Abstract
Direct-acting antivirals (DAA) are now the mainstay of treatment for patients with chronic hepatitis C virus (HCV); however, there is some controversy over whether use of DAAs for HCV, as compared with interferon (IFN)-based regimens, leads to an increased risk of hepatocellular carcinoma (HCC) development. We investigated the association between use of DAAs and subsequent development of HCC in longitudinal data from HCV patients of diverse backgrounds (various ages, ethnicities, and geographic regions) across the US. The design was a retrospective study performed using medical and pharmacy claims from OptumLabs®. HCV treatment exposure was categorized as DAA-only, DAA+IFN, any-DAA, or IFN-only. To account for confounding by indication, inverse probability of treatment weighting was performed. Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI). We identified 5,781 HCV patients with no history of HCC at baseline. Compared with IFN-only regimen, no significant increase in HCC risk was found for use of DAA-only (HR=1.53, 95% CI: 0.73–3.23), DAA+IFN (HR=1.02, 95% CI: 0.51–2.06), or any-DAA (HR=1.04, 95% CI: 0.65–1.65). When stratified by sustained virological response (SVR), we noted a higher HCC risk for DAA-only among patients who achieved SVR post-treatment (HR=7.53, 95% CI: 1.48–38.34), but the CIs were wide, which might be due to the small sample size of the subgroups. Among those who did not achieve SVR, no association was found for use of DAA-only (HR=0.59, 95% CI: 0.19–1.91). These findings do not provide compelling evidence for the conception that use of DAAs for HCV is associated with increased risk of HCC development.
Keywords: direct acting antiviral medications, chronic hepatitis C virus, hepatocellular carcinoma, interferon, DAA, HCV, HCC, IFN
Introduction
Hepatocellular carcinoma (HCC) is a frequently fatal cancer with increasing incidence (1) and mortality (2). Chronic hepatitis C virus (HCV) infection is a major risk factor for HCC in the US (3), with estimated population attributable risk fraction of 20–22% (4,5). Historically, HCV has been treated with interferon (IFN)-based medications, which yields sustained virological response (SVR) in about 50% of patients (6,7). Newly developed direct acting antiviral agents (DAA) are more effective for treating HCV, yielding SVR in >90% of patients (7–9). Thus, DAAs have become the mainstay for HCV treatment (10). Effective treatment of HCV is expected to result in substantial reduction in HCC risk. However, some studies have reported high rates of HCC development in patients treated with DAA (8,11), which has been attributed to the more rapid and more potent eradication of HCV by DAA that may lead to disruption of some protective immune functions against tumor development (11–14).
The suggested link between use of DAA for HCV and the subsequent development of HCC have been a subject of controversy (15–19). At least four studies have reported increased HCC incidence or recurrence following DAA treatment for HCV (8,11,13,20). The reported HCC recurrence rate following DAA use is estimated at around 28% (8,11), while incidence rates for de novo HCC in DAA-treated patients range between 3% and 9% (8,13). However, other studies did not find increased HCC incidence following DAA use (21–25), and some actually found a reduction in HCC risk in patients who achieved DAA-induced SVR (21,22,24,26,27). Because of the conflicting reports, it is not entirely clear whether DAA use is associated with an increased risk or decreased risk of HCC development or has no association with HCC (19,28). Further, it is unclear if these observations are geographically limited. Studies in patient populations that are representative of the entire US population are necessary to evaluate the association between use of DAA for HCV and subsequent development of HCC to inform strategies for risk prevention that are applicable to the broader US population.
We investigated the association between DAA use and risk for HCC development in a retrospective cohort of patients with HCV who were treated with DAA, IFN-based medications, or both using medical and pharmacy claims data from the OptumLabs® Data Warehouse. We performed a head-to-head comparison between DAA-only versus IFN-only treatment for HCV in relation to HCC development following treatment. We further examined HCC risk associated with combined use of DAA and IFN-based medications (i.e., DAA + IFN) or any treatment combination that involves the use of DAA (i.e., any DAA) with each compared to IFN-only regimen. For each comparison, we used inverse probability of treatment weighting (IPTW) to ensure equal distribution of treatment-related factors and HCC risk factors among the treatment groups to control for confounding by indication.
Methods
Data Source and Study Sample
The study involves retrospective analysis of claims data from the OptumLabs® Data Warehouse (OLDW), which includes de-identified information for privately insured and Medicare Advantage enrollees in a large, private, US health plan (29). The database contains longitudinal health information on enrollees, representing a diverse mixture of ages, ethnicities, and geographical regions across the US. The health plan provides comprehensive full insurance coverage for physician, hospital, and prescription drug services (30,31). Enrollees were eligible for the present study if they met the following criteria: (a) diagnosed with HCV between January 1, 2010 and December 31, 2016, (b) did not receive antiviral treatment for HCV before the study start date (2010), (c) received treatment with IFN-based medications, DAA, or both on or after the HCV diagnosis date, (d) had medical and pharmacy coverage in the 12 months prior to antiviral treatment initiation to enable the assessment of baseline characteristics, (e) did not have a diagnosis of HCC or another malignancy prior to the initiation of antiviral treatment, and (f) was not concurrently enrolled in commercial health insurance and Medicare Advantage. Chronic HCV was defined by at least one documented diagnosis based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 070.44 and 070.54 or International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code B18.2, or at least two documented HCV diagnoses on different dates based on the ICD-9-CM codes 070.70, 070.71 and V02.62 or ICD-10-CM codes B19.20, B19.21 and Z22.52 (32).
Antiviral Therapy
Pharmacy and medical (injectable medications) claims for HCV treatment starting from the date of HCV diagnosis to the date of HCC diagnosis or end of follow-up (for those who did not develop HCC), were used to categorize exposure to antiviral treatment as: (a) IFN-only, (b) DAA-only, (c) DAA + IFN, or (d) any DAA. The IFN-only group consisted of individuals who received regular IFN or pegylated-IFN with or without ribavirin and did not receive DAA. The DAA-only group consisted of patients who used any of the following medications (and did not use IFN): boceprevir, daclatasvir dihydrochloride, elbasvir/grazoprevir, glecaprevir/pibrentasvir, ledipasvir/sofosbuvir, ombita/paritap/riton/dasabuvir, ombitasvir/paritaprev/ritonav, simeprevir sodium, sofosbuvir, sofosbuvir/velpatas/voxilaprev, sofosbuvir/velpatasvir, or telaprevir. The DAA + IFN group consisted of patients who used any combination of an IFN-based medication and a DAA. Finally, the any DAA group consisted of the users of DAA-only and DAA + IFN combined into one group.
Other Study Variables
Patient baseline characteristics obtained from the OLDW included date of HCV diagnosis, age at antiviral treatment initiation, gender, race/ethnicity, body mass index (BMI), US census region (Midwest, Northeast, South, West), household income, comorbid conditions, health insurance type (commercial, Medicare Advantage), and smoking history (current, former, never). In addition, time from HCV diagnosis to treatment initiation was calculated. Information on comorbid conditions in the 12 months before HCV treatment initiation (baseline) were used to calculate the Charlson-Deyo Comorbidity Index (33). Data on the following health conditions were obtained using primary and secondary ICD-9 and ICD-10 diagnosis codes: autoimmune hepatitis, hemochromatosis, Wilson’s disease, biliary cirrhosis, cirrhosis (compensated and decompensated), diabetes mellitus, human immunodeficiency virus (HIV), hepatitis B virus (HBV) infection, liver transplantation, alcohol use disorder, and substance use disorder. The ICD codes used to identify these conditions, which have been used widely to derive information about hepatic and comorbid conditions (34–37) and previously validated in a number of studies, are presented in Supplementary Table 1.
Assessment of SVR and HCC Incidence
SVR was defined as undetectable serum HCV viral load performed at least 12 weeks after completion of the antiviral therapy (38). Patients were categorized based on whether they achieved SVR post-treatment or not (yes, no). By design, no patient had a diagnosis of HCC or another malignancy prior to the antiviral therapy for HCV. The incidence of HCC occurring either during or after completion of the antiviral therapy was determined using the ICD-9-CM code 155.0 or ICD-10-CM code C22.0 (34,36). Analyses were restricted to patients with complete data on SVR status post-antiviral treatment with DAA, IFN, or both (Figure 1). Analyses were restricted to patients with complete data on SVR status post-antiviral treatment with DAA, IFN, or both (Figure 1).
Figure 1:
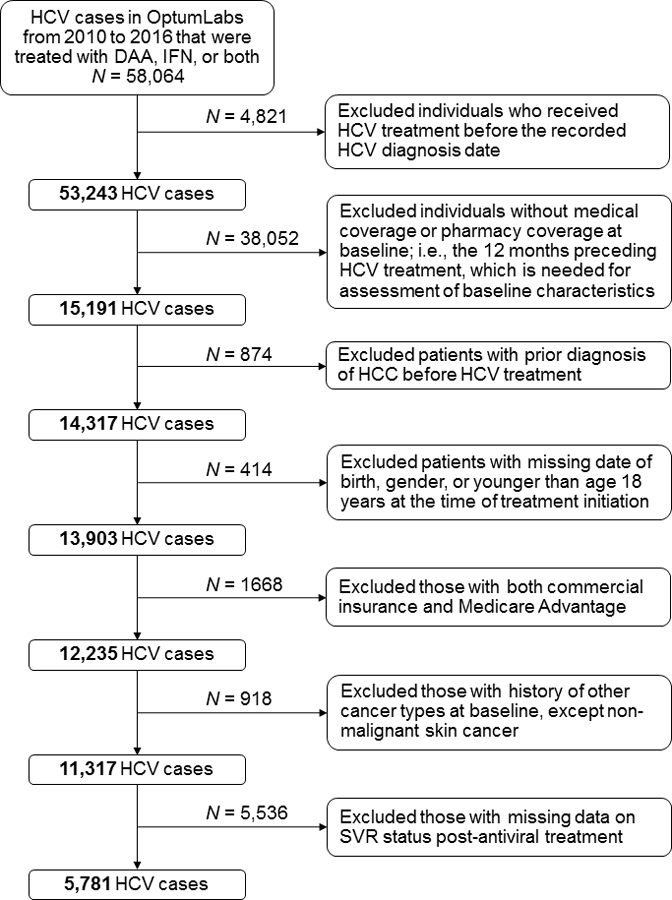
Flow chart showing study sample selection process.
Abbreviations: DAA, direct acting antiviral medication; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN, interferon
Primary Endpoint
The outcome of interest was HCC incidence following antiviral therapy for HCV. Each treatment regimen (DAA-only, DAA + IFN, and any DAA) was compared to IFN-only (reference) to ascertain the risk of HCC development following HCV treatment.
Statistical Analyses
Differences in demographic and clinical attributes at baseline were compared between the treatment groups and summarized as proportions for categorical variables and means for continuous variables using chi-squared tests and t-tests, respectively. Crude event rates for HCC incidence were calculated per 1,000 person-years for each treatment group. Confounding by indication, when the indication for a particular treatment correlates with the probability of receiving that treatment and the indication is also a risk for the outcome and thus produces an imbalance in the outcome probability between treatment groups, is a common constraint of most observational studies (39). To account for confounding by indication, IPTW was performed in an attempt to ensure equitable distribution of treatment-related factors and HCC risk factors among the treatment groups at baseline (i.e., before treatment initiation). Specifically, we estimated propensity scores and weights using generalized boosted models based on 10,000 regression trees for optimal balance among the treatment groups (40). The weights were used to obtain estimates representing cohort average treatment effects. The propensity models included demographics, comorbidities, time-to-treatment initiation and other covariates (Supplementary Table 2). Because the IFN-only group was used as the reference group for all comparisons, separate IPTW was performed between IFN-only and each of the other three treatment groups (DAA-only, DAA +IFN, and any DAA), rather than a single IPTW across all four groups. This was done to ensure a more rigorous weighting and balance of potential confounding factors for each comparison. The success of the balance of treatment-related factors and HCC risk factors was assessed after IPTW using standardized mean differences < 20% for continuous variables and Kolmogorov-Smirnov (K-S) statistic < 0.10 for categorical variables as indication of adequate balance of potential confounding factors (41). Factors that remained unbalanced after IPTW were included in the statistical model as covariates for further adjustment. Cox proportional hazard models were used on the weighted cohorts to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) to ascertain the relative risk of HCC incidence across follow-up for use of DAA-only, DAA + IFN, and any DAA compared with use of IFN-only. Prior to fitting the proportional hazard models, the proportional hazards assumption was assessed for each model using log [-log (survival)] curves and was determined to have been satisfied. Detail results of the IPTW for each comparison, including p-values, standardized mean differences, and K-S statistics are presented in Supplementary Tables 2–4. In addition to analyses of the entire study population, we performed separate subgroup analyses among patients with cirrhosis and those without cirrhosis, and among patients who achieved SVR post-treatment and those who did not achieve SVR. All statistical tests were two-sided and a p-value < 0.05 was considered statistically significant. The statistical analyses were performed using SAS® version 9.4 (SAS Institute, Inc., Cary, North Carolina) or R version 3.5.1 (R Foundation for Statistical Computing Vienna, Austria).
Results
We identified 58,064 patients diagnosed with HCV between 2010 and 2016, with follow-up up to December 31, 2017. Of these patients, we excluded individuals who received antiviral treatment before the documented HCV diagnosis date, those without medical or pharmacy coverage in the 12 months before treatment initiation, individuals who had HCC diagnosis before initiation of the antiviral therapy and those with missing date of birth, gender, or were younger than age 18 years. We further excluded individuals with both commercial health insurance and Medicare Advantage, those with diagnosis of other cancer types at baseline (except non-melanoma skin cancer), and those with missing data on SVR status post-antiviral treatment. These exclusions left a final analytic sample of 5,781 patients with HCV who did not have a prior history of HCC or another malignancy at baseline and had complete data on SVR status (Figure 1).
The cohort was categorized into three groups based on treatment exposure: IFN-only, DAA-only, and DAA + IFN. A fourth group (any DAA) was created by combining patients who received DAA-only or DAA + IFN into one group (DAA-only + [DAA + IFN]). Median follow-up of the entire cohort was 1.7 years. Follow-up was longest for the IFN + DAA (2.5 years), followed by IFN-only (2.2 years), any DAA (1.7 Years), and then DAA-only (1.5 years). One hundred and ten de novo HCC cases developed over the follow-up period, with the highest number of new HCC cases occurring among the DAA-only group (n = 76), followed by the DAA + IFN group (n = 25), and the lowest number among the IFN-only group (n = 9). The combined any DAA group accounted for 101 of the 110 de novo HCC cases. However, the percentages of HCC cases occurring in the DAA-only, the DAA + IFN, or the any DAA group were not significantly different from that of the IFN-only group (p-values >0.05) (Table 1).
Table 1.
Patient characteristics at baseline by treatment group before inverse probability of treatment weighting, N = 5,781.
| IFN only (N=370) |
DAA only (N=4438) |
DAA + IFN (N=973) |
Any DAA a (N=5411) |
p-value b | p-value c | p-value d | |
|---|---|---|---|---|---|---|---|
| HCC Development | 9 (2.4%) | 76 (1.7%) | 25 (2.6%) | 101 (1.9%) | 0.31 | 0.89 | 0.44 |
| Age Groups | <0.01 | <0.01 | <0.01 | ||||
| 18–34 years | 38 (10.3%) | 217 (4.9%) | 63 (6.5%) | 280 (5.2%) | |||
| 35–44 years | 45 (12.2%) | 212 (4.8%) | 89 (9.1%) | 301 (5.6%) | |||
| 45–54 years | 143 (38.6%) | 825 (18.6%) | 332 (34.1%) | 1157 (21.4%) | |||
| 55–64 years | 131 (35.4%) | 2201 (49.6%) | 444 (45.6%) | 2645 (48.9%) | |||
| 65+ years | 13 (3.5%) | 983 (22.1%) | 45 (4.6%) | 1028 (19.0%) | |||
| Mean (SD) | 50.2 (10.4) | 57.8 (10.8) | 52.7 (9.8) | 56.9 (10.8) | |||
| Gender | 0.22 | 0.81 | 0.28 | ||||
| Female | 135 (36.5%) | 1764 (39.7%) | 362 (37.2%) | 2126 (39.3%) | |||
| Male | 235 (63.5%) | 2674 (60.3%) | 611 (62.8%) | 3285 (60.7%) | |||
| Race | <0.01 | <0.01 | <0.01 | ||||
| Asian | 18 (4.9%) | 126 (2.8%) | 16 (1.6%) | 142 (2.6%) | |||
| Black | * | 836 (18.8%) | 181 (18.6%) | 1017 (18.8%) | |||
| Hispanic | 40 (10.8%) | 525 (11.8%) | 126 (12.9%) | 651 (12.0%) | |||
| White | 271 (73.2%) | 2822 (63.6%) | 624 (64.1%) | 3446 (63.7%) | |||
| Unknown | * | 129 (2.9%) | 26 (2.7%) | 155 (2.9%) | |||
| Census Region | <0.01 | <0.01 | <0.01 | ||||
| Midwest | 25 (6.8%) | 467 (10.5%) | 91 (9.4%) | 558 (10.3%) | |||
| Northeast | 21 (5.7%) | 679 (15.3%) | 103 (10.6%) | 782 (14.5%) | |||
| South | 262 (70.8%) | 2670 (60.2%) | 662 (68.0%) | 3332 (61.6%) | |||
| West | 62 (16.8%) | 622 (14.0%) | 117 (12.0%) | 739 (13.7%) | |||
| Median Annual Household Income | <0.01 | 0.10 | <0.01 | ||||
| <$40K | 64 (17.3%) | 1188 (26.8%) | 196 (20.1%) | 1384 (25.6%) | |||
| $40K-$49K | 27 (7.3%) | 374 (8.4%) | 87 (8.9%) | 461 (8.5%) | |||
| $50K-$59K | 43 (11.6%) | 392 (8.8%) | 88 (9.0%) | 480 (8.9%) | |||
| $60K-$74K | 32 (8.6%) | 502 (11.3%) | 108 (11.1%) | 610 (11.3%) | |||
| $75K-$99K | 62 (16.8%) | 603 (13.6%) | 154 (15.8%) | 757 (14.0%) | |||
| $100K+ | 111 (30.0%) | 1034 (23.3%) | 291 (29.9%) | 1325 (24.5%) | |||
| unknown | 31 (8.4%) | 345 (7.8%) | 49 (5.0%) | 394 (7.3%) | |||
| Year of Treatment Initiation | <0.01 | <0.01 | <0.01 | ||||
| 2010 | 127 (34.3%) | 0 (0.0%) | 29 (3.0%) | 29 (0.5%) | |||
| 2011 | 97 (26.2%) | 0 (0.0%) | 238 (24.5%) | 238 (4.4%) | |||
| 2012 | 93 (25.1%) | * | 318 (32.7%) | * | |||
| 2013 | 49 (13.2%) | * | 161 (16.5%) | * | |||
| 2014 | * | 654 (14.7%) | 224 (23.0%) | 878 (16.2%) | |||
| 2015 | * | 1628 (36.7%) | * | * | |||
| 2016 | 0 (0.0%) | 1494 (33.7%) | * | * | |||
| 2017 | 0 (0.0%) | 652 (14.7%) | 0 (0.0%) | 652 (12.0%) | |||
| Charlson index | <0.01 | 0.01 | <0.01 | ||||
| 0 | * | 119 (2.7%) | 20 (2.1%) | 139 (2.6%) | |||
| 1 | 244 (65.9%) | 1923 (43.3%) | 534 (54.9%) | 2457 (45.4%) | |||
| 2 | * | 949 (21.4%) | 205 (21.1%) | 1154 (21.3%) | |||
| 3+ | 63 (17.0%) | 1447 (32.6%) | 214 (22.0%) | 1661 (30.7%) | |||
| Ribavirin | 365 (98.6%) | 710 (16.0%) | 969 (99.6%) | 1679 (31.0%) | <0.01 | <0.06 | <0.01 |
| Comorbidities | |||||||
| Autoimmune hepatitis | 73 (19.7%) | 373 (8.4%) | 171 (17.6%) | 544 (10.1%) | <0.01 | 0.3601 | <0.01 |
| Hemochromatosis | 12 (3.2%) | 64 (1.4%) | 26 (2.7%) | 90 (1.7%) | <0.01 | 0.5728 | 0.0255 |
| Wilson’s disease | * | * | * | * | - | 0.3828 | 0.7115 |
| Biliary cirrhosis | * | * | * | * | 0.21 | 0.3828 | 0.2299 |
| Cirrhosis | 83 (22.4%) | 2841 (64.0%) | 267 (27.4%) | 3108 (57.4%) | <0.01 | 0.0618 | <0.0001 |
| Compensated | 58 (69.9%) | 368 (13.0%) | 178 (66.7%) | 2562 (82.4%) | <0.01 | 0.5854 | 0.0032 |
| Decompensated | 25 (30.1%) | 2473 (87.0%) | 89 (33.3%) | 546 (17.6%) | |||
| Diabetes | 38 (10.3%) | 1047 (23.6%) | 176 (18.1%) | 1223 (22.6%) | <0.01 | <0.01 | <0.01 |
| HIV | 16 (4.3%) | 195 (4.4%) | 30 (3.1%) | 225 (4.2%) | 0.95 | 0.26 | 0.88 |
| Hepatitis B | * | * | * | * | 0.14 | 0.76 | 0.21 |
| Liver transplant | * | * | * | * | 0.23 | 0.27 | 0.23 |
| Alcohol use disorder | 33 (8.9%) | 333 (7.5%) | 77 (7.9%) | 410 (7.6%) | 0.32 | 0.55 | 0.35 |
| Substance use disorder | 45 (12.2%) | 856 (19.3%) | 137 (14.1%) | 993 (18.4%) | <0.01 | 0.36 | <0.01 |
| Insurance type | <0.01 | <0.01 | <0.01 | ||||
| Commercial | 339 (91.6%) | 2542 (57.3%) | 808 (83.0%) | 3350 (61.9%) | |||
| Medicare Advantage | 31 (8.4%) | 1896 (42.7%) | 165 (17.0%) | 2061 (38.1%) | |||
| BMI kg/m2 | 0.23 | 0.74 | 0.34 | ||||
| < 18.5 | * | 42 (0.9%) | * | * | |||
| ≤ 18.5 – 24.9 | * | 318 (7.2%) | * | * | |||
| 25 – 29.9 | 43 (11.6%) | 586 (13.2%) | 112 (11.5%) | 698 (12.9%) | |||
| ≥ 30 | 68 (18.4%) | 929 (20.9%) | 208 (21.4%) | 1137 (21.0%) | |||
| Unknown | 236 (63.8%) | 2563 (57.8%) | 592 (60.8%) | 3155 (58.3%) | |||
| Smoking status | <0.01 | 0.09 | <0.01 | ||||
| Current | 79 (21.4%) | 964 (21.7%) | 245 (25.2%) | 1209 (22.3%) | |||
| Former | 34 (9.2%) | 618 (13.9%) | 110 (11.3%) | 728 (13.5%) | |||
| Never | 22 (5.9%) | 111 (2.5%) | 36 (3.7%) | 147 (2.7%) | |||
| Unknown | 235 (63.5%) | 2745 (61.9%) | 582 (59.8%) | 3327 (61.5%) | |||
| Time from HCV diagnosis to first treatment (months) | <0.01 | <0.01 | <0.01 | ||||
| Mean (SD) | 5.6 (187.6) | 6.1 (555.2) | 18.2 (10.2) | 18.4 (17.3) | |||
| Serologic viral response | <0.01 | <0.21 | <0.01 | ||||
| No | 127 (16.1%) | 689 (7.9%) | 299 (16.8%) | 988 (9.4%) | |||
| Yes | 243 (30.9%) | 3749 (42.9%) | 674 (37.8%) | 4423 (42.0%) |
Any DAA=(DAA-only + (DAA + IFN)).
p-value for DAA only vs. IFN.
p-value for DAA + IFN vs. IFN.
p-value for any DAA vs. IFN
cell size less than 11 counts were suppressed to protect patient confidentiality.
Abbreviations: hepatocellular carcinoma; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN, interferon; INR, international normalized ratio.
Analyses of the baseline demographic and clinical characteristics (before IPTW) show that, on average, patients in the IFN-only group were younger (50 years) than those in the DAA-only (58 years), DAA + IFN (53 years), or the combined any DAA group (57 years), but there were no differences in gender (Table 1). The IFN-only group consisted of a higher proportion of White patients, patients from Southern US, and patients with higher household incomes (≥$75,000 per year) compared with the other treatment groups. Further, the IFN-only group had a fewer percentage of patients with three or more comorbidities but had higher percentage of patients with commercial insurance than the DAA-only, DAA+IFN or the any DAA groups. HCC risk factors, such as cirrhosis (particularly decompensated cirrhosis) and diabetes mellitus were significantly lower among the IFN-only group compared with DAA only, DAA+IFN or any DAA. Time from HCV diagnosis to antiviral treatment was shortest for the IFN-only group (5.6 months), followed by the DAA-only (6.1 months) then DAA + IFN group (18.2 months) and any DAA group (18.4 months). Thirty-one percent of patients in the IFN-only group achieved SVR post-antiviral treatment for HCV, compared with 43% for DAA-only, 38% for DAA +IFN, and 42% for the any DAA group. Other differences in baseline characteristics are presented in Table 1. The extent of balance in the distribution of baseline characteristics between the treatment groups attained after IPTW are presented in Supplementary Tables 2–4.
Crude incidence rates for HCC per 1,000 person-years across follow-up was lowest for the IFN-only group (8.66), followed by DAA + IFN (9.26), any DAA (10.20), and then DAA-only (10.55) (Figure 2). Multivariable Cox proportional hazard modeling was used on the weighted cohort for assessment of HCC events with further adjustment for factors the remained unbalanced after the IPTW for each comparison (Figure 2). The results did not show a significant difference in HCC risk among users of DAA-only (HR=1.53, 95% CI: 0.73–3.23) compared with users of IFN-only. Users of DAA + IFN (HR=1.02, 95% CI: 0.51–2.06) or any DAA (HR=1.04, 95% CI: 0.65–1.65) also did not have higher HCC risk when compared with users of IFN-only. We also performed sensitivity analysis where HCC cases that occurred within 6 months of completing HCV therapy (n=33) were excluded, leaving 77 de novo HCC cases for analyses. The results show no significant association for DAA-only (HR= 3.12, 95% CI: 0.83–9.46) or any DAA (HR=2.11, 95% CI: 0.86–4.29) or the other treatment groups, similar to the overall results. Thus, the cases were retained in the subsequent analyses to maximize samples size.
Figure 2:
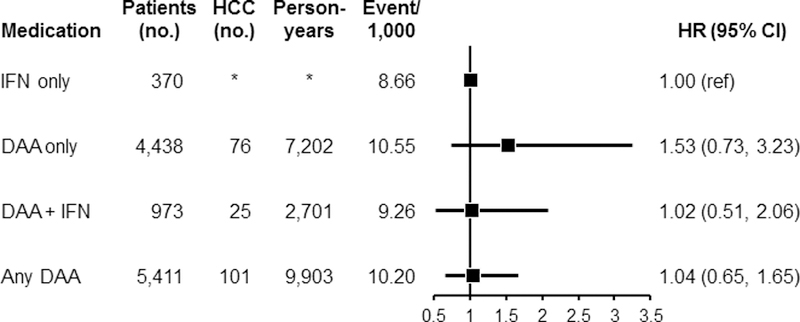
Associations between use of DAA-only, DAA + IFN or any DAA, and risk of HCC. Each group was compared with IFN-only after inverse probability of treatment weighting (IPTW). For the comparison between DAA-only vs. IFN-only, after the IPTW, additional adjustments were performed for gender, race, region, income, ribavirin, comorbidities, insurance type, BMI, smoking status, and SVR attainment. For the comparison between DAA + IFN vs. IFN only, additional adjustments were performed for race, Census region, diabetes, substance abuse disorder, insurance type, and BMI after the IPTW. For the comparison between Any DAA vs. IFN-only, additional adjustments were performed for gender, race, region, income, ribavirin, comorbidities, insurance type, BMI, smoking status, and SVR after the IPTW.
Abbreviations: BMI, body mass index; DAA, direct acting antiviral medication; IFN, interferon; SVR, sustained virological response
In a separate analysis among HCV patients with cirrhosis, no significant difference in HCC risk was observed for those who used DAA-only (HR=0.56, 95% CI: 0.20–1.55), DAA + IFN (HR=0.91, 95% CI: 0.35–2.35), or any DAA (HR=0.75, 95% CI: 0.42–1.35) compared with users of IFN-only (Figure 3). Among HCV patients without cirrhosis, no significant increase in HCC risk was observed for the patients who used DAA-only (HR=1.00, 95% CI: 0.32–3.16), DAA + IFN (HR=1.07, 95% CI: 0.38–2.97), or any DAA (HR=1.11, 95% CI: 0.50–2.45) when compared with users of IFN-only.
Figure 3:
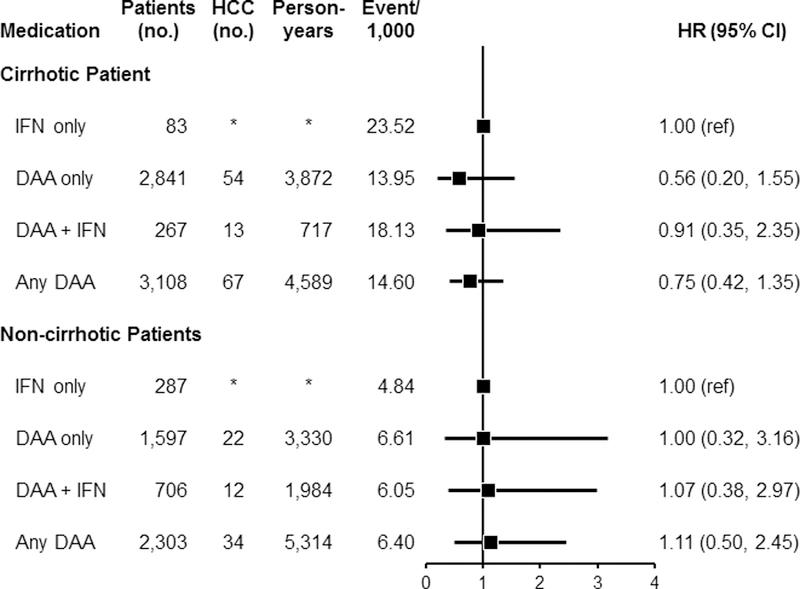
Association between use of antiviral medication for HCV and subsequent development of HCC separately among patients with cirrhosis and those without cirrhosis. Analyses were performed after inverse probability of treatment weighting (IPTW). Among patients with cirrhosis: for the comparison between DAA-only vs. IFN-only, after the IPTW, additional adjustments were performed for age, time from treatment to HCC development, Charlson comorbidity index, race, ribavirin, SVR, substance use, insurance type, and BMI. For the comparison between DAA + IFN vs. IFN-only, additional adjustment was performed for time from treatment to HCC development after IPTW. For the comparison between any DAA and IFN-only, additional adjustments were performed for age, time from treatment to HCC development, Charlson comorbidity index, gender, census region, ribavirin, SVR, insurance type, and smoking status after the IPTW. Among patients without cirrhosis, the same factors were adjusted for each comparison as was done for the patients with cirrhosis.
Abbreviations: DAA, direct acting antiviral medication; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN, interferon, SVR, sustained virological response
For patients who achieved SVR post-antiviral treatment for HCV, the use of DAA-only was associated with an increase in risk of HCC (HR=7.53, 95% CI: 1.48–38.34) compared with IFN-only (Figure 4). Similarly, use of any DAA was associated with increased HCC risk among patients who achieved SVR (HR=3.46, 95% CI: 1.43–8.41), but no association was found for use of DAA + IFN as compared with IFN-only. By contrast, among patients who did not achieve SVR post-treatment, use of DAA-only was not associated with HCC risk (HR=0.59, 95% CI: 0.19–1.91) as was use of DAA + IFN (HR=0.64, 95% CI: 0.26–1.58); however, we noted a lower HCC risk among users of any DAA (HR=0.34, 95% CI: 0.16–0.74). It is important to emphasize that the confidence intervals for the association between DAA only and increased HCC risk is substantially wide (95% CI: 1.48–38.34), indicating unstable effect estimates and reflecting the small sample sizes in the subgroup analyses stratified by SVR.
Figure 4:
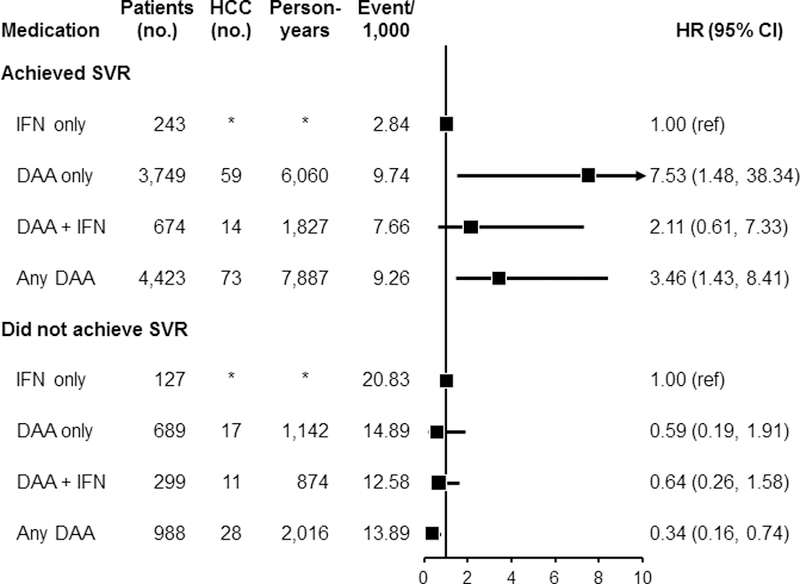
Association between use of antiviral medication for HCV and HCC risk separately among those who achieved sustained virological response (SVR) and those who did not achieve SVR. Analyses were performed after inverse probability of treatment weighting (IPTW) of treatment-related factors. Among patients who achieved SVR: after the IPTW, additional adjustments were performed for age, time from treatment to HCC development, Charlson comorbidity index, race, ribavirin, cirrhosis type, substance use, insurance type, and BMI. For the comparison between DAA + IFN vs. IFN-only, additional adjustment was performed for time from treatment to HCC development after the IPTW. For the comparison between any DAA and IFN-only, additional adjustments were performed for age, time from treatment to HCC development, Charlson comorbidity index, gender, census region, ribavirin, cirrhosis type, insurance type, and smoking status after IPTW. Among patients who did not achieve SVR, the same factors were adjusted for each comparison as was done for those who achieved SVR.
Abbreviations: DAA, direct acting antiviral medication; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN, interferon; SVR, sustained virological response
Discussion
In this study, we investigated whether use of DAA is associated with de novo HCC development in patients with HCV using data from OptumLabs®. We found that use of DAA-only regimen for HCV is not associated with higher risk of HCC development when compared with use of IFN-only regimen. Additional analyses did not show higher HCC risk associated with use of DAA + IFN or any DAA as compared with use of IFN-only regimen. These null associations were observed also among patients with HCV-associated cirrhosis and those without cirrhosis. However, among patients who achieved SVR post-antiviral treatment, HCC risk was higher in those treated with DAA-only as compared with patients treated with IFN-only. By contrast, no association was found for DAA use among patients who did not achieve SVR, and any DAA use was associated with lower risk among those who did not achieve SVR. It is important to note that the confidence intervals for the analyses stratified by SVR status were extremely wide, reflecting the small number of HCC events in each subgroup, which precludes drawing firm conclusions from the SVR-stratified results.
Chronic HCV infection is a major risk factor for HCC, accounting for a significant proportion of HCC cases diagnosed in the US (3,42). Findings from four small studies published in 2016 and 2017 raised considerable concerns about excessive rates of HCC occurring in HCV-infected patients who were treated with DAA (8,11,13,20), which led to controversies over the safety of DAA regarding HCC risk (15). The idea of high rates of HCC occurring after eradication of HCV with DAA is incongruent with the widely-held expert opinion that effective antiviral clearance of HCV should result in substantial reduction in HCC risk. Proposed mechanisms for the reported high rates of HCC in DAA-treated patients include disruption of the body’s anti-tumor immune surveillance system, promotion of angiogenesis, and dysregulation of inflammatory microenvironment in the liver by DAA (11–14,43), but none of these hypotheses have been proven conclusively.
A Spanish study of 58 patients with HCV by Reig et al. (11) and an Italian study of 59 patients with HCV by Conti et al. (8) reported excessive HCC recurrence rates of 27.6% and 28.8%, respectively, following treatment with DAA. The study by Conti et al. included an additional 285 patients with HCV who did not have a prior history of HCC among whom 3.2% developed de novo HCC within six months of receiving DAA (8). Cheung and colleagues examined clinical outcomes after DAA treatment in an English prospective study of 406 patients with HCV-associated decompensated cirrhosis and found that out of 317 patients who achieved DAA-induced SVR, 5% developed incident HCC over a 15-month follow-up (20). A retrospective study in Alabama by Ravi et al. also reported “unusually high rates” of HCC incidence (9.1%) among 66 patients with HCV-associated cirrhosis who were treated with DAA (13). It is worth noting that these studies had small sample sizes, did not perform statistical adjustment for potential confounders, and the findings are not consistent with findings from similar studies with more robust analyses (24,44).
Larger studies with more rigorous methods have since been published (21–24,26,45). In a multicenter prospective study conducted in France among 1,270 patients with HCV-induced compensated cirrhosis, Nahon et al. did not find an increased risk of HCC following receipt of DAA (HR=0.89, 95% CI: 0.46–1.73) when compared with receipt of IFN, after a median follow-up of 67.5 months and with inverse probability weighted adjustment of baseline factors (24). Four retrospective studies also did not find increased HCC risk following DAA treatment of HCV (21–23,26); however, three of these studies were conducted in a Veterans Administrative (VA) population and seem to have overlapping data based on the years covered in the studies (21–23). Among 22,500 VA patients with HCV who were treated with DAA in 2015, Kanwal et al. (21) identified 271 new HCC cases occurring after DAA treatment, with 183 of the HCC cases occurring among patients who achieved DAA-induced SVR. In multivariable analysis with adjustment for baseline characteristics, patients with DAA-induced SVR were found to have lower risk for HCC (HR=0.28, 95% CI: 0.22–0.36) compared with patients who did not achieve SVR post-DAA treatment (21). In a retrospective analysis of 62,354 VA patients diagnosed with HCV between 1999 and 2015 and treated with DAA-only, DAA + IFN or IFN-only, Ioannou et al. (22) found 3,271 new HCC cases occurring after antiviral treatment. Consistent with our findings, receipt of DAA-only (HR=1.12, 95% CI: 0.95–1.32) and DAA + IFN (HR=1.04 95% CI: 0.87–1.26) were not associated with increased risk for HCC after controlling for multiple risk factors. However, in contrast to our findings, attainment of SVR with DAA-only (HR=0.29; 95% CI: 0.23–0.37), DAA + IFN (HR=0.48; 95% CI: 0.32–0.73), or IFN-only (HR=0.32; 95% CI: 0.28–0.37) were each associated with lower HCC compared patients who did not achieve SVR irrespective of treatment type (22). Li et al. also investigated whether DAA use is associated with higher incidence of HCC a retrospective cohort of 17,836 VA patients diagnosed with HCV between 2002 and 2016 (23). They also did not find higher HCC risk in a DAA-only group (HR=1.07; 95% CI: 0.55–2.08) compared with an IFN-only group (23). Of note, the findings for these studies are not necessarily applicable to non-VA populations because of a number of constraints, including use of data from a nearly all-male patient population with high proportions of smokers and individuals with histories of excessive alcohol use (21–23).
Our study, based a national claims data of enrollees with private insurance or Medicare Advantage, allowed us to assess these findings in a more representative US population. Our findings show that compared with receipt of IFN-only for HCV, receipt of DAA-only, DAA + IFN, or any DAA is not associated with increased risk for HCC. The null association between DAA use and HCC risk in the overall sample was again observed in a subgroup of patients with cirrhosis and in a subgroup without cirrhosis. However, we found an increased risk for HCC among patients with DAA-induced SVR compared to patients with IFN-induced SVR. This confirms previous observation that DAA use may ameliorate, but does not eliminate risk of HCC development (19,28).
Our study is limited by the short follow-up, particularly among the DAA-only group (1.4 years), which may have limited the number of HCC events accrued for the analyses. The retrospective design prohibits definitive causal inferences. However, it is noteworthy that our findings are consistent with those from a recent prospective study (24) and from other retrospective studies (22,23). The use of claims data to ascertain baseline patient characteristics, such as cirrhosis status, could be subject to some level of misclassification, but such misclassification is unlikely to result in systematic differences between the treatment groups and therefore may not have a meaningful impact on the findings. We were unable to account for potential differences in HCC surveillance practices across participating sites in OptumLabs®, which may have influenced the study results to some extent. Furthermore, because the study was focused on de novo HCC cases, we excluded patients with prior history of HCC before treatment initiation; thus, we were unable to assess treatment impact on HCC recurrence, as has been done by others (11,46,47). We explored performing a subgroup analyses among patients with compensated cirrhosis and those with decompensated cirrhosis, but the small numbers of HCC events in each of these subgroups would have impeded meaningful interpretation of the resulting data. The use of ICD codes to identify HCV and HCC cases and comorbidities lack of granularity; however, the codes used in this study have been proven to have very high positive predictive values in several studies (34,36). Recent studies based on data from the National Health and Nutrition Examination Survey (NHANES) (48,49) found that about 5 million individuals infected with HCV in the US are not aware of their infection status because they have not been screened for HCV. This limits the generalizability of our finding to only screening-detected HCV patients. Furthermore, some of the HCV patients who developed HCC during the follow-up period may have had subclinical disease at the time of treatment initiation; hence, treatment exposure would not have an impact on such cases of HCC. The results should therefore be interpreted with this in mind. It would be desirable to verify our findings in a much larger sample with longer follow-up than the present study. Another limitation of our study, which is inherent in studies that utilize administrative claims data, is the large numbers of missing data on lab values. A significant number (>40%) of the study population had missing data on lab values. The use of these variables for construction of the IPTW becomes challenging and in our experience would had little impact on the study results even if adjusted in the model. To ensure more homogeneous comparison groups, we did not include the lab values in constructing the IPTW or for adjustment in the model. This could have influence the results to some extent, either toward or away from null. Moreover, information on HCV genotype was not available in the OptumLabs® Database and that could have influence the study results and should be considered in the interpretation of the findings.
Major strengths of the present study include its large overall sample size, the ability to assess and account for differences in baseline characteristics in the year prior to antiviral treatment initiation, and the use of IPTW to control for confounding by indication. Additional strengths include the diverse study population with representation of patients of various ages, ethnicities, and geographical regions in the US, which makes the findings more generalizable to the broader US population.
Despite some reports of increased HCC risk among patients with HCV who are treated with DAA, our findings indicate that DAA treatment for HCV does not increase risk of HCC development when compared with IFN-based treatment for HCV and controlling for multiple baseline risk factors. Lack of adequate control of confounding factors may have accounted for the prior reports of excess HCC incidence in DAA-treated patients.
Supplementary Material
Acknowledgments
Grant support: This study was made possible by funding from NIH and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Abbreviations:
- AFP
alpha-fetoprotein
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CI
confidence intervals
- DAA
direct acting antiviral medications
- HBV
hepatitis B virus
- HCV
chronic hepatitis C virus
- HIV
human immunodeficiency virus
- HR
hazard ratio
- ICD
International Classification of Diseases
- IFN
interferon
- INR
international normalized ratio
- IPTW
inverse probability of treatment weighting
- OLDW
OptumLabs Data Warehouse
- SVR
sustained virological response
- VA
Veterans Affairs
Footnotes
Conflict of Interest: The authors declare no conflict of interest
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians 2018;68(1):7–30 doi 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research 2014;74(11):2913–21 doi 10.1158/0008-5472.can-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatology research : the official journal of the Japan Society of Hepatology 2007;37 Suppl 2:S88–94 doi 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 4.Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016;122(11):1757–65 doi 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. The American journal of gastroenterology 2013;108(8):1314–21 doi 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet (London, England) 2015;385(9973):1124–35 doi 10.1016/s0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung RT, Baumert TF. Curing chronic hepatitis C--the arc of a medical triumph. The New England journal of medicine 2014;370(17):1576–8 doi 10.1056/NEJMp1400986. [DOI] [PubMed] [Google Scholar]
- 8.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65(4):727–33 doi 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Calleja JL, Crespo J, Rincon D, Ruiz-Antoran B, Fernandez I, Perello C, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. Journal of hepatology 2017;66(6):1138–48 doi 10.1016/j.jhep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Baumert TF, Juhling F, Ono A, Hoshida Y Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC medicine 2017;15(1):52 doi 10.1186/s12916-017-0815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65(4):719–26 doi 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Serti E, Park H, Keane M, O’Keefe AC, Rivera E, Liang TJ, et al. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNalpha. Gut 2017;66(4):724–35 doi 10.1136/gutjnl-2015-310033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravi S, Axley P, Jones D, Kodali S, Simpson H, McGuire BM, et al. Unusually High Rates of Hepatocellular Carcinoma After Treatment With Direct-Acting Antiviral Therapy for Hepatitis C Related Cirrhosis. Gastroenterology 2017;152(4):911–2 doi 10.1053/j.gastro.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Villani R, Facciorusso A, Bellanti F, Tamborra R, Piscazzi A, Landriscina M, et al. DAAs Rapidly Reduce Inflammation but Increase Serum VEGF Level: A Rationale for Tumor Risk during Anti-HCV Treatment. PloS one 2016;11(12):e0167934 doi 10.1371/journal.pone.0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nault J-C, Colombo M. Hepatocellular carcinoma and direct acting antiviral treatments: Controversy after the revolution. Journal of Hepatology 2016;65(4):663–5 doi 10.1016/j.jhep.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Kwo PY, Shiffman ML, Bernstein DE. The Cochrane Review Conclusion for Hepatitis C DAA Therapies is Wrong. The American journal of gastroenterology 2018;113(1):2–4 doi 10.1038/ajg.2017.420. [DOI] [PubMed] [Google Scholar]
- 17.Kwo PY, Lacerda MA. After the Direct-acting Antivirals Are Gone, There Is Still Work to Be Done in the Liver. Gastroenterology 2016;151(4):582–4 doi 10.1053/j.gastro.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 18.Ji F, Yeo YH, Wei MT, Ogawa E, Enomoto M, Lee DH, et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis. J Hepatol 2019. doi 10.1016/j.jhep.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Galati G, Muley M, Vigano M, Iavarone M, Vitale A, Dell’Unto C, et al. Occurrence of hepatocellular carcinoma after direct-acting antiviral therapy for hepatitis C virus infection: literature review and risk analysis. Expert Opin Drug Saf 2019;18(7):603–10 doi 10.1080/14740338.2019.1617272. [DOI] [PubMed] [Google Scholar]
- 20.Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 2016;65(4):741–7 doi 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017;153(4):996–1005.e1 doi 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2017. doi 10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, Lo Re V 3rd, et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology (Baltimore, Md) 2018;67(6):2244–53 doi 10.1002/hep.29707. [DOI] [PubMed] [Google Scholar]
- 24.Nahon P, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Incidence of Hepatocellular Carcinoma After Direct Antiviral Therapy for HCV in Patients With Cirrhosis Included in Surveillance Programs. Gastroenterology 2018;155(5):1436–50.e6 doi 10.1053/j.gastro.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Ide T, Koga H, Nakano M, Hashimoto S, Yatsuhashi H, Higuchi N, et al. Direct-acting antiviral agents do not increase the incidence of hepatocellular carcinoma development: a prospective, multicenter study. Hepatol Int 2019;13(3):293–301 doi 10.1007/s12072-019-09939-2. [DOI] [PubMed] [Google Scholar]
- 26.Singer AW, Reddy KR, Telep LE, Osinusi AO, Brainard DM, Buti M, et al. Direct-acting antiviral treatment for hepatitis C virus infection and risk of incident liver cancer: a retrospective cohort study. Alimentary pharmacology & therapeutics 2018;47(9):1278–87 doi 10.1111/apt.14593. [DOI] [PubMed] [Google Scholar]
- 27.Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology 2018;155(2):411–21.e4 doi 10.1053/j.gastro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Singal AG, Lim JK, Kanwal F. AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review. Gastroenterology 2019;156(8):2149–57 doi 10.1053/j.gastro.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health affairs (Project Hope) 2014;33(7):1187–94 doi 10.1377/hlthaff.2014.0038. [DOI] [PubMed] [Google Scholar]
- 30.OptumLabs. OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation Cambridge Mnp, February 2018. PDF. Reproduced with permission from OptumLabs. [Google Scholar]
- 31.Optum 2015. Real World Health Care Experiences Eden Prairie, MN: Optum; https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf. [Google Scholar]
- 32.Yao X, Sangaralingham LR, Ross JS, Shah ND, Talwalkar JA. Adoption of new agents and changes in treatment patterns for hepatitis C: 2010–2014. The American journal of managed care 2016;22(6):e224–32. [PubMed] [Google Scholar]
- 33.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 34.Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology 2011;140(4):1182–8.e1 doi 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology (Baltimore, Md) 2007;46(1):37–47 doi 10.1002/hep.21662. [DOI] [PubMed] [Google Scholar]
- 36.Kramer JR, Kanwal F, Richardson P, Giordano TP, Petersen LA, El-Serag HB. Importance of patient, provider, and facility predictors of hepatitis C virus treatment in veterans: a national study. The American journal of gastroenterology 2011;106(3):483–91 doi 10.1038/ajg.2010.430. [DOI] [PubMed] [Google Scholar]
- 37.Beste LA, Ioannou GN, Larson MS, Chapko M, Dominitz JA. Predictors of early treatment discontinuation among patients with genotype 1 hepatitis C and implications for viral eradication. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2010;8(11):972–8 doi 10.1016/j.cgh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida EM, Sulkowski MS, Gane EJ, Herring RW Jr., Ratziu V, Ding X, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology (Baltimore, Md) 2015;61(1):41–5 doi 10.1002/hep.27366. [DOI] [PubMed] [Google Scholar]
- 39.Salas M, Hotman A, Stricker BH. Confounding by Indication: An Example of Variation in the Use of Epidemiologic Terminology. American journal of epidemiology 1999;149(11):981–3 doi 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 40.McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Statistics in medicine 2013;32(19):3388–414 doi 10.1002/sim.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radice R, Ramsahai R, Grieve R, Kreif N, Sadique Z, Sekhon JS. Evaluating treatment effectiveness in patient subgroups: a comparison of propensity score methods with an automated matching approach. The international journal of biostatistics 2012;8(1):25 doi 10.1515/1557-4679.1382. [DOI] [PubMed] [Google Scholar]
- 42.El-Serag HB. Hepatocellular carcinoma. The New England journal of medicine 2011;365(12):1118–27 doi 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 43.Reig M, Boix L, Marino Z, Torres F, Forns X, Bruix J. Liver Cancer Emergence Associated with Antiviral Treatment: An Immune Surveillance Failure? Seminars in liver disease 2017;37(2):109–18 doi 10.1055/s-0037-1601349. [DOI] [PubMed] [Google Scholar]
- 44.Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol 2016;65(4):734–40 doi 10.1016/j.jhep.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 45.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67(6):1204–12 doi 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 46.Kozbial K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol 2016;65(4):856–8 doi 10.1016/j.jhep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Torres HA, Vauthey JN, Economides MP, Mahale P, Kaseb A. Hepatocellular carcinoma recurrence after treatment with direct-acting antivirals: First, do no harm by withdrawing treatment. J Hepatol 2016;65(4):862–4 doi 10.1016/j.jhep.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 48.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology (Baltimore, Md) 2015;62(5):1353–63 doi 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg ES, Hall EW, Sullivan PS, Sanchez TH, Workowski KA, Ward JW, et al. Estimation of State-Level Prevalence of Hepatitis C Virus Infection, US States and District of Columbia, 2010. Clin Infect Dis 2017;64(11):1573–81 doi 10.1093/cid/cix202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


