Abstract
Objectives In the present study, we examined available articles from online databases to comprehensively investigate the effect of the XPC (xeroderma pigmentosum complementation group C) rs2228000 polymorphism on the risk of different types of clinical cancer.
Methods We conducted a group of overall and subgroup pooling analyses after retrieving the data from four databases (updated till September 2019). The P-value of association, OR (odds ratios), and 95% CI (confidence interval) were calculated.
Results We selected a total of 71 eligible studies with 26835 cancer cases and 37069 controls from the 1186 retrieved articles. There is an enhanced susceptibility for bladder cancer cases under T vs. C [P=0.004; OR (95% CI) = 1.25 (1.07, 1.45)], TT vs. CC [P=0.001; 1.68 (1.25, 2.26)], CT+TT vs. CC [P=0.016; 1.26 (1.04, 1.53)], and TT vs. CC+ CT [P=0.001; 1.49 (1.18, 1.90)] compared with negative controls. Additionally, there is an increased risk of breast cancer under T vs. C, TT vs. CC and TT vs. CC+ CT (P<0.05, OR > 1). Nevertheless, there is a decreased risk of gastric cancer cases in China under T vs. C [P=0.020; 0.92 (0.85, 0.99)], CT vs. CC [P=0.001, 0.83 (0.73, 0.93)], and CT+TT vs. CC [P=0.003, 0.84 (0.76, 0.94)].
Conclusions The TT genotype of XPC rs2228000 may be linked to an increased risk of bladder and breast cancer, whereas the CT genotype is likely to be associated with reduced susceptibility to gastric cancer in the Chinese population.
Keywords: cancer, rs2228000, susceptibility, XPC
Introduction
The human XPC (xeroderma pigmentosum complementation group C) gene is located on chromosome 3p25 and contains 16 exons and 15 introns [1,2]. The human XPC protein with 940 amino acids, encoded by XPC, serves as an essential member within the NER (nucleotide excision repair) pathway [3–5]. The XPC protein is important for the early damage site recognition and DNA repair initiation of NER [3,6,7]. The abnormal expression of the XPC protein was also reportedly linked to the progression of the cancer [3,8].
Within the XPC gene, three common variants, including rs2228000 (C21151T) of exon 8, rs2228001 (A33512C) of exon 15, and poly-AT insertion/deletion polymorphism (PAT−/+) of intron 9, were identified [4,9–11]. XPC rs2228000 results in a substitution of alanine for valine in position 499 (Ala499Val), while rs2228001 leads to a transversion from lysine to glutamine in position 939 (Lys939Gln) [4,9–11]. The present study investigated the potential genetic role of nonsynonymous XPC rs2228000 in the risk of different clinical types of cancer by pooling published studies with inconclusive conclusions.
After retrieving these studies, only three previous meta-analyses with no more than 15 studies in 2008 [12–14] and one meta-analysis with 33 studies in 2013 [15] were performed to assess the genetic association of XPC rs2228000 and the risk of overall cancer. Thus, we enrolled more sample sizes (71 case–control studies) and utilized different analysis strategies for an updated comprehensive evaluation in 2019 through meta-analysis and TSA (trial sequential analysis).
Materials and methods
Case–control study identification
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was utilized for our pooling analysis. In September 2019, we used a series of search terms (shown in Supplementary Table S1) to retrieve from four databases [PubMed, Embase, CChia National Knowledge Infrastructure (CNKI)) and WOS (Web of Science)] to obtain potentially relevant articles. We also designed a group of criteria for the inclusion/exclusion and eligibility assessment of the article. Inclusion criteria were the following: (1) case/control studies; (2) cancer; (3) XPC rs2228000; and (4) genotypic frequency data within both the case and control groups. Exclusion criteria were the following: (1) review; (2) meeting abstract; (3) case reports or family data; (4) meta-analysis; (5) cell, mice, horse, or other species; (6) other gene, disease or variant; (7) lack of specific data; (8) lack of normal group; (9) not in line with HWE (Hardy–Weinberg equilibrium); and (10) cohort.
Basic information collection
We extracted some basic information, including author name, publication year, country, race, genotypic frequency, cancer type, control source, genotyping assay, and sample size, from the selected eligible case–control studies. The P-value of HWE based on the genotypic distribution in the control group was calculated.
Article quality assessment
We utilized two approaches, including the NOS (Newcastle–Ottawa quality assessment scale) system (Supplementary Table S2) [16,17] and the risk-of-bias score system (Supplementary Table S3) [18,19] for the assessment of article quality. The article with an NOS score > 5 and a risk-of-bias score > 9 was considered to be high quality.
Pooling analysis
We used STATA software (Stata Corporation, U.S.A.) to perform the association test in the overall and subgroup meta-analysis, heterogeneity assessment, Begg’s/Egger’s tests (for the publication bias evaluation) and sensitivity analysis (for data stability assessment) [16,17]. The OR (odds ratio), 95% CI (confidence interval) and P-value in a series of association tests under the five genetic models, including T vs. C (allele), TT vs. CC (homozygote), CT vs. CC (heterozygote), CT+TT vs. CC (dominant), and TT vs. CC+CT (recessive), were obtained. In addition, six factors, including race, country, control source, article quality, genotyping assay, and cancer type, were considered in our subgroup analysis.
The high heterogeneity was considered when the I2 value in the I2 test was larger than 50% and the P-value in the Q statistical test was less than 0.05, which led to the use of the DerSimonian–Laird method of the random-effect model. If not, a Mantel–Haenszel method of a fixed-effect model was used for the relatively low heterogeneity between studies.
False-positive report probability
Targeting the positive findings, we also calculated the false-positive report probability (FPRP) and statistical power, as suggested by Wacholder et al. [20]. During analysis, an FPRP cut-off value of 0.2, a power OR of 1.5, and different prior probability levels (0.25, 0.1, 0.01, 0.001, 0.0001) were established. After assessing the research status regarding the association between XPC rs2228000 and cancer risk and referencing the similar publications [21,22], the FPRP value of the positive results less than 0.2 under the prior probability level of 0.1 indicates a noteworthy outcome.
TSA
We also performed the TSA test to evaluate whether further research was needed, referring to some similar publications [23–26]. For the TSA parameter, a type I error probability of 5%, a statistical test power of 80%, and a low bias-based risk ratio reduction were established. Trial Sequential Analysis Viewer software (http://www.ctu.dk/tsa/) was utilized.
Results
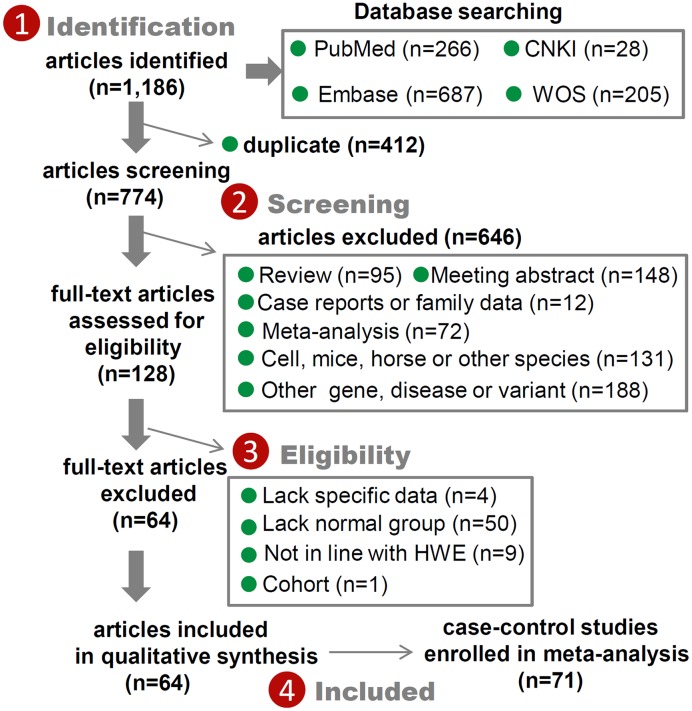
Identification of eligible studies
In total, we obtained 1186 potential eligible articles [PubMed (n=266), Embase [n=687], CNKI (n=28), and WOS (n=205)] and then ruled out another 412 duplicates and 646 improper articles according to our exclusion criteria (detailed information listed in Figure 1). Furthermore, we excluded 64 articles due to the question of ‘lack of specific data or normal group’, ‘not in line with HWE’ or ‘cohort’. Finally, we identified a total of 71 eligible case–control studies from the 64 retrieved articles [1,2,4,10,11,27–85] for pooling analysis. We summarized some basic information in Table 1 and presented the flow chart in Figure 1. All the genotypic distribution of the control group in all studies followed the principle of HWE. Although the NOS scores in all studies were larger than 5 (Supplementary Table S2), the risk-of-bias scores of nine articles (Supplementary Table S3) were less than 9.
Figure 1. Selection process of eligible case–control studies.
Table 1. Basic information of the studies included in the meta-analysis.
| First author | Year | Country | Race | Cases | Cancer type | Control | Control source | Genotyping assay | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | CC | CT | TT | |||||||
| Al-Qadoori | 2019 | Iraq | Asian | 37 | 23 | 2 | Bladder cancer | 31 | 7 | 0 | PB | Gene sequencing |
| An | 2007 | U.S.A. | Caucasian | 445 | 293 | 91 | HNSCC | 454 | 342 | 58 | HB | PCR-RFLP |
| Bai | 2007 | China | Asian | 184 | 193 | 48 | LAC | 446 | 456 | 88 | HB | TaqMan |
| China | Asian | 149 | 149 | 34 | LSCC | 446 | 456 | 88 | HB | TaqMan | ||
| China | Asian | 31 | 25 | 8 | SCLC | 446 | 456 | 88 | HB | TaqMan | ||
| Broberg | 2005 | Sweden | Caucasian | 35 | 20 | 6 | Bladder cancer | 92 | 55 | 8 | PB | MassARRAY |
| Chen | 2013 | China | Asian | 45 | 60 | 26 | Cervical cancer | 101 | 118 | 38 | HB | PCR-RFLP |
| de Verdier | 2010 | Sweden | Caucasian | 138 | 138 | 35 | Bladder cancer | 196 | 124 | 10 | PB | PCR-RFLP |
| Doherty | 2011 | U.S.A. | Mixed | 411 | 257 | 49 | Endometrial cancer | 384 | 278 | 61 | PB | PCR-RFLP/SNaPshot |
| Dong | 2008 | China | Asian | 141 | 90 | 22 | GCA | 272 | 282 | 58 | PB | PCR-RFLP |
| Farnebo | 2015 | Sweden | Caucasian | 89 | 63 | 17 | HNSCC | 219 | 105 | 20 | PB | PCR-RFLP |
| Figl | 2010 | Spain/Germany | Caucasian | 626 | 477 | 81 | Melanoma | 670 | 516 | 88 | PB | TaqMan |
| Garcia | 2006 | Spain | Caucasian | 583 | 440 | 85 | Bladder cancer | 599 | 435 | 75 | HB | SNP500Cancer |
| Guo | 2008 | China | Asian | 156 | 133 | 38 | ESCC | 272 | 282 | 58 | PB | PCR-RFLP |
| He | 2016 | China | Asian | 201 | 198 | 51 | Breast cancer | 228 | 174 | 28 | PB | MassARRAY |
| He | 2012 | China | Asian | 104 | 90 | 16 | Pancreatic cancer | 106 | 85 | 22 | PB | SNaPshot |
| Hu | 2005 | China | Asian | 124 | 171 | 25 | Lung cancer | 158 | 145 | 19 | PB | PCR-PIRA |
| Hua | 2016a | China | Asian | 432 | 531 | 178 | CRC | 429 | 583 | 161 | PB | TaqMan |
| Hua | 2016b | China | Asian | 457 | 524 | 161 | Gastric cancer | 429 | 583 | 161 | PB | TaqMan |
| Huang | 2006 | U.S.A. | Mixed | 397 | 261 | 31 | CRC | 403 | 259 | 41 | HB | SNP500Cancer |
| Ibarrola | 2011 | Spain | Caucasian | 323 | 227 | 49 | Melanoma | 198 | 158 | 23 | PB/HB | MassARRAY |
| Jiao | 2011 | China | Asian | 127 | 177 | 30 | GBC | 163 | 146 | 20 | HB | PCR-RFLP |
| Jorgensen | 2007 | U.S.A. | Caucasian | 153 | 87 | 13 | Breast cancer | 157 | 104 | 14 | PB | TaqMan |
| Kim | 2002 | Korea | Asian | 104 | 102 | 12 | Lung cancer | 77 | 62 | 10 | PB | PCR-RFLP |
| Lee | 2005 | Korea | Asian | 113 | 84 | 13 | LSCC | 223 | 179 | 29 | PB | PCR-RFLP |
| Korea | Asian | 79 | 58 | 4 | LAC | 223 | 179 | 29 | PB | PCR-RFLP | ||
| Korea | Asian | 39 | 28 | 6 | SCLC | 223 | 179 | 29 | PB | PCR-RFLP | ||
| Li | 2006 | U.S.A. | Caucasian | 338 | 214 | 50 | Melanoma | 318 | 248 | 37 | HB | PCR-RFLP |
| Li | 2014 | China | Asian | 92 | 91 | 19 | Gastric cancer | 144 | 153 | 30 | PB | PCR-RFLP |
| Li | 2010 | China | Asian | 163 | 248 | 89 | HCC | 169 | 250 | 88 | HB | TaqMan |
| Liang | 2018 | China | Asian | 98 | 89 | 18 | Pancreatic cancer | 116 | 90 | 24 | HB | SNaPshot |
| Liu | 2016 | China | Asian | 444 | 351 | 96 | Gastric cancer | 424 | 408 | 95 | HB | MassARRAY |
| Liu | 2012 | China | Asian | 242 | 294 | 64 | Bladder cancer | 272 | 285 | 52 | PB | PCR-RFLP |
| Liu | 2019 | China | Asian | 178 | 159 | 54 | Uterine leiomyoma | 183 | 232 | 78 | PB | Sequence Detection System |
| Long | 2010 | China | Asian | 170 | 156 | 35 | GAA | 280 | 274 | 62 | HB | TaqMan |
| McWilliams | 2008 | U.S.A. | Mixed | 246 | 182 | 29 | Pancreatic cancer | 339 | 211 | 32 | HB | SNPstream or Pyrosequencing |
| Monroy | 2011 | U.S.A. | Mixed | 92 | 90 | 8 | HL | 137 | 71 | 10 | PB | MassARRAY |
| Na | 2012 | China | Asian | 213 | 124 | 23 | Breast cancer | 228 | 118 | 14 | HB | MassARRAY |
| Nigam | 2019 | China | Asian | 22 | 22 | 26 | Oral cancer | 69 | 145 | 83 | PB | PCR-RFLP |
| Ozgoz | 2019 | Turkey | Caucasian | 57 | 38 | 7 | Breast cancer | 67 | 26 | 7 | PB | MassARRAY |
| Pan | 2009 | U.S.A | Caucasian | 228 | 129 | 26 | Esophageal cancer | 251 | 178 | 21 | PB | TaqMan |
| Paszkowska | 2015 | Poland | Caucasian | 443 | 269 | 41 | CRC | 548 | 563 | 177 | PB | MassARRAY/Taqman |
| Paszkowska | 2013 | Poland | Caucasian | 245 | 240 | 34 | Melanoma | 548 | 563 | 177 | PB | MassARRAY |
| Perez | 2013 | U.S.A. | Caucasian | 0 | 63 | 115 | Breast cancer | 21 | 131 | 203 | PB | TaqMan |
| Ravegnini | 2016 | Italy | Caucasian | 42 | 34 | 5 | GIST | 90 | 45 | 12 | PB | TaqMan |
| Roberts | 2011 | U.S.A. | Mixed | 167 | 100 | 18 | Breast cancer1 | 317 | 193 | 40 | PB | MassARRAY |
| U.S.A. | Mixed | 437 | 273 | 48 | Breast cancer2 | 793 | 478 | 72 | PB | MassARRAY | ||
| Sak | 2006 | U.K. | Mixed | 279 | 202 | 57 | Bladder cancer | 317 | 210 | 38 | PB/HB | TaqMan |
| Sakoda | 2012 | U.S.A. | Caucasian | 401 | 299 | 43 | Lung cancer | 822 | 566 | 87 | PB | GoldenGate/Taqman |
| Sankhwar | 2016 | India | Asian | 52 | 113 | 69 | Bladder cancer | 87 | 112 | 59 | PB | PCR-RFLP/gene sequencing |
| Santos | 2013 | Portugal | Caucasian | 47 | 55 | 4 | Thyroid cancer | 95 | 98 | 19 | HB | PCR-RFLP |
| Shen | 2006 | U.S.A. | Caucasian | 96 | 50 | 9 | Breast cancer | 91 | 55 | 5 | PB | TaqMan |
| Shen | 2008 | U.S.A. | Mixed | 614 | 385 | 62 | Breast cancer | 632 | 417 | 56 | PB | Fluorescence polarization |
| Shen | 2005 | China | Asian | 56 | 47 | 13 | Lung cancer | 50 | 47 | 13 | PB | TaqMan |
| Slyskova | 2012 | Czech Republic | Caucasian | 36 | 24 | 9 | CRC | 37 | 24 | 3 | PB | PCR-RFLP |
| Smith | 2008 | U.S.A. | Caucasian | 178 | 116 | 23 | Breast cancer | 211 | 161 | 29 | PB | MassARRAY |
| U.S.A. | Others | 44 | 7 | 1 | Breast cancer | 61 | 14 | 0 | PB | MassARRAY | ||
| Steck | 2014 | U.S.A. | Others | 175 | 51 | 2 | CRC | 276 | 47 | 0 | PB | MassARRAY |
| U.S.A. | Caucasian | 177 | 104 | 22 | CRC | 293 | 207 | 35 | PB | MassARRAY | ||
| Tang | 2011 | China | Asian | 40 | 55 | 14 | ALL | 80 | 74 | 15 | PB | MassARRAY |
| Weiss | 2005 | U.S.A. | Mixed | 211 | 129 | 31 | Endometrial cancer | 213 | 166 | 41 | PB | SNaPshot |
| Wu | 2011a | China | Asian | 172 | 195 | 52 | CRC | 315 | 406 | 117 | PB | PCR-RFLP |
| Wu | 2011b | China | Asian | 65 | 86 | 22 | Breast cancer | 69 | 85 | 16 | PB | PCR-RFLP |
| Yang | 2012 | China | Asian | 197 | 322 | 99 | Breast cancer | 235 | 312 | 75 | PB | PCR-RFLP |
| Yang | 2008 | China | Asian | 52 | 73 | 28 | NPC | 76 | 79 | 13 | PB | PCR-RFLP |
| Zhao | 2018 | China | Asian | 46 | 35 | 8 | Ovarian cancer | 127 | 175 | 54 | PB | TaqMan |
| Zheng | 2016 | China | Asian | 111 | 108 | 34 | Neuroblastoma | 205 | 250 | 76 | PB | TaqMan |
| Zhou | 2008 | China | Asian | 103 | 78 | 27 | Ovarian cancer | 118 | 95 | 18 | PB | PCR-RFLP |
| Zhu | 2018 | China | Asian | 64 | 59 | 22 | Nneuroblastoma | 205 | 250 | 76 | PB | TaqMan |
| Zhu | 2008 | China | Asian | 110 | 60 | 18 | ESCC | 83 | 88 | 32 | PB | PCR-RFLP |
| Zhu | 2007 | U.S.A. | Caucasian | 323 | 193 | 30 | Bladder cancer | 310 | 215 | 24 | HB | TaqMan |
Abbreviations: ALL, acute lymphoblastic leukemia; CRC, colorectal cancer; ESCC, esophageal squamous cell carcinoma; GAA, gastric antrum adenocarcinoma; GBC, primary gallbladder adenocarcinoma; GCA, gastric cardiac adenocarcinoma; GIST, gastrointestinal stromal tumour; HB, hospital-based; HCC, hepatocellular carcinoma; HL, Hodgkin lymphoma; HNSCC, head and neck squamous cell carcinoma; LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma; NPC, nasopharyngeal cancer; PB, population-based; PCR, polymerase chain reaction; PIRA, primer-introduced restriction analysis; RFLP, restriction fragment length polymorphism; SCLC, Small cell lung carcinoma; SNP, single nucleotide polymorphism.
1 Premenopausal.
2 Postmenopausal.
Overall meta-analysis
As shown in Table 2, our overall meta-analysis included a total of 71 studies with 26835 cases and 37069 controls. We observed high between-study heterogeneity (Table 2, all I2 > 50%, Pheterogeneity<0.001) and thus utilized the random-effect model for the pooling analysis. After pooling the different studies together, we only detected an increased risk of overall cancers under the TT vs. CC+CT model [Table 2, Passociation=0.023, OR = 1.11, 95% CI = (1.01, 1.22)] but not other models (all Passociation>0.05). These results indicated that XPC rs2228000 does not seem to be statistically associated with susceptibility to cancer.
Table 2. Meta-analysis of XPC rs2228000 and overall cancer risk.
| Genetic model | Sample size | Association | Heterogeneity | Publication bias | ||||
|---|---|---|---|---|---|---|---|---|
| Study | Case/control | Passociation | OR (95% CI) | I2 | Pheterogeneity | PBegg | PEgger | |
| T vs. C | 71 | 26835/37069 | 0.218 | 1.03 (0.98,1.09) | 72.2% | <0.001 | 0.079 | 0.031 |
| TT vs. CC | 71 | 26835/37069 | 0.090 | 1.10 (0.99,1.23) | 64.6% | <0.001 | 0.124 | 0.065 |
| CT vs. CC | 71 | 26835/37069 | 0.588 | 0.98 (0.93,1.04) | 59.1% | <0.001 | 0.093 | 0.046 |
| CT+TT vs. CC | 71 | 26835/37069 | 0.793 | 1.01 (0.95,1.07) | 68.0% | <0.001 | 0.069 | 0.023 |
| TT vs. CC+ CT | 71 | 26835/37069 | 0.023 | 1.11 (1.01,1.22) | 54.1% | <0.001 | 0.493 | 0.230 |
Abbreviations: Passociation, P-value in the association test; Pheterogeneity, P-value in the heterogeneity test; PBegg, P-value in Begg’s test; PEgger, P-value in Egger’s test.
Subgroup analysis
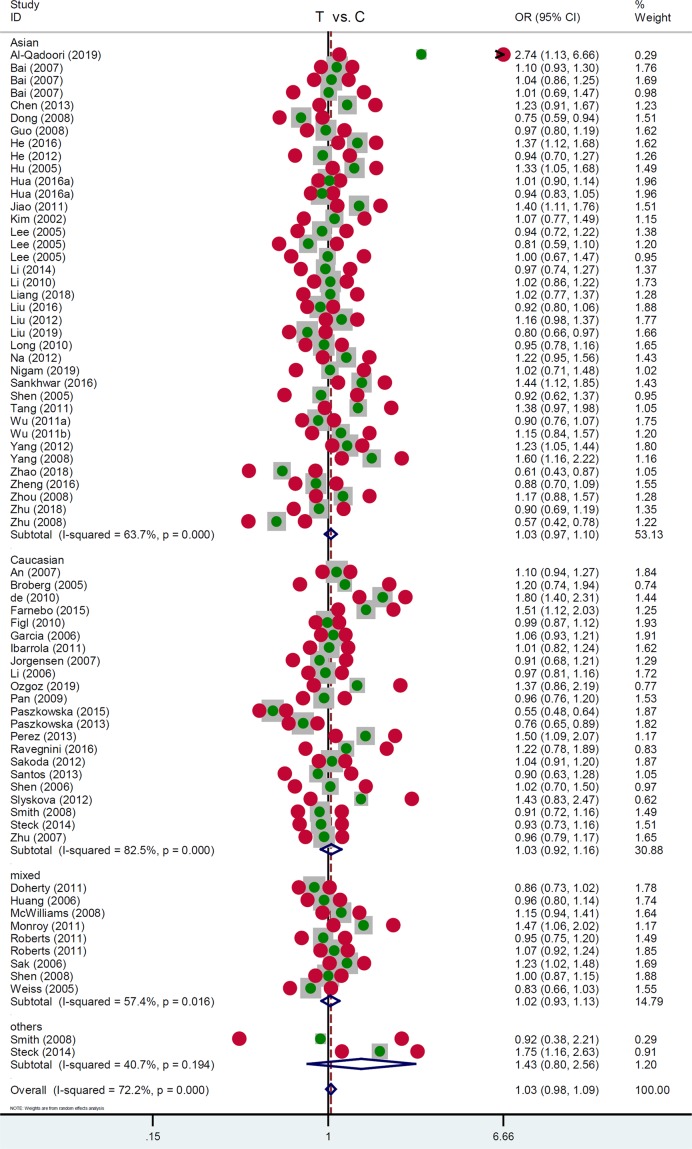
Next, we performed a series of subgroup analyses by the factors of race, control source, country, article quality, and genotyping assay. As shown in Table 3, a total of 38 studies (12118 cases/18124 controls) were included for the subgroup analysis of ‘Asian’, while 22 studies with 9371 cases and 12338 controls were included for the ‘Caucasian’ subgroup. We did not observe a significant difference between cancer cases and negative controls under the most genetic models (Table 3, Passociation>0.05), only apart from the Asian subgroup under the TT vs. CC+CT model [Passociation=0.005, OR = 1.13, 95% CI = (1.04, 1.23)]. Within the subgroup analysis by the factor or control source (PB/HB (population/hospital-based)), an increased risk of cancer was only detected in the ‘HB’ subgroup under TT vs. CC [Table 3, Passociation=0.010, OR = 1.17, 95% CI = (1.04,1.32)] and TT vs. CC+CT [Passociation=0.006, OR = 1.18, 95% CI = (1.05, 1.32)] models but not others (Passociation>0.05). Similarly, we observed negative results in the majority of the subgroup analyses by country, article quality and genotyping assay (Supplementary Table S4). As examples, we presented the forest plots of the subgroup analysis data by the factor of race (Figure 2), control source (Supplementary Figure S1), country (Supplementary Figure S2), article quality (Supplementary Figure S3), and genotyping assay (Supplementary Figure S4) under the T vs. C model.
Table 3. Subgroup analysis data by the factors of race and control source.
| Genetic model | Subgroup | Sample size | Association | ||
|---|---|---|---|---|---|
| Study | Case/Control | Passociation | OR (95% CI) | ||
| T vs. C | Asian | 38 | 12118/18124 | 0.360 | 1.03 (0.97, 1.10) |
| Caucasian | 22 | 9371/12338 | 0.572 | 1.03 (0.92, 1.16) | |
| PB | 52 | 17758/25317 | 0.447 | 1.03 (0.96, 1.10) | |
| HB | 17 | 7940/10808 | 0.109 | 1.04 (0.99, 1.09) | |
| TT vs. CC | Asian | 38 | 12118/18124 | 0.091 | 1.11 (0.98, 1.25) |
| Caucasian | 22 | 9371/12338 | 0.329 | 1.15 (0.87, 1.53) | |
| PB | 52 | 17758/25317 | 0.397 | 1.07 (0.92, 1.23) | |
| HB | 17 | 7940/10808 | 0.010 | 1.17 (1.04, 1.32) | |
| CT vs. CC | Asian | 38 | 12118/18124 | 0.554 | 0.98 (0.90, 1.06) |
| Caucasian | 22 | 9371/12338 | 0.483 | 0.96 (0.86, 1.07) | |
| PB | 52 | 17758/25317 | 0.612 | 0.98 (0.91, 1.06) | |
| HB | 17 | 7940/10808 | 0.857 | 0.99 (0.92, 1.07) | |
| CT+TT vs. CC | Asian | 38 | 12118/18124 | 0.948 | 1.00 (0.92, 1.09) |
| Caucasian | 22 | 9371/12338 | 0.905 | 0.99 (0.88, 1.12) | |
| PB | 52 | 17758/25317 | 0.965 | 1.00 (0.92, 1.09) | |
| HB | 17 | 7940/10808 | 0.581 | 1.02 (0.95, 1.09) | |
| TT vs. CC+ CT | Asian | 38 | 12118/18124 | 0.005 | 1.13 (1.04, 1.23) |
| Caucasian | 22 | 9371/12338 | 0.293 | 1.14 (0.89, 1.45) | |
| PB | 52 | 17758/25317 | 0.219 | 1.08 (0.96, 1.21) | |
| HB | 17 | 7940/10808 | 0.006 | 1.18 (1.05, 1.32) | |
Abbreviations: PB, population-based; Passociation, P-value in the association test.
Figure 2. Subgroup analysis data by the factor of race under the T vs. C model.
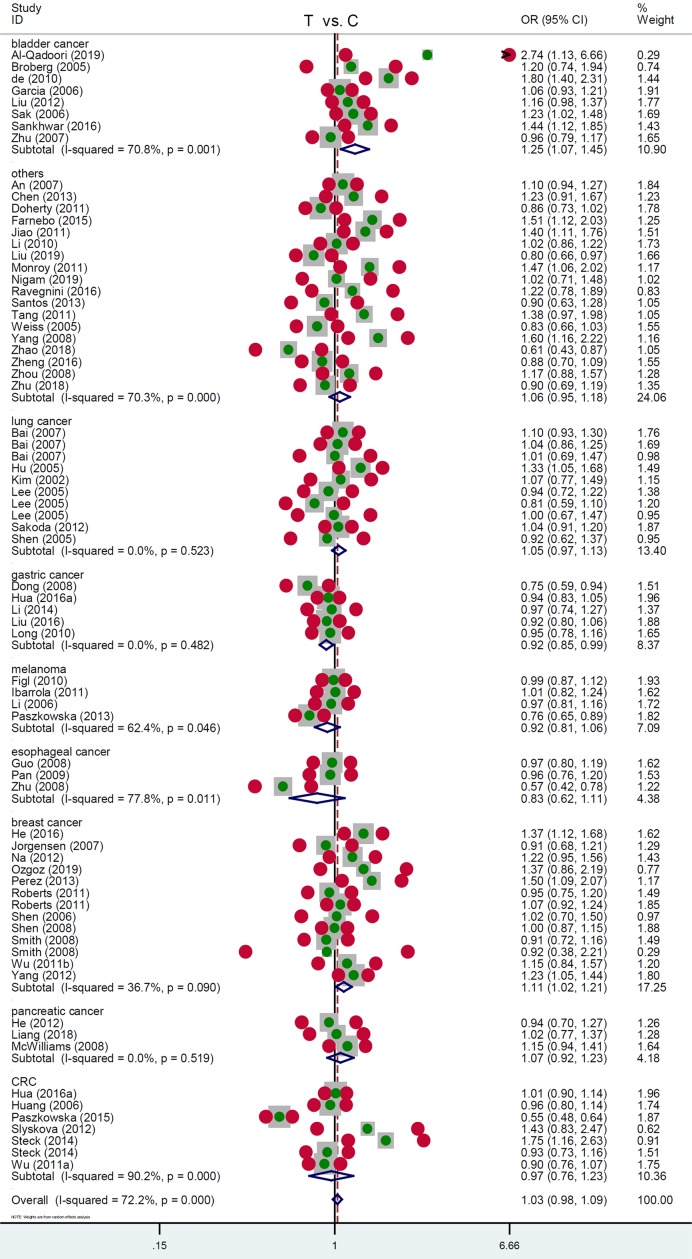
Additionally, we performed a subgroup analysis using the specific cancer type. As shown in Table 4, in the subgroup of ‘bladder cancer’ with 3460 cases and 3613 controls, enhanced susceptibility was detected in bladder cancer cases under T vs. C [Table 4, Passociation=0.004, OR = 1.25, 95% CI = (1.07, 1.45)], TT vs. CC [Passociation=0.001, OR = 1.68, 95% CI = (1.25, 2.26)], CT+TT vs. CC [Passociation=0.016, OR = 1.26, 95% CI = (1.04, 1.53)], TT vs. CC+ CT [Passociation= 0.001, OR = 1.49, 95% CI = (1.18, 1.90)] compared with the negative controls. Additionally, there is an increased risk of breast cancer under T vs. C [Table 4, Passociation=0.018, OR = 1.11, 95% CI = (1.02, 1.21)], TT vs. CC [Passociation=0.003, OR = 1.33, 95% CI = (1.10, 1.60)], and TT vs. CC+ CT [Passociation= 0.001, OR = 1.29, 95% CI = (1.12, 1.48)]. Nevertheless, we observed a decreased risk of gastric cancer in the Chinese population under T vs. C [Table 4, Passociation=0.020, OR = 0.92, 95% CI = (0.85, 0.99)], CT vs. CC [Passociation=0.001, OR = 0.83, 95% CI = (0.73, 0.93)], CT+TT vs. CC [Passociation=0.003, OR = 0.84, 95% CI = (0.76, 0.94)]. The relevant forest plots under different genetic models are presented in Figure 3 (T vs. C), Supplementary Figure S5 (TT vs. CC), Supplementary Figure S6 (CT vs. CC), Supplementary Figure S7 (CT+TT vs. CC), and Supplementary Figure S8 (TT vs. CC+ CT).
Table 4. Subgroup analysis data by the factors of specific cancer type.
| Genetic model | Subgroup | Sample size | Association | ||
|---|---|---|---|---|---|
| Study | Case/Control | Passociation | OR (95% CI) | ||
| T vs. C | Bladder cancer | 8 | 3460/3613 | 0.004 | 1.25 (1.07, 1.45) |
| Lung cancer | 10 | 2642/6319 | 0.222 | 1.05 (0.97, 1.13) | |
| Gastric cancer | 5 | 2849/3655 | 0.020 | 0.92 (0.85, 0.99) | |
| Melanoma | 4 | 2904/3544 | 0.250 | 0.92 (0.81, 1.06) | |
| Esophageal cancer | 3 | 898/1265 | 0.210 | 0.83 (0.62, 1.11) | |
| Breast cancer | 13 | 4762/5937 | 0.018 | 1.11 (1.02, 1.21) | |
| Pancreatic cancer | 3 | 872/1025 | 0.380 | 1.07 (0.92, 1.23) | |
| CRC | 7 | 3602/4924 | 0.776 | 0.97 (0.76, 1.23) | |
| TT vs. CC | Bladder cancer | 8 | 3460/3613 | 0.001 | 1.68 (1.25, 2.26) |
| Lung cancer | 10 | 2642/6319 | 0.252 | 1.11 (0.93, 1.34) | |
| Gastric cancer | 5 | 2849/3655 | 0.361 | 0.93 (0.78, 1.09) | |
| Melanoma | 4 | 2904/3544 | 0.697 | 0.90 (0.55, 1.50) | |
| Esophageal cancer | 3 | 898/1265 | 0.724 | 0.89 (0.46, 1.71) | |
| Breast cancer | 13 | 4762/5937 | 0.003 | 1.33 (1.10, 1.60) | |
| Pancreatic cancer | 3 | 872/1025 | 0.952 | 0.99 (0.69, 1.41) | |
| CRC | 7 | 3602/4924 | 0.588 | 0.87 (0.52, 1.45) | |
| CT vs. CC | Bladder cancer | 8 | 3460/3613 | 0.069 | 1.17 (0.99, 1.39) |
| Lung cancer | 10 | 2642/6319 | 0.368 | 1.05 (0.95, 1.16) | |
| Gastric cancer | 5 | 2849/3655 | 0.001 | 0.83 (0.73, 0.93) | |
| Melanoma | 4 | 2904/3544 | 0.157 | 0.93 (0.83, 1.03) | |
| Esophageal cancer | 3 | 898/1265 | 0.013 | 0.73 (0.57, 0.94) | |
| Breast cancer | 13 | 4762/5937 | 0.418 | 1.04 (0.94, 1.16) | |
| Pancreatic cancer | 3 | 872/1025 | 0.128 | 1.16 (0.96, 1.40) | |
| CRC | 7 | 3602/4924 | 0.405 | 0.91 (0.73, 1.13) | |
| CT+TT vs. CC | Bladder cancer | 8 | 3460/3613 | 0.016 | 1.26 (1.04, 1.53) |
| Lung cancer | 10 | 2642/6319 | 0.282 | 1.05 (0.96, 1.16) | |
| Gastric cancer | 5 | 2849/3655 | 0.003 | 0.84 (0.76, 0.94) | |
| Melanoma | 4 | 2904/3544 | 0.088 | 0.92 (0.83, 1.01) | |
| Esophageal cancer | 3 | 898/1265 | 0.065 | 0.74 (0.54, 1.02) | |
| Breast cancer | 13 | 4762/5937 | 0.175 | 1.08 (0.97, 1.21) | |
| Pancreatic cancer | 3 | 872/1025 | 0.182 | 1.13 (0.94, 1.36) | |
| CRC | 7 | 3602/4924 | 0.563 | 0.93 (0.71, 1.20) | |
| TT vs. CC+ CT | Bladder cancer | 8 | 3460/3613 | 0.001 | 1.49 (1.18, 1.90) |
| Lung cancer | 10 | 2642/6319 | 0.293 | 1.10 (0.92, 1.32) | |
| Gastric cancer | 5 | 2849/3655 | 0.834 | 1.02 (0.87, 1.19) | |
| Melanoma | 4 | 2904/3544 | 0.826 | 0.94 (0.56, 1.59) | |
| Esophageal cancer | 3 | 898/1265 | 0.889 | 1.04 (0.61, 1.78) | |
| Breast cancer | 13 | 4762/5937 | 0.001 | 1.29 (1.12, 1.48) | |
| Pancreatic cancer | 3 | 872/1025 | 0.669 | 0.94 (0.66, 1.31) | |
| CRC | 7 | 3602/4924 | 0.682 | 0.91 (0.58, 1.43) | |
Abbreviations: CRC, colorectal cancer; Passociation, P-value in the association test.
Figure 3. Subgroup analysis data by the factor of cancer type under the T vs. C model.
Moreover, we performed subgroup analysis data for different system cancers. As shown in Supplementary Table S5 and Figure S9 (forest plot data under the allelic model), we observed the same result in the subgroup of ‘urinary system cancer’ as the subgroup of ‘bladder cancer’. There is a reduced cancer risk in the subgroup of ‘reproductive system cancer’ under the models of CT vs. CC [Passociation=0.006, OR = 0.81, 95% CI = (0.70, 0.94)] and CT+TT vs. CC [Passociation=0.041, OR = 0.82, 95% CI = (0.68, 0.99)] and an increased risk in the subgroup of ‘head and neck cancer’ under the TT vs. CC+CT [Passociation=0.024, OR = 1.58, 95% CI = (1.06, 2.34)]. However, no positive association was observed in other subgroups (Supplementary Table S5, Passociation>0.05).
The above results indicated that the TT genotype of XPC rs2228000 seems to be related to a high risk of bladder and breast cancer, whereas the CT genotype is more likely to be associated with reduced susceptibility to gastric cancer in the Chinese population.
Publication bias/sensitivity
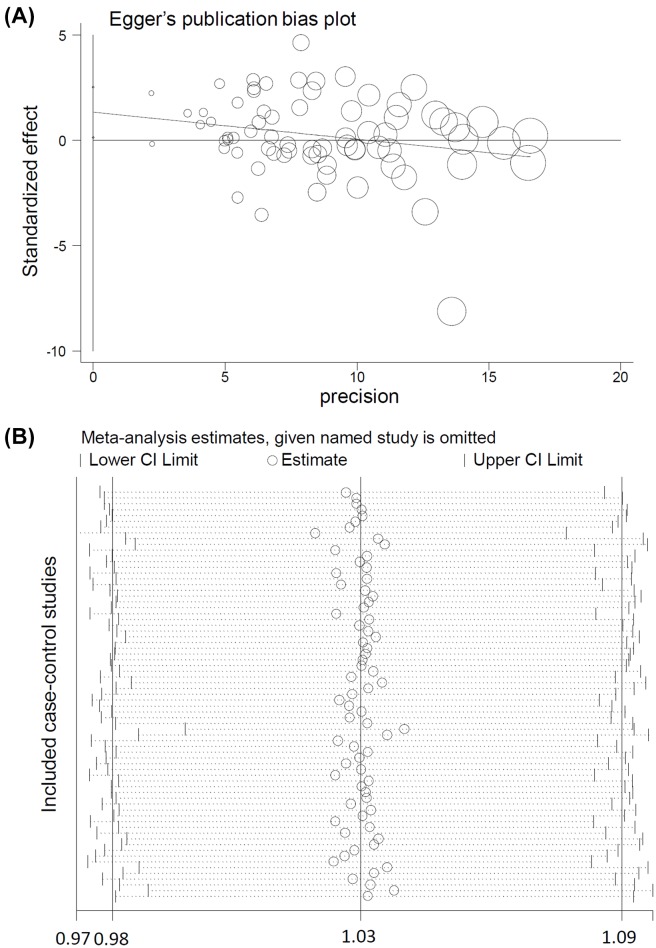
As shown in Table 2, we did not observe a notable publication bias among these comparisons, in that all the PBegg>0.05, PEgger>0.05 apart from the PEgger=0.031 (T vs. C), PEgger=0.046 (CT vs. CC), PEgger=0.023 (CT+TT vs. CC). Figure 4A presents the publication bias plot of Egger’s test under the T vs. C model. In addition, as shown in Figure 4B (allelic model data as example), we also observed relatively stable pooling data through the performance of sensitivity analyses.
Figure 4. Egger’s test plot and the sensitivity analysis data under the T vs. C model.
(A) Egger’s test; (B) sensitivity analysis data.
FPRP/TSA
An FPRP test was conducted to confirm the above positive findings for bladder, breast, and gastric cancers. The FPRP values of positive results at different prior probability levels are shown in Supplementary Table S6. We found that at a prior probability of 0.1 with an OR of 1.5, all the FPRP values were less than 0.2 (Supplementary Table S6, FPRP = 0.028, T vs. C; FPRP = 0.023, TT vs. CC; FPRP = 0.155, CT+TT vs. CC; FPRP = 0.022, TT vs. CC+ CT), indicating a noteworthy association between XPC rs2228000 and the risk of bladder cancer. Similar true positive associations were observed for breast and gastric cancer (Supplementary Table S6, all FPRP < 0.02) at a prior probability of 0.1.
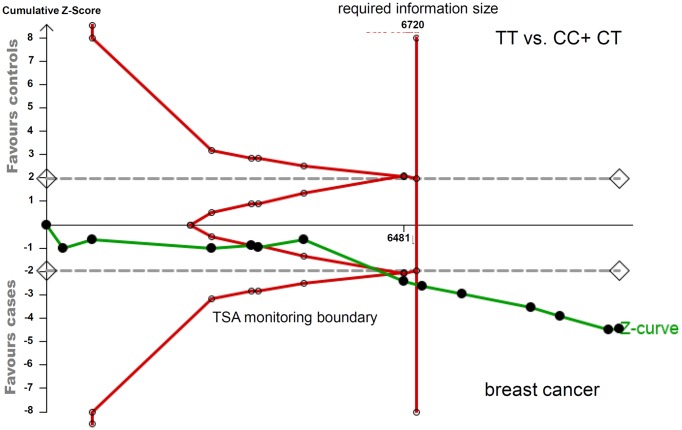
In addition, we also performed the TSA test to assess the robustness of our significant findings. As shown in the TSA data of breast cancer under the TT vs. CC+CT model (Figure 5) and gastric cancer under the CT+TT vs. CC models (Supplementary Figure S10), we found that the cumulative number of participants (Z-curve) met the TSA monitoring boundary and required information size. With regard to the bladder cancer under the TT vs. CC+CT model (Supplementary Figure S11), the cumulative Z-curve crossed with the TSA monitoring boundary, even though it did not reach the required information size. These data therefore indicated the robustness of our conclusions.
Figure 5. TSA for the association between XPC rs2228000 and the risk of breast cancer under the TT vs. CC+CT model.
Discussion
There is a controversial conclusion regarding the genetic impacts of the XPC rs2228000 SNP in the risk of clinical cancer diseases in different publications. For example, XPC rs2228000 was reportedly related to susceptibility to bladder cancer cases in Iraq [27], Sweden [30], or India [67] but not the U.S.A. [85] or Spain [35]. Likewise, XPC rs2228000 was also significantly associated with the risk of breast cancer in a Chinese population [37,78] but not Caucasians or African-Americans in the U.S.A. [72]. Although several meta-analyses of XPC rs2228000 and certain specific cancer types exist [86–92], differences in study enrolment, data extraction, analysis strategy, and result descriptions were observed. We thus conducted a meta-analysis and TSA for a comprehensive assessment regarding the genetic influence of the XPC rs2228000 in the risk of various types of cancer, including bladder cancer, lung cancer, gastric cancer, melanoma, esophageal cancer, breast cancer, pancreatic cancer, and colorectal cancer.
Only three prior meta-analyses with fewer than 15 studies in 2008 [12–14] and one meta-analysis with 33 articles in 2013 [15] were reported to detect the genetic association between XPC rs2228000 and overall cancer risk. In our study, we retrieved four databases (updated till September 2019) to include the potential publication for the pooling analysis. After employing our strict screen strategy, we finally included 64 eligible articles, which contained 71 case–control studies, for the overall meta-analysis and the following subgroup analyses by the factors of race, country, control source, article quality, genotyping assay, and cancer type. Five genetic models, including allelic, homozygotic, heterozygotic, dominant, and recessive models, were utilized. We excluded the improper studies according to the strict requirement of full genotype frequency data and the HWE principle. For instance, there are a total of 33 articles with 14877 cases and 17888 controls [1,28,30–32,36,39,42,44,45,47,48,50,54–56,60,64–66,68–70,72,75–79,82,93–95] for the prior meta-analysis of He et al. in 2013 [15]. In this study, we excluded two articles regarding bladder cancer [95] and cutaneous melanoma [94] because the genotype distribution in the control group is not in line with the HWE, and we added 32 other published articles [4,10,11,27,29,33–35,37,38,40,41,43,46,49,51,52,57–59,61–63,67,71,73,74,80,81,83–85]. Our pooling data from eight case–control studies showed the genetic correlation between XPC rs2228000 and increased risk of bladder cancer under the allelic, homozygotic, heterozygotic, dominant, and recessive models, which is partly consistent with the positive data of He et al. (2013) [15] under the homozygotic and recessive models from four case–control studies. A similar result was obtained for breast cancer, even though four new case–control studies were added, compared with the pooling results of He et al. (2013) [15]. Moreover, we provided assessment evidence regarding the potential impact of XPC rs2228000 on the reduced susceptibility to gastric cancer in the Chinese population. Nevertheless, we did not detect a significant association between XPC rs2228000 and other types of cancer, such as lung cancer, melanoma, pancreatic cancer, or colorectal cancer.
In our study, we performed the FPRP test with a prior probability of 0.1 and an FPRP threshold of 0.2 to check whether the positive findings of breast, bladder, and gastric cancers are noteworthy, considering the potential presence of ‘false positives’. After the FPRP estimation, the genetic association between XPC rs2228000 and the risk of bladder, breast, and gastric cancers risk remain significant at the prior probability level of 0.1. Furthermore, we observed the robustness of our conclusions through the performance of TSA test and sensitivity analyses and the absence of large publication bias by Begg’s/Egger’s test.
Despite these findings, some limitations to this research may still influence the statistical power of analyses of certain types of cancer. Although more than 70 case–control studies were enrolled in the overall meta-analysis, small sample sizes were still included in some subgroup analyses. For example, only two case–control studies [56,74] were included for the subgroup of ‘blood system cancer’, while only two studies [81,83] were enrolled for ‘nervous system cancer’. Therefore, we still cannot rule out the possible genetic role played by XPC rs2228000 in the risk of cancers of the blood or nervous systems. A similar uncertainty also exists in the subgroup analysis of ‘lung cancer’, ‘melanoma’, ‘esophageal cancer’, ‘pancreatic cancer’ and ‘CRC’.
We observed clear between-study heterogeneity, even if articles with low quality are removed. Regarding the available sample size, more factors, such as gender, age, environmental exposure, drinking/smoking status, tumor situations, characteristics, antiepileptic agents, or drug resistance, should be adjusted in future pooling analyses. It would be valuable to carry out an integrated analysis to evaluate the combined role of more XPC polymorphic loci (e.g., rs2228001, PAT−/+) in susceptibility to different types of cancer based on the available evidence.
Conclusions
In general, the TT genotype of XPC rs2228000 may be linked to an increased risk of bladder and breast cancers, whereas the CT genotype is more likely to be associated with a reduced susceptibility to gastric cancer in the Chinese population. Considering the limitations of our study, we need to analyze more publications to verify the genetic impact of XPC rs2228000 in other types of cancer.
Supplementary Material
Acknowledgments
The authors are grateful to American Journal Experts for providing English language editing.
Abbreviations
- CI
confidence interval
- CNKI
China National Knowledge Infrastructure
- FPRP
false-positive report probability
- HB
hospital-based
- HWE
Hardy–Weinberg equilibrium
- NER
nucleotide excision repair
- NOS
Newcastle–Ottawa quality assessment Scale
- OR
odds ratio
- PAT−/+
poly-AT insertion/deletion polymorphism
- TSA
trial sequential analysis
- WOS
Web of Science
- XPC
xeroderma pigmentosum complementation group C
Author Contribution
Y.D., Z.S., and J.Z. conducted the database search and study screening. Y.D., Z.S., and W.G. summarized the evidence, performed the pooling analysis, FPRP, and TSA tests. Y.D. and J.Z. wrote the manuscript. All the authors reviewed and approved the final version.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was in part supported by the National Natural Science Fund Grant [grant numbers 81471761, 81501568]; the Tianjin Science and Technology Support Plan Key Projects [grant number 15ZCZDSY00890]; and the Tianjin Medical University Cancer Hospital Project [grant number 1711].
References
- 1.Bai Y., Xu L., Yang X., Hu Z., Yuan J., Wang F. et al. (2007) Sequence variations in DNA repair gene XPC is associated with lung cancer risk in a Chinese population: a case-control study. BMC Cancer 7, 81 10.1186/1471-2407-7-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An J., Liu Z., Hu Z., Li G., Wang L.E., Sturgis E.M. et al. (2007) Potentially functional single nucleoticle polymorphisms in the core nucleoticle excision repair genes and risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol. Biomarkers Prev. 16, 1633–1638 [DOI] [PubMed] [Google Scholar]
- 3.Chen Z., Yang J., Wang G., Song B., Li J. and Xu Z. (2007) Attenuated expression of xeroderma pigmentosum group C is associated with critical events in human bladder cancer carcinogenesis and progression. Cancer Res. 67, 4578–4585 10.1158/0008-5472.CAN-06-0877 [DOI] [PubMed] [Google Scholar]
- 4.Liang X.H., Yan D., Zhao J.X., Ding W., Xu X.J. and Wang X.Y. (2018) Interaction of polymorphisms in xeroderma pigmentosum group C with cigarette smoking and pancreatic cancer risk. Oncol. Lett. 16, 5631–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsey-Boltz L.A. (2017) Bringing it all together: coupling excision repair to the DNA damage checkpoint. Photochem. Photobiol. 93, 238–244 10.1111/php.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp M.G. and Hu J. (2017) Post excision events in human nucleotide excision repair. Photochem. Photobiol. 93, 178–191 10.1111/php.12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusakabe M., Onishi Y., Tada H., Kurihara F., Kusao K., Furukawa M. et al. (2019) Mechanism and regulation of DNA damage recognition in nucleotide excision repair. Genes Environ. 41, 2 10.1186/s41021-019-0119-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H., Huang Y., Shi J., Zhi Y., Yuan F., Yu J. et al. (2019) XPC deficiency leads to centrosome amplification by inhibiting BRCA1 expression upon cisplatin-mediated DNA damage in human bladder cancer. Cancer Lett. 444, 136–146 10.1016/j.canlet.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 9.Guillem V.M., Cervantes F., Martínez J., Alvarez-Larrán A., Collado M., Camós M. et al. (2010) XPC genetic polymorphisms correlate with the response to imatinib treatment in patients with chronic phase chronic myeloid leukemia. Am. J. Hematol. 85, 482–486 10.1002/ajh.21726 [DOI] [PubMed] [Google Scholar]
- 10.Perez-Mayoral J., Pacheco-Torres A.L., Morales L., Acosta-Rodriguez H., Matta J.L. and Dutil J. (2013) Genetic polymorphisms in RAD23B and XPC modulate DNA repair capacity and breast cancer risk in Puerto Rican women. Mol. Carcinog. 52, E127–E138 10.1002/mc.22056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos L.S., Gomes B.C., Gouveia R., Silva S.N., Azevedo A.P., Camacho V. et al. (2013) The role of CCNH Val270Ala (rs2230641) and other nucleotide excision repair polymorphisms in individual susceptibility to well-differentiated thyroid cancer. Oncol. Rep. 30, 2458–2466 10.3892/or.2013.2702 [DOI] [PubMed] [Google Scholar]
- 12.Francisco G., Menezes P.R., Eluf-Neto J. and Chammas R. (2008) XPC polymorphisms play a role in tissue-specific carcinogenesis: a meta-analysis. Eur. J. Hum. Genet. 16, 724–734 10.1038/ejhg.2008.6 [DOI] [PubMed] [Google Scholar]
- 13.Qiu L., Wang Z., Shi X. and Wang Z. (2008) Associations between XPC polymorphisms and risk of cancers: a meta-analysis. Eur. J. Cancer 44, 2241–2253 10.1016/j.ejca.2008.06.024 [DOI] [PubMed] [Google Scholar]
- 14.Zhang D., Chen C., Fu X., Gu S., Mao Y., Xie Y. et al. (2008) A meta-analysis of DNA repair gene XPC polymorphisms and cancer risk. J. Hum. Genet. 53, 18–33 10.1007/s10038-007-0215-5 [DOI] [PubMed] [Google Scholar]
- 15.He J., Shi T.Y., Zhu M.L., Wang M.Y., Li Q.X. and Wei Q.Y. (2013) Associations of Lys939Gln and Ala499Val polymorphisms of the XPC gene with cancer susceptibility: a meta-analysis. Int. J. Cancer 133, 1765–1775 10.1002/ijc.28089 [DOI] [PubMed] [Google Scholar]
- 16.Jiang C., Xie C., Feng J. and Hao M. (2018) The genetic effect of the ICAM1 (intercellular adhesion molecule 1) rs5498 polymorphism on the susceptibility towards multiple sclerosis. Biosci. Rep. 38, 10.1042/BSR20181642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheng Z., Kang M. and Wang H. (2018) The potential role of MGMT rs12917 polymorphism in cancer risk: an updated pooling analysis with 21010 cases and 34018 controls. Biosci. Rep. 38, 10.1042/BSR20180942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J., Liao X.Y., Zhu J.H., Xue W.Q., Shen G.P., Huang S.Y. et al. (2014) Association of MTHFR C677T and A1298C polymorphisms with non-Hodgkin lymphoma susceptibility: evidence from a meta-analysis. Sci. Rep. 4, 6159 10.1038/srep06159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thakkinstian A., McKay G.J., McEvoy M., Chakravarthy U., Chakrabarti S., Silvestri G. et al. (2011) Systematic review and meta-analysis of the association between complement component 3 and age-related macular degeneration: a HuGE review and meta-analysis. Am. J. Epidemiol. 173, 1365–1379 10.1093/aje/kwr025 [DOI] [PubMed] [Google Scholar]
- 20.Wacholder S., Chanock S., Garcia-Closas M., El Ghormli L. and Rothman N. (2004) Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J. Natl. Cancer Inst. 96, 434–442 10.1093/jnci/djh075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J., Wang M.Y., Qiu L.X., Zhu M.L., Shi T.Y., Zhou X.Y. et al. (2013) Genetic variations of mTORC1 genes and risk of gastric cancer in an Eastern Chinese population. Mol. Carcinog. 52, E70–E79 10.1002/mc.22013 [DOI] [PubMed] [Google Scholar]
- 22.Meng J., Wang S., Zhang M., Fan S., Zhang L. and Liang C. (2018) TP73 G4C14-A4T14 polymorphism and cancer susceptibility: evidence from 36 case-control studies. Biosci. Rep. 38, 10.1042/BSR20181452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu W., Zhuo Z.J., Chen Y.C., Zhu J., Zhao Z., Jia W. et al. (2017) NFKB1 -94insertion/deletion ATTG polymorphism and cancer risk: Evidence from 50 case-control studies. Oncotarget 8, 9806–9822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu M., Tan Z., Luo Z., Hu H., Wu T., Fang S. et al. (2019) Association of the vitamin D metabolism gene GC and CYP27B1 polymorphisms with cancer susceptibility: a meta-analysis and trial sequential analysis. Biosci. Rep. 39, 10.1042/BSR20190368pii: BSR20190368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H., He H., Ojha S.C., Sun C., Fu J., Yan M. et al. (2019) Association of STAT3 and STAT4 polymorphisms with susceptibility to chronic hepatitis B virus infection and risk of hepatocellular carcinoma: a meta-analysis. Biosci. Rep. 39, 10.1042/BSR20190783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakur N., Kumari S. and Mehrotra R. (2018) Association between Cyclin D1 G870A (rs9344) polymorphism and cancer risk in Indian population: meta-analysis and trial sequential analysis. Biosci. Rep. 38, 10.1042/BSR20180694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Qadoori S.A., El-Yassin H.D. and Al-Nasiri U.S. (2019) Genetic association between xeroderma pigmentosum polymorphism rs2228000 with staging and development of bladder cancer. J. Pharm. Sciences Res. 11, 434–439 [Google Scholar]
- 28.Broberg K., Bjork J., Paulsson K., Hoglund M. and Albin M. (2005) Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis 26, 1263–1271 10.1093/carcin/bgi063 [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., Xing X.D., Luo X.P. and Hong X.S. (2013) Relationship between polymorphisms of XPC Lys939Gln and Ala499Val genes and cervical carcinogenesis. Guangdong Med. J. 34, 2032–2035 [Google Scholar]
- 30.de Verdier P.J., Sanyal S., Bermejo J.L., Steineck G., Hemminki K. and Kumar R. (2010) Genotypes, haplotypes and diplotypes of three XPC polymorphisms in urinary-bladder cancer patients. Mutat. Res. 694, 39–44 10.1016/j.mrfmmm.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 31.Doherty J.A., Weiss N.S., Fish S., Fan W., Loomis M.M., Sakoda L.C. et al. (2011) Polymorphisms in nucleotide excision repair genes and endometrial cancer risk. Cancer Epidemiol. Biomarkers Prev. 20, 1873–1882 10.1158/1055-9965.EPI-11-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Z., Guo W., Zhou R., Wan L., Li Y., Wang N. et al. (2008) Polymorphisms of the DNA repair gene XPA and XPC and its correlation with gastric cardiac adenocarcinoma in a high incidence population in North China. J. Clin. Gastroenterol. 42, 910–915 10.1097/MCG.0b013e3180f6262c [DOI] [PubMed] [Google Scholar]
- 33.Farnebo L., Stjernstrom A., Fredrikson M., Ansell A., Garvin S. and Thunell L.K. (2015) DNA repair genes XPC, XPD, XRCC1, and XRCC3 are associated with risk and survival of squamous cell carcinoma of the head and neck. DNA Rep. (Amst.) 31, 64–72 10.1016/j.dnarep.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 34.Figl A., Scherer D., Nagore E., Bermejo J.L., Botella-Estrada R., Gast A. et al. (2010) Single-nucleotide polymorphisms in DNA-repair genes and cutaneous melanoma. Mutat. Res. 702, 8–16 10.1016/j.mrgentox.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Closas M., Malats N., Real F.X., Welch R., Kogevinas M., Chatterjee N. et al. (2006) Genetic variation in the nucleotide excision repair pathway and bladder cancer risk. Cancer Epidemiol. Biomarkers Prev. 15, 536–542 10.1158/1055-9965.EPI-05-0749 [DOI] [PubMed] [Google Scholar]
- 36.Guo W., Zhou R.M., Wan L.L., Wang N., Li Y., Zhang X.J. et al. (2008) Polymorphisms of the DNA repair gene xeroderma pigmentosum groups A and C and risk of esophageal squamous cell carcinoma in a population of high incidence region of North China. J. Cancer Res. Clin. Oncol. 134, 263–270 10.1007/s00432-007-0283-0 [DOI] [PubMed] [Google Scholar]
- 37.He B.S., Xu T., Pan Y.Q., Wang H.J., Cho W.C., Lin K. et al. (2016) Nucleotide excision repair pathway gene polymorphisms are linked to breast cancer risk in a Chinese population. Oncotarget 7, 84872–84882 10.18632/oncotarget.12744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He T.Y. (2012) The association study of genetic polymorphisms in nucleotide excision -repair pathway gene XPC and XPF with genetic susceptibility to pancreatic cancer. XinJiang Med. Univ. 39–40 [Google Scholar]
- 39.Hu Z., Wang Y., Wang X., Liang G., Miao X., Xu Y. et al. (2005) DNA repair gene XPC genotypes/haplotypes and risk of lung cancer in a Chinese population. Int. J. Cancer 115, 478–483 10.1002/ijc.20911 [DOI] [PubMed] [Google Scholar]
- 40.Hua R.X., Zhu J., Jiang D.H., Zhang S.D., Zhang J.B., Xue W.Q. et al. (2016a) Association of XPC gene polymorphisms with colorectal cancer risk in a Southern Chinese population: a case-control study and meta-analysis. Genes (Basel) 7, 1–14 10.3390/genes7100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua R.X., Zhuo Z.J., Shen G.P., Zhu J.H., Zhang S.D., Xue W.Q. et al. (2016b) Polymorphisms in the XPC gene and gastric cancer susceptibility in a Southern Chinese population. Onco Targets Ther. 9, 5513–5519 10.2147/OTT.S113055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang W.Y., Berndt S.I., Kang D., Chatterjee N., Chanock S.J., Yeager M. et al. (2006) Nucleotide excision repair gene polymorphisms and risk of advanced colorectal adenoma: XPC polymorphisms modify smoking-related risk. Cancer Epidemiol. Biomarkers Prev. 15, 306–311 10.1158/1055-9965.EPI-05-0751 [DOI] [PubMed] [Google Scholar]
- 43.Ibarrola-Villava M., Pena-Chilet M., Fernandez L.P., Aviles J.A., Mayor M., Martin-Gonzalez M. et al. (2011) Genetic polymorphisms in DNA repair and oxidative stress pathways associated with malignant melanoma susceptibility. Eur. J. Cancer 47, 2618–2625 10.1016/j.ejca.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 44.Jiao X., Ren J., Chen H., Ma J., Rao S., Huang K. et al. (2011) Ala499Val (C>T) and Lys939Gln (A>C) polymorphisms of the XPC gene: their correlation with the risk of primary gallbladder adenocarcinoma–a case-control study in China. Carcinogenesis 32, 496–501 [DOI] [PubMed] [Google Scholar]
- 45.Jorgensen T.J., Visvanathan K., Ruczinski I., Thuita L., Hoffman S. and Helzlsouer K.J. (2007) Breast cancer risk is not associated with polymorphic forms of xeroderma pigmentosum genes in a cohort of women from Washington County, Maryland. Breast Cancer Res. Treat. 101, 65–71 10.1007/s10549-006-9263-3 [DOI] [PubMed] [Google Scholar]
- 46.Kim K.R., Lee S.Y., Choi J.E., Kim K.M., Jang S.S., Jung C.Y. et al. (2002) Polymorpshisms of XPC gene and risk of primary lung cancer in Koreans. Tuberculosis Respir. Dis. 53, 113–126 10.4046/trd.2002.53.2.113 [DOI] [Google Scholar]
- 47.Lee G.Y., Jang J.S., Lee S.Y., Jeon H.S., Kim K.M., Choi J.E. et al. (2005) XPC polymorphisms and lung cancer risk. Int. J. Cancer 115, 807–813 10.1002/ijc.20900 [DOI] [PubMed] [Google Scholar]
- 48.Li C., Hu Z., Liu Z., Wang L.E., Strom S.S., Gershenwald J.E. et al. (2006) Polymorphisms in the DNA repair genes XPC, XPD, and XPG and risk of cutaneous melanoma: a case-control analysis. Cancer Epidemiol. Biomarkers Prev. 15, 2526–2532 10.1158/1055-9965.EPI-06-0672 [DOI] [PubMed] [Google Scholar]
- 49.Li D., Hou H., Xu C. and Ning T. (2014) Relationship between xpc gene polymorphism and risk of gastric cancer. Shandong Med. J. 54, 24–26 [Google Scholar]
- 50.Li L.M., Zeng X.Y., Ji L., Fan X.J., Li Y.Q., Hu X.H. et al. (2010) Association of XPC and XPG polymorphisms with the risk of hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 18, 271–275 [DOI] [PubMed] [Google Scholar]
- 51.Liu J., Sun L., Xu Q., Tu H., He C., Xing C. et al. (2016) Association of nucleotide excision repair pathway gene polymorphisms with gastric cancer and atrophic gastritis risks. Oncotarget 7, 6972–6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Wang H., Lin T., Wei Q., Zhi Y., Yuan F. et al. (2012) Interactions between cigarette smoking and XPC-PAT genetic polymorphism enhance bladder cancer risk. Oncol. Rep. 28, 337–345 [DOI] [PubMed] [Google Scholar]
- 53.Liu Z.Q., Lu M.Y. and Liu B. (2019) Polymorphisms in XPC gene and risk of uterine leiomyoma in reproductive women. Pathol. Oncol. Res. 10.1007/s12253-019-00720-2 [DOI] [PubMed] [Google Scholar]
- 54.Long X.D., Ma Y., Huang Y.Z., Yi Y., Liang Q.X., Ma A.M. et al. (2010) Genetic polymorphisms in DNA repair genes XPC, XPD, and XRCC4, and susceptibility to Helicobacter pylori infection-related gastric antrum adenocarcinoma in Guangxi population, China. Mol. Carcinog. 49, 611–618 [DOI] [PubMed] [Google Scholar]
- 55.McWilliams R.R., Bamlet W.R., Cunningham J.M., Goode E.L., de Andrade M., Boardman L.A. et al. (2008) Polymorphisms in DNA repair genes, smoking, and pancreatic adenocarcinoma risk. Cancer Res. 68, 4928–4935 10.1158/0008-5472.CAN-07-5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monroy C.M., Cortes A.C., Lopez M., Rourke E., Etzel C.J., Younes A. et al. (2011) Hodgkin lymphoma risk: role of genetic polymorphisms and gene-gene interactions in DNA repair pathways. Mol. Carcinog. 50, 825–834 10.1002/mc.20747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Na R.N. (2012) Retrospective study on the correlation between DNA repair system and its polymorphism with breast cancer. South. Med. Univ. 39–53 [Google Scholar]
- 58.Nigam K., Yadav S.K., Gupta S., Bhatt M.L.B., Samadi F.M. and Sanyal S. (2019) Alteration of the risk of oral pre-cancer and cancer in North Indian population by XPC polymorphism genotypes and haplotypes. Meta Gene 21, 100583 10.1016/j.mgene.2019.100583 [DOI] [Google Scholar]
- 59.Ozgoz A., Hekimler Ozturk K., Yukselturk A., Samli H., Baskan Z., Mutlu Icduygu F. et al. (2019) Genetic variations of DNA repair genes in breast cancer. Pathol. Oncol. Res. 25, 107–114 10.1007/s12253-017-0322-3 [DOI] [PubMed] [Google Scholar]
- 60.Pan J., Lin J., Izzo J.G., Liu Y., Xing J., Huang M. et al. (2009) Genetic susceptibility to esophageal cancer: the role of the nucleotide excision repair pathway. Carcinogenesis 30, 785–792 10.1093/carcin/bgp058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paszkowska-Szczur K., Scott R.J., Gorski B., Cybulski C., Kurzawski G., Dymerska D. et al. (2015) Polymorphisms in nucleotide excision repair genes and susceptibility to colorectal cancer in the Polish population. Mol. Biol. Rep. 42, 755–764 10.1007/s11033-014-3824-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paszkowska-Szczur K., Scott R.J., Serrano-Fernandez P., Mirecka A., Gapska P., Górski B. et al. (2013) Xeroderma pigmentosum genes and melanoma risk. Int. J. Cancer 133, 1094–1100 10.1002/ijc.28123 [DOI] [PubMed] [Google Scholar]
- 63.Ravegnini G., Nannini M., Simeon V., Musti M., Sammarini G., Saponara M. et al. (2016) Polymorphisms in DNA repair genes in gastrointestinal stromal tumours: susceptibility and correlation with tumour characteristics and clinical outcome. Tumour Biol. 37, 13413–13423 10.1007/s13277-016-5276-7 [DOI] [PubMed] [Google Scholar]
- 64.Roberts M.R., Shields P.G., Ambrosone C.B., Nie J., Marian C., Krishnan S.S. et al. (2011) Single-nucleotide polymorphisms in DNA repair genes and association with breast cancer risk in the web study. Carcinogenesis 32, 1223–1230 10.1093/carcin/bgr096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sak S.C., Barrett J.H., Paul A.B., Bishop D.T. and Kiltie A.E. (2006) Comprehensive analysis of 22 XPC polymorphisms and bladder cancer risk. Cancer Epidemiol. Biomarkers Prev. 15, 2537–2541 10.1158/1055-9965.EPI-06-0288 [DOI] [PubMed] [Google Scholar]
- 66.Sakoda L.C., Loomis M.M., Doherty J.A., Julianto L., Barnett M.J., Neuhouser M.L. et al. (2012) Germ line variation in nucleotide excision repair genes and lung cancer risk in smokers. Int. J. Mol. Epidemiol. Genet. 3, 1–17 [PMC free article] [PubMed] [Google Scholar]
- 67.Sankhwar M., Sankhwar S.N., Bansal S.K., Gupta G. and Rajender S. (2016) Polymorphisms in the XPC gene affect urinary bladder cancer risk: a case-control study, meta-analyses and trial sequential analyses. Sci. Rep. 6, 27018 10.1038/srep27018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen J., Desai M., Agrawal M., Kennedy D.O., Senie R.T., Santella R.M. et al. (2006) Polymorphisms in nucleotide excision repair genes and DNA repair capacity phenotype in sisters discordant for breast cancer. Cancer Epidemiol. Biomarkers Prev. 15, 1614–1619 10.1158/1055-9965.EPI-06-0218 [DOI] [PubMed] [Google Scholar]
- 69.Shen J., Gammon M.D., Terry M.B., Teitelbaum S.L., Eng S.M., Neugut A.I. et al. (2008) Xeroderma pigmentosum complementation group C genotypes/diplotypes play no independent or interaction role with polycyclic aromatic hydrocarbons-DNA adducts for breast cancer risk. Eur. J. Cancer 44, 710–717 10.1016/j.ejca.2007.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen M., Berndt S.I., Rothman N., Demarini D.M., Mumford J.L., He X. et al. (2005) Polymorphisms in the DNA nucleotide excision repair genes and lung cancer risk in Xuan Wei, China. Int. J. Cancer 116, 768–773 10.1002/ijc.21117 [DOI] [PubMed] [Google Scholar]
- 71.Slyskova J., Naccarati A., Pardini B., Polakova V., Vodickova L., Smerhovsky Z. et al. (2012) Differences in nucleotide excision repair capacity between newly diagnosed colorectal cancer patients and healthy controls. Mutagenesis 27, 225–232 10.1093/mutage/ger088 [DOI] [PubMed] [Google Scholar]
- 72.Smith T.R., Levine E.A., Freimanis R.I., Akman S.A., Allen G.O., Hoang K.N. et al. (2008) Polygenic model of DNA repair genetic polymorphisms in human breast cancer risk. Carcinogenesis 29, 2132–2138 10.1093/carcin/bgn193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steck S.E., Butler L.M., Keku T., Antwi S., Galanko J., Sandler R.S. et al. (2014) Nucleotide excision repair gene polymorphisms, meat intake and colon cancer risk. Mutat. Res. 762, 24–31 10.1016/j.mrfmmm.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang F.F., Ouyang J., Xu Y., Zhou R.F., Zhou M., Chen B. et al. (2011) The relationship between polymorphism of genes XPA, XPC, XPD, XRCC1 and susceptibility to acute lymphoblastic leukemia. Zhonghua Nei Ke Za Zhi 50, 859–862 [PubMed] [Google Scholar]
- 75.Weiss J.M., Weiss N.S., Ulrich C.M., Doherty J.A., Voigt L.F. and Chen C. (2005) Interindividual variation in nucleotide excision repair genes and risk of endometrial cancer. Cancer Epidemiol. Biomarkers Prev. 14, 2524–2530 10.1158/1055-9965.EPI-05-0414 [DOI] [PubMed] [Google Scholar]
- 76.Wu Y., Jin M., Liu B., Liang X., Yu Y., Li Q. et al. (2011a) The association of XPC polymorphisms and tea drinking with colorectal cancer risk in a Chinese population. Mol. Carcinog. 50, 189–198 10.1002/mc.20704 [DOI] [PubMed] [Google Scholar]
- 77.Wu Y.Y., Ma X.Y., Yao K.Y., Li Q.L. and Chen K. (2011b) Association of XPC gene polymorphisms with breast cancer risk. Zhejiang Da Xue Xue Bao Yi Xue Ban 40, 252–258 [DOI] [PubMed] [Google Scholar]
- 78.Yang X., Liu D., Wu H., Kang H., Pang H., Huang D. et al. (2012) Association of XPC polymorphisms with susceptibility and clinical outcome to chemotherapy in breast cancer patients. Cancer Sci. 103, 1207–1214 10.1111/j.1349-7006.2012.02312.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Z.H., Liang W.B., Jia J., Wei Y.S., Zhou B. and Zhang L. (2008) The xeroderma pigmentosum group C gene polymorphisms and genetic susceptibility of nasopharyngeal carcinoma. Acta Oncol. 47, 379–384 10.1080/02841860701558815 [DOI] [PubMed] [Google Scholar]
- 80.Zhao Z.G., Zhang A.Q., Zhao Y., Xiang J.M., Yu D.Y., Liang Z.W. et al. (2018) The association of polymorphisms in nucleotide excision repair genes with ovarian cancer susceptibility. Biosci. Rep. 38, 1–5 10.1042/BSR20180114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng J., Zhang R., Zhu J., Wang F., Yang T., He J. et al. (2016) Lack of associations between XPC gene polymorphisms and neuroblastoma susceptibility in a Chinese population. Biomed Res. Int. 2016, 10.1155/2016/2932049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou R.M., Wang N. and Guo W. (2008) Correlation of polymorphisms in xeroderma pigmentosum group C to the risk of ovarian carcinoma. Zhonghua Liu Xing Bing Xue Za Zhi 29, 91–92 [PubMed] [Google Scholar]
- 83.Zhu J.H., Fu W., Jia W., Xia H.M., Liu G.C. and Hei J. (2018) Association between NER pathway gene polymorphisms and Wilms tumor risk. Mol. Ther. Nucleic Acids 12, 854–860 10.1016/j.omtn.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu X.L. (2008) Study on relationship between DNA repair genes polymorphism of hOGG1, XPC, XPA and esophageal squamous cell carcinoma susceptibility. Huazhong Univ. Sci. Tech. 22–26 [Google Scholar]
- 85.Zhu Y., Lai M., Yang H., Lin J., Huang M., Grossman H.B. et al. (2007) Genotypes, haplotypes and diplotypes of XPC and risk of bladder cancer. Carcinogenesis 28, 698–703 10.1093/carcin/bgl201 [DOI] [PubMed] [Google Scholar]
- 86.Dai Q.S., Hua R.X., Zeng R.F., Long J.T. and Peng Z.W. (2014) XPC gene polymorphisms contribute to bladder cancer susceptibility: a meta-analysis. Tumour Biol. 35, 447–453 10.1007/s13277-013-1062-y [DOI] [PubMed] [Google Scholar]
- 87.Figl A., Scherer D., Nagore E., Bermejo J.L., Botella-Estrada R., Gast A. et al. (2010) Single-nucleotide polymorphisms in DNA-repair genes and cutaneous melanoma. Mutat. Res. 702, 8–16 10.1016/j.mrgentox.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 88.Jiang X., Zhou L.T., Zhang S.C. and Chen K. (2012) XPC polymorphism increases risk of digestive system cancers: current evidence from a meta-analysis. Chin. J. Cancer Res. 24, 181–189 10.1007/s11670-012-0181-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y., Li Z., Liu N. and Zhang G. (2014) Association between CCND1 and XPC polymorphisms and bladder cancer risk: a meta-analysis based on 15 case-control studies. Tumour Biol. 35, 3155–3165 10.1007/s13277-013-1412-9 [DOI] [PubMed] [Google Scholar]
- 90.Yu G., Wang J., Dong J. and Liu J. (2015) XPC Ala499Val and XPG Asp1104His polymorphisms and digestive system cancer risk: a meta-analysis based on model-free approach. Int. J. Clin. Exp. Med. 8, 6621–6630 [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng W., Cong X.F., Cai W.H., Yang S., Mao C. and Zou H.W. (2011) Current evidences on XPC polymorphisms and breast cancer susceptibility: a meta-analysis. Breast Cancer Res. Treat. 128, 811–815 10.1007/s10549-011-1369-6 [DOI] [PubMed] [Google Scholar]
- 92.Zhu M.L., Hua R.X. and Zheng L. (2014) Associations between polymorphisms of the XPC gene and lung cancer susceptibility: a meta-analysis. Tumour Biol. 35, 2931–2939 10.1007/s13277-013-1377-8 [DOI] [PubMed] [Google Scholar]
- 93.An J., Liu Z., Hu Z., Li G., Wang L.E., Sturgis E.M. et al. (2007) Potentially functional single nucleotide polymorphisms in the core nucleotide excision repair genes and risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol. Biomarkers Prev. 16, 1633–1638 10.1158/1055-9965.EPI-07-0252 [DOI] [PubMed] [Google Scholar]
- 94.Blankenburg S., Konig I.R., Moessner R., Laspe P., Thoms K.M., Krueger U. et al. (2005) No association between three xeroderma pigmentosum group C and one group G gene polymorphisms and risk of cutaneous melanoma. Eur. J. Hum. Genet. 13, 253–255 10.1038/sj.ejhg.5201296 [DOI] [PubMed] [Google Scholar]
- 95.Wu X., Gu J., Grossman H.B., Amos C.I., Etzel C., Huang M. et al. (2006) Bladder cancer predisposition: a multigenic approach to DNA-repair and cell-cycle-control genes. Am. J. Hum. Genet. 78, 464–479 10.1086/500848 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.