Abstract
Modified citrus pectin (MCP) has a low-molecular-weight degree of esterification to allow absorption from the small intestinal epithelium into the circulation. MCP produces pleiotropic effects, including but not limited to its antagonism of galectin-3, which have shown benefit in preclinical and clinical models. Regarding cancer, MCP modulates several rate-limiting steps of the metastatic cascade. MCP can also affect cancer cell resistance to chemotherapy. Regarding fibrotic diseases, MCP modulates many of the steps involved in the pathogenesis of aortic stenosis. MCP also reduces fibrosis to the kidney, liver, and adipose tissue. Other benefits of MCP include detoxification and improved immune function. This review summarizes the pleiotropic effects of MCP.
Keywords: cancer, cardiovascular, fibrosis, galectin, inflammation, pectasol
1. Introduction
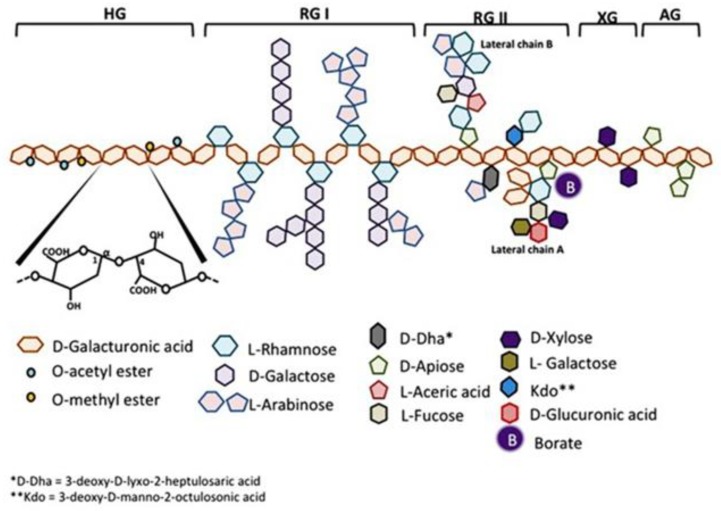
Citrus pectin is a soluble dietary fiber derived from the white pith of citrus fruit peels. Pectin is a large and complex molecule in its natural form, weighing 60–300 kilodalton (kDa), and containing a variable degree of (as much as ~70%) esterification. Pectins are a family of covalently linked galacturonic acid-rich polymers, with three identified central pectic polysaccharides regions: homogalacturonan (HG), rhamnogalacturonan-I (RG-I), and substituted galacturonans (GS). Among the GS is rhamnogalacturonan-II (RG-II), which is distinct from RG-I. RG-II has four types of structurally different oligosaccharides chains composed of 12 kinds of glycosyl residues [1] (Figure 1). Native pectin is not degraded during human digestion, and its large size prevents intestinal absorption [2,3]. However, when citrus pectin is modified (MCP) with a specific pH and heat-controlled enzymatic treatment to yield a product with a low molecular weight of <15 kilodaltons (kDa) and a degree of esterification under 5%, it can be absorbed from the small intestinal epithelium into the circulation [3]. The health benefits of MCP are increasingly recognized and summarized in this review and Table 1.
Figure 1.
Schematic representation of pectin structure. AG, arabinogalactan; HG, homogalacturonan; RG, rhamnogalacturonan; XG, xylogalacturonan. It is reproduced with permission under a Creative Commons Attribution License (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/ from Leclere, L.; Cutsem, PV.; Michiels, C.; Anti-cancer activities of pH- or heat-modified pectin. Front Pharmacol. 2013 Oct 8; 4:128 [1].
Table 1.
Pleiotropic effects of modified citrus pectin.
| Main Indication | Study Type | Disease Model | Species Studied | Reference | Summary of Results |
|---|---|---|---|---|---|
| Cancer | Clinical trial | Circulating tumor cells | Human | [4] | Nutrients with anti-carcinogenic properties could reduce circulating tumor cell count, and included curcumin, garlic, green tea, grape seed, MCP, and medicinal mushroom extract |
| Cancer | Clinical trial | Advanced solid tumors | Human | [5] | Clinical benefits and life quality with far advanced solid tumors |
| Cancer | Clinical trial | Prostate cancer | Human | [6] | PSADT extended in 70% of patients |
| Cancer | Preclinical | Ovarian cancer | In vitro | [7] | MCP enhanced the PTX effect on ovarian cancer cells MCTS through the inhibition of STAT3 activity |
| Cancer | Preclinical | Cisplatin-induced nephrotoxicity | Mouse | [8] | MCP-treated mice demonstrated decreased renal fibrosis and apoptosis |
| Cancer | Preclinical | Colon cancer | In vitro, in vivo, and ex vivo | [9] | MCP inhibition of extracellular Gal-3 decreases colon cancer cell migration |
| Cancer | Preclinical | Prostate cancer and radiation therapy | In vitro | [10] | MCP reduced prostate cancer cell viability and synergistically enhanced cell sensitivity to ionizing radiation |
| Cancer | Preclinical | Bladder cancer | In vitro and mouse | [11] | Remarkable inhibitory effects of MCP on urinary bladder cancer cell proliferation and survival in vitro and in vivo mainly through Gal-3 |
| Cancer | Preclinical | Gastrointestinal cancer | Mouse | [12] | MCP effectively inhibits the growth and metastasis of gastrointestinal cancer cells, partly by down-regulating Bcl-xL and Cyclin B to promote apoptosis and suppress EMT |
| Cancer | Preclinical | Colonic carcinogenesis | Mouse | [13] | Modified L. acidophilus ATCC 4356 cell envelope improved the bioavailability and the anti-(colon) cancer effect of MCP |
| Cancer | Preclinical | Breast and prostate cancer | In vitro | [14] | Inhibits breast/prostate cancer cell migration and synergy with MCP |
| Cancer | Preclinical | Ovarian cancer | In vitro | [15] | MCP synergy with paclitaxel |
| Cancer | Preclinical | Prostate cancer | In vitro | [16] | MCP synergy with doxorubicin |
| Cancer | Preclinical | Prostate cancer | In vitro | [17] | MCP induced cell death and inhibition of the proliferation of prostate cancer |
| Cancer | Preclinical | Liver and colon cancer | Mouse | [18] | MCP inhibits liver metastasis of colon cancer |
| Cardiovascular | Preclinical | Myocardial infarction | Rat | [19] | MCP blockade of Gal-3 can prevent cardiac fibrosis, inflammation, and functional alterations |
| Cardiovascular | Preclinical | Ischemic heart failure | Rabbit | [20] | Perindopril and MCP comparably improve ischemic heart failure in rabbits by downregulating Gal-3 and reducing myocardial fibrosis |
| Cardiovascular | Preclinical | Myocardial fibrosis | Rat, mouse, and human | [21] | MCP -mediated Gal-3 inhibition in mice prevented the profibrotic and proinflammatory effects of cardiotrophin-1 |
| Cardiovascular | Preclinical | Blood-brain barrier disruption | Mouse | [22] | MCP prevents post-Subarachnoid Hemorrhage blood-brain barrier disruption possibly by inhibiting Gal-3, of which the mechanisms may include binding to TLR4 and activating ERK1/2, STAT3, and MMP-9 |
| Cardiovascular | Preclinical | Cardiovascular fibrosis | In vitro, in vivo, and ex vivo | [23] | The pharmacological inhibition of Gal-3 with MCP restored cardiac Prx-4 as well as prohibitin-2 levels and improved oxidative status in spontaneously hypertensive rats |
| Cardiovascular | Preclinical | Cardiac lipotoxicity | Rat | [24] | Gal-3 inhibition with MCP attenuates consequences of cardiac lipotoxicity induced by a high-fat diet, reducing total triglyceride and lysophosphatidylcholine levels |
| Cardiovascular | Preclinical | Abdominal aortic aneurysm | Mouse | [25] | Mice treated with MCP showed decreased aortic dilation, as well as elastin degradation, vascular smooth muscle cell loss, and macrophage content at day 14 post-elastase perfusion compared with control mice |
| Cardiovascular | Preclinical | Atherosclerotic lesions in apoE-deficiency | Mouse | [26] | MCP reduced the size of atherosclerotic lesions by inhibiting the adhesion of leukocytes to endothelial cells |
| Cardiovascular | Preclinical | Aortic stenosis | Rat | [27] | In short-term AS, the increase in myocardial Gal-3 expression associated with cardiac fibrosis and inflammation, alterations that were prevented by Gal-3 blockade with MCP |
| Cardiovascular | Preclinical | Cardiovascular fibrosis and aortic valve calcification | Rat | [28] | MCP treatment prevented the increase in Gal-3, media thickness, fibrosis, and inflammation in the aorta of pressure overload rats |
| Cardiovascular | Preclinical | Aortic stenosis | Human and ex vivo | [29] | Gal-3 expression was blocked in VICs undergoing osteoblastic differentiation using MCP |
| Cardiovascular | Preclinical | Cardiovascular LV fibrosis | Mouse | [30] | MCP reversed induced LV dysfunction of HF with cardiac hyperaldosteronism |
| Cardiovascular | Preclinical | Cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension | Rat | [31] | MCP prevention of inflammation and fibrosis with hypertension |
| Cardiovascular | Preclinical | Heart fibrosis | Rat | [32] | MCP prevention of cardiac fibrosis |
| Cardiovascular | Preclinical | Vascular fibrosis | Rat | [33] | MCP reverses vascular hypertrophy and fibrosis |
| Kidney | Preclinical | Renal damage in spontaneous hypertension | Rat | [34] | The inflammatory mediators (monocyte chemoattractant protein-1, osteopontin, cd68, cd80, cd44, and cd45) were elevated in spontaneously hypertensive rats and attenuated by MCP |
| Kidney | Preclinical | Kidney fibrosis | Rat | [35] | In experimental models of mild kidney damage, the increase in renal Gal-3 expression paralleled with renal fibrosis and inflammation, while these alterations prevented with MCP |
| Kidney | Preclinical | Kidney fibrosis | Rat | [32] | MCP prevention of kidney fibrosis |
| Kidney | Preclinical | Acute kidney disease | In vitro | [36] | MCP inhibits renal fibrosis |
| Obesity | Preclinical | Adipose tissue remodeling | Rat | [37] | Despite no effect on body weight, adipose tissue weights or adiposity, MCP prevented adipose tissue fibrosis, inflammation and the increase in adipocyte differentiation markers in a model of diet-induced obesity |
| Obesity | Preclinical | Adipose tissue remodeling/fibrosis | Rat | [31] | MCP prevented an increase in pericellular collagen, adipose tissue inflammation and differentiation degree of the adipocytes |
| Liver | Preclinical | Liver fibrosis | Rat | [38] | MCP attenuates liver fibrosis through an antioxidant effect, the inhibition of Gal-3, and the induction of apoptosis |
| Detoxification | Clinical trial | Chronic low-level uranium exposure | Human | [39] | MCP, after a post-treatment period of 6 weeks, decreased in fecal excretion of uranium found in 5 of 6 participants |
| Detoxification | Clinical trial | Child lead toxicity | Human | [40] | Detoxification from lead toxicity in hospitalized children |
| Detoxification | Clinical trial | Lead and mercury toxicity | Human | [41] | MCP lowered body burden of lead and or mercury and chronic ailment improvements |
| Detoxification | Clinical trial | Toxic metals | Human | [42] | MCP detoxification of lead, cadmium, arsenic, and mercury |
| Immune | Preclinical | Immuno-modulation | Mouse | [43] | CP and mainly MCP have an immunomodulatory effect on the levels of cytokine secretion in the spleen of mice with a pro-inflammatory potential |
| Immune | Preclinical | Probiotic | Mouse | [44] | The number of fecal lactobacilli in the MCP alginate probiotic-treated mice significantly increased |
| Immune | Preclinical | Shiga toxin producing E. Coli | In vitro | [45] | MCP inhibits adhesion of shiga toxin, reduces shiga toxin cytotoxicity |
| Immune | Preclinical | Inflammation | In vitro | [46] | MCP: Honokiol (9:1) combination induced a synergistic effect on antioxidant activity suggesting that the mixture is significantly more efficient than individual compounds |
| Immune | Preclinical | Staphylococcus aureus | In vitro | [47] | MCP demonstrates in vitro antimicrobial activity alone and combination with cefotaxime against staphylococcus aureus. |
| Immune | Preclinical | Immune activation | Human blood and ex vivo | [48] | MCP significantly activated T-cells and natural killer cells |
Abbreviations. apoE: Apolipoprotein E, AS: Aortic stenosis, CP: Citrus pectin, EMT: Epithelial-mesenchymal transition, ERK1/2: Extracellular signal-related kinase 1/2, Gal-3: Galectin-3, HF: Heart failure, HNK: Honokiol, LV: Left ventricular, MCP: PectaSol-C Modified citrus pectin, MCTS: multicellular tumor spheroid, MMP-9: Matrix metalloproteinase-9, Prx-4: Peroxiredoxin-4, PSADT: Prostate-specific antigen doubling time, PTX: Paclitaxel, STAT3: Signal transducer and activator of transcription 3, TLR4: Toll-like receptor 4, and VIC: Valvular interstitial cell.
2. Galectin-3
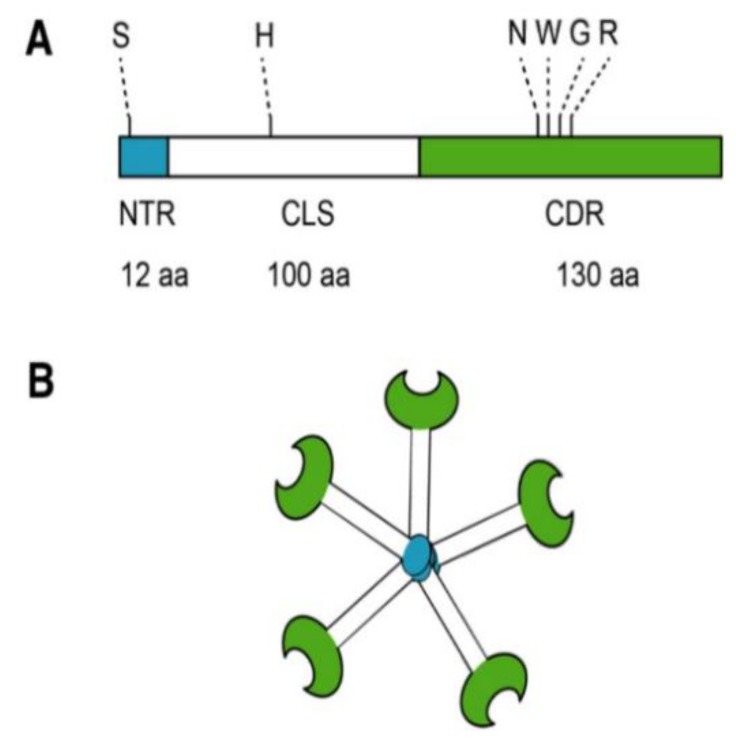
Most biomedical reports of MCP focus specifically on its antagonism of galectin-3 (Gal-3). Located in the nucleus, cytoplasm, outer cell surface, and extracellular space, Gal-3 is a member of a β-galactoside-binding lectin family [49]. Galectin-3 is a unique chimeric galectin containing a single carbohydrate recognition domain (CRD) of 130 amino acids at the COOH terminal with a unique 12 amino acid NH2-terminal domain (NTD). The CRD also contains what has been referred to as the anti-death motif or Asp-Trp-Gly-Arg (NWGR) due to sequence similarity to anti-apoptotic B-cell lymphoma-2 (Bcl-2) protein [50]. In the NTD, the serine 6 can be phosphorylated by casein kinases 1 and 2, contributing to nuclear translocation and a reduction of affinity to its ligands. Connecting the CRD and the NTD is a collagen-like sequence (CLS) domain consisting of about 100 amino acids and contains a Pro-Gly-Ala-Tyr-rich repeat motif. This long tail allows for unique pentameric oligomerization and contains the collagenase cleavable H-domain (Figure 2) [51]. Galectin-3 plays a prominent role in the progression of cancer and fibrotic diseases [52].
Figure 2.
Structure of Gal-3. (A) Gal-3 protein structure consists of an N terminal Domain (NTD), which has an N terminal region of 12 amino acids (aa) and contains a serine 6 (S) phosphorylation site. The carbohydrate recognition domain (CRD) of 130 aa comprises the C-terminal and contains the anti-death motif or Asp-Trp-Gly-Arg (NWGR); (B) Pentameric structure of Gal-3. Reproduced with permission under a Creative Commons Attribution License (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/. Clementy, N.; Piver, E.; Bisson, A.; André, Clémentine, A.; Bernard, A.; Pierre, B.; Fauchier, L.; Babuty, D. (2018). Galectin-3 in Atrial Fibrillation: Mechanisms and Therapeutic Implications. Int J Mol Sci. 2018 Apr; 19(4): 976 [49].
Regarding the former, the damaging effects of Gal-3 are due to its ability to accelerate the rate-limiting steps of metastasis [53]. Regarding the latter, the detrimental effects are due to its ability to bind matrix proteins such as cell surface receptors (integrins), collagen, elastin, and fibronectin and form cross-linking lattices in the extracellular matrix (ECM) [54]. Modified citrus pectin is abundant in β-galactose [53], which allows it to bind tightly to Gal-3 and modulate its bioactivity [55].
3. Cancer
Most of the morbidity and mortality associated with cancer is caused by metastasis, which is the migration of cancer from the site of primary tumor growth to distant organs and tissues. The metastatic cascade contains several rate-limiting steps that are modulated by Gal-3 and, in turn, by MCP as well [53]. The first step for neoplastic cells is to survive apoptosis that is associated with the loss of anchorage (anoikis) following escape from the primary tumor and intravasation. Galectin-3 protects cancer cells from anoikis [56,57] by causing a cell cycle arrest at the late G1 phase, which is an anoikis-insensitive point [56]. MCP has been shown to downregulate cyclin B and cdc2 in human prostatic JCA-1 cells [58], which may cause an accumulation of cancer cells in G2/M, thereby inducing apoptosis.
The next rate-limiting step in metastasis involves tumor cell arrest in distant organ microvasculature. Galectin-3 has been shown to mediate metastatic cell adhesion to the endothelium [59,60,61,62,63]. MCP was demonstrated to inhibit tumor cell adhesion to the endothelium as well as cancer cell homotypic aggregation involved in metastatic cell arrest in distant organs and the formation of intravascular metastatic deposits [59,64,65,66,67,68].
The third rate-limiting step in metastasis involves a forking point where tumor cells can either proliferate inside organ microvessels until the metastatic tumor outgrows the blood vessel and invades distant organ parenchyma [69], or extravasate before starting secondary tumor growth. Invasive propensity involves a series of tumor cell interactions with ECM proteins associated with the basement membrane and target organ stroma. MCP has been shown to reduce Gal-3-mediated tumor cell interactions with ECM proteins such as laminin [66]. Also, citrus pectin polysaccharides dose-dependently decreased the invasion through matrigel of human endothelial cells [67], of MDA-MB-231 human metastatic breast carcinoma cells [70], and human buccal metastatic cells [70].
After the initial parking in distant organs and extravasation, the overwhelming majority of cancer cells undergo apoptosis caused by various factors, and only ~2% survive and lead to micrometastasis [71]. Clonogenicity survival of early metastatic colonies is, therefore, the fourth rate-limiting step in metastasis. Galectin-3 protects cancer cells from different types of apoptosis by acting on mitochondrial pathways [72,73,74]. Some have suggested that MCP could undo Gal-3 anti-apoptotic function, thereby reducing the clonogenicity survival of cancer cells [74]. MCP dose-dependently inhibited the clonogenicity survival of hemangiosarcoma cells, and this was associated with increased tumor cell apoptosis [75].
Micrometastasis that transforms into clinically relevant secondary tumors eventually comes to depend on the development of new blood vessels via angiogenesis, the fifth and final rate-limiting step in metastasis. Galectin-3 promotes angiogenesis by serving as a chemoattractant for endothelial cells and inducing endothelial cell motility, invasion through matrigel, and capillary tube formation [67,76]. MCP was found to thwart chemotaxis of human endothelial cells toward Gal-3 dose-dependently, and inhibit in vitro capillary tube formation by endothelial cells dose-dependently [76]. The administration of MCP also reduced angiogenesis and spontaneous metastasis in vivo in tumor-bearing mice [76].
MCP can also modulate cancer cell resistance to chemotherapy. Most anti-neoplastic drugs work by inducing tumor cell apoptosis via the mitochondrial apoptosis pathway. As mentioned above, Galectin-3 dampens this pathway [77,78,79,80,81]. It also directly affects the sensitivity of cancer cells to chemotherapeutic drugs such as cisplatin [79,81,82], staurosporine [79], etoposide [81], bortezomib [83], dexamethasone [83], and doxorubicin (Dox) [75]. MCP has been demonstrated to reduce Gal-3 anti-apoptotic function and thereby reverse multiple myeloma cell resistance to bortezomib and enhance the response to apoptosis induced by dexamethasone [83]. Also, MCP substantially increased sensitivity to Dox-induced apoptosis in hemangiosarcoma cells [75].
Beyond this, MCP has been shown to induce apoptosis in cancer cells by itself through a caspase-8-to caspase-3 signaling cascade in the absence of change to mitochondrial membrane potential [83].
The reports of the anti-cancer benefits with the administration of MCP have continued. MCP inhibited the growth and metastasis of implanted colon cancer in mouse spleen [84]. It also induced cytotoxicity in both androgen-dependent and -independent prostate cancer cells in vitro [17]. Furthermore, MCP synergizes with Dox in the treatment of prostate carcinoma DU-145 and LNCaP cells by decreasing the viability and proliferation of cells [16]. When combined with paclitaxel (PTX), MCP increased caspase-3 activity and the percentage of human SKOV-3 ovarian cancer cells in subG1 [15]. Also, MCP inhibited the invasive potential of highly metastatic human breast (MDA-MB-231) or prostate (PC-3) cancer cells, when combined with a breast or prostate cellular health supplement, respectively [14]. A MCP-alginate probiotic dramatically inhibited precancerous lesions [13]. MCP reversed epithelial-mesenchymal transition, reduced cell proliferation, and increased suppression of anti-apoptotic proteins (Bcl-xL and survivin), with the collective action of promoting caspases-mediated apoptosis and inhibiting tumor cell growth [12]. MCP also inhibited tumor growth via the induction of cell cycle arrest and apoptosis in urinary bladder cancer cells in vitro and in vivo [11].
Moreover, MCP reduced prostate cancer cell viability and synergistically enhanced cell sensitivity to ionizing radiation [10]. Inhibition with the MCP of extracellular Gal-3 decreased colon cancer cell migration [9]. Also, the MCP abrogation of Gal-3 attenuated kidney tissue apoptosis and protected against the progression of renal fibrosis in cisplatin-induced nephrotoxicity [8]. Synergy with MCP and PTX to kill human ovarian cancer cell line (SKOV3) multicellular tumor spheroid through abrogation of signal transducer and activator of transcription 3 activity reduced expression of its downstream target HIF-1α, reduced integrin mRNA levels, and subsequently decreased AKT activity [7].
The size and domain structures of the MCP affect its anti-cancer properties. Using a more random heat (autoclaving) modification method to produce MCP, the enrichment of de-esterified homogalacturonan oligomers and the type one arabinogalactans (AG-I) and rhamnogalacturonan (RG-I) depletions in MCP smaller than 3 kDa, or the increase in AGI and decrease in RGI in MCP between 10 and 30 kDa, promoted anti-cancer behaviors by inhibiting migration, aggregation, and proliferation of cancer cells [85]. MCP, with its known specification for a low degree of esterification, low molecular mass, and a high percentage of RG-II domains, lends itself to an effective adjuvant oncological and immune therapy.
Clinical trials with the use of MCP have shown positive results. An open-labeled Phase II pilot study evaluated patients with biopsy-confirmed adenocarcinoma of the prostate, which were untreated at baseline, and had low but gradually rising prostate-specific antigen (PSA) levels (<10 ng/mL). Patients took MCP at a dose of 18 capsules per day (14.4 g) for 12 months. PSA doubling time (PSADT) was extended in 70% of patients [6]. The results indicate a slower cancer progression and possibly even prolongation of life.
An initial pilot trial investigated seven patients who had either relapsed after or failed prior treatment for prostate cancer (PSA range 0.63 to 7.50). The daily dosage of MCP was 15 g. A positive response (more than 30% lengthening of PSADT) was found in 4/7 patients, one patient had a partial response, one patient had stable disease, and one patient did not respond. All patients survived a 3-year follow-up [86].
An open labeled clinical trial examined patients with various solid tumors in an advanced state of progression. Treatment cycles consisted of 15 g of MCP daily for eight weeks. Six patients out of 29 (20.7%) had an overall clinical benefit response (pain, functional performance, weight change), as well as an improvement of quality of life. Eleven out of 49 (22.5%) showed stable disease (SD) after two cycles, and six out of 49 patients (12.3%) had SD for a period longer than 24 weeks. One patient suffering from metastasized prostate carcinoma experienced a halving of serum PSA after 16 weeks of therapy, as well as increased clinical benefit and quality of life, and decreased pain [5].
In an additional study, patients with detected circulating tumor cells received integrative therapy, which included advice on diet and exercise, supplementing with MCP, and supplementing with other products, including curcumin, green tea, garlic extract, vitamin D, medicinal mushroom extract, black cumin seed, artemisinin, and other unnamed supplements. Circulating tumor cell count decreased as a result of this integrative therapy [4].
Finally, there are promising interim results of an open-labeled Phase II study evaluating patients with non-castrate non-metastatic biochemically relapsed prostate cancer, presented at the 2019 American Society of Clinical Oncology (ASCO)-Genitourinary (GU) Cancers Symposium. Thirty-four patients consumed MCP at 4.8 g × 3/day for six months. No patient had treatment-related grade 3/4 toxicity, while six patients had grade 1 side effects (gas and bloating). Of these patients, twenty-one (62%) had stabilization or decrease in PSA, and negative scans, 27 patients (79%) had stabilization or improvement in PSADT and no metastases on scans [87]. The study is continuing with those showing benefit at six months staying on treatment for an additional 12 months.
4. Fibrotic Diseases
4.1. Aortic Stenosis
Many of the steps involved in the pathogenesis of aortic stenosis (AS) are modulated by Gal-3 and, therefore, presumably also by MCP [88]. Galectin-3 transforms quiescent fibroblasts into myofibroblasts that produce and secrete matrix proteins such as collagen [89,90]. Galectin-3 also affects collagen maturation and cross-linking [91,92]. It also stimulates pro-inflammatory mediators [93]. In human cardiac fibroblasts, Gal-3 increases the production and secretion of interleukin (IL)-1β, IL-6, monocyte chemoattractant protein-1, collagen type I and type III, and fibronectin [31]. It also increases the activity of metalloproteinases-1, -2, and -9 [31]. Treatment with MCP abrogates these effects. In the vasculature, Gal-3 promotes arterial stiffness by enhancing the production and secretion of pro-fibrotic and pro-inflammatory markers in vascular smooth muscle cells [33]. Again, MCP reverses this effect. In endothelial cells, Gal-3 enhances the expression of inflammatory factors, chemokines, and adhesion molecules [94]. Galectin-3 also affects the cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells, which contributes to plasma membrane retention and promotes angiogenesis [95]. In interstitial valve cells from the aorta, Gal-3 enhances the secretion of inflammatory and fibrotic mediators and increases the expression of calcification mediators [29]. The administration of MCP prevents these effects. MCP also prevented an increase in cardiac Gal-3, and normalized histological and molecular alterations in short-term AS [27].
4.2. Additional Cardiovascular Effects
Galectin-3 inhibition with MCP prevented cardiac inflammation and fibrosis associated with an excess of aldosterone levels independently of blood pressure levels [96]. Galectin-3 antagonism with MCP and aldosterone opposition reversed isoproterenol-induced left ventricular systolic dysfunction, thereby preventing the development of myocardial fibrosis in this mice model with selective cardiac hyperaldosteronism [30]. Blockade by MCP in a pressure overload-induced model, which increases Gal-3, showed amelioration of media thickness, fibrosis, and inflammation in aortic valve calcification [28]. It also reduced the size of atherosclerotic lesion areas by inhibiting the adhesion of leukocytes to endothelial cells [26].
Furthermore, MCP inhibited Gal-3 and decreased experimental abdominal aortic aneurysm development [25]. MCP reduced cardiac lipotoxicity and ameliorated cardiac mitochondrial damage in an obesity model [24]. Also, MCP restored the levels of cardiac peroxiredoxin-4 as well as prohibitin-2 levels and improved oxidative status [23]. Neurological impairments were prevented with MCP on post-aneurysmal subarachnoid hemorrhage, suggesting pleiotropic neuroprotective action, such as anti-neuroinflammatory and anti-apoptotic effects, beyond protecting the blood-brain barrier through inhibiting Gal-3 [22]. Inhibition mediated by the MCP of Gal-3 in mice prevented the pro-fibrotic and pro-inflammatory effects of cardiotrophin-1 [21]. Furthermore, MCP and perindopril comparably improved ischemic heart failure by downregulating Gal-3 and reducing myocardial fibrosis [20]. The blockade of Gal-3 with MCP prevented cardiac fibrosis, inflammation, and functional alterations [19].
4.3. Kidney
In a model of experimental acute kidney injury, all folic acid-treated mice lost weight while their kidneys enlarged secondary to the renal insult; MCP significantly lessened these gross changes, but this was not associated with changes in Gal-3 expression [36]. Via its blocking action on Gal-3, MCP protected against aldosterone-induced cardiac and renal fibrosis and dysfunction [32]. The inhibition of Gal-3 normalized renal Gal-3 levels as well as functional, histological, and molecular alterations in an obese and AS model, preventing renal fibrosis, inflammation, and damage with MCP treatment [35]. Moreover, MCP attenuated early renal impairment in spontaneously hypertensive rats as indicated by reduced albuminuria, improved renal function, and decreased renal fibrosis, epithelial-mesenchymal transition, and inflammation [34].
4.4. Additional Fibrotic Diseases
In a model of diet-induced obesity, MCP prevented adipose tissue fibrosis, inflammation, and the increase in adipocyte differentiation markers despite not affecting body weight, adipose tissue weights, or adiposity [37]. Liver fibrosis was alleviated with MCP and aided in hepatic regeneration, which may be mediated by an antioxidant effect [38].
5. Detoxification
To date, MCP has been used in four clinical studies of detoxification. Treatment with MCP increased urinary excretion of lead, arsenic, and cadmium in healthy volunteers, without side effects or depletion of essential elements [42]. In a case study of five patients, there was an average of 74% reduction in lead or mercury without side effects with the use of MCP alone or with a MCP/alginates combination [41]. Treatment with MCP dramatically decreased the levels of lead in blood and increased the levels of lead in urine in children hospitalized with lead toxicity [40]. Fecal uranium excretion was promoted by MCP/alginate supplement without side effects in a family with low-level chronic exposure from their environment and diet [39].
6. Immune Function
There are many reports of various effects on immunity with MCP. There was significant activation of T-cytotoxic and natural killer (NK) cells in blood cultures by MCP, and the NK-cells demonstrated functionality against K562 leukemic cells in culture. The presence of a low degree of methyl esterification and flexible low-molecular-weight pectin polymer enriched in saturated and unsaturated oligogalacturonic acids appear to be the immunostimulatory carbohydrates in MCP [48]. An additive effect of MCP in combination with cefotaxime against all six methicillin-resistant Staphylococcus aureus (MRSA) strains has been shown [47]. Honokiol, a purified extract from magnolia bark used in traditional Asian medicine and MCP, has been shown to have synergistic antioxidant activity and anti-inflammatory effects [46]. There was an inhibition of toxin-producing Escherichia coli adhesion and reduced Shiga toxin cytotoxicity with MCP [45]. Furthermore, MCP co-administration with live probiotic L. acidophilus ATCC 4356 supplement helped maintain or improve the integrity and population of the intestinal microbiota [44]. Finally, MCP has an immunomodulatory effect on the levels of cytokine secretion in the spleen of mice, which may be regulated by IL-4 [43].
7. Other Galectin-3 Inhibitors
There are other laboratory MCPs prepared by just heat and pH treatment. Heat by autoclaving MCP induced cell death in HepG2 and A549 cells. The induced cell death was different from classical apoptosis because there was no DNA cleavage [97]. Also, the delivery of autoclaved MCP reduced plaque volume in apolipoprotein E-deficient mice [98]. Renal cell carcinoma cells cotreated with autoclaved pectin and arsenic trioxide demonstrated increased apoptosis [99]. Synergistic treatment with S-trans, transfarnesylthiosalicylic acid and pH modified citrus pectin inhibited anaplastic thyroid cells proliferation in vitro by inducing cell cycle arrest and increased apoptosis rate [100]. pH modified citrus pectin also reduced the growth of solid tumors in balb-c mice [57].
Several Gal-3 inhibitors are also in pharmaceutical development. An injectable MCP in pharmaceutical development now abandoned called GCS-100 induced apoptosis in acute myeloid leukemia cells [101]. It also removed cell-surface Gal-3 from CD45, thus rendering diffuse large B-cell lymphoma cells susceptible to chemotherapeutic agents [102]. GCS-100 also detached Gal-3 from tumor-infiltrating lymphocytes and improved the cytotoxicity and secretion of different cytokines [103]. Furthermore, GCS-100 induced the inhibition of proliferation, the accumulation of cells in sub-G1 and G1 phases, and apoptosis with the activation of both the caspase-8 and -9 pathways [104]. GCS-100 enhanced calpain activation, which reduced the proapoptotic effect of Gal-3 [105].
Another Gal-3 inhibitor in pharmaceutical development is an inhalable formulation called TD139, a thiodigalactoside derivative. This inhibitor abrogated the susceptibility to natural killer T-cell-dependent hepatitis [106]. Pretreatment of wild-type C57BL/6 mice with TD139 lowered liver injury and led to milder infiltration of interferon-gamma and interleukin (IL)-17 and -4-producing cluster of differentiation (CD)4(+) T cells, and an increase in the total number of IL-10-producing CD4(+) T cells and F4/80(+) CD206(+) activating macrophages, and prevented the apoptosis of liver-infiltrating mononuclear cells [107]. TD139 blocked transforming growth factor-β-induced β-catenin activation in vitro and in vivo and lowered the late-stage progression of lung fibrosis after treatment with bleomycin [108].
Other pectin carbohydrate-based galectin inhibitors in drug development, injectables GR-MD-02 (galactoarabino-rhamnogalacturonan) and GM-CT-01 (galactomannan) resulted in a lowering of fibrosis with the reduction in the portal and septal Gal-3 positive macrophages and reductions in portal pressure [109]. The treatment resulted in sharp improvement in liver histology, with a significant decrease in non-alcoholic steatohepatitis (NASH) activity and collagen deposition; GM-CT-01 had an intermediate effect between the vehicle and GR-MD-02 [110].
8. Possible New Areas for MCP Research
Galectin-3 plays several prominent roles where there is little to no MCP research to date. One such role is bone pathophysiology [111]. Examples include acting as a cell marker and pro-survival factor in chondrocytes [112], a Runx2 target gene in osteoblasts [113], a cell marker in osteocytes [112], a biomarker for the pro-osteogenic capacity of mesenchymal stem cells [114], and a mediator of cell matrix adhesion in osteoclasts [115].
Galectin-3 has a mixed role in intestinal inflammation. Galectin-3 expression in the small bowel epithelial cells from Crohn’s disease patients is imbalanced and often significantly reduced [116]. Soluble Gal-3 acts as an activator of lamina propria fibroblasts [117]. It is also a substrate for matrix metalloproteinase-7, which may lead to delayed wound healing in chronic intestinal diseases [118]. Acute dextran sodium sulfate-induced colitis was ameliorated by Gal-3 in a mouse model [119]. Similarly, Gal-3 inhibited colonic mucosa inflammation by inducing regulatory T cells [120]. On the other hand, Gal-3 promotes the activation of NOD-like receptor family, pyrin domain containing 3 inflammasome and the production of IL-1β in macrophages [121].
Although the research on the relationship between Gal-3 and diabetes mellitus is also mixed, the overall suggest is that it has a pro-diabetic role. Key findings include the following: the delivery of Gal-3 to mice causes insulin resistance and glucose intolerance, whereas the inhibition of Gal-3, through either genetic or pharmacologic loss of function, improves insulin sensitivity in obese mice. Also, in vitro treatment with Gal-3 enhances macrophage chemotaxis, reduces insulin-stimulated glucose uptake in myocytes and 3T3-L1 adipocytes, and impairs the insulin-mediated suppression of glucose output in primary mouse hepatocytes [122]. In addition, Gal-3 directly activates peroxisome proliferator-activated receptor-γ and leads to adipocyte differentiation in vitro and in vivo [123]. Finally, circulating Gal-3 is positively associated with diabetes prevalence and incidence [124].
9. Conclusions
Clinical studies and preclinical research on the use of MCP have noted wide-ranging benefits. Much of the interest of MCP relates to its antagonism of Gal-3. As the Gal-3 research continues to identify novel mechanisms of disease progression, undoubtedly new benefits will be discovered for MCP. The advantageous effects of MCP are not limited to Gal-3 antagonism; other pleiotropic effects have been researched. Large-scale clinical trials are justified for examining the impact of MCP on robust clinical endpoints.
Acknowledgments
The authors would like to thank John Trepanowski and Barry Wilk for their help in preparing the manuscript.
Author Contributions
A.R. and I.E. contributed to the writing and critical review of the manuscript; all approved the final version of the manuscript.
Funding
There is no funding for this manuscript.
Conflicts of Interest
I.E. is the owner of a dietary supplement company.
References
- 1.Leclere L., Van Cutsem P., Michiels C. Anti-cancer activities of pH-or heat-modified pectin. Front. Pharm. 2013;4:128. doi: 10.3389/fphar.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niture S.K., Refai L. Plant pectin: A potential source for cancer suppression. Am. J. Pharm. Toxicol. 2013;8:9–19. doi: 10.3844/ajptsp.2013.9.19. [DOI] [Google Scholar]
- 3.Courts F.L. Profiling of modified citrus pectin oligosaccharide transport across Caco-2 cell monolayers. PharmaNutrition. 2013;1:22–31. doi: 10.1016/j.phanu.2012.12.001. [DOI] [Google Scholar]
- 4.Ried K., Eng P., Sali A. Screening for circulating tumour cells allows early detection of cancer and monitoring of treatment effectiveness: An observational study. Asian Pac. J. Cancer Prev. 2017;18:2275–2285. doi: 10.4172/2472-0429.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azémar M., Hildenbrand B., Haering B., Heim M.E., Unger C. Clinical benefit in patients with advanced solid tumors treated with modified citrus pectin: A prospective pilot study. Clin. Med. Oncol. 2007;1:73–80. doi: 10.4137/CMO.S285. [DOI] [Google Scholar]
- 6.Guess B., Scholz M., Strum S., Lam R., Johnson H., Jennrich R. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: A phase II pilot study. Prostate Cancer Prostatic Dis. 2003;6:301–304. doi: 10.1038/sj.pcan.4500679. [DOI] [PubMed] [Google Scholar]
- 7.Hossein G., Halvaei S., Heidarian Y., Dehghani-Ghobadi Z., Hassani M., Hosseini H., Naderi N., Sheikh Hassani S. Pectasol-C Modified Citrus Pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019;8:4315–4329. doi: 10.1002/cam4.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H.-Y., Yang S., Li J.-C., Feng J.-X. Galectin 3 inhibition attenuates renal injury progression in cisplatin-induced nephrotoxicity. Biosci. Rep. 2018;38:BSR20181803. doi: 10.1042/BSR20181803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu K.-L., Kuo C.-M., Huang E.-Y., Pan H.-M., Huang C.-C., Chen Y.-F., Hsiao C.-C., Yang K.D. Extracellular galectin-3 facilitates colon cancer cell migration and is related to the epidermal growth factor receptor. Am. J. Transl. Res. 2018;10:2402–2412. [PMC free article] [PubMed] [Google Scholar]
- 10.Conti S., Vexler A., Hagoel L., Kalich-Philosoph L., Corn B.W., Honig N., Shtraus N., Meir Y., Ron I., Eliaz I. Modified citrus pectin as a potential sensitizer for radiotherapy in prostate cancer. Integr. Cancer. 2018;17:1225–1234. doi: 10.1177/1534735418790382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang T., Liu D.-D., Ning H.-M., Liu D., Sun J.-Y., Huang X.-J., Dong Y., Geng M.-Y., Yun S.-F., Yan J. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharm. Sin. 2018;39:1885–1893. doi: 10.1038/s41401-018-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S., Li P., Lu S.-M., Ling Z.-Q. Chemoprevention of low-molecular-weight citrus pectin (LCP) in gastrointestinal cancer cells. Int. J. Biol. Sci. 2016;12:746–756. doi: 10.7150/ijbs.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odun-Ayo F., Mellem J., Naicker T., Reddy L. Chemoprevention of azoxymethane-induced colonic carcinogenesis in Balb/c mice using a modified pectin alginate probiotic. Anticancer Res. 2015;35:4765–4775. [PubMed] [Google Scholar]
- 14.Jiang J., Eliaz I., Sliva D. Synergistic and additive effects of modified citrus pectin with two polybotanical compounds, in the suppression of invasive behavior of human breast and prostate cancer cells. Integr. Cancer. 2013;12:145–152. doi: 10.1177/1534735412442369. [DOI] [PubMed] [Google Scholar]
- 15.Hossein G., Keshavarz M., Ahmadi S., Naderi N. Synergistic effects of PectaSol-C modified citrus pectin an inhibitor of Galectin-3 and paclitaxel on apoptosis of human SKOV-3 ovarian cancer cells. Asian Pac. J. Cancer Prev. 2013;14:7561–7568. doi: 10.7314/APJCP.2013.14.12.7561. [DOI] [PubMed] [Google Scholar]
- 16.Tehranian N., Sepehri H., Mehdipour P., Biramijamal F., Hossein-Nezhad A., Sarrafnejad A., Hajizadeh E. Combination effect of PectaSol and Doxorubicin on viability, cell cycle arrest and apoptosis in DU-145 and LNCaP prostate cancer cell lines. Cell Biol. Int. 2012;36:601–610. doi: 10.1042/CBI20110309. [DOI] [PubMed] [Google Scholar]
- 17.Yan J., Katz A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and-independent prostate cancer cells. Integr. Cancer. 2010;9:197–203. doi: 10.1177/1534735410369672. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y., Chen L., He X., Fan L., Yang G., Chen X., Lin X., Du L., Li Z., Ye H. Enhancement of therapeutic effectiveness by combining liposomal honokiol with cisplatin in ovarian carcinoma. Int. J. Gynecol. Cancer. 2008;18:652–659. doi: 10.1111/j.1525-1438.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 19.Ibarrola J., Matilla L., Martínez-Martínez E., Gueret A., Fernández-Celis A., Henry J.-P., Nicol L., Jaisser F., Mulder P., Ouvrard-Pascaud A. Myocardial Injury After Ischemia/Reperfusion Is Attenuated By Pharmacological Galectin-3 Inhibition. Sci. Rep. 2019;9:9607. doi: 10.1038/s41598-019-46119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S., Li S., Hao X., Zhang Y., Deng W. Perindopril and a galectin-3 inhibitor improve ischemic heart failure in rabbits by reducing Gal-3 expression and myocardial fibrosis. Front. Physiol. 2019;10:267. doi: 10.3389/fphys.2019.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Martínez E., Brugnolaro C., Ibarrola J., Ravassa S., Buonafine M., López B., Fernández-Celis A., Querejeta R., Santamaria E., Fernández-Irigoyen J. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation: Mechanistic Insights and Clinical Implications. Hypertension. 2019;73:602–611. doi: 10.1161/HYPERTENSIONAHA.118.11874. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa H., Liu L., Nakano F., Kawakita F., Kanamaru H., Nakatsuka Y., Okada T., Suzuki H. Modified citrus pectin prevents blood-brain barrier disruption in mouse subarachnoid hemorrhage by inhibiting galectin-3. Stroke. 2018;49:2743–2751. doi: 10.1161/STROKEAHA.118.021757. [DOI] [PubMed] [Google Scholar]
- 23.Ibarrola J., Arrieta V., Sádaba R., Martinez-Martinez E., Garcia-Peña A., Alvarez V., Fernández-Celis A., Gainza A., Santamaría E., Fernández-Irigoyen J. Galectin-3 down-regulates antioxidant peroxiredoxin-4 in human cardiac fibroblasts: A new pathway to induce cardiac damage. Clin. Sci. 2018;132:1471–1485. doi: 10.1042/CS20171389. [DOI] [PubMed] [Google Scholar]
- 24.Marín-Royo G., Gallardo I., Martínez-Martínez E., Gutiérrez B., Jurado-López R., López-Andrés N., Gutiérrez-Tenorio J., Rial E., Bartolomé M.a.V., Nieto M.L. Inhibition of galectin-3 ameliorates the consequences of cardiac lipotoxicity in a rat model of diet-induced obesity. Dis. Model. Mech. 2018;11:dmm032086. doi: 10.1242/dmm.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-García C.-E., Tarin C., Roldan-Montero R., Martinez-Lopez D., Torres-Fonseca M., Lindhot J.S., de Ceniga M.V., Egido J., Lopez-Andres N., Blanco-Colio L.-M. Increased galectin-3 levels are associated with abdominal aortic aneurysm progression and inhibition of galectin-3 decreases elastase-induced AAA development. Clin. Sci. 2017;131:2707–2719. doi: 10.1042/CS20171142. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y., Zhang M., Zhao P., Jia M., Liu B., Jia Q., Guo J., Dou L., Li J. Modified citrus pectin inhibits galectin-3 function to reduce atherosclerotic lesions in apoE-deficient mice. Mol. Med. Rep. 2017;16:647–653. doi: 10.3892/mmr.2017.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arrieta V., Martinez-Martinez E., Ibarrola J., Alvarez V., Sádaba R., Garcia-Peña A., Fernández-Celis A., Cachofeiro V., Rossignol P., López-Andrés N. A role for galectin-3 in the development of early molecular alterations in short-term aortic stenosis. Clin. Sci. 2017;131:935–949. doi: 10.1042/CS20170145. [DOI] [PubMed] [Google Scholar]
- 28.Ibarrola J., Martínez-Martínez E., Sádaba J., Arrieta V., García-Peña A., Álvarez V., Fernández-Celis A., Gainza A., Rossignol P., Cachofeiro Ramos V. Beneficial effects of galectin-3 blockade in vascular and aortic valve alterations in an experimental pressure overload model. Int. J. Mol. Sci. 2017;18:1664. doi: 10.3390/ijms18081664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sádaba J.R., Martínez-Martínez E., Arrieta V., Álvarez V., Fernández-Celis A., Ibarrola J., Melero A., Rossignol P., Cachofeiro V., López-Andrés N. Role for galectin-3 in calcific aortic valve stenosis. J. Am. Heart Assoc. 2016;5:e004360. doi: 10.1161/JAHA.116.004360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vergaro G., Prud’homme M., Fazal L., Merval R., Passino C., Emdin M., Samuel J.-L., Cohen Solal A., Delcayre C. Inhibition of galectin-3 pathway prevents isoproterenol-induced left ventricular dysfunction and fibrosis in mice. Hypertension. 2016;67:606–612. doi: 10.1161/HYPERTENSIONAHA.115.06161. [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Martínez E., López-Ándres N., Jurado-López R., Rousseau E., Bartolomé M.V., Fernández-Celis A., Rossignol P., Islas F., Antequera A., Prieto S. Galectin-3 participates in cardiovascular remodeling associated with obesity. Hypertension. 2015;66:961–969. doi: 10.1161/HYPERTENSIONAHA.115.06032. [DOI] [PubMed] [Google Scholar]
- 32.Calvier L., Martinez-Martinez E., Miana M., Cachofeiro V., Rousseau E., Sádaba J.R., Zannad F., Rossignol P., López-Andrés N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015;3:59–67. doi: 10.1016/j.jchf.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Calvier L., Miana M., Reboul P., Cachofeiro V., Martinez-Martinez E., de Boer R.A., Poirier F., Lacolley P., Zannad F., Rossignol P. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arter. Thromb. Vasc. Biol. 2013;33:67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Martínez E., Ibarrola J., Fernández-Celis A., Calvier L., Leroy C., Cachofeiro V., Rossignol P., López-Andrés N. Galectin-3 pharmacological inhibition attenuates early renal damage in spontaneously hypertensive rats. J. Hypertens. 2018;36:368–376. doi: 10.1097/HJH.0000000000001545. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Martinez E., Ibarrola J., Calvier L., Fernandez-Celis A., Leroy C., Cachofeiro V., Rossignol P., Lopez-Andres N. Galectin-3 blockade reduces renal fibrosis in two normotensive experimental models of renal damage. PLoS ONE. 2016;11:e0166272. doi: 10.1371/journal.pone.0166272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolatsi-Joannou M., Price K.L., Winyard P.J., Long D.A. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS ONE. 2011;6:e18683. doi: 10.1371/journal.pone.0018683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Martinez E., Calvier L., Rossignol P., Rousseau E., Fernandez-Celis A., Jurado-Lopez R., Laville M., Cachofeiro V., Lopez-Andres N. Galectin-3 inhibition prevents adipose tissue remodelling in obesity. Int. J. Obes. (Lond.) 2016;40:1034–1038. doi: 10.1038/ijo.2016.19. [DOI] [PubMed] [Google Scholar]
- 38.Abu-Elsaad N.M., Elkashef W.F. Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Can. J. Physiol. Pharm. 2016;94:554–562. doi: 10.1139/cjpp-2015-0284. [DOI] [PubMed] [Google Scholar]
- 39.Eliaz I., Weil E., Schwarzbach J., Wilk B. Modified Citrus Pectin/Alginate Dietary Supplement Increased Fecal Excretion of Uranium: A Family. Altern. Health Med. 2019;25:20–24. [PubMed] [Google Scholar]
- 40.Zhao Z.Y., Liang L., Fan X., Yu Z., Hotchkiss A.T., Wilk B.J., Eliaz I. The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Altern. Health Med. 2008;14:34–38. [PubMed] [Google Scholar]
- 41.Eliaz I., Weil E., Wilk B. Integrative medicine and the role of modified citrus pectin/alginates in heavy metal chelation and detoxification-five case reports. Komplementmed. 2007;14:358–364. doi: 10.1159/000109829. [DOI] [PubMed] [Google Scholar]
- 42.Eliaz I., Hotchkiss A.T., Fishman M.L., Rode D. The effect of modified citrus pectin on urinary excretion of toxic elements. Phytother. Res. 2006;20:859–864. doi: 10.1002/ptr.1953. [DOI] [PubMed] [Google Scholar]
- 43.Merheb R., Abdel-Massih R.M., Karam M.C. Immunomodulatory effect of natural and modified Citrus pectin on cytokine levels in the spleen of BALB/c mice. Int. J. Biol. Macromol. 2019;121:1–5. doi: 10.1016/j.ijbiomac.2018.09.189. [DOI] [PubMed] [Google Scholar]
- 44.Odun-Ayo F., Mellem J., Reddy L. The effect of modified citrus pectin-probiotic on faecal lactobacilli in Balb/c mice. Food Sci. Technol. 2017;37:478–482. doi: 10.1590/1678-457x.22116. [DOI] [Google Scholar]
- 45.Di R., Vakkalanka M.S., Onumpai C., Chau H.K., White A., Rastall R.A., Yam K., Hotchkiss A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157: H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017;227:245–254. doi: 10.1016/j.foodchem.2017.01.100. [DOI] [PubMed] [Google Scholar]
- 46.Ramachandran C., Wilk B., Melnick S.J., Eliaz I. Synergistic antioxidant and anti-inflammatory effects between modified citrus pectin and honokiol. Evid. Based Complement. Altern. Med. 2017;2017:8379843. doi: 10.1155/2017/8379843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahdouh E., El-Khatib S., Baydoun E., Abdel-Massih R.M. Additive Effect of MCP in Combination with Cefotaxime Against Staphylococcus aureus. Med. Chem. 2017;13:682–688. doi: 10.2174/1573406413666170306112444. [DOI] [PubMed] [Google Scholar]
- 48.Ramachandran C., Wilk B.J., Hotchkiss A., Chau H., Eliaz I., Melnick S.J. Activation of human T-helper/inducer cell, T-cytotoxic cell, B-cell, and natural killer (NK)-cells and induction of natural killer cell activity against K562 chronic myeloid leukemia cells with modified citrus pectin. BMC Complement. Altern. Med. 2011;11:59. doi: 10.1186/1472-6882-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumic J., Dabelic S., Flögel M. Galectin-3: An open-ended story. Biochim. Biophys. Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 50.Harazono Y., Nakajima K., Raz A. Why anti-Bcl-2 clinical trials fail: A solution. Cancer Metastasis Rev. 2014;33:285–294. doi: 10.1007/s10555-013-9450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clementy N., Piver E., Bisson A., Andre C., Bernard A., Pierre B., Fauchier L., Babuty D. Galectin-3 in atrial fibrillation: Mechanisms and therapeutic implications. Int. J. Mol. Sci. 2018;19:976. doi: 10.3390/ijms19040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong R., Zhang M., Hu Q., Zheng S., Soh A., Zheng Y., Yuan H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy. Int. J. Mol. Med. 2018;41:599–614. doi: 10.3892/ijmm.2017.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glinsky V.V., Raz A. Modified citrus pectin anti-metastatic properties: One bullet, multiple targets. Carbohydr. Res. 2009;344:1788–1791. doi: 10.1016/j.carres.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fortuna-Costa A., Gomes A.M., Kozlowski E.O., Stelling M.P., Pavão M.S. Extracellular galectin-3 in tumor progression and metastasis. Front. Oncol. 2014;4:138. doi: 10.3389/fonc.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao X., Zhi Y., Zhang T., Xue H., Wang X., Foday A.D., Tai G., Zhou Y. Analysis of the neutral polysaccharide fraction of MCP and its inhibitory activity on galectin-3. Glycoconj. J. 2012;29:159–165. doi: 10.1007/s10719-012-9382-5. [DOI] [PubMed] [Google Scholar]
- 56.Kim H.-R.C., Lin H.-M., Biliran H., Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999;59:4148–4154. [PubMed] [Google Scholar]
- 57.Hayashi A., Gillen A.C., Lott J.R. Effects of daily oral administration of quercetin chalcone and modified citrus pectin on implanted colon-25 tumor growth in Balb-c mice. Altern. Med. Rev. 2000;5:546–552. [PubMed] [Google Scholar]
- 58.Hsieh T., Wu J.M. Changes in cell growth, cyclin/kinase, endogenous phosphoproteins and nm23 gene expression in human prostatic JCA-1 cells treated with modified citrus pectin. Biochem. Mol. Biol. Int. 1995;37:833–841. [PubMed] [Google Scholar]
- 59.Lehr J.E., Pienta K.J. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J. Natl. Cancer Inst. 1998;90:118–123. doi: 10.1093/jnci/90.2.118. [DOI] [PubMed] [Google Scholar]
- 60.Glinsky V.V., Glinsky G.V., Rittenhouse-Olson K., Huflejt M.E., Glinskii O.V., Deutscher S.L., Quinn T.P. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–4857. [PubMed] [Google Scholar]
- 61.Khaldoyanidi S.K., Glinsky V.V., Sikora L., Glinskii A.B., Mossine V.V., Quinn T.P., Glinsky G.V., Sriramarao P. MDA-MB-435 human breast carcinoma cell homo-and heterotypic adhesion under flow conditions is mediated in part by Thomsen-Friedenreich antigen-galectin-3 interactions. J. Biol. Chem. 2003;278:4127–4134. doi: 10.1074/jbc.M209590200. [DOI] [PubMed] [Google Scholar]
- 62.Glinsky V.V., Glinsky G.V., Glinskii O.V., Huxley V.H., Turk J.R., Mossine V.V., Deutscher S.L., Pienta K.J., Quinn T.P. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. doi: 10.1016/j.urolonc.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Glinskii O.V., Turk J.R., Pienta K.J., Huxley V.H., Glinsky V.V. Evidence of porcine and human endothelium activation by cancer-associated carbohydrates expressed on glycoproteins and tumour cells. J. Physiol. 2004;554:89–99. doi: 10.1113/jphysiol.2003.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Platt D., Raz A. Modulation of the lung colonization of B16-F1 melanoma cells by citrus pectin. J. Natl. Cancer Inst. 1992;84:438–442. doi: 10.1093/jnci/84.6.438. [DOI] [PubMed] [Google Scholar]
- 65.Pienta K.J., Nailk H., Akhtar A., Yamazaki K., Replogle T.S., Lehr J., Donat T.L., Tait L., Hogan V., Raz A. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J. Natl. Cancer Inst. 1995;87:348–353. doi: 10.1093/jnci/87.5.348. [DOI] [PubMed] [Google Scholar]
- 66.Inohara H., Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj. J. 1994;11:527–532. doi: 10.1007/BF00731303. [DOI] [PubMed] [Google Scholar]
- 67.Nangia-Makker P., Hogan V., Honjo Y., Baccarini S., Tait L., Bresalier R., Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 68.Glinskii O.V., Huxley V.H., Glinsky G.V., Pienta K.J., Raz A., Glinsky V.V. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia. 2005;7:522–527. doi: 10.1593/neo.04646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Mehdi A., Tozawa K., Fisher A., Shientag L., Lee A., Muschel R. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: A new model for metastasis. Nat. Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 70.Sathisha U., Jayaram S., Nayaka M.H., Dharmesh S.M. Inhibition of galectin-3 mediated cellular interactions by pectic polysaccharides from dietary sources. Glycoconj. J. 2007;24:497–507. doi: 10.1007/s10719-007-9042-3. [DOI] [PubMed] [Google Scholar]
- 71.Chambers A., Groom A., MacDonald I. Dissemination and Growth of Cancer Cells in Metastatic Sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 72.Nakahara S., Oka N., Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267–275. doi: 10.1007/s10495-005-0801-y. [DOI] [PubMed] [Google Scholar]
- 73.Yang R.-Y., Liu F.-T. Galectins in cell growth and apoptosis. Cell Mol. Life Sci. 2003;60:267–276. doi: 10.1007/s000180300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nangia-Makker P., Nakahara S., Hogan V., Raz A. Galectin-3 in apoptosis, a novel therapeutic target. J. Bioenerg. Biomembr. 2007;39:79–84. doi: 10.1007/s10863-006-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson K.D., Glinskii O.V., Mossine V.V., Turk J.R., Mawhinney T.P., Anthony D.C., Henry C.J., Huxley V.H., Glinsky G.V., Pienta K.J. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia. 2007;9:662–670. doi: 10.1593/neo.07433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nangia-Makker P., Honjo Y., Sarvis R., Akahani S., Hogan V., Pienta K.J., Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matarrese P., Tinari N., Semeraro M.L., Natoli C., Iacobelli S., Malorni W. Galectin-3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett. 2000;473:311–315. doi: 10.1016/S0014-5793(00)01547-7. [DOI] [PubMed] [Google Scholar]
- 78.Akahani S., Nangia-Makker P., Inohara H., Kim H.-R.C., Raz A. Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–5276. [PubMed] [Google Scholar]
- 79.Yu F., Finley R.L., Raz A., Kim H.-R.C. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria A role for synexin in galectin-3 translocation. J. Biol. Chem. 2002;277:15819–15827. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 80.Oka N., Nakahara S., Takenaka Y., Fukumori T., Hogan V., Kanayama H.-O., Yanagawa T., Raz A. Galectin-3 inhibits tumor necrosis factor–related apoptosis-inducing ligand–induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Res. 2005;65:7546–7553. doi: 10.1158/0008-5472.CAN-05-1197. [DOI] [PubMed] [Google Scholar]
- 81.Fukumori T., Oka N., Takenaka Y., Nangia-Makker P., Elsamman E., Kasai T., Shono M., Kanayama H.-O., Ellerhorst J., Lotan R. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res. 2006;66:3114–3119. doi: 10.1158/0008-5472.CAN-05-3750. [DOI] [PubMed] [Google Scholar]
- 82.Oishi T., Itamochi H., Kigawa J., Kanamori Y., Shimada M., Takahashi M., Shimogai R., Kawaguchi W., Sato S., Terakawa N. Galectin-3 may contribute to Cisplatin resistance in clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer. 2007;17:1040–1046. doi: 10.1111/j.1525-1438.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 83.Chauhan D., Li G., Podar K., Hideshima T., Neri P., He D., Mitsiades N., Richardson P., Chang Y., Schindler J. A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Res. 2005;65:8350–8358. doi: 10.1158/0008-5472.CAN-05-0163. [DOI] [PubMed] [Google Scholar]
- 84.Liu H.-Y., Huang Z.-L., Yang G.-H., Lu W.-Q., Yu N.-R. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J. Gastroenterol. 2008;14:7386–7391. doi: 10.3748/wjg.14.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Do Prado S.B.R., Shiga T.M., Harazono Y., Hogan V.A., Raz A., Carpita N.C., Fabi J.P. Migration and proliferation of cancer cells in culture are differentially affected by molecular size of modified citrus pectin. Carbohydr. Polym. 2019;211:141–151. doi: 10.1016/j.carbpol.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strum S., Scholz M., McDermed J., McCulloch M., Eliaz I. Modified citrus pectin slows PSA doubling time: A pilot clinical trial; Proceedings of the International Conference on Diet and Prevention of Cancer; Tampere, Finland. 28 May–1 June 1999. [Google Scholar]
- 87.Keizman D., Frenkel M.A., Peer A., Rosenbaum E., Margel D., Sarid D.L., Neiman V., Gottfried M., Maimon N., Leibovitch I. Effect of pectasol-c modified citrus pectin (P-MCP) treatment (tx) on PSA dynamics in non-metastatic biochemically relapsed prostate cancer (BRPC) patients (pts): Results of a prospective phase II study. J. Clin. Oncol. 2019 doi: 10.1200/JCO.2019.37.15_suppl.e16609. [DOI] [Google Scholar]
- 88.Arrieta V., Sádaba J., Álvarez V., Rodríguez J., López-Andrés N. Galectin-3 as a novel biotarget in cardiovascular alterations associated to development of severe aortic stenosis. Sist. Sanit. Navar. 2019:72347. doi: 10.23938/ASSN.0643. [DOI] [PubMed] [Google Scholar]
- 89.Sharma U.C., Pokharel S., van Brakel T.J., van Berlo J.H., Cleutjens J.P., Schroen B., André S., Crijns H.J., Gabius H.-J., Maessen J. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 90.Yu L., Ruifrok W.P., Meissner M., Bos E.M., van Goor H., Sanjabi B., van der Harst P., Pitt B., Goldstein I.J., Koerts J.A. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ. Heart Fail. 2013;6:107–117. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 91.Ho M.-K., Springer T. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J. Immunol. 1982;128:1221–1228. [PubMed] [Google Scholar]
- 92.De Boer R.A., Van Der Velde A.R., Mueller C., Van Veldhuisen D.J., Anker S.D., Peacock W.F., Adams K.F., Maisel A. Galectin-3: A modifiable risk factor in heart failure. Cardiovasc. Drugs. 2014;28:237–246. doi: 10.1007/s10557-014-6520-2. [DOI] [PubMed] [Google Scholar]
- 93.Papaspyridonos M., McNeill E., de Bono J.P., Smith A., Burnand K.G., Channon K.M., Greaves D.R. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler. Thromb. Vasc. Biol. 2008;28:433–440. doi: 10.1161/ATVBAHA.107.159160. [DOI] [PubMed] [Google Scholar]
- 94.Chen X., Zhang R., Zhang Q., Xu Z., Xu F., Li D., Li Y. Microtia patients: Auricular chondrocyte ECM is promoted by CGF through IGF-1 activation of the IGF-1R/PI3K/AKT pathway. J. Cell. Physiol. 2019;234:21817–21824. doi: 10.1002/jcp.27316. [DOI] [PubMed] [Google Scholar]
- 95.Markowska A.I., Jefferies K.C., Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J. Biol. Chem. 2011;286:29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martínez-Martínez E., Calvier L., Fernández-Celis A., Rousseau E., Jurado-López R., Rossoni L.V., Jaisser F., Zannad F., Rossignol P., Cachofeiro V. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension. 2015;66:767–775. doi: 10.1161/HYPERTENSIONAHA.115.05876. [DOI] [PubMed] [Google Scholar]
- 97.Leclere L., Fransolet M., Cote F., Cambier P., Arnould T., Van Cutsem P., Michiels C. Heat-modified citrus pectin induces apoptosis-like cell death and autophagy in HepG2 and A549 cancer cells. PLoS ONE. 2015;10:e0115831. doi: 10.1371/journal.pone.0115831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.MacKinnon A.C., Liu X., Hadoke P.W., Miller M.R., Newby D.E., Sethi T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology. 2013;23:654–663. doi: 10.1093/glycob/cwt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu Y., Gu X., Gong M., Guo G., Han K., An R. Galectin-3 inhibition sensitizes human renal cell carcinoma cells to arsenic trioxide treatment. Cancer Biol. 2013;14:897–906. doi: 10.4161/cbt.25937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Menachem A., Bodner O., Pastor J., Raz A., Kloog Y. Inhibition of malignant thyroid carcinoma cell proliferation by Ras and galectin-3 inhibitors. Cell Death Discov. 2015;1:15047. doi: 10.1038/cddiscovery.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruvolo P.P., Ruvolo V.R., Benton C.B., AlRawi A., Burks J.K., Schober W., Rolke J., Tidmarsh G., Hail N., Jr., Davis R.E. Combination of galectin inhibitor GCS-100 and BH3 mimetics eliminates both p53 wild type and p53 null AML cells. Biochim. Biophys. Acta. 2016;1863:562–571. doi: 10.1016/j.bbamcr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clark M.C., Pang M., Hsu D.K., Liu F.-T., De Vos S., Gascoyne R.D., Said J., Baum L.G. Galectin-3 binds to CD45 on diffuse large B-cell lymphoma cells to regulate susceptibility to cell death. Blood. 2012;120:4635–4644. doi: 10.1182/blood-2012-06-438234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Demotte N., Wieërs G., Van Der Smissen P., Moser M., Schmidt C., Thielemans K., Squifflet J.-L., Weynand B., Carrasco J., Lurquin C. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Res. 2010;70:7476–7488. doi: 10.1158/0008-5472.CAN-10-0761. [DOI] [PubMed] [Google Scholar]
- 104.Streetly M.J., Maharaj L., Joel S., Schey S.A., Gribben J.G., Cotter F.E. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood. 2010;115:3939–3948. doi: 10.1182/blood-2009-10-251660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y., Nangia-Makker P., Balan V., Hogan V., Raz A. Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell Death Dis. 2010;1:e101. doi: 10.1038/cddis.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Volarevic V., Markovic B.S., Bojic S., Stojanovic M., Nilsson U., Leffler H., Besra G.S., Arsenijevic N., Paunovic V., Trajkovic V. Gal-3 regulates the capacity of dendritic cells to promote NKT-cell-induced liver injury. Eur. J. Immunol. 2015;45:531–543. doi: 10.1002/eji.201444849. [DOI] [PubMed] [Google Scholar]
- 107.Volarevic V., Milovanovic M., Ljujic B., Pejnovic N., Arsenijevic N., Nilsson U., Leffler H., Lukic M.L. Galectin-3 deficiency prevents concanavalin A–induced hepatitis in mice. Hepatology. 2012;55:1954–1964. doi: 10.1002/hep.25542. [DOI] [PubMed] [Google Scholar]
- 108.MacKinnon A.C., Gibbons M.A., Farnworth S.L., Leffler H., Nilsson U.J., Delaine T., Simpson A.J., Forbes S.J., Hirani N., Gauldie J. Regulation of transforming growth factor-β1–driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012;185:537–546. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Traber P.G., Chou H., Zomer E., Hong F., Klyosov A., Fiel M.-I., Friedman S.L. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE. 2013;8:e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Traber P.G., Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS ONE. 2013;8:e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iacobini C., Fantauzzi C.B., Pugliese G., Menini S. Role of galectin-3 in bone cell differentiation, bone pathophysiology and vascular osteogenesis. Int. J. Mol. Sci. 2017;18:2481. doi: 10.3390/ijms18112481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Colnot C., Sidhu S., Poirier F., Balmain N. Cellular and subcellular distribution of galectin-3 in the epiphyseal cartilage and bone of fetal and neonatal mice. Cell. Mol. Biol. (Noisy-Le-Grandfrance) 1999;45:1191–1202. [PubMed] [Google Scholar]
- 113.Stock M., Schäfer H., Stricker S., Gross G., Mundlos S., Otto F. Expression of galectin-3 in skeletal tissues is controlled by Runx2. J. Biol. Chem. 2003;278:17360–17367. doi: 10.1074/jbc.M207631200. [DOI] [PubMed] [Google Scholar]
- 114.Weilner S., Keider V., Winter M., Harreither E., Salzer B., Weiss F., Schraml E., Messner P., Pietschmann P., Hildner F. Vesicular Galectin-3 levels decrease with donor age and contribute to the reduced osteo-inductive potential of human plasma derived extracellular vesicles. Aging (Albany N. Y.) 2016;8:16. doi: 10.18632/aging.100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Niida S., Amizuka N., Hara F., Ozawa H., Kodama H. Expression of Mac-2 antigen in the preosteoclast and osteoclast identified in the op/op mouse injected with macrophage colony-stimulating factor. J. Bone Miner. Res. 1994;9:873–881. doi: 10.1002/jbmr.5650090613. [DOI] [PubMed] [Google Scholar]
- 116.Jensen-Jarolim E., Gscheidlinger R., Oberhuber G., Neuchrist C., Lucas T., Bises G., Radauer C., Willheim M., Scheiner O., Liu F.-T. The constitutive expression of galectin-3 is downregulated in the intestinal epithelia of Crohn’s disease patients, and tumour necrosis factor alpha decreases the level of galectin-3-specific mRNA in HCT-8 cells. Eur. J. Gastroenterol. Hepatol. 2002;14:145–152. doi: 10.1097/00042737-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 117.Lippert E., Falk W., Bataille F., Kähne T., Naumann M., Goeke M., Herfarth H., Schoelmerich J., Rogler G. Soluble galectin-3 is a strong, colonic epithelial-cell-derived, lamina propria fibroblast-stimulating factor. Gut. 2007;56:43–51. doi: 10.1136/gut.2005.081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Puthenedam M., Wu F., Shetye A., Michaels A., Rhee K.-J., Kwon J.H. Matrilysin-1 (MMP7) cleaves galectin-3 and inhibits wound healing in intestinal epithelial cells. Inflamm. Bowel Dis. 2010;17:260–267. doi: 10.1002/ibd.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lippert E., Stieber-Gunckel M., Dunger N., Falk W., Obermeier F., Kunst C. Galectin-3 modulates experimental colitis. Digestion. 2015;92:45–53. doi: 10.1159/000431312. [DOI] [PubMed] [Google Scholar]
- 120.Tsai H.-F., Wu C.-S., Chen Y.-L., Liao H.-J., Chyuan I.-T., Hsu P.-N. Galectin-3 suppresses mucosal inflammation and reduces disease severity in experimental colitis. J. Mol. Med. 2016;94:545–556. doi: 10.1007/s00109-015-1368-x. [DOI] [PubMed] [Google Scholar]
- 121.Simovic Markovic B., Nikolic A., Gazdic M., Bojic S., Vucicevic L., Kosic M., Mitrovic S., Milosavljevic M., Besra G., Trajkovic V. Galectin-3 plays an important pro-inflammatory role in the induction phase of acute colitis by promoting activation of NLRP3 inflammasome and production of IL-1β in macrophages. J. Crohn’s Colitis. 2016;10:593–606. doi: 10.1093/ecco-jcc/jjw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li P., Liu S., Lu M., Bandyopadhyay G., Oh D., Imamura T., Johnson A.M., Sears D., Shen Z., Cui B. Hematopoietic-derived galectin-3 causes cellular and systemic insulin resistance. Cell. 2016;167:973–984. doi: 10.1016/j.cell.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baek J.H., Kim S.J., Kang H.G., Lee H.W., Kim J.H., Hwang K.A., Song J., Chun K.H. Galectin-3 activates PPARγ and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology. 2015;156:147–156. doi: 10.1210/en.2014-1374. [DOI] [PubMed] [Google Scholar]
- 124.Vora A., de Lemos J.A., Ayers C., Grodin J.L., Lingvay I. Association of Galectin-3 with Diabetes Mellitus in the Dallas Heart Study. J. Clin. Endocrinol. Metab. 2019;104:4449–4458. doi: 10.1210/jc.2019-00398. [DOI] [PubMed] [Google Scholar]