Abstract
Many filamentous ascomycetes develop three-dimensional fruiting bodies for production and dispersal of sexual spores. Fruiting bodies are among the most complex structures differentiated by ascomycetes; however, the molecular mechanisms underlying this process are insufficiently understood. Previous comparative transcriptomics analyses of fruiting body development in different ascomycetes suggested that there might be a core set of genes that are transcriptionally regulated in a similar manner across species. Conserved patterns of gene expression can be indicative of functional relevance, and therefore such a set of genes might constitute promising candidates for functional analyses. In this study, we have sequenced the genome of the Pezizomycete Ascodesmis nigricans, and performed comparative transcriptomics of developing fruiting bodies of this fungus, the Pezizomycete Pyronema confluens, and the Sordariomycete Sordaria macrospora. With only 27 Mb, the A. nigricans genome is the smallest Pezizomycete genome sequenced to date. Comparative transcriptomics indicated that gene expression patterns in developing fruiting bodies of the three species are more similar to each other than to nonsexual hyphae of the same species. An analysis of 83 genes that are upregulated only during fruiting body development in all three species revealed 23 genes encoding proteins with predicted roles in vesicle transport, the endomembrane system, or transport across membranes, and 13 genes encoding proteins with predicted roles in chromatin organization or the regulation of gene expression. Among four genes chosen for functional analysis by deletion in S. macrospora, three were shown to be involved in fruiting body formation, including two predicted chromatin modifier genes.
Keywords: fruiting body development, Ascodesmis nigricans, Sordaria macrospora, Pyronema confluens, comparative transcriptomics
THE ability to develop complex multicellular structures evolved several times independently in eukaryotes (Knoll 2011; Niklas 2014). Within the fungi (Eumycota), complex multicellular structures evolved at least twice and possibly up to 11 times. Fungal multicellular structures are often involved in sexual development, e.g., the fruiting bodies of basidiomycetes and filamentous ascomycetes, which most likely evolved independently (Knoll 2011; Nagy 2017; Nagy et al. 2018; Varga et al. 2019). Fruiting bodies function in the production and dispersal of sexual spores, and contain a number of cell types that are not found in vegetative mycelium (Kües 2000; Bistis et al. 2003; Han 2009; Lord and Read 2011; Pöggeler et al. 2018). The molecular mechanisms regulating fruiting body development in filamentous ascomycetes have been studied in recent decades mostly using model organisms from the Sordariomycetes or Eurotiomycetes, e.g., Neurospora crassa, Sordaria macrospora, Fusarium graminearum (Gibberella zeae), Trichoderma reesei, and Aspergillus nidulans, which are able to produce fruiting bodies under laboratory conditions and are amenable to classical and molecular genetics (Pöggeler et al. 2018). With the advent of next generation sequencing techniques, sequencing of genomes and transcriptomes of nonmodel species became feasible, allowing comparative genomics and transcriptomics analyses of fruiting body development in different fungal groups (Nowrousian 2014, 2018). In a previous study, we sequenced the genome and several transcriptomes of different developmental stages from Pyronema confluens, which belongs to the early-diverging lineage of Pezizomycetes (Traeger et al. 2013). A comparative analysis of P. confluens transcriptome data with transcriptomes from different developmental stages of S. macrospora suggested that gene expression during sexual development might be conserved to some degree, and that similar tissues from different species might have more similar expression patterns than different tissues within a species (Teichert et al. 2012; Traeger et al. 2013). However, at the time of this analysis, fruiting body–specific transcriptomes were available for S. macrospora, while for P. confluens, only total sexual mycelia were analyzed, which contain fruiting bodies and the surrounding nonsexual hyphae. Recently, fruiting body–specific transcriptomes were generated for P. confluens (Murat et al. 2018), and in the present study, we sequenced the genome and several transcriptomes for the Pezizomycete Ascodesmis nigricans, including fruiting body transcriptomes that were used for a comparative study with S. macrospora and P. confluens.
Like P. confluens, A. nigricans is a member of the Pezizomycetes, an early-diverging group of filamentous ascomycetes. The Pezizomycetes form fruiting bodies called apothecia, which are often disk-like in appearance with the spore-containing asci (meiosporangia) exposed on top of the fruiting body. However, several Pezizomycetes lineages harbor ectomycorrhizal truffle species that form subterranean fruiting bodies with a complex morphology (Hansen and Pfister 2006; Murat et al. 2018). Only few Pezizomycetes are able to produce fruiting bodies under laboratory conditions. This has hampered the genetic and molecular analysis of sexual development in this group. An exception is P. confluens, which is able to produce fruiting bodies in the laboratory within 1 week (Claussen 1912; Moore and Korf 1963; Traeger et al. 2013). A. nigricans also produces fruiting bodies under laboratory conditions, and similar to P. confluens, this species is homothallic (self-fertile) and therefore does not need a mating partner for sexual development (Obrist 1961; Van Brummelen 1981). A. nigricans is a coprophilic fungus (Obrist 1961), and in this it is similar to the Sordariomycete S. macrospora (Kück et al. 2009), whereas P. confluens is a soil-living saprobe (Seaver 1909). Under laboratory conditions, the three species A. nigricans, P. confluens, and S. macrospora display very similar life cycles as they are all homothallic and able to form fruiting bodies within a week. Furthermore, none of the three species forms conidia (asexual spores); therefore, changes in gene expression patterns during sexual reproduction are not obscured by changes related to asexual sporulation. Thus, they are suitable model organisms for a comparative study of gene expression during fruiting body development in filamentous ascomycetes.
Another reason for sequencing the A. nigricans genome was the analysis of its genome size and repeat content. Previous studies of eight Pezizomycetes genomes showed that they are overall rather large for filamentous fungi, the smallest genomes being those of saprotrophic species (48–60 Mb for Morchella importuna, P. confluens, and Ascobolus immersus), whereas five analyzed truffle species have genomes ranging from 63 to 192 Mb, due to repeat expansion (Martin et al. 2010a; Traeger et al. 2013; Murat et al. 2018). However, so far the sequenced genomes cover mostly two of the three major phylogenetic lineages within the Pezizomycetes, with the third lineage represented only by the genome of P. confluens (Hansen and Pfister 2006; Murat et al. 2018). A. nigricans is also a member of this third lineage, even though it is only distantly related to P. confluens (Hansen and Pfister 2006). Therefore, analysis of the A. nigricans genome will improve the phylogenetic coverage for Pezizomycetes genomes, and also improve the coverage of Pezizomycetes with a nonmycorrhizal lifestyle.
Another point of interest in the A. nigricans genome is the organization of the mating type (MAT) locus. MAT loci in filamentous ascomycetes contain various genes that are central regulators of sexual development. In heterothallic (self-sterile) ascomycetes, each strain possesses one of two nonallelic versions (idiomorphs) of a single MAT locus, named MAT1-1 and MAT1-2. These loci usually contain (among others) the MAT1-1-1 and MAT1-2-1 genes, which encode transcription factors with a conserved alpha domain and high-mobility group domain, respectively. In contrast, homothallic ascomycetes carry both MAT loci within a single genome. The two loci can be fused together, located within close proximity, or located on separate chromosomes (Debuchy et al. 2010; Billiard et al. 2011; Bennett and Turgeon 2016; Pöggeler et al. 2018). In P. confluens, homologs of the core MAT genes MAT1-1-1 and MAT1-2-1 were found, as expected for a homothallic ascomycete. However, other genes that are often part of the MAT loci in other ascomycetes were neither found near MAT1-1-1 or MAT1-2-1 in this species, nor in the MAT loci of the heterothallic Pezizomycete Tuber melanosporum (Rubini et al. 2011; Traeger et al. 2013). In addition, of the two genes apn2 and sla2 that often flank the MAT locus in more derived lineages of filamentous ascomycetes (Pöggeler et al. 2018), only apn2 was identified in proximity to the P. confluens MAT locus, whereas none of these genes flank the MAT loci of T. melanosporum (Rubini et al. 2011; Traeger et al. 2013). It is not clear if the MAT loci of T. melanosporum and P. confluens represent basal or derived MAT configurations, therefore the analysis of additional Pezizomycetes MAT loci is of great interest for the analysis of the evolution of sexual development in fungi.
In this study, we sequenced the genome of A. nigricans, and generated transcriptomes for vegetative and sexual mycelia, as well as for developing fruiting bodies that were isolated from the surrounding mycelium by laser microdissection. The transcriptomics data were used for a comparative analysis with RNA-sequencing (RNA-seq) data from mycelia and developing fruiting bodies of P. confluens and S. macrospora to identify conserved core groups of genes that are differentially regulated during sexual development. Several differentially expressed genes were functionally characterized to address their roles during fruiting body morphogenesis by generating corresponding deletion mutants in S. macrospora.
Materials and Methods
Strains, culture conditions, and genetic crosses
A. nigricans and S. macrospora strains used in this study are given in Table 1. A. nigricans was grown on cornmeal medium (Biomalz-Mais-Medium; BMM) (Esser 1982), rabbit food agar medium (RFA; 25 g of rabbit food pellets were boiled in 1 liter of distilled water., set to cool for 30 min, filtered through cotton, and autoclaved), or V8 medium (50 ml vegetable juice per liter, pH 5.2) at 25°. S. macrospora was grown on cornmeal medium (BMM) or a Sordaria minimal medium (Sordaria Westergaard’s medium; SWG) at 25°, as described (Esser 1982; Nowrousian et al. 2005). Both media support vigorous fruiting body formation. Transformation protocols and protocols for genetic crosses for S. macrospora were as described previously (Esser 1982; Nowrousian et al. 1999; Dirschnabel et al. 2014). To observe hyphal fusions, strains were grown on minimal medium (MM) with cellophane, which allows sparse hyphal growth for better visualization of individual hyphae (Rech et al. 2007). For microscopy, strains were inoculated for 2–10 days on glass slides with a thin layer of BMM with 0.8% agar (Engh et al. 2007). Quantification of linear growth was performed on BMM or SWG using petri dishes with inoculation at the edge. The growth front was marked over 3–5 days every 24 hr, and experiments were performed in triplicate.
Table 1. Fungal strains used in this study.
| Strain | Relevant genotype and phenotype | Reference or source |
|---|---|---|
| Ascodesmis nigricans strains | ||
| CBS 389.68 | Wild type | CBS-KNAW |
| CBS 704.96 | Wild type | CBS-KNAW |
| CBS 114.53 | Wild type | CBS-KNAW |
| CBS 163.74 | Wild type | CBS-KNAW |
| Sordaria macrospora strains | ||
| Wild type | Wild type | AMBa |
| Fus | Spore color mutant | Nowrousian et al. (2012) |
| S96888 | Δku70 | Pöggeler and Kück (2006) |
| S110115 | Δrtt106; fertile | Gesing et al. (2012) |
| S110235 | Δcac2; fertile | Gesing et al. (2012) |
| S123704 | Δcrc1; fertile | Schumacher et al. (2018) |
| S111081 | Δrtt106, Δcac2, fus; fertile | Schumacher et al. (2018) |
| S111094 | Δrtt106, Δcac2; fertile | Schumacher et al. (2018) |
| S128347 | Δcrc1, Δrtt106; fertile | Schumacher et al. 2018) |
| S128175 | Δcrc1, Δcac2, fus; fertile | Schumacher et al. (2018) |
| S155732 | ∆scm1, fus; fertile | This study |
| S155906 | ∆scm1; fertile | This study |
| S156325 | ∆scm1, ∆cac2; fertile | This study |
| S156391 | ∆scm1, ∆crc1, fus; fertile | This study |
| S156436 | ∆scm1, ∆ rtt106; fertile | This study |
| RL1637 | ∆cac2, ∆crc1, ∆rtt106; sterile | This study |
| RL1648 | ∆crc1, ∆rtt106, ∆scm1; partially fertile | This study |
| RL1737 | ∆cac2, ∆crc1, ∆scm1; sterile | This study |
| RL1738 | ∆cac2, ∆crc1, ∆scm1; sterile | This study |
| RL1761 | ∆cac2, ∆crc1, ∆scm1; sterile | This study |
| RL1987 | ∆cac2, ∆rtt106, ∆scm1; sterile | This study |
| RL1923 | ∆cac2, ∆crc1, ∆rtt106, ∆scm1; sterile | This study |
| RL1924 | ∆cac2, ∆crc1, ∆rtt106, ∆scm1; sterile | This study |
| RL1957 | ∆cac2, ∆crc1, ∆rtt106, ∆scm1; sterile | This study |
| S153858 | ∆spt3; sterile | This study |
| S155241 | ∆spt3; sterile | This study |
| RL1164 | ∆spt3 + pOE_1829.3_GFP; fertile | This study |
| RL1184 | ∆spt3 + pOE_1829.3_GFP; fertile | This study |
| RL1493 | ∆spt3 + pN_1829.3_GFP; partially fertile | This study |
| RL1509 | ∆spt3 + pN_1829.3_GFP; partially fertile | This study |
| SJBK 1 AS8 | ∆aod5; fertile | This study |
| SJBK 19.2 AS9 | ∆aod5 + pSMAC_06113_EGFP; fertile | This study |
AMB: culture collection Allgemeine und Molekulare Botanik, Ruhr-Universität, Bochum, Germany.
DNA preparation, sequencing, and assembly of the A. nigricans genome
Genomic DNA from A. nigricans strain CBS 389.68 was prepared for sequencing as described for P. confluens (Traeger et al. 2013). One 270 bp insert library (2 × 150 bp paired-end sequencing) and one 4 kb mate-pair library (2 × 100 bp paired-end sequencing) were sequenced on an Illumina HiSeq 2500. Illumina fastq files were filtered for artifacts/process contamination. Postprocessed genomic reads were assembled with AllPathsLG v.R49403 (Gnerre et al. 2011).
Genome annotation and analysis of repeat content
RNA-seq reads for annotation (for RNA preparation and sequencing, see below) were assembled into consensus sequences using Rnnotator v.3.3.2 (Martin et al. 2010b). The assembled consensus RNA sequence data were mapped to genome assembly using alignments of 90% identity and 85% coverage or higher to assess genome completeness at 97.91%. The genome was annotated using the JGI Annotation pipeline and made available via JGI fungal genome portal MycoCosm (jgi.doe.gov/fungi) (Grigoriev et al. 2014).
Analysis of transposable elements and other repeats in the A. nigricans genome assembly was performed as described (Traeger et al. 2013) with RepeatMasker (A.F.A. Smit, R. Hubley, P. Green; unpublished data; www.repeatmasker.org) based on the RepbaseUpdate library (Jurka et al. 2005) and a library of de novo–identified A. nigricans repeat consensus sequences that was generated by RepeatModeler (A.F.A. Smit, R. Hubley; unpublished data; www.repeatmasker.org/RepeatModeler.html). An overview of assembly and annotation statistics is given in Table 2.
Table 2. Genome assembly statistics for three Pezizomycetes.
| A. nigricans | P. confluensa | T. magnatumb | |
|---|---|---|---|
| Assembly size (Mb) | 27 | 50 | 192 |
| No. of scaffolds | 176 | 1588 | 1283 |
| N50 (Mb) | 0.49 | 0.14 | 1.81 |
| Repeats (Mb) | 1 | 6 | 111 |
| Repeats (%) | 4 | 12 | 58 |
| Predicted genes | 9622 | 13,369 | 9433 |
| Coding regions (Mb) | 12.1 | 14.6 | 11.5 |
| Coding regions (%) | 44.3 | 29.2 | 6.0 |
| Introns (Mb) | 1.5 | 2.5 | 2.4 |
| Introns (%) | 5.6 | 5.1 | 1.2 |
Genome data from Traeger et al. (2013).
Genome data from Murat et al. (2018),
Laser microdissection, RNA preparation, and RNA-seq
For RNA preparation, A. nigricans strain CBS 389.68 was grown in liquid RFA or V8 medium as surface cultures (in petri dishes without shaking) or from submerged cultures (in 100 ml flasks shaken at 130 rpm) at 25°. RNA preparation was performed as described (Nowrousian and Kück 2006). For annotation purposes, total RNA from mycelia grown for 3 and 5 days as surface cultures in RFA and V8 was combined and sequenced on an Illumina HiSeq 2000 (2 × 150 bp paired-end sequencing). For quantification of gene expression, RNA was extracted from total vegetative and sexual mycelia, as well as from young fruiting bodies isolated by laser microdissection. Total vegetative and sexual mycelia were obtained by growing A. nigricans as described above in submerged cultures and surface cultures, respectively, in 20 ml RFA medium for 4 days at 25°. For laser microdissection of young fruiting bodies, A. nigricans was grown on microdissection slides coated with 150–200 µl RFA (with 0.8% agar) for 3 days at 25°. Fixation of slides, laser microdissection, RNA preparation, and linear RNA amplification were as described (Teichert et al. 2012). Approximately 230 microdissected young fruiting bodies were combined for each RNA extraction. For each condition (vegetative mycelium, sexual mycelium, and young fruiting bodies), two independent biological replicates were performed. The corresponding RNAs were sequenced on an Illumina HiSeq 2500 (51 bp single-end sequencing) by GATC Biotech AG (Konstanz, Germany).
Synteny analysis
An orthology-based analysis of synteny was performed as described before (Traeger et al. 2013), by determining orthologs for all A. nigricans proteins in the predicted proteomes of P. confluens and T. melanosporum by reciprocal Basic Local Alignment Search Tool (BLAST) analysis (Altschul et al. 1997), and using custom-made Perl scripts based on BioPerl modules (Stajich et al. 2002) to determine the positions of corresponding orthologous genes on sequenced contigs.
Phylogenomics analysis
The predicted proteomes of A. nigricans and the following 19 other fungal species were used for the reconstruction of the phylome using the phylomeDB pipeline (Huerta-Cepas et al. 2011): Agaricus bisporus (Morin et al. 2012), Arthrobotrys oligospora (Yang et al. 2011), Blumeria graminis (Spanu et al. 2010), Coccidioides immitis (Sharpton et al. 2009), Emericella nidulans (Galagan et al. 2005), F. graminearum (Cuomo et al. 2007), Laccaria bicolor (Martin et al. 2008), Mycosphaerella graminicola (Goodwin et al. 2011), Neosartorya fischeri (Fedorova et al. 2008), N. crassa (Galagan et al. 2003), Phaeosphaeria nodorum (Hane et al. 2007), P. confluens (Traeger et al. 2013), Saccharomyces cerevisiae (Goffeau et al. 1996), Schizosaccharomyces pombe (Wood et al. 2002), Sclerotinia sclerotiorum (Amselem et al. 2011), S. macrospora (Nowrousian et al. 2010), Taphrina deformans (Cissé et al. 2013), T. melanosporum (Murat et al. 2018), and Yarrowia lipolytica (Dujon et al. 2004). All alignments and trees are available in phylomeDB (www.phylomeDB.org) (Huerta-Cepas et al. 2014). For each gene encoded in A. nigricans, a Smith–Waterman search was performed against a proteome database containing the proteome information of the selected species. We used an e-value threshold of <1e−05 and a continuous overlap of 50% over the query sequence for the detection of homologs. We limited the number of hits included in a tree to the closest 150 homologs per gene. We used three different aligners for the multiple sequence alignments of the homologous sequences (forward and reversed versions of the sequences): MUSCLE (Edgar 2004), MAFFT (Katoh et al. 2005), and KALIGN (Lassmann and Sonnhammer 2005). The final six alignments were combined using M-COFFEE (Wallace et al. 2006) and then trimAl to trim the alignment (consistency cut-off of 0.16667 and -gt >0.1) (Capella-Gutierrez et al. 2009). We used PhyML v.3 for maximum-likelihood (ML) trees (Guindon et al. 2010). Branch support was analyzed using an approximate likelihood ratio parametric test based on a chi-square distribution. We used a discrete gamma distribution with three rates categories in all the cases (estimating the gamma parameter from the data). We scanned this phylome using a previously described algorithm for duplication detection (Huerta-Cepas et al. 2010). Using FatiGO (Al-Shahrour et al. 2007), we analyzed the gene enrichment of the genes duplicated at each branch of the species tree. To reconstruct the species tree, 143 genes that had one-to-one orthologs in each of the selected species were trimmed and then the alignments were concatenated. The final alignment had 108,319 nucleotide positions. To reconstruct the ML species tree for each alignment we used RaxML v.7.2.6, model Protgammalg, and 100 bootstrap support (Stamatakis 2006). Finally, a consensus tree using Phylip and a supertree using Duptree (Wehe et al. 2008), with a parsimony strategy from all single gene trees, was created.
Quantitative analysis of gene expression in A. nigricans based on RNA-seq data, and comparative transcriptomics analysis of A. nigricans, P. confluens, and S. macrospora
Analysis of RNA-seq data from A. nigricans was done as described previously, with minor modifications (Teichert et al. 2012; Traeger et al. 2013). Briefly, reads were trimmed with custom-made Perl programs to remove reads with nondetermined nucleotides, remove polyA or polyT stretches from end and start of reads, respectively, and trim reads from 3′ and 5′ ends until a base quality of ≥10 was reached. Trimmed reads of at least 40 bases were used for mapping to the A. nigricans genome using TopHat v.2.0.11 (Trapnell et al. 2010). Reads mapping to annotated features were counted as described (Teichert et al. 2012), and quantitative analysis of gene expression was performed with DESeq2 (Love et al. 2014).
For comparative transcriptomics analyses of the three species A. nigricans, P. confluens, and S. macrospora, orthologs between A. nigricans and the other two species were determined by reciprocal BLAST analysis. Read counts for each ortholog in the three species were obtained from RNA-seq data from this study as well as previous analyses of P. confluens (Traeger et al. 2013; Murat et al. 2018) and S. macrospora (Teichert et al. 2012). RNA-seq samples included in the analysis are given in Table 3. A combined analysis of read counts for all orthologs in all conditions was performed with DESeq2 (Love et al. 2014).
Table 3. RNA-seq data analyzed in this study.
| Condition | GEO accession number | Reference |
|---|---|---|
| Ascodesmis nigricans | ||
| Vegetative mycelium | GSE92315 | This study |
| Sexual myceliuma | GSE92315 | This study |
| Protoapotheciab | GSE92315 | This study |
| Pyronema confluens | ||
| Vegmixc | GSE41631 | Traeger et al. (2013) |
| DDc | GSE41631 | Traeger et al. (2013) |
| Sexual myceliuma | GSE41631 | Traeger et al. (2013) |
| Protoapotheciab | GSE61274 | Murat et al. (2018) |
| Sordaria macrospora | ||
| Vegetative mycelium | GSE33668 | Teichert et al. (2012) |
| Sexual myceliuma | GSE33668 | Teichert et al. (2012) |
| Wild-type protoperitheciab | GSE33668 | Teichert et al. (2012) |
For each condition, two independent biological replicates were analyzed, except for P. confluens protoapothecia, where three independent biological replicates were analyzed. GEO, Gene Expression Omnibus; DD, constant darkness.
Sexual mycelium represents mycelia including embedded developing fruiting bodies.
Protoapothecia and protoperithecia are young fruiting bodies isolated by laser microdissection.
Conditions vegmix (combined RNA from several growth conditions that allow only vegetative growth) and DD (growth in darkness) represent mycelia that cannot develop sexual structures.
Analysis of the mating type region in several A. nigricans strains
DNA fragments from the mating type regions of the A. nigricans wild-type strains given in Table 1 were amplified with primer combinations Anig_mat1/Anig_mat2, Anig_mat3/Anig_mat4, and Anig_mat5/Anig_mat6 (Supplemental Material, Table S1) and sequenced with Sanger sequencing (Eurofins Genomics, Ebersberg, Germany). The resulting overlapping fragments of 1 kb each cover the MAT1-1-1 gene and flanking regions of 0.7 kb upstream and 0.8 kb downstream.
Cloning procedures
Plasmids for generating gene deletion strains and complementation experiments in S. macrospora were cloned by homologous recombination in yeast as described (Colot et al. 2006). Oligonucleotides used for generating PCR products for cloning procedures are given in Table S1, and plasmids are given in Table S2. Deletion cassettes for SMAC_01829 (spt3), SMAC_04946 (scm1), SMAC_06113 (aod5), and SMAC_06770 were generated by amplifying ∼1 kb genomic regions upstream and downstream of the corresponding genes or including coding regions if the neighboring genes are closer than 1 kb (for spt3 and SMAC_06770). PCR fragments were then cloned to flank the hph gene conferring hygromycin resistance (Nowrousian and Cebula 2005). Plasmid pN_1829.3-GFP contains the spt3 and egfp open reading frames flanked by the spt3 5′-untranslated region (UTR) and 440 bp upstream of the 5′-UTR, and the 3′-UTR and 144 bp downstream of the 3′-UTR in pRSnat, which confers nourseothricin resistance in S. macrospora (Klix et al. 2010). Plasmids pOE_1829.3-GFP and pSMAC_06113_EGFP carry the open reading frames of spt3 and aod5, respectively, in fusion with a C-terminal egfp under control of the A. nidulans gpd promoter and trpC terminator.
Generation of gene deletion strains in S. macrospora
Deletion strains for SMAC_01829 (spt3), SMAC_04946 (scm1), SMAC_06113 (aod5), and SMAC_06770 were generated by transforming the deletion cassette (upstream and downstream regions flanking the hph gene, obtained by restriction digest of the corresponding gene deletion plasmid and gel elution) into a Δku70 strain, as described previously (Pöggeler and Kück 2006). Hygromycin-resistant primary transformants were verified for insertion of the deletion cassette by PCR and Southern blot analysis, and knockout strains were crossed against the spore color mutant fus (Nowrousian et al. 2012) to obtain homokaryotic ascospore isolates carrying the deletion allele in a genetic background without the Δku70 allele.
Stereomicroscopy and microscopy
For top and side view of cultures, stereomicroscope Stemi 2000-C (Zeiss, Jena, Germany) was used. Images were captured with an AxioCam ERc5s (Zeiss) and Zen2Core (v.2.5; Zeiss). Fluorescence and light microscopic investigations were carried out with an AxioImager microscope (Zeiss). Fluorescence was studied using Chroma (Bellows Falls, VT) filter set 41017 (HQ470/40, HQ525/50, Q495lp) for detection of EGFP, and set 49008 (EG560/40x, ET630/75m, T585lp) for the detection of mRFP. Images were captured with a Photometrix Cool SnapHQ camera (Roper Scientific) and MetaMorph (Universal Imaging). Recorded images were edited with MetaMorph and Adobe Photoshop CS6. Light microscopy of ascus rosettes and ascospores were carried out with AxioPhot (Zeiss) and an AxioCam. ZEN (v.2.3, blue edition; Zeiss) was used as software for taking images.
Data availability
Raw sequence data generated in this study were submitted to the NCBI Sequence Read Archive (A. nigricans genome sequencing and transcriptome sequencing for annotation, accession numbers SRP082924 and SRP082925) and Gene Expression Omnibus databases (A. nigricans transcriptome data, accession number GSE92315). The A. nigricans whole-genome shotgun project has been deposited at DNA Databank of Japan (DDBJ)/European Molecular Biology Laboratory (EMBL)/GenBank under the accession number SSHT00000000. The version described in this manuscript is version SSHT01000000. Supplemental material available at figshare: https://doi.org/10.25386/genetics.9891440.
Results
Sequencing and assembly of the A. nigricans genome
A. nigricans is a homothallic Pezizomycete that produces fruiting bodies within a week under laboratory conditions (Figure 1). However, while P. confluens needs light for fruiting body formation (Claussen 1912; Traeger et al. 2013), A. nigricans can form fruiting bodies independent of light (Figure S1), and under laboratory conditions has a life cycle that is very similar to those of P. confluens and S. macrospora (Figure S2), making it a suitable species to be included in comparative transcriptomics analyses of fruiting body formation.
Figure 1.
Life cycle of A. nigricans under continuous illumination and laboratory conditions. Strain CBS 389.68 was grown on microscopic slides with RFA medium (with 0.8% agar) for 1–6 days in constant light. After 1 day, a mycelium of septated hyphae is formed. After 2 days, apothecia initials can be observed that contain swollen young asci after 3 days (arrows). Immature hyaline spores can be observed within asci after 4 days. Spores become pigmented during maturation after 5 days. Mature spores are released from eight-spore asci after 6 days. Development of mycelium and apothecia is the same in constant darkness (Figure S1). Bar for all images, 20 µm.
The genome of A. nigricans strain CBS 389.68 was sequenced as part of the 1000 Fungal Genomes project (http://1000.fungalgenomes.org) (Grigoriev et al. 2011, 2014). The assembly consists of 176 scaffolds with a total size of 27 Mb and 9622 predicted protein-coding genes (Table 2). BLASTP searches with a eukaryotic core gene set were used to determine completeness of the gene space as described previously (Parra et al. 2009). All of the 248 single-copy core genes were present among the predicted A. nigricans genes, suggesting that the assembly covers the complete gene space. With 27 Mb, the A. nigricans genome is the smallest Pezizomycete genome sequenced to date. However, it contains about the same number of genes with a similar amount of coding sequence as the over seven times larger genome of T. magnatum, the largest Pezizomycete genome currently known (Murat et al. 2018), as well as the genome of P. confluens, the closest sequenced relative of A. nigricans (Table 2). Part of the smaller genome size of A. nigricans can be attributed to much fewer repeat sequences compared to other Pezizomycetes (Table 2). Furthermore, intron sequences also cover less sequence space in the A. nigricans genome than in other Pezizomycetes (Table 2). Overall, the A. nigricans genome is more compact with respect to noncoding features than other Pezizomycetes genomes, but retains the same coding capacity.
To assess the evolution of A. nigricans genes and their homologs across 19 other sequenced fungi, we reconstructed their evolutionary histories using the phylomeDB pipeline (Huerta-Cepas et al. 2011). We reconstructed the evolutionary relationship of the selected species based on concatenating the alignments of 143 genes that were present in a single copy in all the species analyzed and building a supertree combining all individual gene trees from the phylome (see Material and Methods). The resulting phylogeny confirms that P. confluens and A. nigricans are sister species within the Pezizomycetes, with the Tuber species, represented by T. melanosporum, on a separate branch within the Pezizomycetes lineage (Figure 2).
Figure 2.
Species tree of 20 fungal species based on phylome reconstruction. The species tree was built based on 143 single-copy, widespread genes (see Materials and Methods for details). All nodes are maximally supported by 100% bootstrap. The scale bar gives substitutions per site.
An analysis of synteny between the genomes of A. nigricans and other Pezizomycetes showed little conservation in gene order, both at the level of scaffolds as well as for small genomic regions of two or three genes (Figure S3). Interestingly, the number of syntenic gene pairs or triplets that A. nigricans shares with P. confluens is lower than the same numbers for P. confluens and T. melanosporum, even though A. nigricans and P. confluens are more closely related to each other than to T. melanosporum (Figure 2). One possible explanation might be that the reduction of genome size observed in A. nigricans was achieved through extensive genome restructuring involving multiple translocations.
Analysis of the mating type locus of A. nigricans
The genome of the homothallic Pezizomycete P. confluens contains the two MAT genes MAT1-1-1 and MAT1-2-1, which is typical in homothallic ascomycetes (Traeger et al. 2013). In contrast, the A. nigricans genome contains only one MAT gene, namely MAT1-1-1 (Figure 3). TBLASTN searches in the A. nigricans genome also failed to discover a MAT1-2-1 homolog. Interestingly, the A. nigricans MAT1-1-1 gene is located in the vicinity of two genes, APN2 and locus tag 50832, that are linked to MAT1-2-1 in P. confluens (Figure 3). Furthermore, several repeat regions are flanking the MAT gene as well as APN2 in A. nigricans (Figure 3). One hypothesis to explain these findings might be that a common ancestor of A. nigricans and P. confluens carried a MAT locus with both MAT1-1-1 and MAT1-2-1, and that a recombination/duplication event separated the MAT genes in P. confluens, whereas repeat-induced recombination led to the deletion of MAT1-2-1 in A. nigricans.
Figure 3.
Comparison of the mating type loci of A. nigricans and P. confluens. Orthologs of two genes that are linked to MAT1-2-1 in P. confluens (APN2, shown in yellow, and PCON_08388, shown in green) are linked to MAT1-1-1 in A. nigricans. No MAT1-2-1 homolog was detected in A. nigricans. Genes shown in white do not have orthologs within the mating type regions. Repeat regions around the A. nigricans MAT locus are shown in red. The region around the A. nigricans MAT1-1-1 amplified by PCR from several A. nigricans strains is indicated by a horizontal black bar. The predicted genes encoding helicase domain proteins adjacent to MAT1-1-1 were manually annotated on scaffold 13 with the coordinates [join (17159..17356,17407..17700)] and [join (19850..21059,21107..21250,21298..22613)].
To verify that the region occupied by MAT1-1-1 in strain CBS 389.68 is the same in other A. nigricans strains, the region between the genes flanking MAT1-1-1 was amplified by PCR from four A. nigricans wild-type strains (including CBS 389.68; Figure 3 and Table 1) and sequenced by Sanger sequencing. All four strains carry the MAT1-1-1 gene in this genomic location, therefore this MAT configuration is present in all analyzed A. nigricans strains so far.
Genes for secondary metabolism in A. nigricans
Most genomes of higher filamentous ascomycetes carry multiple genes for the biosynthesis of polyketides and nonribosomal peptides, two major classes of secondary metabolites in fungi (Kroken et al. 2003; Bushley and Turgeon 2010; Teichert and Nowrousian 2011; Brakhage 2013; Keller 2019). However, previous analyses of the P. confluens genome revealed only seven nonribosomal peptide synthase (NRPS) genes and one polyketide synthase (PKS) gene in this species, much fewer than in the genomes of higher filamentous ascomycetes (Traeger et al. 2013). An analysis of the predicted A. nigricans proteins revealed five putative NRPS, but no PKS (Table S3). Thus, A. nigricans lacks even a homolog for the single type I PKS gene present in the P. confluens genome, and the single type III PKS gene present in the genomes of higher filamentous ascomycetes is missing in both P. confluens and A. nigricans.
One of the five NRPS genes in the A. nigricans genome encodes a siderophore NRPS also found in other fungal genomes (Table S3). There are three putative alpha-aminoadipate reductase (AAR) NRPSs, which are typical fungal NRPSs involved in amino acid biosynthesis. Most fungi have only one AAR gene; an exception is P. confluens, with five genes (Bushley and Turgeon 2010; Traeger et al. 2013). Thus, the three AAR gene homologs in A. nigricans suggest that this gene family expansion might be present throughout the P. confluens/A. nigricans lineage of Pezizomycetes. The fifth NRPS gene in A. nigricans, proteinId396591, encodes a putative NRPS of unknown function (Table S3). There is one NRPS of unknown function encoded in P. confluens, too, but its domain architecture is different from proteinId396591, therefore these genes might not be orthologs.
Comparative transcriptomics of fruiting body development in A. nigricans, P. confluens, and S. macrospora
To analyze global changes in gene expression during sexual development in A. nigricans, we sequenced transcriptomes from three developmental stages by RNA-seq (Table 3 and Table S4). To obtain total vegetative mycelia, A. nigricans was grown in submerged cultures, which prevents the formation of sexual structures. For total sexual mycelia, A. nigricans was grown as surface cultures, and the developing fruiting bodies as well as the surrounding nonsexual mycelium was harvested for RNA extraction. To obtain RNA solely from developing fruiting bodies, we used laser microdissection to isolate young fruiting bodies from the surrounding mycelium as described previously (Teichert et al. 2012). RNA-seq data from similar developmental stages are available for P. confluens and S. macrospora (Teichert et al. 2012; Traeger et al. 2013; Murat et al. 2018) (Table 3), and we used these for comparative transcriptomics analyses with A. nigricans.
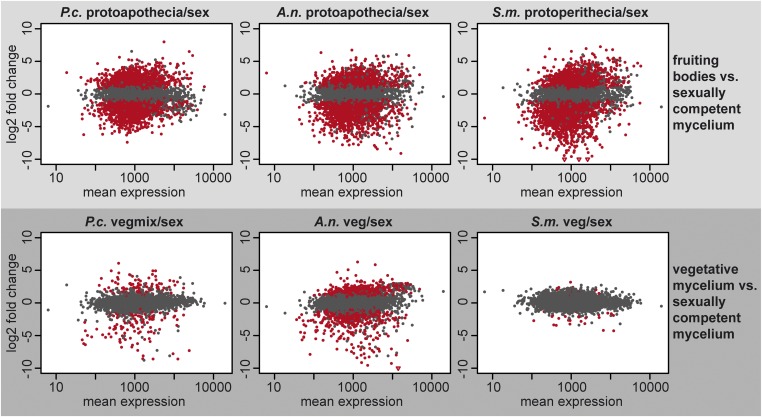
To address the question if orthologous genes in A. nigricans and P. confluens (Pezizomycetes) as well as in S. macrospora (Sordariomycetes) show similar expression patterns during fruiting body formation, we analyzed gene expression for the 4791 genes for which putative orthologs were found in all three species (Table S5). In a previous study of S. macrospora, expression patterns in developing fruiting bodies differed much more from total vegetative and total sexual mycelia than the total mycelial samples differed from each other (Teichert et al. 2012). This trend is confirmed when analyzing data from orthologs in the three species (Figure 4). In all cases, the number of differentially expressed genes is much higher when comparing fruiting body samples vs. sexually competent mycelium than in a comparison of vegetative vs. sexually competent mycelium. These data suggest that the expression patterns in sexual mycelia are dominated by the nonsexual hyphae that make up the bulk of the mycelium rather than by the developing fruiting bodies. The results also indicate that fruiting bodies of filamentous ascomycetes significantly restructure their transcriptome in the transition from vegetative hyphal growth to the development of fruiting bodies.
Figure 4.
Comparative analysis of gene expression during development in A. nigricans (A.n.), P. confluens (P.c.), and S. macrospora (S.m.). The graphs show log2 fold change values vs. mean expression for all genes with orthologs in all three species. In each graph, expression during fruiting body formation (protoapothecia or protoperithecia) or expression during vegetative growth (veg or vegmix) is compared to expression in total sexual mycelium from the respective species. The analysis was done with DESeq2, genes in red are genes that are differentially expressed with an adjusted P-value <0.1.
Clustering of correlation coefficients based on gene expression ratios for comparisons of fruiting bodies or vegetative mycelium vs. sexual mycelium showed that comparisons involving fruiting bodies for all three species group together and are separated from the comparisons of vegetative vs. sexual mycelia (Figure S4). This confirms the trend described above, namely that fruiting bodies have distinct transcriptomes compared to nonsexual hyphae (Figure 4). Furthermore, it suggests that there might be conserved gene expression patterns during sexual development in filamentous ascomycetes.
Next, we identified genes that are differentially regulated in developing fruiting bodies in all three species. There are 83 genes that are upregulated, and 114 genes that are downregulated in developing fruiting bodies of all three species, but not differentially regulated in other comparisons (Figure 5 and Table S6). Among the downregulated genes are 16 genes with predicted functions in protein synthesis or turnover, and another 16 genes with predicted roles in protein phosphorylation/dephosphorylation or signal transduction (Table S6). It is possible that downregulation of such genes is an essential step during fruiting body formation, and in-depth analyses of these genes might be of interest for future studies. However, in this study we focused on the genes that are upregulated specifically in developing fruiting bodies in all three species, as these might have conserved roles in sexual development in filamentous ascomycetes. An analysis of putative functions based on conserved domains among the upregulated genes showed that there are 23 genes encoding proteins with predicted roles in vesicle transport, the endomembrane system, or transport across membranes. This group of genes might be of interest for future functional analyses.
Figure 5.
Expression ratios of orthologs that are up- or downregulated in young fruiting bodies of A. nigricans (A.n.), P. confluens (P.c.), and S. macrospora (S.m.), but not differentially regulated in other conditions. The heatmaps were generated based on hierarchical clustering of log2 fold changes. The heatmap on the left shows genes that are up- or downregulated in young fruiting bodies, the heatmap on the right shows only genes that are upregulated in young fruiting bodies. The corresponding S. macrospora locus tags for selected genes are indicated on the right. Locus tags shown in gray correspond to genes that are predicted to be involved in vesicle transport, the endomembrane system, or transport across membranes. Locus tags shown in black correspond to genes predicted to be involved in chromatin organization or regulation of gene expression.
Among the upregulated genes during fruiting body formation in A. nigricans, P. confluens, and S. macrospora are also 13 genes encoding proteins with predicted roles in chromatin organization or the regulation of gene expression (Figure 5 and Table S6). As the transition from vegetative growth to fruiting body development requires a drastic restructuring of the transcriptome, transcription factors and chromatin modifiers are expected to play pivotal roles in this transition. A number of specific transcription factors have already been shown to be involved in sexual development in filamentous ascomycetes, whereas the role of chromatin modifiers in this process is less well understood (Nowrousian 2018; Pöggeler et al. 2018). To learn more about the roles of genes with evolutionary conserved expression patterns, we chose four of these genes for functional analysis through gene deletion.
Functional analysis of genes with evolutionary conserved expression patterns during development
Functional analysis of four genes with conserved expression patterns was carried out in S. macrospora, because for this filamentous ascomycete molecular techniques like transformation and gene deletion systems are available (Engh et al. 2010; Teichert et al. 2014). The candidates were chosen from the genes with conserved upregulation during sexual development based on their predicted functions in other species or presence of conserved domains. Among the four genes that were chosen for deletion in S. macrospora, one (SMAC_06770) has a predicted function within the endomembrane system, whereas the other three (SMAC_01829, SMAC_04946, and SMAC_06113) are predicted to be involved in regulating transcription or chromatin organization.
SMAC_06770 encodes a homolog to the S. cerevisiae ALG11 gene, which encodes glycolipid 2-alpha-mannosyltransferase, an enzyme involved in protein glycosylation in the endoplasmic reticulum through formation of glycosylation intermediates on the cytosolic side of the endoplasmic reticulum (Cipollo et al. 2001). Deletion of ALG11 in S. cerevisiae leads to poor growth at 25°, and a temperature-sensitive lethality at 37° (Cipollo et al. 2001). Deletion of SMAC_06770 in S. macrospora resulted in transformants that grew very poorly and were unable to form fruiting bodies (Figure S5). Thus, the gene appears to be involved in basic cellular processes besides sexual development in S. macrospora, similar to S. cerevisiae.
SMAC_06113 is orthologous to the N. crassa aod-5 gene, which regulates transcription of the gene encoding alternative oxidase (Chae et al. 2007; Chae and Nargang 2009). AOD-5 consists of two domains, a GAL-4 like domain at the N-terminus and a central PAS domain that might be involved in protein-protein interactions. Deletion of SMAC_06113 in S. macrospora did not result in any defects in sexual developmental under laboratory conditions. The strain was fully fertile, similar to the wild type (Figures S6 and S7). Because of the homology of SMAC_06113 to N. crassa aod-5, we tested growth of the deletion strain and complemented transformants on antimycin A. This drug inhibits the electron transport through complex III in mitochondria and, consequently, alternative oxidase expression is induced for respiration (Descheneau et al. 2005). Similar to N. crassa aod mutants, the S. macrospora SMAC_06113 deletion strain was not able to grow in presents of antimycin A, in contrast to the wild type and a complemented strain (Figure S7). Therefore, SMAC_06113 was named aod5 (alternative oxidase 5). Fluorescence microscopy with strains expressing an aod5-egfp fusion showed that aod5 localizes to the nucleus, as expected for a transcription factor (Figure S7).
Earlier studies with chromatin modifiers asf1, cac2, crc1, and rtt106 revealed only asf1 as essential for sexual reproduction in S. macrospora, whereas cac2 and rtt106 might have redundant function under nutrient deprivation (Gesing et al. 2012; Schumacher et al. 2018). Here, we chose another putative chromatin modifier encoded by SMAC_04946 for functional analysis. SMAC_04946 encodes a protein with a conserved SAS4 domain. In S. cerevisiae, Sas4 is described as part of the SAS complex (something about silencing) together with Sas2 and Sas5 (Sutton et al. 2003), and was found to interact with Asf1p (Osada et al. 2001). However, DELTA-BLASTp searches did not reveal clear homologs for Sas2 and Sas5 in S. macrospora, and the SAS4 domain is the only part of the SMAC_04946 protein that is conserved in S. macrospora compared to yeast. Hence, we named the gene scm1 (sas4-domain chromatin modifier) and analyzed if deletion of scm1 results in any phenotype (Figure S8). Similar to the deletion of several other chromatin modifiers (cac2, crc1, and rtt106) (Gesing et al. 2012; Schumacher et al. 2018), the ∆scm1 mutant was fertile after 7 days on BMM and SWG (Figure 6). To address whether there might be redundancy of SCM1 and other chromatin modifiers, we generated double-deletion strains by genetic crossing (Figure S9). However, none of the double mutants of scm1 with cac2, crc1, or rtt106 had a developmental phenotype (Figure 6). This is similar to double mutants involving cac2, crc1, or rtt106, which were generated previously, and all of which are fertile on BMM medium (Schumacher et al. 2018) (Figure S10). Therefore, we performed crosses to obtain triple- and quadruple-deletion strains (Figure S9). All possible triple-mutant combinations of scm1, cac2, crc1, and rtt106 showed at least reduced fertility up to sterility (Figure 6). While the ∆crc1/∆rtt106/∆scm1 mutant formed perithecia and even discharged some spores, all triple mutants with ∆cac2 background are sterile. The triple mutants ∆scm1/∆cac2/∆rtt106 and ∆cac2/∆crc1/∆rtt106 formed few immature fruiting bodies without a perithecial neck, sometimes with a few immature spores inside (Figure 6). However, the spores were not discharged even after 21 days on BMM. ∆scm1∆cac2∆crc1 formed only protoperithecia. The quadruple mutant showed a phenotype comparable to so-called pro mutants (Teichert et al. 2014), forming only small protoperithecia (Figure 6).
Figure 6.
Phenotypes of single, double, triple, and quadruple chromatin-modifier mutants of S. macrospora. The strains were grown for 7 days on BMM. Gene deletion of scm1 results in a fully fertile strain, which only sometimes forms perithecia lying on the side. Double-deletion strains of scm1 with cac2, crc2, or rtt106 are also fully fertile after 7 days (the Δscm1/Δcrc1/fus mutant produces brown ascospores due to the presence of the spore color mutation fus). Triple and quadruple chromatin-modifier deletion strains showed reduced fertility up to sterility. While Δscm1/Δcrc1/Δrtt106 was able to form perithecia and discharge spores, all three triple mutants containing Δcac2 were sterile. Although sometimes forming immature fruiting bodies with few spores inside, Δscm1/Δcac2/Δrtt106 and Δcac2/Δcrc1/Δrtt106 never discharged spores (strains were observed for 21 days). Δscm1/Δcac2/Δcrc1 forms few enlarged protoperithecia, but no spores. The quadruple mutant showed a phenotype comparable to so-called pro mutants forming only protoperithecia, and therefore is sterile. Scale bars for top and side view, 500 µm; scale bars for ascus rosettes and spores, 100 µm.
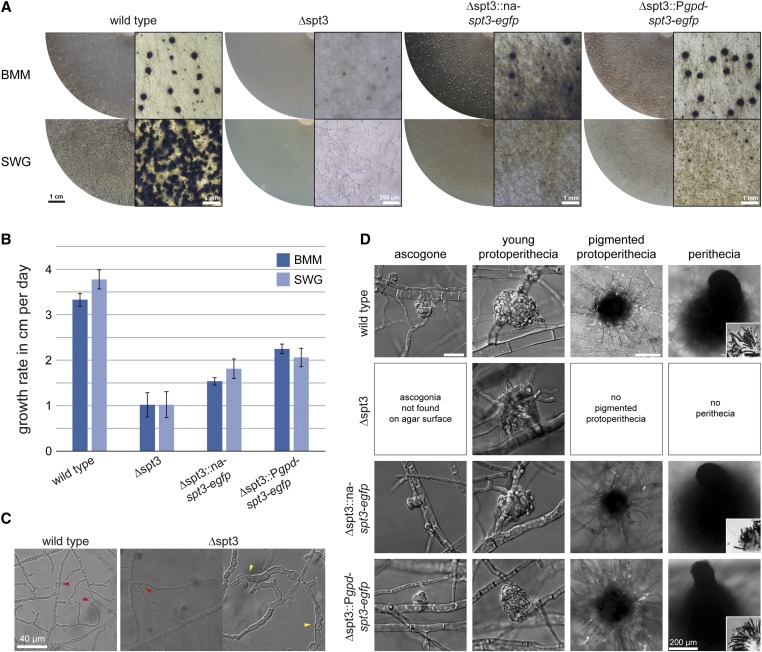
The fourth gene we chose for further analysis was SMAC_01829 encoding a homolog to the SPT3 subunit of the SAGA complex, a conserved eukaryotic transcriptional coactivator complex (Spedale et al. 2012; Helmlinger and Tora 2017). The SAGA complex is well characterized in yeast, and for filamentous fungi a deletion strain of spt3 was analyzed in F. graminearum (Timmers and Tora 2005; Gao et al. 2014). In S. macrospora, deletion of spt3 results in a most conspicuous phenotype (Figure 7 and Figure S11). ∆spt3 strains grow significantly slower than the wild type on both full medium (BMM) and minimal medium (SWG) (Figure 7B). The ∆spt3 mutant is still able to undergo hyphal fusion (Figure 7C); however, hyphal morphology is different from the wild type in older hyphae, with intrahyphal growth occurring in swollen hyphae (Figure 7C). Besides the vegetative phenotype, deletion of spt3 leads to sterility with only few nonpigmented, often submerged protoperithecia (Figure 7, A and D). Hyphae that make up the protoperithecia are less densely packed than in the wild type. The formation of fruiting bodies and ascospores was restored in complemented transformants on BMM with spt3 under native and constitutive promoter within 10 days (Figure 7, A and D). However, only complemented strains with spt3 under a constitutive promotor were able to discharge spores. On SWG medium, complementation did not result in fertile strains even after 14 days, but resulted in formation of more pigmented protoperithecia and few perithecia (Figure 7A). The growth rate was also only partially restored in complemented transformants (Figure 7C). The transformants carry ectopically integrated complementation plasmids, and it is possible that the native chromatin environment is required for a fully functional spt3.
Figure 7.
Phenotypic characterization of S. macrospora ∆spt3 and complemented strains. (A) Overview of strains grown on BMM and SWG for 7 and 14 days (details on the right for each strain). ∆spt3 is sterile on both media and forms only few nonpigmented protoperithecia. Complemented strains under native promoter (∆spt3::na-spt3-egfp) and constitutive promoter (∆spt3::Pgpd-spt3-egfp) form perithecia on BMM, but need longer (10 days compared to 7 days in the wild type) to become fertile and discharge spores. On minimal medium (SWG), complemented strains did not form mature perithecia even after 14 days. (B) The growth rate of ∆spt3 is significantly reduced on BMM and SWG compared to the wild type. Complemented strains grow faster than the mutant strain, but not as fast as the wild type. (C) Hyphal fusion and hyphal morphology of ∆spt3. The mutant strain is able to form hyphal anastomoses (red arrowheads). In older mycelium, ∆spt3 forms enlarged hyphae, which start to grow into dead hyphae (intrahyphal growth, yellow arrowheads). (D) Detail of fruiting body development on BMM. Protoperithecia of ∆spt3 are nonpigmented and less compact than wild-type protoperithecia. Ascogonia were not found on the agar surface, where they are formed in the wild type, because protoperithecia in the mutant were mostly formed below the agar surface. Consequently, ascogonia are present within the agar, but difficult to detect there due to their small size and lack of pigmentation. The deletion strain never formed pigmented protoperithecia or perithecia. The complemented strains formed perithecia after 8–10 days. Only the complemented strain with spt3 expressed from a constitutive promoter discharged spores after 10 days; however, both complemented strains formed spores within the perithecia. Scale bar for ascogonia and young protoperithecia, 20 µm; scale bar for pigmented protoperithecia and perithecia, 100 µm unless indicated otherwise; scale bar for ascus rosettes, 40 µm.
Discussion
The A. nigricans genome is small and gene-dense
Fruiting body morphogenesis in ascomycetes is a complex process that requires the concerted action of a large number of genes. Molecular studies with several model organisms have led to the identification of many such developmental genes, but the degree to which fruiting body development is conserved at the morphological and molecular level is not yet clear (Pöggeler et al. 2018). One way to address this question is by comparative transcriptomics to test if gene expression patterns are conserved across species. In this study, we sequenced the genome of the Pezizomycete A. nigricans, and generated several transcriptomes that were used in comparative transcriptomics analyses with two other ascomycetes.
Compared to previously sequenced Pezizomycete genomes, the A. nigricans genome is rather small. With 27 Mb, it is only about half the size of the M. importuna genome, the smallest of the previously sequenced Pezizomycete genomes (Murat et al. 2018). However, it has retained a coding capacity similar to other, much larger Pezizomycete genomes. The size differences are caused mainly by a higher amount of repeats in other Pezizomycetes, but noncoding regions like introns also make up a smaller part of the genome in A. nigricans. The differences in genome size could be explained by the expansion of repeats and noncoding regions including introns in the other Pezizomycetes, or by genome reduction processes specific to A. nigricans, or both. The finding that microsynteny is higher between P. confluens and T. melanosporum than between P. confluens and the more closely related A. nigricans might support a hypothesis of genome size reduction involving major restructuring in A. nigricans.
One group of genes usually present in the genomes of filamentous ascomycetes, but absent in A. nigricans, are PKS genes. This is unusual even for Pezizomycetes, which have fewer secondary metabolism genes than other Pezizomycotina, with T. melanosporum harboring two, and P. confluens containing only one PKS gene (Martin et al. 2010a; Teichert and Nowrousian 2011; Traeger et al. 2013). Given their phylogenetic relationships, the most parsimonious explanation would be the presence of (at least) one PKS gene in the common ancestor of Pezizomycetes, which was lost in the lineage leading to A. nigricans. The NRPS gene content of A. nigricans is more typical of filamentous ascomycetes, even though the number of NRPS genes is small, similar to other Pezizomycetes.
The mating type locus of the homothallic A. nigricans contains a single MAT1-1-1 gene
Another unusual feature of the A. nigricans genome is its mating type region. A. nigricans is homothallic, and most homothallic filamentous ascomycetes harbor a MAT1-1-1 gene and a MAT1-2-1 gene in their genome. However, there is no indication of a MAT1-2-1 gene in the A. nigricans genome, and the MAT1-1-1 region is the same in three additional strains analyzed. Thus, A. nigricans apparently manages sexual reproduction with a single idiomorph carrying a single MAT gene. While unusual, there are other cases of such unisexual mating in filamentous ascomycetes, where all nuclei carry the same single MAT idiomorph (Bennett and Turgeon 2016). One example is Neurospora africana, a homothallic species that carries a MAT1-1 idiomorph, but no MAT1-2-related gene, and similar findings were made for several other homothallic Neurospora species, and possibly for homothallic species of the Dothideomycete genus Stemphylium (Glass et al. 1988, 1990; Inderbitzin et al. 2005; Wik et al. 2008; Gioti et al. 2012). In the homothallic Sordariomycete Huntiella moniliformis, unisexual reproduction takes place with just a MAT1-2 idiomorph (Wilson et al. 2015). In the homothallic S. macrospora, MAT1-1-1 is present, but dispensable for sexual development, whereas MAT1-2-1 is required together with MAT1-1-2 (Pöggeler et al. 2006b; Klix et al. 2010). Unisexual mating can also occur in heterothallic species, if one or both mating types are capable of sexual reproduction on their own. This was demonstrated, for example, for the MAT A mating type of the Sordariomycete Sordaria brevicollis, for MATa cells of the ascomycete yeast Candida albicans, and for MATα cells of the basidiomycete Cryptococcus neoformans (Robertson et al. 1998; Lin et al. 2005; Alby et al. 2009). Thus, it might be possible that A. nigricans is heterothallic with the ability of (at least) one mating type to undergo unisexual mating because currently, only four strains have been analyzed for their mating types, making it possible that additional mating types exist in the population. Another hypothesis to explain the single-gene mating type locus of A. nigricans might be that the species is indeed homothallic, and that the loss of the MAT1-2-1 gene might be related to a reduction in morphological complexity of the fruiting body. It has been hypothesized previously that the morphologically simple fruiting bodies of the Pyronema and Ascodesmis lineages are reduced forms that evolved independently from more complex apothecia in other Pezizomycete lineages (Hansen and Pfister 2006). Since mating type genes can have functions other than the actual mating (Böhm et al. 2013; Bennett and Turgeon 2016), it is possible that a less complex fruiting body morphology can be sustained with a reduced complement of mating type genes.
Comparative transcriptomics of fruiting body development in three ascomycetes reveals conserved patterns of gene expression
Comparative transcriptomics can be used to identify conserved patterns of gene expression in different species, or conversely, to identify species-specific expression patterns that might help to explain, for example, morphological differences between species (Stuart et al. 2003; Brawand et al. 2011; Romero et al. 2012). In fungi, the latter approach was applied in comparative transcriptomics studies of Sordariomycete species from the Fusarium and Neurospora lineages. While expression patterns for many groups of genes or functional categories were similar, distinct differences in gene expression could be used to identify genes involved in species-specific morphological transitions (Sikhakolli et al. 2012; Lehr et al. 2014; Trail et al. 2017). In basidiomycete mushrooms, several comparative transcriptomics studies revealed a certain degree of conservation of gene expression during mushroom formation in several Agaricomycetes, including genes for cell wall remodeling, adhesion, signal transduction, transcription factors, and protein degradation (Ohm et al. 2010; Morin et al. 2012; Plaza et al. 2014; Almási et al. 2019; Krizsán et al. 2019). To address the question if conserved patterns of gene expression can be found during fruiting body development in distantly related filamentous ascomycetes, we compared transcriptomes from mycelia and young fruiting bodies from A. nigricans, P. confluens, and S. macrospora. The three species represent different Pezizomycotina lineages, but have similar lifestyles in that they are homothallic and do not produce any asexual spores, facilitating sexual development–specific transcriptome analyses. Our results indicate that transcriptomes of developing fruiting bodies are distinct from mycelial samples in all three species, and furthermore, are more similar between species than fruiting body transcriptomes are compared to mycelial samples from the same species. This confirms preliminary results based on comparisons of mycelia of P. confluens with fruiting bodies and mycelia of S. macrospora (Traeger et al. 2013). Similar tissue- or development-specific conserved expression patterns of protein-coding genes have been noted previously in animals (Necsulea and Kaessmann 2014; Levin et al. 2016; Marlétaz et al. 2018).
To identify genes that might play a role in fruiting body development, we identified genes that were upregulated during fruiting body development in all three analyzed species, but which were not differentially regulated in other analyzed conditions. Among the 83 identified genes, 23 encode proteins with predicted roles in vesicle transport, the endomembrane system, or transport across membranes. Genes that encode proteins involved in cellular transport were also enriched among genes that are expressed during fruiting body development of three Neurospora species (Lehr et al. 2014). Interestingly, a recent study on Neolecta irregularis, a member of the early-diverging ascomycete group of Taphrinomycetes, showed that genes involved in the functions of diverse endomembrane systems are conserved in N. irregularis and the Pezizomycotina (filamentous ascomycetes), all of which form fruiting bodies, but not in ascomycete yeasts that do not form fruiting bodies (Nguyen et al. 2017). The fruiting bodies of Neolecta and the Pezizomycotina most likely evolved independently, but based on a common set of genes in the last common ancestor of ascomycetes. It is possible that the evolution of complex multicellular structures with similar functions selected for similar cellular machineries (Nguyen et al. 2017). One reason might be that fruiting body formation requires a metabolically “competent” mycelium that transfers nutrients to the developing fruiting body (Wessels 1993; Pöggeler et al. 2006a). Such a transfer might need a specialized complement of genes managing the transport of large amounts of nutrients. Another, not mutually exclusive, explanation could be the requirement for building cells with specialized cell wall structures, e.g., asci, ascospores, or the nonsexual cells of the fruiting body. Again, specialized groups of genes involved in transport processes might be required for these purposes.
In addition to genes involved in transport processes, 13 genes with predicted roles in chromatin organization or the regulation of gene expression are among the genes upregulated during fruiting body development in the three species. The differentiation of fruiting bodies entails a drastic restructuring of the transcriptome, as evidenced by the greatly different transcriptome profiles of fruiting bodies and nonsexual mycelia. Thus, it is likely that the combined actions of chromatin modifiers and specific transcription factors prepare the cells for the transition to sexual development (Pöggeler et al. 2018). Enrichment of genes involved in transcription was also found among genes preferentially expressed during fruiting body morphogenesis of three Neurospora species, and in a comparative transcriptomics analysis of S. macrospora and F. graminearum (Gesing et al. 2012; Lehr et al. 2014). Genes involved in transcription might in turn regulate the expression of genes important for cell differentiation, e.g., genes for managing endomembrane systems as described above. In S. macrospora, the transcription factor gene pro44 was found to be upregulated during fruiting body development (Teichert et al. 2012). Subsequent transcriptome analysis of a pro44 deletion mutant showed that genes involved in cellular transport were downregulated in developing fruiting bodies of the mutant strain (Schumacher et al. 2018).
Functional characterization of genes with conserved expression patterns reveals roles in fruiting body development
Our functional characterization of four genes with evolutionary conserved transcriptional upregulation during fruiting body formation showed that three of them indeed play a role during sexual development. The exception is aod5, the homolog of N. crassa aod-5, which encodes a transcription factor involved in regulating the expression of the alternative oxidase gene aod-1 (Chae et al. 2007; Chae and Nargang 2009). The corresponding S. macrospora aod5 mutant shows a growth defect on antimycin A, similar to N. crassa, but no defects in sexual development. In N. crassa, AOD-5 interacts with another transcription factor, AOD-2, to activate aod-1 transcription (Chae et al. 2007; Chae and Nargang 2009), and one might speculate that an aod-2 homolog in S. macrospora might carry out some functions of aod5 during development. However, the aod-2 ortholog of S. macrospora, SMAC_04081, is not transcriptionally upregulated during development, in contrast to aod5.
For the other genes that were functionally characterized, involvement in fruiting body differentiation could be confirmed. However, for one of the genes, the putative glycolipid 2-alpha-mannosyltransferase SMAC_06770, sterility of the deletion mutant accompanies a severe growth defect. A growth phenotype was also reported for the corresponding S. cerevisiae mutant (Cipollo et al. 2001). The sterility of the S. macrospora deletion strains might therefore not be a specific effect related to development but caused by the overall growth defect.
Deletion of the putative chromatin modifier gene scm1 did not result in a developmental phenotype in a single mutant or double mutants with chromatin modifier genes cac2, crc1, and rtt106. However, analysis of all possible triple mutants as well as the quadruple mutant revealed developmental defects ranging from impaired spore formation and discharge to complete lack of perithecia and spore production. While cac2 and rtt106 are homologs to histone H3/H4 chaperones of other eukaryotes, and crc1 is predicted to encode a subunit of the chromatin remodeling complexes RSC or SWI/SNR (Wilson et al. 2006; Avvakumov et al. 2011; Schumacher et al. 2018), the molecular role of scm1 is not yet clear. However, it is unlikely that the four chromatin modifiers act in the same protein complexes or regulatory pathways, therefore the lack of developmental phenotypes in the double mutants might indicate that there is a certain redundancy in the molecular mechanisms priming chromatin for its cellular functions. Another, not mutually exclusive, explanation might be that the chromatin structure needs to be drastically reshaped for successful fruiting body development, and that this restructuring needs most, but not all chromatin-modifying activities to be available. Future experiments using techniques like Hi-C to analyze three-dimensional chromatin organization during development will help to address these hypotheses (Mota-Gómez and Lupiáñez 2019).
The deletion mutant of the gene for the predicted SAGA complex subunit SPT3 is sterile in addition to a mycelial growth defect, and these phenotypes are similar to the spt3 mutant of F. graminearum (Gao et al. 2014). The SAGA complex is a multisubunit transcriptional coactivator that performs multiple functions, e.g., histone modification and interaction with transcriptional activators (Spedale et al. 2012; Helmlinger and Tora 2017). These activities are carried out by distinct modules within the complex, and SPT3 is part of the TBP (TATA-binding protein) binding module (Helmlinger and Tora 2017). The modularity of the complex allows sharing of the modules between SAGA and other complexes (Helmlinger and Tora 2017), and additional studies will be required to address the role of transcriptional coactivator complexes and other chromatin modifiers during sexual development in fungi.
Acknowledgments
The authors would like to thank Swenja Henne, Silke Nimtz, and Susanne Schlewinski for excellent technical assistance; Ines Teichert for sharing plasmids; Ulrich Kück and Christopher Grefen for support at the Department of General and Molecular Botany/Molecular and Cellular Botany at the Ruhr-University Bochum; Francis Martin for sharing data on T. melanosporum before publication; and Joseph Spatafora and the 1000 Fungal Genomes Project for making the sequencing of the A. nigricans genome possible. This work was funded by the German Research Foundation (grant NO407/7-1 to M.N.). The work conducted by the US Department of Energy (DOE) Joint Genome Institute, a DOE Office of Science User Facility, is supported by the Office of Science of the US DOE under contract no. DE-AC02-05CH11231.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.9891440.
Communicating editor: J. Stajich
Literature Cited
- Alby K., Schaefer D., and Bennett R. J., 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460: 890–893. 10.1038/nature08252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almási É., Sahu N., Krizsán K., Bálint B., Kovács G. M. et al. , 2019. Comparative genomics reveals unique wood-decay strategies and fruiting body development in the Schizophyllaceae. New Phytol. 224: 902–915. 10.1111/nph.16032 [DOI] [PubMed] [Google Scholar]
- Al-Shahrour F., Minguez P., Tárraga J., Medina I., Alloza E. et al. , 2007. FatiGO +: a functional profiling tool for genomic data. Integration of functional annotation, regulatory motifs and interaction data with microarray experiments. Nucleic Acids Res. 35: W91–W96. 10.1093/nar/gkm260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Madden T. L., Schaffer A. A., Zhang J., Zhang Z. et al. , 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amselem J., Cuomo C. A., Van Kan J. L., Viaud M., Benito E. P. et al. , 2011. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 7: e1002230 10.1371/journal.pgen.1002230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvakumov N., Nourani A., and Côté J., 2011. Histone chaperones: modulators of chromatin marks. Mol. Cell 41: 502–514. 10.1016/j.molcel.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Bennett R. J., and Turgeon B. G., 2016. Fungal sex: the ascomycota. Microbiol. Spectr. 4: FUNK-0005-2016. 10.1128/microbiolspec.FUNK-0005-2016 [DOI] [PubMed] [Google Scholar]
- Billiard S., López-Villavicencio M., Devier B., Hood M. E., Fairhead C. et al. , 2011. Having sex, yes, but with whom? Inferences from fungi on the evolution of anisogamy and mating types. Biol. Rev. Camb. Philos. Soc. 86: 421–442. 10.1111/j.1469-185X.2010.00153.x [DOI] [PubMed] [Google Scholar]
- Bistis G. N., Perkins D. D., and Read N. D., 2003. Different cell types in Neurospora crassa. Fungal Genet. Newsl. 50: 17–19. [Google Scholar]
- Böhm J., Hoff B., O’gorman C. M., Wolfers S., Klix V. et al. , 2013. Sexual reproduction and mating-type mediated strain development in the penicillin-producing fungus Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA 110: 1476–1481. 10.1073/pnas.1217943110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakhage A. A., 2013. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 11: 21–32. 10.1038/nrmicro2916 [DOI] [PubMed] [Google Scholar]
- Brawand D., Soumillon M., Necsulea A., Julien P., Csardi G. et al. , 2011. The evolution of gene expression levels in mammalian organs. Nature 478: 343–348. 10.1038/nature10532 [DOI] [PubMed] [Google Scholar]
- Bushley K. E., and Turgeon B. G., 2010. Phylogenomics reveals subfamilies of fungal nonribosomal peptide synthetases and their evolutionary relationships. BMC Evol. Biol. 10: 26 10.1186/1471-2148-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutierrez S., Silla-Martinez J. M., and Gabaldón T., 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinf. 25: 1972–1973. 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae M. S., and Nargang F. E., 2009. Investigation of regulatory factors required for alternative oxidase production in Neurospora crassa. Physiol. Plant. 137: 407–418. 10.1111/j.1399-3054.2009.01239.x [DOI] [PubMed] [Google Scholar]
- Chae M. S., Nargang C. E., Cleary I. A., Lin C. C., Todd A. T. et al. , 2007. Two zinc-cluster transcription factors control induction of alternative oxidase in Neurospora crassa. Genetics 177: 1997–2006. 10.1534/genetics.107.078212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollo J. F., Trimble R. B., Chi J. H., Yan Q., and Dean N., 2001. The yeast ALG11 gene specifies addition of the terminal alpha 1,2-Man to the Man5GlcNAc2-PP-dolichol N-glycosylation intermediate formed on the cytosolic side of the endoplasmic reticulum. J. Biol. Chem. 276: 21828–21840. 10.1074/jbc.M010896200 [DOI] [PubMed] [Google Scholar]
- Cissé O. H., Almeida J. M., Fonseca A., Kumar A. A., Salojärvi J. et al. , 2013. Genome sequencing of the plant pathogen Taphrina deformans, the causal agent of peach leaf curl. MBio 4: e00055-13 10.1128/mBio.00055-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen P., 1912. Zur Entwicklungsgeschichte der Ascomyceten: Pyromena confluens. Zeitschrift für Botanik. 4: 1–63. [Google Scholar]
- Colot H. V., Park G., Turner G. E., Ringelberg C., Crew C. M. et al. , 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 103: 10352–10357 (erratum: Proc. Natl. Acad. Sci. USA 103:16614). 10.1073/pnas.0601456103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo C. A., Güldener U., Xu J. R., Trail F., Turgeon B. G. et al. , 2007. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317: 1400–1402. 10.1126/science.1143708 [DOI] [PubMed] [Google Scholar]
- Debuchy R., Berteaux-Leceleir V., and Silar P., 2010. Mating systems and sexual morphogenesis in ascomycetes, pp. 501–535 in Cellular and Molecular Biology of Filamentous Fungi, edited by Borkovich K. A. and Ebbole D. J.. ASM Press, Washington, DC: 10.1128/9781555816636.ch33 [DOI] [Google Scholar]
- Descheneau A. T., Cleary I. A., and Nargang F. E., 2005. Genetic evidence for a regulatory pathway controlling alternative oxidase production in Neurospora crassa. Genetics 169: 123–135. 10.1534/genetics.104.034017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirschnabel D. E., Nowrousian M., Cano-Domínguez N., Aguirre J., Teichert I. et al. , 2014. New insights into the roles of NADPH oxidases in sexual development and ascospore germination in Sordaria macrospora. Genetics 196: 729–744. 10.1534/genetics.113.159368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B., Sherman D., Fischer G., Durrens P., Casaregola S. et al. , 2004. Genome evolution in yeasts. Nature 430: 35–44. 10.1038/nature02579 [DOI] [PubMed] [Google Scholar]
- Edgar R. C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh I., Würtz C., Witzel-Schlömp K., Zhang H. Y., Hoff B. et al. , 2007. The WW domain protein PRO40 is required for fungal fertility and associates with Woronin bodies. Eukaryot. Cell 6: 831–843. 10.1128/EC.00269-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh I., Nowrousian M., and Kück U., 2010. Sordaria macrospora, a model organism to study fungal cellular development. Eur. J. Cell Biol. 89: 864–872. [DOI] [PubMed] [Google Scholar]
- Esser K., 1982. Cryptogams - Cyanobacteria, Algae, Fungi, Lichens. Cambridge University Press, London. [Google Scholar]
- Fedorova N. D., Khaldi N., Joardar V. S., Maiti R., Amedeo P. et al. , 2008. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 4: e1000046 10.1371/journal.pgen.1000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan J. E., Calvo S. E., Borkovich K. A., Selker E. U., Read N. D. et al. , 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422: 859–868. 10.1038/nature01554 [DOI] [PubMed] [Google Scholar]
- Galagan J. E., Calvo S. E., Cuomo C., Ma L.-J., Wortman J. R. et al. , 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438: 1105–1115. 10.1038/nature04341 [DOI] [PubMed] [Google Scholar]
- Gao T., Zheng Z., Hou Y., and Zhou M., 2014. Transcription factors spt3 and spt8 are associated with conidiation, mycelium growth, and pathogenicity in Fusarium graminearum. FEMS Microbiol. Lett. 351: 42–50. 10.1111/1574-6968.12350 [DOI] [PubMed] [Google Scholar]
- Gesing S., Schindler D., Fränzel B., Wolters D., and Nowrousian M., 2012. The histone chaperone ASF1 is essential for sexual development in the filamentous fungus Sordaria macrospora. Mol. Microbiol. 84: 748–765. 10.1111/j.1365-2958.2012.08058.x [DOI] [PubMed] [Google Scholar]
- Gioti A., Mushegian A. A., Strandberg R., Stajich J. E., and Johannesson H., 2012. Unidirectional evolutionary transitions in fungal mating systems and the role of transposable elements. Mol. Biol. Evol. 29: 3215–3226. 10.1093/molbev/mss132 [DOI] [PubMed] [Google Scholar]
- Glass N. L., Vollmer S. J., Staben C., Grotelueschen J., Metzenberg R. L. et al. , 1988. DNAs of the two mating-type alleles of Neurospora crassa are highly dissimilar. Science 241: 570–573. 10.1126/science.2840740 [DOI] [PubMed] [Google Scholar]
- Glass N. L., Metzenberg R. L., and Raju N. B., 1990. Homothallic Sordariaceae from nature: the absence of strains containing only the a mating type sequence. Exp. Mycol. 14: 274–289. 10.1016/0147-5975(90)90025-O [DOI] [Google Scholar]
- Gnerre S., Maccallum I., Przybylski D., Ribeiro F. J., Burton J. N. et al. , 2011. High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc. Natl. Acad. Sci. USA 108: 1513–1518. 10.1073/pnas.1017351108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A., Barrell B. G., Bussey H., Davis R. W., Dujon B. et al. , 1996. Life with 6000 genes. Science 274: 546–567. 10.1126/science.274.5287.546 [DOI] [PubMed] [Google Scholar]
- Goodwin S. B., M’barek S. B., Dhillon B., Wittenberg A. H., Crane C. F. et al. , 2011. Finished genome of the fungal wheat pathogen Mycosphaerella graminicola reveals dispensome structure, chromosome plasticity, and stealth pathogenesis. PLoS Genet. 7: e1002070 10.1371/journal.pgen.1002070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I. V., Cullen D., Goodwin S. B., Hibbett D., Jeffries T. W. et al. , 2011. Fueling the future with fungal genomics. Mycology 2: 192–209. [Google Scholar]
- Grigoriev I. V., Nikitin R., Haridas S., Kuo A., Ohm R. et al. , 2014. MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res. 42: D699–D704. 10.1093/nar/gkt1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S., Dufayard J. F., Lefort V., Anisimova M., Hordijk W. et al. , 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59: 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- Han K. H., 2009. Molecular genetics of Emericella nidulans sexual development. Mycobiology 37: 171–182. 10.4489/MYCO.2009.37.3.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane J. K., Lowe R. G. T., Solomon P. S., Tan K.-C., Schoch C. L. et al. , 2007. Dothideomycete plant interactions illuminated by genome sequencing and EST analysis of the wheat pathogen Stagonospora nodorum. Plant Cell 19: 3347–3368. 10.1105/tpc.107.052829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., and Pfister D. H., 2006. Systematics of the Pezizomycetes - the operculate discomycetes. Mycologia 98: 1029–1040. [DOI] [PubMed] [Google Scholar]
- Helmlinger D., and Tora L., 2017. Sharing the SAGA. Trends Biochem. Sci. 42: 850–861. 10.1016/j.tibs.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas J., Dopazo J., and Gabaldón T., 2010. ETE: a python environment for tree exploration. Bioinf. 11: 24 10.1186/1471-2105-11-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas J., Capella-Gutierrez S., Pryszcz L. P., Denisov I., Kormes D. et al. , 2011. PhylomeDB v3.0: an expanding repository of genome-wide collections of trees, alignments and phylogeny-based orthology and paralogy predictions. Nucleic Acids Res. 39: D556–D560. 10.1093/nar/gkq1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas J., Capella-Gutiérrez S., Pryszcz L. P., Marcet-Houben M., and Gabaldón T., 2014. PhylomeDB v4: zooming into the plurality of evolutionary histories of a genome. Nucleic Acids Res. 42: D897–D902. 10.1093/nar/gkt1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderbitzin P., Harkness J., Turgeon B. G., and Berbee M. L., 2005. Lateral transfer of mating system in Stemphylium. Proc. Natl. Acad. Sci. USA 102: 11390–11395. 10.1073/pnas.0501918102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J., Kapitonov V. V., Pavlicek A., Klonowski P., Kohany O. et al. , 2005. Repbase update, a database of eukyrotic repetitive elements. Cytogenet. Genome Res. 110: 462–467. 10.1159/000084979 [DOI] [PubMed] [Google Scholar]
- Katoh K., Kuma K., Toh H., and Miyata T., 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 33: 511–518. 10.1093/nar/gki198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller N. P., 2019. Fungal secondary metabolism: regulation, function and drug discovery. Nat. Rev. Microbiol. 17: 167–180. 10.1038/s41579-018-0121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klix V., Nowrousian M., Ringelberg C., Loros J. J., Dunlap J. C. et al. , 2010. Functional characterization of MAT1–1-specific mating-type genes in the homothallic ascomycete Sordaria macrospora provides new insights into essential and non-essential sexual regulators. Eukaryot. Cell 9: 894–905. 10.1128/EC.00019-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll A. H., 2011. The multiple origins of complex multicellularity. Annu. Rev. Earth Planet. Sci. 39: 217–239. 10.1146/annurev.earth.031208.100209 [DOI] [Google Scholar]
- Krizsán K., Almási É., Merényi Z., Sahu N., Virágh M. et al. , 2019. Transcriptomic atlas of mushroom development reveals conserved genes behind complex multicellularity in fungi. Proc. Natl. Acad. Sci. USA 116: 7409–7418. 10.1073/pnas.1817822116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroken S., Glass N. L., Taylor J. W., Yoder O. C., and Turgeon B. G., 2003. Phylogenomic analyis of type I polyketide synthase genes in pathogenic and saprobic ascomycetes. Proc. Natl. Acad. Sci. USA 100: 15670–15675. 10.1073/pnas.2532165100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kück U., Pöggeler S., Nowrousian M., Nolting N., and Engh I., 2009. Sordaria macrospora, a model system for fungal development, pp. 17–39 in The Mycota XV, Physiology and Genetics, edited by Anke T. and Weber D.. Springer, Berlin, Heidelberg. [Google Scholar]
- Kües U., 2000. Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol. Mol. Biol. Rev. 64: 316–353. 10.1128/MMBR.64.2.316-353.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann T., and Sonnhammer E. L., 2005. Kalign–an accurate and fast multiple sequence alignment algorithm. BMC Genomics 6: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr N. A., Wang Z., Li N., Hewitt D. A., López-Giráldez F. et al. , 2014. Gene expression differences among three Neurospora species reveal genes required for sexual reproduction in Neurospora crassa. PLoS One 9: e110398 10.1371/journal.pone.0110398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Anavy L., Cole A. G., Winter E., Mostov N. et al. , 2016. The mid-developmental transition and the evolution of animal body plans. Nature 531: 637–641. 10.1038/nature16994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Hull C. M., and Heitman J., 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434: 1017–1021. 10.1038/nature03448 [DOI] [PubMed] [Google Scholar]
- Lord K. M., and Read N. D., 2011. Perithecium morphogenesis in Sordaria macrospora. Fungal Genet. Biol. 48: 388–399. 10.1016/j.fgb.2010.11.009 [DOI] [PubMed] [Google Scholar]
- Love M. I., Huber W., and Anders S., 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15: 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlétaz F., Firbas P. N., Maeso I., Tena J. J., Bogdanovic O. et al. , 2018. Amphioxus functional genomics and the origins of vertebrate gene regulation. Nature 564: 64–70. 10.1038/s41586-018-0734-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F., Aerts A., Ahren D., Brun A., Danchin E. G. J. et al. , 2008. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452: 88–92. 10.1038/nature06556 [DOI] [PubMed] [Google Scholar]
- Martin F., Kohler A., Murat C., Balestrini R., Coutinho P. M. et al. , 2010a Périgord black truffle genome uncovers evolutionary origins and mechanisms of symbiosis. Nature 464: 1033–1038. 10.1038/nature08867 [DOI] [PubMed] [Google Scholar]
- Martin J., Bruno V., Fang Z., Meng X., Blow M. et al. , 2010b Rnnotator: an automated de novo transcriptome assembly pipeline from stranded RNA-Seq reads. BMC Genomics 11: 663 10.1186/1471-2164-11-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. J., and Korf R. P., 1963. The genus Pyronema. Bull. Torrey Bot. Club 90: 33–42. 10.2307/2482857 [DOI] [Google Scholar]
- Morin E., Kohler A., Baker A. R., Foulongne-Oriol M., Lombard V. et al. , 2012. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc. Natl. Acad. Sci. USA 109: 17501–17506 [corrigenda: Proc. Natl. Acad. Sci. USA 110:4146 (2013)]. 10.1073/pnas.1206847109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota-Gómez I., and Lupiáñez D. G., 2019. A (3D-nuclear) space odyssey: making sense of Hi-C maps. Genes (Basel) 10: E415. 10.3390/genes10060415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat C., Payen T., Noel B., Kuo A., Morin E. et al. , 2018. Pezizomycetes genomes reveal the molecular basis of ectomycorrhizal truffle lifestyle. Nat. Ecol. Evol. 2: 1956–1965. 10.1038/s41559-018-0710-4 [DOI] [PubMed] [Google Scholar]
- Nagy L. G., 2017. Evolution: complex multicellular life with 5,500 genes. Curr. Biol. 27: R609–R612. 10.1016/j.cub.2017.04.032 [DOI] [PubMed] [Google Scholar]
- Nagy L. G., Kovács G. M., and Krizsán K., 2018. Complex multicellularity in fungi: evolutionary convergence, single origin, or both. Biol. Rev. Camb. Philos. Soc. 93: 1778–1794. 10.1111/brv.12418 [DOI] [PubMed] [Google Scholar]
- Necsulea A., and Kaessmann H., 2014. Evolutionary dynamics of coding and non-coding transcriptomes. Nat. Rev. Genet. 15: 734–748. 10.1038/nrg3802 [DOI] [PubMed] [Google Scholar]
- Nguyen T. A., Cissé O. H., Wong J. Y., Zheng P., Hewitt D. et al. , 2017. Innovation and constraint leading to complex multicellularity in the Ascomycota. Nat. Commun. 8: 14444 10.1038/ncomms14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas K. J., 2014. The evolutionary-developmental origins of multicellularity. Am. J. Bot. 101: 6–25. 10.3732/ajb.1300314 [DOI] [PubMed] [Google Scholar]
- Nowrousian M., 2014. Genomics and transcriptomics to analyze fruiting body development, pp. 149–172 in The Mycota XIII. Fungal Genomics, Ed. 2, edited by Nowrousian M. Springer, Berlin, Heidelberg: 10.1007/978-3-642-45218-5_7 [DOI] [Google Scholar]
- Nowrousian M., 2018. Genomics and transcriptomics to study fruiting body development: an update. Fungal Biol. Rev. 32: 231–235. 10.1016/j.fbr.2018.02.004 [DOI] [Google Scholar]
- Nowrousian M., and Cebula P., 2005. The gene for a lectin-like protein is transcriptionally activated during sexual development, but is not essential for fruiting body formation in the filamentous fungus Sordaria macrospora. BMC Microbiol. 5: 64 10.1186/1471-2180-5-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrousian M., and Kück U., 2006. Comparative gene expression analysis of fruiting body development in two filamentous fungi. FEMS Microbiol. Lett. 257: 328–335. 10.1111/j.1574-6968.2006.00192.x [DOI] [PubMed] [Google Scholar]
- Nowrousian M., Masloff S., Pöggeler S., and Kück U., 1999. Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP citrate lyase activity. Mol. Cell. Biol. 19: 450–460. 10.1128/MCB.19.1.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrousian M., Ringelberg C., Dunlap J. C., Loros J. J., and Kück U., 2005. Cross-species microarray hybridization to identify developmentally regulated genes in the filamentous fungus Sordaria macrospora. Mol. Genet. Genomics 273: 137–149. 10.1007/s00438-005-1118-9 [DOI] [PubMed] [Google Scholar]
- Nowrousian M., Stajich J. E., Chu M., Engh I., Espagne E. et al. , 2010. De novo assembly of a 40 Mb eukaryotic genome from short sequence reads: Sordaria macrospora, a model organism for fungal morphogenesis. PLoS Genet. 6: e1000891 10.1371/journal.pgen.1000891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrousian M., Teichert I., Masloff S., and Kück U., 2012. Whole-genome sequencing of Sordaria macrospora mutants identifies developmental genes. G3 (Bethesda) 2: 261–270. 10.1534/g3.111.001479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist W., 1961. The genus Ascodesmis. Can. J. Bot. 39: 943–953. 10.1139/b61-079 [DOI] [Google Scholar]
- Ohm R. A., De Jong J. F., Lugones L. G., Aerts A., Kothe E. et al. , 2010. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 28: 957–963. 10.1038/nbt.1643 [DOI] [PubMed] [Google Scholar]
- Osada S., Sutton A., Muster N., Brown C. E., Yates J. R. R. et al. , 2001. The yeast SAS (something about silencing) protein complex contains a MYST-type putative acetyltransferase and functions with chromatin assembly factor ASF1. Genes Dev. 15: 3155–3168. 10.1101/gad.907201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra G., Bradnam K., Ning Z., Keane T., and Korf I., 2009. Assessing the gene space in draft genomes. Nucleic Acids Res. 37: 289–297. 10.1093/nar/gkn916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza D. F., Lin C. W., Van Der Velden N. S., Aebi M., and Künzler M., 2014. Comparative transcriptomics of the model mushroom Coprinopsis cinerea reveals tissue-specific armories and a conserved circuitry for sexual development. BMC Genomics 15: 492 10.1186/1471-2164-15-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöggeler S., and Kück U., 2006. Highly efficient generation of signal transduction knockout mutants using a fungal strain deficient in the mammalian ku70 ortholog. Gene 378: 1–10. 10.1016/j.gene.2006.03.020 [DOI] [PubMed] [Google Scholar]
- Pöggeler S., Nowrousian M., and Kück U., 2006a Fruiting-body development in ascomycetes, pp. 325–355 in The Mycota I, edited by Kües U. and Fischer R.. Springer, Berlin, Heidelberg. [Google Scholar]
- Pöggeler S., Nowrousian M., Ringelberg C., Loros J. J., Dunlap J. C. et al. , 2006b Microarray and real time PCR analyses reveal mating type-dependent gene expression in a homothallic fungus. Mol. Genet. Genomics 275: 492–503. 10.1007/s00438-006-0107-y [DOI] [PubMed] [Google Scholar]
- Pöggeler S., Nowrousian M., Teichert I., Beier A., and Kück U., 2018. Fruiting body development in ascomycetes, in The Mycota XV, Physiology and Genetics, Ed. 2, edited by Anke T. and Schüffler A.. Springer, Berlin, Heidelberg: 10.1007/978-3-319-71740-1_1 [DOI] [Google Scholar]
- Rech C., Engh I., and Kück U., 2007. Detection of hyphal fusion in filamentous fungi using differently fluorescene-labeled histones. Curr. Genet. 52: 259–266. 10.1007/s00294-007-0158-6 [DOI] [PubMed] [Google Scholar]
- Robertson S. J., Bond J., and Read N. D., 1998. Homothallism and heterothallism in Sordaria brevicollis. Mycol. Res. 102: 1215–1223. 10.1017/S0953756298006297 [DOI] [Google Scholar]
- Romero I. G., Ruvinsky I., and Gilad Y., 2012. Comparative studies of gene expression and the evolution of gene regulation. Nat. Rev. Genet. 13: 505–516. 10.1038/nrg3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubini A., Belfiori B., Riccioni C., Tisserant E., Arcioni S. et al. , 2011. Isolation and characterization of MAT genes in the symbiotic ascomycete Tuber melanosporum. New Phytol. 189: 710–722. 10.1111/j.1469-8137.2010.03492.x [DOI] [PubMed] [Google Scholar]
- Schumacher D. I., Lütkenhaus R., Altegoer F., Teichert I., Kück U. et al. , 2018. The transcription factor PRO44 and the histone chaperone ASF1 regulate distinct aspects of multicellular development in the filamentous fungus Sordaria macrospora. BMC Genet. 19: 112 10.1186/s12863-018-0702-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver F. J., 1909. Studies in pyrophilous fungi - I. The occurence and cultivation of Pyronema. Mycologia 1: 131–139. [Google Scholar]
- Sharpton T. J., Stajich J. E., Rounsley S. D., Gardner M. J., Wortman J. R. et al. , 2009. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 19: 1722–1731. 10.1101/gr.087551.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikhakolli U. R., López-Giráldez F., Li N., Common R., Townsend J. P. et al. , 2012. Transcriptome analyses during fruiting body formation in Fusarium graminearum and Fusarium verticillioides reflect species life history and ecology. Fungal Genet. Biol. 49: 663–673. 10.1016/j.fgb.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Spanu P. D., Abbott J. C., Amselem J., Burgis T. A., Soanes D. M., 2010. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science 330: 1543–1546. 10.1126/science.1194573 [DOI] [PubMed] [Google Scholar]
- Spedale G., Timmers H. T., and Pijnappel W. W., 2012. ATAC-king the complexity of SAGA during evolution. Genes Dev. 26: 527–541. 10.1101/gad.184705.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stajich J. E., Block D., Boulez K., Brenner S. E., Chervitz S. A. et al. , 2002. The bioperl toolkit: perl modules for the life sciences. Genome Res. 12: 1611–1618. 10.1101/gr.361602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A., 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinf. 22: 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Segal E., Koller D., and Kim S. K., 2003. A gene-coexpression network for global discovery of conserved genetic modules. Science 302: 249–255. 10.1126/science.1087447 [DOI] [PubMed] [Google Scholar]
- Sutton A., Shia W. J., Band D., Kaufman P. D., Osada S. et al. , 2003. Sas4 and Sas5 are required for the histone acetyltransferase activity of Sas2 in the SAS complex. J. Biol. Chem. 278: 16887–16892. 10.1074/jbc.M210709200 [DOI] [PubMed] [Google Scholar]
- Teichert I., and Nowrousian M., 2011. Evolution of genes for secondary metabolism in fungi, pp. 231–255 in Evolution of fungi and fungal-like organisms, The Mycota XIV, edited by Pöggeler S. and Wöstemeyer J.. Springer-Verlag, Berlin, Heidelberg: 10.1007/978-3-642-19974-5_10 [DOI] [Google Scholar]
- Teichert I., Wolff G., Kück U., and Nowrousian M., 2012. Combining laser microdissection and RNA-seq to chart the transcriptional landscape of fungal development. BMC Genomics 13: 511 10.1186/1471-2164-13-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert I., Nowrousian M., Pöggeler S., and Kück U., 2014. The filamentous fungus Sordaria macrospora as a genetic model to study fruiting body development. Adv. Genet. 87: 199–244. 10.1016/B978-0-12-800149-3.00004-4 [DOI] [PubMed] [Google Scholar]
- Timmers H. T., and Tora L., 2005. SAGA unveiled. Trends Biochem. Sci. 30: 7–10. 10.1016/j.tibs.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Traeger S., Altegoer F., Freitag M., Gabaldon T., Kempken F. et al. , 2013. The genome and development-dependent transcriptomes of Pyronema confluens: a window into fungal evolution. PLoS Genet. 9: e1003820 10.1371/journal.pgen.1003820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trail F., Wang Z., Stefanko K., Cubba C., and Townsend J. P., 2017. The ancestral levels of transcription and the evolution of sexual phenotypes in filamentous fungi. PLoS Genet. 13: e1006867 10.1371/journal.pgen.1006867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G. et al. , 2010. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28: 511–515. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brummelen J., 1981. The genus Ascodesmis (Pezizales, Ascomycetes). Persoonia 11: 333–358. [Google Scholar]
- Varga T., Krizsán K., Földi C., Dima B., Sánchez-García M. et al. , 2019. Megaphylogeny resolves global patterns of mushroom evolution. Nat. Ecol. Evol. 3: 668–678. 10.1038/s41559-019-0834-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace I. M., O’sullivan O., Higgins D. G., and Notredame C., 2006. M-Coffee: combining multiple sequence alignment methods with T-Coffee. Nucleic Acids Res. 34: 1692–1699. 10.1093/nar/gkl091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehe A., Bansal M. S., Burleigh J. G., and Eulenstein O., 2008. DupTree: a program for large-scale phylogenetic analyses using gene tree parsimony. Bioinf. 24: 1540–1541. 10.1093/bioinformatics/btn230 [DOI] [PubMed] [Google Scholar]
- Wessels J. G. H., 1993. Fruiting in the higher fungi. Adv. Microb. Physiol. 34: 147–202. 10.1016/S0065-2911(08)60029-6 [DOI] [PubMed] [Google Scholar]
- Wik L., Karlsson M., and Johannesson H., 2008. The evolutionary trajectory of the mating-type (mat) genes in Neurospora relates to reproductive behavior of taxa. BMC Evol. Biol. 8: 109 10.1186/1471-2148-8-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. M., Godlonton T., Van Der Nest M. A., Wilken P. M., Wingfield M. J. et al. , 2015. Unisexual reproduction in Huntiella moniliformis. Fungal Genet. Biol. 80: 1–9. 10.1016/j.fgb.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Wilson B., Erdjument-Bromage H., Tempst P., and Cairns B. R., 2006. The RSC chromatin remodeling complex bears an essential fungal-specific protein module with broad functional roles. Genetics 172: 795–809. 10.1534/genetics.105.047589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood V., Gwilliam R., Rajandream M. A., Lyne M., Lyne R. et al. , 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415: 871–880 [corrigenda: Nature 421:94 (2003)]. 10.1038/nature724 [DOI] [PubMed] [Google Scholar]
- Yang J., Wang L., Ji X., Feng Y., Li X. et al. , 2011. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog. 7: e1002179 10.1371/journal.ppat.1002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw sequence data generated in this study were submitted to the NCBI Sequence Read Archive (A. nigricans genome sequencing and transcriptome sequencing for annotation, accession numbers SRP082924 and SRP082925) and Gene Expression Omnibus databases (A. nigricans transcriptome data, accession number GSE92315). The A. nigricans whole-genome shotgun project has been deposited at DNA Databank of Japan (DDBJ)/European Molecular Biology Laboratory (EMBL)/GenBank under the accession number SSHT00000000. The version described in this manuscript is version SSHT01000000. Supplemental material available at figshare: https://doi.org/10.25386/genetics.9891440.