, we could only rely on self-report for the duration of abstinence. The BD group also had more regular cannabis users than the HC group. Although earlier studies did not observe dopaminergic differences with chronic cannabis use (Urban et al., 2012), a more recent study with severe cannabis dependence did find a decrease in dopamine release (van de Giessen et al., 2017). The subjects in the current study neither met criteria for a cannabis use disorder nor exhibited chronic or heavy use, thus we would not expect this to affect our results.
Our procedure for imaging the D2 receptor and dopamine release cannot inform us about intracellular dopamine stores, only receptor availability and dopamine release in the synapse. Thus, any changes in dopaminergic stores that did not affect receptor availability or neurotransmitter transmission would not have been detected by this study. We also did not study any expectancy effects using placebos or substance cues, which has been recently reported to alter dopamine transmission (Wang et al., 2019). In our study, non-specific binding was not measured. Thus, it is possible that the lack of a between group difference in BPND or ΔBPND could have resulted from differences in non-specific binding. Additionally, we did not measure methylphenidate levels. However, our challenge procedure has been previously shown to decrease dopamine BPND, and methylphenidate levels have not been shown to correlate with dopamine release (Volkow et al., 2001)
5. Conclusion
In this sample of 18–25-year-old binge drinkers without a diagnosis of a substance use disorder, there were no significant differences in D2/3 receptor binding potential or methylphenidate-induced dopamine release. Our findings are in contrast to PET findings in participants with AUD and in drug users with a strong family history of a substance use disorder, where decreased measures of BPND and ΔBPND have been observed. Further study should examine the changes in the dopaminergic system in binge drinkers with a strong family history of substance use disorders.
Role of funding source
This work was supported by the National Institutes of Health grants T32 DA007294 (JMW) and R01 AA017648 (DM [Columbia site]).
Footnotes
Declaration of Competing Interest
None.
References
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC, 2004. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addiction 99, 1520–1528. [DOI] [PubMed] [Google Scholar]
- Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M, 2014. Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction. Biol. Psychiatry 76, 23–30. [DOI] [PubMed] [Google Scholar]
- Hasin D, Paykin A, Endicott J, 2001. Course of DSM-IV alcohol dependence in a community sample: effects of parental history and binge drinking. Alcohol. Clin. Exp. Res 25, 411–414. [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF, 2007. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 64, 830–842. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P, 2005. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am. J. Psychiatry 162, 1515–1520. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P, 2004. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am. J. Psychiatry 161, 1783–1789. [DOI] [PubMed] [Google Scholar]
- Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P, Ruotsalainen U, 1994. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology 116, 285–290. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE, 2007. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab 27, 1533–1539. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP, 1996. Simplified reference tissue model for PET receptor studies. NeuroImage 4, 153–158. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Sobell LC, Cooper AM, Sobell MB, 1982. Comparison of two techniques to obtain retrospective reports of drinking behavior from alcohol abusers. Addict. Behav 7, 33–38. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E, 2011. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am. J. Psychiatry 168, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A, 2005. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol. Psychiatry 58, 779–786. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M, 2003. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J. Cereb. Blood Flow Metab 23, 285–300. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL, 2004. Actual causes of death in the United States, 2000. JAMA 291, 1238–1245. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2015. College Drinking. National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2016. Rethinking Drinking: Alcohol and Your Health. National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD. [Google Scholar]
- Ravan S, Martinez D, Slifstein M, Abi-Dargham A, 2014. Molecular imaging in alcohol dependence. Handb. Clin. Neurol 125, 293–311. [DOI] [PubMed] [Google Scholar]
- Rominger A, Cumming P, Xiong G, Koller G, Boning G, Wulff M, Zwergal A, Forster S, Reilhac A, Munk O, Soyka M, Wangler B, Bartenstein P, la Fougere C, Pogarell O, 2012. [18F]Fallypride PET measurement of striatal and extrastriatal dopamine D 2/3 receptor availability in recently abstinent alcoholics. Addict. Biol 17, 490–503. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2016. Results From the 2015 National Survery on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M, 1993. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption— II. Addiction 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Sirin SR, 2005. Socioeconomic status and academic achievement: a meta-analytic review of research. Rev. Educ. Res 75, 417–453. [Google Scholar]
- Slifstein M, Laruelle M, 2001. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl. Med. Biol 28, 595–608. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D, 2014. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology 76, 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, Haney M, Abi-Dargham A, 2012. Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol. Psychiatry 71, 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Giessen E, Weinstein JJ, Cassidy CM, Haney M, Dong Z, Ghazzaoui R, Ojeil N, Kegeles LS, Xu X, Vadhan NP, Volkow ND, Slifstein M, Abi-Dargham A, 2017. Deficits in striatal dopamine release in cannabis dependence. Mol. Psychiatry 22, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D, 2001. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J. Neurosci 21, Rc121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K, 1996. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol. Clin. Exp. Res 20, 1594–1598. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N, 2002. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 116, 163–172. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, 2007. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J. Neurosci 27, 12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Wiers CE, Shumay E, Tomasi D, Yuan K, Wong CT, Logan J, Fowler JS, Volkow ND, 2019. Expectation effects on brain dopamine responses to methylphenidate in cocaine use disorder. Transl. Psychiatry 9, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]