Abstract
Background:
Assessing the prevalence and level of exposure (dose) of tobacco and marijuana use is important in studies of harm from use of these substances. We used biochemical analysis of urine to quantitatively assess exposure to nicotine and delta 9-tetrahydrocannabinol (THC) in adolescents receiving medical care in a public hospital
Methods:
Participants were 686 adolescents between 12 and 21 years old seen at Zuckerberg San Francisco General Hospital between 2012 and 2014. Urine samples were assayed using high sensitivity liquid chromatographic assays for cotinine, a major metabolite of nicotine, and 11-nor-9-carboxy-delta 9-THC (THC-COOH), a major metabolite of THC. A commonly used immunoassay screen for THC-COOH was also performed.
Results:
The THC-COOH immunoassay substantially underestimated THC exposure, as measured with the high sensitivity assay. THC use was detected in 25% of participants, with higher prevalence with increasing age and in non-Hispanic blacks. Active tobacco smokers had an 80% prevalence of THC use (odds ratio for cigarette smoking predicting THC use 13.2). Urine cotinine and THC-COOH were significantly correlated (r = 0.60).
Conclusions:
The use of a high sensitivity chromatographic urine assay provides a much more complete picture of adolescent tobacco use compared to a commonly used immunoassay. The immunoassay provides high specificity but moderate sensitivity. We confirm high concordance of tobacco and marijuana use and the high predictive value of cigarette smoking in predicting marijuana use, and provide novel data on the quantitative correlation between level of exposure to nicotine and THC. Quantitative screening of nicotine and THC exposure may enhance our understanding of addiction and harm from single and dual product use.
Keywords: Adolescents, Young Adults, Nicotine, Cannabis, Marijuana, Urine Screening, Analytical Chemistry
1. Introduction
Cannabis and tobacco use are common among adolescents in the U.S. In this paper, we will refer to marijuana use rather than cannabis use since we will be discussing exposure to the psychoactive drug delta-9-tetrahydrocannabinol (THC), and some cannabis products contain the non-psychoactive cannabinoids without THC. Nationally representative Monitoring the Future survey data from 2017 indicates that lifetime prevalence of marijuana use in middle and high schoolers was 29%, with 15% using in the past 30-days (Cerda et al., 2018). A Washington State survey from 2014 to 2016 similarly found an 18% report of past 30-day marijuana use in students from grades 8 to 12 (Dilley et al., 2019). Recreational marijuana was legalized in Washington in 2012. Tobacco cigarette lifetime prevalence was 17%, with 5% smoking within the past 30-days. In surveys of adolescents, cigarette smokers are much more likely to smoke marijuana than those who do not use tobacco products (Ramo et al., 2012; Schauer et al., 2017a; Schauer et al., 2017b). Some adolescents smoke tobacco and marijuana together in the form of blunts - hollowed out cigars filled with marijuana (Cooper and Haney, 2009; Schauer and Peters, 2018). Others smoke mixtures of tobacco and marijuana (mulling, spliffs).
A high prevalence of co-use of tobacco and marijuana in adolescents has raised a number of health concerns (Meier and Hatsukami, 2016). Co-users of tobacco and marijuana are more likely to experience mental health problems compared to those who use either alone (Peters et al., 2014). Co-users are less likely to quit marijuana use and are more likely to experience cannabis use disorder (Ream et al., 2008; Schauer et al., 2017a; Schauer et al., 2017b). Conversely, some research indicates that co-users have more difficulty quitting smoking cigarettes (Strong et al., 2018). Human laboratory studies have found that withdrawal symptoms are more severe in dual users (Vandrey et al., 2008). Animal studies report that nicotine augments pharmacologic responses to THC, including increasing the rewarding effects, attenuation of development of tolerance, enhancement of antagonist-precipitated withdrawal and long-lasting brain biochemical changes (Ponzoni et al., 2019; Valjent et al., 2002). Genetic studies indicate a common genetic predisposition to tobacco and marijuana use, and cannabinoid receptor genes appears to be related to nicotine dependence (Chen et al., 2008; Evans et al., 2016).
Assessing tobacco and marijuana exposure, both prevalence and level of exposure (dose) in adolescents is important in epidemiologic studies of harm from nicotine and cannabis, particularly as marijuana use is being legalized in more and more states in the U.S. and around the world. Most data on the prevalence of marijuana and tobacco use come from self-reports, which may suffer from misreporting. Few studies have used biochemical screening to assess exposure. In a study of 12–19 year olds receiving medical care in an outpatient clinic, urine samples were screened via an immunoassay and found that 24% of the sample were positive for THC (Silber et al., 1987). Tobacco cigarette smoking status was not asked or analyzed biochemically. In children ages 1 month to 2 years hospitalized for bronchiolitis, 16% had evidence of marijuana exposure; and exposure was more likely when there was biochemical evidence of tobacco exposure (Wilson et al., 2017). Another study screened hospitalized children with a parent in smoking cessation treatment for THC in urine, finding that 46% of children had detectable levels of 11-nor-9-carboxy-THC (THC-COOH), a primary metabolite of THC that can detect both active and passive marijuana smoke exposure (Wilson et al., 2018). Cotinine, the primary proximate metabolite of nicotine, was also measured in urine; cotinine and THC-COOH levels were not significantly correlated.
We have published studies on biochemical screening for active and passive cigarette smoking in adolescents attending pediatric clinics in a public hospital in San Francisco (Benowitz et al., 2017; Benowitz et al., 2018; Dempsey et al., 2012). Here we present data on biochemically-determined marijuana use in the same population. Our aims were to compare high sensitivity quantitative testing vs a commonly used immunoassay screening test and self-report of THC use, examine demographics of marijuana exposure, and determine qualitative and quantitative aspects of the concordance of marijuana and tobacco use.
Biochemical assessment of marijuana involved measurement of the THC metabolite THC-COOH in urine. THC-COOH has a relatively long half-life (28–60 hr, depending on sampling time frame) and typical urine screening tests remain positive for 30 hours after a single use and for as long as 30 days after abstinence in a heavy regular marijuana user (Huestis and Cone, 1998). Biochemical assessment of tobacco use was performed by measuring levels in urine of cotinine, the main proximate metabolite of nicotine which has a half-life averaging 16 hr, and the tobacco-specific nitrosamine, 4-(methylnitrosamino)-l-(3-pyridyl)-1-butanol (NNAL), which has a half-life of 10–16 days and is a biomarker of long term tobacco use (Benowitz et al., 2017).
2. Materials and Methods
2.1. Participants and Study Procedures
The participants came from two studies, including 686 adolescents or young adults between 12 and 21 years old, who received care at the Children’s Health Center (CHC) at Zuckerberg San Francisco General Hospital Center (ZSFG). ZSFG is the county hospital for San Francisco and serves an economically disadvantaged population (Benowitz et al., 2017; Benowitz et al., 2018). The study was conducted from 2013 to 2014, prior to the legalization of recreational marijuana in California. Adolescents attending the CHC in 2013 included 62.4% Latino, 15.9% non-Hispanic Black (African American), 11.0% Asian, 5.1% White and 5.5% other race/ethnicity children.
In the first study, adolescents (n=462) who had surplus urine during a clinic visit for both sick and well care were included. Urine samples were collected for clinical indications, including urinary tract or sexually-transmitted infection screening and diagnosis, abdominal pain evaluation, trauma and pregnancy screening. There was no direct patient contact, and after chart review, all patient identifiers were deleted from the database and research charts. This study was conducted anonymously and participants were not consented. In the second study, adolescents (n=224) participated in a consented study in which they were asked to provide urine samples and complete a questionnaire regarding tobacco use, secondhand smoke exposure, and the use of marijuana products, including blunts, and alcohol. Participants were told that their study data would not be shared with their doctors or other health care providers. Each question had an option not to answer.
Information on race/ethnicity, sex, age and self-reported tobacco use history (in the first study reported in the medical record or, in the second study, by self-report) was collected on all participants. These studies were approved by the Institutional Review Board at the University of California San Francisco.
2.2. Analytical Chemistry
Urine THC screening (termed “THC screen” in this paper) was performed via a competitive enzyme immunoassay (DRI tm Cannabinoid (THC) Assay, Thermo Scientific) on an ADVIA 1800 Chemistry System (Siemens). This test measures THC-COOH with a recommended reporting cut-off of 50 ng/ml. The immunoassay cross-reacts to varying extents with THC metabolites, including THC-COOH. THC-COOH and THC-COOH glucuronide (THC-COOH-gluc) were measured by LC-MS/MS, with limits of quantitation of 0.5 and 2.5 ng/ml, respectively. Oasis PRiME HLB 96-well μElution Plate (3 mg Sorbent per Well, Waters, Milford, MA) was used for solid phase extraction (SPE). To each 200 μL urine, 20 μL IS working solution was added. 180 μl of sample was applied to the plate. All wells of SPE plate were then washed twice with 200 μl, 80% ammonium acetate (10 mM)/20% methanol: acetonitrile (15:85). Samples were eluted twice with 25 μl acetonitrile. 50 μl of ammonium acetate (10 mM) was added to all samples before injection. The chromatographic separation was achieved using a Phenomenex KineteX® C18 (50 × 3 mm, 2.6 μm) column coupled with a Phenomenex SecurityGuard C18 guard column (3 mm × 2.0 mm). Mobile phase A was 10 mM ammonium acetate and mobile phase B was methanol:acetonitrile (15:85). The injection volume was 20 μL and the flow rate was 0.5 mL/min. The gradient condition was: (time/minute, % mobile phase B): (0, 40), (0.5, 50), (2, 75), (2.5, 75), (2.6, 98), (4, 98), (4.1, 98) and (6.5, 40). The mass spectrometer was operated in negative ion ESI mode using multiple reaction monitoring (MRM). The source parameters included curtain gas of 45 psi, ion spray voltage of −4000 V, ion source temperature of 550, and medium collision gas.
The bioanalytical method was validated in accordance with current U.S. FDA guidelines including selectivity, linearity, intra- and interday precision and accuracy, recovery and matrix effect (“Guidance for Industry, Bioanalytical Method Validation,” 2001). The linearity was tested by the use of spiked standards in urine over the range from 0.5–500.0 ng/mL for THC-COOH, and 2.5–500.0 ng/ml for THC-COOH-gluc. The R2 values are over 0.999 for analytes, demonstrating the good linearity of the method. The inter- and intra-day precision and accuracy of the method were better than 14.2 % at the lower limit of quantification (LLOQ) and better than 9.5 % over the remainder of the linear range. The absolute recoveries were above 54.9%. The matrix effects were lower than 24.1%. This validated method has been used to quantify THC-COOH and THC-COOH-gluc levels in 686 urine samples. Urine cotinine and NNAL were measured by LC-MS/MS with limits of detection of 0.05 ng/ml and 0.25 pg/ml, respectively (Jacob et al., 2008; Jacob et al., 2011).
2.3. Data Analysis/Statistics
As described in previous publications in this study population, we categorized urine cotinine levels in urine as follows: Active smoking for values >30 ng/ml, significant SHS exposure as 0.25 to 30 ng/ml, and light SHS and/or thirdhand smoke (THS) exposure as 0.05 to 0.25 ng/ml (Benowitz et al., 2017; Benowitz et al., 2018). Including covariate categories of race/ethnicity, sex (male and female) and age (12–14, 15–16, 17–18, 19–21 years), we present a descriptive analysis of THC detection frequencies (%) and median (IQR) for THC-COOH concentrations. We tested differences in THC-COOH detection frequency with the χ2 test or fisher’s exact test as appropriate. To evaluate whether absolute measured values of THC-COOH differed across covariates we used Kruskal-Wallis tests. Spearman’s correlations were used to describe the correlation of tobacco biomarkers with THC-COOH and this relationship between smoking status and THC-COOH detection was further assessed using logistic regression. Both an unadjusted and an age- and race category-adjusted model of the odds ratio for THC-COOH detection given active smoking are provided with 95% confidence intervals. All statistical analyses were carried out using SAS v. 9.4 (SAS Institute, Inc. Cary, NC, USA). All statistical tests were considered significant at p < 0.05.
3. Results
Among urine samples with levels above the detection limit (n=173), the mean (SD) and median (IQR) THC-COOH and THC-COOH-gluc concentrations by the chromatographic method were 42.0 (191.5), 4.3 (1.0 – 16.3) and 73.7 (144.6), 11.3 (3.6 – 71.0) ng/ml, respectively. Because of the possibility of spontaneous deconjugation of THC-COOH-gluc in stored urine, we combined the free and glucuronide concentrations (molar sum) to present data on total THC-COOH (termed “Total THC-COOH” in this paper).
Among the full study group (n=686), 11.5% had a positive THC immunoassay screen and 25.2% were positive by total THC-COOH, the latter defined as above limit of quantitation (Table 1). The quantitative urine THC-COOH assay was much more sensitive than the immunoassay THC screen (Table 1). Of those who were positive by screen, 98.7% were positive by total THC-COOH. Of those who were negative by screen, 15.1% were positive by total THC-COOH. Using the THC-COOH assay as the gold standard, the sensitivity and specificity of the THC screen is 46.7 % and 99.8 %, respectively.
Table 1:
Assay Concordance
| THC-COOH | ||||
|---|---|---|---|---|
| n (%) | Not Detected | Detected | Total | |
| Screen THC | Negative |
501 (74.9) |
89 (13.3) |
590 (88.2) |
| Positive |
1 (0.1) |
78 (11.7) |
79 (11.8) |
|
| Total |
502 (75.0) |
167 (25.0) |
669 | |
| Sensitivity* (95% CI) | 46.7 (39.0 – 54.6) | |||
| Specificity* (95% CI) | 99.8 (98.7 – 99.9) | |||
Screen THC Positive (>50) w/ THC-COOH detected (>0.59) as gold standard
For the full study group, prevalence of THC exposure based on THC-COOH increased with age, and was highest in Black and Mixed/Other groups, but did not differ by sex (Table 2). Among those who were positive for THC-COOH, absolute levels of THC-COOH followed a similar pattern, with highest levels in older adolescents, in Blacks and Mixed/Other group, and in males.
Table 2:
THC-COOH Detection Frequency and Concentrations in Participants
| THC Screen Positive | THC-COOH Detected | Total THC-COOH | |||||
|---|---|---|---|---|---|---|---|
| N | Frequency (%) | p | Frequency (%) | p | Median (IQR) | p | |
| All Participants | 686 | 79 (11.5) | - | 173 (25.2) | - | 12.3 (4.0–81.1) | - |
| Sex | |||||||
| Female | 399 | 44 (11.0) | 0.80 | 110 (27.6) | 0.11 | 9.3 (3.7–44.9) | 0.044 |
| Male | 287 | 35 (12.2) | 63 (22.0) | 32.0 (4.8–143.5) | |||
| Age | |||||||
| 12–14 | 170 | 4 (2.4) | <0.001 | 16 (9.4) | <0.001 | 3.9 (3.1–8.7) | <0.001 |
| 15–16 | 255 | 30 (11.8) | 65 (25.5) | 12.3 (3.0–50.4) | |||
| 17–18 | 212 | 32 (15.1) | 75 (35.4) | 11.9 (4.2–110.2) | |||
| 19–21 | 49 | 13 (26.5) | 17 (34.7) | 120.1 (18.6–430.4) | |||
| Race | |||||||
| Asian | 70 | 4 (5.7) | <0.001 | 9 (12.9) | <0.001 | 9.4 (2.8–14.4) | 0.18 |
| Black | 131 | 34 (26.0) | 62 (47.3) | 19.4 (4.9–79.5) | |||
| Latino | 390 | 23 (5.9) | 71 (18.2) | 7.3 (3.1–47.8) | |||
| Mixed/Other | 80 | 15 (18.8) | 26 (32.5) | 17.7 (6.0–141.9) | |||
| White | 15 | 3 (20.0) | 5 (33.3) | 37.9 (5.2–353.6) | |||
| Cotinine | |||||||
| BLQ | 106 | 1 (0.9) | <0.001 | 3 (2.8) | <0.001 | 7.6 (7.4–83.1) | <0.001 |
| 0.05–> 0.25 | 230 | 4 (1.7) | 20 (8.7) | 3.2 (2.4–12.7) | |||
| 0.25–> 30 | 283 | 27 (9.5) | 96 (33.9) | 7.0 (3.3–19.2) | |||
| > 30 | 66 | 47 (71.2) | 54 (81.8) | 92.4 (27.0–219.4) | |||
BLQ: Below limit of Quantitation; IQR: Inter-quartile Range; p values report results from Chi-squared or Fisher’s Exact tests for detection frequencies and Kruskal-Wallis Tests for Total THC-COOH
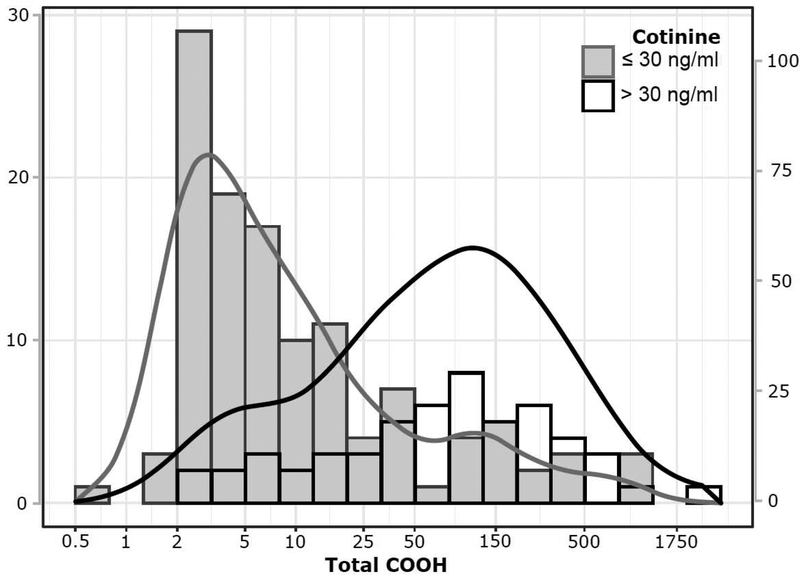
Based on a urine cotinine cut-point of 30 ng/ml, 66 participants (9.6%) were determined to be active tobacco cigarette smokers. Frequency distributions for total THC-COOH among smokers and non-smokers are shown in Fig 1. The prevalence of THC exposure was much higher among adolescent tobacco cigarette smokers compared to those who did not use tobacco (Table 2, Fig 2). Among biochemically-determined cigarette smokers, 81.8% were positive for marijuana use by total THC-COOH and 71.2% were positive using the THC screen. The proportion of THC-positives did not vary significantly by sex or age, but was highest in Black and Mixed/Other groups. The absolute levels of THC-COOH were much higher in cigarette smokers compared to non-smokers, and increased with age (Table 3). Comparison by race/ethnicity was not possible due to small numbers in some groups.
Figure 1:
Log-Scale Distribution of Total THC-COOH values in urine from adolescents classified as active smokers (Cotinine >30 ng/ml) and nonsmokers (Cotinine ≤30 ng/ml) with kernel density overlay. The scale of the left axis describes absolute frequencies while that of the right describes proportions within each class
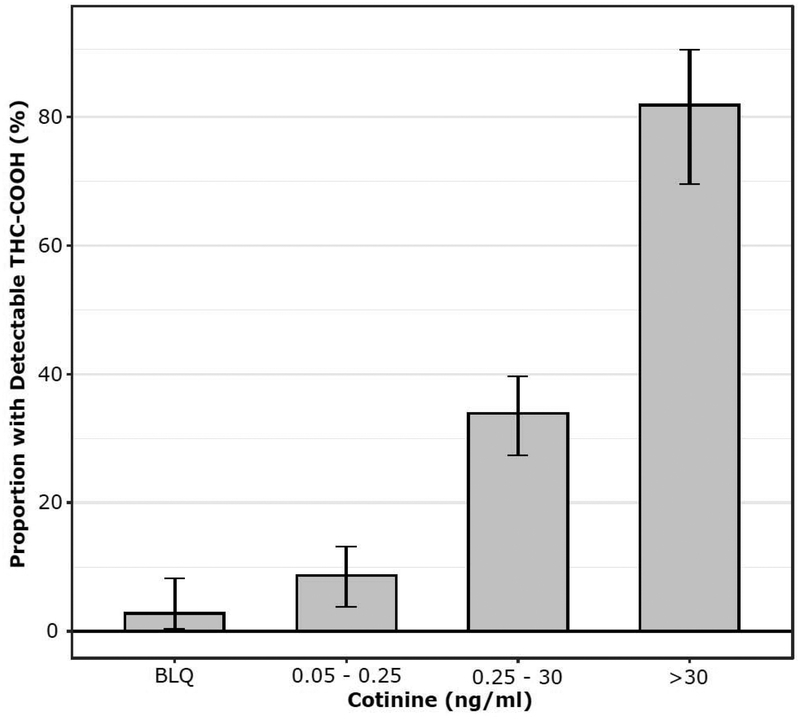
Figure 2:
Proportion of urine samples with detectable levels of THC-COOH across categories of cotinine exposure
Table 3:
Detection Frequency and THC-COOH Concentration in Active Tobacco Users (Cotinine >30)
| Screen THC Positive | THC-COOH Detected | Total THC-COOH | ||
|---|---|---|---|---|
| N | Frequency (%) | Frequency (%) | Median (IQR) | |
| All Participants | 66 | 47 (71) | 54 (82) | 92.4 (27.0–219.4) |
| Sex | ||||
| Female | 39 | 27 (69) | 33 (85) | 120.1 (35.3–241.0) |
| Male | 27 | 20 (74) | 21 (78) | 77 (26.2–134.9) |
| Age | ||||
| 12–14 | 5 | 3 (60) | 4 (80) | 30.7 (14.0–135.7) |
| 15–16 | 23 | 20 (87) | 20 (87) | 50.2 (28.6–115.8) |
| 17–18 | 29 | 16 (55) | 22 (76) | 107.1 (15.5–218.0) |
| 19–21 | 9 | 8 (89) | 8 (89) | 219.4 (166.4–469.7) |
| Race | ||||
| Asian | 1 | 1 (100) | 1 (100) | 114.4 (-) |
| Black | 33 | 27 (82) | 32 (97) | 72.8 (0.5–137.8) |
| Latino | 14 | 6 (43) | 7 (50) | 181.9 (8.2–221.2) |
| Mixed/Other | 13 | 11 (85) | 11 (85) | 57.9 (7.8–381.6) |
| White | 5 | 2 (40) | 3 (60) | 353.6 (79.4–400.0) |
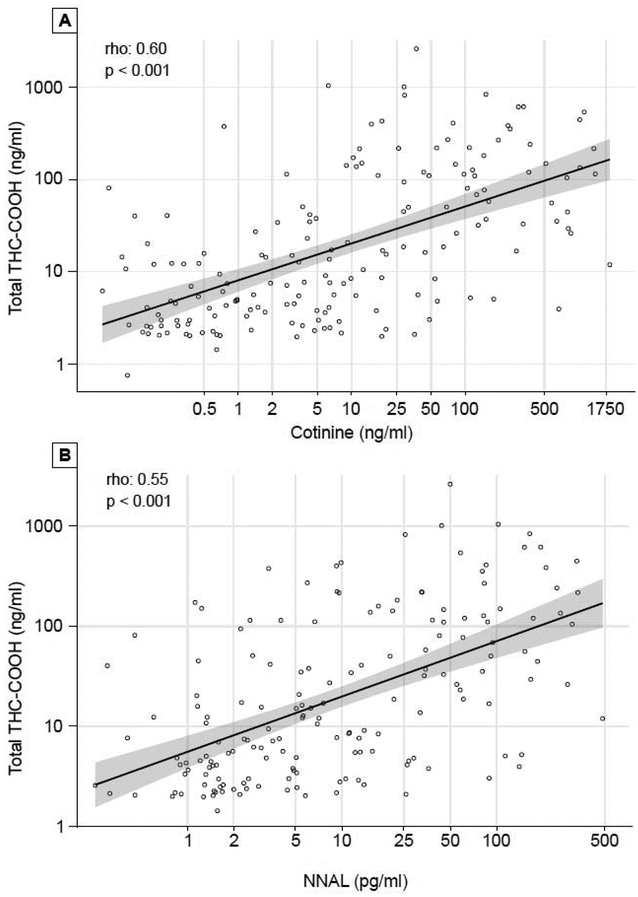
Using urine cotinine, we were also able to examine the relationship between extent of secondhand tobacco smoke (SHS) exposure to THC use (Table 1, Fig 2). Based on THC-COOH, the prevalence of THC exposure was 2.8% among those with no tobacco smoke exposure, 8.7% with light SHS exposure, 33.9% with heavy SHS exposure, and as noted above 81.8% in active smokers (p <0.001). As shown in Fig 3, across all levels of tobacco exposure, there was a moderately strong quantitative correlation between tobacco use and THC biomarkers, with r = 0.60, p <0.001 for cotinine and r = 0.55 for NNAL, respectively, vs total THC-COOH (both p < 0.001). The odds ratio (95% C. I.) for a urine cotinine level of > 30 ng/ml as a predictor of COOH-THC positivity was 18.9 (CI: 10.1 – 38.1) unadjusted and 13.2 (CI: 6.9 – 27) after adjusting for age and race (Black).
Figure 3:
Correlation of Cannabis and Tobacco Exposure (A, Urine Cotinine. B, Urine NNAL) in Dual-Exposed Adolescents.
In a subset of participants (n=224) we were able to compare self-report of various self-reported behaviors to THC biomarker levels (Table 4). For those who reported marijuana use in the past three days (n=24), 91.7% were positive and 8.3% negative for total THC-COOH. For those who reported marijuana use in the past 3 months (n=52), 53.8% were positive and 46.2% negative for THC-COOH. Among all self-reported ever marijuana users (n=83), 77% had smoked marijuana in the form of blunts (but were not asked about use of other forms of marijuana as well). For those who reported blunt use in the past 30 days (n=27), 74.1% were positive THC-COOH. For those who reported alcohol use in the past 3 months (n=62), 32.3% were positive; while among those who report alcohol use in the past 3 days (n=11), 54.5% were positive for THC-COOH.
Table 4:
Self-Report Substance Use (Study 2)
| Self-Reported Substance Use | N | THC-COOH Detected n (%) | p |
|---|---|---|---|
| No 170 | |||
| No 196 | |||
| No 10 | |||
| No 25 | |||
| No 160 | |||
| No 210 |
NOTE: For each question, a range of 2 to 4 participants chose not to respond.
4. Discussion
Our study provides novel information in several areas. We show that the commonly used urine immunoassay screen for THC exposure with a cut-off of 50 ng/ml substantially underestimates actual THC exposure, as measured by a sensitive chromatographic method. On the other hand, in a subset of adolescents in whom use of marijuana was queried, misreporting was relatively low, with only 4.6% and 1.8% of those who reported no marijuana use in the past 3 days and 3 months, respectively, biochemically positive for THC exposure. Thus, the routine urine THC screen has a high degree of specificity, but only a moderate degree of sensitivity. The THC screen presumably has lower sensitivity because many urines had THC-COOH values between 3 and 50 ng/ml. It should be noted that other immunoassays might have lower cut-off values and have a higher levels of sensitivity.
We found that 25% of adolescents had biochemical evidence of marijuana use, which is slightly higher than the 15 to 17% self-reported prevalence of past month use reported in the Monitoring the Future study and in a recent survey in Washington State (Cerda et al., 2018; Dilley et al., 2019), but similar to the 24% biochemically-assessed exposure in adolescents attending a hospital clinics by Silber et al in Washington D.C. in 1987 (Silber et al., 1987). Consistent with other studies, we found an increased prevalence of marijuana use with increasing age and in Blacks, compared with other racial/ethnic groups. Black adolescents in particular have a much higher use of use of blunts compared to other groups, which may account for the higher prevalence of THC exposure (Schauer and Peters, 2018).
We found an extremely high biochemically-determined prevalence (80%) of THC use in active cigarettes smokers. A strong association between tobacco and marijuana use was expected based on other studies of self-reported behaviors, but the strength of the association was remarkable. The odds ratio of active cigarette smoking predicting THC use was 13.2 (adjusted). Thus, cigarette smoking was a strong surrogate marker for marijuana use. Whether this remains the case as the prevalence of cigarette smoking declines in youth in the future and the extent to which the use of electronic cigarettes in youth are predictive of marijuana use remain to be determined.
Another novel finding was the association between the level of SHS exposure, determined by urine cotinine, and biochemically-determined prevalence of THC use, with a progressive increase in prevalence from no exposure to light, and to heavy SHS exposure, and to active smoking. A limitation of our study is that we cannot tell if the source of nicotine exposure is cigarette smoking or use of electronic cigarettes. Additionally, we cannot determine if cotinine levels consistent with high levels of SHS exposure actually represent low-level nicotine exposure from SHS or intermittent non-daily smoking vaping, or whether the low levels of cotinine reflect the use of blunts. One laboratory study reported no detectable plasma nicotine after three puffs of a blunt, suggesting that cotinine levels attributable to blunt use would be low (Cooper and Haney, 2009). Across all categories of tobacco exposure as indicated by either urine cotinine or NNAL, there was a moderately strong quantitative correlation between tobacco biomarkers and level of THC exposure. The pharmacologic bases and health implications of this association remain to be explored.
As expected, a history of use of blunts in the past 30 days was associated with a high prevalence of biochemically-determined marijuana use (74%). A history of alcohol use in the past 3 days was also associated with a high prevalence of marijuana use (54%). The concordance of marijuana and alcohol use has been well described in other studies (Schlienz and Lee, 2018; Terry-McElrath et al., 2013).
Limitations of our study include the use of a convenience sample of adolescents seeking medical care in an urban public hospital outpatient clinics in one city. Our study was conducted before recreational use of marijuana was legalized in California. The prevalence of marijuana use might be even higher now that recreational marijuana is legal. Further, we did not collect data on level of dependence on tobacco or marijuana.
5. Conclusion
The conclusions and implications of our study are as follows. The use of high sensitivity quantitative analysis for total THC-COOH provides a much more complete picture of adolescent marijuana use compared to the usual immunoassay screening method. This is probably due to relatively infrequent use of marijuana in adolescents, such that urine THC-COOH levels fall below the urine screen cutoff in many individuals. We also find that while 92% of participants who reported marijuana use in the past 3 days were biochemically confirmed as exposed, only 54% of those who reported use in past 3 months were biochemically positive. Thus, a combination of biochemical screening and self-report may be necessary to fully characterize marijuana use patterns. We confirm the high concordance in prevalence between cigarette smoking and marijuana use, and provide novel data on the dose-response relationships between the level of nicotine exposure associated with SHS and active smoking with prevalence and levels of exposure to THC in adolescents. We suggest that quantitative screening for THC and nicotine exposure will provide greater insight into patterns and doses of co-substance use, and may enhance our understanding of dose-related aspects of addiction and pathophysiology of adverse health effects associated with dual use. A caveat is that high sensitivity chromatographic screening is expected to cost 4–5 times more than commonly used immunoassay methods.
Highlights.
There is known to be a high concordance of tobacco and marijuana use in adolescents
Co-use has been demonstrated to increase risk of addiction and mental illness
Biochemical analysis of adolescent urine confirms a high biomarker concordance
Correlation between nicotine and THC exposure levels in youth is further quantified
Novel data suggests a strong predictive value of smoking tobacco in marijuana use
Acknowledgements
The authors thank Sandra Tinetti, Daisy Mendoza and the nurses at the ZSFG CHC for conducting the clinical studies and Trisha Mao, Lita Ramos and Lawrence Chan for performing the analytical chemistry.
Role of Funding Source
Research reported in this paper was supported by the Flight Attendants Medical Research Institute, and the National Institute on Drug Abuse (NIDA) under award number DA012393 and the National Center for Research Resources (NCRR) under award RR026437. The funding sources were not involved in study design, in collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit this article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Flight Attendants Medical Research Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Benowitz is a consultant to Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has been a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to disclose.
References
- Benowitz NL, Jain S, Dempsey DA, Nardone N, Helen GS, Jacob P 3rd, 2017. Urine Cotinine Screening Detects Nearly Ubiquitous Tobacco Smoke Exposure in Urban Adolescents. Nicotine Tob. Res 19, 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Nardone N, Jain S, Dempsey DA, Addo N, St Helen G, Jacob P 3rd, 2018. Comparison of Urine 4-(Methylnitrosamino)-1-(3)Pyridyl-1-Butanol and Cotinine for Assessment of Active and Passive Smoke Exposure in Urban Adolescents. Cancer Epidemiol. Biomark. Prev 27, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda M, Sarvet AL, Wall M, Feng T, Keyes KM, Galea S, Häsin DS, 2018. Medical marijuana laws and adolescent use of marijuana and other substances: Alcohol, cigarettes, prescription drugs, and other illicit drugs. Drug Alcohol Depend. 183, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS, 2008. Cannabinoid receptor 1 gene association with nicotine dependence. Arch. Gen. Psychiatr 65, 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M, 2009. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 103,107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DA, Meyers MJ, Oh SS, Nguyen EA, Fuentes-Afflick E, Wu AH, Jacob P, Benowitz NL, 2012. Determination of tobacco smoke exposure by plasma cotinine levels in infants and children attending urban public hospital clinics. Arch. Pediatr. Adolesc. Med 166, 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley JA, Richardson SM, Kilmer B, Pacula RL, Segawa MB, Cerda M, 2019. Prevalence of Cannabis Use in Youths After Legalization in Washington State. JAMA Pediatr. 173, 192–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Sutton SK, Jentink KG, Lin HY, Park JY, Drobes DJ, 2016. Cannabinoid receptor 1 (CNR1) gene variant moderates neural index of cognitive disruption during nicotine withdrawal. Genes. Brain Behav. 15, 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ, 1998. Urinary excretion half-life of 11-nor-9-carboxy-delta9-tetrahydrocannabinol in humans. Ther. Drug Monit. 20, 570–576. [DOI] [PubMed] [Google Scholar]
- Jacob P 3rd, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL, 2008. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal. Chem 80, 8115–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL, 2011. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J. Chromatrogr. B 879, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier E, Hatsukami DK, 2016. A review of the additive health risk of cannabis and tobacco co-use. Drug Alcohol Depend. 166, 6–12. [DOI] [PubMed] [Google Scholar]
- Peters EN, Schwartz RP, Wang S, O’Grady KE, Blanco C, 2014. Psychiatric, psychosocial, and physical health correlates of co-occurring cannabis use disorders and nicotine dependence. Drug Alcohol Depend. 134, 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Braida D, Zoli M, Clementi F, Viani P, Sala M, Gotti C, 2019. Increased sensitivity to Delta(9)-THC-induced rewarding effects after seven-week exposure to electronic and tobacco cigarettes in mice. Eur. Neuropsychopharmacol 29, 566–576. [DOI] [PubMed] [Google Scholar]
- Ramo DE, Liu H, Prochaska JJ, 2012. Tobacco and marijuana use among adolescents and young adults: a systematic review of their co-use. Clin. Psychol. Rev 32, 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream GL, Benoit E, Johnson BD, Dunlap E, 2008. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 95, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer GL, King BA, McAfee TA, 2017a. Prevalence, correlates, and trends in tobacco use and cessation among current, former, and never adult marijuana users with a history of tobacco use, 2005–2014. Addict. Behav 73, 165–171. [DOI] [PubMed] [Google Scholar]
- Schauer GL, Peters EN, 2018. Correlates and trends in youth co-use of marijuana and tobacco in the United States, 2005–2014. Drug Alcohol Depend. 185, 238–244. [DOI] [PubMed] [Google Scholar]
- Schauer GL, Rosenberry ZR, Peters EN, 2017b. Marijuana and tobacco co-administration in blunts, spliffs, and mulled cigarettes: A systematic literature review. Addict. Behav 64, 200–211. [DOI] [PubMed] [Google Scholar]
- Schlienz NJ, Lee DC, 2018. Co-use of cannabis, tobacco, and alcohol during adolescence: policy and regulatory implications. Int. Rev. Psychiatry 30, 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber TJ, Getson P, Ridley S, Iosefsohn M, Hicks JM, 1987. Adolescent marijuana use: concordance between questionnaire and immunoassay for cannabinoid metabolites. J. Pediatr 111, 299–302. [DOI] [PubMed] [Google Scholar]
- Strong DR, Myers MG, Pulvers K, Noble M, Brikmanis K, Doran N, 2018. Marijuana use among US tobacco users: Findings from wave 1 of the population assessment of tobacco health (PATH) study. Drug Alcohol Depend. 186, 16–22. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, O’Malley PM, Johnston LD, 2013. Simultaneous alcohol and marijuana use among U.S. high school seniors from 1976 to 2011 : trends, reasons, and situations. Drug Alcohol Depend. 133, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R, 2002. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br. J. Pharmacol 135, 564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A, 2008. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 92, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KM, Torok MR, Wei B, Wang L, Lowary M, Blount BC, 2018. Marijuana and Tobacco Coexposure in Hospitalized Children. Pediatrics. 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KM, Torok MR, Wei B, Wang L, Robinson M, Sosnoff CS, Blount BC, 2017. Detecting biomarkers of secondhand marijuana smoke in young children. Pediatr. Res 81, 589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]