Abstract
Atherosclerosis, the main contributor to coronary heart disease, is characterised by an accumulation of lipids such as cholesterol in the arterial wall. Reverse cholesterol transport (RCT) reduces cholesterol via its conversion into bile acids (BAs). During RCT in non-hepatic peripheral tissues, cholesterol is transferred to high-density lipoprotein (HDL) particles and returned to the liver for conversion into BAs predominantly via the rate-limiting enzyme, cholesterol 7 α-hydroxylase (CYP7A1). Numerous reports have described that polyphenol induced increases in BA excretion and corresponding reductions in total and LDL cholesterol in animal and in-vitro studies, but the process whereby this occurs has not been extensively reviewed. There are three main mechanisms by which BA excretion can be augmented: (1) increased expression of CYP7A1; (2) reduced expression of intestinal BA transporters; and (3) changes in the gut microbiota. Here we summarise the BA metabolic pathways focusing on CYP7A1, how its gene is regulated via transcription factors, diurnal rhythms, and microRNAs. Importantly, we will address the following questions: (1) Can polyphenols enhance BA secretion by modulating the CYP7A1 biosynthetic pathway? (2) Can polyphenols alter the BA pool via changes in the gut microbiota? (3) Which polyphenols are the most promising candidates for future research? We conclude that while in rodents some polyphenols induce CYP7A1 expression predominantly by the LXRα pathway, in human cells, this may occur through FXR, NF-KB, and ERK signalling. Additionally, gut microbiota is important for the de-conjugation and excretion of BAs. Puerarin, resveratrol, and quercetin are promising candidates for further research in this area.
Keywords: atherosclerosis, reverse cholesterol transport, diurnal rhythms, microRNA, ASBT, flavonoid, phenolic acid, catechin, anthocyanin
1. Introduction
Atherosclerosis, the main contributor to coronary heart disease, is characterised by an accumulation of lipids in the arterial wall [1]. Polyphenols have been shown to confer beneficial effects against cardio-metabolic diseases. Among the mechanisms proposed for their beneficial effects is the alteration of bile acid metabolism. Here, we give a comprehensive review of research on the molecular mechanisms through which polyphenols exert their beneficial effects focusing on CYP7A1 and bile acid metabolism. The key points raised in this review include:
Polyphenols have been shown to have a wide range of beneficial effects, of note red wine, rich in flavonoids, phenolic polymers and resveratrol are promising as possible targets for further investigation.
- As well as giving a state-of- the-art review on the mechanisms through which polyphenols exert their beneficial effects focussing on CYP7A1 and bile acid metabolism, critical points that will be of benefit to clinical nutritionists, academic experts in the area of bioactive food compounds, and possible stakeholders have also been raised in this review namely;
- While polyphenols do have effects on bile acid metabolism, it should be born in mind that species differences, time of cull, dose, and length of treatment can also affect the results and as such, may lead to discrepancies between studies. As such, there is a need to standardise animal studies, ensuring that these factors are correctly reported.
- Polyphenols confer their beneficial effects partly through altering the microbiome, thus they could have useful prebiotic-like functions.
- There is a lack of data on the effects conferred by the parent compound versus those of their metabolic products, and future studies should aim to determine the effects of both the parent compounds and their metabolites, with particular emphasis on the gut microbiota.
- Further studies are required in humans to substantiate the mechanisms proposed using animal and cell culture models.
1.1. Reverse Cholesterol Transport
The reverse cholesterol transport (RCT) pathway removes excess cholesterol from the peripheral system including from lipid laden macrophages, thus preventing foam cell accumulation during atherosclerosis development. In this process, cholesterol is returned to the liver where it is converted into BAs predominantly via the cytochrome P450 enzyme, cholesterol 7 α-hydroxylase (CYP7A1), for subsequent excretion into the faeces (Figure 1).
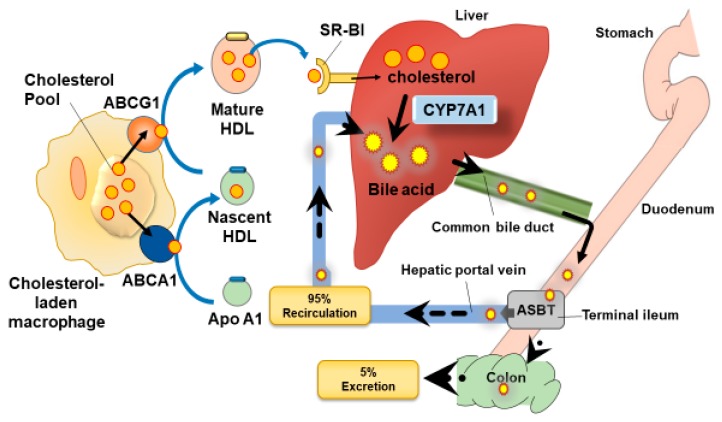
Figure 1.
The enterohepatic re-circulation of bile acids via reverse cholesterol transport. Cholesterol laden macrophages in the arterial wall deliver cholesterol via the ABCA1 transporter to lipid free apoA-I, preventing foam cell formation and also forming nascent HDL particles. Further lipidation of the nascent HDLs occurs via ABCG1. Cholesterol is delivered to the liver from mature HDL particles via specific HDL cholesterol efflux (CE) uptake by a scavenger receptor class B type I (SR-BI). In the liver, cholesterol is converted into BAs predominantly by the CYP7A1 neutral (classic) pathway. The BAs travel via the bile duct to the intestine, where they are de-conjugated via the bacteria and excreted or re-circulated (95%), usually in their conjugated form via passive diffusion or via active transport via the apical sodium dependent BA transporter (ASBT).
In humans, approximately 500 mg/day of cholesterol is converted into bile acids (Bas) in the liver and lost in the faeces [2]. Two pathways, namely the neutral (classic) and the alternative (acidic) pathways, are involved in cholesterol metabolism, with CYP7A1 being the rate-limiting enzyme in the former, and CYP7A1 being responsible for cholesterol metabolism in the latter. Both pathways result in the formation of the primary BAs chenodeoxycholic acid (CDCA) and cholic acid (CA), the neutral pathway being the most predominant [3]. The acidic pathway only contributes 9% and 25% of total BAs in humans and mice, respectively [4]. The biochemistry of BA biosynthesis is reviewed elsewhere [2,5] and is summarised in Figure 2.
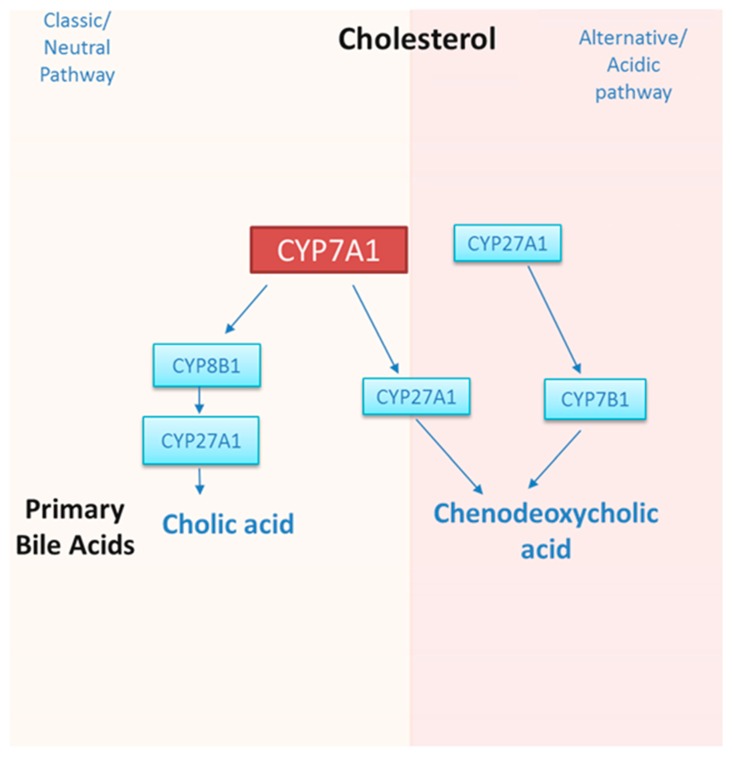
Figure 2.
A summary of the main enzymes involved in the classic and alternative bile acid biosynthesis pathways. The classic pathway is controlled by the rate limiting enzyme CYP7A1 and the alternative pathway is controlled by CYP27A1; both pathways culminate in the production of cholic acid (CA) and chenodexoycholic acid (CDCA), the ratio of which depends on the activity of CYP8B1. Briefly, bile-acid biosynthesis begins with the modification of the ring structure of cholesterol, which involves oxidation and shortening of the side chain [2]. In the classic pathway, cholesterol is converted into 7 α-hydroxycholesterol by CYP7A1 and in subsequent steps, cytochrome P450 Family 8 Subfamily B Member 1a (CYP8B1) and sterol 27-hydroxylase (CYP27A1) are required for the synthesis of cholic acid (CA). Without CYP8B1, the product is chenodeoxycholic acid (CDCA), which is formed via the activity of CYP27A1 alone. The acidic pathway (or alternative pathway) is initiated by CYP27A1 and relies on 25-hydroxycholesterol 7-alpha-hydroxylase (CYP7B1) to produce CDCA.
Before excretion into bile, primary BAs are conjugated with amino acids glycine and to a lesser degree taurine in humans [6]. In rodents, taurine is almost exclusively used for conjugation [6]. This increases solubility, minimises passive absorption, and makes the BAs resistant to cleavage by pancreatic carboxypeptidase. BAs are secreted from the gallbladder via the bile duct into the intestine and can be metabolised into their respective secondary BAs via the gut microbiota, which are also responsible for de-conjugation. Conjugated BAs are actively exported from the liver via the bile salt export pump (BSEP)/ABCB11, which in humans preferentially transports conjugated BAs, but can also transport unconjugated BAs [7]. The main function of BAs is to facilitate digestion in the gut and their synthesis inadvertently regulates lipid concentrations. A list of all known BAs and their abbreviations is given in Supplementary Table S1. BAs are reabsorbed in the intestine by either passive diffusion or by active transport, which occurs via the apical sodium BA transporter (ASBT) in the terminal portion of the ileum [8]. Once reabsorbed, BAs travel through the hepatic portal vein and are returned to the liver via specific transporters, with approximately 95% of BAs being recirculated back to the gallbladder in a process termed enterohepatic circulation, and the rest is excreted in the faeces (Figure 1). Typically, cholesterol synthesis equals its secretion as BAs; however, this equilibrium can be disturbed during disease states or by diet [9].
1.2. Regulation of CYP7A1 by Dietary Cholesterol, Circadian Rhythm, Transcription Factors, and microRNAs
Diet can regulate BA synthesis, particularly the intake of a cholesterol rich diet. In wild-type mice, feeding a high cholesterol diet stimulated BA synthesis by activating LXRα to induce CYP7A1 gene transcription [10,11,12]. Cholesterol treatment of HepG2 cells also moderately induces CYP7A1 gene expression [13]. In contrast, another study using HepG2 cells, cholesterol dose dependently decreased CYP7A1 expression [14], which is supported by a mouse study [15]. Although it has been suggested that mice have a different response to cholesterol when compared to humans, here, comparable effects were observed on BA pool size, faecal BA excretion, and plasma cholesterol levels between humans, transgenic mice, and wild-type CYP7A1 mice. This suggests that another mechanism other than LXR signalling alters BAs on a high cholesterol diet, perhaps the microbiota [16]. There are also differences between rodents and humans, for example LXRα-CYP7A1 signalling, is redundant in humans due to the lack of LXRE in the human CYP7A1 gene promoter, but this pathway is well known in rodent models. Nevertheless, FXR signalling is homologous between mice and humans and is a well-studied mechanism of CYP7A1 control. It is logical that cholesterol, the substrate for CYP7A1, would increase CYP7A1 levels, but the data published to date are equivocal and may depend on the presence of a functional LXRα receptor.
One key mechanism is the circadian regulation of CYP7A1. Cyp7a1 levels in mice have been shown to peak mid-morning to noon, although this is also influenced by the fed and non-fed state [17]. This is particularly problematic when animals treated with different diets are culled at different times over the day as this would dramatically alter the results and lead to erroneous interpretation [17]. The circadian control of Cyp7a1, along with other clock associated genes, has also been shown to parallel changes in triglyceride and total cholesterol [18]. A summary of circadian genes involved in the regulation of Cyp7a1 is shown in Table 1. Cyp7a1 is also regulated by microRNAs, which are non-coding RNAs of 15–25 bases that bind to complementary sequences in the 3′-UTR regions of target mRNA to repress translation. The effects and targets of microRNAs on Cyp7a1 and cholesterol metabolism are also summarised in Table 1. The concept that diet can regulate BA synthesis is not a new one, in fact fibre is well known to regulate BA synthesis [19,20,21,22,23], however, the idea that bio-actives within food, particularly polyphenols, can increase CYP7A1 without increased food consumption is an exciting field and will be considered in the following sections.
Table 1.
A summary of the transcription factors and microRNAs involved in Cyp7a1 gene regulation in humans and rodents.
| Factor | Humans/Human Cell Lines | Rodents | Ref. |
|---|---|---|---|
| Farnesoid X receptor, retinoic acid receptor and small heterodimer partner (FXR-RXR and SHP) |
FXR is highly expressed in both the liver and ileum tissue. Ligand binding to FXR allows translocation from the cytoplasm to the nucleus to bind RXR at FXR-response elements. FXR is not able to bind to CYP7A1 but can bind to the promoter of the SHP gene. SHP represses CYP7A1 gene expression by binding to human α-fetoprotein transcription factor (FTF). FXR-RXR complex also binds illeal FGF19 (humans) or FGFR4 (mice) translocates to the liver, activates FGR4 which inhibits c CYP7A1 transcription. | The same as humans, however, FTF is called liver receptor homolog-1 (LRH-1) in mice. | [24,25,26] |
| Pregnane X receptor (PXR) | PXR activation by specific bile acids such as lithocholic acid (LCA) leads to the repression of bile acid synthesis by binding and inactivating the transcription factor, hepatocyte nuclear factor 4 alpha (HNF4α) so that it can no-longer bind to its transcriptional co-activator, proliferator-activated receptor γ co-activator 1-α (PGC1α) to induce CYP7A1 transcription. | [27] | |
| Liver X receptor α (LXRα) | LXRα cannot bind to the human CYP7A1 promoter due to an alteration of the DR4 motif in the BARE-I sequence. Therefore, in humans LXRα does not play a role in the regulation of CYP7A1 gene expression. | Unlike in humans, LXRα can directly bind to the Cyp7a1 promoter to upregulate expression. | [28] |
| Hepatocyte nuclear factor 4 alpha/Peroxisome proliferator-activated receptor γ co-activator 1-α (HNF4α /PGC1α) |
HNF4α is a transcription factor that upregulates CYP7A1 by directly binding to its promoter along with the trans-activator PGC-1α. | Same as humans. | [29,30,31,32] |
| Peroxisome Proliferator Activated Receptor Alpha (PPARα) | In vitro PPARα over-expression in human liver cells has been shown to reduce CYP7A1gene expression. However, when activators of PPARα where added to non-over-expressing cells a moderate amount of inhibition was observed. | PPARα knock out mice did not show altered Cyp7a1 levels. | [31,33] |
| Peroxisome Proliferator Activated Receptor gamma (PPARγ) | PPARγ activation induced CYP7A1 expression in HepG2 cells | [34] | |
| Forkhead box protein O1 (FoxO1) | FoxO1 is an in-direct suppressor of CYP7A1, there is no binding site for FoxO1 on the human CYP7A11 promoter. FoxO1 inhibits CYP7A1 by inhibiting expression of HNF-4α and PGC-1α TFs. | FoxO1 has the opposite function in mice and upregulates Cyp7a1 directly by binding to its promoter. | [35,36] |
| Nuclear receptor subfamily 1, group D, member 1 (NR1D1 or Rev-Erba) | NR | Competes for the promoter of the clock gene Bmal1 and mediate the circadian regulation of Cyp7a1 | [37,38]. |
| Per1 and Per2 | NR | Genetic ablation in mice disrupts normal BA control, increases serum BA and in parallel reduces dbp and Cyp7a1 expression in both rats and mice | [39,40]. |
| D site albumin promoter binding protein (DBP) | Gain-of-function studies have shown that DBP serves as a circadian activator of CYP7A1 transcription. | Cyp7a1 peaks after dark | [37,41,42] |
| Enhancer binding protein C/EBPβ-LAP | Binds cyp7a1 promoter site at DBP | [39] | |
| miR-33 | Located in the intron sequence of SREBP and regulates Cyp7a1 expression possibly synergistically to control hepatic cholesterol metabolism and BA synthesis | [43,44,45,46] | |
| miR-144-3p and miR-99a-3p | Target CYP7A1 and other non-alcoholic fatty liver disease related genes | [43] | |
| miR-122 and miR-422 | Cyp7a1 also has recognition sequences for miR-122 and miR-422 in its 3′-UTR. A synthetic miR-122 mimic inhibits CYP7A1expression in vitro and miR-122 inhibition has shown the opposite effect | [44] | |
| miR-24 and miR-34 | Indirectly decrease CYP7A1 by decreasing HNF4α transcription factor | [47] | |
| miR-17 | Leads a reduction in CYP7A1 mRNA expression | Reduces Cyp7a1 mRNA expression accompanied by hepatic steatosis | [30]. |
2. Current Status of Knowledge: Polyphenols That Regulate Bile Acid Synthesis and CYP7A1
Numerous studies have assessed the effects of polyphenols on BA excretion and CYP7A1 expression and the mechanisms by which they alter BA excretion and CYP7A1expression have been summarised in Supplementary Table S2; these were mostly obtained from rodent studies showing a depletion of hepatic cholesterol when fed polyphenols, which in some instances is linked to an increase in CYP7A1 activity and LXRα signalling. Polyphenols are subdivided into different categories: flavonoids, isoflavonoids, lignans, stilbenes, phenolic acids, and phenolic polymers (Supplementary Figure S1). It is worth noting that all types of polyphenols are only partially bioavailable and subject to both phase-2 conjugation and catabolism by the gut microbiota, and the reader is referred to some excellent reviews of the bioavailability and metabolism of polyphenols and how these processes can affect their biological activities [48,49,50,51,52]. Currently, evidence for whether the known effects of polyphenols on BA metabolism and CYP7A1 are mediated by the parent polyphenols or their metabolites, or a combination of both is limited; this is an important area for future research. There are multiple pathways through which polyphenols confer their beneficial effects including those involved in inflammation. These have been substantially reviewed elsewhere [53,54] Another biological activity of polyphenols is as antioxidant molecules that have the potential to mop up free radicals. Although this has been somewhat challenging to prove unequivocally in humans, it is notable that there is a significant body of evidence to show that consumption of specific polyphenols such as those in olive oil can effectively reduce the levels of oxidised LDL [55]. In the following sections, we review the effects of each sub-group of polyphenols on CYP7A1 expression, BA metabolism/excretion, and comment on the mechanism of action. We then further discuss the emerging research that polyphenols can mediate cholesterol efflux and microbial changes in the gut, subsequently modulating bile acid excretion.
2.1. Flavonoids
Flavonoids are water soluble polyphenolic molecules that consist of six major subgroups: flavanols (catechins), anthocyanins, flavonols, flavanones, and flavones (see Supplementary Figure S1). The effects of flavonoids on the HDL delivery of cholesterol to the liver via RCT have been recently reviewed [56], however, the effects of polyphenols on bile acid biosynthesis and efflux was not described.
2.1.1. Flavanols
Flavanol-rich green tea has been shown to increase BA excretion, however, the link to CYP7A1 is tentative. For example, Lung Chen tea has been shown to reduce serum cholesterol and increase faecal BA excretion in hypercholesteraemic rats, without altering Cyp7a1 enzyme activity [57]. Cyp7a1 gene expression was not changed in C57BL mice fed green tea extract (GTE) over six weeks [15]. Despite this, there was an increase in circulating BAs and excreted faecal cholesterol, although this was only apparent once normalised to the amount of faeces excreted per day as the mice on the GTE produced more faeces overall [15]. Interestingly, in the same study, GTE dramatically increased Cyp27a1 mRNA expression in mice fed a high cholesterol diet, but not in mice fed a normal chow diet; indicating a shift from the neutral pathway to the acidic pathway only in the presence of high cholesterol [15]. Several studies have shown an increase in Cyp7a1 expression in response to catechin treatment. For example, in rats, epigallocatechin gallate (EGCG) decreased bile acid-independent bile flow but not excretion, whilst increasing Cyp7a1 mRNA expression and increasing circulating BAs [58]. EGCG increased Cyp7a1 protein levels in C57BL/6 mouse livers, however, BAs were not measured [59]. Finally, an in vitro study using HepG2 cells has shown that catechins increase CYP7A1mRNA levels with epicatechin gallate showing the greatest fold induction [60]. In contrast, EGCG is also known to be an activator of FXR signalling [61], which would in turn supress CYP7A1expression. Studies in our lab confirm a reduction of CYP7A1 gene expression in human liver HepG2 cells treated with 5 µM EGCG in the absence of serum, which is consistent with the hypothesis that EGCG is an activator of farnesoid X receptor (FXR) signalling (unpublished data). Interestingly, in the presence of chenodeoxycholic acid, another FXR inhibitor, EGCG acts as a suppressor of FXR signalling in vitro [61]. CYP7A1 levels were not measured, but it would be fascinating to explore the role of EGCG on CYP7A1expression in the presence and absence of FXR inhibitors.
2.1.2. Anthocyanins
There are very limited data on the effects of anthocyanins on BAs. In one study, red pericarp glutinous rice, rich in anthocyanins, induced Cyp7a1 expression when fed to hypercholesterolemic C57BL/6 mice when compared to brown rice [62]. A decrease in hepatic cholesterol was also observed although faecal cholesterol or BAs were not measured [62]. Lingonberries, which contain high levels of anthocyanins, have been shown to increase Cyp7a1 expression and decrease atherosclerotic plaque and triglyceride concentration in ApoE-/- mice on a high fat diet [63]. The relative faecal abundance of the bacterial genera Bacteroides, Parabacteroides, and Clostridium were also increased [63]. In type-2 diabetic mice, Buckwheat sprouts, which contain cyanidin 3-O-glucoside (C-3-G) [64] and C-3-G alone, increased faecal BAs and Cyp7a1 mRNA expression in the liver whilst correspondingly decreasing serum cholesterol, liver cholesterol, and triglycerides and consequently reduced atherosclerosis [65,66]. However, buckwheat protein has also been investigated as the active cholesterol lowering component [67]. High doses of polyphenol-rich Lonicera caerulea berry extract, which contains mainly anthocyanins, significantly upregulated Cyp7a1 gene expression, reduced the expression of SREBP-1C, SREBP2, miR-33, and miR-122 and caused a reduction in cholesterol, LDL, and triglyceride in Sprague Dawley rats [68]. To our knowledge, all the published anthocyanin data that have examined changes in BA levels or Cyp7a1 expression have utilised mouse models. However, human cells may respond differently, as in mouse primary hepatocytes, C-3-G treatment increased Cyp7a1 expression in an LXRα dependent way [66]. Indeed, using HepG2 cells, we showed that C-3-G does not increase CYP7A1 mRNA expression (data unpublished), perhaps because human cells do not possess functional LXRα.
2.1.3. Flavonols
Quercetin, a flavonol found in capers, has been shown to elevate hepatic Cyp7a1 as well as LXRα at both mRNA and protein levels in male Wistar rats along with increased secretion of BAs and total hepatic BAs [69]. Importantly, quercetin also increased the expression of hepatic ATP binding cassette transporter G1 (ABCG1) mRNA and protein expression, indicating that quercetin may be involved in the regulation of hepatic cholesterol efflux [69]. Black bean seed coat extract predominantly containing quercetin 3-O-glucoside significantly stimulated the expression of Cyp7a1 protein in the liver and faecal BAs in C57BL mice [70]. A combination of quercetin and leucodelphinidin or quercetin with Banyan tree, derived leucopelargonin and leucocyanin significantly increased hepatic and faecal BAs in hypercholesteraemic rats and correspondingly reduced L-LDL cholesterol with the former also increasing HDL [71,72]. Overall, there is some evidence from animal studies that quercetin alone or in combination with other flavonoids can induce BA excretion via induction of CYP7A1. Other flavonols such as kaempferol have been shown to increase hepatic CYP7A1, faecal cholesterol, and BAs through mechanisms that may involve its binding to LXRα [73]. Human interventions and in vitro studies are still required to examine the role of quercetin and kaempferol on Cyp7a1 and lipid metabolism.
2.1.4. Flavanones, Flavones, and Isoflavones
Naringin (a flavanone) occurs naturally in citrus fruits, especially in grapefruit, where it is responsible for the fruit’s bitter taste. Naringin, has been shown to induce LDL-receptor and CYP7A1 expression in HepG2 cells through the NF-κB and ERK signalling pathway as well as through PPARy, which occurs in a dose-dependent manor [74,75]. Its aglycone naringenin has been shown to be a partial agonist of LXRα in cells transfected with a reporter construct [74]. In contrast, using computer modelling of tetrahydro-flavanones (cryptochinones A–D), it was shown that flavanones may behave as FXR agonists to decrease CYP7A1 mRNA expression [76]. The only one study that investigated the effects of flavones from a leaf extract of Xanthosoma sagittifolium showed no effects on total Bas, but reduced secondary BAs in rats [77,78].
When administered to mice and rats, an isoflavonoid puerarin (from arrowroot) increases hepatic Cyp7a1 expression and suppresses serum and hepatic cholesterol, although in HepG2 cells, no changes were observed [79,80,81]. Additionally, Xuezhikang, an isoflavone-rich extract of red-yeast-rice and Erythrina lysistemon also rich in isoflavones, increased hepatic Cyp7a1 expression and BA excretion in high-fat fed mice and in ovariectomised rats, respectively [82,83]. The bacterial isoflavone metabolite equol has also been shown to alter BA metabolism by increasing hepatic CYP7A1 mRNA levels in the chicken embryos liver [84]. Soymilk and fermented soymilk, which contain both isoflavones, bioactive proteins, and peptides, have also been shown to attenuate hepatic cholesterol and triglycerides levels and increase hepatic Cyp7a1 gene expression in Sprague Dawley rats [85]. It is not clear whether the effects can be attributed to either the flavonoids or the proteins/peptides or both, as soy protein alongside a high-fat diet also increased hepatic Cyp7a1 mRNA in male Syrian Golden hamsters [86]. In addition, soy protein isolate has been shown to induce Cyp7a1 hepatic expression and reduce hepatic cholesterol in rats [87]. The only study in humans showed no effects [81].
2.2. Stillbenes
The anti-atherosclerotic effects of resveratrol (3,5,4′-trihydroxy-trans-stilbene), which is found in the skin of grapes, blueberries, raspberries, and mulberries, have been widely studied in mouse models and human cell lines [88]. In mice on a high fat diet and in HepG2 cells, resveratrol increased CYP7A1 mRNA expression with activity being increased in the former [89]. Similar results were observed in HepG2 cells treated with resveratrol or resveratrol-glucuronides along with a concurrent decrease in cholesterol content and an increase in bile salt export protein (BSEP), respectively [90]. In alpha-Naphthylisothiocyanate (ANIT) induced liver injury in rats, resveratrol restored FXR and Cyp7a1 expression and BA secretion [91]. Donryu rats implanted with an ascites hepatoma cell line and given resveratrol showed a dose-dependent reduction in serum cholesterol and excreted BAs [92]. Thus, supporting the evidence that resveratrol or its metabolites (aglycone and glucuronide), which are bioavailable in bile and plasma, 4 to 8 h after administration, may be functional bio-actives [93]. Resveratrol may confer its beneficial effects through FXR signalling targeting SIRT1, which acetylates FXR and prevents its binding with RXRα, consequently inhibiting its binding and activation of the Cyp7a1 repressor Shp [94,95]. Resveratrol has also been shown to alter the gut microbiota profile in C57BL/6J and ApoE-/- mice as well as bile salt hydrolase activity and de-conjugation, thus increasing faecal excretion. This was associated with the repression of enterohepatic FXR and FGF15 signalling and increased Cyp7a1 expression and hepatic BA synthesis [96]. In antibiotic treated mice, resveratrol has no such effects, indicating the importance of gut microbiota in resveratrol mediated effects [96].
2.3. Phenolic Acids and Phenolic Polypmers
Phenolic acids such as vanillic, caffeic, ferulic, and gallic acid are found in high concentrations in berries, tea, whole grains, and wine. A fermented Chinese tea, which contains a range of phenolic acids including catechin and gallic acid, has been shown to reduce LDL cholesterol in a 3-month double-blind randomised study of health and hypercholesterolemic patients, although BA levels were not measured [97,98,99]. Similarly, in hypercholesteraemic rats, an 8-week administration of the tea increased faecal BAs, but not Cyp7a1 expression [57]. A separate study showed decreases in serum LDL and total cholesterol in hypercholesteraemic rats after 3-weeks of feeding [100]. Indeed, a phenolic acid, chlorogenic acid, found in green tea, increased in Cyp7a1 mRNA expression in 129/Sv mice, indicating that at least some of the phenolic acid components are bioactive [101].
The beneficial effects of some phenolic polymers found in raisins, grapes, and wine (high in fibre but also tannins (proanthocyanidins)) in humans are however questionable as two human studies from the same lab showed that consuming 80–120 g raisins over a two to nine week period decreased total faecal BAs, and in another study, when tartaric acid was fed to participants, no effects on BAs were observed [102,103,104]. In contrast, 2% tannin polymers from raisins significantly increased faecal BAs and lowered LDL cholesterol in mice, although tannin monomers did not have any effect [105]. Using an in vitro study, Camire et al. proposed that the fibre in chopped raisins act as a BA sequestrant to prevent enterohepatic re-circulation, although the role of polymeric tannins cannot be ruled out as gallic acid does directly bind to taurocholic, taurodeoxycholic, and glycodeoxycholic BAs in vitro, and ellagic acid has been shown to induce genes involved in BA synthesis in mice [106,107,108]. It is therefore evident that some raisin constituents may promote BA secretion in rodents, tannin polymers (but not monomers) being among them, although their effects in humans is yet to be assessed.
2.4. Grape Juice, Wine and Grape Seed Extract
In-vivo and vitro effects of grapes, wine, and grape seed extract (GPSE) on BA secretion have recently been reviewed with moderate increase in Cyp7a1 after GPSE treatments of hamsters on a 0.1% cholesterol diet while red wine procyanidins induced hepatic Cyp7a1 in Wistar rats along with reduced LDL-C [109,110,111]. Grape juice fed to rats also increased primary BA but reduced secondary BA in the intestinal contents. Interestingly faecal counts of Lactobacillus and Bifdobaceterium were also increased [112]. In FXR knockout mice, GPSE induced faecal BA output and downregulated genes involved in intestinal BA absorption and transport. This correlated with increased Cyp7a1 mRNA expression in the liver, decreased circulating LDL cholesterol, and decreased intestinal Fgf15 expression [113]. In contrast, fisetin a flavonol from red wine, decreased Cyp7a1 mRNA expression in Sprague Dawley rats, when compared to controls of mice fed a high fat diet [114]. Despite this, the rats still exhibited decreased plasma total cholesterol and LDL-cholesterol, along with decreased hepatic cholesterol content [114]. Perhaps the alternative pathway regulated by CYP27A1 may be responsible for altered BA secretion in this instance. Procyanidins may also have long-term effects; for example, in the alteration of reverse cholesterol transport in the adult offspring after intake of grape procyanidins during gestation and lactation [115]. Overall, the evidence suggests GPSE induces Cyp7a1 mRNA expression and BA secretion into faeces. There is less evidence for wine and grape juice, although the existing publications support the same trend presented with GPSE. Once again, studies in humans are lacking.
3. Polyphenol Mediated Mechanisms of Action
Understanding the mechanisms through which polyphenols modulate CYP7A1 to regulate cholesterol and bile acid metabolism is important and this review has highlighted that multiple pathways may be involved. These include regulation through the NF-Kβ/ERK and SIRT-RXR-FXR (LXR) signalling pathways, modification of circadian rhythm associated genes, reverse cholesterol transport, and bile salt hydrolyses (Figure 3). While there is limited experimental data on the effects of polyphenols on microRNAs and CYP7A1expression, using a database that predicts the target for microRNAs in messenger RNA in rats, several microRNAs have been recognised to target CYP7A1, and some of these microRNAs have been shown to be modulated by polyphenol supplementation (Figure 3). Polyphenols have also been shown to regulate intestinal BA transporters and alter the gut microbial composition (Figure 3) to regulate the excretion of BAs as will be discussed in Section 3.1 and Section 3.2.
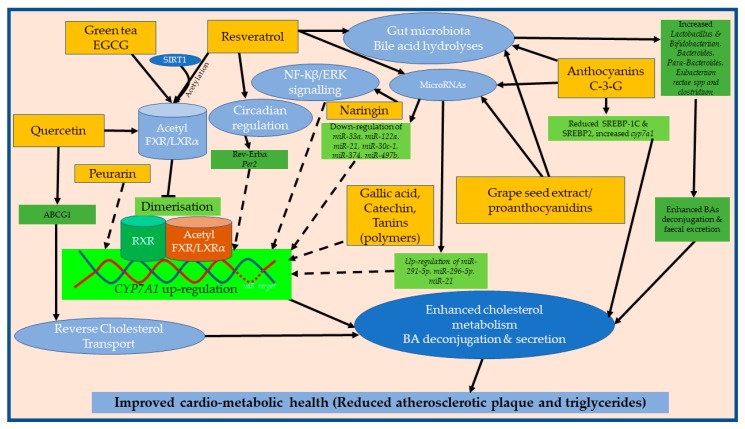
Figure 3.
Putative mechanisms through which polyphenols modulate CYP7A1 to promote cholesterol metabolism and bile acid deconjugation, consequently attenuating atherosclerosis plaque development. Resveratrol, epigallocatechin gallate (EGCG), and quercetin increase cyp7a1 by employing SIRT1 to acetylate FXR/LXRα; preventing its dimerisation with RXR, which then inhibits its binding and activation of CYP7A1 repressor [75,91]. Resveratrol can activate circadian proteins [116,117] and genes that subsequently upregulate cyp7a1 and together with C-3-G, procyanidins, and naringin, can downregulate or upregulate certain microRNAs to promote CYP7A1 expression [44,118,119,120,121]. Alternatively, these may also increase bile acid hydrolases and certain gut microbiota species involved in the deconjugation and excretion of bile acids [122,123,124]. Dashed lines indicate mechanisms not completely known.
3.1. Control of the Apical Sodium Dependent Bile Acid Transporter (ASBT) by Polyphenols
ASBT is expressed in the ileum and the terminal portion of the small intestine, and is responsible for the re-circulation of 95% of BAs back to the liver [125]. Blocking BA enterohepatic circulation by interrupting the ASBT pathway increases hepatic BA synthesis to compensate for BAs lost in the faeces [126]. Consequently, the concentration of liver cholesterol decreases causing the activation of compensatory mechanisms to maintain cellular cholesterol homeostasis such as increased hepatic uptake of LDL cholesterol, thereby, reducing circulating cholesterol levels [126]. Therefore, ASBT is an attractive therapeutic target to lower LDL cholesterol [127]. Inhibition of ASBT by SC-435, a potent ASBT inhibitor, was found to increase feacal BA excretion, upregulate Cyp7a1 gene expression, decrease total cholesterol, and reduce the aortic lesion area in Apo E-/- mice [126]. Dietary cholesterol has direct effects on ASBT function and expression [128]. In vitro, 25-hydroxycholesterol, and to a lesser extent 22-and 24-hydroxycholesterol, reduced ASBT function and mRNA levels in Caco2 cells [129]. Moreover, 25-hydroxycholesterol significantly reduced the relative activity of the human ASBT promoter in a dose-dependent manner [129]. On the other hand, 100 µM cholesterol produced no inhibitory effect on sodium-dependent taurocholate uptake into COS-7 cells that were transiently transfected with ASBT [130]. In vivo, feeding 1% cholesterol to C57BL/6J female mice for two weeks decreased ASBT mRNA abundance by 54% when compared to control, while there was no change in ASBT protein expression in Sprague Dawley rats fed with 2% cholesterol for 10 days [130,131]. It is unlikely that the differences in these two studies were due to doses given, as in the later study, a much higher dose was given, which should be more effective than a 1% cholesterol dose. Possibly, the feeding duration could have caused these differences. However, in the later study using rats, changes in other genes such as cyp7a1 were observed, indicating that there was ample feeding time to alter gene expression. As the time of cull was not specified in the later study, circadian gene expression changes may explain the observed differences [132]. Species differences could not be ruled out either, as in the same study that used rats, 2% cholesterol significantly increased ASBT protein expression in rabbits. Although cholesterol is not a dietary bioactive, the study results are applicable as polyphenols may alter cholesterol levels, which can further alter gene expression. However, for cholesterol, the results were varied, with a trend towards an enhanced cholesterol diet leading to a decrease in the expression of ASBT. Several reports have investigated the effects of dietary bioactives on the active transport of conjugated BAs via ASBT and these are summarised in Supplementary Table S3 The evidence that polyphenols such as resveratrol alter the gut microbiota to increase faecal BAs is further explored in the following sections of the review.
3.2. Changes in Gut Microbiota Can Increase Bile Acid Excretion: Action of Polyphenols
Microbial metabolism and de-conjugation of BAs lead to increased diversity within the BA pool and in general, a more hydrophobic population. Increasing the hydrophobic nature of the BAs makes them less readily re-absorbed and more easily excreted into faeces. De-conjugation of BAs is catalysed by bile salt hydrolase (BSH) enzymes and genes coding for BSH have been detected in the main bacterial genera: Firmicutes, Bacteroidetes, and Actinobacteria [133]. In fact, BSH is enriched in the gut microbiota and helps the bacteria survive and colonise the gastrointestinal tract [134]. De-conjugated primary BAs that are not re-absorbed by the enterocytes enter the colon, where they are metabolised through 7-dehydroxylation into secondary BAs. 7-dehydroxylation of the primary BAs, CDCA, and CA can occur due to accessibility of the hydroxyl group, leading to DCA and LCA production. Bacteria that have 7-dehydroxylation activity are members of the Firmicutes phylum (Clostridium and Eubacterium) and these bacteria have a BA-inducible (BAI) gene. The bacteria are essential for the formation of secondary BAs and this is demonstrated in germ free mice, which have lower levels of secondary BAs and a smaller BA pool [135,136]. Germ-free animals accumulate cholesterol at higher levels [137], have higher levels of conjugated BAs, and significantly reduced excretion of faecal BAs [136,138].
The gut microbiota can also regulate BA metabolism by reducing the levels of tauro beta-muricholic acid (tbMCA), a naturally occurring FXR antagonist, and FXR antagonism increases Cyp7a1 expression [135]. This has been further confirmed in a separate study, where an anti-oxidant, tempol, was used to suppress FXR signalling, which increased the levels of tbMCA [139]. Specifically, tempol decreased the genera Lactobacillus and Clostridium, which was accompanied by decreased BSH activity [139]. Oral feeding of Lactobacillus plantarum to mice resulted in significant reduction in LDL-C, increased faecal BA excretion, increased hepatic BA synthesis, and increased gene and protein expression of Cyp7a1 [140]. Recently, the glycine conjugate of bMCA (GbMCA) has also been found to be an FXR antagonist, which in contrast to TbMCA, was found to be resistant to faecal microbial BSH [141]. However, at present, it is not known whether TbMCA or GbMCA can antagonise FXR signalling in humans and how the human microbiota, which is more adapted to glycine conjugated BAs, metabolises GbMCA.
Human dietary interventions have shown that certain probiotics can reduce blood cholesterol by altering the microbial environment to increase BAs in the faeces [142,143]. The Lactobacilus reuteri strain, NCIMB 30242, which is known for its BSH activity reduced cholesterol levels in hypercholesterolaemic participants (>100) when fed an encapsulated form in a yoghurt. In a second study using the same strain of bacteria, but lyophylised, they found similar results and most importantly found that the participants had higher levels of de-conjugated BAs in their faeces [143]. In addition, live Lactobacillus reuteri with BSH activity when fed to pigs on a high fat, high cholesterol, low fibre diet reduced total and LDL-cholesterol concentrations [144]. Therefore, the interesting question is whether diet can alter the composition of the microbiota to decrease cholesterol levels.
Polyphenols have recently emerged as modulators of the gut microbiota [145]. Tea polyphenols have been shown to modulate the composition of the gut microbial community through the inhibition of pathogenic bacteria (Clostridium perfringens, Clostridium difficile, and Bacteroides) [146]. In the same study, no changes to the beneficial bacteria (Clostridium, Bifidobacterium, and Lactobacillus) were found [146], whereas, rats fed a diet containing tea polyphenols or gallotannins had reduced amounts of secondary BAs in the faeces, which suggests a reduction in bacteria with BSH activity [147]. Accordingly, in a study using rats, the supplementation of a high fat diet with catechin, curcumin, caffeic acid, rutin, ellagic acid, or quercetin reduced the concentration of secondary BAs in the faeces [148]. This suggests that the amount of de-conjugating bacteria were also reduced in the rats on these diets [148]. However, when tea flavan-3-ol monomers such as catechin were fed to humans, there was an enhanced growth of members of Clostridium coccoides, particularly Eubacterium rectale, which are known to break down BAs to secondary BAs [149]. Interestingly, Eubacterium rectale spp. has been shown to play a probiotic role of butyrate synthesis from carbohydrates [150,151,152] and other members of the Clostridium coccoides family are known to have high levels of BA 7α-dehydroxylating activity, which break down primary BAs to secondary BAs [151].
A human intervention study has shown that consumption of red wine polyphenols significantly increased the number of Bacteroides, Bifidobacterium, Enterococcus, Prevotella, Bacteroides uniformis, Eggerthellalenta, and Blautia coccoides-E. rectale. Correspondingly, total cholesterol was significantly decreased and most importantly, changes in cholesterol concentrations were linked to changes in the Bifidobacteria number, Bifidobacteria were probiotic, and some groups had BSH activity, although BSH activity was not measured [150]. This corresponded to a study in rats supplemented with a de-alcoholised, proanthocyanidin-rich red wine extract over 16-weeks, where the bacterial composition changed from a predominance of Bacteroides, Clostridium, and Propionibacterium to a prevalence of Bacteroides, Lactobacillus, and Bifidobacterium [153]. Interestingly, in another study, red wine tannins had no effect on the conversion of primary to secondary BAs in the faeces of rats [154].
In a human intervention study, the consumption of a wild blueberry drink, rich in polyphenols significantly increased the amount of Bifidobacterium, some Bifidobacteria are known to have BSH activity [155]. Proanthocyanidin-rich extract from grape seeds fed to healthy adults for two weeks also significantly increased the number of intestinal Bifidobacteria [156]. In addition, resveratrol, which is found in wine and grapes, also increased faecal counts of Bifidobacterium and increased Lactobacillus in a rat model, and in humans, it increased α-diversity such as Barnesiella levels that are associated with gut health and may improve cholesterol metabolism [157,158]. In one study, which substituted the water fed to rats for grape, apple, or beetroot juice, a higher concentration of primary BAs but lower concentration of secondary BAs was found in the intestinal contents along with increased amounts of cholesterol and its metabolites. Intriguingly, this corresponded to increased faecal counts of bacteria with BSH activity, Lactobacillus and Bifidobacterium [112]. Raspberry pomace containing seeds fed to rats on a high fat diet also reduced the amount of secondary BAs (LCA and DCA) in the cecum, but interestingly, the seedless fraction did not have the same effect [159]. Grape seed flour has been shown to alter the gut microbiota of male Golden Syrian hamsters. Hamsters were fed a high-fat (HF) control diet or a HF diet supplemented with 10% partially defatted grape seed flour. The Chardonnay diet altered the numbers of total bacteria and relative abundances of Bifidobacterium, Lactobacillus, and Firmicutes in the faeces, which were significantly lower than the control group. This was accompanied by decreased intestinal FGF15 expression and increased liver Cyp7a1 gene expression [160]. It was suggested that alteration of the intestinal microbiota may regulate BA metabolism, but BAs were not measured in this study.
4. Conclusions
4.1. The Limitations of Existing Models
Rodent models are useful for studying the effects of polyphenols on faecal BAs, liver, and circulating cholesterol levels, but the evidence that this occurs through the regulation of CYP7A1 in humans should be examined with caution, as human cells lack a LXRα response element in the human CYP7A1 promoter. Nevertheless, the effects of polyphenols on FXR signalling are similar in mice and humans with studies showing that polyphenols affect this signalling pathway in both species [94,95]. The reverse cholesterol transport pathway and the NF-κB and ERK signalling pathway are also both inducible by polyphenols in humans and mice [69,75,161,162], providing a degree of confidence that effects observed in mouse models would also be observed in humans. Thus, indicating great potential for observations in rodent models to inform future translational studies in human. Human cell lines are the obvious next best choice for functional studies, but previous research has shown that cell density can affect the expression of CYP7A1mRNA [163], and it has been shown that CYP7A1 expression is dependent on hepatocyte differentiation [164]. Specifically, cells which are more terminally differentiated are more responsive to hormones or BA conjugates when compared to less differentiated cells [13]. CYP7A1 mRNA levels significantly increased in HepG2 cells cultured over time [14]. In addition, the presence of Fetal Bovine Serum (FBS) inhibits CYP7A1expression due to the presence of bovine BAs [14]. The removal of serum was shown to stimulate CYP7A1 mRNA levels in HepG2 cells [13]. A factor in the serum such as calf BAs may be directly repressing expression of Cyp7a1. Alternatively, serum may influence hepatocyte differentiation, as serum-free medium has been found to promote a more differentiated phenotype than serum-containing medium [165,166]. Additionally, the presence of LDL cholesterol can affect the impact on CYP7A1. For example, when HepG2 cells were incubated in serum free medium with or without a red grape juice (RGJ) extract, different results were observed. In cells exposed to LDL-C, RGJ caused a marked reduction in the expression of CYP7A1 expression, however, when LDL-C was absent, increased CYP7A1 levels were observed on treatment with RGJ [167]. Therefore, care in the interpretation of both human and animal studies is required. Additionally, cells lines do not represent a multi-organ regulation of cholesterol and bile acid metabolism as do animal studies. Another factor that may greatly lead to disparities between experiments may be the concentrations of polyphenols used, some of which may not be physiologically relevant.
4.2. The Most Promising Polyphenol Candidates for Future Studies
Puerarin, an isoflavonoid, is a promising candidate for further studies. Rodent studies from three separate labs have shown that puerarin increases hepatic Cyp7a1 expression [79,80,81]. However, when liver HepG2 cells were treated with increasing doses of puerarin, there was no effect, highlighting the disparity between human and animal studies [81]. The predominant mechanism for BA induction via puerarin in mice appears to be LXRα driven, which suggests that BA induction may not occur in human cells via the same pathway. This suggests that BA induction via polyphenols is not always LXRα driven. Quercetin has also been shown to regulate cholesterol metabolism via LXRα signalling as well as reverse cholesterol transport, which also occurs in humans, making it a promising candidate for further studies in humans [161]. There are documented mechanisms whereby polyphenols can induce CYP7A1 expression that are not driven by LXRα. For example, NF-κB and ERK signalling induces CYP7A1expression in human cells treated with naringin, a flavanone-7-O-glycoside [75]. In addition, attenuated FXR signalling by resveratrol, a stilbene, has been shown to induce CYP7A1 mRNA expression in human HepG2 liver cells [89,90,95]. In conclusion, there is some promising research that polyphenols may regulate CYP7A1 expression. In addition, modification of the gut bacteria appears to be an important factor in the enhancement of BAs in the faeces, through the enrichment of bacteria with BSH activity. Red wine, rich in phenolic polymers and resveratrol, has been particularly well documented at increasing beneficial bacteria [150,153,157]. In particular, resveratrol increased levels of Lactobacillus and Bifidobacterium in mice and bacterial α-diversity in humans. These bacteria have enhanced BSH activity, which enables increased BA de-conjugation to promote faecal excretion of BAs [96]. Further studies are required to ascertain whether the parent polyphenols or their metabolites are responsible for the alterations in the gut microbiota and whether the effects seen in animal studies can be replicated in humans to inform future clinical studies.
Abbreviations
| BA | bile acid |
| Cyp7a1 | cholesterol 7 alpha-hydroxylase |
| RCT | reverse cholesterol transport |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| FXR | farnesoid X receptor |
| BSH | bile salt hydrolase |
| HF | high-fat |
| CDCA | chenodeoxycholic acid |
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/11/2588/s1, Figure S1: The categories and structures of dietary polyphenols. Polyphenols are subdivided into different categories: flavonoids, isoflavonoids, lignans, stilbenes, phenolic acids, and phenolic polymers. Table S1: A list of all the known BAs from rodents and humans with their abbreviations used in the review. * Dehydrocholic acid is a synthetic BA, manufactured by the oxidation of cholic acid. Table S2: The published studies that have determined whether dietary polyphenols can alter BA excretion and Cyp7a1 expression. NR = not reported. Table S3: Effects of polyphenols on ASBT protein and gene expression. NR = not reported.
Author Contributions
K.F.C. coordinated wrote the draft of the manuscript, H.T.A. wrote the manuscript and contributed to the figures, P.E.D. contributed to writing and finalising the manuscript for submission, and P.A.K. contributed to the writing, proofreading, and finalisation of the manuscript for publication.
Funding
This research was funded by the Biotechnology and Biological Sciences Research Council (UK) through the Institute Strategic Programme Grants (‘Food and Health’—Grant No. BB/J004545/1, and ‘Food Innovation and Health’—Grant No. BB/R012512/1) to the Quadram Institute. Hassan Aboufarrag was funded by the Newton-Mosharafa Scholarship Fund from the Egyptian Ministry of Higher Education (Cultural Affairs and Mission sector), the British Council and the British Embassy in Egypt. All authors have read and approved the final manuscript. KC/PK planned and wrote the manuscript, HA/PD contributed to the text, HA/KC prepared the figures.
Conflicts of Interest
K.F.C. Chambers, H.T.A. Aboufarrag, P.E.D., P.A.K., no conflicts of interest.
References
- 1.Gistera A., Hansson G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017;13:368–380. doi: 10.1038/nrneph.2017.51. [DOI] [PubMed] [Google Scholar]
- 2.Russell D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz M., Russell D.W., Dietschy J.M., Turley S.D. Marked reduction in bile acid synthesis in cholesterol 7alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 1998;39:1833–1843. [PubMed] [Google Scholar]
- 4.Duane W.C., Javitt N.B. 27-hydroxycholesterol: Production rates in normal human subjects. J. Lipid Res. 1999;40:1194–1199. [PubMed] [Google Scholar]
- 5.De Aguiar Vallim T.Q., Tarling E.J., Edwards P.A. Pleiotropic Roles of Bile Acids in Metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falany C.N., Johnson M.R., Barnes S., Diasio R.B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA: Amino acid N-acyltransferase. J. Biol. Chem. 1994;269:19375–19379. [PubMed] [Google Scholar]
- 7.Hayashi H., Takada T., Suzuki H., Onuki R., Hofmann A.F., Sugiyama Y. Transport by vesicles of glycine- and taurine-conjugated bile salts and taurolithocholate 3-sulfate: A comparison of human BSEP with rat Bsep. Biochim. Biophys. Acta. 2005;1738:54–62. doi: 10.1016/j.bbalip.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Dawson P.A., Lan T., Rao A. Bile acid transporters. J. Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlton-Menys V., Durrington P.N. Human cholesterol metabolism and therapeutic molecules. Exp. Physiol. 2008;93:27–42. doi: 10.1113/expphysiol.2006.035147. [DOI] [PubMed] [Google Scholar]
- 10.Peet D.J., Turley S.D., Ma W., Janowski B.A., Lobaccaro J.M., Hammer R.E., Mangelsdorf D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/S0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 11.Jelinek D.F., Andersson S., Slaughter C.A., Russell D.W. Cloning and regulation of cholesterol 7 alpha-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis. J. Biol. Chem. 1990;265:8190–8197. [PMC free article] [PubMed] [Google Scholar]
- 12.Noshiro M., Nishimoto M., Okuda K. Rat liver cholesterol 7 alpha-hydroxylase. Pretranslational regulation for circadian rhythm. J. Biol. Chem. 1990;265:10036–10041. [PubMed] [Google Scholar]
- 13.Taniguchi T., Chen J., Cooper A.D. Regulation of cholesterol 7 alpha-hydroxylase gene expression in Hep-G2 cells. Effect of serum, bile salts, and coordinate and noncoordinate regulation with other sterol-responsive genes. J. Biol. Chem. 1994;269:10071–10078. [PubMed] [Google Scholar]
- 14.Andreou E.R. Analysis of CYP7A1 Gene Regulation in HepG2 Cells by Reverse-Transcriptase Polymerase Chain Reaction. University of Toronto; Toronto, ON, Canada: 1997. [Google Scholar]
- 15.Hirsch N., Konstantinov A., Anavi S., Aronis A., Hagay Z., Madar Z., Tirosh O. Prolonged feeding with green tea polyphenols exacerbates cholesterol-induced fatty liver disease in mice. Mol. Nutr. Food Res. 2016 doi: 10.1002/mnfr.201600221. [DOI] [PubMed] [Google Scholar]
- 16.Tiemann M., Han Z., Soccio R., Bollineni J., Shefer S., Sehayek E., Breslow J.L. Cholesterol feeding of mice expressing cholesterol 7alpha-hydroxylase increases bile acid pool size despite decreased enzyme activity. Proc. Natl. Acad. Sci. USA. 2004;101:1846–1851. doi: 10.1073/pnas.0308426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggink H.M., Oosterman J.E., De Goede P., De Vries E.M., Foppen E., Koehorst M., Groen A.K., Boelen A., Romijn J.A., La Fleur S.E., et al. Complex interaction between circadian rhythm and diet on bile acid homeostasis in male rats. Chronobiol. Int. 2017;34:1339–1353. doi: 10.1080/07420528.2017.1363226. [DOI] [PubMed] [Google Scholar]
- 18.Chen P.P., Zhang R.Y., Mou L.J., Li X.W., Qin Y., Li X.M. An impaired hepatic clock system effects lipid metabolism in rats with nephropathy. Int. J. Mol. Med. 2018;42:2720–2736. doi: 10.3892/ijmm.2018.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Story J.A., Watterson J.J., Matheson H.B., Furumoto E.J. Dietary Fiber and Bile-Acid Metabolism. Adv. Exp. Med. Biol. 1990;270:43–48. doi: 10.1007/978-1-4684-5784-1_5. [DOI] [PubMed] [Google Scholar]
- 20.Ide T. Food Hydrocolloids. Springer; Boston, MA, USA: 1993. Dietary Fiber-Induced Changes in Bile-Acid Conjugation and Taurine Metabolism in Rats; pp. 491–496. [Google Scholar]
- 21.Locket P., Gallaher D. The Effect of Fiber Source and Fat Level on Bile-Acid Metabolism in the Rat. Faseb J. 1988;2:A862. [Google Scholar]
- 22.Fadden K., Hill M.J., Owen R.W. Effect of fibre on bile acid metabolism by human faecal bacteria in batch and continuous culture. Eur. J. Cancer Prev. 1997;6:175–194. [PubMed] [Google Scholar]
- 23.Kritchevsky D. Influence of Dietary Fiber on Bile-Acid Metabolism. Lipids. 1978;13:982–985. doi: 10.1007/BF02533860. [DOI] [PubMed] [Google Scholar]
- 24.Calkin A.C., Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee E.A., Lee D.I., Kim H.Y., Ahn S.H., Seong H.R., Jung W.H., Kim K.Y., Kim S., Rhee S.D. Cyp7a1 is continuously increased with disrupted Fxr-mediated feedback inhibition in hypercholesterolemic TALLYHO/Jng mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:20–25. doi: 10.1016/j.bbalip.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Chiang J.Y., Kimmel R., Weinberger C. Stroup D: Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J. Biol. Chem. 2000;275:10918–10924. doi: 10.1074/jbc.275.15.10918. [DOI] [PubMed] [Google Scholar]
- 27.Li T., Chiang J.Y. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G74–G84. doi: 10.1152/ajpgi.00258.2004. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin B., Watson M.A., Kim H., Miao J., Kemper J.K., Kliewer S.A. Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver X receptor-alpha. Mol. Endocrinol. 2003;17:386–394. doi: 10.1210/me.2002-0246. [DOI] [PubMed] [Google Scholar]
- 29.Shin D.J., Campos J.A., Gil G., Osborne T.F. PGC-1alpha activates CYP7A1 and bile acid biosynthesis. J. Biol. Chem. 2003;278:50047–50052. doi: 10.1074/jbc.M309736200. [DOI] [PubMed] [Google Scholar]
- 30.Kir S., Zhang Y., Gerard R.D., Kliewer S.A., Mangelsdorf D.J. Nuclear receptors HNF4alpha and LRH-1 cooperate in regulating Cyp7a1 in vivo. J. Biol. Chem. 2012;287:41334–41341. doi: 10.1074/jbc.M112.421834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrapodi M., Chiang J.Y. Peroxisome proliferator-activated receptor alpha (PPARalpha) and agonist inhibit cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J. Lipid Res. 2000;41:514–520. [PubMed] [Google Scholar]
- 32.Inoue Y., Yu A.M., Yim S.H., Ma X., Krausz K.W., Inoue J., Xiang C.C., Brownstein M.J., Eggertsen G., Bjorkhem I., et al. Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4alpha. J. Lipid Res. 2006;47:215–227. doi: 10.1194/jlr.M500430-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel D.D., Knight B.L., Soutar A.K., Gibbons G.F., Wade D.P. The effect of peroxisome-proliferator-activated receptor-alpha on the activity of the cholesterol 7 alpha-hydroxylase gene. Pt 3Biochem. J. 2000;351:747–753. doi: 10.1042/bj3510747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duan Y., Chen Y., Hu W., Li X., Yang X., Zhou X., Yin Z., Kong D., Yao Z., Hajjar D.P., et al. Peroxisome Proliferator-activated receptor gamma activation by ligands and dephosphorylation induces proprotein convertase subtilisin kexin type 9 and low density lipoprotein receptor expression. J. Biol. Chem. 2012;287:23667–23677. doi: 10.1074/jbc.M112.350181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T., Kong X., Owsley E., Ellis E., Strom S., Chiang J.Y. Insulin regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes: Roles of forkhead box O1 and sterol regulatory element-binding protein 1c. J. Biol. Chem. 2006;281:28745–28754. doi: 10.1074/jbc.M605815200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park W.H., Pak Y.K. Insulin-dependent suppression of cholesterol 7alpha-hydroxylase is a possible link between glucose and cholesterol metabolisms. Exp. Mol. Med. 2011;43:571–579. doi: 10.3858/emm.2011.43.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Martelot G., Claudel T., Gatfield D., Schaad O., Kornmann B., Lo Sasso G., Moschetta A., Schibler U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T., Zhao M., Lu D., Wang S., Yu F., Guo L., Wen S., Wu B. REV-ERBalpha Regulates CYP7A1 Through Repression of Liver Receptor Homolog-1. Drug Metab. Dispos. 2018;46:248–258. doi: 10.1124/dmd.117.078105. [DOI] [PubMed] [Google Scholar]
- 39.Lee Y.H., Alberta J.A., Gonzalez F.J., Waxman D.J. Multiple, functional DBP sites on the promoter of the cholesterol 7 alpha-hydroxylase P450 gene, CYP7. Proposed role in diurnal regulation of liver gene expression. J. Biol. Chem. 1994;269:14681–14689. [PubMed] [Google Scholar]
- 40.Ogawa A., Yano M., Tsujinaka T., Morimoto T., Morita S., Taniguchi M., Shiozaki H., Okamoto K., Sato S., Monden M. Modulation of circadian expression of D-site binding protein by the schedule of parenteral nutrition in rat liver. Hepatology. 1997;26:1580–1586. doi: 10.1002/hep.510260628. [DOI] [PubMed] [Google Scholar]
- 41.Berard A.M., Dumon M.F., Darmon M. Dietary fish oil up-regulates cholesterol 7alpha-hydroxylase mRNA in mouse liver leading to an increase in bile acid and cholesterol excretion. FEBS Lett. 2004;559:125–128. doi: 10.1016/S0014-5793(04)00049-3. [DOI] [PubMed] [Google Scholar]
- 42.Chiang J.Y.L. Bile acids: Regulation of synthesis. J. Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li T., Francl J.M., Boehme S., Chiang J.Y. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7alpha-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology. 2013;58:1111–1121. doi: 10.1002/hep.26427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song K.H., Li T., Owsley E., Chiang J.Y. A putative role of micro RNA in regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes. J. Lipid Res. 2010;51:2223–2233. doi: 10.1194/jlr.M004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigues P.M., Afonso M.B., Simao A.L., Carvalho C.C., Trindade A., Duarte A., Borralho P.M., Machado M.V., Cortez-Pinto H., Rodrigues C.M., et al. miR-21 ablation and obeticholic acid ameliorate nonalcoholic steatohepatitis in mice. Cell Death Dis. 2017;8:e2748. doi: 10.1038/cddis.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong R., Lv X., Liu F. MiRNA-17 encoded by the miR-17-92 cluster increases the potential for steatosis in hepatoma cells by targeting CYP7A1. Cell Mol. Biol. Lett. 2018;23:16. doi: 10.1186/s11658-018-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takagi S., Nakajima M., Kida K., Yamaura Y., Fukami T., Yokoi T. MicroRNAs regulate human hepatocyte nuclear factor 4alpha, modulating the expression of metabolic enzymes and cell cycle. J. Biol. Chem. 2010;285:4415–4422. doi: 10.1074/jbc.M109.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D., Gao Q., Wang T., Zhao G., Qian F., Huang J., Wang H., Zhang X., Wang Y. Green tea infusion protects against alcoholic liver injury by attenuating inflammation and regulating the PI3K/Akt/eNOS pathway in C57BL/6 mice. Food Funct. 2017;8:3165–3177. doi: 10.1039/C7FO00791D. [DOI] [PubMed] [Google Scholar]
- 49.Kawabata K., Yoshioka Y., Terao J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teng H., Chen L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019;59:2040–2051. doi: 10.1080/10408398.2018.1437023. [DOI] [PubMed] [Google Scholar]
- 51.D’Archivio M., Filesi C., Vari R., Scazzocchio B., Masella R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010;11:1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manach C., Williamson G., Morand C., Scalbert A., Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 53.Yahfoufi N., Alsadi N., Jambi M., Matar C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients. 2018;10:1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu A.J. Antagonism by bioactive polyphenols against inflammation: A systematic view. Inflamm. Allergy Drug Targets. 2014;13:34–64. doi: 10.2174/1871528112666131119211002. [DOI] [PubMed] [Google Scholar]
- 55.EFSA Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage. EFSA J. 2011 doi: 10.2903/j.efsa.2011.2033. [DOI] [Google Scholar]
- 56.Millar C.L., Duclos Q., Blesso C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv. Nutr. 2017;8:226–239. doi: 10.3945/an.116.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang T.T., Koo M.W. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci. 2000;66:411–423. doi: 10.1016/S0024-3205(99)00607-4. [DOI] [PubMed] [Google Scholar]
- 58.Hirsova P., Karlasova G., Dolezelova E., Cermanova J., Zagorova M., Kadova Z., Hroch M., Sispera L., Tomsik P., Lenicek M., et al. Cholestatic effect of epigallocatechin gallate in rats is mediated via decreased expression of Mrp2. Toxicology. 2013;303:9–15. doi: 10.1016/j.tox.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 59.Shan D., Fang Y., Ye Y., Liu J. EGCG reducing the susceptibility to cholesterol gallstone formation through the regulation of inflammation. Biomed. Pharmacother. 2008;62:677–683. doi: 10.1016/j.biopha.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Lee M.S., Park J.Y., Freake H., Kwun I.S., Kim Y. Green tea catechin enhances cholesterol 7alpha-hydroxylase gene expression in HepG2 cells. Br. J. Nutr. 2008;99:1182–1185. doi: 10.1017/S0007114507864816. [DOI] [PubMed] [Google Scholar]
- 61.Li G., Lin W., Araya J.J., Chen T., Timmermann B.N., Guo G.L. A tea catechin, epigallocatechin-3-gallate, is a unique modulator of the farnesoid X receptor. Toxicol. Appl. Pharmacol. 2012;258:268–274. doi: 10.1016/j.taap.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park Y., Park E.M., Kim E.H., Chung I.M. Hypocholesterolemic metabolism of dietary red pericarp glutinous rice rich in phenolic compounds in mice fed a high cholesterol diet. Nutr. Res. Pract. 2014;8:632–637. doi: 10.4162/nrp.2014.8.6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matziouridou C., Marungruang N., Nguyen T.D., Nyman M., Fak F. Lingonberries reduce atherosclerosis in Apoe(-/-) mice in association with altered gut microbiota composition and improved lipid profile. Mol. Nutr. Food Res. 2016;60:1150–1160. doi: 10.1002/mnfr.201500738. [DOI] [PubMed] [Google Scholar]
- 64.Kim S.J., Maeda T., Sarker M.Z., Takigawa S., Matsuura-Endo C., Yamauchi H., Mukasa Y., Saito K., Hashimoto N., Noda T., et al. Identification of anthocyanins in the sprouts of buckwheat. J. Agric. Food Chem. 2007;55:6314–6318. doi: 10.1021/jf0704716. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe M., Ayugase J. Effects of buckwheat sprouts on plasma and hepatic parameters in type 2 diabetic db/db mice. J. Food Sci. 2010;75:H294–H299. doi: 10.1111/j.1750-3841.2010.01853.x. [DOI] [PubMed] [Google Scholar]
- 66.Wang D., Xia M., Gao S., Li D., Zhang Y., Jin T., Ling W. Cyanidin-3-O-beta-glucoside upregulates hepatic cholesterol 7alpha-hydroxylase expression and reduces hypercholesterolemia in mice. Mol. Nutr. Food Res. 2012;56:610–621. doi: 10.1002/mnfr.201100659. [DOI] [PubMed] [Google Scholar]
- 67.Zhang C., Zhang R., Li Y.M., Liang N., Zhao Y., Zhu H., He Z., Liu J., Hao W., Jiao R., et al. Cholesterol-Lowering Activity of Tartary Buckwheat Protein. J. Agric. Food Chem. 2017;65:1900–1906. doi: 10.1021/acs.jafc.7b00066. [DOI] [PubMed] [Google Scholar]
- 68.Huang J.B., Feng S.M., Liu A.N., Dai Z.Q., Wang H., Reuhl K., Lu W.Y., Yang C.S. Green Tea Polyphenol EGCG Alleviates Metabolic Abnormality and Fatty Liver by Decreasing Bile Acid and Lipid Absorption in Mice. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M., Xie Z., Gao W., Pu L., Wei J., Guo C. Quercetin regulates hepatic cholesterol metabolism by promoting cholesterol-to-bile acid conversion and cholesterol efflux in rats. Nutr. Res. 2016;36:271–279. doi: 10.1016/j.nutres.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 70.Chavez-Santoscoy R.A., Gutierrez-Uribe J.A., Granados O., Torre-Villalvazo I., Serna-Saldivar S.O., Torres N., Palacios-González B., Tovar A.R. Flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats modulate lipid metabolism and biliary cholesterol secretion in C57BL/6 mice. Br. J. Nutr. 2014;112:886–899. doi: 10.1017/S0007114514001536. [DOI] [PubMed] [Google Scholar]
- 71.Daniel R.S., Devi K.S., Augusti K.T., Sudhakaran Nair C.R. Mechanism of action of antiatherogenic and related effects of Ficus bengalensis Linn. flavonoids in experimental animals. Indian J. Exp. Biol. 2003;41:296–303. [PubMed] [Google Scholar]
- 72.Mathew B.C., Yoseph B.A., Dessale T., Daniel R.S., Alemayehu A., Campbell I.W., Augusti K.T. Hypolipidaemic effect of Leucodelphinidin derivative from Ficus bengalensis Linn on Cholesterol fed rats. Res. J. Chem. Sci. 2012;2:54–60. [Google Scholar]
- 73.Hoang M.H., Jia Y., Mok B., Jun H.J., Hwang K.Y., Lee S.J. Kaempferol ameliorates symptoms of metabolic syndrome by regulating activities of liver X receptor-beta. J. Nutr. Biochem. 2015;26:868–875. doi: 10.1016/j.jnutbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 74.Goldwasser J., Cohen P.Y., Yang E., Balaguer P., Yarmush M.L., Nahmias Y. Transcriptional Regulation of Human and Rat Hepatic Lipid Metabolism by the Grapefruit Flavonoid Naringenin: Role of PPARα, PPARγ and LXRα. PLoS ONE. 2010;5:e12399. doi: 10.1371/journal.pone.0012399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang J., Wang C., Peng J., Li W., Jin Y., Liu Q., Meng Q., Liu K., Sun H. Naringin regulates cholesterol homeostasis and inhibits inflammation via modulating NF-kappaB and ERK signaling pathways in vitro. Die Pharm. 2016;71:101–108. [PubMed] [Google Scholar]
- 76.Lin H.R., Chou T.H., Huang D.W., Chen I.S. Cryptochinones from Cryptocarya chinensis act as farnesoid X receptor agonists. Bioorg. Med. Chem. Lett. 2014;24:4181–4186. doi: 10.1016/j.bmcl.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 77.de Almeida Jackix E., Monteiro E.B., Raposo H.F., Vanzela E.C., Amaya-Farfan J. Taioba (Xanthosoma sagittifolium) leaves: Nutrient composition and physiological effects on healthy rats. J. Food Sci. 2013;78:H1929–H1934. doi: 10.1111/1750-3841.12301. [DOI] [PubMed] [Google Scholar]
- 78.Picerno P., Mencherini T., Lauro M.R., Barbato F., Aquino R. Phenolic Constituents and Antioxidant Properties of Xanthosoma violaceum Leaves. J. Agric. Food Chem. 2003;51:6423–6428. doi: 10.1021/jf030284h. [DOI] [PubMed] [Google Scholar]
- 79.Ma J.Q., Ding J., Zhao H., Liu C.M. Puerarin attenuates carbon tetrachloride-induced liver oxidative stress and hyperlipidaemia in mouse by JNK/c-Jun/CYP7A1 pathway. Basic Clin. Pharmacol. Toxicol. 2014;115:389–395. doi: 10.1111/bcpt.12245. [DOI] [PubMed] [Google Scholar]
- 80.Yan L.P., Chan S.W., Chan A.S., Chen S.L., Ma X.J., Xu H.X. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci. 2006;79:324–330. doi: 10.1016/j.lfs.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 81.Chung M.J., Sung N.-J., Park C.-S., Kweon D.-K., Mantovani A., Moon T.-W., Lee S.-J., Park K.-H. Antioxidative and hypocholesterolemic activities of water-soluble puerarin glycosides in HepG2 cells and in C57 BL/6J mice. Eur. J. Pharmacol. 2008;578:159–170. doi: 10.1016/j.ejphar.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 82.Feng D., Sun J.G., Sun R.B., Ou-Yang B.C., Yao L., Aa J.Y., Zhou F., Zhang J.W., Zhang J., Wang G.J. Isoflavones and phytosterols contained in Xuezhikang capsules modulate cholesterol homeostasis in high-fat diet mice. Acta Pharmacol. Sin. 2015;36:1462–1472. doi: 10.1038/aps.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mvondo M.A., Njamen D., Kretzschmar G., Imma Bader M., Tanee Fomum S., Wandji J., Vollmer G. Alpinumisoflavone and abyssinone V 4’-methylether derived from Erythrina lysistemon (Fabaceae) promote HDL-cholesterol synthesis and prevent cholesterol gallstone formation in ovariectomized rats. J. Pharm. pharmacol. 2015;67:990–996. doi: 10.1111/jphp.12386. [DOI] [PubMed] [Google Scholar]
- 84.Ni Y.D., Wei X.J., Zhang C.X., Zhong Y., Lu L.Z., Grossmann R., Zhao R.Q. The effect of equol injection in ovo on lipid metabolism and hepatic lipogenic gene expression in broilers. Anim. Int. J. Anim. Biosci. 2012;6:1444–1450. doi: 10.1017/S1751731112000468. [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi M., Harada T., Takagi N., Tsuzuki K., Sugawara M., Fukuda M. Effects of lactic acid-fermented soymilk on lipid metabolism-related gene expression in rat liver. Biosci. Biotechnol. Biochem. 2012;76:19–24. doi: 10.1271/bbb.100354. [DOI] [PubMed] [Google Scholar]
- 86.Zhang H., Bartley G.E., Mitchell C.R., Zhang H., Yokoyama W. Lower weight gain and hepatic lipid content in hamsters fed high fat diets supplemented with white rice protein, brown rice protein, soy protein, and their hydrolysates. J. Agric. Food Chem. 2011;59:10927–10933. doi: 10.1021/jf202721z. [DOI] [PubMed] [Google Scholar]
- 87.Laurent T., Okuda Y., Chijimatsu T., Umeki M., Kobayashi S., Kataoka Y., Tatsuguchi I., Mochizuki S., Oda H. Freshwater Clam Extract Ameliorates Triglyceride and Cholesterol Metabolism through the Expression of Genes Involved in Hepatic Lipogenesis and Cholesterol Degradation in Rats. Evid.-Based Complement. Altern. Med. eCAM. 2013;2013:830684. doi: 10.1155/2013/830684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroon P.A., Iyer A., Chunduri P., Chan V., Brown L. The cardiovascular nutrapharmacology of resveratrol: Pharmacokinetics, molecular mechanisms and therapeutic potential. Curr. Med. Chem. 2010;17:2442–2455. doi: 10.2174/092986710791556032. [DOI] [PubMed] [Google Scholar]
- 89.Chen Q., Wang E., Ma L., Zhai P. Dietary resveratrol increases the expression of hepatic 7alpha-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis. 2012;11:56. doi: 10.1186/1476-511X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shao D., Wang Y., Huang Q., Shi J., Yang H., Pan Z., Jin M., Zhao H., Xu X. Cholesterol-Lowering Effects and Mechanisms in View of Bile Acid Pathway of Resveratrol and Resveratrol Glucuronides. J. Food Sci. 2016 doi: 10.1111/1750-3841.13528. [DOI] [PubMed] [Google Scholar]
- 91.Wang T., Zhou Z.X., Sun L.X., Li X., Xu Z.M., Chen M., Zhao G.L., Jiang Z.Z., Zhang L.Y. Resveratrol effectively attenuates alpha-naphthyl-isothiocyanate-induced acute cholestasis and liver injury through choleretic and anti-inflammatory mechanisms. Acta Pharmacol. Sin. 2014;35:1527–1536. doi: 10.1038/aps.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miura D., Miura Y., Yagasaki K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003;73:1393–1400. doi: 10.1016/S0024-3205(03)00469-7. [DOI] [PubMed] [Google Scholar]
- 93.Marier J.F., Vachon P., Gritsas A., Zhang J., Moreau J.P., Ducharme M.P. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exp. Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 94.Qiao Y., Sun J., Xia S., Tang X., Shi Y., Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5:1241–1249. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- 95.Kemper J.K., Xiao Z., Ponugoti B., Miao J., Fang S., Kanamaluru D., Tsang S., Wu S.Y., Chiang C.M., Veenstra T.D. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen M.L., Yi L., Zhang Y., Zhou X., Ran L., Yang J., Zhu J.D., Zhang Q.Y., Mi M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. MBio. 2016;7:e02210–e02215. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fujita H., Yamagami T. Antihypercholesterolemic effect of Chinese black tea extract in human subjects with borderline hypercholesterolemia. Nutr. Res. 2008;28:450–456. doi: 10.1016/j.nutres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H.M., Wang C.F., Shen S.M., Wang G.L., Liu P., Liu Z.M., Wang Y.Y., Du S.S., Liu Z.L., Deng Z.W. Antioxidant phenolic compounds from Pu-erh tea. Molecules. 2012;17:14037–14045. doi: 10.3390/molecules171214037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujita H., Yamagami T. Efficacy and safety of Chinese black tea (Pu-Ehr) extract in healthy and hypercholesterolemic subjects. Ann. Nutr. Metab. 2008;53:33–42. doi: 10.1159/000153006. [DOI] [PubMed] [Google Scholar]
- 100.Fujita H., Yamagami T. Extract of black tea (pu-ehr) inhibits postprandial rise in serum cholesterol in mice, and with long term use reduces serum cholesterol and low density lipoprotein levels and renal fat weight in rats. Phytother. Res. PTR. 2008;22:1275–1281. doi: 10.1002/ptr.2477. [DOI] [PubMed] [Google Scholar]
- 101.Tan Z., Luo M., Yang J., Cheng Y., Huang J., Lu C., Song D., Ye M., Dai M., Gonzalez F.J., et al. Chlorogenic acid inhibits cholestatic liver injury induced by alpha-naphthylisothiocyanate: Involvement of STAT3 and NFkappaB signalling regulation. J. Pharm. Pharmacol. 2016;68:1203–1213. doi: 10.1111/jphp.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spiller G.A., Story J.A., Furumoto E.J., Chezem J.C., Spiller M. Effect of tartaric acid and dietary fibre from sun-dried raisins on colonic function and on bile acid and volatile fatty acid excretion in healthy adults. Br. J. Nutr. 2003;90:803–807. doi: 10.1079/BJN2003966. [DOI] [PubMed] [Google Scholar]
- 103.Spiller G.A., Story J.A., Lodics T.A., Pollack M., Monyan S., Butterfield G., Spiller M. Effect of sun-dried raisins on bile acid excretion, intestinal transit time, and fecal weight: A dose-response study. J. Med. Food. 2003;6:87–91. doi: 10.1089/109662003322233477. [DOI] [PubMed] [Google Scholar]
- 104.Williamson G., Carughi A. Polyphenol content and health benefits of raisins. Nutr. Res. 2010;30:511–519. doi: 10.1016/j.nutres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 105.Tebib K., Besancon P., Rouanet J.M. Dietary grape seed tannins affect lipoproteins, lipoprotein lipases and tissue lipids in rats fed hypercholesterolemic diets. J. Nutr. 1994;124:2451–2457. doi: 10.1093/jn/124.12.2451. [DOI] [PubMed] [Google Scholar]
- 106.Camire M.E., Dougherty M.P. Raisin dietary fiber composition and in vitro bile acid binding. J. Agric. Food Chem. 2003;51:834–837. doi: 10.1021/jf025923n. [DOI] [PubMed] [Google Scholar]
- 107.Ngamukote S., Makynen K., Thilawech T., Adisakwattana S. Cholesterol-lowering activity of the major polyphenols in grape seed. Molecules. 2011;16:5054–5061. doi: 10.3390/molecules16065054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao R., Yasuoka A., Kamei A., Ushiama S., Kitagawa Y., Rogi T., Shibata H., Abe K., Misaka T. Nuclear receptor-mediated alleviation of alcoholic fatty liver by polyphenols contained in alcoholic beverages. PLoS ONE. 2014;9:e87142. doi: 10.1371/journal.pone.0087142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiao R., Zhang Z., Yu H., Huang Y., Chen Z.Y. Hypocholesterolemic activity of grape seed proanthocyanidin is mediated by enhancement of bile acid excretion and up-regulation of CYP7A1. J. Nutr. Biochem. 2010;21:1134–1139. doi: 10.1016/j.jnutbio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 110.Downing L.E., Edgar D., Ellison P.A., Ricketts M.L. Mechanistic insight into nuclear receptor-mediated regulation of bile acid metabolism and lipid homeostasis by grape seed procyanidin extract (GSPE) Cell Biochem. Funct. 2017;35:12–32. doi: 10.1002/cbf.3247. [DOI] [PubMed] [Google Scholar]
- 111.Del Bas J.M., Fernandez-Larrea J., Blay M., Ardevol A., Salvado M.J., Arola L., Blade C. Grape seed procyanidins improve atherosclerotic risk index and induce liver CYP7A1 and SHP expression in healthy rats. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005;19:479–481. doi: 10.1096/fj.04-3095fje. [DOI] [PubMed] [Google Scholar]
- 112.Sembries S., Dongowski G., Mehrlander K., Will F., Dietrich H. Physiological effects of extraction juices from apple, grape, and red beet pomaces in rats. J. Agric. Food Chem. 2006;54:10269–10280. doi: 10.1021/jf0618168. [DOI] [PubMed] [Google Scholar]
- 113.Heidker R.M., Caiozzi G.C., Ricketts M.L. Dietary procyanidins selectively modulate intestinal farnesoid X receptor-regulated gene expression to alter enterohepatic bile acid recirculation: Elucidation of a novel mechanism to reduce triglyceridemia. Mol. Nutr. Food Res. 2016;60:727–736. doi: 10.1002/mnfr.201500795. [DOI] [PubMed] [Google Scholar]
- 114.Shin M.J., Cho Y., Moon J., Jeon H.J., Lee S.M., Chung J.H. Hypocholesterolemic effect of daily fisetin supplementation in high fat fed Sprague-Dawley rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;57:84–90. doi: 10.1016/j.fct.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 115.Del Bas J.M., Crescenti A., Arola-Arnal A., Oms-Oliu G., Arola L., Caimari A. Intake of grape procyanidins during gestation and lactation impairs reverse cholesterol transport and increases atherogenic risk indexes in adult offspring. J. Nutr. Biochem. 2015;26:1670–1677. doi: 10.1016/j.jnutbio.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 116.Oike H., Kobori M. Resveratrol regulates circadian clock genes in Rat-1 fibroblast cells. Biosci. Biotechnol. Biochem. 2008;72:3038–3040. doi: 10.1271/bbb.80426. [DOI] [PubMed] [Google Scholar]
- 117.Miranda J., Portillo M.P., Madrid J.A., Arias N., Macarulla M.T., Garaulet M. Effects of resveratrol on changes induced by high-fat feeding on clock genes in rats. Br. J. Nutr. 2013;110:1421–1428. doi: 10.1017/S0007114513000755. [DOI] [PubMed] [Google Scholar]
- 118.Baselga-Escudero L., Blade C., Ribas-Latre A., Casanova E., Salvado M.J., Arola L., Arola-Arnal A. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats. Mol. Nutr. Food Res. 2012;56:1636–1646. doi: 10.1002/mnfr.201200237. [DOI] [PubMed] [Google Scholar]
- 119.Baselga-Escudero L., Blade C., Ribas-Latre A., Casanova E., Suarez M., Torres J.L., Salvado M.J., Arola L., Arola-Arnal A. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells. Nucleic Acids Res. 2014;42:882–892. doi: 10.1093/nar/gkt1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jahanafrooz Z., Motamed N., Bakhshandeh B. Effects of miR-21 downregulation and silibinin treatment in breast cancer cell lines. Cytotechnology. 2017 doi: 10.1007/s10616-017-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Su X., Zhang J., Wang H., Xu J., He J., Liu L., Zhang T., Chen R., Kang J. Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and miRNA Regulation in the Polyphenols of 16 Blueberry Samples from China. Molecules. 2017;22:312. doi: 10.3390/molecules22020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Milenkovic D., Deval C., Gouranton E., Landrier J.F., Scalbert A., Morand C., Mazur A. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: A new mechanism of the action of polyphenols. PLoS ONE. 2012;7:e29837. doi: 10.1371/journal.pone.0029837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shimoyama M.D.P.J., Hayman G.T., Laulederkind S.J., Liu W., Nigam R., Petri V., Smith J.R., Tutaj M., Wang S.J., Worthey E., et al. The Rat Genome Database 2015: Genomic, phenotypic and environmental variations and disease. Nucleic Acids Res. 2014;43:D743–D750. doi: 10.1093/nar/gku1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arola-Arnal A., Blade C. Proanthocyanidins modulate microRNA expression in human HepG2 cells. PLoS ONE. 2011;6:e25982. doi: 10.1371/journal.pone.0025982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rao A., Kosters A., Mells J.E., Zhang W., Setchell K.D., Amanso A.M., Wynn G.M., Xu T., Keller B.T., Yin H., et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl. Med. 2016;8:357ra122. doi: 10.1126/scitranslmed.aaf4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bhat B.G., Rapp S.R., Beaudry J.A., Napawan N., Butteiger D.N., Hall K.A., Null C.L., Luo Y., Keller B.T. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE-/-mice by SC-435. J. Lipid Res. 2003;44:1614–1621. doi: 10.1194/jlr.M200469-JLR200. [DOI] [PubMed] [Google Scholar]
- 127.Huff M.W., Telford D.E., Edwards J.Y., Burnett J.R., Barrett P.H.R., Rapp S.R., Napawan N., Keller B.T. Inhibition of the Apical Sodium-Dependent Bile Acid Transporter Reduces LDL Cholesterol and ApoB by Enhanced Plasma Clearance of LDL ApoB. Arterioscler. Thromb. Vasc. Biol. 2002;22:1884–1891. doi: 10.1161/01.ATV.0000035390.87288.26. [DOI] [PubMed] [Google Scholar]
- 128.Thomas C., Landrier J.F., Gaillard D., Grober J., Monnot M.C., Athias A., Besnard P. Cholesterol dependent downregulation of mouse and human apical sodium dependent bile acid transporter (ASBT) gene expression: Molecular mechanism and physiological consequences. Gut. 2006;55:1321–1331. doi: 10.1136/gut.2005.085555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alrefai W.A., Sarwar Z., Tyagi S., Saksena S., Dudeja P.K., Gill R.K. Cholesterol modulates human intestinal sodium-dependent bile acid transporter. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G978–G985. doi: 10.1152/ajpgi.00379.2004. [DOI] [PubMed] [Google Scholar]
- 130.Xu G., Shneider B.L., Shefer S., Nguyen L.B., Batta A.K., Tint G.S., Arrese M., Thevananther S., Ma L., Stengelin S., et al. Ileal bile acid transport regulates bile acid pool, synthesis, and plasma cholesterol levels differently in cholesterol-fed rats and rabbits. J. Lipid Res. 2000;41:298–304. [PubMed] [Google Scholar]
- 131.Torchia E.C., Cheema S.K., Agellon L.B. Coordinate regulation of bile acid biosynthetic and recovery pathways. Biochem. Biophys. Res. Commun. 1996;225:128–133. doi: 10.1006/bbrc.1996.1141. [DOI] [PubMed] [Google Scholar]
- 132.Zhang Y.K., Guo G.L., Klaassen C.D. Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLoS ONE. 2011;6:e16683. doi: 10.1371/journal.pone.0016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Odermatt A., Da Cunha T., Penno C.A., Chandsawangbhuwana C., Reichert C., Wolf A., Dong M., Baker M.E. Hepatic reduction of the secondary bile acid 7-oxolithocholic acid is mediated by 11beta-hydroxysteroid dehydrogenase 1. Biochem. J. 2011;436:621–629. doi: 10.1042/BJ20110022. [DOI] [PubMed] [Google Scholar]
- 134.Jones B.V., Begley M., Hill C., Gahan C.G.M., Marchesi J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA. 2008;105:13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sayin S.I., Wahlstrom A., Felin J., Jantti S., Marschall H.U., Bamberg K., Angelin B., Hyotylainen T., Oresic M., Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 136.Wostmann B.S. Intestinal bile acids and cholesterol absorption in the germfree rat. J. Nutr. 1973;103:982–990. doi: 10.1093/jn/103.7.982. [DOI] [PubMed] [Google Scholar]
- 137.Kellogg T.F., Wostmann B.S. Fecal neutral steroids and bile acids from germfree rats. J. Lipid Res. 1969;10:495–503. [PubMed] [Google Scholar]
- 138.Madsen D., Beaver M., Chang L., Bruckner-Kardoss E., Wostmann B. Analysis of bile acids in conventional and germfree rats. J. Lipid Res. 1976;17:107–111. [PubMed] [Google Scholar]
- 139.Li F., Jiang C., Krausz K.W., Li Y., Albert I., Hao H., Fabre K.M., Mitchell J.B., Patterson A.D., Gonzalez F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jeun J., Kim S., Cho S.Y., Jun H.J., Park H.J., Seo J.G., Chung M.J., Lee S.J. Hypocholesterolemic effects of Lactobacillus plantarum KCTC3928 by increased bile acid excretion in C57BL/6 mice. Nutrition. 2010;26:321–330. doi: 10.1016/j.nut.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 141.Jiang C., Xie C., Lv Y., Li J., Krausz K.W., Shi J., Brocker C.N., Desai D., Amin S.G., Bisson W.H., et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jones M.L., Martoni C.J., Parent M., Prakash S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br. J. Nutr. 2012;107:1505–1513. doi: 10.1017/S0007114511004703. [DOI] [PubMed] [Google Scholar]
- 143.Jones M.L., Martoni C.J., Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur. J. Clin. Nutr. 2012;66:1234–1241. doi: 10.1038/ejcn.2012.126. [DOI] [PubMed] [Google Scholar]
- 144.De Smet I., De Boever P., Verstraete W. Cholesterol lowering in pigs through enhanced bacterial bile salt hydrolase activity. Br. J. Nutr. 1998;79:185–194. doi: 10.1079/BJN19980030. [DOI] [PubMed] [Google Scholar]
- 145.Espín J.C., González-Sarrías A., Tomás-Barberán F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017 doi: 10.1016/j.bcp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 146.Lee H.C., Jenner A.M., Low C.S., Lee Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 147.Nakamura Y., Kaihara A., Yoshii K., Tsumura Y., Ishimitsu S., Tonogai Y. Effects of the Oral Administration of Green Tea Polyphenol and Tannic Acid on Serum and Hepatic Lipid Contents and Fecal Steroid Excretion in Rats. J. Health Sci. 2001;47:107–117. doi: 10.1248/jhs.47.107. [DOI] [Google Scholar]
- 148.Han Y., Haraguchi T., Iwanaga S., Tomotake H., Okazaki Y., Mineo S., Moriyama A., Inoue J., Kato N. Consumption of some polyphenols reduces fecal deoxycholic acid and lithocholic acid, the secondary bile acids of risk factors of colon cancer. J. Agric. Food Chem. 2009;57:8587–8590. doi: 10.1021/jf900393k. [DOI] [PubMed] [Google Scholar]
- 149.Tzounis X., Vulevic J., Kuhnle G.G., George T., Leonczak J., Gibson G.R., Kwik-Uribe C., Spencer J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008;99:782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 150.Queipo-Ortuno M.I., Boto-Ordonez M., Murri M., Gomez-Zumaquero J.M., Clemente-Postigo M., Estruch R., Cardona Diaz F., Andres-Lacueva C., Tinahones F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012;95:1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 151.Louis P., Flint H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 152.Scott K.P., Martin J.C., Duncan S.H., Flint H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. Fems Microbiol. Ecol. 2014;87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 153.Dolara P., Luceri C., Filippo C.D., Femia A.P., Giovannelli L., Caderni G., Cecchini C., Silvi S., Orpianesi C., Cresci A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005;591:237–246. doi: 10.1016/j.mrfmmm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 154.Caderni G., Remy S., Cheynier V., Morozzi G., Dolara P. Effect of complex polyphenols on colon carcinogenesis. Eur. J. Nutr. 1999;38:126–132. doi: 10.1007/s003940050053. [DOI] [PubMed] [Google Scholar]
- 155.Vendrame S., Guglielmetti S., Riso P., Arioli S., Klimis-Zacas D., Porrini M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011;59:12815–12820. doi: 10.1021/jf2028686. [DOI] [PubMed] [Google Scholar]
- 156.Yamakoshi J., Tokutake S., Kikuchi M., Kubota Y., Konishi H., Mitsuoka T. Effect of Proanthocyanidin-Rich Extract from Grape Seeds on Human Fecal Flora and Fecal Odor. Microb. Ecol. Health Dis. 2001;13:25–31. doi: 10.1080/089106001750071672. [DOI] [Google Scholar]
- 157.Larrosa M., Yanez-Gascon M.J., Selma M.V., Gonzalez-Sarrias A., Toti S., Ceron J.J., Tomas-Barberan F., Dolara P., Espin J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009;57:2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 158.Le Roy C.I., Wells P.M., Si J., Raes J., Bell J.T., Spector T.D. Red Wine Consumption Associated With Increased Gut Microbiota alpha-diversity in 3 Independent Cohorts. Gastroenterology. 2019 doi: 10.1053/j.gastro.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 159.Fotschki B., Juskiewicz J., Jurgonski A., Rigby N., Sojka M., Kolodziejczyk K., Mackie A., Zdunczyk Z. Raspberry pomace alters cecal microbial activity and reduces secondary bile acids in rats fed a high-fat diet. J. Nutr. Biochem. 2017;46:13–20. doi: 10.1016/j.jnutbio.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 160.Kim H., Kim D.H., Seo K.H., Chon J.W., Nah S.Y., Bartley G.E., Arvik T., Lipson R., Yokoyama W. Modulation of the intestinal microbiota is associated with lower plasma cholesterol and weight gain in hamsters fed chardonnay grape seed flour. J. Agric. Food Chem. 2015;63:1460–1467. doi: 10.1021/jf5026373. [DOI] [PubMed] [Google Scholar]
- 161.Ren K., Jiang T., Zhao G.J. Quercetin induces the selective uptake of HDL-cholesterol via promoting SR-BI expression and the activation of the PPARgamma/LXRalpha pathway. Food Funct. 2018;9:624–635. doi: 10.1039/C7FO01107E. [DOI] [PubMed] [Google Scholar]
- 162.Hou D.X., Luo D., Tanigawa S., Hashimoto F., Uto T., Masuzaki S., Fujii M., Sakata Y. Prodelphinidin B-4 3′-O-gallate, a tea polyphenol, is involved in the inhibition of COX-2 and iNOS via the downregulation of TAK1-NIF-kappa B pathway. Biochem. Pharmacol. 2007;74:742–751. doi: 10.1016/j.bcp.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 163.Crestani M., Galli G., Chiang J.Y. Genomic cloning, sequencing, and analysis of the hamster cholesterol 7 alpha-hydroxylase gene (CYP7) Arch. Biochem. Biophys. 1993;306:451–460. doi: 10.1006/abbi.1993.1537. [DOI] [PubMed] [Google Scholar]
- 164.Ramirez M.I., Karaoglu D., Haro D., Barillas C., Bashirzadeh R., Gil G. Cholesterol and bile acids regulate cholesterol 7 alpha-hydroxylase expression at the transcriptional level in culture and in transgenic mice. Mol. Cell. Biol. 1994;14:2809–2821. doi: 10.1128/MCB.14.4.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gatmaitan Z., Jefferson D.M., Ruiz-Opazo N., Biempica L., Arias I.M., Dudas G., Leinwand L.A., Reid L.M. Regulation of growth and differentiation of a rat hepatoma cell line by the synergistic interactions of hormones and collagenous substrata. J. Cell Biol. 1983;97:1179–1190. doi: 10.1083/jcb.97.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Enat R., Jefferson D.M., Ruiz-Opazo N., Gatmaitan Z., Leinwand L.A., Reid L.M. Hepatocyte proliferation in vitro: Its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc. Natl. Acad. Sci. USA. 1984;81:1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Davalos A., Fernandez-Hernando C., Cerrato F., Martinez-Botas J., Gomez-Coronado D., Gomez-Cordoves C., Lasuncion M.A. Red grape juice polyphenols alter cholesterol homeostasis and increase LDL-receptor activity in human cells in vitro. J. Nutr. 2006;136:1766–1773. doi: 10.1093/jn/136.7.1766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.