Abstract
Fx-5A peptide complex (Fx-5A), a High Density Lipoproteins (HDL) mimetic, has been shown to reduce atherosclerosis. The safety and toxicokinetics of Fx-5A administered IV by 30 min infusion at 8, 25 or 75 mg/kg body weight or vehicle, once every other day for 27 days, were assessed in cynomolgus monkeys. The Fx-5A was well tolerated at all doses. At the highest dose, there were statistically significant effects on hematology and clinical chemistry parameters that were considered non-adverse. Dose-dependent recoverable non-adverse erythrocytes morphological changes (acanthocytes, echinocytes, spherocytes, microcytes, and/or schistocytes) were observed. Fx-5A was not hemolytic in in-vitro fresh NHP or human blood assay. There were no Fx-5A-related statistically significant changes for any cardiovascular function, ECG or respiratory parameters, when compared to control. In addition, there were no Fx-5A-related effects on organ weights, macroscopic or microscopic endpoints. Finally, Fx-5A exhibited sporadic non-appreciable detection of anti-Fx-5A antibodies and a dose-dependent linear toxicokinetics with T1/2 value ranges from 2.7 to 6.2 hrs. In conclusion, the No Observed Adverse Effect Level was considered to be 75 mg/kg/day with associated exposures average Cmax and AUC0-last of 453 μg/mL and 2232 hr·μg/mL, respectively, on Day 27.
Keywords: Atherosclerosis, HDL mimetic, Fx-5A peptide complex, safety, Cynomolgus Monkeys
1. INTRODUCTION
Cholesterol associated with High Density Lipoprotiens (HDL) are inversely related to Coronary Heart Disease (CHD) and hence HDL has been a target for drug development. HDL has several potential anti-atherogenic functions, but the best understood is its ability to promote the efflux of cellular cholesterol, and hence promote reverse cholesterol transport, the pathway by which excess cellular cholesterol is removed from peripheral cells and returned to the liver for exretion or conversion to bile salts (Remaley et al., 2008).
There are several synthetic or reconstituted form of HDL that have been produced and shown to rapidly reduce atherosclerotic plaque in animal models and have been tested in early stage clinical trials (Remaley et al., 2008; Uehara et al., 2015). HDL mimetic therapy can be separated into 2 general approaches, one that uses the full length human apolipoprotein A-I (ApoA-I), the main associated with HDL, or those that use apoA-I mimetic peptides to make reconstituted HDL. Besides wild type ApoA1, ApoA1- Milano, a natural mutation, and trimeric forms of ApoA1 have been developed. Clinical studies with reconstituted and purified ApoA1 (CSL-111) were discontinued based on safety concerns, but it has been reformulated (CSL-112) (Gille et al., 2014; Tardif et al., 2007). CSL-112 was well tolerated and is now in phase III clinical trials (https://clinicaltrials.gov/ct2/show/NCT03473223?term=CSL-112&rank=1). MDCO-216, a complex of dimeric recombinant ApoA-I Milano (apoA-IM) and palmitoyl-oleoyl-phosphatidylcholine (POPC), was administered to cynomolgus monkeys at 30, 100, and 300 mg/kg every other day for a total of 21 infusions (Kempen et al., 2013). Free cholesterol (FC) and phospholipids (PL) were strongly increased, and HDL-cholesterol (HDL-C), ApoA-I, and ApoA-II were strongly decreased (Kempen et al., 2013). In early clinical trials, ApoA1 Milano also showed promises; a single infusion of MDCO-216 (ApoA-1 Milano/ POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease and was well tolerated (Kallend et al., 2016). A later larger study, however, failed to show reduction in atherosclerotic plaque as determined by Intravascular Ultrasound (https://clinicaltrials.gov/ct2/show/NCT02678923?term=apoAI+milano&rank=1). ApoA-I, was also fused to the trimerization domain of tetranectin (TN) and complexed with phospholipids to generate an HDL mimetic (lipidated TN-ApoA-I) with reduced renal clearance and enhanced efficacy (Regenass-Lechner et al., 2016). Cynomolgus monkeys received 24-h IV infusions of control, 100mg/kg or 400mg/kg lipidated TN-ApoA-I every 4 days for 3 weeks, followed by a 6-week recovery period. The multiple infusions of lipidated TN-ApoA-I resulted in high immunogenicity, lipid accumulation and were not well tolerated in nonhuman primates and hence not further developed (Regenass-Lechner et al., 2016). CER-001, a recombinant human ApoA-I pre-beta HDL mimetic, is a complex between recombinant human ApoA-I and two phospholipids (dipalmitoyl phosphatidylcholine and sphingomyelin). CER-001 has been shown to be safe and well tolerated and is currently in a phase III clinical trial for patients with a genetic HDL deficiency ("TANGO") (https://clinicaltrials.gov/ct2/show/NCT02697136 ). CER-001 related publications have reported results in patients post-acute coronary syndrome (post-ACS, CHI-SQUARE study, CARAT study) (Andrews et al., 2017; Nicholls et al., 2018), patients with familial hypercholesterolemia (FH, MODE study) (Hovingh et al., 2015), patients with HDL deficiencies (SAMBA study) (Kootte et al., 2015), and patients with advanced atherosclerosis (LOCATION Study) (Zheng et al., 2016).
Several small (<40 residues) peptide mimetics of ApoA-I have also been previously developed and examined in animals and humans. ESP24218 produced by Esperion was the first apolipoprotein mimetic peptide to reach clinical development (Khan et al., 2003; Miles et al., 2004). This peptide was combined with two naturally occurring phospholipids, sphingomyelin and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC0) and the resulting complex is referred to as ETC-642. A phase I clinical study of ETC-642 was performed in 2002 (Khan et al., 2003; Miles et al., 2004). It was a single-dose intravenous infusion study of 28 patients with stable coronary artery disease and was designed to determine the safety and tolerability of 0.1, 0.3, 1, 3, and 10 mg/kg dose levels of ETC-642 (Khan et al., 2003). After a 4week observation period, ETC-642 was considered to be safe and well tolerated at all dose levels tested (Khan et al., 2003). The development of ETC-642 was shortly terminated in 2006 when Esperion was acquired by Pfizer (http://www.medscape.com/viewarticle/581528). D-4F and L-4F (two enantiomer) are amphipathic peptide, which promote the remodeling of endogenous HDL, the formation of pre-beta HDL (Navab et al., 2004) and possess antiinflammatory properties apparently by the sequestration of oxidized lipids (Anantharamaiah et al., 2007). D-4F was advanced into phase I clinical trials in 2008 and found to be well tolerated (Bloedon et al., 2008). A phase I clinical trial of either subcutaneous (SC) or IV administration of L-4F has been performed (Buga et al., 2010). L-4F was well tolerated when administered IV for seven daily doses with the dose range of 3–100 mg and SC for 28 daily dose of 10 and 30 mg. However, there was no apparent improvement of the HDL inflammatory index, paraoxonase activity, or other lipid measures or inflammatory markers. Subsequent animal studies with both D-4F and L-4F indicate that the main site of action of these peptides may be in the gut in blocking the absorption and/or production of oxidized lipids (Remaley, 2013), which could be a reason for the lack of an effect observed after IV administration in the phase I clinical trial.
The Fx-5A is produced by combining sphingomyelin with the 5A peptide, which is a bihelical ApoA-I mimetic peptide (Sethi et al., 2008; Tabet et al., 2010). Synthetic lipid complexes made with 5A are very effective for specifically effluxing cholesterol by the ABCA1 transporter, thus enhancing the first step in the reverse cholesterol transport pathway. Fx-5A has been shown to reduce the progression of atherosclerosis in rabbits and mice (Amar et al., 2010). Fx-5A also displays anti-inflammatory and antioxidant properties in-vivo and in-vitro (Tabet et al., 2010). Therefore, Fx-5A was considered a strong candidate for further drug development. Indeed, to support a phase I study, the safety profile and pharmacokinetic of Fx-5A was assessed in this study in cynomolgus monkeys.
2. METHODS AND MATERIALS
2.1. Fx-5A AND VEHICLE INFORMATION:
Fx-5A is a liquid solution containing peptide 5A (15 mg/mL) with egg sphingomyelin (ESM) (21.50 mg/mL)] in vehicle 20mM sodium phosphate, pH 5.1. Molar Ratio of peptide 5A to ESM was 1:8. Purity of peptide 5A (by RP-HPLC) was 97.7% with a bacterial entotoxin <0.025 EU/mg and Bioburden level < 10CFU/g. Purity of sphingomyelin (COATSOME NM-10, NOF Corporation, Japan) was 99.9% with bacterial endotoxin level 0.0 EU/g and Bioburden level < 1 CFU/g
2.2. Species & Strain:
Nonhuman Primate (NHP)/Cynomolgus, approximately 3–4 years old from Charles River. The NHPs were individually housed in an environmentally monitored, well-ventilated room maintained at a temperature of approximately 64 – 84 °F and a relative humidity of approximately 30% – 70%. Fluorescent lighting provided illumination approximately 12 hours per day. The NHPs were offered commercial 2050C Teklad Certified Global Primate Diet twice daily, with approximately half the recommended daily ration available at each feeding interval. In addition, the diet was supplemented with fresh fruit and/or treats offered daily. The quantity of the daily ration was sufficient to meet nutritional requirements. Water was supplied ad libitum, during the pre-study and study periods. The NHPs were acclimated/quarantined for a minimum of 32 days. Prior to study start, the animals were observed for general health and acceptability for use in this study. The following minimum pre-study testing was completed: TB testing, hematology and serology screening, and fecal culture and flotation test. Only animals deemed healthy were included in this study.
2.3. 28-Day IV Toxicity Study of Fx-5A followed by a 14-Day Recovery Period
2.3.1. RANDOMIZATION AND GROUP ASSIGNMENT:
To obtain groups comparable by body weight, all animals were assigned to their group using a computer-generated randomization procedure. The body weights required for randomization were determined during Week-1. After randomization, animals were assigned to their respective groups as indicated in Table 1:
Table 1.
Study Design
| Group | 5A Peptidea Dose (mg/kg/dose) | Fx-5A Dose Volumed (mL/kg)c | Number of NHPs at Study start (M+F) | Terminal Necropsy (M+F) | |
|---|---|---|---|---|---|
| Day 29 | Day 42 | ||||
| 1 | 0 | 5.000b | 10 (5+5) | 6 (3+3) | 4 (2+2) |
| 2 | 8 | 0.533 | 10 (5+5) | 6 (3+3) | 4 (2+2) |
| 3 | 25 | 1.667 | 10 (5+5) | 6 (3+3) | 4 (2+2) |
| 4 | 75 | 5.000 | 10 (5+5) | 6 (3+3) | 4 (2+2) |
dose is expressed in 5A peptide equivalent.
Volume of vehicle (20mM sodium phosphate, pH 5.1) injected
The actual dose volume (mL/kg) administered to each monkey was based on that animal’s most recent body weight
[peptide 5A (15 mg/mL) with egg sphingomyelin (ESM) (21.50 mg/mL)].
Animals were trained/acclimated to the restraint chair prior to the day of dosing and/or blood collection. NHPs were administered ketamine (10–50 mg/kg) intramuscularly in preparation for ECG and blood pressure collection, respiratory rate collection, ophthalmoscopic examination, clinical pathology collections as needed, and euthanasia.
Starting on Day 1, each animal received the appropriate treatment, as detailed in the Table 1 by IV infusion (30 minutes) once every other day for 14 consecutive doses at a dose volume based on the weight of the animal. Animals were fasted overnight prior to collection of blood samples for clinical pathology evaluation and prior to day of necropsy.
All animals were observed at least twice daily during the pre-study and study periods for signs of mortality and moribundity. All animals were observed once daily for clinical signs of toxicity without removing the animal from the cage. Each animal was removed from its cage and examined for clinical signs of toxicity daily (approximately 30 minutes after completion of dosing on dosing days), or more often as clinical signs warranted. Baseline body temperatures were taken once on Day −2 to −3. Body temperatures were measured and recorded on Days 1 and 27, approximately 2 hours after dosing. Body weights were obtained during Week-1 (randomization), prior to dosing on Day 1, and continued weekly throughout the duration of the study. Body weights were obtained prior to that day’s treatment, blood collection, and/or euthanasia. Food consumption was qualitatively measured daily, beginning on Day 1. The NHPs were sedated and had ophthalmoscopic examinations performed prior to initiation of treatment (Week -1) and prior to necropsy.
2.3.2. PLASMA 5A PEPTIDE LEVELS AND TOXICOKINETICS:
Plasma levels of 5A peptide in NHP was determined by using reverse-phase liquid chromatography with tandem mass spectrometry detection (LC-MS/MS) and Labeled 5A peptide as the internal standard (ThermoFisher, Waltham, MA). Blood samples were collected from the NHP using tubes containing lithium heparin as the anticoagulant. Blood samples were drawn at eight time points prior to dosing (0 hr) and at the following time points: immediately post dose completion, and approximately 0.5, 1, 2, 4, 8, and 24 hours post dose completion) on Days 1–2 and 27–28. Upon collection, each blood sample was mixed by gentle inversion, placed on ice, and subsequently centrifuged to separate plasma. Plasma 5A peptide level data was subjected to pharmacokinetic analysis using WinNonlin® (Version 6.3; Pharsight, A Certara Company; Cary, NC). Parameters calculated included at a minimum Cmax, Tmax, AUC, and half-life.
2.3.3. ANTI-DRUG ANTIBODY (ADA) ANALYSIS:
5A peptide, Acetate Salt (Bachem, Torrance, CA), was diluted to 0.5μg/ml in PBS and coated on 96-well flat-bottom plates (Costar EIA/RIA plates, high binding, Corning, NY). Rabbit anti-5A positive control antibody used was from Capralogics, Hardwick, MA. Blood samples (EDTA tubes) were collected during Day −2, and on Days 29, and 42. The appearance of ADA were correlated with any pharmacological and/or toxicological changes. The ADA assay was used to detect antibodies generated against Fx-5A in the serum.
2.3.4. MACROSCOPIC AND MICROSCOPIC PATHOLOGY:
Macroscopic Observations:
Included, an examination of the external surfaces of the body, all orifices of the body, and the cranial, thoracic, abdominal, and pelvic cavities and their contents. Eyes were fixed in Davidson's Solution, testes and epididymides were fixed in modified Davidson’s solution, and all other tissue/organ samples were preserved in 10% neutral buffered formalin for histopathological evaluation. The following tissues, as well as all gross lesions, were collected: Adrenal gland; Aorta; Bone (femur, stifle joint with articular surface); Bone marrow (sternum); Bone marrow (rib, costochondrial junction); Bone marrow (smear-sternum); Brain; Bronchus (mainstem); Epididymidis; Esophagus; Eye; Gallbladder; Heart; Intestine (large, cecum); Intestine (large, colon); Intestine (large, rectum); Intestine (small, duodenum); Intestine (small, jejunum); Intestine (small, ileum); Kidney; Larynx; Liver; Lungs with bronchi; Lymph node (bronchial); Lymph node (mandibular); Lymph node (mesenteric); Mammary gland; Nerve (optic); Ovary with oviducts; Pancreas; Pituitary gland; Prostate; Salivary gland (mandibular); Salivary gland, parotid; Salivary gland (sublingual); Nerve (sciatic); Skeletal muscle; Skin (abdominal); Spinal cord (cervical); Spinal cord (lumbar); Spinal cord (thoracic); Spleen; Stomach; Testis; Thymus; Thyroid gland/Parathyroid gland; Tongue; Tonsil; Trachea; Ureter; Urinary bladder; Uterus (Vagina).
Samples of each tissue listed above were taken for histological evaluation.
Organ Weights:
The organs (Adrenal gland; Brain; Heart; Kidney; Liver; Lungs with bronchi; Ovary with oviducts; Pituitary gland; Prostate; Spleen; Testis; Thymus; Thyroid/parathyroid gland) from each main study animal euthanized at scheduled necropsy and/or euthanized as moribund, were weighed before samples are collected for microscopic examination. All organs, except the adrenal, pituitary, and thyroid with parathyroid, were weighed prior to fixation. Paired organs were weighed together and their combined weight was used for the calculation of organ-to-body and organ-to-brain weight ratios.
Histology:
All tissues from control and high dose group animals (Day 29), as well as any gross lesions were processed to slides. Only target tissues and any gross lesions from the control and high dose groups (Day 42) and low and mid groups were processed to slides. The fixed tissues were trimmed, processed, and microtomed (approximately 5-μm sections). Tissue sections were mounted on glass slides, stained with hematoxylin and eosin, and a coverslip was applied. Special stains may be used at the discretion of the pathologist when necessary to establish a diagnosis. Use of any special stain was documented.
Microscopic Observations:
All slides were submitted to a veterinary pathologist for evaluation. Records of gross findings for a specimen from postmortem observations were available to the pathologist when examining that specimen histopathologically. All lesions were categorized either as test article related or non-test article related. Each lesion was listed and coded for the most specific topographic and morphologic diagnoses, severity, and distribution. A four-step grading system was used to rank the severity of microscopic lesions for comparison among groups.
2.4. Fx-5A SINGLE DOSE IV INFUSION CARDIOVASCULAR AND PULMONARY SAFETY PHARMACOLOGY STUDY IN NHP
On a given dosing day, each animal was administered either the vehicle control or Fx-5A via intravenous infusion at the dose levels indicated in Table 2:
Table 2.
Group Assignment
| Animal | Treatment | 5A Peptide a | Fx-5A |
|---|---|---|---|
| Numbers (Sex) | Dose Level (mg/kg) | Dose Volumed (mL/kg)c | |
| 101 to 103 (Males) | Control | 0 | 5.0b |
| Low Dose | 8 | 0.5 | |
| 151 to 155 (Females) | Mid Dose | 25 | 1.7 |
| High Dose | 75 | 5.0 | |
dose is expressed in 5A peptide equivalent.
Volume of vehicule (20mM sodium phosphate, pH 5.1) injected
The actual dose volume (mL/kg) administered to each monkey was based on that animal’s most recent body weight.
[peptide 5A (15 mg/mL) with egg sphingomyelin (ESM) (21.50 mg/mL)].
Each animal selected for dosing was dosed a total of four times with at least seven days of washout between dosing. The dose assignments were determined using the Latin-Square crossover design. All animals received all three doses and control in a manner that maximized the number of dosing combinations as specified below.
2.4.1. Telemetry Data Collection:
The Dataquest A.R.T. telemetry software collected systemic arterial blood pressures (systolic, diastolic and mean), heart rate, core body temperature, and ECG data continuously (except when the animals were out-of-range of the receivers for study-driven activities), at least 24 hours before the first dosing event through at least 48 hours following the last dosing event.
2.4.2. Respiratory Data Collection:
Following this initial calm-down period, baseline data were collected for at least 30 minutes. Respiratory data collection continued for approximately three hours (± 30 minutes) following dosing.
Clinical Observations:
Prior to Day 1, cage side observations were performed at least once daily. During dosing, cage side observations were performed at least twice daily. On days of dosing, cage side observations were performed prior to dosing and at least once following respiratory data collection once animals were returned to their home cage. Body weights were recorded prior to animal selection and on each day of dose administration (Days 1, 8, 15, and 22), prior to dosing. Blood was collected for hematology, serum chemistry, and coagulation prior to dosing on Days 1, 8, 15, and 22.
2.5. In-Vitro Testing of Hemolytic Potential of Fx-5A in Blood
The Fx-5A particle contains the lipid sphingomyelin. Excess lipid in blood have the potential to interfere with the hemoglobin measurement that is the key to the hemolytic potential assay by falsely increasing the measured hemoglobin concentration (Stockham and Scott, 2008). Because of the possibility that sphingomyelin may interfere with the hemoglobin assay, the hemolytic potential assessment described in this protocol was done with a positive control that allowed assessment of interference in the assay that used rat blood.
In-vitro hemolytic potential was assessed using heparinized rat, NHP, and human blood. Only fresh NHP and human blood was tested in the assay and both fresh and commercially purchased rat blood were collected from fasted animals. The assays with fresh rat and NHP blood and with the commercially purchased fresh human blood used samples from three different individuals per species; the assay with commercially purchased rat blood used a single blood sample from one animal, as described below.
The assay with NHP blood used freshly-drawn samples that were collected from animals in the in-house NHP colony and were used within 3 hours after collection. The assay with human blood used commercially purchased samples that were drawn on the same morning that the assay was conducted and were used with 3 hours after collection. The instrument used during the conduct of this study was the HemoCue Plasma/Low Hb Photometer. The instrument’s primary function was the measurement of hemoglobin concentration.
2.6. STATISTICAL ANALYSIS:
Descriptive statistics (group means and standard deviations) were calculated, as appropriate. Comparative statistical analyses of the body weight, clinical pathology, and organ weight data were performed for differences between groups was performed using Provantis. Data were evaluated for normality using the Shapiro-Wilk test and homogeneity of variance using the Levene test. Based on the results of these tests, a parametric or a non-parametric one-way ANOVA was performed followed by a post hoc pairwise test (e.g., Dunnett’s, Wilcoxon’s) as appropriate. The level of significance is p <0.05 (p <0.01 for normality and variance tests). Student t-test’s were performed using Graphpad Prism version 5.04 via a two tailed, unpaired t-test at an alpha level of p≤0.05. For the hemoglobin data values obtained from animals using fresh blood, a Student's t-test was used to evaluate differences between Fx-5A and negative control at an alpha level of p≤0.05. For the hemoglobin data values obtained from commercially purchased blood from a single animal, a Student's t-test was used to look for differences between the Fx-5A and negative control at an alpha level of p≤0.05.
3. RESULTS
3.1. 28-Day IV Toxicity Study of Fx-5A in NHP followed by a 14-Day Recovery Period
All animals survived to scheduled study termination. Few clinical observations were noted in the control groups and were not considered Fx-5A- related, even when noted in the treated animals: distended abdomen, scab on hind limb, sore/ulcer at the dose administration site, mass, discoloration, swelling, and tremor. The tremor was thought to be due to the administration of Fx-5A and the vehicle control at room temperature, which could have lowered the body temperature and caused a normal shivering response. Clinical observations noted in the Fx-5A -treated animals but not seen in the control animals included loose stool, diarrhea, blood in feces (which was considered potentially due to prolapse), alopecia, wounds, and abnormal breathing were considered incidental and not Fx-5A -related due to lack of dose response and single or sporadic occurrences. Body temperatures in the study were in the normal range, with the exception of two animals at the 25 mg/kg/dose group, with temperatures on Day 27 as 103.8 ºC and 103.0ºC. These two body temperatures were not considered to be Fx-5A-related since increased temperatures were not observed in the high dose group (75 mg/kg/dose), there were no other abnormally high temperature readings on the study, and the temperature range of 103ºC are not uncommon where the capture of the animal is stressful. There were no Fx-5A -related effects on body weight, food consumption or on ophthalmology.
3.1.1. CLINICAL PATHOLOGY:
Several clinical chemistry lab changes were observed at the 75 mg/kg/dose level. On Day 2, male NHPs, showed a decrease in total protein (TP) (−6.17%) and Alb (albumin) (−15.0%) and an increase in Chol (Cholesterol) (117%), and Trig (triglycerides) (33.08%). On Day 15, a decrease in ALB (−14.22%) and increase in Chol (43.51%) was evident and on Day 29, a decrease in ALB (−12.27%) and an increase in Chol (55.09%) was noted. For female NHPs, there was an increase in the markers associated with liver functions on Day 2 including AST, ALT, ALP, and GGT (Table 3), as well as an increase in Chol (81.53%) (Table 4).
Table 3.
Summary of Day 2 Clinical Chemistry Results: Females
| Groups | 5A Peptide (mg/kg) | BUN (mg/dL) | Crea (mg/dL) | AST (U/L) | ALT (U/L) | ALP (U/L) | GGT (U/L) | LD (U/L) | CK (U/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | Mean | 15.94 | 0.54 | 174.2 | 91.8 | 246.2 | 39.4 | 986.4 | 3178.2 |
| SD | 2.67 | 0.09 | 204.2 | 78.2 | 15.2 | 16.3 | 1150.8 | 5033.0 | ||
| 2 | 8 | Mean | 15.50 | 0.50 | 146.0 | 83.6 | 215.0 | 43.0 | 660.6 | 2286.8 |
| SD | 3.03 | 0.10 | 70.8 | 32.0 | 23.1 | 16.7 | 216.2 | 1617.4 | ||
| 3 | 25 | Mean | 17.52 | 0.52 | 172.4 | 90.2 | 192.8 | 46.4 | 779.8 | 3052.4 |
| SD | 2.77 | 0.11 | 80.4 | 49.0 | 41.6 | 9.9 | 286.3 | 4307.7 | ||
| 4 | 75 | Mean | 23.42 | 0.76 | 2331.4* | 1051.6* | 319.0* | 139.2* | 12669.2 | 163348.2 |
| SD | 20.81 | 0.70 | 3129.2 | 976.8 | 45.9 | 136.1 | 20013.9 | 262263.9 | ||
[Test: Dunnett 2 Sided p < 0.05]
Table 4.
Summary of Cholesterol (mg/dL) levels
| Groups | 5A Peptide (mg/kg) | Day 2 | Day 15 | Day 29 | Day 42 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |||
| 1 | 0 | Mean | 88.0 | 114.8 | 98.4 | 139.4 | 104.2 | 134.6 | 84.0 | 146.5 |
| SD | 26.3 | 26.7 | 30.4 | 24.8 | 30.2 | 19.5 | 18.4 | 9.2 | ||
| 2 | 8 | Mean | 91.2 | 101.6 | 102.6 | 125.0 | 104.8 | 123.0 | 126.5 | 156.0 |
| SD | 12.9 | 25.9 | 15.9 | 29.4 | 14.0 | 30.6 | 13.4 | 15.6 | ||
| 3 | 25 | Mean | 110.4 | 101.8 | 116.0 | 111.0 | 110.6 | 111.8 | 106.5 | 107.0 |
| SD | 18.4 | 12.8 | 23.6 | 18.7 | 20.5 | 11.9 | 13.4 | 11.3 | ||
| 4 | 75 | Mean | 191.0* | 208.4* | 174.2* | 144.6 | 161.6* | 132.0 | 109.0 | 95.5* |
| SD | 44.1 | 47.7 | 52.4 | 53.3 | 37.6 | 42.8 | 25.5 | 4.9 | ||
[Test: Dunnett 2 Sided p < 0.05]
Table 4 summarizes the Chol and serum enzyme changes in all groups, males and females from Day 2 to day 42. The increase in cholesterol changes was expected based on previous findings showing that it mobilizes tissue cholesterol and increases cholesterol on HDL (Amar et al., 2010; Sethi et al., 2008). At relatively high doses, the Fx-5A has also been shown to increase total plasma triglycerides (Amar et al., 2015).
On Day 15, increases in ALT and GGT were observed (Table 5), as well as on Day 29, for ALT (226.6 U/L). There was no dose response increase in ALT levels so it is possible that the increase in ALT was in part a result of animal handling. It is worthy of note that the levels of AST, ALT, ALP, and GGT observed on Day 2 did not progress during subsequent dose administrations. There were no clear test article-related changes in BILA levels in the monkeys.
Table 5.
Summary of Day 15 Clinical Chemistry Results: Females
| Groups | 5A Peptide(mg/kg) | BUN (mg/dL) | Crea (mg/dL) | AST (U/L) | ALT (U/L) | ALP (U/L) | GGT (U/L) | LD (U/L) | CK (U/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | Mean | 19.04 | 0.64 | 48.6 | 39.8 | 289.4 | 45.4 | 496.4 | 224.2 |
| SD | 2.39 | 0.13 | 27.6 | 11.1 | 34.3 | 18.7 | 302.4 | 144.3 | ||
| 2 | 8 | Mean | 18.42 | 0.64 | 36.8 | 35.6 | 246.0 | 47.0 | 323.6 | 975.4 |
| SD | 2.23 | 0.09 | 18.3 | 9.0 | 37.1 | 17.0 | 134.2 | 1673.8 | ||
| 3 | 25 | Mean | 18.36 | 0.64 | 38.4 | 57.0 | 201.4* | 51.0 | 391.0 | 155.4 |
| SD | 3.05 | 0.17 | 10.6 | 38.1 | 25.2 | 10.7 | 56.6 | 28.3 | ||
| 4 | 75 | Mean | 17.18 | 0.62 | 48.0 | 231.4* | 306.0 | 99.8* | 505.8 | 178.2 |
| SD | 4.31 | 0.16 | 16.2 | 234.4 | 81.4 | 55.9 | 145.5 | 60.1 | ||
[Test: Dunnett 2 Sided p < 0.05]
Fx-5A had no apparent effects on coagulation and urinalysis parameters. Fx-5A-related effects were observed for some of the hematology parameters tested as described below, with the most changes apparent in animals tested at the 75 mg/kg/dose level. Unless otherwise noted, the changes in parameters listed in this section were statistically different compared to the vehicle treated animals. For male NHPs, animals on Day 2 had an increase in MCV levels (6.72%) at the 8 mg/kg/dose level, whereas, on Day 15, there was a decrease in RBC (− 6.34%) at the 25 mg/kg/dose level, declines in HGB (−11.34%) and MCHC (− 4.95%) at the 75 mg/kg/dose level and increases in PLT (48.4%) (Table 6). On Day 29, there were increases in PLT (75.5%) and LUC (137.5%) at the 75 mg/kg/dose level. Male NHPs also showed an increase in WBC (90.816%) on Day 42 compared to controls. In female NHPs, there were no statistically significant changes in hematologic parameters on Day 2. On Day 15, decreases in HGB (−14.80%), MCH (−11.47%), and MCHC (−6.78%) and increases in PLT (70.0%) were observed at the 75 mg/kg/dose level (Table 7). On Day 29, females also demonstrated decreases in HGB (−14.78%), MCH (−12.69%), and MCHC (−7.54%) and increases in PLT (53.5%) (Table 8) and Retic (Abs) (140.0%) at the 75 mg/kg/dose level. There is no definitive cause for the the increase of PLT seen in the study.
Table 6.
Summary of Day 15 Hematology Results: Males
| Groups | 5A peptide (mg/kg) | WBC (10^3/mm3) | RBC (10^6/mm3) | HGB (g/dL) | HCT (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PLT (10^3/mm3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | Mean | 9.374 | 5.428 | 12.70 | 40.24 | 74.16 | 23.42 | 31.54 | 499.8 |
| SD | 1.920 | 0.323 | 0.69 | 2.59 | 1.42 | 0.80 | 0.78 | 70.5 | ||
| 2 | 8 | Mean | 14.348 | 5.084 | 12.26 | 40.30 | 79.34* | 24.12 | 30.40 | 483.2 |
| SD | 4.691 | 0.309 | 0.75 | 1.84 | 1.97 | 0.53 | 0.87 | 106.8 | ||
| 3 | 25 | Mean | 11.900 | 4.862* | 11.60 | 38.32 | 78.96* | 23.88 | 30.24 | 422.8 |
| SD | 2.414 | 0.250 | 0.73 | 1.45 | 2.86 | 1.36 | 0.83 | 69.7 | ||
| 4 | 75 | Mean | 14.268 | 5.010 | 11.26* | 37.54 | 74.82 | 22.42 | 29.98* | 741.6* |
| SD | 4.494 | 0.200 | 0.80 | 3.18 | 4.46 | 1.21 | 0.86 | 161.6 | ||
[Test: Dunnett 2 Sided p < 0.05]
Table 7.
Summary of Day 15 Hematology Results: Females
| Groups | 5A Peptide (mg/kg) | WBC (10^3/mm3) | RBC (10^6/mm3) | HGB (g/dL) | HCT (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PLT (10^3/mm3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | Mean | 10.556 | 5.080 | 12.16 | 39.34 | 77.82 | 24.06 | 30.96 | 516.2 |
| SD | 1.871 | 0.604 | 0.91 | 2.81 | 4.66 | 1.51 | 0.46 | 135.4 | ||
| 2 | 8 | Mean | 13.246 | 4.966 | 11.80 | 39.04 | 78.86 | 23.88 | 30.26 | 486.6 |
| SD | 3.043 | 0.499 | 0.80 | 2.60 | 3.28 | 1.09 | 0.24 | 90.2 | ||
| 3 | 25 | Mean | 13.738 | 4.760 | 11.06 | 36.66 | 76.92 | 23.22 | 30.20 | 484.6 |
| SD | 4.764 | 0.324 | 0.93 | 3.14 | 2.10 | 1.28 | 1.16 | 101.8 | ||
| 4 | 75 | Mean | 13.494 | 4.858 | 10.36* | 35.90 | 73.88 | 21.30* | 28.86* | 877.6* |
| SD | 1.217 | 0.116 | 0.28 | 1.22 | 2.43 | 0.24 | 0.67 | 164.1 | ||
[Test: Dunnett 2 Sided p < 0.05]
Table 8.
Summary of Day 29 Hematology Results: Females
| Groups | 5A Peptide (mg/kg) | WBC (10^3/mm3) | RBC (10^6/mm3) | HGB (g/dL) | HCT (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PLT (10^3/mm3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | Mean | 10.660 | 4.806 | 11.50 | 35.60 | 74.48 | 24.12 | 32.38 | 482.0 |
| SD | 3.837 | 0.573 | 0.88 | 2.30 | 4.35 | 1.48 | 0.85 | 87.4 | ||
| 2 | 8 | Mean | 12.892 | 4.678 | 11.22 | 35.66 | 76.44 | 24.08 | 31.50 | 395.4 |
| SD | 4.763 | 0.559 | 1.00 | 2.93 | 3.27 | 1.08 | 0.44 | 48.3 | ||
| 3 | 25 | Mean | 11.260 | 4.556 | 10.54 | 34.14 | 74.84 | 23.16 | 30.94* | 380.8 |
| SD | 2.532 | 0.297 | 0.69 | 2.74 | 2.43 | 1.03 | 1.11 | 60.0 | ||
| 4 | 75 | Mean | 11.672 | 4.648 | 9.80* | 32.72 | 70.40 | 21.06* | 29.94* | 740.0* |
| SD | 2.537 | 0.355 | 0.67 | 2.19 | 1.39 | 0.42 | 0.32 | 154.2 | ||
[Test: Dunnett 2 Sided p < 0.05]
Examination of hematology slides demonstrated the dose-dependent formation of abnormal erythrocyte morphologies, including acanthocytes, echinocytes, spherocytes, microcytes, and/or schistocytes at all dose levels of Fx-5A. The prevalence of these changes was higher in males. There was an increase in frequency of these effects from Day 2 to Day 29, with evidence of improvement on Day 42 for all effects except the occurrence of microcytes (data not shown). In all groups, including the vehicle control group, there was evidence of regeneration based upon increases in absolute reticulocyte counts, as well as an increase in the incidence of polychromasia, and possibly in the degree of anisocytosis for the erythrocytes. This may be a combination of an Fx-5A related effect and from multiple bleeds during the study. However, in females, the combination of the increase in reticulocytes and the higher incidence of polychromasia in the 75 mg/kg/dose level compared to the vehicle control animals suggests an Fx-5A-related regenerative effect. Overall, there were no noted adverse effects of abnormal red blood cell morphologies on the health of the animals. It should be noted that the abnormal red blood cell morphologies are qualitatively scored and compared by frequency of observed cell morphologies and, thus, they are not subject to statistical analysis.
3.1.2. PLASMA DRUG LEVEL AND TOXICOKINETICS:
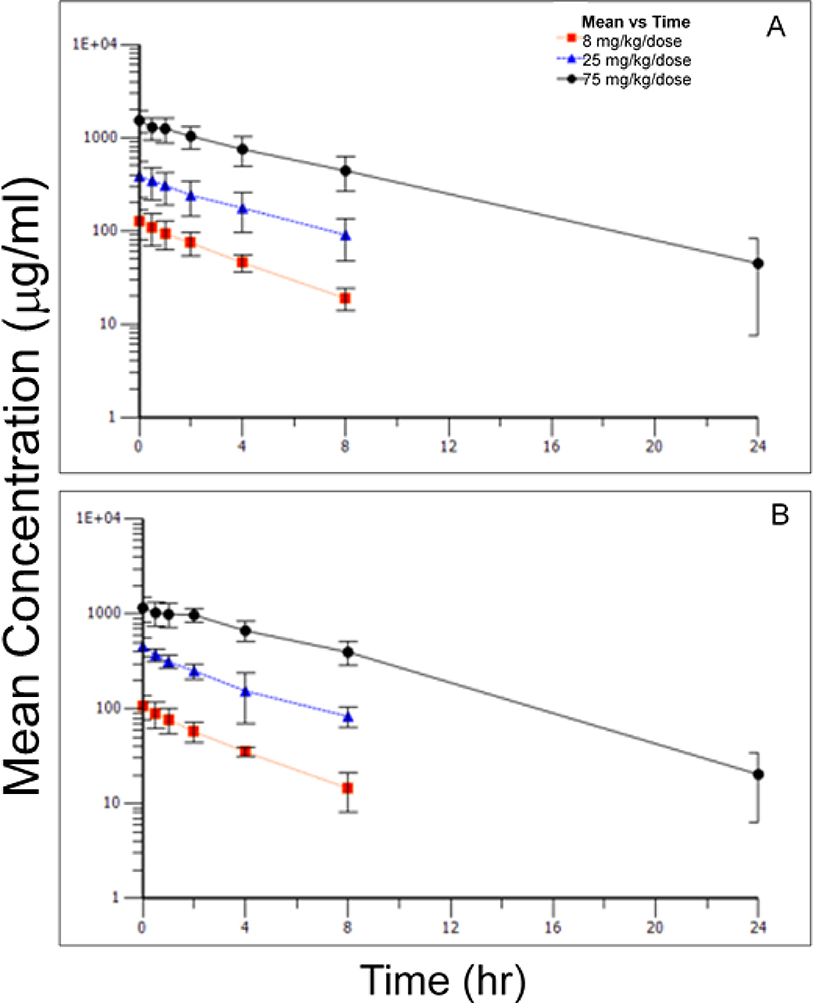
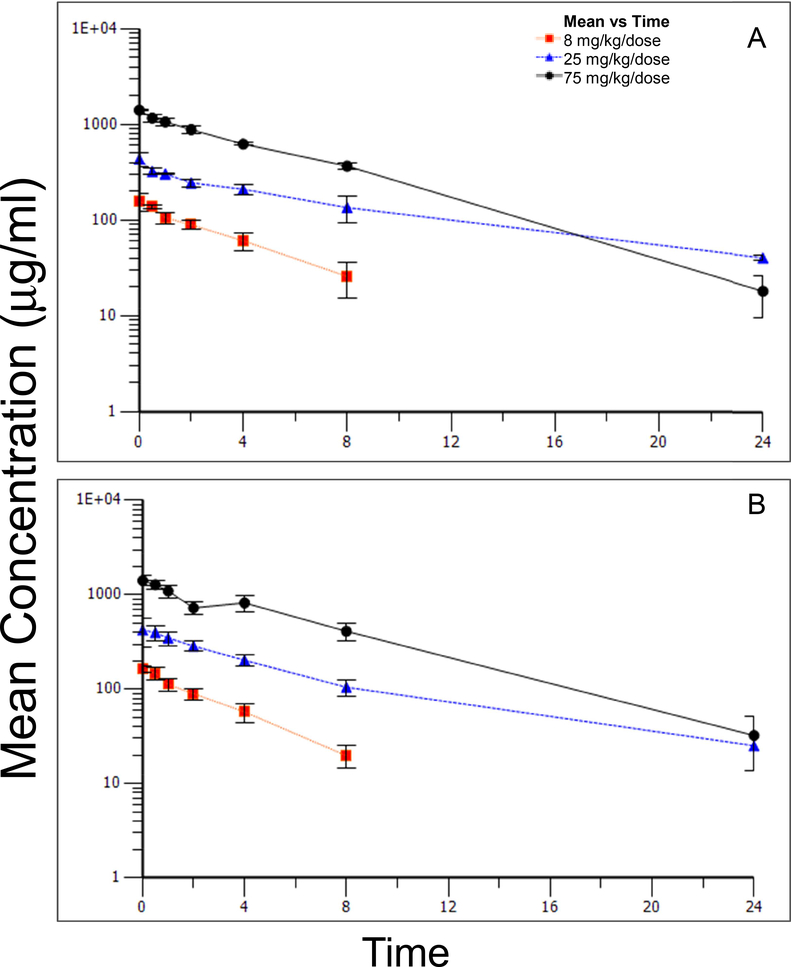
Table 9 presented the toxicokinetic parameters derived from plasma concentrations of the 5A peptide. Plasma concentration versus time curves for Days 1 and Day 27 are presented in Figure 1 and Figure 2, respectively. No sex-related differences in toxicokinetic parameters and, thus, the level of exposure, were observed following administration of the initial IV dose of 8, 25, or 75 mg/kg/dose on Day 1. With few exceptions, the maximum concentration (Cmax) of 5A peptide in plasma was observed at the end of the infusion (0 hours). Cmax and AUC (AUClast and AUCINF) values for 5A peptide increased in proportion to the increase in dose levels.
Table 9:
Toxicokinetic Parameters
| Sex | Group | 5A Peptide (mg/kg) | Day | Animal | Cmaxa (μg/mL) | Tmaxb (hr) | T1/2c (hr) | AUClastd (hr·μg/mL) | AUCINFe (hr·μg/mL) | Clf (mL/hr/kg) | Vssg (mL/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | 2 | 8 | 1 | Mean | 125 | 0 | 3.8 | 430 | 541 | 15 | 53 |
| SD | 44 | 0 | 2.4 | 106 | 58 | 2 | 1 | ||||
| Female | 2 | 8 | 27 | Mean | 159 | 0 | 3.2 | 540 | 573 | 14 | 54 |
| SD | 32 | 0 | 0.8 | 68 | 66 | 2 | 1 | ||||
| Female | 3 | 25 | 1 | Mean | 392 | 0 | 4.0 | 1544 | NA | NA | NA |
| SD | 153 | 0 | 0.7 | 649 | NA | NA | NA | ||||
| Female | 3 | 25 | 27 | Mean | 436 | 0 | 6.2 | 2472 | 3791 | 7 | 76 |
| SD | 72 | 0 | 1.9 | 987 | 16 | 0 | 4 | ||||
| Female | 4 | 75 | 1 | Mean | 1535 | 0 | 4.2 | 8484 | 11364 | 7 | 44 |
| SD | 396 | 0 | 0.9 | 3900 | 2517 | 2 | 5 | ||||
| Female | 4 | 75 | 27 | Mean | 1412 | 0 | 4.0 | 7101 | 7651 | 10 | 57 |
| SD | 38 | 0 | 0.5 | 1017 | 310 | 1 | 5 | ||||
| Male | 2 | 8 | 1 | Mean | 108 | 0 | 3.8 | 324 | 415 | 19 | 65 |
| SD | 32 | 0 | 3.1 | 76 | 40 | 2 | 5 | ||||
| Male | 2 | 8 | 27 | Mean | 164 | 0 | 2.7 | 527 | 607 | 13 | 52 |
| SD | 15 | 0 | 0.2 | 73 | 97 | 2 | 5 | ||||
| Male | 3 | 25 | 1 | Mean | 459 | 0 | 3.7 | 1504 | NA | NA | NA |
| SD | 102 | 0 | 0.5 | 402 | NA | NA | NA | ||||
| Male | 3 | 25 | 27 | Mean | 470 | 0 | 4.7 | 1992 | NA | NA | NA |
| SD | 106 | 0 | 1.4 | 589 | NA | NA | NA | ||||
| Male | 4 | 75 | 1 | Mean | 1232 | 1 | 3.8 | 7474 | 8506 | 9 | 53 |
| SD | 286 | 1 | 0.6 | 2196 | 1008 | 1 | 7 | ||||
| Male | 4 | 75 | 27 | Mean | 1416 | 0 | 4.2 | 8424 | 8633 | 9 | 57 |
| SD | 177 | 0 | 0.7 | 1318 | 1459 | 2 | 3 |
NA: Not applicable
NR: Not reported; the percent of the AUCINF value that was extrapolated was > 20%
Maximum observed concentration in plasma
Time of maximum observed concentration in plasma; the end of the infusion was designated as 0 hours.
Half-life of the terminal phase of elimination from plasma
Area under the plasma concentration versus time curve calculated from 0 to the last time point 5A peptide was quantifiable in plasma
Area under the plasma concentration versus time curve calculated from 0 to infinity
Total body clearance
Volume of distribution at steady state
FIGURE 1.
Mean concentrations (±standard deviation) of 5A peptide in plasma over time following IV administration of 8, 25, or 75 mg/kg/dose to female (A) and male (B) non-human primates: Day 1.
FIGURE 2.
Mean concentrations (±standard deviation) of 5A peptide in plasma over time following IV administration of 8, 25, or 75 mg/kg/dose to female (A) and male (B) non-human primates: Day 27.
Elimination half-life of 5A peptide was similar between sexes and across dose groups; group mean values ranged from 3.7 to 4.2 hours. In addition, both the total body clearance and volume of distribution at steady state of 5A peptide appeared to be similar between sexes and across dose levels. Mean clearance values observed for male and female animals ranged from 7 mL/kg/hr to 19 ml/kg/hr. The mean volume of distribution of 5A peptide at steady state was between 44 mL/kg and 65 mL/kg for male and female animals. The plasma concentration versus time profile for this animal suggested that the animal may not have received its complete dose intravascularly. No changes were noted in the toxicokinetic parameters observed in male and female animals after dosing every other day for 14 doses. Linear kinetics were demonstrated in both sexes and across all groups.
3.1.3. MACROSCOPIC AND MICROSCOPIC OBSERVATIONS:
No test article related macroscopic or microscopic lesions were observed on Day 29 or Day 42. On day 29, a statistically significant increases in the pituitary-to-brain and pituitary-to-body weight ratios were observed in male NHP in the 75 mg/kg/dose Fx-5A group when compared to the vehicle control group. Because no direct macroscopic or microscopic correlation to the increases in relative pituitary weights were observed, the changes in relative pituitary weights were thus not thought to be related to the administration of Fx-5A.
3.1.4. ANTI-DRUG ANTIBODY ANALYSIS:
Anti-Fx-5A peptide antibodies were sporadic but non-appreciable after dosing of the NHPs. Five of the samples from pre-dose had titers of 100 and one had a titer of 200 with none of these seven samples showing increased titers at later time points. There was one sample from the vehicle only treated group that had a titer of 100 on day −2. At Day 29, only one sample had a titer of 100 with the rest of the samples having titers of less than 100. At Day 42, eleven of sixteen total samples tested had titers of less than 100, one sample had a titer of 100 and four of the samples had titers of 200.
3.2. Fx-5A SINGLE DOSE IV INFUSION CARDIOVASCULAR AND PULMONARY SAFETY PHARMACOLOGY STUDY IN NHPs
There were no test article-related changes for any cardiovascular function, body temperature or ECG parameters for the 8 mg/kg dose group during the study (In-Box and In-Cage) as compared to control. There were no statistically significant changes in systemic blood pressure (systolic, diastolic and mean arterial pressure) or heart rate data noted for this study in the isolation box at the 8, 25 and 75 mg/kg dose levels. Statistically-significant decrease in body temperature ranged from 0.3 to 0.5°C during this time period as compared to control. These changes in body temperature were not considered to be physiologically or toxicologically significant and the animal’s body temperature went back to levels similar to control animals when they were returned to their home cages.
There were no changes in ECG interval data for QRS or QTcB interval data for the 8, 25 and 75 mg/kg dose levels for this study in the isolation boxes. There were increases in RR, PR and QT interval data noted. These interval data changes were not considered to be physiologically or toxicologically significant and when the QT interval data were corrected by heart rate (using the Bazett’s formula) all statistical significance disappeared.
There were no changes in systemic blood pressure (systolic, diastolic and mean arterial pressure), heart rate, or body temperature data at the 8, 25 and 75 mg/kg dose levels while the animals were in their home cages, that was considered physiologically or toxicologically significant. There were no changes in respiratory parameters (respiration rate, tidal volume or minute volume) noted on this study.
3.3. In-Vitro Testing of Hemolytic Potential of Fx-5A in Rat, NHP, and Human Blood
The hemoglobin readings from the vehicle samples were lower than the readings of the negative control samples from the same animals(s) when using both the commercial and fresh blood, indicating that the vehicle did not have a hemolytic effect. The hemoglobin levels in the positive control samples demonstrated that the 1% saponin produced significant lysis and that the assay was working as expected.
Hemoglobin levels detected in samples of commercially purchased blood were statistically different (p< 0.0001) between the negative controls versus the Fx-5A test samples. The hemoglobin levels in the dilution control were not statistically different than those in the sphingomyelin control (p= 0.8911), indicating that the presence of Fx-5A did not interfere with the measurement of the hemoglobin content. Taken together, the results indicate the Fx-5A was hemolytic when using commercially purchased blood.
Hemoglobin levels detected in samples using fresh rat, NHP, and human blood were not statistically different between the negative control versus the Fx-5A test samples (p= 0.069). These data indicate that the Fx-5A was not hemolytic when using freshly drawn blood from any of the three species tested. The difference between the results of commercial rat blood (drawn the day prior and shipped refrigerated overnight) versus freshly drawn rat blood indicates the possibility that the time period and handling of commercial blood may have compromised the integrity of red blood cells. This is supported by the fact that the mean hemoglobin value in the negative control samples of commercially purchased blood (266.7 mg/dL) was higher than the mean hemoglobin level in the freshly drawn blood (63.33 mg/dL) where a post-hoc t-test demonstrated statistical significance (p< 0.0001).
4. DISCUSSION
Several reconstituted HDL formulations have been shown to convincingly reduce atherosclerosis in animal models but none have yet been definitively shown to have benefit in humans (Remaley et al., 2008; Sviridov and Remaley, 2015). Very few full length protein and/or smaller ApoA-I peptide mimetics were tested side-by-side and the variability in doses, route of administration, animal models, patient populations and other conditions make comparisons of the various mimetics difficult. Thus, there is need to do considerable more development work in this area to produce a form of reconstituted HDL that is suitable for reducing atherosclosis in humans.
The use of ApoA-I mimetic peptides over full-length protein offers several potential advantages (Sviridov and Remaley, 2015). First and most importantly because of the relatively large dose used in some of the clinical trials (45 mg/kg) the cost of such therapy is quite expensive in terms of either purifying the ApoA-I protein from plasma or producing it recombinantly. The other is the ease to modify ApoA-I mimetic peptides to enhance their properties, which was done in the case of the Fx-5A for increasing its specificity for effluxing cholesterol by the ABCA1 transporter and reducing its cytotoxicity (Sethi et al., 2008). Combining 5A peptide with a variety of phospholipids has been shown to attenuate the development of atherosclerotic plaque in preclinical models of atherosclerosis, including APOE deficient mice (Amar et al., 2010). It also impairs macrophage recruitment and foam cell formation in the rabbit collar injury model (Tabet et al., 2010). Furthermore, Fx-5A catalyzes reverse cholesterol transport in vivo and is effective in reducing the progression of atherosclerosis in rabbits and mice with no signs of toxicity (Amar et al., 2010); therefore, we considered Fx-5A a strong candidate for further drug development.
In this study, Fx-5A was well tolerated by NHPs at all doses with no effects on survival, body weight, food consumption, ophthalmology, ECG, pulmonary function, blood pressure, urinalysis, and blood coagulation parameters. There were no Fx-5A related macroscopic and microscopic lesions and no impact on organ weights. Fx-5A was also not hemolytic under the conditions tested in the assay when fresh blood samples were used and there was only occasional detection of ADA response.
There were Fx-5A-related hematological effects especially at the 75 mg/kg/dose level, with statistically significant effects including a decrease in HGB for both sexes on Day 15 and for females on Day 29 and a dose-dependent occurrence of changes in red blood cell morphologies. HGB levels for males and females on Day 15 were on the low end of published historical control data (Andrade et al., 2004; Hall, 2007; Magden, 2015) but were not out of the normal range and would not be considered anemic. For females on Day 29, three of the five HGB values were below 10 g/dL with an average of 9.81 g/dL for the five animals, which are outside the range of published data (Andrade et al., 2004; Hall, 2007; Magden, 2015) and would be considered anemic. Most of the impacted parameters recovered after the 14 day recovery period, with the exception that microcytes, which were still evident at the Day 42 clinical pathology interval. Overall, there were no noted major adverse effects on HGB and other RBC measures blood cell morphologies on the health of the animals.
Changes in clinical chemistry parameters at the 75 mg/kg/dose level, including markers of liver function, were transient and in case of plasma cholesterol changes were expected based on the mechanism of action of the Fx-5A. The pharmacokinetic data obtained after dose administration on Day 1 and Day 27 suggested that 5A peptide exhibited dose dependent linear kinetics in both sexes and across all groups, and that repeated dosing with the complex did not impact the toxicokinetic.
Because there were no adverse toxicological effects, and the hematologic effects were not evident after a 2-week recovery period, the No Observed Adverse Effect Level (NOAEL) under the conditions of this study was considered to be 75 mg/kg/day, with associated exposures, average male and female Cmax and AUC0-last of 453 μg/mL and 2232 hr·μg/mL, respectively, on day 27 after every other day dosing. In summary, Fx-5A like other reconstituted HDL formulations appeared to be relatively safe and well tolerated in a NHP toxicology study and support the future development of this agent.
5. Conclusion
Fx-5A, an HDL mimetic, was well tolerated by NHPs and the NOAEL was considered to be 75 mg/kg/day (the highest dose tested) with average Cmax and AUC0-last of 453 μg/mL and 2232 hr·μg/mL, respectively, when administred as 30 min infusion once everyother day for 27 days.
Supplementary Material
Highlights:
Fx-5A was not hemolytic in in-vitro fresh NHP or human blood assay.
Dose-dependent recoverable erythrocytes morphological changes were observed.
Fx-5A produced non-adverse hematological and clinical chemistry effects.
Fx- 5A exhibited dose-dependent linear toxicokinetics with T1/2 of 2.7 – 6.2 hr.
NOAEL was 75 mg/kg/day for 30 min iv infusion every other day for 27 day schedule.
Acknowledgments:
The authors thank the Study Director, Richard. D. Guarino and other Southern Research Institute staff, Birmingham, Alabama for conducting the study.
Funding: This work was supported by (NCATS/NIH, USA) (Contract No. HHSN261200800001E) under BrIDGs Program.
ABBREVIATIONS:
- Fx-5A
Fx-5A Peptide Complex
- ApoA-I
Human apolipoprotein A-I
- HDL
High Density Lipoproteins
- LDL
Low Density Lipoproteins
- ADA
Anti-Drug Antibody
- NHP
Nonhuman Primate
- ECG
Electrocardiogram
- IV
Intravenous
- PLT
Platelets
- WBC
White Blood Cells
- HCT
Hematocrit
- MCV
Mean corpuscular volume
- RBC
Red Blood Cells
- HGB
Hemoglobin
- MCH
Mean corpuscular hemoglobin
- MCHC
Mean corpuscular HGB conc
- LUC
Large unstained cells
- Retic (Abs)
Reticulocyte (Abs)
- BUN
Urea Nitrogen
- Crea
Creatinine
- CK
Creatine Kinase
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- LD
lactate dehydrogenase LD
- GGT
gamma glutamyl transferase
- ALB
Albumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amar MJ, et al. , 2010. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Ther. 334, 634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar MJ, et al. , 2015. A novel apolipoprotein C-II mimetic peptide that activates lipoprotein lipase and decreases serum triglycerides in apolipoprotein E-knockout mice. J Pharmacol Exp Ther. 352, 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharamaiah GM, et al. , 2007. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J Lipid Res. 48, 1915–23. [DOI] [PubMed] [Google Scholar]
- Andrade MC, et al. , 2004. Biologic data of Macaca mulatta, Macaca fascicularis, and Saimiri sciureus used for research at the Fiocruz primate center. Mem Inst Oswaldo Cruz. 99, 581–9. [PubMed] [Google Scholar]
- Andrews J, et al. , 2017. Effect of serial infusions of reconstituted high-density lipoprotein (CER-001) on coronary atherosclerosis: rationale and design of the CARAT study. Cardiovasc Diagn Ther. 7, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloedon LT, et al. , 2008. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D4F in high-risk cardiovascular patients. J Lipid Res. 49, 1344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga GM, et al. , 2010. L-4F alters hyperlipidemic (but not healthy) mouse plasma to reduce platelet aggregation. Arterioscler Thromb Vasc Biol. 30, 283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille A, et al. , 2014. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 34, 2106–14. [DOI] [PubMed] [Google Scholar]
- Hall R, 2007. Clinical pathology of laboratory animals. Taylor & Francis, Boca Raton, FL. [Google Scholar]
- Hovingh GK, et al. , 2015. The effect of an apolipoprotein A-I-containing high-density lipoprotein-mimetic particle (CER-001) on carotid artery wall thickness in patients with homozygous familial hypercholesterolemia: The Modifying Orphan Disease Evaluation (MODE) study. Am Heart J. 169, 736742.e1. [DOI] [PubMed] [Google Scholar]
- Kallend DG, et al. , 2016. A single infusion of MDCO-216 (ApoA-1 Milano/POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease. Eur Heart J Cardiovasc Pharmacother. 2, 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen HJ, et al. , 2013. Effect of repeated apoA-IMilano/POPC infusion on lipids, (apo)lipoproteins, and serum cholesterol efflux capacity in cynomolgus monkeys. J Lipid Res. 54, 2341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, et al. , 2003. Single-dose intravenous infusion of ETC-642, a 22-Mer ApoA-I analogue and phospholipids complex, elevates HDL-C in atherosclerosis patients. Circulation. 108, 563–564. [Google Scholar]
- Kootte RS, et al. , 2015. Effect of open-label infusion of an apoA-I-containing particle (CER-001) on RCT and artery wall thickness in patients with FHA. J Lipid Res. 56, 703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magden E, 2015. Nonhuman Primates in Laboratory Animal Medicine. pp. 805. [Google Scholar]
- Miles J, et al. , Single-dose tolerability, pharmacokinetics, and cholesterol mobilization in HDL-C fraction following intravenous administration of ETC-642, a 22-mer ApoA-I analogue and phospholipids complex, in atherosclerosis patients, Proc. ATVB24 2004, E19–E19. [Google Scholar]
- Navab M, et al. , 2004. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves highdensity lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 109, 3215–20. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, et al. , 2018. Effect of Serial Infusions of CER-001, a Pre-beta High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial. JAMA Cardiol. 3, 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenass-Lechner F, et al. , 2016. Immunogenicity, Inflammation, and Lipid Accumulation in Cynomolgus Monkeys Infused with a Lipidated Tetranectin-ApoA-I Fusion Protein. Toxicol Sci. 150, 378–89. [DOI] [PubMed] [Google Scholar]
- Remaley AT, 2013. Tomatoes, lysophosphatidic acid, and the small intestine: new pieces in the puzzle of apolipoprotein mimetic peptides? J Lipid Res. 54, 3223–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaley AT, et al. , 2008. HDL-replacement therapy: mechanism of action, types of agents and potential clinical indications. Expert Rev Cardiovasc Ther. 6, 1203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi AA, et al. , 2008. Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J Biol Chem. 283, 32273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockham SL, Scott MA, 2008. Erythrocytes in: Scott S. a., (Ed.), Fundamentals of Veterinary Clinical Pathology. Blackwell Publishing, pp. 157–158. [Google Scholar]
- Sviridov D, Remaley AT, 2015. High-density lipoprotein mimetics: promises and challenges. Biochem J. 472, 249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabet F, et al. , 2010. The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol. 30, 246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif JC, et al. , 2007. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. Jama. 297, 1675–82. [DOI] [PubMed] [Google Scholar]
- Uehara Y, et al. , 2015. High-Density Lipoprotein-Targeted Therapy and Apolipoprotein A-I Mimetic Peptides. Circ J 79, 2523–8. [DOI] [PubMed] [Google Scholar]
- Zheng KH, et al. , 2016. HDL mimetic CER-001 targets atherosclerotic plaques in patients. Atherosclerosis. 251, 381–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.