Abstract
While the role of hyperhomocysteinemia in cardiovascular pathogenesis continuously draws attention, deficiency of hydrogen sulfide (H2S) has been growingly implicated in cardiovascular diseases. Generation of H2S is closely associated with the metabolism of homocysteine via key enzymes such as cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). The level of homocysteine and H2S is regulated by each other. Metabolic switch in the activity of CBS and CSE may occur with a resultant operating preference change of these enzymes in homocysteine and H2S metabolism. This paper presented an overview regarding (1) linkage between the metabolism of homocysteine and H2S, (2) mutual regulation of homocysteine and H2S, (3) imbalance of homocysteine and H2S in cardiovascular disorders, (4) mechanisms underlying the protective effect of H2S against homocysteine-induced vascular injury, and (5) the current status of homocysteine-lowering and H2S-based therapies for cardiovascular disease. The metabolic imbalance of homocysteine and H2S renders H2S/homocysteine ratio a potentially reliable biomarker for cardiovascular disease and development of drugs or interventions targeting the interplay between homocysteine and H2S to maintain the endogenous balance of these two molecules may hold an even bigger promise for management of vascular disorders than targeting homocysteine or H2S alone.
1. Association between H2S Generation and Homocysteine Metabolism
1.1. Biosynthesis and Catabolism of Homocysteine
Homocysteine is a nonproteinogenic, sulfur-containing amino acid formed during metabolism of the essential amino acid methionine. Plasma level of homocysteine is determined by a balance between its biosynthesis and catabolism, which in healthy subjects is below 15 μmol/L. Synthesis of homocysteine via transmethylation of methionine is catalyzed by enzymes namely S-adenosylmethionine (SAM) synthetase, methyltransferase (MT), and S-adenosylhomocysteine (SAH) hydrolase in three sequential steps: formation of SAM by SAM synthetase-catalyzed reaction of methionine and ATP, conversion of SAM to SAH by methyl transfer reaction catalyzed by MT, and SAH metabolized to adenosine and homocysteine by SAH hydrolase [1, 2].
Homocysteine is catabolized by two means: remethylation to methionine and transsulfuration to cysteine. Remethylation of homocysteine involves folate/vitamin B12-dependent and vitamin B12-independent mechanisms. The former uses N-5-methyl tetrahydrofolate (THF) as a methyl group donor under catalysis of the vitamin B12-dependent enzyme methionine synthase (MS), while the later relies on the methyl group donated by betaine and requires betaine-homocysteine S-MT for catalysis. Transsulfuration of homocysteine is catalyzed by the vitamin B6-dependent enzymes: cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). CBS converts homocysteine and serine into cystathionine, which is taken up by CSE to generate cysteine [1, 2]. CBS and CSE are also the major enzymes responsible for the biogenesis of hydrogen sulfide (H2S), a gasotransmitter known for its regulatory role in many physiological processes [3]. A small portion of homocysteine, approximately 5~10% of the total daily cellular production that is not metabolized within the cell, is exported to the plasma compartment and such baseline value is maintained in healthy human subjects by a constant clearance through the kidney [4].
1.2. H2S Biogenesis and Catabolism
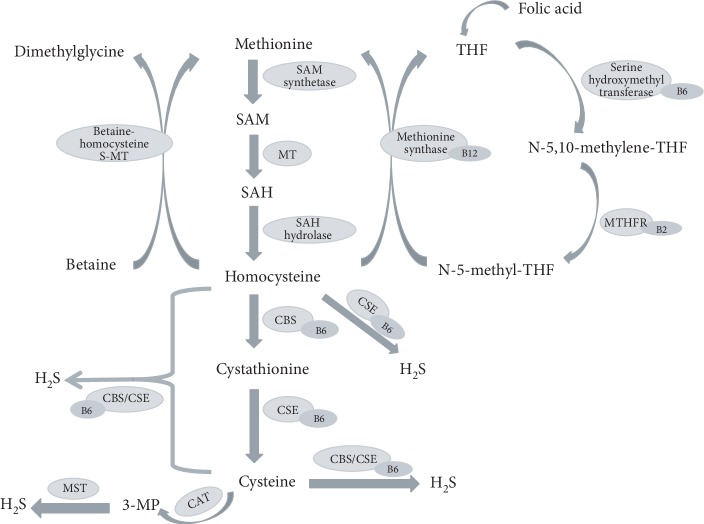
H2S is endogenously generated in mammalian tissues via independent reactions catalyzed by CBS, CSE, and 3-mercaptopyruvate sulfurtransferase (MST) [5, 6]. CBS produces H2S from cysteine via a β-elimination reaction, and CSE generates H2S via α,β-elimination of cysteine (cysteine + H2O ⟶ serine + H2S). Both CBS and CSE can catalyze β-replacement reaction, which condenses two cysteine molecules, or catalyze the condensation reaction of homocysteine with cysteine through β- or γ-replacement, to produce H2S [7] (cysteine + cysteine ⟶ lanthionine + H2S; cysteine + homocysteine ⟶ cystathionine + H2S). In addition, both CBS and CSE can catalyze cysteine α,β-elimination to yield cysteine persulfide that ultimately may generate H2S [7] (cysteine ⟶ cysteine persulfide + pyruvate + NH3 ⟶ H2S). It was reported that human CBS is much more active at producing H2S by a β-replacement reaction than by a β-elimination reaction [8]. CSE alone may produce H2S via homocysteine α, γ-elimination (homocysteine + H2O ⟶ homoserine + H2S ⟶ α-ketobutyrate + NH3) or γ-replacement (homocysteine + homocysteine ⟶ homolanthionine + H2S) [9]. MST is another H2S-generating enzyme that is tightly associated with cysteine metabolism. It acts in conjunction with cysteine aminotransferase (CAT) to produce H2S. MST produces H2S from 3-mercaptopyruvate (3-MP), which is generated by CAT from cysteine in the presence of α-ketoglutarate [10, 11] (cysteine + α-ketoglutarate ⟶ 3-MP + glutamate, 3-MP + MST ⟶ MST persulfide intermediate + pyruvate, MST persulfide intermediate + thiol-containing substrates (RSH) or reduced thioredoxin ⟶ disulfide (RSSR) or oxidized thioredoxin + H2S). A recent study provided evidence suggesting that in addition to L-cysteine, D-cysteine also serves a substrate for 3-MP generation thereof H2S production, particularly in the cerebellum and kidney [12]. Figure 1 schematically describes the association between H2S generation and homocysteine metabolism.
Figure 1.
Schematic overview of the association between homocysteine and H2S. Homocysteine is biosynthesized from methionine by S-adenosylmethionine (SAM) synthetase, methyltransferase (MT), and S-adenosylhomocysteine (SAH) hydrolase in sequential steps. Homocysteine can be either remethylated to methionine through folate/vitamin B12-dependent or vitamin B12-independent mechanisms, or transsulfurated to cysteine under the catalysis of cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) that requires vitamin B6 as an enzyme cofactor. Homocysteine and cysteine are substrates for H2S production, and the generation of H2S is catalyzed by CBS, CSE, and 3-mercaptopyruvate sulfurtransferase (MST). THF: tetrahydrofolate; 3-MP: 3-mercaptopyruvate; CAT: cysteine aminotransferase; MTHFR: N-5,10-methylenetetrahydrofolate reductase.
Although the expression of CBS, CSE, and MST shows tissue-specific dominance, i.e., CBS and MST predominate in the brain and kidney and CSE abundantly exists in the liver and in vascular and nonvascular smooth muscle, these H2S-producing enzymes are generally ubiquitously expressed in mammalian tissues with H2S produced to impact a wide range of cellular processes [13]. All three enzymes are found to be expressed in vascular endothelial and smooth muscle cells [14, 15], which is the biochemical basis underlying the pathophysiological significance of H2S in vasculatures.
2. Influence of Hyperhomocysteinemia on H2S Metabolism
Hyperhomocysteinemia occurs as a result of increased synthesis and/or decreased catabolism (remethylation and transsulfuration) of homocysteine. In recognition of severity and pathogenic mechanisms, hyperhomocysteinemia is categorized into three classes as mild, moderate, and severe hyperhomocysteinemia with plasma homocysteine level ranging from 15 to 30 μmol/L, 31 to 100 μmol/L, and >100 μmol/L, respectively [16]. Nutritional deficiency of folic acid and vitamin B6 and/or B12 and renal insufficiency often cause relatively mild hyperhomocysteinemia, while genetic disorders such as mutations in N-5,10-methylenetetrahydrofolate reductase (MTHFR) and CBS may result in moderate and severe hyperhomocysteinemia [17, 18].
H2S biosynthesis is affected by hyperhomocysteinemia. Under normal conditions, plasma total cysteine in humans (∼250 μmol/L) is much higher than the concentration of total homocysteine (∼6-15 μmol/L) [19]. Chiku and colleagues reported that at physiological concentrations of homocysteine and cysteine, approximately 70% of H2S is produced from cysteine through CBS-/CSE-catalyzed α,β-elimination of cysteine. However, in the state of homocysteine accumulation, homocysteine substitutes cysteine becoming a significant source of H2S in moderate and the principal source of H2S in severe hyperhomocysteinemia. As a result, H2S generated by CSE through the α,γ-elimination and γ-replacement reactions of homocysteine is dramatically enhanced [9]. In contrast to CSE, CBS-catalyzed H2S-generating reactions are insensitive to the grade of hyperhomocysteinemia [20]. Such difference suggests that CSE may be primarily responsible for H2S production change under conditions of hyperhomocysteinemia.
It was reported that increase of homocysteine causes a decrease of H2S production. For example, plasma H2S level was found to be lowered in hyperhomocysteinemic mice [21], and intracerebroventricular injection of homocysteine in rats resulted in decreased generation of endogenous H2S in the hippocampus [22]. Reduction of H2S was also observed in cells exposed to homocysteine [21, 23]. The decrease of H2S was attributed to suppressed expression/activity of the H2S-generating enzymes CBS [22, 23] and CSE [21]. Further studies demonstrated that homocysteine-induced transcriptional repression of CSE in macrophages is a result of increased DNA methyltransferase expression and DNA hypermethylation in CSE promoter region [21]. Homocysteine was also found to capture H2S anion to form homocysteine persulfide [24], which weakened the cardioprotective effect of H2S in hyperhomocysteinemic animals subjected to ischemia-reperfusion injury.
Despite accumulating evidence in support of the inhibitory effect of homocysteine on H2S generation, there were anomalous reports of elevated H2S levels in hyperhomocysteinemia. Compared with healthy volunteers, hyperhomocysteinemic patients with MTHFR C677T polymorphism showed a significantly higher content of H2S in either platelets or plasma [25]. Similarly, in hyperhomocysteinemic mice bearing MTHFR or CBS mutations, H2S level in retinas was markedly increased, as compared to wild-type animals [26]. The mechanisms underlying the elevated H2S level in these studies remain elusive, though the investigators ruled out the possibility of upregulation of CSE and MST [26].
3. Regulation of Homocysteine Metabolism by H2S
On the one hand, homocysteine regulates H2S production; on the other hand, the level of homocysteine is regulated by H2S. It was observed that intraperitoneal injection of H2S gas saturation solution significantly reduces plasma total homocysteine concentration in hyperhomocysteinemic rat induced by subcutaneous injection of homocysteine [27]. Treating myoblasts with the H2S donor sodium hydrosulfide (NaHS) significantly lowered homocysteine content in the cell. Conversely, deficient endogenous H2S production induced by CSE siRNA was concomitant with the increased homocysteine level [28]. Oral administration of a new H2S-releasing compound ACS94 to healthy rats increases the concentration of circulating H2S and decreases homocysteine level in plasma and organs [29]. In an in vitro study of mouse brain endothelial cells, Tyagi and colleagues found that NaHS decreases homocysteine accumulation in cells exposed to high concentration of methionine, concluding that H2S is a potent inhibitor of homocysteine formation [30].
In a recent study of the antihypertrophic effect of H2S against homocysteine on cardiomyocytes, Nandi and associates observed differential effects of H2S and homocysteine on the expression of CBS and CSE along with a finding of a negative feedback regulation between these two enzymes [31]. Elevated levels of homocysteine downregulated CBS but upregulated CSE whereas H2S downregulated CSE but upregulated CBS in cardiomyocytes, indicating the negative feedback between CBS and CSE, which can be influenced by hyperhomocysteinemia or H2S. The direct regulation of CSE by CBS was further confirmed in CBS-deficient hyperhomocysteinemic animals. As compared to CBS+/+ siblings, CBS+/− mice exhibit upregulated CSE in the heart, suggesting that CBS deficiency induces CSE. Further mechanistic exploration revealed that homocysteine-induced CBS deficiency enhances the activity of specificity protein-1 (SP1), an inducer for CSE, and downregulates miR-133, a SP-1 targeting microRNA. On the contrary, H2S suppresses CSE by inhibiting SP1 directly and also indirectly by inducing miR-133a, which consequently leads to CBS upregulation.
4. Imbalance of Homocysteine and H2S in Cardiovascular Disease
Considering both evidence of defective and enhanced H2S production under hyperhomocysteinemic conditions and the interplay between homocysteine and H2S, the change of H2S/homocysteine ratio may be more valuable than the absolute concentration change of H2S and homocysteine in depicting the role of these metabolites in disease pathogenesis. Hypertensive children in comparison to normotensive children showed significantly lower plasma H2S/homocysteine ratio due to increased homocysteine concentration and decreased H2S level, and a negative correlation existed between systolic blood pressure and the plasma H2S/homocysteine ratio [32]. Similarly, decreased levels of H2S and increased levels of homocysteine were shown to be significantly negatively correlated in pulmonary hypertension associated with congenital heart disease, which was attributed to decreased expressions of MTHFR and CSE along with vitamin B12 deficiency [33]. He and colleagues found that although patients with chronic obstructive pulmonary disease (COPD) and concomitant cardiovascular disease (CVD) have higher H2S and homocysteine levels than those without CVD but only COPD, the H2S/homocysteine ratio in serum from COPD + CVD patients was significantly lower than that from the COPD group, and such ratio was positively correlated with lung function [34]. These studies supported the notion of metabolic imbalance of homocysteine and H2S in cardiovascular pathologies and as comparing to homocysteine or H2S alone, the ratio of H2S to homocysteine may be a more reliable biomarker to predict risk of cardiovascular disease.
5. Role of Hyperhomocysteinemia and H2S Deficiency in Vascular Pathologies
Hyperhomocysteinemia is recognized as an independent risk factor for cardiovascular, cerebrovascular, and peripheral artery disease and its association with atherosclerosis, hypertension, coronary artery disease, stroke, etc. has been well-documented [35]. For example, in patients with coronary artery disease, the plasma homocysteine level was revealed as a strong predictor of cardiovascular mortality [36], and the severity of atherosclerosis was demonstrated to be correlated with the plasma level of homocysteine [37]. Also, evidence in support of the role of H2S deficiency in vascular disorders such as hypertension and atherosclerosis keeps growing [38, 39]. A couple of newly published review articles addressed vascular biology of H2S [40] and the mechanisms of vascular injury induced by hyperhomocysteinemia [41]. The role of oxidative stress, endoplasmic reticulum (ER) stress, and regulation of DNA methylation in homocysteine-induced endothelial dysfunction and vascular inflammation has been discussed in depth [41]. In light of the linkage between homocysteine and H2S, the focus of the ensuing sections is to discuss the underlying basis of vasoprotection conferred by H2S against hyperhomocysteinemia.
6. H2S Antagonizes Homocysteine-Induced Vascular Injury: Role of NO Signaling
Conversion of homocysteine to H2S has been found to improve renovascular function in hyperhomocysteinemia. In the presence of a high level of homocysteine, compared with nontransfected renal arteries, arteries transfected with CBS, CSE, and MST triple genes generated more H2S and were more responsive to endothelium-dependent vasodilator, accompanied by an increased expression of eNOS protein [42]. Reduced caveolin-1 expression also contributes to increased eNOS activity, as demonstrated in CBS+/− hyperhomocysteinemic mice receiving H2S treatment who showed attenuation in renovascular smooth muscle cell proliferation and decrease in blood pressure [43]. Previous in vitro and in vivo studies of hyperhomocysteinemia have attributed the inhibition of eNOS and the consequent reduction of nitric oxide (NO) bioavailability to homocysteine-induced eNOS gene inhibition, eNOS inactivation (decreased phosphorylation at activating site and increased phosphorylation at inhibitory site), eNOS uncoupling, and arginase activation [44–47]. Szabo in his recent review article summarized the mechanisms by which H2S enhances eNOS-NO signaling, including increasing eNOS mRNA synthesis, stimulating eNOS activity via Ca2+ mobilization and Akt-mediated phosphorylation, direct sulfhydration of eNOS, and maintaining soluble guanylate cyclase (sGC) in an NO-activatable state, reacting with cGMP to yield PDE5-resistant 8-SH-cGMP and inhibiting PDE5 activity [48]. Out of a pile of evidence suggesting H2S-induced stimulation of eNOS-NO, there is rebuttal evidence. In a latest study using CSE–/– mice, Szijártó and colleagues demonstrated that lack of CSE-produced H2S is associated with higher NO bioavailability in peripheral arteries. They attributed this to a decrease in NO scavenging, which occurs through direct interaction of H2S and NO resulting in nitroxyl (HNO) formation [49].
Not only H2S regulates NO production/activity, NO also influences H2S-induced response. eNOS−/− mice exhibited significantly enhanced relaxation to L-cysteine in carotid arteries whereas overexpression of eNOS suppressed L-cysteine-induced relaxation, which suggested that endogenously produced H2S can compensate for impaired vasodilatory responses when NO is deficient while eNOS/NO abundance limits endogenous H2S-induced vascular responses [50]. The cross-talk between H2S and NO in different grades of hyperhomocysteinemia is worthy of study, which will help us gain a comprehensive understanding of the role of these two important gasotransmitters in vascular pathology associated with hyperhomocysteinemia.
7. H2S Antagonizes Homocysteine-Induced Vascular Injury: Role of Oxidative Stress
Oxidative stress has been strongly implicated in vascular injury and remodeling in hyperhomocysteinemia. Homocysteine induces oxidative stress through multiple mechanisms, including (1) homocysteine autooxidation. When homocysteine binds via a disulfide bridge with plasma proteins (mainly albumin), or other low-molecular plasma thiols, or a second homocysteine molecule, autooxidation of the free thiol group of homocysteine occurs, leading to generation of hydrogen peroxide (H2O2) and reactive radical oxygen species, superoxide and hydroxyl radical; (2) imbalance between oxidant and antioxidant enzymes, e.g., activation of NADPH oxidases and inhibition of superoxide dismutase (SOD); (3) eNOS-dependent generation of superoxide anion via eNOS uncoupling, which may be triggered by homocysteine-induced decrease of tetrahydrobiopterin (BH4) [45, 51, 52].
H2S inhibits homocysteine-induced oxidative stress. In vitro cell culture experiments demonstrated that H2S precursor NaHS effectively lowered reactive oxygen/nitrogen species (ROS/RNS) production and normalized redox enzyme levels in vascular smooth muscle and endothelial cells subjected to homocysteine exposure [30, 53]. Further investigation revealed the contributing role of mitochondria in homocysteine-induced ROS production and imbalance of NOX-4 and SOD-2, and the antioxidative and antimitotoxic properties of H2S in mediating endothelial protection [54]. In mice which accepted intracerebral injection of homocysteine, treatment with NaHS significantly ameliorated cerebrovascular dysfunction and neurodegeneration. The protection was associated with suppressed oxidative stress, indicated by decreased malondialdehyde and increased glutathione [55]. Similar results were also obtained in hyperhomocysteinemic mice orally taking homocysteine. Restoration of plasma H2S level by H2S supplementation ameliorated homocysteine-induced neurovascular remodeling with concomitant decreases in superoxide and nitrite and increases in SOD, catalase, glutathione, etc. [56] The ability of H2S in enhancing the activity of γ-glutamylcysteine synthetase, the committing step in the synthesis of glutathione; and upregulating transport of cysteine, the rate-limiting substrate of glutathione synthesis [57] may provide an explanation for the increased level of this antioxidant in hyperhomocysteinemic mice treated with NaHS. A recent study suggested that H2S may also function as a heme-redox-intermediate-scavenging antioxidant [58]. H2S mitigates hemoglobin oxidation which thereby inhibits oxidized hemoglobin-induced lipid peroxidation and the consequent atherosclerotic lesion in the vessel wall in both human and mouse. Such reduction of hemoglobin oxidation species may take part in the cytoprotective effects conferred by H2S against homocysteine since homocysteine has been shown to enhance hemoglobin oxidation [59].
It has been well-known that oxidative stress can trigger inflammatory responses and these two constitute a mutual reinforcing system in the development of atherosclerosis. By focusing on matrix metalloproteinases (MMPs) in atherosclerosis, Vacek and coworkers reviewed the evidence that H2S may deactivate homocysteine-induced MMP activities, resulting in a decrease of smooth muscle proliferation and suppression of vascular inflammation and remodeling [60]. The finding of alleviation of inflammatory responses via antioxidant-dependent inhibition of MMPs enriched our understanding of the vascular protection conferred by H2S in hyperhomocysteinemia.
8. Role of Subcellular Machineries in Homocysteine-Induced Oxidative Stress: Effect of H2S
8.1. Mitochondrion
Being a major source of endogenous ROS, mitochondria significantly contributes to excessive ROS generation induced by homocysteine and have been shown to be an essential target for H2S. In studies of mouse brain endothelial cells (bEnd3), Kamat and colleagues demonstrated that homocysteine induces upregulation of N-methyl D-aspartate (NMDA-R1), a receptor for homocysteine by increasing DNA methylation, leading to NOX-4 overexpression and mitochondrial superoxide production. NaHS treatment downregulated NMDA-R1 expression, maintained mitochondrial integrity, and attenuated mitochondrial redox stress caused by homocysteine. Amelioration of mitochondrial toxicity by NaHS through antagonizing NMDA-R1 protects the integrity and function of endothelial cells, shown by preserved eNOS and endothelin-1 expression. Moreover, experiments through modulating CSE further demonstrated the role of endogenous H2S in inhibiting mitochondrial superoxide generation and mitochondrial toxicity [54]. In vitro studies of isolated mitochondria from mouse aortic endothelial cells showed that homocysteine increased ROS production, particularly H2O2, in the mitochondria, which was associated with increased mitophagy. Gene delivery of CBS, CSE, or MST to the cells inhibited ROS production and mitigated mitophagy [42].
8.2. Endoplasmic Reticulum
While prone to oxidative damages, endoplasmic reticulum (ER) is also a ROS generator. As characterized by an elevated ratio of oxidized to reduced glutathione (GSSG/GSH), the lumen of ER is an oxidizing environment enriched with protein disulfide isomerase (PDI) and ER oxidoreductase (ERO)-1α, which allows the proper native disulfide bond formation and the resultant proper protein folding (polypeptide rearrangement to reach the native conformational state of the protein). During disulfide bond-dependent protein folding, electrons are transferred from the target cysteine residues to molecular oxygen, generating H2O2; however, in stressed ER, nonnative disulfide bond formation is increased, resulting in GSH consumption as a protective mechanism, which leads to GSH depletion contributing to excessive ROS generation and consequent development of oxidative stress [61]. The role of ER stress and the cross-talk between ER stress and oxidative stress in mediating endothelial dysfunction have been suggested in several pathological conditions including homocysteine exposure [62–65]. There are several lines of evidence suggesting that H2S could prevent homocysteine-induced ER stress [66–69], though these results were not derived from studies of vessels but from the skeletal muscle, neural system, and cardiomyocytes, it is very likely that the anti-ER stress capacity of H2S is also involved in its protective effect on vasculatures in hyperhomocysteinemic conditions, which however warrants further investigation.
In a recent study, Kabil and colleagues demonstrated that ER stress induces CSE and causes inhibition of CBS by binding with CO, a product of heme oxygenase-1 in response to ER stress, leading to a build-up of homocysteine and a decrease in cystathionine, which combined to flip the operating preference of CSE from cystathionine to cysteine thus favors the production of H2S [70]. As homocysteine is known to act as an ER stress inducer, the metabolic switch in the activity of transsulfuration pathway enzymes in response to ER stress and the consequent increase of H2S synthesis may serve as an endogenous cardioprotective mechanism in hyperhomocysteinemia.
We recently demonstrated that ER stress mediates homocysteine-induced vascular dysfunction via suppressing calcium-activated potassium (KCa) channels [71–73]. ER stress inhibited the cell surface expression of intermediate and small conductance KCa (IKCa and SKCa) channels in endothelium [71] and enhanced the ubiquitin ligase-mediated loss of the β1 subunit of large conductance KCa (BKCa) channels in smooth muscle cells of coronary arteries [72, 73]. Previous studies showed that H2S may augment the KCa channel current in vascular cells and activation of KCa channels is involved in H2S-induced vasodilatation [74, 75]. It would therefore be intriguing to unravel whether preserving KCa channel activity from ER stress takes part in H2S-conferred vascular protection against homocysteine, which may enrich our molecular-level understanding of the impact of H2S-homocysteine imbalance and the beneficial role of H2S in hyperhomocysteinemia.
9. H2S Antagonizes Homocysteine-Induced Vascular Injury: Other Potential Mechanisms?
As studies on the vascular effect of homocysteine keep advancing, more mechanistic linkage between H2S and homocysteine in the regulation of vascular function may be uncovered. For example, H2S is known to inhibit the angiotensin II type I receptor (Ang II/AT1R) pathway to regulate vascular function [76]. Recently, direct interaction and activation of AT1R by homocysteine were demonstrated to aggregate vascular injury [77]. Whether AT1R is a molecular basis underpinning vascular protection conferred by H2S against homocysteine therefore is a topic worthy of investigation. In addition, considering latest evidence regarding homocysteine-induced enhancement of T-type Ca2+ currents [78] and the inhibitory effect of H2S on CaV1.2 channels in vascular smooth muscle cells [79], modulation of voltage-dependent Ca2+ channels might also be a mechanism involved in H2S-mediated vascular protection in hyperhomocysteinemia although this warrants further investigation.
10. Homocysteine-Lowering Strategies and H2S Therapeutics in Treatment of Cardiovascular Disease
Despite the association between hyperhomocysteinemia and cardiovascular disease, randomized, placebo-controlled clinic trials evaluating the efficacy of homocysteine-lowering treatment, i.e., folic acid or/and B-vitamin supplementation, yielded inconsistent results; some favoring the effectiveness, e.g, slowing the progression of subclinical atherosclerosis and stroke, and improving endothelial function in coronary artery disease [80–83], while others showing no beneficial or only minor effect on the risk of major cardiovascular events in patients with vascular disease [84–86]. The causes leading to such contradictories remain not well understood, which might be related to the basal level of plasma folate, whether or not the patients on antiplatelet therapy, and the MTHFR C677T genotypes. Administration of folic acid and B-vitamin showed less effectiveness in lowering plasma homocysteine in subjects with normally high folate consumption before the treatment [87]. Suggestions have been made for clinical trials of homocysteine-lowering interventions via dietary supplementation with folic acid and B-vitamin to be conducted in regions where foods are not commonly fortified with folate [88]. In a randomized double-blind, placebo-controlled trial, Hankey and colleagues uncovered an interaction between antiplatelet therapy and the effect of folic acid/B-vitamin-based homocysteine-lowering therapy on major vascular events in patients with stroke or transient ischemic attack. They found that B-vitamins had no significant effect on the primary outcome in participants taking antiplatelet drugs at baseline whereas participants not taking antiplatelet drugs significantly benefited from the B-vitamin supplementation [89]. Recently, the China Stroke Primary Prevention Trial assessed individual variation in response to homocysteine-lowering interventions and suggested the effect modification by MTHFR polymorphisms. Compared with MTHFR677CC and CT genotypes, participants with the MTHFR677TT genotype exhibited a more prominent L-shaped curve between homocysteine and serum folate levels and required higher folate levels to eliminate the differences in homocysteine level by genotypes [90]. The influence of MTHFR C677T genotypes on the efficacy of folic acid and vitamin B12 in lowering homocysteine concentrations was also observed in hemodialysis patients [91]. A series of experiment performed in a mouse model containing a transgene (Tg-I278T), the most common mutation found in CBS-deficient patients, showed an entirely different response with regard to homocysteine-lowering diet as compared to the normal controls [92], which gives more support to the concept of gene-diet interaction in disease treatment. In addition, whether the newly found mutations of CBS in hyperhomocysteinemic patients, such as c.467T>C; p.Leu156Pro and c.808_810del; p.Glu270del, have impact on the therapeutic efficiency may need to be studied [93]. Taken together, well-constructed trials with consideration of the abovementioned factors are needed to provide conclusive answers to the clinical effectiveness of homocysteine-lowering strategies in reducing the incidence of cardiovascular complications.
As evidence concerning the safety and effectiveness of H2S-releasing therapeutics in animal models of cardiovascular disease keeps growing, attempts have been made to develop H2S-based drugs for human use, and the promise is now being demonstrated in clinical trials. In both spontaneously or two-kidney one-clip hypertensive rats, NaHS treatment significantly lowered the mean arterial pressure and improved vasodilatation [94, 95]. Activation of eNOS through PPARδ/PI3K/Akt or PPARδ/AMPK signaling [94] and restoration of NO bioavailability by decreasing the plasma level of the NOS inhibitor NG monomethyl-l-arginine were revealed as underlying mechanisms [95]. Further studies of renal arteries from hypertensive patients and human umbilical vein endothelial cells subjected to angiotensin II exposure confirmed the protective effect of NaHS on endothelium and eNOS-NO functionality, supporting the potential of H2S-releasing drugs in the treatment of hypertension [94]. Nevertheless, clinical use of NaHS seems to be impractical due to its short half-life and toxicity, and novel H2S donors with enhanced efficacy and reduced toxicity are needed to realize H2S-based therapies. In a recent review article, Wallace and colleagues introduced the few H2S-releasing drugs that have progressed into clinical trials, such as H2S prodrug SG1002, an inorganic mixture (sodium polysulthionate) containing S8, Na2SO4, Na2S2O3, Na2S3O6, Na2S4O6, and Na2S5O6 for heart failure, and ATB-346, a nonsteroidal anti-inflammatory drug derived from naproxen but coupled to an H2S-releasing moiety for arthritis [96]. Alleviation of cardiac remodeling and afterload by SG1002 was recently proven in the CBS+/- hyperhomocysteinemic mouse model [97]. By discussing the signaling pathways influenced by H2S-dependent sulfhydration that attenuates DNA damage, oxidative stress, and eNOS inhibition, Li et al. gave an overview of the development of H2S donors and the evolution of H2S therapeutics in cardiovascular disease with presenting the hypothesis that H2S may serve as a dual protector for both the heart and the kidney in cardiorenal syndrome [98]. The development of H2S-based therapy shall benefit from new techniques/materials that are able to control the amount of H2S released from the donor drug. The effectiveness of a synthesized peptide-based H2S-releasing hydrogel in reducing intimal hyperplasia of the vein grafts from patients undergoing bypass surgery is an example [99].
11. Summary and Future Perspectives
Homocysteine and H2S are both metabolites of sulfur-containing amino acids. Transsulfuration of homocysteine to cysteine is catalyzed by CBS and CSE, which are also key enzymes producing H2S from homocysteine and/or cysteine. Biogenesis of homocysteine and H2S is regulated by each other. H2S protects against homocysteine-induced vascular injury through multiple mechanisms including normalizing NO functionality by regulating eNOS signaling and alleviating oxidative stress and inflammation via restoration of redox balance, in which maintaining the structural and functional integrity of mitochondrion and ER plays a significant role. Recent findings of compromised ion channel activity in particular KCa channel activity in response to ER stress caused by homocysteine added new mechanistic insight into homocysteine-induced vascular injury and raised a topic worthy of investigation whether preserving ion channel activity by protecting ER takes part in H2S-conferred protection against homocysteine on vasculatures. Metabolic imbalance of homocysteine and H2S has been implicated in several types of cardiovascular disorders, which renders homocysteine-lowering and H2S-enhancing strategies promising therapeutics for cardiovascular disease. Furthermore, H2S/homocysteine ratio in comparison with homocysteine or H2S level alone may be a more reliable biomarker for cardiovascular-disease risk prediction. Clinical trials assessing the effectiveness of homocysteine-lowering interventions, i.e., folic acid or/and B-vitamin supplementation in reducing the incidence of cardiovascular complications should take into account the influence of dietary folate intake and antiplatelet treatment. Further consideration of MTHFR C677T and CBS genotypes is required for precision of homocysteine-lowering interventions in hyperhomocysteinemic individuals. Development of novel H2S-generating compounds with controlled-release properties and oral bioavailability is essential for clinical application of H2S therapies that are so far still at a very early stage. More importantly, development of drugs or interventions targeting the interplay between homocysteine and H2S to maintain the endogenous balance of these two molecules may hold even bigger promise for management of cardiovascular disorders.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (81870227) and Tianjin Municipal Science and Technology Commission (18PTZWHZ00060).
Abbreviations
- MST:
3-Mercaptopyruvate sulfurtransferase
- 3-MP:
3-Mercaptopyruvate
- KCa channels:
Calcium-activated potassium channels
- CVD:
Cardiovascular disease
- COPD:
Chronic obstructive pulmonary disease
- CBS:
Cystathionine β-synthase
- CSE:
Cystathionine γ-lyase
- CAT:
Cysteine aminotransferase
- ER:
Endoplasmic reticulum
- (ERO)-1α:
ER oxidoreductase-1α
- H2O2:
Hydrogen peroxide
- H2S:
Hydrogen sulfide
- MMPs:
Matrix metalloproteinases
- MS:
Methionine synthase
- MT:
Methyltransferase
- MTHFR:
N-5,10-methylenetetrahydrofolate reductase
- NO:
Nitric oxide
- NMDA-R1:
N-methyl D-aspartate receptor
- PDI:
Protein disulfide isomerase
- SAH hydrolase:
S-Adenosylhomocysteine hydrolase
- SAM synthetase:
S-Adenosylmethionine synthetase
- NaHS:
Sodium hydrosulfide
- SP1:
Specificity protein-1
- SOD:
Superoxide dismutase
- BH4:
Tetrahydrobiopterin
- THF:
Tetrahydrofolate.
Conflicts of Interest
Both authors declare that they have no conflicts of interest.
References
- 1.Schalinske K. L., Smazal A. L. Homocysteine imbalance: a pathological metabolic marker. Advances in Nutrition. 2012;3(6):755–762. doi: 10.3945/an.112.002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skovierova H., Vidomanova E., Mahmood S., et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. International Journal of Molecular Sciences. 2016;17(10):p. 1733. doi: 10.3390/ijms17101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.di Masi A., Ascenzi P. H2s: A “double face” molecule in health and disease. Biofactors. 2013;39(2):186–196. doi: 10.1002/biof.1061. [DOI] [PubMed] [Google Scholar]
- 4.Friedman A. N., Bostom A. G., Selhub J., Levey A. S., Rosenberg I. H. The kidney and homocysteine metabolism. Journal of the American Society of Nephrology. 2001;12(10):2181–2189. doi: 10.1681/ASN.V12102181. [DOI] [PubMed] [Google Scholar]
- 5.Paul B. D., Snyder S. H. H2S signalling through protein sulfhydration and beyond. Nature Reviews Molecular Cell Biology. 2012;13(8):499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41(1):113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 7.Giuffre A., Vicente J. B. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxidative Medicine and Cellular Longevity. 2018;2018:31. doi: 10.1155/2018/6290931.6290931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Jhee K. H., Kruger W. D. Production of the neuromodulator h2s by cystathionine beta-synthase via the condensation of cysteine and homocysteine. Journal of Biological Chemistry. 2004;279(50):52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- 9.Chiku T., Padovani D., Zhu W., Singh S., Vitvitsky V., Banerjee R. H2s biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. Journal of Biological Chemistry. 2009;284(17):11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibuya N., Tanaka M., Yoshida M., et al. 3-mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxidants & Redox Signaling. 2009;11(4):703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 11.Singh S., Banerjee R. PLP-dependent H2S Biogenesis. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2011;1814(11):1518–1527. doi: 10.1016/j.bbapap.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibuya N., Koike S., Tanaka M., et al. A novel pathway for the production of hydrogen sulfide from d-cysteine in mammalian cells. Nature Communications. 2013;4(1):p. 1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 13.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiological Reviews. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 14.Chertok V. M., Kotsyuba A. E. Distribution of h2s synthesis enzymes in the walls of cerebral arteries in rats. Bulletin of Experimental Biology and Medicine. 2012;154(1):104–107. doi: 10.1007/s10517-012-1886-2. [DOI] [PubMed] [Google Scholar]
- 15.Shibuya N., Mikami Y., Kimura Y., Nagahara N., Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. Journal of Biochemistry. 2009;146(5):623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 16.Handy D. E., Loscalzo J. Homocysteine and atherothrombosis: diagnosis and treatment. Current Atherosclerosis Reports. 2003;5(4):276–283. doi: 10.1007/s11883-003-0050-x. [DOI] [PubMed] [Google Scholar]
- 17.Liaugaudas G., Jacques P. F., Selhub J., Rosenberg I. H., Bostom A. G. Renal insufficiency, vitamin B12 Status, and population attributable risk for mild hyperhomocysteinemia among coronary artery disease patients in the era of folic acid-fortified cereal grain flour. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(5):849–851. doi: 10.1161/01.ATV.21.5.849. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen J. T., Gaustadnes M., Stabler S. P., Allen R. H., Mudd S. H., Hvas A. M. Molecular and biochemical investigations of patients with intermediate or severe hyperhomocysteinemia. Molecular Genetics and Metabolism. 2016;117(3):344–350. doi: 10.1016/j.ymgme.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Ueland P. M. Homocysteine species as components of plasma redox thiol status. Clinical Chemistry. 1995;41(3):340–342. [PubMed] [Google Scholar]
- 20.Singh S., Padovani D., Leslie R. A., Chiku T., Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to h2s biogenesis via alternative trans-sulfuration reactions. Journal of Biological Chemistry. 2009;284(33):22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J. J., Li Q., Du H. P., et al. Homocysteine triggers inflammatory responses in macrophages through inhibiting cse-h2s signaling via DNA hypermethylation of cse promoter. International Journal of Molecular Sciences. 2015;16(12):12560–12577. doi: 10.3390/ijms160612560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M. H., Tang J. P., Zhang P., et al. Disturbance of endogenous hydrogen sulfide generation and endoplasmic reticulum stress in hippocampus are involved in homocysteine-induced defect in learning and memory of rats. Behav Brain Res. 2014;262:35–41. doi: 10.1016/j.bbr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Tang X. Q., Shen X. T., Huang Y. E., et al. Inhibition of endogenous hydrogen sulfide generation is associated with homocysteine-induced neurotoxicity: Role of erk1/2 activation. Journal of Molecular Neuroscience. 2011;45(1):60–67. doi: 10.1007/s12031-010-9477-z. [DOI] [PubMed] [Google Scholar]
- 24.Nakano S., Ishii I., Shinmura K., et al. Hyperhomocysteinemia abrogates fasting-induced cardioprotection against ischemia/reperfusion by limiting bioavailability of hydrogen sulfide anions. Journal of Molecular Medicine. 2015;93(8):879–889. doi: 10.1007/s00109-015-1271-5. [DOI] [PubMed] [Google Scholar]
- 25.d'Emmanuele di Villa Bianca R., Mitidieri E., Di Minno M. N. D., et al. Hydrogen sulphide pathway contributes to the enhanced human platelet aggregation in hyperhomocysteinemia. Proceedings of the National Academy of Sciences. 2013;110(39):15812–15817. doi: 10.1073/pnas.1309049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui X., Navneet S., Wang J., et al. Analysis of mthfr, cbs, glutathione, taurine, and hydrogen sulfide levels in retinas of hyperhomocysteinemic mice. Investigative Opthalmology & Visual Science. 2017;58(4):1954–1963. doi: 10.1167/iovs.16-21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang L., Geng B., Yu F., et al. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids. 2008;34(4):573–585. doi: 10.1007/s00726-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 28.Parsanathan R., Jain S. K. Hydrogen sulfide increases glutathione biosynthesis, and glucose uptake and utilisation in C2C12mouse myotubes. Free Radical Research. 2018;52(2):288–303. doi: 10.1080/10715762.2018.1431626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giustarini D., Tazzari V., Bassanini I., Rossi R., Sparatore A. The new h2s-releasing compound acs94 exerts protective effects through the modulation of thiol homoeostasis. Journal of Enzyme Inhibition and Medicinal Chemistry. 2018;33(1):1392–1404. doi: 10.1080/14756366.2018.1509211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyagi N., Moshal K. S., Sen U., et al. H2s protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal. 2009;11(1):25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nandi S. S., Mishra P. K. H2s and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Scientific Reports. 2017;7(1):p. 3639. doi: 10.1038/s41598-017-03776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L., Ingrid S., Ding Y. G., et al. Imbalance of endogenous homocysteine and hydrogen sulfide metabolic pathway in essential hypertensive children. Chinese Medical Journal. 2007;120(5):389–393. doi: 10.1097/00029330-200703010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Sun L., Sun S., Li Y., et al. Potential biomarkers predicting risk of pulmonary hypertension in congenital heart disease: The role of homocysteine and hydrogen sulfide. Chinese Medical Journal. 2014;127(5):893–899. [PubMed] [Google Scholar]
- 34.He Y., Liu S., Zhang Z., et al. Imbalance of endogenous hydrogen sulfide and homocysteine in chronic obstructive pulmonary disease combined with cardiovascular disease. Frontiers in Pharmacology. 2017;8:p. 624. doi: 10.3389/fphar.2017.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganguly P., Alam S. F. Role of homocysteine in the development of cardiovascular disease. Nutrition Journal. 2015;14(1):p. 6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nygard O., Nordrehaug J. E., Refsum H., Ueland P. M., Farstad M., Vollset S. E. Plasma homocysteine levels and mortality in patients with coronary artery disease. New England Journal of Medicine. 1997;337(4):230–237. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 37.Montalescot G., Ankri A., Chadefaux-Vekemans B., et al. Plasma homocysteine and the extent of atherosclerosis in patients with coronary artery disease1. International Journal of Cardiology. 1997;60(3):295–300. doi: 10.1016/S0167-5273(97)00099-5. [DOI] [PubMed] [Google Scholar]
- 38.Mani S., Li H., Untereiner A., et al. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127(25):2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 39.Possomato-Vieira J. S., Gonçalves-Rizzi V. H., do Nascimento R. A., et al. Clinical and experimental evidences of hydrogen sulfide involvement in lead-induced hypertension. BioMed Research International. 2018;2018:13. doi: 10.1155/2018/4627391.4627391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanagy N. L., Szabo C., Papapetropoulos A. Vascular biology of hydrogen sulfide. American Journal of Physiology-Cell Physiology. 2017;312(5):C537–C549. doi: 10.1152/ajpcell.00329.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Y., Wang X., Kong W. Hyperhomocysteinaemia and vascular injury: Advances in mechanisms and drug targets. British Journal of Pharmacology. 2018;175(8):1173–1189. doi: 10.1111/bph.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sen U., Sathnur P. B., Kundu S., et al. Increased endogenous h2s generation by cbs, cse, and 3mst gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. American Journal of Physiology-Cell Physiology. 2012;303(1):C41–C51. doi: 10.1152/ajpcell.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pushpakumar S., Kundu S., Sen U. Hydrogen sulfide protects hyperhomocysteinemia-induced renal damage by modulation of caveolin and enos interaction. Scientific Reports. 2019;9(1):p. 2223. doi: 10.1038/s41598-018-38467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Q., Xue H. M., Underwood M. J., Yu C. M. Mechanistic studies of ave3085 against homocysteine in endothelial protection. Cardiovascular Drugs and Therapy. 2013;27(6):511–520. doi: 10.1007/s10557-013-6478-5. [DOI] [PubMed] [Google Scholar]
- 45.Xie X., Zhang Z., Wang X., et al. Stachydrine protects enos uncoupling and ameliorates endothelial dysfunction induced by homocysteine. Molecular Medicine. 2018;24(1):p. 10. doi: 10.1186/s10020-018-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma S., Singh M., Sharma P. L. Mechanism of hyperhomocysteinemia-induced vascular endothelium dysfunction - possible dysregulation of phosphatidylinositol-3-kinase and its downstream phosphoinositide dependent kinase and protein kinase b. European Journal of Pharmacology. 2013;721(1-3):365–372. doi: 10.1016/j.ejphar.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 47.Yan T. T., Li Q., Zhang X. H., et al. Homocysteine impaired endothelial function through compromised vascular endothelial growth factor/akt/endothelial nitric oxide synthase signalling. Clinical and Experimental Pharmacology and Physiology. 2010;37(11):1071–1077. doi: 10.1111/j.1440-1681.2010.05438.x. [DOI] [PubMed] [Google Scholar]
- 48.Szabo C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. American Journal of Physiology-Cell Physiology. 2017;312(1):C3–C15. doi: 10.1152/ajpcell.00282.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szijártó I. A., Markó L., Filipovic M. R., et al. Cystathionine γ-Lyase–Produced hydrogen sulfide controls endothelial no bioavailability and blood pressure. Hypertension. 2018;71(6):1210–1217. doi: 10.1161/HYPERTENSIONAHA.117.10562. [DOI] [PubMed] [Google Scholar]
- 50.Ertuna E., Loot A. E., Fleming I., Yetik-Anacak G. The role of enos on the compensatory regulation of vascular tonus by h2s in mouse carotid arteries. Nitric Oxide. 2017;69:45–50. doi: 10.1016/j.niox.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 51.McCully K. S. Chemical pathology of homocysteine. Iv. Excitotoxicity, oxidative stress, endothelial dysfunction, and inflammation. Annals of Clinical and Laboratory Science. 2009;39(3):219–232. [PubMed] [Google Scholar]
- 52.Petras M., Tatarkova Z., Kovalska M., et al. Hyperhomocysteinemia as a risk factor for the neuronal system disorders. Journal of Physiology and Pharmacology. 2014;65(1):15–23. [PubMed] [Google Scholar]
- 53.Yan S.-K., Chang T., Wang H., Wu L., Wang R., Meng Q. H. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 2006;351(2):485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 54.Kamat P. K., Kalani A., Tyagi S. C., Tyagi N. Hydrogen sulfide epigenetically attenuates homocysteine-induced mitochondrial toxicity mediated through nmda receptor in mouse brain endothelial (bend3) cells. Journal of Cellular Physiology. 2015;230(2):378–394. doi: 10.1002/jcp.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamat P. K., Kalani A., Givvimani S., Sathnur P. B., Tyagi S. C., Tyagi N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience. 2013;252:302–319. doi: 10.1016/j.neuroscience.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nath N., Prasad H. K., Kumar M. Cerebroprotective effects of hydrogen sulfide in homocysteine-induced neurovascular permeability: Involvement of oxidative stress, arginase, and matrix metalloproteinase-9. Journal of Cellular Physiology. 2018;234(3):3007–3019. doi: 10.1002/jcp.27120. [DOI] [PubMed] [Google Scholar]
- 57.Kimura Y., Kimura H. Hydrogen sulfide protects neurons from oxidative stress. The FASEB Journal. 2004;18(10):1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 58.Potor L., Nagy P., Méhes G., et al. Hydrogen sulfide abrogates hemoglobin-lipid interaction in atherosclerotic lesion. Oxidative Medicine and Cellular Longevity. 2018;2018:16. doi: 10.1155/2018/3812568.3812568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Awasthi V., Yadav V. R., Goins B., Phillips W. T. Modulation of oxidative stability of haemoglobin inside liposome-encapsulated haemoglobin. Journal of Microencapsulation. 2013;30(5):471–478. doi: 10.3109/02652048.2012.752535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vacek T. P., Rehman S., Neamtu D., Yu S., Givimani S., Tyagi S. C. Matrix metalloproteinases in atherosclerosis: role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vascular Health and Risk Management. 2015;11:173–183. doi: 10.2147/VHRM.S68415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Vlies D., Makkinje M., Jansens A., et al. Oxidation of er resident proteins upon oxidative stress: Effects of altering cellular redox/antioxidant status and implications for protein maturation. Antioxidants & Redox Signaling. 2003;5(4):381–387. doi: 10.1089/152308603768295113. [DOI] [PubMed] [Google Scholar]
- 62.Cimellaro A., Perticone M., Fiorentino T. V., Sciacqua A., Hribal M. L. Role of endoplasmic reticulum stress in endothelial dysfunction. Nutrition, Metabolism and Cardiovascular Diseases. 2016;26(10):863–871. doi: 10.1016/j.numecd.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 63.Hu H., Wang C., Jin Y., et al. Alpha-lipoic acid defends homocysteine-induced endoplasmic reticulum and oxidative stress in haecs. Biomedicine & Pharmacotherapy. 2016;80:63–72. doi: 10.1016/j.biopha.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Wu S., Gao X., Yang S., Meng M., Yang X., Ge B. The role of endoplasmic reticulum stress in endothelial dysfunction induced by homocysteine thiolactone. Fundamental & Clinical Pharmacology. 2015;29(3):252–259. doi: 10.1111/fcp.12101. [DOI] [PubMed] [Google Scholar]
- 65.Wu X., Zhang L., Miao Y., et al. Homocysteine causes vascular endothelial dysfunction by disrupting endoplasmic reticulum redox homeostasis. Redox Biology. 2019;20:46–59. doi: 10.1016/j.redox.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang C. Y., Zou W., Liang X. Y., et al. Hydrogen sulfide prevents homocysteine-induced endoplasmic reticulum stress in pc12 cells by upregulating SIRT-1. Molecular Medicine Reports. 2017;16(3):3587–3593. doi: 10.3892/mmr.2017.7004. [DOI] [PubMed] [Google Scholar]
- 67.Wei H.-j., Xu J.-h., Li M.-h., et al. Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the bdnf-trkb pathway. Acta Pharmacologica Sinica. 2014;35(6):707–715. doi: 10.1038/aps.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei H., Zhang R., Jin H., et al. Hydrogen sulfide attenuates hyperhomocysteinemia-induced cardiomyocytic endoplasmic reticulum stress in rats. Antioxidants & Redox Signaling. 2010;12(9):1079–1091. doi: 10.1089/ars.2009.2898. [DOI] [PubMed] [Google Scholar]
- 69.Majumder A., Singh M., George A. K., Tyagi S. C. Restoration of skeletal muscle homeostasis by hydrogen sulfide during hyperhomocysteinemia-mediated oxidative/er stress condition. Canadian Journal of Physiology and Pharmacology. 2019;97(6):441–456. doi: 10.1139/cjpp-2018-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kabil O., Yadav V., Banerjee R. Heme-dependent metabolite switching regulates h2s synthesis in response to endoplasmic reticulum (er) stress. Journal of Biological Chemistry. 2016;291(32):16418–16423. doi: 10.1074/jbc.C116.742213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X. C., Sun W. T., Yu C. M., et al. Er stress mediates homocysteine-induced endothelial dysfunction: Modulation of ikca and skca channels. Atherosclerosis. 2015;242(1):191–198. doi: 10.1016/j.atherosclerosis.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Sun W. T., Wang X. C., Mak S. K., et al. Activation of perk branch of er stress mediates homocysteine-induced BK<sub>Ca</sub> channel dysfunction in coronary artery via foxo3a-dependent regulation of atrogin-1. Oncotarget. 2017;8(31):51462–51477. doi: 10.18632/oncotarget.17721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun W. T., Wang X. C., Novakovic A., Wang J., He G. W., Yang Q. Protection of dilator function of coronary arteries from homocysteine by tetramethylpyrazine: role of er stress in modulation of bkca channels. Vascular Pharmacology. 2019;113:27–37. doi: 10.1016/j.vph.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Kutz J. L., Greaney J. L., Santhanam L., Alexander L. M. Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature. The Journal of Physiology. 2015;593(9):2121–2129. doi: 10.1113/JP270054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang M., Hu Y., Fan Y., et al. Involvement of hydrogen sulfide in endothelium-derived relaxing factor-mediated responses in rat cerebral arteries. Journal of Vascular Research. 2016;53(3-4):172–185. doi: 10.1159/000448712. [DOI] [PubMed] [Google Scholar]
- 76.Liu S.-Y., Duan X.-C., Jin S., et al. Hydrogen Sulfide Improves Myocardial Remodeling via Downregulated Angiotensin II/AT1R Pathway in Renovascular Hypertensive Rats. American Journal of Hypertension. 2017;30(1):67–74. doi: 10.1093/ajh/hpw104. [DOI] [PubMed] [Google Scholar]
- 77.Li T., Yu B., Liu Z., et al. Homocysteine directly interacts and activates the angiotensin ii type i receptor to aggravate vascular injury. Nature Communications. 2018;9(1):p. 11. doi: 10.1038/s41467-017-02401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gaifullina A. S., Lazniewska J., Gerasimova E. V., et al. A potential role for t-type calcium channels in homocysteinemia-induced peripheral neuropathy. Pain. 2019:p. 1. doi: 10.1097/j.pain.0000000000001669. [DOI] [PubMed] [Google Scholar]
- 79.Dai L., Qian Y., Zhou J., Zhu C., Jin L., Li S. Hydrogen sulfide inhibited l-type calcium channels (cav1.2) via up-regulation of the channel sulfhydration in vascular smooth muscle cells. European Journal of Pharmacology. 2019;858, article 172455 doi: 10.1016/j.ejphar.2019.172455. [DOI] [PubMed] [Google Scholar]
- 80.Vermeulen E. G. J., Stehouwer C. D. A., Twisk J. W. R., et al. Effect of homocysteine-lowering treatment with folic acid plus vitamin b6 on progression of subclinical atherosclerosis: A randomised, placebo-controlled trial. The Lancet. 2000;355(9203):517–522. doi: 10.1016/S0140-6736(99)07391-2. [DOI] [PubMed] [Google Scholar]
- 81.Liu X., Shi M., Xia F., et al. The china stroke secondary prevention trial (csspt) protocol: a double-blinded, randomized, controlled trial of combined folic acid and b vitamins for secondary prevention of stroke. International Journal of Stroke. 2015;10(2):264–268. doi: 10.1111/ijs.12017. [DOI] [PubMed] [Google Scholar]
- 82.Yi X., Zhou Y., Jiang D., Li X., Guo Y., Jiang X. Efficacy of folic acid supplementation on endothelial function and plasma homocysteine concentration in coronary artery disease: a meta-analysis of randomized controlled trials. Experimental and Therapeutic Medicine. 2014;7(5):1100–1110. doi: 10.3892/etm.2014.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y., Tian T., Zhang H., Gao L., Zhou X. The effect of homocysteine-lowering therapy with folic acid on flow-mediated vasodilation in patients with coronary artery disease: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;235(1):31–35. doi: 10.1016/j.atherosclerosis.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 84.Ebbing M., Bleie Ø., Ueland P. M., et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering b vitamins after coronary Angiography. JAMA. 2008;300(7):795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]
- 85.Hu S., Ren L., Wang Y., et al. Homocysteine-lowering therapy and early functional outcomes of ischemic patients with h-type hypertension: a retrospective analysis of cnsr. Australasian Physical & Engineering Sciences in Medicine. 2015;38(4):785–791. doi: 10.1007/s13246-015-0406-x. [DOI] [PubMed] [Google Scholar]
- 86.Martí-Carvajal A. J., Solà I., Lathyris D., Dayer M., Cochrane Heart Group Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database of Systematic Reviews. 2017;8, article CD006612 doi: 10.1002/14651858.cd006612.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng R., Xu C. H., Xu Y. N., Wang Y. L., Wang M. The effect of folate fortification on folic acid-based homocysteine-lowering intervention and stroke risk: A meta-analysis. Public Health Nutrition. 2015;18(8):1514–1521. doi: 10.1017/S1368980014002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holmes M. V., Newcombe P., Hubacek J. A., et al. Effect modification by population dietary folate on the association between mthfr genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. The Lancet. 2011;378(9791):584–594. doi: 10.1016/S0140-6736(11)60872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hankey G. J., Eikelboom J. W., Yi Q., et al. Antiplatelet therapy and the effects of b vitamins in patients with previous stroke or transient ischaemic attack: a post-hoc subanalysis of vitatops, a randomised, placebo-controlled trial. The Lancet Neurology. 2012;11(6):512–520. doi: 10.1016/s1474-4422(12)70091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang X., Qin X., Yang W., et al. MTHFRGene and serum folate interaction on serum homocysteine Lowering. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(3):679–685. doi: 10.1161/ATVBAHA.117.310211. [DOI] [PubMed] [Google Scholar]
- 91.Achour O., Elmtaoua S., Zellama D., et al. The c677t mthfr genotypes influence the efficacy of b9 and b12 vitamins supplementation to lowering plasma total homocysteine in hemodialysis. Journal of Nephrology. 2016;29(5):691–698. doi: 10.1007/s40620-015-0235-8. [DOI] [PubMed] [Google Scholar]
- 92.Kruger W. D., Gupta S. The effect of dietary modulation of sulfur amino acids on cystathionine β synthase-deficient mice. Annals of the New York Academy of Sciences. 2016;1363(1):80–90. doi: 10.1111/nyas.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ibrahim S., Maqbool S., Azam M., Iqbal M. P., Qamar R. Cbs mutations and mtfhr snps causative of hyperhomocysteinemia in pakistani children. Molecular Biology Reports. 2018;45(3):353–360. doi: 10.1007/s11033-018-4169-9. [DOI] [PubMed] [Google Scholar]
- 94.Xiao L., Dong J. H., Teng X., et al. Hydrogen sulfide improves endothelial dysfunction in hypertension by activating peroxisome proliferator-activated receptor delta/endothelial nitric oxide synthase signaling. Journal of Hypertension. 2018;36(3):651–665. doi: 10.1097/HJH.0000000000001605. [DOI] [PubMed] [Google Scholar]
- 95.Tain Y.-L., Hsu C.-N., Lu P.-C. Early short-term treatment with exogenous hydrogen sulfide postpones the transition from prehypertension to hypertension in spontaneously hypertensive rat. Clinical and Experimental Hypertension. 2017;40(1):58–64. doi: 10.1080/10641963.2017.1313847. [DOI] [PubMed] [Google Scholar]
- 96.Wallace J. L., Vaughan D., Dicay M., MacNaughton W. K., de Nucci G. Hydrogen sulfide-releasing therapeutics: translation to the clinic. Antioxidants & Redox Signaling. 2018;28(16):1533–1540. doi: 10.1089/ars.2017.7068. [DOI] [PubMed] [Google Scholar]
- 97.Kar S., Shahshahan H. R., Kambis T. N., et al. Hydrogen sulfide ameliorates homocysteine-induced cardiac remodeling and dysfunction. Frontiers in Physiology. 2019;10:p. 598. doi: 10.3389/fphys.2019.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Z., Polhemus D. J., Lefer D. J. Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease. Circulation Research. 2018;123(5):590–600. doi: 10.1161/CIRCRESAHA.118.311134. [DOI] [PubMed] [Google Scholar]
- 99.Longchamp A., Kaur K., Macabrey D., et al. Hydrogen sulfide-releasing peptide hydrogel limits the development of intimal hyperplasia in human vein segments. Acta Biomaterialia. 2019;97:374–384. doi: 10.1016/j.actbio.2019.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]