Abstract
Transporters are transmembrane proteins that mediate the selective translocation of solutes across biological membranes. Recently, we have shown that specific interactions with plasma membrane phospholipids are essential for the formation and/or stability of functional dimers of the purine transporter UapA, a prototypic eukaryotic member of the ubiquitous nucleobase ascorbate transporter (NAT) family. Here, we provide strong evidence that distinct interactions of UapA with membrane lipids are essential for ab initio formation of functional dimers in the ER, or ER exit and further subcellular trafficking. Through genetic screens, we identify mutations that restore defects in dimer formation and/or trafficking. Suppressors of defective dimerization restore ab initio formation of UapA dimers in the ER. Most of these suppressors are located in the movable core domain, but also in the core-dimerization interface and in residues of the dimerization domain exposed to lipids. Molecular dynamics suggest that the majority of suppressors stabilize interhelical interactions in the core domain and thus assist the formation of functional UapA dimers. Among suppressors restoring dimerization, a specific mutation, T401P, was also isolated independently as a suppressor restoring trafficking, suggesting that stabilization of the core domain restores function by sustaining structural defects caused by the abolishment of essential interactions with specific lipids. Importantly, the introduction of mutations topologically equivalent to T401P into a rat homolog of UapA, namely rSNBT1, permitted the functional expression of a mammalian NAT in Aspergillus nidulans. Thus, our results provide a potential route for the functional expression and manipulation of mammalian transporters in the model Aspergillus system.
Keywords: Aspergillus nidulans, UapA, nucleobase, NAT, rSNBT1
TRANSPORTERS are essential transmembrane proteins that catalyze the uptake or efflux of metabolites, nutrients, ions, and drugs across biological membranes. Transporter malfunction, due to genetic mutations or metabolic defects, results in significant cellular or organismal disruption (César-Razquin et al. 2015) (http://www.tcdb.org/). Despite their biological and apparent medical importance, knowledge on structure–function relationships in transporters is limited compared to extramembrane hydrophilic proteins. This is in part due to complexity associated with their translocation and cotranslational folding into a membrane lipid bilayer [the ER in eukaryotes or the plasma membrane (PM) in prokaryotes]. Additionally, in eukaryotes, transporters follow specific membrane trafficking, turnover, or recycling routes, which add further complications in understanding their mechanisms of regulating expression, function, and turnover (Lauwers et al. 2010; MacGurn et al. 2012; Herrmann and Spang 2015; Rabouille 2017; Bouris et al. 2019). A further contributory factor is their functional and structural dependence on specific membrane lipids, something has is only recently begun to be explored in detail (Bechara and Robinson 2015; Gupta et al. 2017). Further complications for transporter study arise from difficulties in expressing sufficient quantities for downstream structural studies, functional reconstitution in proteoliposomes, or in measuring their kinetics in intact cells where the presence of similar transporters with overlapping specificities complicates the analysis (Rigaud and Lévy 2003).
One of the best studied eukaryotic transporters is the UapA xanthine–uric acid/H+ symporter of the filamentous ascomycete Aspergillus nidulans (Diallinas 2014, 2016). This is due to the development of rigorous genetic, biochemical, and in vivo cellular approaches, uniquely available in the model system of A. nidulans, and, more recently, structural and biophysical studies. The high-resolution, inward-facing structure of a conformationally locked mutant of UapA (G411VΔ1–11) revealed that UapA is a closely associated dimer (Alguel et al. 2016). Coexpression experiments with wild-type and nonfunctional UapA mutants revealed a dominant negative effect of the nonfunctional mutants, indicating functional interdependence of individual protomers in the UapA dimer and that the dimer is the functional unit (Alguel et al. 2016). In the UapA dimer each monomeric unit consists of a core domain that hosts the substrate-binding site and a dimerization domain that includes elements crucial for substrate specificity. Molecular dynamics (MD) simulations together with comparison with other related proteins suggested that UapA functions via an elevator mechanism, as reported for a range of other transporters (Arakawa et al. 2015; Geertsma et al. 2015; Thurtle-Schmidt and Stroud 2016; Yu et al. 2017; Huynh et al. 2018; Chang et al. 2019). Upon substrate binding, the core domain moves against the relatively immobile dimerization domain to transport the substrate from one side of the membrane to the other (Alguel et al. 2016; Drew and Boudker 2016). Interestingly, UapA substrate specificity is also determined by the proper formation of the dimer, as a specific Arg residue (Arg481) from one monomeric unit dynamically controls the substrate translocation trajectory in the opposite monomer (Alguel et al. 2016). Recently, native mass spectrometry (MS) of purified UapA, combined with MD, mutagenesis, and functional analyses, established that the membrane lipids, phosphatidylinositol (PI), and phosphatidylethanolamine (PE) have a critical role in stabilizing the functional UapA dimer (Pyle et al. 2018). More specifically, it has been shown that UapA delipidation during purification causes dissociation of the dimer into monomers, but subsequent addition of PI or PE both recovers lipid binding and reforms the UapA dimer. MD simulations predicted possible lipid-binding sites near the UapA dimer interface, and subsequent mutational studies confirmed that Arg287, Arg478, and Arg479 act as the lipid-binding residues involved in the formation of UapA dimers and are absolutely necessary for transport activity (Pyle et al. 2018). Importantly, however, while triple-alanine replacement of these arginines leads to total lack of UapA function, both native MS and bifluorescence complementation (BiFC) assays have indicated that a fraction of UapA can still dimerize (Martzoukou et al. 2015; Pyle et al. 2018). Thus, lipid binding might not be an absolute requirement for dimer formation, but is essential for the formation and/or stability of functional dimers. The total lack of function of the lipid-binding site UapA mutant (R287A/R478A/R479A) further suggests that specific interactions with lipids are also necessary for the mechanism of transport per se. Rather surprisingly, the GFP-tagged inactive triple arginine mutant can still properly traffic to the PM, which means that either monomeric UapA translocates normally to the PM, or that the UapA dimer is initially formed in the ER and traffics to the PM, but then becomes unstable and dissociates into nonfunctional monomers. Noticeably, the triple arginine mutant is also sufficiently folded and stable to allow isolation and native MS analysis, which is also compatible with sorting to the PM. As MD simulations further predicted that lipids could also bind to the outermost, membrane-facing regions of the core domains of the UapA dimer (Pyle et al. 2018), further investigation was needed to fully understand the role of membrane lipids in UapA folding, subcellular traffic, and transport function.
Here, we investigate further the role of residues Arg287, Arg478, and Arg479 in UapA dimer formation and/or stability, and study the role of additional putative interactions of UapA with membrane lipids. Using mutational and functional analyses we confirm that Arg287, Arg478, and Arg479 are essential for ab initio dimerization in the ER, but redundant for membrane traffic, whereas interactions between distinct peripheral residues (Lys73, Arg133, and Arg421) and lipids are essential for proper folding, ER exit, and membrane traffic. Additionally, using genetic screens for suppressors of mutations affecting putative lipid-binding residues, which could in principle lead to residues critical for structure–function relationships in UapA or to proteins assisting UapA sorting to the PM, we obtained intragenic suppressors in UapA that apparently compensate for the “lost” lipid interactions in the original mutants. Importantly, using information on a specific core residue that proved to have a key role in stabilizing UapA, Thr401, we genetically manipulate and achieve the functional expression, for the first time, of a mammalian homolog of UapA in A. nidulans. This opens the way for the functional expression of mammalian transporters, that belong to the nucleobase ascorbate transporter (NAT) family, including those essential for vitamin C transport in humans (Yamamoto et al. 2010; Yasujima et al. 2018), in the model Aspergillus system.
Materials and Methods
Media, strains, and growth conditions
Standard complete (CM) and minimal media (MM) for A. nidulans growth were used. Media and supplemented auxotrophies were used at the concentrations given in http://www.fgsc.net. Glucose 1% (w/v) was used as a carbon source, and 10 mM sodium nitrate (NO3−) or 10 mM proline was used as nitrogen source. Nucleobases and analogs were used at the following final concentrations: 5-fluorouracil (5FU) 100 μΜ, uric acid 0.5 mM, xanthine 1 mM, adenine 2 mM, thymine 2 mM, hypoxanthine 2 mM, and uracil 2 mM. All media and chemical reagents were obtained from Sigma (Sigma Chemical), St. Louis, MO (Life Science Chemilab SA, Athens, Hellas) or AppliChem (Bioline Scientific SA, Athens, Hellas). A ΔazgA ΔuapA ΔuapC::AfpyrG pabaA1 argB2 mutant strain, named Δ3, was the recipient strain in transformations with plasmids carrying uapA alleles, based on complementation of the arginine auxotrophy argB2. A ΔfurD::riboB ΔfurA::riboB ΔfcyB::argB ΔazgA ΔuapA ΔuapC::AfpyrG ΔcntA::riboB pabaA1 pantoB100 mutant strain, named Δ7, was the recipient strain in transformations with plasmids carrying the rSNBT1 wild-type and mutated alleles, based on complementation of the pantothenic acid auxotrophy strain pantoB100 (Krypotou and Diallinas 2014). A. nidulans protoplast isolation and transformation were performed as previously described (Koukaki et al. 2003). Growth tests were performed at 25 or 37° for 48 hr at pH 6.8.
Standard molecular biology manipulations and plasmid construction
Genomic DNA extraction from A. nidulans was performed as described on the Fungal Genetics Stock Center website (http://www.fgsc.net). Plasmids, prepared in Eschericia coli, and DNA restriction or PCR fragments, were purified from agarose 1% gels with the Nucleospin Plasmid Kit or Nucleospin ExtractII kit, according to the manufacturer’s instructions (Macherey-Nagel, Düren, Germany Lab Supplies Scientific SA, Athens, Hellas). Standard PCR reactions were performed using KAPATaq DNA polymerase (Kapa Biosystems). PCR products used for cloning, sequencing, and reintroduction by transformation in A. nidulans were amplified by a high-fidelity KAPA HiFi HotStart Ready Mix (Kapa Biosystems) polymerase. DNA sequences were determined by VBC-Genomics (Vienna, Austria). Site-directed mutagenesis was carried out according to the instructions accompanying the Quik-Change Site-Directed Mutagenesis Kit (Agilent Technologies and Stratagene, La Jolla, CA). The principal vector used for UapA mutants was pAN510-GFP carrying a gfp-tagged uapA gene as a template (Koukaki et al. 2005), and for rSNBT1 mutants was a modified pGEM-T-easy vector carrying a version of the gpdA promoter, the trpC 39 termination region, and the panB selection marker (Pantazopoulou et al. 2007). For BiFC analyses, the N-terminal half of yellow fluorescent protein (YFPn; 154 amino acids of YFP) or the C-terminal half of YFP (YFPc; 86 amino acids of YFP) was amplified from plasmids PDV7 and PDV8 (Krypotou et al. 2015), and cloned into pAN510exp-alcAp or pAN520exp-alcAp (Takeshita et al. 2008) followed by cloning of the uapA ORF. UapA or rSNBT1 mutations were constructed by oligonucleotide-directed mutagenesis, or appropriate forward and reverse primers (Supplemental Material, Table S2). Transformants arising from single-copy integration events with intact UapA ORFs were identified by PCR analysis.
Uptake assays
Kinetic analysis of UapA or rSNBT1 activity was measured by estimating uptake rates of 3H-xanthine or 3H-uracil uptake respectively (40–80 Ci mmol−1; Moravek Biochemicals, Brea, CA, USA), as previously described in (Krypotou and Diallinas 2014). In brief, 3H-xanthine or 3H-uracil uptake, or competition by excess of other unlabeled substrates, was assayed in A. nidulans conidiospores germinating for 4 hr at 37°, at 140 rpm, in liquid MM, pH 6.8. Initial velocities were measured on 107 conidiospores/100 μl by incubation with concentrations of 0.2–2.0 μΜ of radiolabeled substrates at 37°. The time of incubation was defined through time-course experiments and the period of time when each transporter showed linear increased activity was chosen. All transport assays were carried out in triplicate. SD was <20%. Results were analyzed using GraphPad Prism software.
Isolation and characterization of suppressor mutations
Suppressor mutations of 109 conidiospores of strains R287A/R478A/R479A or K73A/R133A/R421A were obtained after 3 min and 45 sec exposure at a standard distance of 20 cm from an Osram HNS30 UV-B/C lamp, and subsequent selection of colonies capable of growing on MM containing uric acid as a sole nitrogen source, at 25°. Spores from positive colonies were collected after 6–8 days and further isolated on the same selective medium that was used to obtain the original colonies. Genomic DNA from 24 purified colonies was isolated, and the uapA ORF was amplified and sequenced. In all cases, the amplified fragments contained a new single missense mutation.
Epifluorescence microscopy
Samples for standard epifluorescence microscopy were prepared as previously described (Gournas et al. 2010; Karachaliou et al. 2013). In brief, sterile 35-mm l-dishes with glass bottoms (Ibidi, Gräfelfing, Germany) containing liquid minimal media supplemented with NaNO3 and 0.1% glucose were inoculated from a spore solution, and incubated for 18 hr at 25° or for 8 hr at 37° (rSNBT1). The samples were observed on an Axioplan Zeiss (Carl Zeiss, Thornwood, NY) phase contrast epifluorescent microscope and the resulting images were acquired with a Zeiss-MRC5 digital camera using AxioVs40 V4.40.0 software. Image processing and contrast adjustment were made using ZEN 2012 software, while further processing of the TIFF files was done using Adobe Photoshop CS3 software for brightness adjustment, rotation, and alignment.
Homology modeling of rSNBT1
The construction of a structural model of rSNBT1 was based on the crystal structure of the UapA in the inward-open conformation [Protein Data Bank (PDB) entry 5I6C]. For this, we utilized the alignment shown in Figure S5. The final model was built using PRIME software with an energy-based algorithm (Jacobson et al. 2004). A loop refinement routine was also implemented.
Induced-fit docking of uracil on rSNBT1
Protein preparation using OPLS2005 force field (Banks et al. 2005) and molecular docking was performed with the Schrödinger Suite 2018. After protein structure alignment with the crystal structure of UapA (PDB 5I6C), the binding pocket was defined by residues Phe124, Glu347, Tyr395, Ser396, and Glu397. Uracil was docked on the final structure from Homology Modeling, using the induced-fit docking protocol (Schrödinger Release 2018-1: Schrödinger Suite 2018-1 Induced Fit Docking protocol; Glide, Schrödinger, LLC, New York, NY, 2018; Prime, Schrödinger, LLC, New York, NY, 2018), which is intended to circumvent the inflexible binding site, and accounts for the side chain and backbone movements upon ligand binding (Sherman et al. 2006).
MD
UapA (wild-type or mutated when discussed) or rSNBT1 homolog dimers were inserted into a lipid bilayer using the CHARMM-GUI tool (Wu et al. 2014, http://www.charmm-gui.org). The resulting system was explicitly solvated using the transferable intermolecular potential with 3 points (TIP3P) water model (Jorgensen et al. 1983), and neutralized by the addition of Na+ and Cl− counter ions at concentrations of 0.15 cM. The lipid bilayer utilized was composed of 20% ergosterol, 9% POPC, 12% DYPC, 9% YOPC, 6% POPE, 3% DYPE, 5% YOPE, 3% DOPE, 19% POPI, and 14% PYPI, as described previously (http://www.charmm-gui.org/?doc=archive&lib=lipid, Pyle et al. 2018). All UapA mutations were constructed by utilizing the CHARMM-GUI’s initial step “PDB Manipulation Options.” The N-terminal residues were always methylated and the C-terminal residues were always amidated. MD simulations were performed with GROMACS 2018 (Abraham et al. 2015) using the all-atom force field CHARMM36 (Huang and MacKerell 2013). Periodic boundary conditions were used. Long-range electrostatic interactions were treated with the Particle Mesh Ewald method. Nonbonded interactions were described with a Lennard–Jones potential, with a cut-off distance of 1 nm and an integration step of two femtoseconds (fs) was implemented. The system was progressively minimized and equilibrated using the GROMACS input scripts generated by CHARMM-GUI, and the temperature and pressure were held at 303.15° Kelvin and 1 bar, respectively (Lee et al. 2016). The resulting equilibrated structures were then used as an initial condition for the production runs of 100 nsec with all the constraints turned off. Production runs were subsequently analyzed using GROMACS tools, and all images and videos were prepared using VMD software (Humphrey et al. 1996).
In silico mutation of I157F, L192F, and L431F on UapA
Starting from the crystal structure of UapA, manual mutation of I157F, L192F, and L431F was performed using the “mutation” command on Maestro v11.5 (Schrödinger Release 2018-1). Each resulting structure was inserted to Protein Preparation Wizard Workflow as implemented on Maestro v11.5. Restrained minimization was converged when heavy atom root-mean-square deviation (RMSD) was >1 Å.
UapA K73A/R133A/R421A (T401P) expression and purification
Expression and purification of the UapA K73A/R133A/R421A and K73A/R133A/R421A/T401P mutants was carried out as described previously (Pyle et al. 2018). In brief, the mutants were individually expressed as C-terminally GFP8His-tagged constructs in Saccharomyces cerevisiae FGY217 cells (12L), using vector pDDGFP2. Yeast cells were incubated at 30° with shaking at 300 rpm to an OD600 of 0.6. Galactose was then added to the cultures to a final concentration of 2% to induce UapA expression. After incubation for a further 22 hr, the cells were harvested by centrifugation and resuspended. Cells were broken in a Constant Systems cell disruptor and the membranes isolated by centrifugation. The membranes were resuspended, flash-frozen, and stored at −80°. Membranes were solubilized for 1 hr in n-dodecyl-β-D-maltoside. Unsolubilized membranes were removed by centrifugation. The supernatant was incubated with Ni2+-NTA resin for 2 hr. The His-tagged UapA bound to the resin was then washed with buffers containing 10 and 30 mM imidazole to remove contaminants. UapA was eluted with buffer containing 250 mM imidazole before overnight dialysis to dilute the imidazole from the protein sample. During dialysis, the protein was cleaved using a His-tagged TEV protease. The sample was run through a His-trap column, from which UapA was eluted in the flow through, to remove the His-tagged GFP and TEV. The sample was then loaded onto a size-exclusion chromatography column. Fractions containing monodisperse UapA were analyzed by SDS-PAGE and concentrated to 20 μM, flash frozen, and stored at −80°.
Native MS of UapA
Native MS of UapA K73A/R133A/R421A/T401P was carried out as described previously (Pyle et al. 2018). In brief, UapA was buffer exchanged into MS buffer [250 mM EDDA (pH 6.3), 0.014% DDMLA (v/v), and 10 mM L-serine] to a UapA concentration of 20 μM using Micro Bio-Spin 6 columns (Bio-Rad, Hercules, CA). UapA was loaded into gold-coated capillaries and the protein sprayed into a Synapt G2-Si (Waters Associates, Milford, MA) by nanoelectrospray ionization. The following conditions were used in the mass spectrometer for optimal peak resolution: capillary voltage +1.3–1.5 kV, sampling cone voltage 150 V, trap collision energy (CE) 200 V, transfer CE 0 V, backing pressure 3.88 mbar, trap and transfer pressure (argon) 1.72e-2 mbar, and ion mobility cell pressure (nitrogen) 2.58 mbar. The mass spectrometer was calibrated using cesium iodide. Spectra were recorded and processed using Masslynx 4.1 software (Waters Associates). The relative abundances of each oligomeric state were quantified by UniDec (Marty et al. 2015) as described previously (Pyle et al. 2018).
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplemental material available at figshare: https://doi.org/10.25386/genetics.9177566.
Results
Residues Arg287, Arg 479, and Arg479 are crucial for ab initio dimerization of UapA in the ER
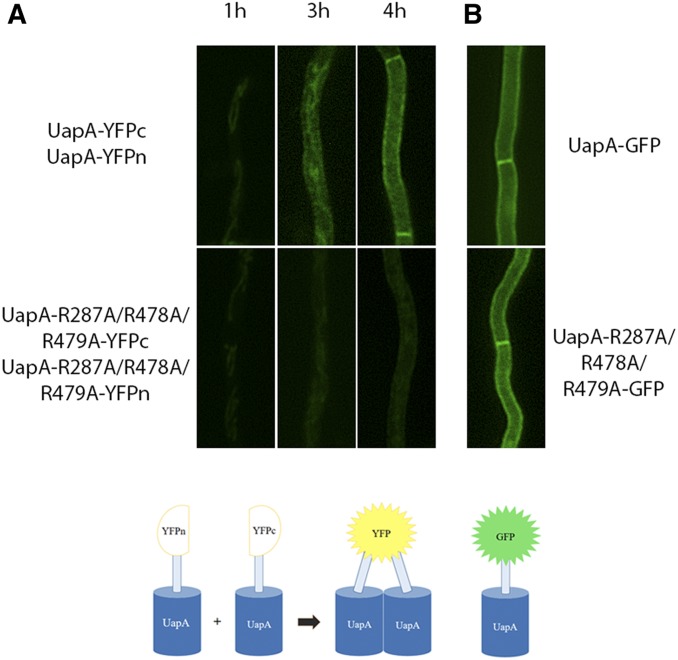
We have previously shown that arginine residues 287, 478, and 479 are essential for phospholipid-dependent functional dimerization of UapA at the PM (Pyle et al. 2018). To further understand the basis of the functional defect in the triple R287A/R478A/R479A mutant, we examined whether loss of dimerization occurs ab initio at the level of the ER or whether what we have previously observed was due to instability of UapA dimers at the PM. For this, we used a previously described BiFC assay (Martzoukou et al. 2015), adapted to follow the sorting and subcellular localization of de novo-made UapA. This was based on time-course experiments following the subcellular localization of de novo-made alcAp-UapA-GFP, which showed that after 1 hr of transcriptional derepression UapA-GFP was hardly visible, but at 2–3 hr it labeled the ER and at 4 hr appeared mostly in the PM (Bouris et al. 2019). Using this system, we followed reconstitution of split-YFP, via UapA dimerization, in a strain containing two copies of the alcAp-uapA gene, tagged at either the N- or the C-part of the yfp ORF (Martzoukou et al. 2015). To follow de novo-made UapA in young mycelia, we repressed the transcription of alcAp-uapA-yfpn and alcAp-uapA-yfpc overnight (16 hr in MM at 25° in glucose MM), a period to allow conidiospore germination and young hyphae development, and then shifted the culture to fructose-derepression medium for 1–4 hr of growth. We performed this assay using wild-type UapA and the triple R287A/R478A/R479A mutant. Figure 1A shows that in the wild-type control strain, our assay detected early reconstitution of split-YFP fluorescence at the ER network at 3 hr, but failed to do so in the triple R287A/R478A/R479A mutant, where only a weak signal was observed. After 4 hr of expression, the totality of wild-type UapA fluorescent signal marks the PM, whereas a weak cytoplasmic fluorescent signal and very low cortical localization is observed in the R287A/R478A/R479A mutant. This shows that Ala substitutions of the three Arg residues led to significant reduction of apparent UapA dimerization at the ER membrane, and further suggests that specific contacts with ER lipids might be a prerequisite for dimerization. Surprisingly, the interactions of R287, R478, and R479 with lipids and dimerization proved redundant for sorting of the mutant UapA to the PM, as judged by the normal PM localization of R287A/R478A/R479A tagged with intact GFP (Figure 1B). These findings suggest that in the R287A/R478A/R479A mutant nonfunctional UapA monomers or partially misfolded dimers can still be secreted to the PM.
Figure 1.
Residues Arg287, Arg 479, and Arg479 are crucial for ab initio dimerization of UapA in the ER. (A) Bimolecular complementation analysis based on reconstitution of split-yellow fluorescent protein (YFP) of de novo-expressed wild-type or R287A/R478A/R479A mutant, expressed via the alcAp regulatable promoter in the presence of derepressive carbon and nitrogen, and two copies of the uapA gene, one tagged with the N-terminal and the other with the C-terminal part of the yfp ORF (UapA-YFPc:UapA-YFPn and UapA-R287A/R478A/R479A-YFPc:UapA-R287A/R478A/R479A-YFPn). Notice the progressive appearance of a clear fluorescent signal in wild-type UapA, first associated with the ER membrane network (3 hr) and finally at the plasma membrane (4 hr). In contrast, in the R287A/R478A/R479A mutant, fluorescence remains extremely low, just above the level of detection. (B) Localization of wild-type or R287A/R478A/R479A mutant UapA tagged with GFP after 4 hr of transcription derepression, showing that the mutant can normally translocate in the plasma membrane despite a very low apparent ability to dimerize, as shown in (A). The lower panel shows schematically that dimerization of UapA is required for detection of a fluorescent signal from reconstituted split-YFP, while a fluorescent signal from GFP-tagged UapA does not distinguish monomers from dimers.
Genetic suppressors of R287A/R478A/R479A map in the core or dimerization domains
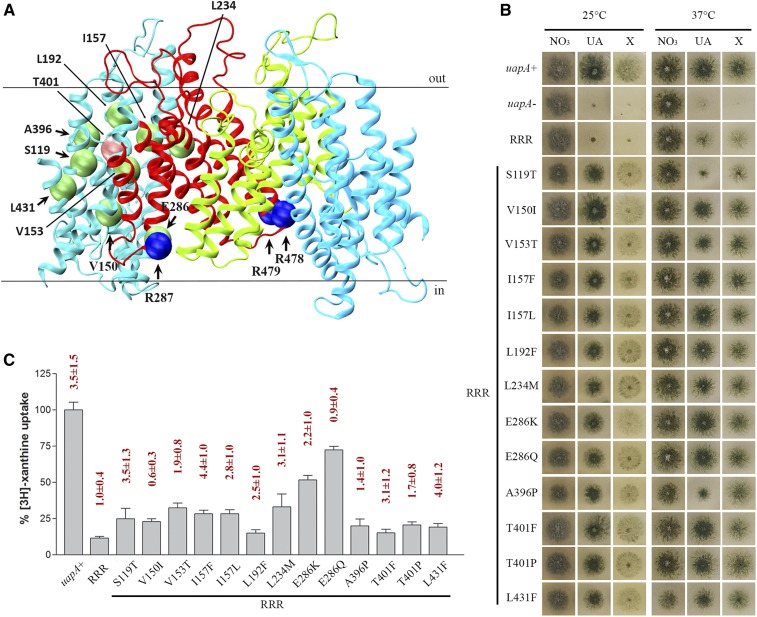
To further understand the molecular basis of how UapA–phospholipid interactions affect the functional dimerization of UapA, we isolated genetic suppressors restoring UapA-mediated growth on uric acid in the R287A/R478A/R479A triple mutant at 25°, a temperature at which this mutant does not grow on media containing UapA substrates. We purified 38 apparent suppressors and sequenced the ORF of the uapA gene. All 38 contained the original mutation (R287A/R478A/R479A) plus an extra point mutation, apparently the one that suppresses the growth defect on uric acid. Table S1 summarizes the identity and frequency of isolation of all suppressors, which concerned 13 distinct single-amino acid substitutions in 11 different residues. Eight of the 13 suppressors were isolated more than once, showing that mutagenesis was fairly saturated and also confirming that the amino acid changes detected are responsible for suppression. Figure 2A shows the topology of suppressor mutations in the UapA structure.
Figure 2.
Genetic suppressors of R287A/R478A/R479A localized in the core and the dimerization domain of UapA partially restore UapA function. (A) Topology of amino acids modified in R287A/R478A/R479A suppressors. Core domains are colored light blue, and dimerization domains red and green for clarity. Mutated amino acids in the original mutant strain are shown with blue spheres, and in the suppressors with green and pink (T401) spheres. (B) Growth tests of R287A/R478A/R479A suppressors on UapA physiological substrates. Control strains are strains with total genetic deletions in all major purine transporters (ΔACZ; negative control), referred in the figure as uapA-, and a ΔACZ transformant expressing wild-type uapA–gfp (uapA+; positive control). All suppressor strains and the original R287A/R478A/R479A strain are isogenic to the negative and positive control strains, and express UapA from single-gene copies of uapA tagged with GFP. All strains were grown in minimal media containing 10 mM nitrate (NO3), 0.5 mM uric acid (UA), or 1 mM xanthine (X) as a nitrogen source at 25° (left panel) or 37° (right panel). RRR depicts the R287A/R478A/R479A original genetic background. (C) Relative 3H-xanthine transport rates of R287A/R478A/R479A and R287A/R478A/R479A suppressors expressed as percentages of initial uptake rates (V) compared to the wild-type (uapA+) rate. Km values (μΜ) for xanthine are shown at the top of histograms. Results are averages of three measurements for each concentration point. SD was 20%.
The 13 distinct suppressors grew well, albeit slightly less than an isogenic strain expressing wild-type UapA, on uric acid or xanthine (Figure 2B). Among the suppressors, S119T scored as a thermosensitive mutant, growing very weakly on both UapA substrates at 37°, similar to the original R287A/R478A/R479A mutant. Direct uptake assays, measuring the transport rate of radiolabeled xanthine (Krypotou and Diallinas 2014), were used to estimate the effect of the suppressor mutation on UapA transport kinetics. Figure 2C shows that in most suppressors, UapA transport rates were reestablished at ∼15–30% of the wild-type protein, a level known to be the threshold for conferring visible UapA-mediated growth on uric acid or xanthine. Highest transport rates were obtained in suppressors E286Q (∼70%) and E286K (∼51%). In fact, the relative uptake differences of some suppressors (L192F, A396P, T401P, T401F, or L431F) compared with the original R287A/R478A/R479A mutant were marginal. It should be noted that in growth tests, purines were added to concentrations at the 1–2 mM level to be used as nitrogen sources, while in uptake experiments, radiolabeled xanthine was used at a submicromolar range (0.3–0.5 μΜ) for technical reasons. Thus, any mutation that causes significant reduction in substrate affinity might score as an apparent loss-of-function mutation in uptake experiments, but still can allow normal growth on the relative substrate when this is supplied at millimolar concentrations. To test whether the low apparent transport capacity of suppressors was due to reduced affinity for xanthine, we estimated the approximate Km of several of the suppressors relative to the original R287A/R478A/R479A mutation or a wild-type UapA control. We found no significant reduction of affinity for xanthine in all suppressors tested (Figure 2C, on top of histograms).
Genetic suppressors of R287A/R478A/R479A reestablish UapA dimerization
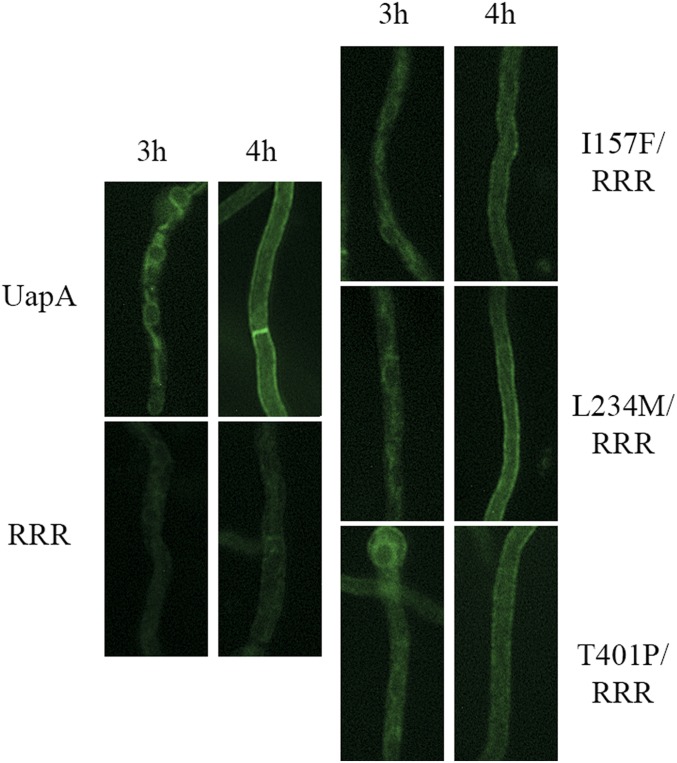
As phospholipid binding has been shown to be essential for the formation of functional UapA dimers, the suppressors of R287A/R478A/R479A could either restore functional dimerization or confer transport activity to UapA monomers. To test these two alternatives, we performed BiFC assays to follow UapA dimerization of de novo-made UapA in selected suppressors (I157F, L234M, and T401P) and control strains, as described earlier for the original R287A/R478A/R479A mutant. Figure 3 shows significant reconstitution of split-YFP fluorescence, and thus apparent dimerization, at both the ER and the PM in all of the three suppressors studied, a picture similar to what is seen in wild-type cells. This confirms that suppressors restored function by restoring early dimerization of UapA at the ER.
Figure 3.
Genetic suppressors R287A/R478A/R479A reestablish UapA dimerization. Bimolecular complementation analysis based on reconstitution of split-YFP of de novo-expressed UapA, R287A/R478A/R479A, and selected R287A/R478A/R479A suppressors, namely I157F, L234M, and T401P. Details of expression are as in Figure 1. Notice that all suppressors analyzed clearly restore, at least partially, the strength of the fluorescent signal detected in the ER and the plasma membrane, when compared to the original R287A/R478A/R479A mutant (RRR).
MD provides a structural rationale for the effect of suppressor mutations on UapA stability and function
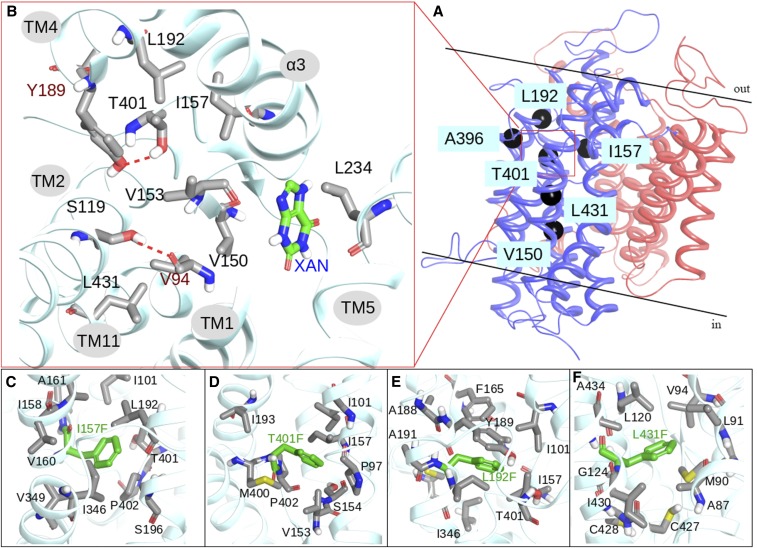
Rather surprisingly, 10 out of the 13 mutations concerned residues located in transmembrane segments (TMSs) of the movable core domain (i.e., in TMS2, TMS3, TMS4, TMS9, TMS10, or TMS11). The core domain consists of two layers of transmembrane helices: TMS1, TMS3, TMS8, and TMS10 in the inner part in contact with the dimerization domain, and TMS2, TMS4, TMS9, and TMS11 in the outer part in contact with the membrane lipids. These two layers are stabilized through an extended network of hydrophobic interactions and certain key polar interactions, which are mainly related to Asp388. Most suppressor mutations are related to the network of the hydrophobic interactions and are located on a virtual diagonal intersecting the core domain in the space between the two transmembrane layers (Figure 4, A and B). The UapA crystal structure (PDB 5i6c) shows that Leu192 interacts with Tyr189 (TMS4), Τhr401 (TMS10), Ιle157 (TMS3), Phe165 (TMS3), Ile101 (TMS1), Ile193 (TMS4), and Ile346 (TMS8). Similarly, Ile157 interacts with Ile101 (TMS1), Τhr401 (TMS10), Pro402 (TMS10), Val349 (TMS8), Ile346 (TMS8), and Leu192 (TMS4). Thr401 is located in the center between the two TMS layers of the core domain and interacts with Tyr189 (TMS4), Leu192 (TMS4), Ile193 (TMS4), Val153 (TMS3), Ιle157 (TMS3), and Ile101 (TMS1). Val153 is surrounded by Thr401 (TMS10), Pro97 (TMS1), Val94 (TMS1), Met400 (TMS10), Ser119 (TMS2), Met403 (TMS10), and Cys123 (TMS2). On the opposite side, the Ser119 side chain is located in the middle between Val153 (TMS3), Val94 (TMS1), Met400 (TMS10), and Cys123 (TMS2). Finally, Leu431 is located between Ala87 (TMS1), Met90 (TMS1), and Leu120 (TMS2). Contrary to the above-mentioned residues, Leu234 is located in the dimerization domain, interacting mainly with Ile158 in TMS3. The suppressor mutations—and more particularly L192F, T401F, L431F, S119T, and V153T—seem to enhance the above-mentioned interactions and mainly stabilize the core domain. To verify this hypothesis, models of UapA, including selected suppressor mutations, were constructed and subjected to geometry optimization and short MD calculations. As shown in Figure 4, C–F, the phenyl moieties of the mutated residues I157F, T401F, and L192F are accommodated in the space between the other lipophilic residues, increasing hydrophobic interactions between TMS8, TMS3, TMS4, and TMS10. The introduction of a methyl group in the S119T mutant will also enhance, albeit to a lower degree, interactions with Val153 (TMS3) and Met400 (TMS10). Finally, the polar mutation V153T introduces a new hydrogen bond with Ser119, which already interacts with the backbone of Val94, creating a hydrogen bond network between TMS3, TMS2, and TMS1.
Figure 4.
Molecular dynamics provide a structural rationale for the effect of suppressor mutations on UapA stability and function. (A) Ribbon representation of UapA monomer. The core domain is colored blue and the gate domain red. Topologies of type I suppressors on UapA crystal structure are depicted with black spheres. (B) Detailed view of the topology of T401 and amino acids within 4 Å, including residues mutated in suppressors in black lettering (see text). The substrate (xanthine) is also shown. (C)–(F). Detailed view of I157F, T401F, L192F, and L431F mutations and amino acids within 4 Å.
Arg133 and Arg421 are essential for ER exit, function, and lipid-dependent stability
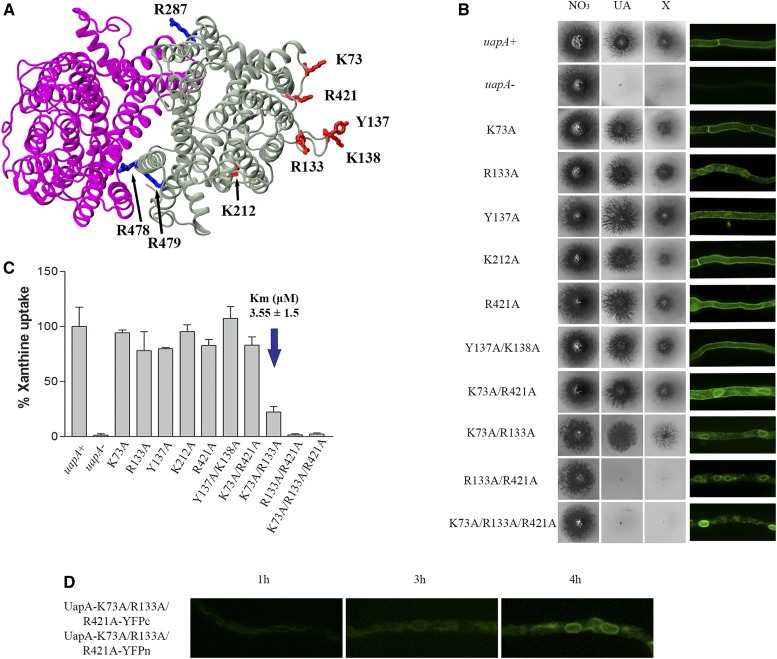
In addition to Arg287, Arg478, and Arg479 forming a lipid-binding site at the dimer interface of UapA, MD simulations identified other, cytosolic-facing residues located on the outside of the core domain that have the potential to form interactions with specific or annular lipids (Pyle et al. 2018). These residues, shown in Figure 5A, are Lys73 in the N-terminus just upstream of TMS1; Arg133, Tyr137, and Lys138 in loop L2; Lys212 in helix 1H of L4; and Arg421 in the border of L10 with TMS11. Of these residues, Arg421 is highly conserved in all NATs, whereas Lys73, Arg133, and Lys212 are well conserved in fungal NATs. Tyr137 and Lys138 are not conserved in other NATs (Figure S1).
Figure 5.
Arg133 and Arg421 are essential for ER exit, function, and lipid-dependent stability. (A) Topology of the predicted lipid-binding sites near the dimer interface (blue) and those in the membrane-facing regions (red) of the UapA dimer. (B) Growth tests of A. nidulans strains in minimal media supplemented with nitrate (NO3), uric acid (UA), or xanthine (X) as a nitrogen source at 37° (left panel). Control strains and concentrations of supplements are as in Figure 2. All UapA mutant strains are isogenic to the negative and positive control strains, expressing uapA alleles tagged with gfp. Inverted fluorescence microscopy images show localization of the GFP-tagged UapA constructs (ight panel). (C) Relative 3H-xanthine transport rates of UapA mutants expressed as percentages of initial uptake rates (V) compared to the wild-type (uapA+) rate. 3H-xanthine uptakes were performed at 37°. The Km value (μM) for xanthine for mutant K73A/R133A is indicated by a blue arrow. The results are averages of three measurements for each concentration point. SD was 20%. (D) Bimolecular complementation analysis of the K73A/R133A/R421A UapA mutant, performed as previously described in Figure 1 and Figure 3. Notice that the K73A/R133A/R421A mutant retains the ability to reconstitute a fluorescent signal in the ER, but not in the plasma membrane.
To investigate the potential functional role of the above residues, we constructed mutants expressing all single-, and selected double- and triple-Ala substitutions. Figure 5B shows that the single mutations did not affect growth on the UapA substrates (xanthine or uric acid). Of the four double mutants constructed, Y137A/K138A and K73A/R421A exhibited no UapA-related growth defect, while mutant K73A/R133A specifically showed significantly reduced growth on xanthine. Mutant R133A/R421A scored as an apparent total loss-of-function mutant, as it did not grow on either uric acid or xanthine. Finally, the triple K73A/R133A/R421A mutant also scored as a total loss-of-function mutant. Overall, Arg133 and Arg421 proved very important for UapA function, while Lys73 (when present in the context of R133A) was critical for UapA specificity for xanthine, but not for uric acid. For each mutant version of UapA we also assessed localization to the PM, compared to the wild-type UapA, using the GFP epitope attached to UapA. This analysis (right panel in Figure 5B) showed that most single mutations and Y137A/R138A, which led to no defect in UapA transport activity, allowed normal localization of UapA in the PM, as was expected. On the other hand, R133A and K73A/R421A mutants showed partial UapA retention in perinuclear ER membranes. Finally, mutants with apparently defective (K73A/R133A) or lost (R133A/R421A and K73A/R133A/R421A) transport activity showed partial or total retention in the ER. Growth tests and subcellular localization were in good agreement with measurements of rates of radiolabeled xanthine accumulation, which confirmed that the simultaneous presence of Arg133 and Arg421 is essential for transport activity, whereas Lys73 is critical for xanthine uptake only when Arg133 is also replaced by Ala (Figure 5C). For the double mutant K73A/R133A, which showed reduced growth on xanthine, we also measured the Km for xanthine and showed that this was very close to that of the wild-type UapA (3.6 vs. 5 ± 2 μΜ), suggesting that reduced growth on xanthine is not assigned to reduced substrate binding.
We investigated whether the lack of UapA sorting out of the ER in the triple K73A/R133A/R421A mutant is related to its ability to dimerize. For this, we employed BiFC assays, as described before for the R287A/R478A/R479A mutant. Figure 5D shows that, rather surprisingly, the K73A/R133A/R421A mutant could apparently dimerize in the ER, as a strong fluorescence signal was reconstituted associated with the ER membrane network. This was in line with western blot analysis that detected persisting dimeric forms of K73A/R133A/R421A (Figure S2). These findings suggested that UapA dimerization is not sufficient for ER exit and further sorting to the PM. This is also in agreement with the observation that a lack or reduction of dimerization, seen in the R287A/R478A/R479A mutant (Pyle et al. 2018), did not interfere with proper ER exit and sorting to the PM. Thus, functional dimerization and trafficking processes, seemingly affected by distinct lipid-interacting residues, are not necessarily related. Noticeably, attempts to purify the K73A/R133A/R421A protein showed that this version of UapA is prone to aggregation and is highly unstable (Figure S3). Given that the K73A/R133A/R421A protein is very stable in total extracts, the instability observed upon purification is consistent with the removal of lipids.
Substitution T401P partially restores the lipid-dependent functional defects of K73A/R133A/R421A
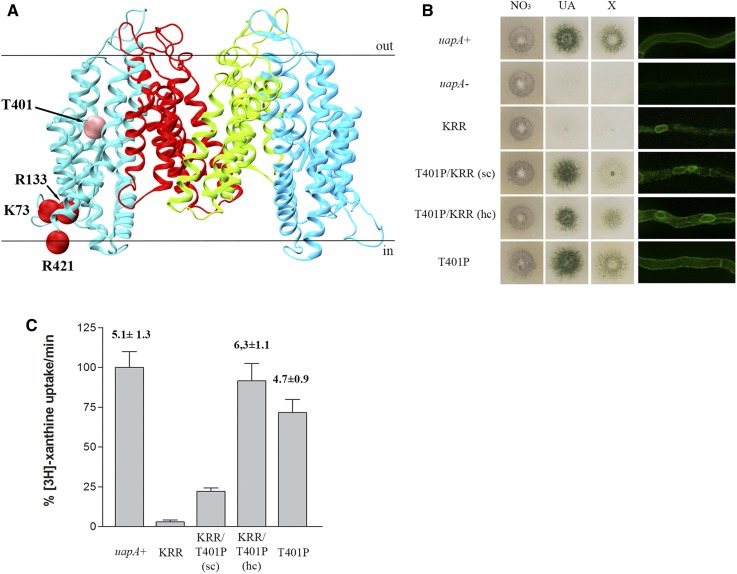
We used a genetic approach to understand how residues Lys73, Arg133, and Arg421 might affect UapA sorting to the PM by isolating suppressor mutations that restored UapA-mediated growth on uric acid in the mutant K73A/R133A/R421A, as described earlier for the isolation suppressors of the R287A/R478A/R479A mutant. We obtained, purified, and sequenced the uapA ORF from nine suppressors. Rather surprisingly, all proved to include the same single mutation, namely T401P, in addition to the original K73A/R133A/R421A triple mutation (Figure 6A). Noticeably, T401P was also isolated among the suppressors of the dimerization-defective R287A/R478A/R479A mutant. Growth tests showed that although T401P confers normal growth on xanthine and uric acid in the context of K73A/R133A/R421A (Figure 6B), this occurs by only partial restoration of UapA-mediated transport of these purines (Figure 6C). By targeted mutagenesis, we also constructed plasmid vectors carrying K73A/R133A/R421A/T401P and T401P alone, introduced them in the A. nidulans strain lacking endogenous nucleobase transporters, and analyzed several purified transformants. Those carrying the quadruple mutation K73A/R133A/R421A/T401P behaved as the original suppressor, confirming that T401P is the causative mutation suppressing the lack of function in R287A/R478A/R479A. Transformants expressing UapA-T401P behaved nearly as well as a wild-type UapA control, showing 70% transport rates, and normal growth on xanthine or uric acid. Epifluorescence microscopy of K73A/R133A/R421A/T401P and T401P was in line with growth tests and uptake transport measurements. In particular, T401P, in the genetic context of K73A/R133A/R421A, partially restored sorting of UapA to the PM, while when present by itself it did not affect UapA localization to the PM (right panel in Figure 6B). High-copy transformants expressing K73A/R133A/R421A/T401P further confirmed that a major fraction of the mutant UapA translocates to the PM, despite some persistent retention in the ER (see Figure 6B). The positive effect of T401P in the context of K73A/R133A/R421A was further shown by the isolation of fairly stable, detergent-solubilized K73A/R133A/R421A/T401P protein. Native MS further showed that the purified K73A/R133A/R421A/T401P protein, despite being mostly monomeric, also formed a distinct population of dimers (Figure S4). As mentioned above, although attempts were made to isolate and analyze the K73A/R133A/R421A mutant, the construct aggregated completely during purification indicating partial or total misfolding of this protein. Thus, all evidence showed that T401P not only favors functional UapA dimerization in the R287A/R478A/R479A context, but also partially restores ER exit, sorting, and function in the K73A/R133A/R421A context.
Figure 6.
Substitution T401P partially restores the lipid-dependent functional defects of K73A/R133A/R421A. (A) Topologies of amino acids modified in K73A/R133A/R421A suppressors. Core domains are colored light blue, and dimerization domains red and green. Mutated amino acids in the original strain are shown with red spheres and in the suppressor with pink spheres. (B) Growth tests of K73A/R133A/R421A suppressors in minimal media supplemented with nitrate (NO3), uric acid (UA), and xanthine (X) as nitrogen sources at 37° (left panel). Control strains and supplement concentrations are as in previous Figures. All mutants are isogenic to the negative and positive control strains, expressing uapA alleles tagged with GFP. Inverted fluorescence microscopy images show localization of the GFP-tagged UapA constructs (right panel). KRR depicts K73A/R133A/R421A, sc depicts single copy transformants and hc depicts high copy transformants. (C) Relative 3H-xanthine transport rates of UapA mutant versions expressed as percentages of initial uptake rates (V) compared to the wild-type (uapA+) rate. 3H-xanthine uptakes were performed at 37°. Km values (μM) for xanthine are shown. Results are averages of three measurements for each concentration point. SD was 20%.
Manipulation of a residue topologically equivalent to T401P leads to functional expression of a mammalian NAT homolog in A. nidulans
While A. nidulans or S. cerevisiae have been successfully used to functionally express plant solute transporters [see early examples in Schachtman et al. (1997), von Wirén et al. (2000), and Fischer et al. (2002)], including a UapA homolog (Argyrou et al. 2001), long-standing efforts of our group and many others have failed to functionally express metazoan solute transporters in model fungi. In all cases, metazoan transporters are retained in the ER of fungi and often elicit an unfolded protein response. The simplest explanation for this outcome is that the membrane environment of the fungal ER is incompatible with packaging metazoan transporters into COPII secretory vesicles (Borgese 2016). Based on this idea, and the fact that T401 might be key in restoring lipid-dependent defects in UapA subcellular sorting and function, seemingly by increasing the compactness of the core domain of UapA, we thought we might achieve the functional expression of metazoan NATs in A. nidulans by manipulating similar key residues involved in interactions with lipids.
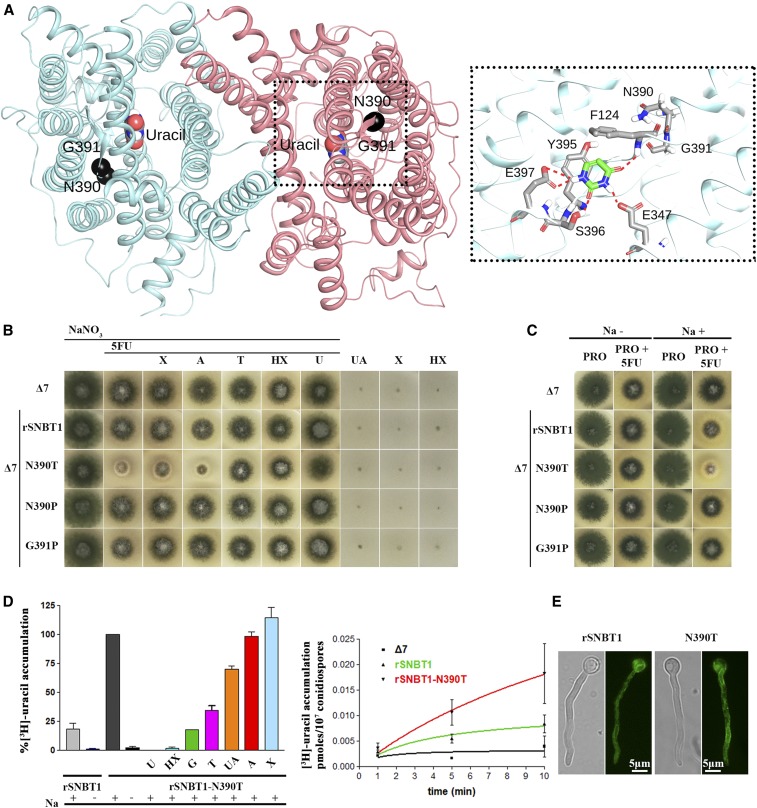
To test this idea, we used a characterized NAT homolog from rat, rSNBT1, which similarly to UapA acts as nucleobase transporter (Yamamoto et al. 2010; Yasujima et al. 2018). Noticeably, UapA and rSNBT1 have different specificities and cation dependence, as rSNBT1 is a rather promiscuous Na+ symporter specific for pyrimidines (uracil and thymine) and most purines (hypoxanthine, guanine, xanthine, and uric acid), while UapA is quite specific for xanthine and uric acid, and utilizes H+ for cotransport. Previous attempts to express rSNBT1 or rSNBT1/UapA chimeric transporters in A. nidulans or S. cerevisiae have failed, always due to total ER retention (A. Kourkoulou, C. Gournas, S. Amillis, B. Byrne, and G. Diallinas, unpublished data). To putatively identify residue(s) in rSNBT1 that are topologically and functionally equivalent to T401 of UapA, we built a structural model by homology threading using the available UapA crystal structure (Figure 7A and Figure S5). Candidate residues, equivalent to T401 in UapA, proved to be Asn390 and Gly391. Both are part of a conserved, short sequence motif, Gly-Thr-Gly-Asn390-Gly391, which is present in all metazoan NAT members irrespective of their specificity (Kourkoulou et al. 2018). In ascomycetes, the analogous sequence motif is Φ-Thr-Φ-Thr-Pro (Φ stands for aliphatic amino acid), while in basidiomycetes and other more primitive fungi it is less well conserved, being Φ-Thr-Φ-Thr/Ser/Ala/Pro-Pro. In other words, what clearly distinguishes metazoans from fungi in this region is the replacement of the Asn residue (390 in rSNBT1) with Thr, and the last Gly (391 in rSNBT1) with a Pro.
Figure 7.
Manipulation of a residue topologically equivalent to T401P leads to functional expression of a mammalian NAT homolog in A. nidulans. (A) Homology modeling of the topology of rSNBT1, constructed using, as described in the Materials and Methods, the inward-facing conformation of the crystal structure of the UapA dimer. The two mutated and functionally analyzed residues, N390 and G391, are shown as black spheres. The location of uracil, the major substrate of rSNBT1, is also depicted, as determined by dynamic docking (left panel). In the right panel, a zoomed-out picture of the substrate-binding site depicting the major interactions of uracil with specific residues. (B) Growth tests of isogenic A. nidulans strains expressing single-copy wild-type rSNBT1 or its mutated versions rSNBT1-N390T, rSNBT1-N390P, and rSNBT1-G391P. A negative control strain (i.e., the recipient Δ7 strain that has null activity for nucleobase transport; see text) is included for comparison. Growth tests were performed at 37° on minimal media supplemented with Na+ (100 mM NaCl); 10 mM NaNO3 was used as a control nitrogen source unrelated to purine transport activities in all tests scoring resistance/sensitivity to 5FU (rows 1–7). Rows 3–7 represent in vivo competition assays scoring the ability of excess purines (2 mM) to compete with the uptake of 5FU (100 μΜ), and thus revert 5FU sensitivity. Χ is xanthine, A is adenine, T is Thymine, HX is hypoxanthine, and U is uracil. Notice that T, HX, and U competed with 5FU uptake and suppressed sensitivity. Growth was also scored on minimal media containing UA (uric acid), X, or HX as sole nitrogen sources, none of which supported growth of the strains tested (three last rows). (C) Growth tests of A. nidulans of the same strains as in (A), on minimal media plus proline as a sole nitrogen source, supplemented or not with 100 mM NaCl. Notice that rSNBT1-N390T-mediated 5FU sensitivity is dependent on the presence of Na+ supplementation. Notice also that in the presence of proline as a nitrogen source, the wild-type rSNBT1 allele confers very moderate sensitivity to 5FU. (D) Left panel: 3H-uracil (0.1 μΜ) accumulation in strains expressing rSNBT1 and rSNBT1-N390T, performed in the presence or absence of 100 mM Na+, and in the presence or absence of excess (2 mM) unlabeled nucleobases, after a period of 10 min incubation with radiolabeled substrate. 3H-uracil accumulation in rSNBT1-N390T in the presence of 100 mM Na+ and absence of unlabeled nucleobase is arbitrarily taken as 100%. Right panel: relative 3H-uracil (0.1 μΜ) transport accumulation in Δ7 (negative control), rSNBT1, or rSNBT1-N390T strains as a time course. Uptake results are averages of three measurements for each concentration point. SD was 20%. (E) Inverted fluorescence microscopy images showing the subcellular localization of the GFP-tagged rSNBT1 and rSNBT1-N390T constructs. Notice that the strains used for microscopy are identical to those used in growth tests and uptake assays, as in all cases rSNBT1 sequences were tagged C-terminally with GFP (see Materials and Methods).
Based on the above observations, we constructed and analyzed isogenic A. nidulans strains expressing the wild-type tSNBT1, or mutated versions with substitutions N390P, N390T, or G391P, in a genetic background lacking all endogenous transporters related to purine or pyrimidine uptake (see Materials and Methods). Strains expressing the wild-type form or mutations N390P and G391P did not grow on purines, and were resistant to 5FU (i.e., a test for scoring uracil uptake), similarly to the recipient negative control strain lacking all endogenous nucleobase transporters. In contrast, the strain expressing rSNBT1-N390T showed clear sensitivity to 5FU and, although it could not grow on any purine, 5FU sensitivity could be competed in the presence of excess purines or pyrimidines that are known rSNBT1 substrates (e.g., hypoxanthine, uracil, or thymine) (Figure 7B). Importantly, rSNBT1-dependent 5FU sensitivity was Na+-dependent (Figure 7C), compatible with the physiological mechanism of functioning of rSNBT1 in rat (Yamamoto et al. 2010; Yasujima et al. 2018).
To further confirm that the phenotype observed in the relative transformants is due uniquely to the genetically introduced rSNBT1-N390T protein, we also analyzed the meiotic progeny of an rSNBT1-N390T transformant. A. nidulans undergoing meiosis during a process called “selfing” (van den Brink-van der Laan et al. 2004) are prone to high recombination rates that often lead to the loss of sequences introduced by transformation. Figure S6 shows that in an analysis of 28 meiotic progenital colonies of an original rSNBT1-N390T transformant, 21 colonies conserved the original sensitivity to 5FU, while 7 colonies appeared to be 5FU-resistant. Subsequent epifluorescence analysis of selected colonies showed that in all cases 5FU sensitivity was conserved, a fluorescent signal from the rSNBT1-N390T protein tagged with GFP was also conserved. In contrast, all selected colonies that acquired resistance to 5FU lost the fluorescent signal of the rSNBT1-N390T-GFP (not shown).
Finally, we also performed direct measurements of radiolabeled uracil accumulation or competition in the strain expressing rSNBT1-N390T, which further confirmed the functionality of the rat transporter in A. nidulans (Figure 7D). Noticeably, the low apparent transport capacity of rSNBT1-N390T with respect to some of its substrates (e.g., hypoxanthine or uric acid) in A. nidulans is very probably due by the observation that mutation N390T restores sorting of rSNBT1 to the PM only partially (Figure 7E).
Discussion
It is becoming well established that the physicochemical nature of lipid bilayers and the specific lipid composition of membranes affect transporter folding, oligomerization, subcellular trafficking, function, and turnover (van den Brink-van der Laan et al. 2004; Koshy et al. 2013; Laganowsky et al. 2014; Koshy and Ziegler 2015; Martens et al. 2016; Gupta et al. 2017; Henrich et al. 2017; Landreh et al. 2017). For transporters conforming to the 5 + 5 inverted repeat or LeuT fold, similarities in structurally resolved lipid–protein interactions suggest common ways in which transporter structure and function are supported by lipid interactions (Drew and Boudker 2016). These are likely to include stabilization of the inverted repeat topology, but also mechanistic roles as major determinants of the alternating access mechanism of secondary transporters. Noticeably however, the great majority of studies on transporter–lipid interactions have to date focused on prokaryotic transporters.
To our knowledge, our previous study on UapA–lipid interactions still remains the only one focusing on a eukaryotic transporter (Pyle et al. 2018). In brief, we have shown that UapA, which primarily exists as a dimer, dissociates into monomers upon removal of tightly bound lipids, and that the dimer can be recovered by the addition of PI or PE. Furthermore, as mutagenesis of tentative lipid-binding Arg residues 287, 478, and 479, predicted by MD, abolished lipid binding and function, we have proposed that PI and PE bind at specific sites in the dimer interface, and thus stabilize the dimeric functional form of UapA (Pyle et al. 2018). The total lack of transport activity in R287A/R478A/R479A, despite the fact that in the mutant a degree of dimerization and normal sorting to the PM was still evident, suggested that lipid binding may also be directly essential for the mechanism of transport. Here, we studied further the role of Arg287, Arg478, and Arg479, and in parallel investigated the role of binding of lipids at specific peripheral residues of UapA. We showed that Arg287, Arg478, and Arg479 are essential for early de novo formation in the ER membrane, a process absolutely essential for transport activity, albeit not for sorting to the PM. In parallel, we identified distinct positively charged residues (Lys73, Arg133, and Arg421), exposed to the PM membrane bilayer, which are essential for ER exit, sorting to the PM, and transport activity, but apparently not essential for the initial formation of dimers in the ER. Thus, the two sets of positively charged residues define two distinct sites of interaction of UapA with membrane lipids, both essential for function, albeit due to different reasons. The distinct defects caused by Ala substitutions at the dimer interface or those exposed to the inner side of the PM bilayer are well supported by epifluorescence microscopy, BiFC assays, and native MS. Thus, while substitutions of Arg287, Arg478, and Arg479 did not affect UapA stability and sorting to the PM, substitutions of Lys73, Arg133, and Arg421 led to significant protein instability and ER retention. Interestingly, in no case did the mutant UapA versions studied elicit an unfolded protein response (results not shown), suggesting that they probably do not lead to significant misfolding. This is particularly interesting in the case of the K73A/R133A/R421A mutant, which is totally blocked within the ER membrane. This observation further suggests that interactions with specific peripheral lipids might be crucial for specific packaging into COPII secretory vesicles and ER exit.
The most original finding of the present work stems from the isolation of genetic suppressors that partially restore defects caused due to modified interactions of UapA with specific lipids at the dimer interface. In principle, genetic reversion of the lost apparent activity of UapA in the original mutants could have led us to either intragenic or extragenic suppressors, the latter probably concerning proteins assisting ER exit and/or sorting of UapA to the PM. However, we did not obtain extragenic suppressors. The reason for that might be found in our recent findings that show that the only currently known factors needed for UapA sorting to the PM are COPII components, actin, and the clathrin heavy chain, all proteins essential for cell viability. Most of the suppressors isolated mapped in the center of the core domain, and less frequently in the dimerization domain. No rational approach or MD studies could have predicted the functional importance of the residues identified via unbiased genetics. How these residues might correct defects in lipid binding became possible a posteriori with the help of MD and by taking into account the biophysical nature of residues introduced by suppressor mutations.
We can classify suppressors of the original dimerization mutant, R287A/R478A/R479A, into three types. Type I, which contains the majority of suppressors, map in the center of the core domain in TMS2, TMS3, TMS4, TMS9, TMS10, and TMS11. All, except one, introduce residues with increased hydrophobicity and/or aromaticity (V150I, I157F, I157L, L192F, A396P, T401P, T401F, or L431F). Only V153T introduces a polar residue, while S119T replaces a polar residue with a longer residue of similar properties. MD suggested that all these changes increase the strength of the relative TMS interactions, and thus the compactness of the core domain and the stability of the protein. Type II includes L234M in TMS5 in the middle plane of the dimerization domain. This mutation could increase the strength of interactions between the dimerization and core domains. Type III includes E286Q and E286K at the end of the cytoplasmic-facing part of TMS6. The most logical scenario for these last two suppressors is that they replace directly the interactions with lipids of the nearby mutated Arg287 (i.e., in R287A). This is also in line with the fact that these are the strongest isolated suppressors in respect to UapA transport activity. Thus, our findings, especially those concerning type I and II suppressors, strongly suggest that by stabilizing the core, which is the motile part of the monomeric units that undergoes dynamic up-and-down elevator-like sliding, the dimer is also stabilized and thus function is restored.
Interestingly, all isolated suppressors of the trafficking mutant K73A/R133A/R421A proved to correspond to substitution T401P, a mutation that also restored the dimerization mutant R287A/R478A/R479A. How this is achieved remains quite unclear, mostly because we still do not understand the molecular basis of the trafficking defect in the original mutant. Based on BiFC assays, western blot analysis, and native MS, R287A/R478A/R479A was shown to form dimers, but these seem much more unstable and less abundant with respect to monomers compared to wild-type UapA (Pyle et al. 2018). Thus, despite the distinct defects caused by K73A/R133A/R421A and R287A/R478A/R479A triple mutations in UapA subcellular localization, both seem to affect the stability and function of UapA via abolition of essential but distinct interactions with specific lipids. Apparently, mutation K73A/R133A/R421A was more critical than R287A/R478A/R479A for the packaging of UapA into COPII secretory vesicles and ER exit (Bouris et al. 2019), which might in turn suggest that specific peripheral lipid interactions are more important for the trafficking of UapA and other structurally similar eukaryotic transporters. In line with our results, it has been recently suggested that lipid binding around domain interfaces of the prokaryotic NhaA Na+/H+ exchanger are also involved in stabilizing the core domain during the conformational transitions required for transport by the elevator mechanism (Landreh et al. 2017). Thus, it has been speculated that elevator-type antiporters use a subset of specific peripheral or annular lipids as structural support to facilitate large-scale conformational changes within the membrane. Other recent studies using native MS and functional assays have also demonstrated that protein–lipid interactions play a crucial role in stabilizing the dimer form of prokaryotic transporters conforming to the 7 + 7 inverted repeat topology found in transporters using the sliding elevator mechanism of transport (Arakawa et al. 2015; Gupta et al. 2017).
An impressive finding of our work was that a single mutation, T401P, proved to be a key residue in restoring defective interactions with specific lipids either at the dimer interface or peripherally. We made use of this information and achieved, for the first time, the functional expression of a rat homolog of UapA, rSNBT1, in A. nidulans. The successful expression of rSNBT1 in A. nidulans strongly supports the idea that the bottleneck in expressing metazoan transporters in fungi is proper folding in an environment of heterologous membrane lipid composition. Our achievement opens the way for further manipulations, via rational design or unbiased genetic screens, of lipid-binding residues in transporters for their functional expression and manipulation in genetically tractable model fungal systems, such as A. nidulans or S. cerevisiae. Additionally, the successful functional expression of metazoan homologs in A. nidulans leads to new routes for identifying and studying the evolution of novel functions and substrate specificities in the NAT family, such as, for example, understanding how human NAT homologs have evolved to become specific for vitamin C rather than nucleobases (Kourkoulou et al. 2018).
Acknowledgments
This work was supported by a Stavros S. Niarchos Foundation grant to A.K. and G.D., by computational time granted from the Greek Research and Technology Network in the National High-Performing Computing facility (ARIS) under project number NCS1_Mechanism (pr006040), and a Biotechnology and Biosciences Research Council grant (B.B./N016467/1). E.P. is the recipient of an Imperial College London Institute of Chemical Biology Engineering and Physical Sc.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.9177566.
Communicating editor: M. Schuldiner
Literature Cited
- Abraham M. J., Murtola T., Schulz R., Páll S., Smith J. C. et al. , 2015. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2: 19–25. 10.1016/j.softx.2015.06.001 [DOI] [Google Scholar]
- Alguel Y., Amillis S., Leung J., Lambrinidis G., Capaldi S. et al. , 2016. Structure of eukaryotic purine/H(+) symporter UapA suggests a role for homodimerization in transport activity. Nat. Commun. 7: 11336 10.1038/ncomms11336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa T., Kobayashi-Yurugi T., Alguel Y., Iwanari H., Hatae H. et al. , 2015. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science 350: 680–684. 10.1126/science.aaa4335 [DOI] [PubMed] [Google Scholar]
- Argyrou E., Sophianopoulou V., Schultes N., and Diallinas G., 2001. Functional characterization of a maize purine transporter by expression in Aspergillus nidulans. Plant Cell 13: 953–964. 10.1105/tpc.13.4.953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J. L., Beard H. S., Cao Y., Cho A. E., Damm W. et al. , 2005. Integrated modeling program, applied chemical theory (IMPACT). J. Comput. Chem. 26: 1752–1780. 10.1002/jcc.20292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara C., and Robinson C. V., 2015. Different modes of lipid binding to membrane proteins probed by mass spectrometry. J. Am. Chem. Soc. 137: 5240–5247. 10.1021/jacs.5b00420 [DOI] [PubMed] [Google Scholar]
- Borgese N., 2016. Getting membrane proteins on and off the shuttle bus between the endoplasmic reticulum and the Golgi complex. J. Cell Sci. 129: 1537–1545. 10.1242/jcs.183335 [DOI] [PubMed] [Google Scholar]
- Bouris V, O. Martzoukou, S. Amillis, and G. Diallinas, 2019 Nutrient transporter translocation to the plasma membrane via a Golgi-independent unconventional route. bioRxiv. Available at: 10.1101/540203 10.1101/540203 [DOI]
- César-Razquin A., Snijder B., Frappier-Brinton T., Isserlin R., Gyimesi G. et al. , 2015. A call for systematic Research on solute carriers. Cell 162: 478–487. 10.1016/j.cell.2015.07.022 [DOI] [PubMed] [Google Scholar]
- Chang Y. N., Jaumann E. A., Reichel K., Hartmann J., Oliver D. et al. , 2019. Structural basis for functional interactions in dimers of SLC26 transporters. Nat. Commun. 10: 2032 10.1038/s41467-019-10001-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallinas G., 2014. Understanding transporter specificity and the discrete appearance of channel-like gating domains in transporters. Front. Pharmacol. 5: 207 10.3389/fphar.2014.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallinas G., 2016. Dissection of transporter function: from genetics to structure. Trends Genet. 32: 576–590. 10.1016/j.tig.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Drew D., and Boudker O., 2016. Shared molecular mechanisms of membrane transporters. Annu. Rev. Biochem. 85: 543–572. 10.1146/annurev-biochem-060815-014520 [DOI] [PubMed] [Google Scholar]
- Fischer W. N., Loo D. D., Koch W., Ludewig U., Boorer K. J. et al. , 2002. Low and high affinity amino acid H+-cotransporters for cellular import of neutral and charged amino acids. Plant J. 29: 717–731. 10.1046/j.1365-313X.2002.01248.x [DOI] [PubMed] [Google Scholar]
- Geertsma E. R., Chang Y. N., Shaik F. R., Neldner Y., Pardon E. et al. , 2015. Structure of a prokaryotic fumarate transporter reveals the architecture of the SLC26 family. Nat. Struct. Mol. Biol. 22: 803–808 [corrigenda: Nat. Struct. Mol. Biol. 23: 462 (2016)]. 10.1038/nsmb.3091 [DOI] [PubMed] [Google Scholar]
- Gournas C., Amillis S., Vlanti A., and Diallinas G., 2010. Transport-dependent endocytosis and turnover of a uric acid-xanthine permease. Mol. Microbiol. 75: 246–260. 10.1111/j.1365-2958.2009.06997.x [DOI] [PubMed] [Google Scholar]
- Gupta K., Donlan J. A. C., Hopper J. T. S., Uzdavinys P., Landreh M. et al. , 2017. The role of interfacial lipids in stabilizing membrane protein oligomers. Nature 541: 421–424. 10.1038/nature20820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich E., Peetz O., Hein C., Laguerre A., Hoffmann B. et al. , 2017. Analyzing native membrane protein assembly in nanodiscs by combined non-covalent mass spectrometry and synthetic biology. Elife 6: e20954. 10.7554/eLife.20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. M., and Spang A., 2015. Intracellular parcel service: current issues in intracellular membrane trafficking. Methods Mol. Biol. 1270: 1–12. 10.1007/978-1-4939-2309-0_1 [DOI] [PubMed] [Google Scholar]
- Huang J., and MacKerell A. D. Jr, 2013. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34: 2135–2145. 10.1002/jcc.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W., Dalke A., and Schulten K., 1996. Vmd - visual molecular dynamics. J. Mol. Graph. 14: 33–38. 10.1016/0263-7855(96)00018-5 [DOI] [PubMed] [Google Scholar]
- Huynh K. W., Jiang J., Abuladze N., Tsirulnikov K., Kao L. et al. , 2018. CryoEM structure of the human SLC4A4 sodium-coupled acid-base transporter NBCe1. Nat. Commun. 9: 900 10.1038/s41467-018-03271-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. P., Pincus D. L., Rapp C. S., Day T. J., Honig B. et al. , 2004. A hierarchical approach to all-atom protein loop prediction. Proteins 55: 351–367. 10.1002/prot.10613 [DOI] [PubMed] [Google Scholar]
- Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., and Klein M. L., 1983. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79: 926–935. 10.1063/1.445869 [DOI] [Google Scholar]
- Karachaliou M., Amillis S., Evangelinos M., Kokotos A. C., Yalelis V. et al. , 2013. The arrestin-like protein ArtA is essential for ubiquitination and endocytosis of the UapA transporter in response to both broad-range and specific signals. Mol. Microbiol. 88: 301–317. 10.1111/mmi.12184 [DOI] [PubMed] [Google Scholar]
- Koshy C., and Ziegler C., 2015. Structural insights into functional lipid-protein interactions in secondary transporters. Biochim. Biophys. Acta 1850: 476–487. 10.1016/j.bbagen.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Koshy C., Schweikhard E. S., Gärtner R. M., Perez C., Yildiz O. et al. , 2013. Structural evidence for functional lipid interactions in the betaine transporter BetP. EMBO J. 32: 3096–3105. 10.1038/emboj.2013.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukaki M., Giannoutsou E., Karagouni A., and Diallinas G., 2003. A novel improved method for Aspergillus nidulans transformation. J. Microbiol. Methods 55: 687–695. 10.1016/S0167-7012(03)00208-2 [DOI] [PubMed] [Google Scholar]
- Koukaki M., Vlanti A., Goudela S., Pantazopoulou A., Gioule H. et al. , 2005. The nucleobase-ascorbate transporter (NAT) signature motif in UapA defines the function of the purine translocation pathway. J. Mol. Biol. 350: 499–513. 10.1016/j.jmb.2005.04.076 [DOI] [PubMed] [Google Scholar]
- Kourkoulou A., Pittis A. A., and Diallinas G., 2018. Evolution of substrate specificity in the Nucleobase-Ascorbate Transporter (NAT) protein family. Microb. Cell 5: 280–292. 10.15698/mic2018.06.636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krypotou E., and Diallinas G., 2014. Transport assays in filamentous fungi: kinetic characterization of the UapC purine transporter of Aspergillus nidulans. Fungal Genet. Biol. 63: 1–8. 10.1016/j.fgb.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Krypotou E., Evangelidis T., Bobonis J., Pittis A. A., Gabaldón T. et al. , 2015. Origin, diversification and substrate specificity in the family of NCS1/FUR transporters. Mol. Microbiol. 96: 927–950. 10.1111/mmi.12982 [DOI] [PubMed] [Google Scholar]
- Laganowsky A., Reading E., Allison T. M., Ulmschneider M. B., Degiacomi M. T. et al. , 2014. Membrane proteins bind lipids selectively to modulate their structure and function. Nature 510: 172–175. 10.1038/nature13419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreh M., Marklund E. G., Uzdavinys P., Degiacomi M. T., Coincon M. et al. , 2017. Integrating mass spectrometry with MD simulations reveals the role of lipids in Na+/H+ antiporters. Nat. Commun. 8: 13993 10.1038/ncomms13993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwers E., Erpapazoglou Z., Haguenauer-Tsapis R., and André B., 2010. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 20: 196–204. 10.1016/j.tcb.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Lee J., Cheng X., Swails J. M., Yeom M. S., Eastman P. K. et al. , 2016. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 12: 405–413. 10.1021/acs.jctc.5b00935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGurn J. A., Hsu P. C., and Emr S. D., 2012. Ubiquitin and membrane protein turnover: from cradle to grave. Annu. Rev. Biochem. 81: 231–259. 10.1146/annurev-biochem-060210-093619 [DOI] [PubMed] [Google Scholar]
- Martens C., Stein R. A., Masureel M., Roth A., Mishra S. et al. , 2016. Lipids modulate the conformational dynamics of a secondary multidrug transporter. Nat. Struct. Mol. Biol. 23: 744–751. 10.1038/nsmb.3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty M. T., Baldwin A. J., Marklund E. G., Hochberg G. K., Benesch J. L. et al. , 2015. Bayesian deconvolution of mass and ion mobility spectra: from binary interactions to polydisperse ensembles. Anal. Chem. 87: 4370–4376. 10.1021/acs.analchem.5b00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martzoukou O., Karachaliou M., Yalelis V., Leung J., Byrne B. et al. , 2015. Oligomerization of the UapA purine transporter is critical for ER-exit, plasma membrane localization and turnover. J. Mol. Biol. 427: 2679–2696. 10.1016/j.jmb.2015.05.021 [DOI] [PubMed] [Google Scholar]
- Pantazopoulou A., Lemuh N. D., Hatzinikolaou D. G., Drevet C., Cecchetto G. et al. , 2007. Differential physiological and developmental expression of the UapA and AzgA purine transporters in Aspergillus nidulans. Fungal Genet. Biol. 44: 627–640. 10.1016/j.fgb.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Pyle E., Kalli A. C., Amillis S., Hall Z., Lau A. M. et al. , 2018. Structural lipids enable the formation of functional oligomers of the eukaryotic purine symporter UapA. Cell Chem. Biol. 25: 840–848.e4. 10.1016/j.chembiol.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C., 2017. Retriever fetches integrins from endosomes. Nat. Cell Biol. 19: 1144–1146. 10.1038/ncb3612 [DOI] [PubMed] [Google Scholar]
- Rigaud J. L., and Lévy D., 2003. Reconstitution of membrane proteins into liposomes. Methods Enzymol. 372: 65–86. 10.1016/S0076-6879(03)72004-7 [DOI] [PubMed] [Google Scholar]
- Schachtman D. P., Kumar R., Schroeder J. I., and Marsh E. L., 1997. Molecular and functional characterization of a novel low-affinity cation transporter (LCT1) in higher plants. Proc. Natl. Acad. Sci. USA 94: 11079–11084. 10.1073/pnas.94.20.11079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman W., Day T., Jacobson M. P., Friesner R. A., and Farid R., 2006. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 49: 534–553. 10.1021/jm050540c [DOI] [PubMed] [Google Scholar]
- Takeshita N., Higashitsuji Y., Konzack S., and Fischer R., 2008. Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 19: 339–351. 10.1091/mbc.e07-06-0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurtle-Schmidt B. H., and Stroud R. M., 2016. Structure of Bor1 supports an elevator transport mechanism for SLC4 anion exchangers. Proc. Natl. Acad. Sci. USA 113: 10542–10546. 10.1073/pnas.1612603113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink-van der Laan E., Killian J. A., and de Kruijff B., 2004. Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim. Biophys. Acta 1666: 275–288. 10.1016/j.bbamem.2004.06.010 [DOI] [PubMed] [Google Scholar]
- von Wirén N., Lauter F. R., Ninnemann O., Gillissen B., Walch-Liu P. et al. , 2000. Differential regulation of three functional ammonium transporter genes by nitrogen in root hairs and by light in leaves of tomato. Plant J. 21: 167–175. 10.1046/j.1365-313x.2000.00665.x [DOI] [PubMed] [Google Scholar]
- Wu E. L., Cheng X., Jo S., Rui H., Song K. C. et al. , 2014. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35: 1997–2004. 10.1002/jcc.23702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Inoue K., Murata T., Kamigaso S., Yasujima T. et al. , 2010. Identification and functional characterization of the first nucleobase transporter in mammals: implication in the species difference in the intestinal absorption mechanism of nucleobases and their analogs between higher primates and other mammals. J. Biol. Chem. 285: 6522–6531. 10.1074/jbc.M109.032961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasujima T., Murata C., Mimura Y., Murata T., Ohkubo M. et al. , 2018. Urate transport function of rat sodium-dependent nucleobase transporter 1. Physiol. Rep. 6: e13714 10.14814/phy2.13714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Yang G., Yan C., Baylon J. L., Jiang J. et al. , 2017. Dimeric structure of the uracil proton symporter UraA provides mechanistic insights into the SLC4/23/26 transporters. Cell Res. 27: 1020–1033. 10.1038/cr.2017.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplemental material available at figshare: https://doi.org/10.25386/genetics.9177566.