Abstract
Background:
Aberrant Myc expression plays a critical role in various tumors, including non-Hodgkin lymphoma (NHL). Myc-positive lymphoma is clinically aggressive, more resistant to chemotherapy, and associated with high mortality.
Objective:
The current study aimed to show inhibition of aurora A kinase (AURKA) may overcome resistance to chemotherapy and improve outcomes in Myc-overexpressing lymphoma.
Methods:
Myc-overexpressing lymphoma cell lines were evaluated by trypan blue, annexin V/propidium iodide staining, and western blotting for cytotoxicity, cell cycle, apoptosis, and Myc-associated protein expression, respectively, in the presence of cyclophosphamide with or without MLN8237, an AURKA inhibitor. Immunofluorescence for apoptosis-inducing factor (AIF) and acridine orange staining were used to analyze levels of autophagy. EμMyc genetically modified mouse model and xenograft models bearing Myc-overexpressing lymphoma cells were used to determine the efficacy of cyclophosphamide, MLN8237, or the combination in chemosensitive and chemoresistant tumors.
Results:
In our in vitro experiments using chemoresistant lymphoma cells, MLN8237 and cyclophosphamide showed synergistic effects. Mice bearing lymphoma xenograft had rapid disease progression with median survival of ~ 35 days when treated with cyclophosphamide alone. In contrast, the combination of cyclophosphamide and MLN8237 induced complete tumor regression in all mice, which led to improvement in survival compared to the single agent control (p=0.022). Kinome analysis of tumors treated with MLN8237 showed global suppression of various kinases.
Conclusion:
Our data demonstrate that AURKA inhibition induces synthetic lethality and overcomes chemoresistance in Myc-overexpressing lymphoma. The combination of MLN8237 and conventional chemotherapy showed promising safety and anti-tumor activities in preclinical models of Myc-positive NHL.
1. Introduction
Non-Hodgkin lymphoma (NHL) is the sixth most common type of cancer, with over 70,000 new cases diagnosed annually in the U.S.1 The survival of patients with NHL has improved dramatically over the past two decades mainly because of advancements in chemoimmunotherapy, including anti-CD20 antibodies, and supportive care. Nonetheless, more than one third of patients with aggressive NHL eventually relapse after initial therapy, and the disease becomes progressively more resistant to treatment.2,3 Notably, abnormal expression of Myc has been associated with significantly inferior survivals in patients with aggressive NHL even in the immunotherapy era.4,5 Rearrangement of Myc proto-oncogene into the immunoglobulin heavy chain resulting in aberrant Myc expression is the major genetic event in approximately 10 to 15% of diffuse large B-cell lymphoma (DLBCL), and survival outcomes are especially poor when Myc rearrangements are paired with Bcl-2 or Bcl-6 rearrangements as in double-hit lymphomas.4–6 Myc deregulation is not restricted to rearrangements of the Myc locus but also involves mechanisms that target its expression through gene amplifications or deregulation of upstream genes in the Myc pathway.7 Myc overexpression has been reported in up to 30% of DLBCL cases and also has been associated with poor clinical outcomes.8–11 Moreover, Myc-abnormality retains its strong negative prognostic significance in patients with relapsed or refractory DLBCL with dismal survival rates despite aggressive treatments, including hematopoietic stem cell transplant.12 Even in pediatric patients with DLBCL, who fare better than adults, the presence of Myc rearrangement has been associated with reduced event-free survival by approximately 6 fold.13 Clearly, novel treatment paradigms are needed to improve survival outcomes of patients with Myc-associated NHL.
Myc is an attractive target for anticancer therapy because of its function as a central transcriptional oncogenic switch that regulates a large variety of cellular functions, especially in cell growth and survival.14,15 The importance of Myc-expression in tumor cell survival has been demonstrated in a number of studies, where conditional expression of Myc resulted in various malignancies, and deactivation of Myc led to spontaneous regression in cancers regardless of the tumor type, demonstrating Myc-dependence of these tumors.16–18 In addition, Myc-induced tumors generally exhibit continued dependence upon this oncogene, a phenomenon known as oncogene addiction.19 Notably, sustained tumor regression after brief inactivation of Myc has been reported in conditional transgenic lymphoma models.20 Despite the fact that Myc was the first gene to be associated with the pathogenesis of lymphoma, no effective therapy has been discovered to date against Myc-associated NHL for several reasons. First, the diverse mechanisms driving its aberrant expression make pharmacologic inhibition of Myc extremely challenging.21 In addition, as a nuclear transcriptional factor, Myc offers no obvious binding site and lacks specific enzymatic activity, which makes direct inhibition of Myc even more problematic.22 A few small molecule inhibitors targeting the protein-protein or protein-DNA interaction in Myc-regulated transcription have been developed, but these agents are still in very early phases of development.23,24 A number of signaling pathways and regulatory mechanisms are involved in keeping Myc expression under tight control, and new ideas for therapeutic interventions for Myc-expressing tumors derive from the involvement of these pathways in Myc signaling.
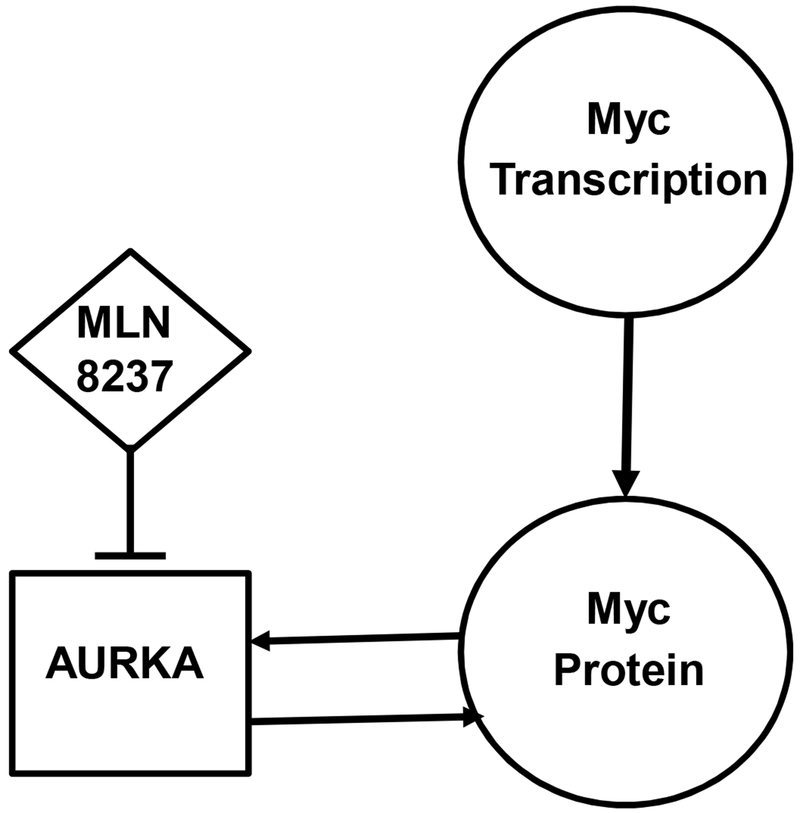
Aurora A Kinase (AURKA) represents one of the key pathways in treatment of Myc-associated NHL (Figure 1). Amplification and overexpression of AURKA is observed in various human tumors, including lymphoma, and is frequently associated with tumor progression as well as resistance to chemotherapy.25 It has been reported that expression of AURKA is directly induced by Myc, and AURKA is essential for the maintenance of Myc-driven lymphoma, suggesting that AURKA could be one of the primary therapeutic targets in Myc-associated NHL.26 Recently, it has been shown that AURKA plays a role in stabilizing Myc protein, and inhibition of AURKA induces Myc protein degradation.27 These data provide strong rationale for targeting AURKA to overcome chemoresistance in Myc-overexpressing lymphoma.
Figure 1.
A schematic diagram of Myc and Aurora A Kinase (AURKA) pathways. Myc directly induces expression of AURKA, which in turn stabilizes Myc protein. Inhibition of AURKA induces Myc protein degradation.
2. Materials and Methods
2.1. Cell lines, Reagents
Raji and Ramos cells were purchased from ATCC (Manassas, VA, USA) and cultured in RPMI1640 supplemented with 10% heat-inactivated FBS, 100IU/ml penicillin G, 100ug/ml streptomycin, and 2mM L-glutamine at 37°C and 5% CO2. Cyclophosphamide was purchased from Sigma-Aldrich (C7397; St. Louis, MO, USA). AURKA inhibitor MLN8237 was purchased from Selleck Chemicals (S1133; Houston, TX, USA) and ChemieTek (CT-M8237; Indianapolis, IN, USA). Nocodazole was purchased from Sigma-Aldrich to induce expression of phosphorylated AURK proteins.28 In vitro experiments were performed at least in 3 repetitions.
2.2. Cytotoxicity, Cell Cycle, and Apoptosis Assay
To test cell viability after drug treatment, cells were plated at 104 cells/ml in 96-well plates and treated with Cyclophosphamide and/or MLN8237 for 72h. Cell viability was assessed by Trypan Blue staining. For cell cycle assays, 106-107 cells were collected by centrifugation, washed twice in cold PBS, and stained with Fixable Viability Dye eFluor780 (eBioscience, San Diego, CA, USA). Cells were washed, resuspended in PBS and fixed with 0.5-1ml ethanol. Cells were reconstituted by resuspending in 1ml PBS, and samples were run on Miltenyi MACSQuant VYB (Bergisch Gladbach, Germany) and analysis done using FlowJo software (Ashland, OR, USA). For apoptosis, cells were collected by centrifugation and stained for AnnexinV-FITC and PI using Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis Kit (Life Technologies; Carlsbad, CA, USA) according to manufacturer’s instructions.
2.3. Immunoblot and Antibodies
Cells were treated with 100nM MLN8237 and 1mM cyclophosphamide for 24-48h. To detect Phospho-Aurora Kinases, cells were treated with 60nM nocodazole for 16h, followed by 100nM MLN8237 and/or 1mM cyclophosphamide for 2h. Lysate was obtained, and equivalent amounts of protein were loaded onto 10% SDS-PAGE gels. The gels were transferred to nitrocellulose and incubated with primary and secondary antibodies purchased from Cell Signaling Technology, as previously described. Primary antibodies used: Mdm2 (D12), p53 (DO-7), actin (2Q1055; Santa Cruz Biotechnology, Inc.; Dallas, TX, USA), c-Myc (Y69; Abcam, Cambridge, UK), P-Rb (D20B12), Mdr1 (E1Y7B), AurkA (D3V7T), AurkB (3094), P-AurkA/B/C (D13A11), P-Histone H3 (Ser10; D7N8E), P-Src Family Y416 (D49G4), LC3B (E5Q2K), Bcl2 (124), Bax (D2E11), GAPDH (D16H11), AIF (D39D2), TBP (D5C9H), Caspase 3 (9662), Caspase 7 (C7), tubulin (2148; Cell Signaling Technology, Danvers, MA, USA).
2.4. Immunofluorescence for Apoptosis-inducing Factor (AIF)
Cells were treated with cyclophosphamide (5 mM), MLN8237 (100 nM), or in combination for 48 hours. Cells were washed three times with PBS and resuspended to a concentration of 10,000 cells per 1 ml. Cells were then fixed with 4% (v/v) paraformaldehyde for 30 min and covered with 10% (v/v) goat serum followed by incubation with diluted primary antibody. Cells were then probed with Alexa Fluor 488 secondary antibodies (Invitrogen, Carlsbad, CA, USA). Fluorescent signals were detected using a confocal fluorescence microscope (Zeiss LSM 780, Zeiss, Jena, Germany).
2.5. Detection of Acidic Vesicular Organelles (AVOs) by Acridine Orange (AO) Staining
Cells were treated with cyclophosphamide (5 mM), MLN8237 (100 nM), or in combination for 48 hours, and acridine orange (Sigma) was added. Detection of fluorescence signal was performed with a fluorescence microscope (Nikon Eclipse 80i) equipped with a 450–490-nm band-pass blue excitation filter, a 515-nm long-pass barrier filter, and a digital camera (Nikon DS-5Mc, Tokyo, Japan).
2.6. Multiplexed inhibitor bead (MIB) chromatography, mass spectrometry (MS), and analysis
MIB/MS assays were performed as described previously.29 Briefly, Snap-frozen Raji xenograft tumors were crushed by mortar and pestle in ice-cold multiplexed inhibitor bead (MIB) lysis buffer supplemented with complete protease inhibitor cocktail (Roche, Basel, Switzerland) and 1% phosphatase inhibitor cocktails 2 and 3 (Sigma). Equal amounts of total protein (3mg) were gravity-flowed over MIB columns in high salt MIB lysis (1 mol/L NaCl). The MIB columns consisted of 175-μL mixture of six type I kinase inhibitors (CTx-0294885, VI-16832, PP58, Purvalanol B, UNC-21474, and UNC-8088A) custom-synthesized with hydrocarbon linkers and covalently linked to ECH-Sepharose (or EAH-Sepharose for Purvalanol B) beads as previously described. Columns were washed, and bound protein was eluted twice with 0.5% SDS, 1% β-mercaptoethanol, 100 mmol/L Tris-HCl. Samples were then precipitated by methanol/chloroform, dried in a speed-vac and resuspended in 50 mmol/L HEPES. Tryptic digests were performed overnight at 37°C, extracted four times with 1 mL ethyl acetate to remove detergent, dried in a speed-vac, and peptides further cleaned using C-18 spin columns according to manufacturer’s protocol (Pierce, Dallas, TX, USA). Peptides were resuspended, and 40-50% of final peptide suspension was run on an Easy nLC-1000 through a Thermo Easy-Spray column on a 180min gradient. Spectra were searched and quantitated against the Swiss-Prot human database using MaxQuant LFQ software with default parameters and the human SwissProt/Uniprot database. Missing log2(LFQ) intensities were imputed across the matrix if at least 3 valid values were identified in at least 1 treatment group. Unsupervised hierarchical clustering and heatmaps were generated using Morpheus software (The Broad Institute, Cambridge, MA, USA).
2.7. Genetically engineered mouse model (GEMM) and xenograft studies
EμMyc mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were randomly assigned to 4 groups (control, MLN8237, cyclophosphamide, and MLN8237 plus cyclophosphamide) as soon as palpable lymph nodes were detected. For the xenograft models, FoxN1Nu athymic mice (6-8 weeks old) were obtained and housed in the UNC animal facility after approval of the experimental protocol by the Institutional Animal Care and Use Committee (IACUC). In vivo experiments were conducted following the UNC IACUC institutional guidelines. Ramos or Raji cells (10 x 106) were injected subcutaneously in the right flank 10 days prior to experiments to produce lymphoma xenografts measuring ~ 10 mm in diameter. Both in the EμMyc and xenograft models, treatment groups of 4 to 12 mice each received cyclophosphamide (100 mg/kg/week via intraperitoneal injection), MLN8237 (50 mg/kg/day in chow), or the combination of cyclophosphamide and MLN8237. The control group received saline. Mice were assessed every few days for tumor volume measurements, weight change, and general appearance. Mice were euthanized if xenografts exceeded 10% of total body weight, caused obvious discomfort or impaired ambulation, or if mice lost more than 30% of their baseline body weight. The survival time was estimated using the method of Kaplan-Meier, and the tumor response assessment was performed using the paired t test. P value of < 0.05 was deemed statistically significant.
3. Results
3.1. AURKA inhibitor suppresses growth of Myc-overexpressing tumors in EμMyc mice as well as chemosensitive and chemoresistant human lymphoma cells
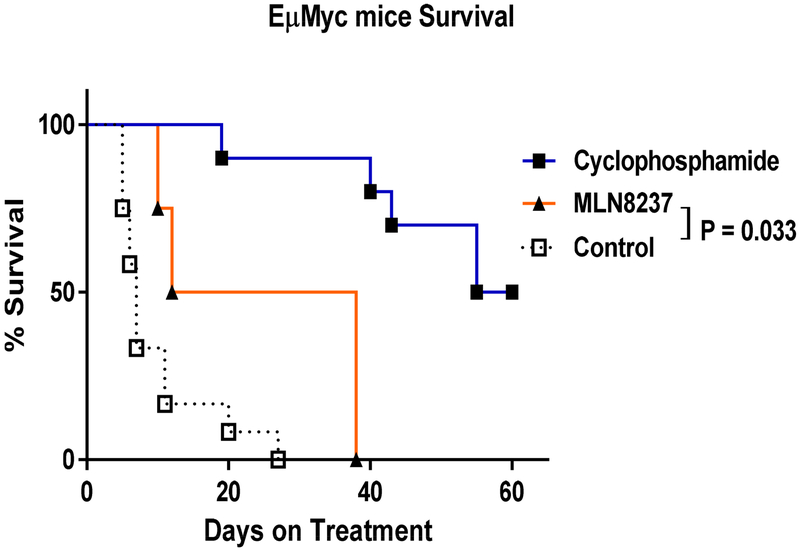
Given the importance of AURKA in Myc-overexpressing cells, we first tested MLN8237 in pilot studies using EμMyc genetically-engineered mouse model (GEMM), which generates Myc-overexpressing lymphoma spontaneously. At the presence of palpable lymph nodes, mice were randomly assigned to the no treatment, cyclophosphamide-treated (100 mg/kg via intraperitoneal injection weekly up to 6 times), or MLN8237-treated (20 mg/kg/day orally up to 4 weeks) groups (n = 4 to 12), and survival rates were compared (Fig. 2). The median survival for mice treated with cyclophosphamide and MLN8237 were 58 and 25 days, respectively, compared to 7 days in untreated mice, hence significantly improving median survival of the treated mice (p < 0.0001).
Figure 2.
Survival of EμMyc mice treated with cyclophosphamide or MLN8237. Mice each received cyclophosphamide (100 mg/kg/week via intraperitoneal injection), MLN8237 (50 mg/kg/day orally), or the combination of cyclophosphamide and MLN8237 when palpable tumors were first palpated. The control group received no treatment.
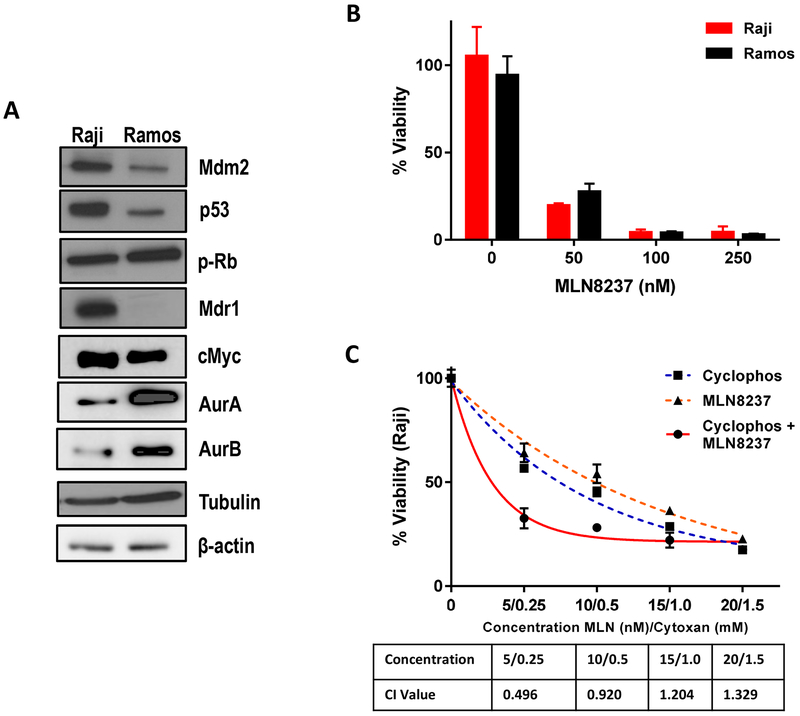
Raji and Ramos human lymphoma cell lines, which overexpress Myc, were then identified as chemoresistant and chemosensitive tumors, respectively, based on our previous pilot xenograft experiments. Raji cells were characterized by increased expression of multidrug resistant protein 1 (MDR1) and mutated p53 (Fig. 3a).31,32 The total Aurora kinase and Myc expressions were not associated with drug sensitivity to cyclophosphamide. Both cell lines were similarly sensitive to MLN8237 with maximum cytotoxicity achieved at ~ 100 nM (Fig. 3b). Combined treatment with MLN8237 and cyclophosphamide induced more significant cell growth inhibition as compared to treatment with the single agent alone in chemoresistant Raji cells (Fig. 3c). The combination index (CI) values were less than 1 at various concentrations, indicating that MLN8237 and cyclophosphamide were synergistic.
Figure 3.
Baseline characteristics of chemosensitive (Ramos) and chemoresistant (Raji) Myc-positive cell lines. (a) Protein expression patterns, including MDR1 and P53, in Ramos and Raji cells. (b) Cytotoxicity assay of MLN8237 in Ramos and Raji cells. (c) Cytotoxicity assay of cyclophosphamide, MLN8237, or the combination in chemoresistant Raji cells.
3.2. Inhibition of AURKA mediates G2/M arrest and induces caspase-independent cell death
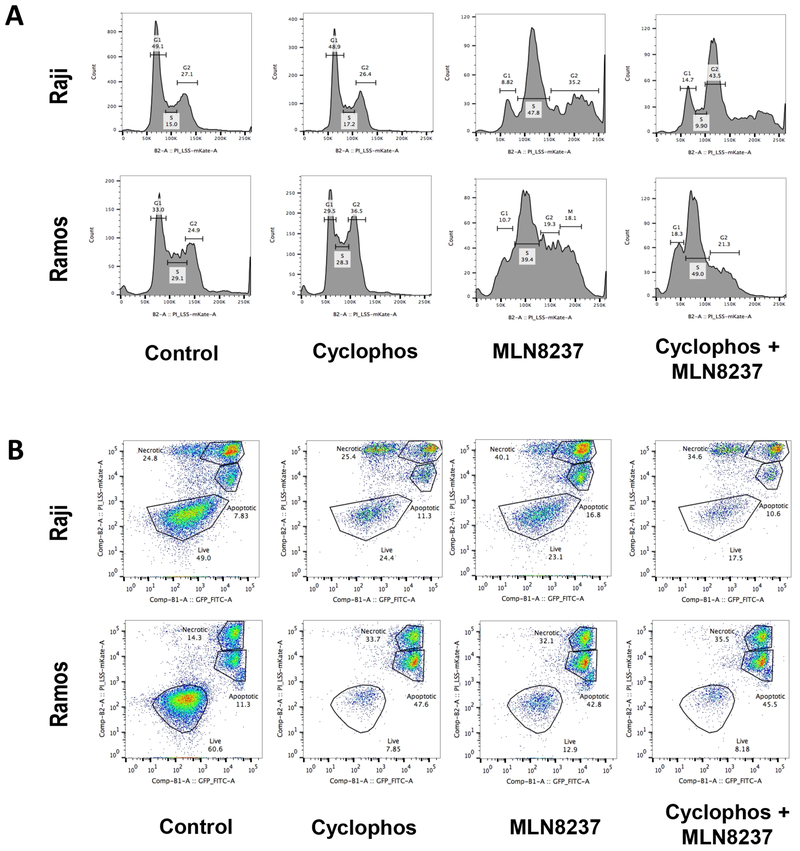
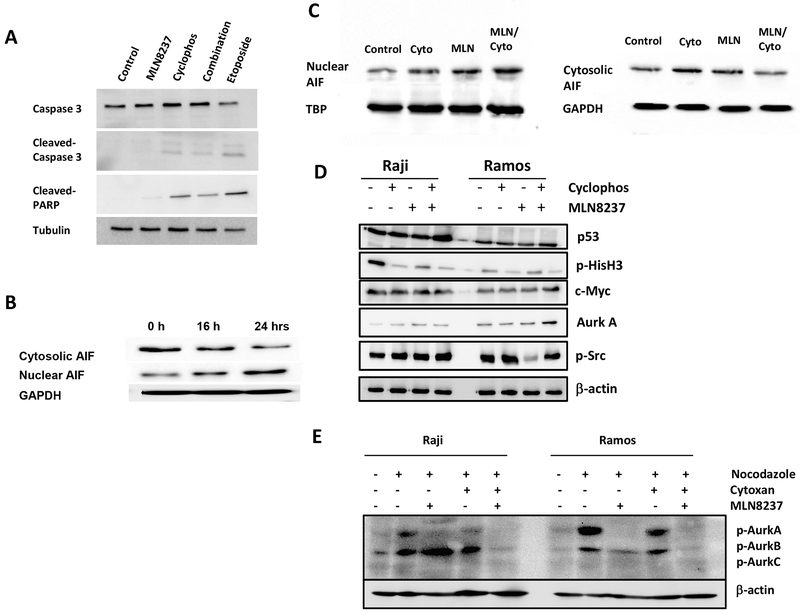
As expected, MLN8237 induced pronounced cell cycle arrest in G2/M phase, resulting in polyploidy, in both Raji and Ramos cell lines (Fig. 4a). To evaluate the effect of AURKA inhibitor on cell death, Raji and Ramos cells were treated with cyclophosphamide (1 mM), MLN8237 (100 nM), or the combination. Both cyclophosphamide and MLN8237 induced cell death independently and in combination in Myc-overexpressing cells (Fig. 4b). Synergistic or additive effect of the combination was only seen in chemoresistant Raji cells, but not in chemosensitive Ramos cells, as expected. Caspase assays by immunoblotting demonstrated that AURKA inhibition by MLN8237 induced caspase-independent cell death (Fig. 5a). The nuclear apoptosis inducing factor (AIF) protein level increased over time while the cytosolic/mitochondrial AIF level decreased when Raji cells were treated with MLN8237, further supporting a caspase-independent cell death process associated with AURKA inhibition (Fig. 5b, c). The effect on the AIF levels were more pronounced when cyclophosphamide was combined with MLN8237 (Fig 5a,b). Immunoblot assays revealed no significant changes in Myc, total AURKA, and p53 levels while p-Histone3-Ser10, p-AURKA, and p-AURKB levels decreased when Raji and Ramos cells were treated with MLN8237 for 24 hours (Fig. 5d,e).
Figure 4.
The effect of AURKA inhibition and/or cyclophosphamide on cell cycle and apoptosis in chemosensitive (Ramos) and chemoresistant (Raji) Myc-positive cell lines. (a) DNA profiles of Ramos or Raji cells treated with PBS control, cyclophosphamide, MLN8237, or combination of cyclophosphamide and MLN8237. (b) Induction of apoptosis measured by flow cytometry with Annexin V FITC in Ramos or Raji cells treated with PBS control, cyclophosphamide, MLN8237, or combination of cyclophosphamide and MLN8237.
Figure 5.
AURKA inhibition induces caspase-independent apoptosis. (a) Induction of caspase-3 and PARP cleavage by immunoblotting in Raji cells treated with PBS (negative control), cyclophosphamide, MLN8237, combination of cyclophosphamide and MLN8237, or etoposide (positive control) at 24 hours. (b) Nuclear and cytosolic AIF in Raji cells treated with PBS control, cyclophosphamide, MLN8237, or combination of cyclophosphamide and MLN8237. (c) Cytosolic and nuclear AIF levels over time in Raji cells treated with MLN8237 at 8, 16, and 24 hours. (d) c-Myc, total AURKA, Src, and p53 protein levels in Raji cells treated with PBS control, cyclophosphamide, or MLN8237 at 24 hours. (e) p-AURK A, B, and C levels in Raji cells treated with PBS control, nocodazole (p-AURK inducer), cyclophosphamide, and/or MLN8237 at 24 hours.
3.3. AURKA inhibitor reverses autophagy induced by chemotherapy
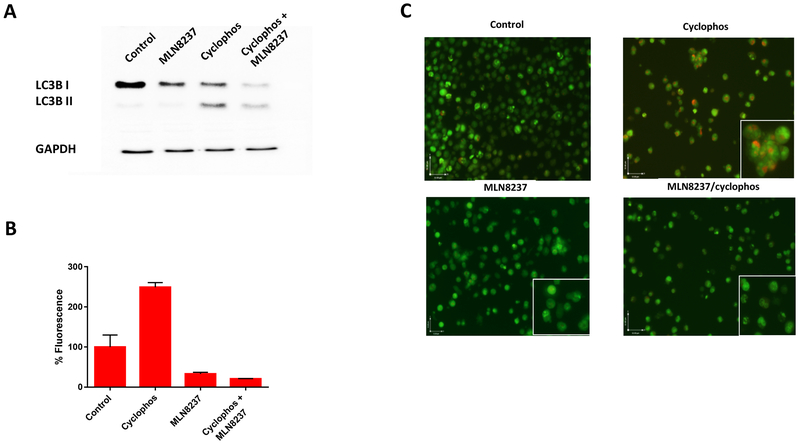
Raji cells exhibited increased protein expression of LC3B-II, indicating increased autophagy, when treated with cyclophosphamide for 48 hours (Fig. 6a). The increased LC3B-II level was abolished by the co-treatment of MLN8237. The autophagy activity was further evaluated by measuring autophagic flux in Raji cells using a cationic amphiphilic tracer dye that selectively labels autophagic vacuoles. The % fluorescence of autophagy influx significantly increased when the cells were treated with cyclophosphamide, but treatment with MLN8237 with or without cyclophosphamide dramatically decreased the % fluorescence of autophagy influx (Fig. 6b). Lastly, Raji cells were stained with acridine orange, which showed accumulation of acidic vesicular organelles when treated with cyclophosphamide (Fig. 6c). The acridine orange staining was not apparent when cells were treated with MLN8237 even in the presence of cyclophosphamide, further supporting the evidence that MLN8237 abrogates the cellular response by tumors, which may induce autophagy as a survival mechanism to overcome cytotoxic effect of cyclophosphamide.
Figure 6.
AURKA inhibition reverses autophagy. (a) LC3B-II levels by immunoblotting in chemoresistant (Raji) cells treated with PBS (negative control), cyclophosphamide, MLN8237, or combination of cyclophosphamide and MLN8237, or etoposide (positive control) at 48 hours. (b) % Fluorescence of autophagy influx measured by flow cytometry. (c) Representative photomicrograph (400 X) of autophagy levels in Raji cells stained with after treatment acridine orange staining with PBS (negative control), cyclophosphamide, MLN8237, or combination of cyclophosphamide and MLN8237. The accumulation of acidic vesicular organelles, which emit bright red/orange fluorescence correspond to autophagy activity.
3.4. AURKA inhibition results in global suppression of kinome activity in Myc-overexpressing tumor
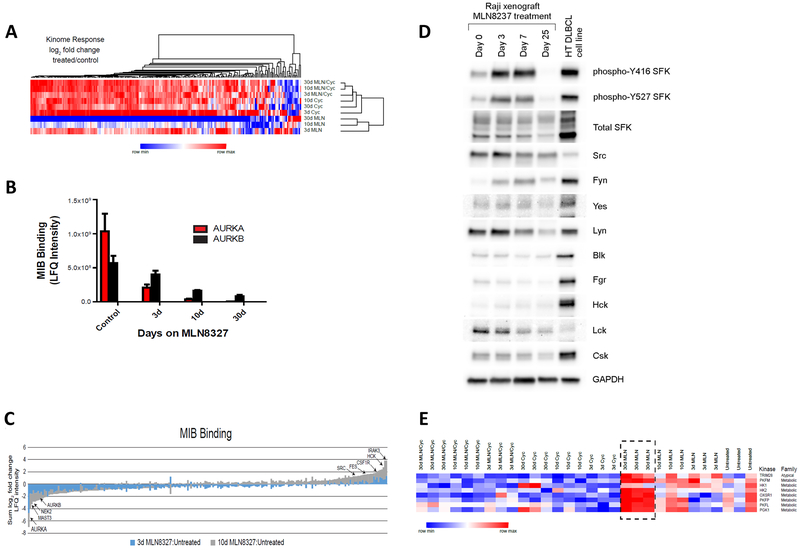
Raji tumors (n = 3) were collected and kinome profiles were assessed by multiplexed inhibitor bead/mass spectrometry (MIB/MS) after treatment with MLN8237, cyclophosphamide, or the combination. Binding to the type I kinase inhibitor beads is influenced by kinase activity, expression, and relative affinity for the inhibitors.29,33 The kinome profiles clustered strongly based on the type and duration of treatment received (Fig. 7a). MLN8237 induced profound suppression of kinase binding while cyclophosphamide was associated with overall increased MIB binding. As expected, AURKA and AURKB binding decreased with longer durations of treatment with MLN8237, and AURKA level was undetectable after 30 days of MLN8237 therapy (Fig. 7b,c). In addition to AURKA and AURKB, binding of the mitotic kinase NEK2 and microtubule-associated serine/threonine kinase (MAST3) was substantially lost in response to MLN8237 therapy (Fig. 7d). The kinome profiles were further evaluated to identify any potential compensatory mechanisms by tumor cells in response to AURKA inhibition, and multiple kinases emerged as potential candidates. Notably, Src kinase level increased significantly by Day 3 of MLN8237 therapy although the level decreased after Day 7 (Fig. 7c, d). A number of immune-associated Src family kinase members, including Fes and Hck, as well as IRAK3 and CSF1R followed the same pattern, suggesting a potential role of initial B-cell receptor pathway activation in response to AURK inhibition (Fig. 7d). Only a small number of kinases were activated after a prolonged exposure to MLN8237, and most activated kinases were involved in metabolic pathways, such as phosphofructokinases and pyruvate kinase (Fig. 7e), which warrants future investigations.
Figure 7.
Ex-vivo kinome profile and protein expression of Raji xenograft tumors. (a) MIB/MS kinome profile of Raji xenograft tumors on treatment with cyclophosphamide, MLN8237, or combination of cyclophosphamide and MLN8237 for 3, 10, or 30 days. Average, mean-centered log2 fold change was used for unsupervised hierarchical clustering. (b) MIB binding (LFQ intensity) focused on AURK A and B in Raji xenograft tumors on treatment with MLN8237 for 3, 10, or 30 days. (c) The sum log2 fold changes in response to MLN8237 treatment on days 3 and 10 in Raji xenograft tumors are shown as a stacked bar plot. (d) Src family protein expression levels by immunoblotting in Raji xenograft tumors on treatment with MLN8237 for 0, 3, 7, or 25 days. HT DLBCL cell line was used as a positive control for Src overexpression. (e) Kinome expression profile (log2 fold change versus untreated) focused on the kinases upregulated in response to MLN8237 treatment on days 3, 10, and 30 in Raji xenograft tumors.
3.5. AURKA inhibitor suppresses growth of Myc-overexpressing tumors in lymphoma xenograft models and overcomes chemoresistance.
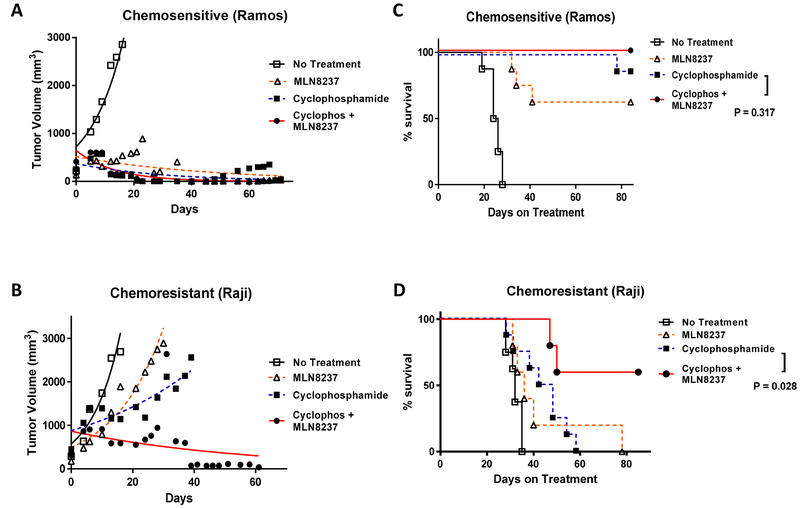
In vivo studies were performed to evaluate the therapeutic efficacy of MLN8237 using two different xenograft models with chemosensitive (Ramos) and chemoresistant (Raji) tumors. Female athymic mice were injected subcutaneously with tumor cells (10 × 106), resulting in development of large palpable tumors (~100 mm3) 7 to 10 days after tumor cell inoculation. Mice were randomized into 4 different treatment groups. Treatment groups of mice (n = 5 to 10) each received saline (negative control), cyclophosphamide (100 mg/kg via intraperitoneal injection weekly up to 6 times), MLN8237 (50 mg/kg/day orally up to 4 weeks), or the combination of cyclophosphamide and MLN8237. As expected, the untreated control mice showed exponential growth of the tumor, requiring euthanasia in all mice by day 35. In the chemosensitive Ramos xenograft model, the mean tumor volumes for cyclophosphamide, MLN8237, and the combination groups were 169.4 ± 20.9, 238.9 ± 52.5, and 125.5 ± 42.4 mm3, respectively, on day 19 with no statistical differences among the treatment groups (Fig. 8a). In comparison, mice bearing Raji xenograft showed only modest tumor regression after a single agent therapy with either cyclophosphamide or MLN8237 with the mean tumor volumes of 1499 ± 154.6 and 1587 ± 290 mm3, respectively, on day 21 (Fig. 8b). However, when mice with Raji tumors were treated with the combination of cyclophosphamide and MLN8237, the mean tumor volumes were significantly smaller with the mean tumor volume of 530.9 ± 122.9 mm3 (p = < 0.0001). Complete tumor regressions were achieved in 60% of mice bearing Raji tumors by Day 60 of the combined therapy. Furthermore, survival rates of mice with the chemosensitive and chemoresistant tumors were evaluated (Fig. 8c, d). Cyclophosphamide alone was sufficient to extend the survival in the majority of mice bearing Ramos xenograft, resembling the survival pattern of the EμMyc genetically engineered mouse model. The addition of MLN8237 to cyclophosphamide did not improve the survival rate in the chemosensitive model (p = 0.317). In the chemoresistant Raji xenograft model, a single agent therapy with either cyclophosphamide or MLN8237 only induced modest improvement in survival with the median survival of 45 and 36 days, respectively, compared to the control (32 days). The addition of MLN8237 to cyclophosphamide significantly improved the survival of mice bearing Raji tumors compared to the single agent cyclophosphamide (p = 0.028). The median survival was not reached at Day 80 for mice treated with the combination therapy in the chemoresistant Raji xenograft model. There was no evidence of significant toxicity or weight loss in all treated mice (data not shown), and all deaths were due to tumor progression.
Figure 8.
Tumor response and survival of mice bearing lymphoma xenografts. Tumor growth of (a) chemosensitive (Ramos) and (b) chemoresistant (Raji) xenografts in athymic treated with saline (negative control, ◻), cyclophosphamide (∎), MLN8237 (Δ), or combination of cyclophosphamide and MLN8237 (●). The data points represent mean values with the standard error of the mean (SEM) curves. The tumor response assessment was performed using the paired t test. P value of < 0.05 was deemed statistically significant. Survival of mice bearing (c) chemosensitive (Ramos) and (d) chemoresistant (Raji) xenografts treated with saline (negative control, ◻), cyclophosphamide (∎), MLN8237 (Δ), or combination of cyclophosphamide and MLN8237 (●). The survival time was estimated using the method of Kaplan-Meier.
4. Discussion
Aberrant expression of Myc proto-oncogene is the major genetic event in Burkitt lymphoma (BL) as well as other subtypes of NHL, including DLBCL. BL and DLBCL have distinct mutational patterns, and unlike BL, Myc rearrangements usually arise as part of complex karyotypic alterations in DLBCL, conferring chemoresistance and poor prognosis.34,35 The 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms subsequently incorporated a new category of lymphoma, termed high-grade B-cell lymphoma with translocations involving Myc and Bcl-2 or Bcl-6, which is also called double-hit lymphomas.36 Myc amplifications may occur without gene rearrangements, and Myc overexpression, especially in the presence of Bcl-2 overexpression, has been also associated with worse survival outcomes.37 Given the role of Myc as an oncogenic transcriptional factor, targeting signaling pathways involved in Myc expression is an attractive approach for treating aggressive lymphoma harboring Myc overexpression.
The Aurora kinases, a family of serine/threonine kinases, have been closely linked to Myc expression in a number of ways. AURKA and AURKB have shown to be upregulated by Myc and play important roles in maintaining the malignant state in Myc-overexpressing tumors.26,38 MLN8237 is a small molecule inhibitor of AURKA, which has been shown to induce defects in mitotic spindle assembly, cell cycle arrest at G2/M phase, and cell death in various tumors, including lymphoma.28,39,40 Recent data have also shown that Myc sensitizes tumor cells to antimitotic agents by regulating expression of the apoptotic network involved in mitotic cell fate.41 Therefore, targeting AURKA by MLN8237 would be especially relevant in tumors with high expression of Myc. In the current study, we demonstrated that MLN8237 overcame chemoresistance in Myc-overexpressing tumors via caspase-independent cell death and inhibition of autophagy. MLN8237 demonstrated significant therapeutic activity in tumor cells regardless of their MDR protein and p53 mutational status. This is in agreement with studies showing AURKA as a potential drug target in chemoresistant solid tumors, such as triple-negative breast cancer, hepatocellular carcinoma, and neuroblastoma.27,42,43 A pan-AURK inhibitor, AMG900, showed anti-tumor activity in chemoresistant tumor cells with g-glycoprotein expression,43 and in a similar fashion, MLN8237 demonstrated significant anti-tumor activity in chemoresistant lymphoma cells irrespective of the MDR1 expression status in the current study. Alkylating agents and AURK inhibitors are thought to share similar cytotoxic mechanisms by inducing mitotic catastrophe via DNA damage and cytokinesis failure, respectively.44 Consistent with results from previous studies,28,45 AURK inhibition by MLN8237 induced cell cycle arrest at the G2/M phase in Myc-overexpressing lymphoma cells in our study.
We further evaluated potential mechanisms by which AURK inhibition might overcome chemoresistance. Interestingly, we detected no significant short-term changes in Myc protein expression or caspase activation triggered by MLN8237 within 24 hours of treatment in vitro. However, nuclear AIF level increased while cytosolic AIF level decreased when the tumor cells were treated with MLN8237, further supporting that AURKA inhibition induces cell death in a caspase independent manner. Autophagy is a complex process for degradation of damaged proteins and recycling of intracellular components. The role of autophagy in tumor remains poorly understood although it is known to be dysregulated in a number of malignancies, including lymphoma.46 Autophagy may suppress tumorigenesis in some settings, but it could also enhance stress tolerance by the tumor to enhance survival under adverse conditions, such as chemotherapy.47 In addition, induction of autophagy has been associated with tumor survival and chemoresistance in multiple tumor models.48,49 In our lymphoma model, treatment with cyclophosphamide induced autophagy, supported by the increased levels of LC3B-II, autophagy influx, and acridine orange stains. However, addition of MLN8237 reversed this phenomenon and normalized the autophagy activity in the cyclophosphamide-treated tumor cells, indicating that reversal of autophagy by AURK inhibition might play a role in overcoming chemoresistance by tumor cells. Furthermore, we evaluated the kinome response to AURKA inhibition by Myc-overexpressing lymphoma by MIB/MS assay. As expected, MLN8237 most prominently reduced the activities of AURKs as well as other cell cycle-associated kinases. More notably, there was a global suppression of kinases (> 90% of kinases evaluated) with exceptions of few kinases involved in metabolic pathways, which became activated in response to AURK inhibition. Expression of Myc is known to play a critical role in tumor metabolism, but the role of AURK in tumor metabolism has not been established. Further studies are needed to examine if tumor cells respond to AURK inhibition by activating alternative metabolic pathways as a survival mechanism.
Lastly, MLN8237 in combination with cyclophosphamide was evaluated in murine Myc-positive lymphoma models. Myc-rearrangement, especially in the presence of concurrent Bcl-2 and/or Bcl-6 rearrangements, is associated with extremely poor prognosis in aggressive lymphoma. The estimated survival for patients with double-hit lymphoma is approximately 30% when treated with R-CHOP (rituximab, cyclophosphamide, vincristine, prednisone), which is considered standard for most aggressive B-NHL types, indicating the chemoresistant nature of the disease. In our genetically engineered mouse and xenograft models bearing Myc-positive lymphoma, MLN8237 demonstrated strong anti-tumor activities. Based on these encouraging preclinical data, we conducted a phase 1/1b clinical trial to investigate the safety and efficacy of MLN8237 in combination with R-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) in patients with Myc-positive aggressive B-cell NHL, defined by the presence of Myc and Bcl-2 or Bcl-6 rearrangements (ClinicalTrials.gov #). Unfortunately, the study was discontinued prematurely when the study sponsor suspended the development of MLN8237 across all indications. However, one patient with double-hit lymphoma on this clinical study completed the entire course of therapy with no non-hematologic adverse events ≥ grade 3. The treatment led to a complete response and ongoing sustained remission for > 24 months. Although the clinical development of MLN8237 is currently on hold, other AURK inhibitors are under investigation in various phases of preclinical and clinical studies. For example, AMG-900 (Amgen, Thousand Oaks, CA) demonstrated manageable toxicity with anti-tumor activity in patients with relapsed or refractory solid tumor.50 ENMD-2076 (CASI Pharmaceuticals, Rockville, MD) is a multi-kinase inhibitor targeting various pathways, including AURKA, and a phase 2 study of the drug was completed in patients with relapsed or refractory solid tumor (ClinicalTrials.gov #). ABT348 is another multi-kinase inhibitor with an inhibitory effect on AURK, and it is currently in phase 2 studies for relapsed or refractory solid tumors (ClinicalTrials.gov #). Lastly, AZD2811 (AstraZeneca, Cambridge, UK) is a nanoparticle formulation of an AURKB inhibitor, and it is currently in phase 1 trials for use in hematological and solid tumors (ClinicalTrials.gov # and ).
5. Conclusion
In summary, AURKA inhibition by MLN8237 induces synthetic lethality and overcomes chemoresistance in Myc-overexpressing tumors. The synergistic effect was mainly through caspase-independent apoptotic pathway and reversal of autophagy in response to conventional chemotherapy drug. The combination of MLN8237 and a cyclophosphamide-containing chemotherapy showed promising safety and anti-tumor activities in animal models of Myc-positive B-cell NHL, and further investigation of this combination is warranted.
Key Points:
MLN8237, an aurora A kinase inhibitor, demonstrated anti-tumor activity in Myc-overexpressing lymphoma cells regardless of their sensitivity to chemotherapy.
AURKA inhibition induces caspase-independent apoptosis by inhibiting autophagy by the tumor in response to chemotherapy.
MLN8237 and cyclophosphamide show synergistic effect and improve outcomes in preclinical animal models of Myc-overexpressing lymphoma.
Acknowledgments
FUNDING: This study was funded by the American Cancer Society Mentored Research Scholar Grant in Tumor Biology and Genomics (126601-MRSG-14-215-01-TBG).
CONFLICT OF INTEREST: Steven Park reports receiving research funding from Seattle Genetics, Teva, Takeda, and BMS, membership of speakers bureau for Gilead and Seattle Genetics, and membership on Board of Directors or advisory committee for Rafael Pharmaceuticals, BMS, and Teva (advisory boards). Carolina Lin, Natalie Ren, Steven Angus, Dirk Dittmer, Michael Foote, Trevor Parton, Aadra Bhatt, Yuri Fedoriw, Daniel Roth, Marissa Cann, Gary Johnson, and Blossom Damania have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2015. CA Cancer J Clin 65:5–29, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Fisher RI, Gaynor ER, Dahlberg S, et al. : Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 328:1002–6, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Vose JM: Current approaches to the management of non-Hodgkin’s lymphoma. Semin Oncol 25:483–91, 1998 [PubMed] [Google Scholar]
- 4.Barrans S, Crouch S, Smith A, et al. : Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 28:3360–5, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Savage KJ, Johnson NA, Ben-Neriah S, et al. : MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 114:3533–7, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Klapper W, Stoecklein H, Zeynalova S, et al. : Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B-cell lymphomas treated within randomized trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Leukemia 22:2226–9, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Nesbit CE, Tersak JM, Prochownik EV: MYC oncogenes and human neoplastic disease. Oncogene 18:3004–16, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Chang CC, Liu YC, Cleveland RP, et al. : Expression of c-Myc and p53 correlates with clinical outcome in diffuse large B-cell lymphomas. Am J Clin Pathol 113:512–8, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Pagnano KB, Vassallo J, Lorand-Metze I, et al. : p53, Mdm2, and c-Myc overexpression is associated with a poor prognosis in aggressive non-Hodgkin’s lymphomas. Am J Hematol 67:84–92, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Saez AI, Artiga MJ, Romero C, et al. : Development of a real-time reverse transcription polymerase chain reaction assay for c-myc expression that allows the identification of a subset of c-myc+ diffuse large B-cell lymphoma. Lab Invest 83:143–52, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Rimsza LM, Leblanc ML, Unger JM, et al. : Gene expression predicts overall survival in paraffin-embedded tissues of diffuse large B-cell lymphoma treated with R-CHOP. Blood 112:3425–33, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuccuini W, Briere J, Mounier N, et al. : MYC+ diffuse large B-cell lymphoma is not salvaged by classical R-ICE or R-DHAP followed by BEAM plus autologous stem cell transplantation. Blood 119:4619–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirel HA, Cairo MS, Heerema NA, et al. : Specific cytogenetic abnormalities are associated with a significantly inferior outcome in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Leukemia 23:323–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CY, Loven J, Rahl PB, et al. : Transcriptional Amplification in Tumor Cells with Elevated c-Myc. Cell 151:56–67, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie Z, Hu G, Wei G, et al. : c-Myc Is a Universal Amplifier of Expressed Genes in Lymphocytes and Embryonic Stem Cells. Cell 151:68–79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felsher DW, Bishop JM: Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell 4:199–207, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Felsher DW, Bishop JM: Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc Natl Acad Sci U S A 96:3940–4, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinkovic D, Marinkovic T, Mahr B, et al. : Reversible lymphomagenesis in conditionally c-MYC expressing mice. Int J Cancer 110:336–42, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Weinstein IB: Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science 297:63–4, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Giuriato S, Ryeom S, Fan AC, et al. : Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proc Natl Acad Sci U S A 103:16266–71, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalla-Favera R, Bregni M, Erikson J, et al. : Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A 79:7824–7, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair SK, Burley SK: X-ray structures of Myc-Max and Mad-Max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors. Cell 112:193–205, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Jeong KC, Ahn KO, Yang CH: Small-molecule inhibitors of c-Myc transcriptional factor suppress proliferation and induce apoptosis of promyelocytic leukemia cell via cell cycle arrest. Mol Biosyst 6:1503–9 [DOI] [PubMed] [Google Scholar]
- 24.Prochownik EV, Vogt PK: Therapeutic Targeting of Myc. Genes Cancer 1:650–659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hata T, Furukawa T, Sunamura M, et al. : RNA interference targeting aurora kinase a uppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res 65:2899–905, 2005 [DOI] [PubMed] [Google Scholar]
- 26.den Hollander J, Rimpi S, Doherty JR, et al. : Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood 116:1498–505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dauch D, Rudalska R, Cossa G, et al. : A MYC-aurora kinase A protein complex represents an actionable drug target in p53-altered liver cancer. Nat Med 22:744–53, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Gorgun G, Calabrese E, Hideshima T, et al. : A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood 115:5202–13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan JS, Whittle MC, Nakamura K, et al. : Dynamic Reprogramming of the Kinome in Response to Targeted MEK Inhibition in Triple-Negative Breast Cancer. Cell 149:307–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson WH, Grossbard ML, Pittaluga S, et al. : Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood 99:2685–93, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Duthu A, Debuire B, Romano J, et al. : p53 mutations in Raji cells: characterization and localization relative to other Burkitt’s lymphomas. Oncogene 7:2161–7, 1992 [PubMed] [Google Scholar]
- 32.Vousden KH, Crook T, Farrell PJ: Biological activities of p53 mutants in Burkitt’s lymphoma cells. J Gen Virol 74 (Pt 5):803–10, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Zawistowski JS, Bevill SM, Goulet DR, et al. : Enhancer Remodeling during Adaptive Bypass to MEK Inhibition Is Attenuated by Pharmacologic Targeting of the P-TEFb Complex. Cancer Discov 7:302–321, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hummel M, Bentink S, Berger H, et al. : A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med 354:2419–30, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Love C, Sun Z, Jima D, et al. : The genetic landscape of mutations in Burkitt lymphoma. Nat Genet 44:1321–5, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swerdlow SH, Campo E, Pileri SA, et al. : The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375–90, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson NA, Slack GW, Savage KJ, et al. : Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30:3452–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz RJ, Golbourn B, Faria C, et al. : Mechanism of action and therapeutic efficacy of Aurora kinase B inhibition in MYC overexpressing medulloblastoma. Oncotarget 6:3359–74, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahadevan D, Stejskal A, Cooke LS, et al. : Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non-Hodgkin lymphoma. Clin Cancer Res 18:2210–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sehdev V, Peng D, Soutto M, et al. : The aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells. Mol Cancer Ther 11:763–74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Topham C, Tighe A, Ly P, et al. : MYC Is a Major Determinant of Mitotic Cell Fate. Cancer Cell 28:129–40, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaelis M, Selt F, Rothweiler F, et al. : Aurora kinases as targets in drug-resistant neuroblastoma cells. PLoS One 9:e108758, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payton M, Bush TL, Chung G, et al. : Preclinical evaluation of AMG 900, a novel potent and highly selective pan-aurora kinase inhibitor with activity in taxane-resistant tumor cell lines. Cancer Res 70:9846–54, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Mc Gee MM: Targeting the Mitotic Catastrophe Signaling Pathway in Cancer. Mediators Inflamm 2015:146282, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li JP, Yang YX, Liu QL, et al. : The investigational Aurora kinase A inhibitor alisertib (MLN8237) induces cell cycle G2/M arrest, apoptosis, and autophagy via p38 MAPK and Akt/mTOR signaling pathways in human breast cancer cells. Drug Des Devel Ther 9:1627–52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.McCarthy A, Marzec J, Clear A, et al. : Dysregulation of autophagy in human follicular lymphoma is independent of overexpression of BCL-2. Oncotarget 5:11653–68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Degenhardt K, Mathew R, Beaudoin B, et al. : Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10:51–64, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belounis A, Nyalendo C, Le Gall R, et al. : Autophagy is associated with chemoresistance in neuroblastoma. BMC Cancer 16:891, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sui X, Chen R, Wang Z, et al. : Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4:e838, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carducci M, Shaheen M, Markman B, et al. : A phase 1, first-in-human study of AMG 900, an orally administered pan-Aurora kinase inhibitor, in adult patients with advanced solid tumors. Invest New Drugs 36:1060–1071, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]