Abstract
Autologous stem cell transplantation (ASCT) is an integral component of the therapeutic arsenal in multiple myeloma. Given that overall survival (OS) is comparable between patients receiving up-front or delayed ASCT, some opt to collect stem cells and postpone transplant until the time of disease progression (i.e. salvage ASCT). It is unknown if induction should be repeated prior to salvage ASCT, or if patients should proceed directly. We identified 234 patients who underwent salvage ASCT at our institution: 188 (80%) were re-induced, whereas 46 (20%) proceeded directly without re-induction. There was no significant difference in time to next therapy (TNT) or OS from Day 0 between the two groups. Patients who were re-induced had a nonsignificant trend towards a higher rate of complete response post-ASCT (45% vs. 33%, p= .12). In multivariate models, re-induction did not affect TNT/OS. In the subgroup of 188 patients who were re-induced, patients with relapsed/refractory disease at the time of ASCT had significantly shorter TNT/OS compared to patients with deeper pre-ASCT responses. In summary, there was no survival difference for patients who were re-induced before salvage ASCT. However, many factors affect the decision to re-induce, and prospective studies would be required to discern its role definitively.
Keywords: autologous stem cell transplant, multiple myeloma, salvage, re-induction
INTRODUCTION
While multiple myeloma (MM) remains to a large extent incurable,1, 2 novel therapies, i.e. proteasome inhibitors (PI), immunomodulatory drugs (IMiD), and monoclonal antibodies have markedly extended survival.3,4 Each wave of new therapies has sparked a re-examination of the role of autologous stem cell transplantation (ASCT), which has been a hallmark of disease management for decades.5–7 However, ASCT has largely withstood the test of time as a distinct, highly effective treatment modality,8, 9 and MM remains the most common indication for ASCT in the United States.10, 11
The timing of ASCT during the treatment of MM remains an open question.12 The Intergroupe Francophone du Myélome (IFM) 2009 trial demonstrated that patients receiving up-front ASCT have improved progression-free survival (PFS), but similar overall survival (OS) compared to patients where ASCT was only offered after first disease progression (172 patients in the latter arm received second-line therapy; 136/172 [79%] underwent delayed ASCT).8 These findings are consistent with other retrospective reports showing no OS benefit for patients receiving early versus delayed ASCT.13, 14 As such, it is not uncommon to store stem cells and delay ASCT until later in the disease course.15
While the prognostic significance of achieving a durable complete response (CR), particularly after ASCT, is well documented,16–18 the impact of the depth of pre-ASCT response on post-ASCT survival is limited. One study using the Center for International Blood and Marrow Transplant Research (CIBMTR) database demonstrated that in patients achieving less than a partial response (PR) after induction, additional therapy before up-front ASCT did not improve PFS or OS from the date of stem cell infusion (Day 0).19
There is no data regarding the impact of re-treating with induction prior to salvage ASCT, i.e. when transplant is delayed until after disease progression. It should be noted that in the aforementioned IFM 2009 trial, the 136 patients who underwent delayed transplant received mostly PI- or IMiD-based regimens before salvage ASCT.8 However, it is unknown if patients who are re-induced have superior outcomes compared to patients proceeding directly to salvage ASCT.
While undoubtedly many factors, such as age, the kinetics of progression, clinical symptoms attributed to relapse, prior disease responsiveness, and patient preference affect the choice to re-induce prior to salvage ASCT, the paucity of literature on the topic motivated the present study. Further, we sought to determine prognostic factors for survival after salvage ASCT in the era of novel therapies.
METHODS
We identified 380 patients with MM who underwent their first ASCT >12 months after diagnosis from January 1, 2006 to January 1, 2017 at Mayo Clinic Rochester. 121 had a delayed transplant, but no progression prior to ASCT and were excluded. Another 17 patients had less than a PR after their initial treatment, and thus were deemed primary refractory and were excluded. Finally, 8 patients were excluded because they received a planned tandem ASCT. We did not exclude 22 patients who went on to receive a second salvage ASCT later in their treatment course, i.e. at least 12 months after their first ASCT. A total of 234 patients undergoing their first ASCT were included in the present study, which was approved by our Institutional Review Board, in accordance with the Declaration of Helsinki.
Patients were diagnosed according to contemporaneous International Myeloma Working Group (IMWG) criteria.20, 21 Likewise, the IMWG Uniform Response Criteria were used.22, 23 Post-ASCT response was determined on Day +60 or after, with the best-response until progression also captured. The international staging system (ISS) was used at diagnosis.24 High-risk cytogenetics were defined as t(4;14), t(14;16), t(14;20) and del 17p/monosomy 17. Serum free light chain ratios outside the range of 0.26 to 1.65 were considered abnormal.25
In the manuscript, the date of progression after which salvage ASCT was deemed necessary is referred to as T0. Further, “pre-ASCT” refers to the laboratory findings immediately prior to Day 0. We examined the use of re-induction after T0, prior to Day 0, as outlined in Figure 1.
Figure 1.

a. Schematic demonstrating the two study groups. T0 represents the date of progression, after which salvage ASCT was deemed necessary. After T0, patients received either re-induction with new anti-myeloma (MM) therapies (n=188), or proceeded directly to salvage ASCT without re-induction (n=46). “Pre-ASCT” refers to the laboratory findings immediately prior to the date of stem cell infusion (Day 0).
We categorized progression (T0) “on-therapy” in patients who were on continuous therapy or completed anti-myeloma therapy within the preceding 60 days, and thus met the criteria for refractory disease.26 Progression >60 days after stopping therapy was defined as progression (T0) “off-therapy.” Duration of response (DOR) prior to T0 was defined as the time from achievement of at least PR to T0. Lines of therapy were defined according to Rajkumar et al.27 For the assessment of treatment response after re-induction (prior to salvage ASCT), relapsed/refractory (R/R) disease was defined as meeting progression criteria while on re-induction therapy, or progressing within 60 days of stopping treatment in patients who at least achieved at least a minimal response.27
Time to next therapy (TNT) was defined as the time from Day 0 to the initiation of a new therapy, but not including planned consolidation or maintenance. Patients who were alive and had not received a new therapy after ASCT, or died without receiving another therapy, were censored at the time of last follow-up, as of May 15, 2018. Progression-free survival (PFS) was defined as the time from ASCT to disease progression or death, with patients alive and progression-free censored at the time of last follow-up. Overall survival (OS) was defined as the time from Day 0 to death from any cause, with patients who remained alive censored at the time of last follow-up. TNT, PFS, and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazard models were used for TNT/OS. Logistic regression models were used to determine factors associated with best-response post-ASCT. Variables with p< .10 in univariate models were included in multivariate models. Median follow-up time was calculated using the reverse Kaplan-Meier method.28 The Mann-Whitney U test and Fisher’s Exact test were used to compared continuous and categorical variables, respectively. Statistical analysis was completed using JMP 14.0 (SAS Institute Inc., Cary, NC, USA) and EZR 1.36.29
RESULTS
Characteristics at diagnosis and T0
Two hundred and thirty-four patients underwent salvage ASCT: 188 (80%) were re-induced after T0, whereas 46 (20%) proceeded directly without re-induction. Baseline characteristics were comparable between the two groups at diagnosis (Table 1), including age, ISS stage, and presence of high-risk cytogenetics. Initial induction regimens and deepest response prior to T0 were also similar.
Table 1.
Characteristics at diagnosis and T0, both for the entire cohort (n=234) and split by treatment group, i.e. patients that proceeded directly to salvage ASCT (n=46) or received re-induction (n=188).
| Characteristic | Entire cohort n=234 | Direct to ASCT n=46 | Re-induction n=188 | P |
|---|---|---|---|---|
| Age at diagnosis, median (range) | 60 (27-75) | 61 (27-70) | 60 (36-75) | .35 |
| Male, n (%) | 136 (58) | 29 (63) | 107 (57) | .51 |
| Type of Myeloma, n (%) | ||||
| IgG | 125 (53) | 27 (59) | 98 (52) | .52 |
| IgA | 54 (23) | 7 (15) | 47 (25) | |
| Light chain | 47 (20) | 10 (22) | 37 (20) | |
| Other | 8 (3) | 2 (4) | 6 (3) | |
| ISS at diagnosis, n (%) | ||||
| I | 68 (29) | 13 (28) | 55 (29) | .37 |
| II | 63 (27) | 17 (37) | 46 (24) | |
| III | 42 (18) | 7 (15) | 35 (19) | |
| Missing data | 61 (26) | 9 (20) | 52 (28) | |
| FISH cytogenetics at diagnosis, n (%) | ||||
| Standard risk | 136 (58) | 29 (63) | 107 (57) | .55 |
| High risk | 33 (14) | 4 (9) | 29 (15) | |
| Missing data | 65 (28) | 13 (28) | 52 (28) | |
| Time from diagnosis to stem cell collection in months, median (range) | 9.2 (0.2-102.0) | 8.2 (3.7-65.1) | 11.2 (0.2-102.0) | .0082 |
| >12 months, n (%) | 103 (44) | 12 (26) | 91 (48) | .0077 |
| ≤12 months, n (%) | 131 (56) | 34 (74) | 97 (52) | |
| Bone marrow plasma cell % at stem cell collection, median (range) | 5 (0-90) | 5 (0-80) | 5 (0-90) | .58 |
| >10%, n(%) | 71 (32) | 15 (33) | 56 (30) | .83 |
| ≤10%, n(%) | 153 (68) | 30 (65) | 123 (65) | |
| Missing data, n(%) | 10 (4) | 1 (2) | 9 (5) | |
| Time from diagnosis to T0 in months, median (range)* | 26.5 (3.1-103.9) | 20.4 (7.5-85.7) | 27.3 (3.1-103.9) | .42 |
| Duration of response (DOR) prior to T0 in months, median (range)* | 15.2 (1.9-99.3) | 18.5 (3.6-83.9) | 14.7 (1.9-99.3) | .12 |
| Induction regimen(s) prior to T0, n (%)* | ||||
| 1 Line | 151 (65) | 34 (74) | 117 (62) | .37 |
| Triplet | 48 | 12 | 36 | |
| Doublet | 98 | 18 | 80 | |
| Other | 3 | 2 | 1 | |
| No novel agents | 2 | 2 | 0 | |
| 2 lines | 50 (21) | 7 (15) | 43 (23) | |
| ≥3 lines | 33 (14) | 5 (11) | 28 (15) | |
| Deepest response prior to T0, n (%)* | ||||
| sCR | 16 (7) | 4 (9) | 12 (6) | .45 |
| CR | 44 (19) | 7 (15) | 37 (20) | |
| VGPR | 97 (41) | 16 (35) | 81 (43) | |
| PR | 77 (33) | 19 (41) | 58 (31) | |
| Time since most recent treatment prior to T0, n (%)* | ||||
| ≤60 days (“on-therapy”) | 156 (67) | 35 (76) | 121 (64) | .16 |
| >60 days (“off-therapy”) | 78 (33) | 11 (24) | 67 (36) | |
| Days off therapy to T0, median (range) | 324 (65-2979) | 333 (65-1817) | 287 (80-2979) | .98 |
| Classification of Progression at T0, n(%)* | ||||
| Biochemical† | 192 (82) | 43 (94) | 149 (79) | .0347 |
| Clinical only (e.g. new bone lesion or plasmacytoma) | 37 (16) | 2 (4) | 35 (19) | |
| Free light chain increase only‡ | 5 (2) | 1 (2) | 4 (2) | |
| Serum M-protein present at T0, n (%)*§ | 171 (73) | 37 (80) | 134 (71) | .27 |
| Serum M-protein at T0 (g/dL), median (range)*§ | 1.1 (0.01-7.2) | 1.1 (0.01-2.7) | 1.05 (0.01-7.2) | .52 |
Bold denotes p<.05
T0 is the date of progression, after which salvage ASCT was deemed necessary.
M-protein increase (n=148); free light chain increase if light chain myeloma (n=41); bone marrow plasma cell increase if non-secretory (n=3).
Although not meeting strict IMWG progression criteria,26 free light chain increases in these patients were treated as a biochemical progression, i.e. free light chain escape.
Among patients with measurable serum M-protein. Unmeasurable fragments counted as 0.01 g/dL.
Bone marrow plasma cell (BMPC) percentage at stem cell collection was comparable between the two groups (5% vs. 5%, p= .58). However, patients in the re-induction group had a longer time from diagnosis to stem cell collection (median 11.2 vs. 8.2 months, p= .0082). There were also trends towards more patients in the re-induction group having been treated with >1 line of therapy prior to T0 (38% vs. 26%, p= .17) and having a shorter DOR prior to T0 (14.7 vs. 18.5 months, p= .12).
Overall, the majority (82%) of patients met biochemical IMWG progression criteria at T0 (Table 1). More patients in the re-induction group met clinical progression criteria only, e.g. from new or expanding bone lesions or plasmacytomas (19% vs. 4%, p= .0347).
Re-induction regimens
Among the 188 patients who were re-induced, most (n=134, 71%) received one line of therapy (Table 2). Sixty-six of these received a triplet regimen. Thirty-four patients (18%) received two lines of re-induction; twenty-four of these received ≥1 alkylating agent. Finally, 20 patients (11%) received ≥3 lines of re-induction; nearly all (n=18) received ≥1 alkylating agent, and eight were treated with VD PACE-like regimens. Just 6/188 patients (3%) overall were re-induced with a monoclonal antibody-containing regimen.
Table 2.
Regimens used in the patients (n=188) who were re-induced prior to salvage ASCT.
| Re-induction therapy* | n (%) |
|---|---|
| 1 line | 134 (71) |
| Triplet-regimen | 66 |
| CyBorD | 35 |
| VRD | 18 |
| Other (e.g. KRd) | 13 |
| Doublet-regimen | 56 |
| Rd | 27 |
| Vd | 22 |
| Other (e.g. Kd) | 7 |
| Alkylating agent (besides CyBorD), VAD, or VD PACE | 8 |
| Daratumumab/Elotuzumab-containing regimen | 4 |
| 2 lines | 34 (18) |
| ≥1 Alkylating agent | 24 |
| Daratumumab/Elotuzumab-containing regimen | 2 |
| Other | 8 |
| ≥3 lines | 20 (11) |
| ≥1 Alkylating agent | 18 |
| VD PACE-like regimen | 8 |
| Other | 2 |
Re-induction regimens:
CyBorD = cyclophosphamide + bortezomib + dexamethasone
VRd = bortezomib + lenalidomide + dexamethasone
KRd = carflilzomib + lenalidomide + dexamethasone
Rd = lenalidomide + dexamethasone
Vd = bortezomib + dexamethasone
Kd = carfilzomib + dexamethasone
Alkylating agent = melphalan, bendamustine, or cyclophosphamide.
VAD = bortezomib + doxorubicin + dexamethasone.
VD PACE = bortezomib + dexamethasone + cisplatin + doxorubicin + cyclophosphamide + etoposide +/− thalidomide.
Pre-ASCT characteristics
Characteristics immediately prior to ASCT (“pre-ASCT”) (Table 3) were comparable. As expected, the re-induction group had a longer time from T0 to ASCT (5.3 vs. 2.3 months, p< .0001). Intuitively, the direct to ASCT group had a higher pre-ASCT BMPC percentage (median 15% vs. 5%, p= .0018) and a trend towards a greater serum M-protein (median 1.3 vs. 0.8 g/dL, p= .07).
Table 3.
Characteristics immediately prior to ASCT (“pre-ASCT”), both for the entire cohort (n=234) and split by treatment group, i.e. patients that proceeded directly to salvage ASCT (n=46) or received re-induction (n=188).
| Characteristic | Entire cohort n=234 | Direct to ASCT n=46 | Re-induction n=188 | P |
|---|---|---|---|---|
| Age at ASCT, median (range) | 64 (29-76) | 64 (29-74) | 63.5 (39-76) | .58 |
| Time period of ASCT, n (%) | ||||
| January 1, 2013 and after | 106 (45) | 17 (37) | 89 (47) | .25 |
| December 31, 2012 and before | 128 (55) | 29 (63) | 99 (53) | |
| Time from T0 to ASCT in months, median (range)* | 5.3 (0.2-37.0) | 2.3 (0.2-37.0) | 5.9 (1.2-18.0) | <.0001 |
| Serum creatinine (mg/dL), median (range)† | 1.0 (0.5-6.8) | 1.0 (0.5-5.1) | 0.9 (0.5-6.8) | .15 |
| Serum M-protein present, n (%)† | 179 (76) | 38 (83) | 141 (75) | .33 |
| Serum M-protein (g/dL), median (range)†§ | 0.8 (0.01-6.1) | 1.3 (0.01-6.1) | 0.8 (0.01-5.9) | .07 |
| Urine M-protein present, n (%)† | 148 (67) | 35 (78) | 113 (64) | .11 |
| Abnormal serum free light chain ratio, n (%)† | 187 (82) | 42 (91) | 145 (79) | .09 |
| Serum albumin (g/dL), median (range)† | 3.4 (2.4-4.1) | 3.4 (2.8-4.1) | 3.4 (2.4-4.1) | .80 |
| Serum B-2-microglobulin (µg/mL), median (range)† | 2.9 (1.2-32.1) | 3.2 (1.6-19.2) | 2.8 (1.2-32.1) | .17 |
| Abnormal B2M (≥3.5 µg/mL), n (%) | 70 (31) | 18 (39) | 52 (28) | .21 |
| Serum LDH (IU/L), median (range)† | 185 (100-819) | 174 (100-380) | 189 (105-819) | .13 |
| Abnormal LDH (≥222 IU/L), n (%) | 52 (23) | 8 (17) | 44 (24) | .43 |
| Bone marrow plasma cell %, median (range)† | 5 (0-95) | 15 (0-80) | 5 (0-95) | .0018 |
| Disease status relative to T0, n (%)*† | ||||
| ≥VGPR (after 1-2 lines‡) | 46 (20) | 0 | 46 (24) | <.0001 |
| PR or Stable Disease (after 1-2 lines‡) | 78 (33) | 0 | 78 (41) | |
| Relapsed/Refractory (and/or ≥3 lines‡) | 110 (47) | 46 (100) | 64 (34) | |
| Melphalan dose, n (%) | ||||
| 200 mg/m2 | 190 (81) | 38 (83) | 152 (81) | .35 |
| Reduced-dose (e.g. 140 mg/m2) | 39 (17) | 6 (13) | 33 (18) | |
| Other (e.g. +proteasome inhibitor) | 5 (2) | 2 (4) | 3 (2) | |
| CD34+ cell dose (×106 cells/kg), median (range) | 4.19 (2.06-12.69) | 4.19 (2.48-10.96) | 4.19 (2.06-12.69) | .70 |
| Maintenance post-ASCT, n (%) | 82 (35) | 15 (33) | 67 (36) | .58 |
| Consolidation post-ASCT, n (%) | 6 (3) | 0 | 6 (3) | .60 |
Bold denotes p<.05
T0 is the date of progression, after which salvage ASCT was deemed necessary.
Pre-ASCT, i.e. laboratory findings immediately prior to the date of stem cell infusion (Day 0).
Lines of re-induction therapy after T0, prior to salvage ASCT.
Among patients with measurable serum M-protein. Unmeasurable fragments counted as 0.01 g/dL.
Responses to re-induction prior to salvage ASCT
Relative to T0, 116/188 patients (62%) responded to re-induction: stringent complete response (sCR) in 8 (4%), CR in 8 (4%), VGPR in 39 (21%), and PR in 61 (32%). Another 37/188 patients (20%) had stable disease (SD) after re-induction. 35/188 patients (19%) met progression criteria relative to T0 on re-induction therapy and were deemed R/R. Furthermore, 15/116 patients with an initial response (≥PR) progressed on-therapy prior to ASCT and were classified as R/R. Of the remaining 138 patients with ≥SD that did not progress prior to ASCT, 14/138 were treated with ≥3 lines of re-induction therapy and were included in the R/R group. Thus, at the time of salvage ASCT, the R/R group contained 64/188 (34%) patients, while 78/188 (41%) had PR or SD, and 46/188 (24%) had ≥VGPR (Table 3).
Best-responses after salvage ASCT
The re-induction and direct to ASCT groups had similar Day +100 (Supplemental Figure 1) and best-responses (Figure 2a) after transplant, with a trend towards deeper best-responses in the re-induction group: 84/188 patients (45%) achieved ≥CR vs. 15/46 (33%) in the direct to ASCT group (p= .18). Notably, the usage of post-ASCT maintenance and consolidation were similar between the re-induction and direct to ASCT groups (Table 3). In a multivariate logistic regression model for best-response ≥CR post-ASCT (Supplementary Table 1), deepest response prior to T0, (≤VGPR vs. ≥CR) and BMPC percentage at stem cell collection (>10% vs. ≤10%) were associated with a significantly lower relative odds of achieving ≥CR post-ASCT.
Figure 2.
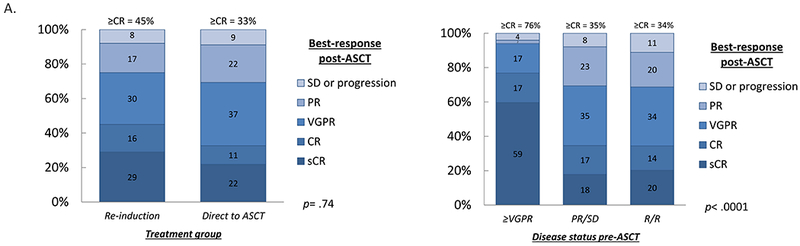
a. Best-response post-salvage ASCT in the patients who received re-induction (n=188) or proceeded directly to salvage ASCT (n=46).
b. Best-response post-salvage ASCT in patients (n=188) who received re-induction, split by disease status pre-ASCT: ≥VGPR (n=46), partial response (PR) or stable disease (SD) (n=78), or relapsed/refractory (R/R) (n=64). Numbers indicate percentages. Proportions were compared with Fisher’s exact test and the Kruskal–Wallis test, respectively.
When examining the 188 patients who were re-induced, patients with ≥VGPR pre-ASCT had significantly deeper best-responses after transplant, with 35/46 (76%) achieving ≥CR (Figure 2b). Just 27/78 (35%) of patients with PR/SD pre-ASCT achieved ≥CR; of the patients with R/R disease pre-ASCT, 22/64 (34%) achieved ≥CR (p< .0001).
Survival comparison between re-induction and direct to ASCT groups
The median TNT (mTNT) for the entire cohort from Day 0 was 17.2 months (95% CI 14.8–19.7); median OS (mOS) was 50.4 months (95% CI 43.3–60.7). With a median follow-up of 60.9 months (95% CI 55.0–68.9), TNT, PFS, and OS were comparable between the re-induction and direct to ASCT groups (Figure 3a–b and Supplementary Figure 2).
Figure 3.

a. Time to next therapy (TNT) from Day 0 amongst the entire cohort, split by whether patients received re-induction after T0, prior to salvage ASCT.
b. Overall survival (OS) from Day 0 amongst the entire cohort, split by whether patients received re-induction after T0, prior to salvage ASCT.
c. Time to Next Therapy (TNT) from Day 0 amongst patients who underwent re-induction prior to salvage ASCT (n=188), split by disease status pre-ASCT.
d. Overall survival (OS) from Day 0 amongst patients who underwent re-induction prior to salvage ASCT (n=188), split by disease status pre-ASCT. Kaplan-Meier curves were compared with the log-rank test.
Survival in the re-induction group
In a subgroup analysis of the 188 patients who were re-induced, disease status pre-ASCT significantly affected survival (Figure 3c–d). TNT at 1-year post-ASCT was 79.2% in the ≥VGPR group, 62.1% in the PR/SD group, and 45.7% in the R/R group (p= .0003). mOS was 86.1, 50.5, and 33.0 months for the three groups, respectively (p= .0033). Interestingly, time from diagnosis to T0 was comparable between the three groups (27 vs. 28 vs. 26 months, respectively, p= .26). However, the R/R group had a significantly more patients with high-risk cytogenetics (34% vs. 15% and 15% in the PR/SD and ≥VGPR groups, respectively, p= .0039) and shorter DOR prior to T0 (10.5 vs. 18.5 and 16.6 months, respectively, p= .0011) (Supplementary Tables 2 and 3). The median pre-ASCT BMPC percentage in the R/R group was 26% (vs. 7% and 1% in the PR/SD and ≥VGPR groups, respectively, p< .0001).
When separating by disease status pre-ASCT (≥SD vs. R/R) and best-response post-ASCT (sCR vs. CR/VGPR vs. ≤PR), patients achieving a best-response of sCR in both the ≥SD and R/R groups had longer TNT and OS (Figure 4a–b), which were comparable. However, when examining patients with a best-response post-ASCT of CR/VGPR, the pre-ASCT R/R group fared significantly worse than the pre-ASCT ≥SD group (mTNT 11.3 vs. 17.0 months, p= .0022; mOS 30.8 vs. 57.7 months, respectively, p= .04). Further, patients in the pre-ASCT R/R group who achieved a best-response post-ASCT of ≤PR had very poor survival compared to the pre-ASCT ≥SD group (mTNT 4.7 vs. 9.6 months, p= .05; mOS 13.1 vs. 48.1 months, respectively, p= .0071).
Figure 4.
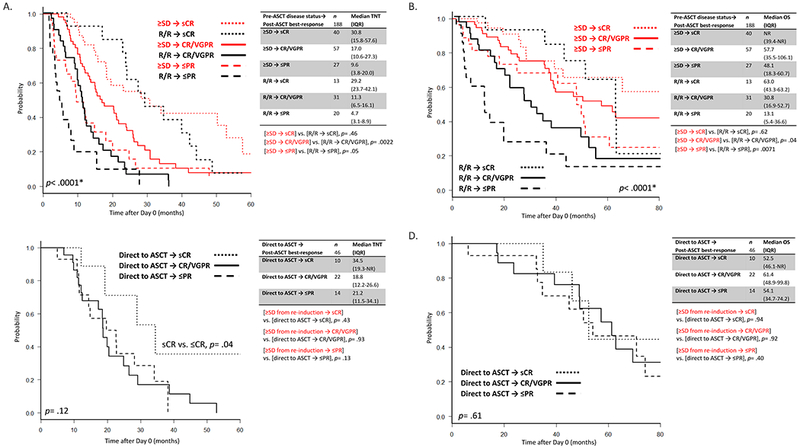
a. Time to Next Therapy (TNT) from Day 0 amongst patients who received re-induction after T0 (n=188), split by disease status pre-ASCT and best-response post-ASCT.
b. Overall survival (OS) from Day 0 amongst patients who received re-induction after T0 (n=188), split by disease status pre-ASCT and best-response post-ASCT.
c. Time to Next Therapy (TNT) from Day 0 amongst patients who proceeded directly to ASCT without re-induction after T0 (n=46), split by best-response post-ASCT.
d. Overall survival (OS) from Day 0 amongst patients who proceeded directly to ASCT without re-induction after T0 (n=46), split by best-response post-ASCT. Kaplan-Meier curves were compared with the log-rank test.
Survival in the direct to ASCT group
When examining the 46 patients in the direct to ASCT group (Figure 4c), patients with a best-response post-ASCT of sCR had significantly longer TNT (34.5 vs. 19.7 months in patients with best-response ≤CR, p= .04). However, OS was similar regardless of best-response (Figure 4d). Interestingly, unlike to the re-induction cohort, patients in the direct to ASCT group who achieved a best-response ≤PR had overlapping TNT/OS with patients who achieved CR/VGPR (Figure 4c–d). Survival stratified by best-response post-ASCT was comparable between patients who proceeded directly to ASCT and those in the re-induction group who had ≥SD pre-ASCT, save for a trend towards longer TNT in patients with a best-response ≤PR in the direct to ASCT group (21.2 vs. 9.6 months in the ≥SD after re-induction group, p= .13) (Figure 4a and 4c).
Univariate and multivariate analyses for TNT and OS
In a univariate analysis for TNT, time period of ASCT (before January 1, 2013 vs. after), high-risk cytogenetics (at diagnosis), ISS (III vs. I or II, at diagnosis), DOR prior to T0, number of lines of therapy prior to T0 and BMPC percentage at stem cell collection (>10% vs. ≤10%) were significant (Table 4). In a multivariate analysis which included these variables, and treatment with re-induction, only high-risk cytogenetics predicted for shorter TNT (hazard ratio [HR] 2.16, 95% CI 1.27–3.66, p= .004). Notably, re-induction did not significantly affect TNT in the multivariate analysis.
Table 4.
Univariate and multivariate cox proportional hazard models for time to next therapy (TNT) and overall survival (OS) post-salvage ASCT.
| Time to Next Therapy (TNT) | Overall Survival (OS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | n/N (%) | Univariate HR (95% CI) | P | Multivariate HR (95% CI) | P | Univariate HR (95% CI) | P | Multivariate HR (95% CI) | P |
| Age at ASCT* | - | 0.99 (0.97-1.01) | .35 | 1.00 (0.98-1.02) | .82 | ||||
| Date of ASCT, Before January 1, 2013 vs. after | 128/234 (55) | 1.36 (1.01-1.84) | .04 | 1.44 (0.92-2.27) | .11 | 1.94 (1.23-3.06) | .004 | 1.90 (1.00-3.63) | .05 |
| High risk FISH cytogenetics† | 33/169 (20) | 1.94 (1.28-2.92) | .002 | 2.16 (1.27-3.66) | .004 | 1.71 (1.03-2.82) | .04 | 2.11 (1.14-3.89) | .02 |
| ISS†, III vs. I or II | 42/173 (24) | 1.61 (1.11-2.35) | .01 | 1.31 (0.80-2.17) | .28 | 1.56 (0.97-2.49) | .06 | 1.96 (1.02-3.77) | .0437 |
| Duration of response (DOR) prior to T0*‡ | - | 0.99 (0.99-0.99) | .006 | 0.99 (0.99-1.00) | .62 | 0.99 (0.99-0.99) | <.001 | 0.99 (0.99-1.00) | .17 |
| # of lines of therapy prior to T0*‡ | - | 1.18 (1.02-1.35) | .02 | 1.12 (0.86-1.43) | .39 | 1.30 (1.09-1.54) | .003 | 1.19 (0.85-1.64) | .30 |
| Deepest response prior to T0‡, ≥CR vs. ≤VGPR | 60/234 (26) | 0.92 (0.65-1.29) | .65 | 1.00 (0.64-1.50) | 1.0 | ||||
| Bone marrow plasma cell percentage at stem cell collection, >10% vs. ≤10% | 71/224 (32) | 1.37 (1.01-1.87) | .05 | 1.16 (0.72-1.88) | .55 | 1.49 (1.04-2.16) | .03 | 1.02 (0.58-1.79) | .96 |
| Time from diagnosis to stem cell collection, >12 vs. ≤12 months | 103/234 (44) | 1.07 (0.80-1.43) | .65 | 1.16 (0.82-1.66) | .40 | ||||
| Progression (T0)‡, on-therapy vs. off-therapy | 156/234 (67) | 1.28 (0.95-1.75) | .11 | 1.77 (1.18-2.64) | .005 | 1.52 (0.82-2.82) | .19 | ||
| Progression (T0)‡, Clinical vs. biochemical only | 37/234 (16) | 1.29 (0.84-1.96) | .24 | 1.18 (0.75-1.85) | .49 | ||||
| Treatment group§, Re-induction vs. direct to ASCT | 188/234 (80) | 1.35 (0.93-1.95) | .11 | 1.30 (0.76-2.22) | .34 | 1.32 (0.85-2.05) | .22 | 1.21 (0.64-2.30) | .56 |
A forward selection Cox regression model was used, whereby any variable with p< .10 in univariate analysis was included in the multivariate model.
Bold denotes p< .05 in multivariate models.
Hazard Ratio (HR) denotes per-unit change in the regressor.
At diagnosis.
T0 is the date of progression, after which salvage ASCT was deemed necessary.
Treatment group was forced into the model to determine the impact of re-induction on TNT/OS after adjusting for other significant variables.
In a univariate analysis for OS, time period of ASCT, high-risk cytogenetics, ISS, DOR prior to T0, number of lines of therapy prior to T0, BMPC percentage at stem cell collection and progression (T0) on-therapy were significant (Table 4). When these variables, and treatment with re-induction, were included in a multivariate model, significant predictors for shorter OS included ISS at diagnosis (III vs. I or II, HR 1.96, 95% CI 1.02–3.77, p= .0437) and high-risk cytogenetics (HR 2.11, 95% CI 1.14–3.89, p= .02). Consistent with the above findings, re-induction did not significantly affect OS.
Univariate and multivariate analyses were also performed in the subgroup of 188 patients treated with re-induction prior to salvage ASCT (Supplementary Table 4). Again, high-risk cytogenetics predicted for shorter TNT (HR 2.37, 95% CI 1.28–4.38, p= .01) after adjusting for several factors including disease status pre-ASCT. For OS, disease status pre-ASCT (R/R vs. ≥SD, HR 1.81, 95% CI 1.01–3.27, p= .0477) was significant in the multivariate analysis.
Survival in patients with one line of induction prior to T0
We performed a sub-group analysis of the 125 patients who received only one line of induction prior to T0 and also met criteria for IMWG biochemical progression at T0 (as opposed to clinical-only relapse). There were 93 and 32 patients in the re-induction and direct to ASCT groups, respectively. Median time from diagnosis to T0 was 24.6 and 22.2 months, respectively (p= .98). Survival post-transplant in the re-induction group did not significantly differ from the direct to ASCT group (mTNT 17.0 vs. 19.9, p= .65; mOS 50.9 vs. 69.4 months, respectively, p= .53) (Supplementary Figure 3).
DISCUSSION
High-dose melphalan followed by ASCT is the single most effective mono-therapy for the treatment of MM.9, 30, 31 It retains activity in the salvage setting, and is cost-effective in era of novel agents.32 In the present study, we examined the impact of re-induction prior to salvage ASCT. Most patients (n=188, 80%) received re-induction; 46 (20%) proceeded directly to salvage ASCT. Re-induction facilitated 124/188 patients (65%) entering transplant with ≥SD, as opposed to 46/46 (100%) entering transplant with active R/R disease in the direct to ASCT group. Re-induction also resulted in a non-significant trend towards more patients (45% vs. 33%, p= .18) achieving a best-response ≥CR after ASCT. However, post-ASCT survival was comparable between the two groups, even after adjusting for multiple pre-transplant factors. Further, when restricting the analysis to the 125 patients who received one line of induction prior to T0 and met biochemical progression criteria, there remained no survival advantage post-ASCT for patients who were re-induced.
It is possible that there were fundamental differences between the two treatment groups that were not accounted for, perhaps signaled by the greater proportion of clinical relapses in the re-induction group, or the trend towards longer TNT in patients achieving a best-response ≤PR in the direct to ASCT group. It is conceivable that some patients in the direct to ASCT group had an indolent or MGUS-like phenotype.17, 33 Another caveat is that the choice to re-induce was dependent on the assessment of the treating physicians, and not based on any specific criteria. For some patients, the decision may have been pragmatic rather than clinical, e.g. to accommodate waiting for insurance approval. Finally, the re-induction regimens per se were quite heterogeneous. As such, we are cautious about our conclusions, as there may indeed be a subset of patients who benefit from re-induction. However, a significant concern is that some patients who are re-induced with the intent to achieve a response prior to transplant may never actually make it to salvage ASCT because of physical decline, infections, and/or clinical manifestations of relapse.34
In a subgroup analysis, we examined patients who were re-induced to determine the prognostic effect of response to re-induction therapy. Interestingly, patients with better responses to re-induction pre-ASCT had superior survival post-ASCT. Also, as expected, deeper best-responses post-ASCT correlated with longer survival. It is impossible to know if the prognostic benefit of deeper responses to re-induction were a marker of chemo-sensitivity/indolent biology or a direct effect of gaining better disease control; only a randomized trial could answer this question. Interestingly, while it hasn’t been studied extensively, the Medical Research Council Myeloma IX trial showed that in patients achieving minimal-residual disease (MRD)-negativity after up-front ASCT, disease status pre-ASCT (MRD+ vs. MRD−) had no effect on OS post-ASCT.35
Our study highlights that patients who were refractory to re-induction and/or those with high-risk cytogenetics constitute a group in need of more intensive post-ASCT interventions. Further, there was a trend towards the date of transplant being a predictor for OS in the multivariate analysis, likely reflecting the efficacy of contemporary triplet induction regimens, post-transplant maintenance, and more effective salvage therapies.
It is important to consider that future updates of the IFM 2009 trial may show an OS benefit for up-front ASCT >5 years from diagnosis. If so, delayed ASCT might become less common, and perhaps the questions addressed by our study may be more relevant for patients proceeding to a second ASCT. Even though this is an effective salvage therapy,36, 37 we did not specifically study second salvage ASCT and it is unknown if our conclusions apply in that setting.
In summary, we did not find a survival detriment for patients who proceeded directly to salvage ASCT with R/R disease, compared to patients who were re-induced. Although based on retrospective data, our conclusions suggest that patients with more indolent biochemical progressions may be able to proceed directly to high-dose melphalan followed by ASCT. However, a sizable fraction of patients may need re-induction to obtain disease control, particularly in the case of aggressive, clinical relapses. Future studies re-examining this question may be warranted given the recent Food and Drug Administration approvals of robust, monoclonal antibody-based regimens for R/R disease. The role of MRD status in determining pre- and post-ASCT therapy is also highly of interest.
Supplementary Material
Acknowledgements
We would like to acknowledge the patients and their families. K.M. was supported by CTSA Grant Number TL1TR002380 from the National Center for Advancing Translational Sciences (NCATS).
Footnotes
Parts of these data were presented on Poster 4613 at the American Society of Hematology Annual Meeting in San Diego, California on December 3rd, 2018.
Conflicts of Interest
S.K. has obtained research support for clinical trials from Celgene, Millennium, Novartis, Janssen, and Sanofi. A.D. has received research support for clinical trials from Pfizer, Jannsen, Millennium, Alnylam, and Celgene. M.A.G. has received research support from ISIS and Prothena, and honoraria from Celgene, Millennium Pharmaceuticals, and Novartis. The remaining authors declare no conflict of interest.
Supplementary Information
Supplementary information is available at Bone Marrow Transplantation’s website.
REFERENCES
- 1.Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV et al. Multiple myeloma. Nat Rev Dis Primers. 2017; 3: 17046. [DOI] [PubMed] [Google Scholar]
- 2.Ravi P, Kumar SK, Cerhan JR, Maurer MJ, Dingli D, Ansell SM et al. Defining cure in multiple myeloma: a comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J. 2018; 8(3): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014; 28(5): 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidiqi MH, Aljama MA, Bin Riaz I, Dispenzieri A, Muchtar E, Buadi FK et al. Bortezomib, lenalidomide, and dexamethasone (VRd) followed by autologous stem cell transplant for multiple myeloma. Blood Cancer J. 2018; 8(11): 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996; 335(2): 91–97. [DOI] [PubMed] [Google Scholar]
- 6.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-Dose Chemotherapy with Hematopoietic Stem-Cell Rescue for Multiple Myeloma. N Engl J Med. 2003; 348(19): 1875–1883. [DOI] [PubMed] [Google Scholar]
- 7.Mahajan S, Tandon N, Kumar S. The evolution of stem-cell transplantation in multiple myeloma. Ther Adv Hematol. 2018; 9(5): 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017; 376(14): 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonsalves WI, Buadi FK, Ailawadhi S, Bergsagel PL, Chanan Khan AA, Dingli D et al. Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement. Bone Marrow Transplant 2018; e-pub ahead of print 9 July 2018; doi: 10.1038/s41409-018-0264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Souza A, Lee S, Zhu X, Pasquini M. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant. 2017; 23(9): 1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah N, Callander N, Ganguly S, Gul Z, Hamadani M, Costa L et al. Hematopoietic Stem Cell Transplantation for Multiple Myeloma: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015; 21(7): 1155–1166. [DOI] [PubMed] [Google Scholar]
- 12.Kumar SK, Buadi FK, Rajkumar SV. Pros and cons of frontline autologous transplant in multiple myeloma: the debate over timing. Blood. 2019; 133(7):652–659. [DOI] [PubMed] [Google Scholar]
- 13.Dunavin NC, Wei L, Elder P, Phillips GS, Benson DM Jr., Hofmeister CC et al. Early versus delayed autologous stem cell transplant in patients receiving novel therapies for multiple myeloma. Leuk Lymphoma. 2013; 54(8): 1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar SK, Lacy MQ, Dispenzieri A, Buadi FK, Hayman SR, Dingli D et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012; 118(6): 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson PG, Laubach JP, Munshi NC, Anderson KC. Early or delayed transplantation for multiple myeloma in the era of novel therapy: does one size fit all? Hematology Am Soc Hematol Educ Program. 2014; 2014(1): 255–261. [DOI] [PubMed] [Google Scholar]
- 16.Sidana S, Tandon N, Dispenzieri A, Gertz MA, Buadi FK, Lacy MQ et al. Relapse after complete response in newly diagnosed multiple myeloma: implications of duration of response and patterns of relapse. Leukemia. 2019; 33(3):730–738. [DOI] [PubMed] [Google Scholar]
- 17.Lahuerta JJ, Paiva B, Vidriales MB, Cordón L, Cedena MT, Puig N et al. Depth of Response in Multiple Myeloma: A Pooled Analysis of Three PETHEMA/GEM Clinical Trials. J Clin Oncol. 2017; 35(25): 2900–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013; 31(36): 4529–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vij R, Kumar S, Zhang MJ, Zhong X, Huang J, Dispenzieri A et al. Impact of pretransplant therapy and depth of disease response before autologous transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2015; 21(2): 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15(12): e538–e548. [DOI] [PubMed] [Google Scholar]
- 21.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003; 121(5): 749–757. [PubMed] [Google Scholar]
- 22.Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia. 2006; 20: 1467–1473. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17(8): e328–e346. [DOI] [PubMed] [Google Scholar]
- 24.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J et al. International staging system for multiple myeloma. J Clin Oncol. 2005; 23(15): 3412–3420. [DOI] [PubMed] [Google Scholar]
- 25.Dispenzieri A, Kyle R, Merlini G, Miguel J, Ludwig H, Hajek R et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009; 23(2): 215–224. [DOI] [PubMed] [Google Scholar]
- 26.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011; 117(18): 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajkumar SV, Richardson P, San Miguel JF. Guidelines for determination of the number of prior lines of therapy in multiple myeloma. Blood. 2015; 126(7): 921–922. [DOI] [PubMed] [Google Scholar]
- 28.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996; 17(4): 343–346. [DOI] [PubMed] [Google Scholar]
- 29.Kanda Y Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48(3): 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rhee F, Giralt S, Barlogie B. The future of autologous stem cell transplantation in myeloma. Blood. 2014; 124(3): 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahajan S, Tandon N, Kumar S. The evolution of stem-cell transplantation in multiple myeloma. Ther Adv Hematol. 2018; 9(5): 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah GL, Winn AN, Lin P-J, Klein A, Sprague KA, Smith HP et al. Cost-Effectiveness of Autologous Hematopoietic Stem Cell Transplantation for Elderly Patients with Multiple Myeloma using the Surveillance, Epidemiology, and End Results-Medicare Database. Biol Blood Marrow Transplant. 2015; 21(10): 1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paiva B, Vídriales MB, Rosiñol L, Martínez-López J, Mateos MV, Ocio EM et al. A multiparameter flow cytometry immunophenotypic algorithm for the identification of newly diagnosed symptomatic myeloma with an MGUS-like signature and long-term disease control. Leukemia. 2013; 27: 2056–2061. [DOI] [PubMed] [Google Scholar]
- 34.Kansagra A, Gonsalves WI, Gertz MA, Buadi FK, Dingli D, Dispenzieri A et al. Analysis of Clinical Factors and Outcomes Associated with Nonuse of Collected Peripheral Blood Stem Cells for Autologous Stem Cell Transplants in Transplant-Eligible Patients with Multiple Myeloma. Biol Blood Marrow Transplant. 2018; 24(10): 2127–2132. [DOI] [PubMed] [Google Scholar]
- 35.Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013; 31(20): 2540–2547. [DOI] [PubMed] [Google Scholar]
- 36.Sellner L, Heiss C, Benner A, Raab MS, Hillengass J, Hose D et al. Autologous retransplantation for patients with recurrent multiple myeloma: a single-center experience with 200 patients. Cancer. 2013; 119(13): 2438–2446. [DOI] [PubMed] [Google Scholar]
- 37.Gonsalves WI, Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK et al. Second auto-SCT for treatment of relapsed multiple myeloma. Bone Marrow Transplant. 2013; 48(4): 568–573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


