Abstract
The promise of growing tissues to replace or improve the function of failing ones, a practice often referred to as regenerative medicine, has been driven in recent years by the development of stem cells and cell lines. Stem cells are typically cultured outside the body to increase cell number or differentiate the cells into mature cell types. In order to maximize the regenerative potential of these cells, there is a need to understand cell-material interactions that direct cell behavior and cell-material dynamics. Most synthetic surfaces used for growth and differentiation of cells in the lab are impractical and cost prohibitive in clinical labs. This review focuses on the modification of low cost polymer substrates that are already widely used for cell culture so that they may be used to control and understand cell-material interactions. In addition, we discuss the ability of cells to exert dynamic control over the microenvironment leading to a more complex, less controlled surface.
Introduction
The therapeutic potential of stem cells is leading to their increased use in clinical trials for treatment of wounds, disease, and as scientific models for drug discovery and toxicology. A major barrier to the reproducibility of cell-based treatments and models is the limited control over cell behavior. In vitro, cells interact with soluble signals, such as growth factors, and the extracellular matrix (ECM), which is tissue-specific and consists of insoluble proteins and glycosaminoglycans. Evidence in the last decade suggests that the properties of the insoluble microenvironment can guide cell behaviors such as adhesion, proliferation, morphology and differentiation [1–4]. The combination of these soluble and insoluble signals regulates cell behavior [5, 6]. While insoluble matrix components can be derived from animal sources, clinical application of stem cells will benefit from chemically defined and animal-product-free culture materials to reduce potential immunogenicity and batch-to-batch variability [7]. In particular, stem cells can internalize components of animal-derived culture materials, which have been shown to cause anaphylactic reactions and the production of anti-serum antibodies in clinical treatments [8, 9]. Understanding and selectively presenting parts of the in vivo extracellular matrix in a cell culture setting can potentially improve the success of stem cell therapies and help answer fundamental scientific questions.
The overwhelming majority of cell culture is performed on 2D polymer surfaces (e.g. tissue culture polystyrene), which can non-specifically adsorb serum-borne proteins and thereby provide adhesion sites for cells [10]. These plastic cell culture materials are low cost, scalable (e.g. T flasks, stacked cultures), and have low batch-to-batch variation. However, plastic cell culture materials typically cannot be used to control cell-material interactions. While a series of chemistries have been developed to coat plastic, glass, and metal substrates, achieving control over the cell-surface interactions and maintaining long-term substrate stability in cell culture conditions remains a challenge [11–14]. This concise review presents a subset of recent advances and challenges in the modification of polymer substrates to control cell behavior (Figure 1). We address surface functionalization of cell culture systems, emerging chemically defined surfaces amenable to biological functionalization, and the ongoing challenge of understanding and controlling time-dependent cell-surface interactions.
Figure 1.
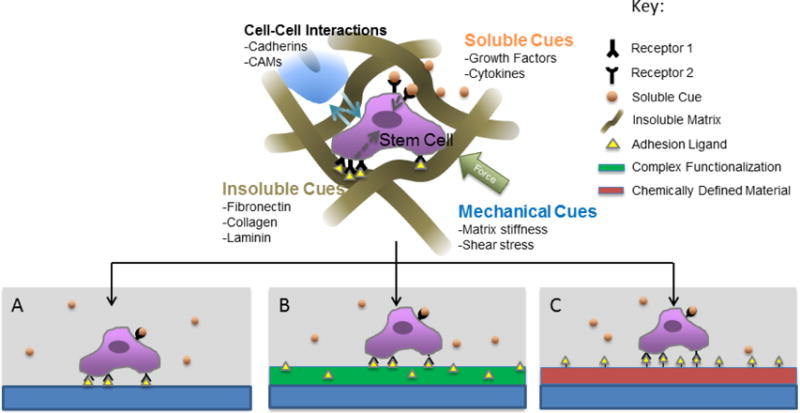
Cell culture systems can reproduce aspects of the cell microenvironment. A) Cells cultured on untreated TCPS adhere to adsorbed serum proteins and interact with soluble signals B) Complex biological functionalization adds insoluble cues derived from the extracellular matrix. C) Chemically defined, functionalizable materials (red) enable tailored presentation of bioactive ligands
Biological Functionalization
Most mammalian cells are adherent, meaning they must attach to a surface to survive. In order to achieve this adhesion, traditional cell culture relies on the adsorption of serum proteins from the media to the solid surface. Protein adsorption occurs within seconds through non-covalent interactions between the protein and the material [10]. This use of adsorbed proteins to achieve cell-surface adhesion has the advantage of simplicity; however, it is not without drawbacks. Proteins that are adsorbed to a surface can denature, and may change in α-helix content, β-sheet content, and structural rigidity [15, 16]. In addition, studies on the kinetics of protein adsorption in a two-component system are rare, dependent on many parameters (including diffusion, components, competitive adsorption, pH, temperature, and others) and are ineffective at accurately predicting adsorption in multi-component systems [17–19]. Thus, it is difficult to predict the concentration and conformation of adsorbed proteins in a cell culture environment.
Coatings that form the cell-material interface can also be composed of individually purified glycoproteins (e.g. fibronectin) or heterogeneous mixtures of proteins (e.g. Matrigel). The orientation of these adsorbed proteins is not easily controlled [20]. The availability of integrin-binding Arg-Gly-Asp (RGD) cell adhesion motifs in Fibronectin coatings, for example, is dependent on the surface chemistry of the material to which fibronectin has adsorbed. This, in turn, may influence cell adhesion and behavior [21–23]. A study by Ba et al. covalently anchored fibronectin via reactions through thiols or primary amines on amine-functionalized polystyrene in an attempt to restrict the orientation of fibronectin on the surface. These reactions increased the retention of fibronectin to the surface, resulting in higher amounts of immobilized fibronectin and a resistance to deformation, but did not increase the availability of binding sites [24]. Klotzsch et al. used single-molecule imaging of fibronectin to track the relative distance between four available cysteines labeled with Cy3B and tracked using photobleaching and total internal reflectance spectroscopy. The average distance between the four sites increased from 33 nm to 43 nm upon fibronectin adsorption to glass in denaturing conditions, indicating a conformational change in the molecule. In the same study, the intra-label distance ranged from 24nm to over 51nm upon denaturing when fibronectin was adsorbed to glass that had been rendered more or less hydrophobic through plasma cleaning, pyrolysis, and chemical silanization [25]. Recently, Lin et al. demonstrated that the adsorption force between fibronectin and the underlying material can affect the morphology of cells grown on the surface [26]. Taken together, studies to date demonstrate that while protein coatings can enable cells to interact with a material, they do not allow for a high level of control over the orientation, density and availability of cell adhesion epitopes.
Synthetic Biological Functionalization of Polymer Substrates
To better control the concentration and identity of biologically active sequences on the cell culture surface, the polymer substrate can be modified with a non-fouling layer and short peptide sequences derived from ECM proteins – like collagen, fibronectin, and laminin – or growth factor mimicking peptides [2, 3, 27–30]. A variety of synthetic approaches have been developed with a goal of generating cell culture substrates that: (a) require low concentrations of peptides to reduce cost and complexity; (b) are functionalized via peptide coupling chemistry which is efficient in aqueous media; (c) are scalable over large surface areas or complex geometries with uniform coverage; and (d) are stable during long-term (days-weeks) culture of cells. Modification of polymer substrates presents some additional, unique challenges when compared to modification of glass or metal substrates. Polymeric materials often must be modified in aqueous or ethanolic solutions, and in some cases crosslinked at low temperature or using UV irradiation in order to prevent damage to the polymer.
Many polymer substrates lack the reactive groups required for direct covalent functionalization with peptides. These substrates require additional functionalization steps, such as a plasma treatment, silanization or other chemical treatment, or coating with a reactive layer. Treatment of TCPS with allylamine, for example, adds primary amines to the substrate, which can then covalently couple to PEG-N-hydrocysuccinimide (NHS) containing copolymers to form a crosslinked coating [31]. Qian et al. used chemical vapor deposition to coat TCPS with an initiator molecule which initiated growth of poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] (PMEDSAH) brushes via atom transfer radical polymerization (ATRP) for embryonic stem cell culture [32]. This study compared the ATRP coatings to PMEDSAH polymerized by UV-ozone initiated free radical polymerization, first demonstrated in Nandivada et al. [33]. Lavanant et al. used a simple photobromination reaction to modify polyethylene and thereby polymerize polyethylene glycol methacrylate brushes in a water/ethanol mixture.[34] The resulting brushes were robust in a one month water stability test and facilitated cell adhesion through RGD peptides.
Coating a substrate with an insoluble layer may also provide an appealing method to introduce functional groups for subsequent non-fouling brush growth or peptide immobilization [35, 36]. An illustrative example of this is polydopamine which can coat many surface types and have been used for both zwitterionic polymer brush growth and peptide attachment [37, 38]. In another coating approach, Schmitt et.al recently reported the synthesis of a PEG-based random copolymer in solution that was then spin-coated onto an untreated TCPS substrate [28–30]. The polymer coating contained azlactone functional groups for peptide attachment in aqueous solution at room temperature and low concentration (1mM in aqueous solution) with no activation step. The resultant thin film permitted peptide surface density quantification (12.6 pmol/cm2) by X-ray photoelectron spectroscopy and RGD-mediated cell attachment.
Time-Dependent Surface Remodeling
In vivo, cells and the ECM are typically in a state of dynamic remodeling. In vitro, scientists have made significant progress in understanding and manipulating the initial characteristics of the cell culture substrate, prior to cell culture. However, despite the progress that has been made in generating and characterizing chemically defined surfaces for cell culture, there is still only a limited understanding of the dynamics of cell-surface interactions over time. In particular, while surfaces can be designed to present a single epitope (e.g. a cell adhesion peptide) for binding of cell surface receptors, it is not clear how long the cell has access to that epitope. Do cell adhesion epitopes rapidly become unavailable due to peptide-material bond scission, peptide instability, protein adsorption, cell-mediated ECM protein secretion, or other mechanisms (Figure 2)? This is an intriguing, poorly addressed question in cell culture applications. In the following paragraphs we introduce these dynamic mechanisms at the cell-material interface, and discuss the limited insights gained to date regarding the putative dynamics of cell-substrate interactions.
Figure 2.
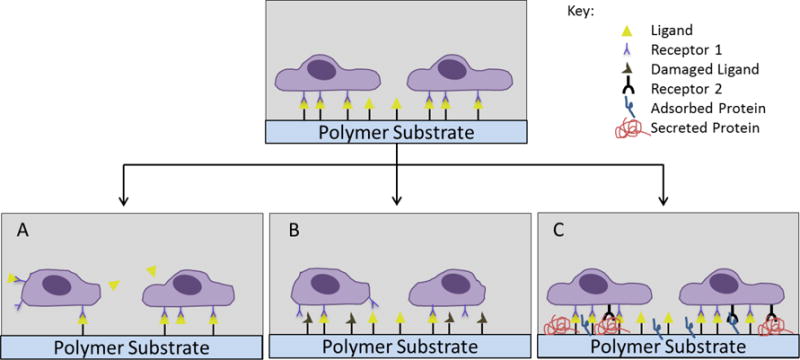
Over time, the cell-material interface can be dynamically altered through (A) removal of a cell-adhesive ligand from the surface, (B) degradation/denaturation of the ligand, and (C) fouling over time due to secreted or adsorbed molecules.
Common peptide-polymer bonds include amide, carbamate, alkyl sulfide, thioester, triazole, ester, thioether, and disulfide bonds, which may be vulnerable to hydrolysis, displacement, or protease-mediated degradation [39]. Carbamate [40], thioester [41], and ester [42] bonds are generally susceptible to hydrolytic degradation at physiological pH, while amide bonds are more stable [28, 29]. Studies on the effect of polymer-peptide bond stability in cell culture systems are limited, although degradation of thioester, amide, and carbamate linkers has been observed on PEG thin films using cell-based assays [28, 29].
In addition to cleavage of peptide epitopes from the culture surface, the peptide itself can be subject to degradation or damage in the culture environment via oxidation, deamidation, reduction and hydrolysis [43]. Methionine (Met), cysteine (Cys), histidine (His), tryptophan (Trp) and tyrosine (Tyr) are all subject to oxidation in the presence of oxygen radicals or alkaline pH [43, 44]. Oxidation of these amino acids can also be triggered by visible light in the presence of oxygen, resulting in a decreased biological activity of the peptide, or, in the case of whole proteins, changes to the secondary and tertiary structure [45]. Met and Cys are susceptible to oxidation at their sulfur atoms, while His, Trp and Tyr undergo oxidation of their aromatic rings [44]. In the case of cysteine, this can lead to unwanted disulfide bonds [46]. Glutamine and asparagine residues are susceptible to deamidation, resulting in isomerization or racemization of the residue [47, 48]. Fragmentation of peptides via amide hydrolysis is possible, usually occurring at Asp-Gly and Asp-Pro sequences, but amide hydrolysis is not likely to occur near physiological pH (pH 7–8) [49]. Taken together, studies performed to date, and reviewed in more detail elsewhere [50], indicate that changes to the primary structure of peptides or whole proteins are likely to occur during cell culture on functionalized biomaterials. These changes can result in altered biological activity over time, which may result in unintended changes in the cell microenvironment. However, if changes in peptide and protein stability can be understood and harnessed they may provide adaptable mechanisms for intentional, transient presentation of cell-interactive epitopes.
Cell-mediated remodeling of synthetic surfaces is another source of dynamic variation. Many cell types have been shown to synthesize ECM molecules in vitro, including collagen, elastin, laminin, fibronectin, aggrecan, decorin, glycosaminoglycans, and calcium deposits. [51–57] These cell-secreted matrices can then be decellularized and used to direct stem cell differentiation [58–60], which provides evidence that cells interact with cell-secreted ECM molecules. Even in environments designed to be “non-fouling” or “low-fouling”, protein adsorption is commonly observed [61, 62], and degradation of the initially non-fouling surfaces can reduce their ability to resist protein adsorption [63]. Cells can also dynamically modify their surrounding ECM by enzymatic degradation in concert with ECM molecule secretion. Cell-secreted or cell-associated matrix-metalloproteases (MMP) can mediate cell-mediated remodeling of the microenvironment in vitro. [64-68] However, the dynamic interplay of ECM protein secretion, protein adsorption, and cell-mediated ECM degradation is poorly understood. For example, quantitative understanding of how long a cell can engage with the initial substrate before cell-secreted ECM molecules or metalloprotease activity significantly alter the initial surface is lacking.
Concluding Remarks
Recent studies have made significant progress toward controlling the initial cell-surface interface, and understanding the effect that this may have on cell behavior. While the initial conditions of these types of substrates are thoroughly examined, they may be susceptible to increasingly complex modifications after hours to days of cell culture. The quantitative characterization of cell secreted ECM molecules, protein adsorption through surface degradation, and degradation of functional peptides on cell culture materials is likely to be a highly significant area of study that is as of yet largely unexplored. In order to maintain control and biological relevance in vitro, there is a need to create cell culture surfaces that are designed for real-time characterization of polymer stability, peptide stability, ECM molecule secretion, protein adsorption, and cell-mediated ECM degradation/remodeling. Ultimately, a clearer understanding of cell-substrate dynamics may lead to innovative approaches to dynamically adapt to cell behavior in a controlled and predictable manner.
Acknowledgments
This research was funded by the National Science Foundation (NSF DMR 1306482), the National Institutes of Health (R01HL093282), and the Environmental Protection Agency (STAR grant no. 83573701).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Maheshwari G, et al. Cell adhesion and motility depend on nanoscale RGD clustering. Journal of Cell Science. 2000;113(10):1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 2.Lin E, et al. Peptide microarray patterning for controlling and monitoring cell growth. Acta Biomaterialia. doi: 10.1016/j.actbio.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Li L, et al. Spatial control of cell fate using synthetic surfaces to potentiate TGF-β signaling. Proceedings of the National Academy of Sciences. 2011;108(29):11745–11750. doi: 10.1073/pnas.1101454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotech. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 5.Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnology Advances. 2014;32(2):462–484. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutolf MP, Blau HM. Artificial Stem Cell Niches. Advanced materials (Deerfield Beach, Fla) 2009;21(32–33):3255–3268. doi: 10.1002/adma.200802582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng X, et al. Proteomic Analysis for the Assessment of Different Lots of Fetal Bovine Serum as a Raw Material for Cell Culture. Part IV. Application of Proteomics to the Manufacture of Biological Drugs. Biotechnology Progress. 2006;22(5):1294–1300. doi: 10.1021/bp060121o. [DOI] [PubMed] [Google Scholar]
- 8.Mackensen A, et al. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000;49(3):152–6. doi: 10.1007/s002620050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spees JL, et al. Internalized Antigens Must Be Removed to Prepare Hypoimmunogenic Mesenchymal Stem Cells for Cell and Gene Therapy[ast] Mol Ther. 2004;9(5):747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Mrksich M. A surface chemistry approach to studying cell adhesion. Chemical Society Reviews. 2000;29(4):267–273. [Google Scholar]
- 11.Saha K, et al. Surface Creasing Instability of Soft Polyacrylamide Cell Culture Substrates. Biophysical Journal. 2010;99(12):L94–L96. doi: 10.1016/j.bpj.2010.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tugulu S, Klok HA. Stability and Nonfouling Properties of Poly(poly(ethylene glycol) methacrylate) Brushes under Cell Culture Conditions. Biomacromolecules. 2008;9(3):906–912. doi: 10.1021/bm701293g. [DOI] [PubMed] [Google Scholar]
- 13.Sha J, et al. Sequential Nucleophilic Substitutions Permit Orthogonal Click Functionalization of Multicomponent PEG Brushes. Biomacromolecules. 2013;14(9):3294–3303. doi: 10.1021/bm400900r. [DOI] [PubMed] [Google Scholar]
- 14.Hudalla GA, Murphy WL. Chemically well-defined self-assembled monolayers for cell culture: toward mimicking the natural ECM. Soft Matter. 2011;7(20):9561–9571. doi: 10.1039/C1SM05596H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethuraman A, et al. Protein unfolding at interfaces: Slow dynamics of α-helix to β-sheet transition. Proteins: Structure, Function, and Bioinformatics. 2004;56(4):669–678. doi: 10.1002/prot.20183. [DOI] [PubMed] [Google Scholar]
- 16.Renner L, et al. Fibronectin Displacement at Polymer Surfaces. Langmuir. 2005;21(10):4571–4577. doi: 10.1021/la046801n. [DOI] [PubMed] [Google Scholar]
- 17.Cano T, Offringa N, Willson RC. The effectiveness of three multi-component binding models in describing the binary competitive equilibrium adsorption of two cytochrome b5 mutants. Journal of Chromatography A. 2007;1144(2):197–202. doi: 10.1016/j.chroma.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Yang K. Analysis of mass transport models based on Maxwell– Stefan theory and Fick’s law for protein uptake to porous anion exchanger. Separation and Purification Technology. 2008;60(2):180–189. [Google Scholar]
- 19.Yang K. Dynamic binary protein adsorption in ion-exchange media depicted with a parallel diffusion model derived from Maxwell–Stefan theory. Chemical Engineering Science. 2016;139:163–172. [Google Scholar]
- 20.Daly SM, Przybycien TM, Tilton RD. Coverage-Dependent Orientation of Lysozyme Adsorbed on Silica. Langmuir. 2003;19(9):3848–3857. [Google Scholar]
- 21.Iuliano DJ, Saavedra SS, Truskey GA. Effect of the conformation and orientation of adsorbed fibronectin on endothelial cell spreading and the strength of adhesion. Journal of Biomedical Materials Research. 1993;27(8):1103–1113. doi: 10.1002/jbm.820270816. [DOI] [PubMed] [Google Scholar]
- 22.Lewandowska K, et al. Cell-Type-Specific adhesion mechanisms mediated by fibronectin adsorbed to chemically derivatized substrata. Journal of Biomedical Materials Research. 1992;26(10):1343–1363. doi: 10.1002/jbm.820261007. [DOI] [PubMed] [Google Scholar]
- 23.Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. Journal of Biomedical Materials Research Part A. 2003;66A(2):247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 24•.Ba OM, et al. Protein covalent immobilization via its scarce thiol versus abundant amine groups: Effect on orientation, cell binding domain exposure and conformational lability. Colloids and Surfaces B: Biointerfaces. 2015;134:73–80. doi: 10.1016/j.colsurfb.2015.06.009. Surface immobilization of fibronectin was achieved through specific reactions at primary amines and free thiols, increasing the stability of the adsorbed protein. [DOI] [PubMed] [Google Scholar]
- 25•.Klotzsch E, et al. Conformational distribution of surface-adsorbed fibronectin molecules explored by single molecule localization microscopy. Biomaterials Science. 2014;2(6):883–892. doi: 10.1039/c3bm60262a. FTIR was used to observe the relative separation of fluorescently tagged cysteines within a fibronectin molecule during adsorption. [DOI] [PubMed] [Google Scholar]
- 26•.Lin M, et al. Adsorption Force of Fibronectin on Various Surface Chemistries and Its Vital Role in Osteoblast Adhesion. Biomacromolecules. 2015;16(3):973–984. doi: 10.1021/bm501873g. Various surface chemistries adsorbed to fibronectin with different forces. This change in adsorption force played a key role in osteoblast adhesion. [DOI] [PubMed] [Google Scholar]
- 27.Koepsel JT, et al. Combinatorial screening of chemically defined human mesenchymal stem cell culture substrates. Journal of materials chemistry. 2012;22(37):19474–19481. doi: 10.1039/C2JM32242K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt SK, Murphy WL, Gopalan P. Crosslinked PEG mats for peptide immobilization and stem cell adhesion. Journal of Materials Chemistry B. 2013;1(9):1349–1360. doi: 10.1039/c2tb00253a. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt SK, et al. Polyethylene Glycol Coatings on Plastic Substrates for Chemically Defined Stem Cell Culture. Advanced Healthcare Materials. 2015 doi: 10.1002/adhm.201500191. p.n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Schmitt SK, et al. Peptide Conjugation to a Polymer Coating via Native Chemical Ligation of Azlactones for Cell Culture. Biomacromolecules. 2016 doi: 10.1021/acs.biomac.5b01682. Vinyl dimethyl azlactone, as a part of a PEG-based copolymer culture system, undergoes native chemical ligation with N-terminal cysteine molecules to create an amide bond at low concentration and room temperature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ameringer T, et al. Polymer coatings that display specific biological signals while preventing nonspecific interactions. Journal of Biomedical Materials Research Part A. 2012;100(2):370–379. doi: 10.1002/jbm.a.33194. [DOI] [PubMed] [Google Scholar]
- 32••.Qian X, et al. Enhancement of the propagation of human embryonic stem cells by modifications in the gel architecture of PMEDSAH polymer coatings. Biomaterials. 2014;35(36):9581–9590. doi: 10.1016/j.biomaterials.2014.08.015. Chemical vapor deposition and UV-ozone techniques were used to deposit initiator molecules for PMEDSAH polymerization on a wide variety of substrates. Demonstrated the effect of polymerization time and gel architecture on the maintenance of ESCs pluripotency during expansion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nandivada H, et al. Fabrication of synthetic polymer coatings and their use in feeder-free culture of human embryonic stem cells. Nat Protocols. 2011;6(7):1037–1043. doi: 10.1038/nprot.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavanant L, et al. A Facile Strategy for the Modification of Polyethylene Substrates with Non-Fouling, Bioactive Poly (poly (ethylene glycol) methacrylate) Brushes. Macromolecular bioscience. 2010;10(1):101–108. doi: 10.1002/mabi.200900205. [DOI] [PubMed] [Google Scholar]
- 35••.Telford AM, Neto C, Meagher L. Robust grafting of PEG-methacrylate brushes from polymeric coatings. Polymer. 2013;54(21):5490–5498. PEG brushes are grown from polymeric macroinitiator films that can be applied to any surface shape or composition. [Google Scholar]
- 36.Sweat DP, et al. A Single-Component Inimer Containing Cross-Linkable Ultrathin Polymer Coating for Dense Polymer Brush Growth. Langmuir. 2013;29(11):3805–3812. doi: 10.1021/la305060z. [DOI] [PubMed] [Google Scholar]
- 37.Kuang J, Messersmith PB. Universal Surface-Initiated Polymerization of Antifouling Zwitterionic Brushes Using a Mussel-Mimetic Peptide Initiator. Langmuir. 2012;28(18):7258–7266. doi: 10.1021/la300738e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang K, et al. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials. 2012;33(29):6952–6964. doi: 10.1016/j.biomaterials.2012.06.067. [DOI] [PubMed] [Google Scholar]
- 39.Canalle LA, Lowik DWPM, van Hest JCM. Polypeptide-polymer bioconjugates. Chemical Society Reviews. 2010;39(1):329–353. doi: 10.1039/b807871h. [DOI] [PubMed] [Google Scholar]
- 40.Dworak A, et al. Degradable polymeric nanoparticles by aggregation of thermoresponsive polymers and “click” chemistry. Nanoscale. 2015;7(40):16823–16833. doi: 10.1039/c5nr04448k. [DOI] [PubMed] [Google Scholar]
- 41.Thapa P, et al. Native Chemical Ligation: A Boon to Peptide Chemistry. Molecules. 2014;19(9):14461. doi: 10.3390/molecules190914461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charton M. Steric effects. I. Esterification and acid-catalyzed hydrolysis of esters. Journal of the American Chemical Society. 1975;97(6):1552–1556. [Google Scholar]
- 43.Manning MC, Patel K, Borchardt RT. Stability of Protein Pharmaceuticals. Pharmaceutical Research. 611:903–918. doi: 10.1023/a:1015929109894. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Schöneich C, Borchardt RT. Chemical instability of protein pharmaceuticals: Mechanisms of oxidation and strategies for stabilization. Biotechnology and Bioengineering. 1995;48(5):490–500. doi: 10.1002/bit.260480511. [DOI] [PubMed] [Google Scholar]
- 45.Kerwin BA, Remmele RL. Protect from light: Photodegradation and protein biologics. Journal of Pharmaceutical Sciences. 2007;96(6):1468–1479. doi: 10.1002/jps.20815. [DOI] [PubMed] [Google Scholar]
- 46.Astafieva IV, Eberlein GA, John Wang Y. Absolute on-line molecular mass analysis of basic fibroblast growth factor and its multimers by reversed-phase liquid chromatography with multi-angle laser light scattering detection. Journal of Chromatography A. 1996;740(2):215–229. doi: 10.1016/0021-9673(96)00134-3. [DOI] [PubMed] [Google Scholar]
- 47.Shahrokh Z, et al. Major Degradation Products of Basic Fibroblast Growth Factor: Detection of Succinimide and Iso-aspartate in Place of Aspartate15. Pharmaceutical Research. 117:936–944. doi: 10.1023/a:1018962732067. [DOI] [PubMed] [Google Scholar]
- 48.Geiger T, Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. Journal of Biological Chemistry. 1987;262(2):785–794. [PubMed] [Google Scholar]
- 49.Schrier JA, et al. Degradation Pathways for Recombinant Human Macrophage Colony-Stimulating Factor in Aqueous Solution. Pharmaceutical Research. 107:933–944. doi: 10.1023/a:1018990001310. [DOI] [PubMed] [Google Scholar]
- 50.Jaipa Patel RK, Tunga Rashbehari, Ritter Nadine M, Tunga Binita S. Stability Considerations for Biopharmaceuticals, Part 1: Overview of Protein and Peptide Degradation Pathways. BioProcess International. 2011 [Google Scholar]
- 51.Bosnakovski DM, Morimichi, Kim Gonhung, Takagi Satoshi, Okumura Masahiro, Fujinaga Toru. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnology and Bioengineering. 2006;93(6):1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 52.Amable P, et al. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Research & Therapy. 2014;5(2):53. doi: 10.1186/scrt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bahramsoltani M, et al. Angiogenesis and Collagen Type IV Expression in Different Endothelial Cell Culture Systems. Anatomia, Histologia, Embryologia. 2014;43(2):103–115. doi: 10.1111/ahe.12052. [DOI] [PubMed] [Google Scholar]
- 54.van der Smissen A, et al. Artificial extracellular matrices support cell growth and matrix synthesis of human dermal fibroblasts in macroporous 3D scaffolds. Journal of Tissue Engineering and Regenerative Medicine. 2015 doi: 10.1002/term.2037. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 55.Mann BK, et al. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20(23–24):2281–2286. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 56.Wu Y, et al. Ascorbic acid promotes extracellular matrix deposition while preserving valve interstitial cell quiescence within 3D hydrogel scaffolds. Journal of Tissue Engineering and Regenerative Medicine. 2015 doi: 10.1002/term.2093. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elhadj S, Mousa SA, Forsten-Williams K. Chronic pulsatile shear stress impacts synthesis of proteoglycans by endothelial cells: Effect on platelet aggregation and coagulation. Journal of Cellular Biochemistry. 2002;86(2):239–250. doi: 10.1002/jcb.10226. [DOI] [PubMed] [Google Scholar]
- 58.Decaris ML, Leach JK. Design of Experiments Approach to Engineer Cell-Secreted Matrices for Directing Osteogenic Differentiation. Annals of Biomedical Engineering. 2010;39(4):1174–1185. doi: 10.1007/s10439-010-0217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pham QP, et al. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29(18):2729–2739. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 60.Datta N, et al. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26(9):971–977. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Ryu JY, et al. New Antifouling Platform Characterized by Single-Molecule Imaging. ACS Applied Materials & Interfaces. 2014;6(5):3553–3558. doi: 10.1021/am4057387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F, et al. Modification of gold surface by grafting of poly(ethylene glycol) for reduction in protein adsorption and platelet adhesion. Journal of Biomaterials Science, Polymer Edition. 2001;12(5):515–531. doi: 10.1163/156856201300194252. [DOI] [PubMed] [Google Scholar]
- 63.Herold DA, Keil K, Bruns DE. Oxidation of polyethylene glycols by alcohol dehydrogenase. Biochemical Pharmacology. 1989;38(1):73–76. doi: 10.1016/0006-2952(89)90151-2. [DOI] [PubMed] [Google Scholar]
- 64.Kloxin AM, et al. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu Y, et al. PEG-based hydrogels with tunable degradation characteristics to control delivery of marrow stromal cells for tendon overuse injuries. Acta Biomaterialia. 2011;7(3):959–966. doi: 10.1016/j.actbio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Wilson MJ, et al. Hydrogels with well-defined peptide-hydrogel spacing and concentration: impact on epithelial cell behavior. Soft Matter. 2012;8(2):390–398. doi: 10.1039/C1SM06589K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eyrich D, et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28(1):55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]


