Abstract
Essential for physiology, transient receptor potential (TRP) channels constitute a large and diverse family of cation channels functioning as cellular sensors responding to a vast array of physical and chemical stimuli. Detailed understanding of the inner workings of TRP channels has been hampered by a lack of atomic structures, though structural biology of TRP channels has been an enthusiastic endeavor since their molecular identification two decades ago. These multi-domain integral membrane proteins, exhibiting complex polymodal gating behavior, have been a challenge for traditional X-ray crystallography, which requires formation of well-ordered protein crystals. X-ray structures remain limited to a few TRP channel proteins to date. Fortunately, recent breakthroughs in single-particle cryo-electron microscopy (cryo-EM) have enabled rapid growth of the number of TRP channel structures, providing tremendous insights into channel gating and regulation mechanisms and serving as foundations for further mechanistic investigations. This brief review focuses on recent exciting developments in structural biology of a subset of TRP channels, the calcium-permeable, non-selective and thermosensitive vanilloid subfamily of TRP channels (TRPV1–4), and the permeation and gating mechanisms revealed by structures.
Keywords: Ion channel, TRP channel, TRPV, ThermoTRPV, cryo-EM, crystallography
Graphical Abstract

1. Introduction
TRP channels are central to numerous biological processes by sensing a multitude of environmental cues and physiological stimuli [1–5]. Consequently, mutations of human TRP channel genes have been associated with a wide spectrum of inherited diseases in the musculoskeletal, cardiovascular, and nervous systems [6]. On the basis of sequence similarity, the mammalian TRP channel subunit genes can be divided into six subfamilies - TRPA, TRPC, TRPM, TRPML, TRPP, and TRPV [2]. Functional TRP channels are composed of homo- or hetero- tetramers, which are architecturally related to voltage-gated ion channels (VGIC) such as voltage-gated sodium (NaV), potassium (KV), and calcium channels (CaV). All TRP channel subunits consist of a transmembrane domain (TMD) with six membrane-spanning helices S1–S6, and intracellular amino- and carboxyl- termini [1]. A characteristic of TRP channels is the polymodal gating behavior; these channels are activated by a plethora of physical and chemical stimuli, including membrane voltage, temperature, force, and numerous endogenous and exogenous ligands [1,2]. Thus TRP channels are particularly capable of sensing multiple environmental cues and integrating them into cellular signaling events including calcium signaling [1,2].
Among the six TRPV subfamily members, TRPV1–4 are non-selective, Ca2+-permeable cation channels intrinsically sensitive to warm to hot temperatures (thermoTRPVs) [7–11]. Broadly expressed, these thermally activated channels play important roles in multiple physiological processes, including thermoregulation, osmoregulation and nociception [12–19]. In addition to temperature activation, TRPV1–4 channels are also activated or modulated by a rich array of natural products, synthetic chemicals, and endogenous ligands. For instance, the founding member TRPV1 is activated by extracellular protons, tarantula peptide toxins, and plant products such as capsaicin from hot chili pepper, piperine from black pepper and gingerol from ginger [7,20–24]. 2-Aminoethoxydiphenyl borate (2-APB) activates wild-type TRPV1–3 channels and a TRPV4 variant with mutations of two cytoplasmic residues [25,26]. Cannabinoids from the plant Cannabis sativa and cannabinoid analogs have been identified as agonists for TRPV1–4 channels [27–30]. TRPV4 is activated by plant extract bisandrographolide A from Andrographis paniculata, synthetic compounds GSK1016790A and phorbol ester 4α-PDD, and endogenous lipid metabolites [31–34]. Additionally, mechanical stimuli have been reported to activate TRPV2 and TRPV4 channels [17–19,35–37].
To better understand the underlying gating mechanisms by these diverse stimuli and to develop efficient rational therapeutics, we would need high-resolution structures of TRP channels at the atomic level. X-ray crystallography is a well-established technique for atomic structure determination if well-ordered protein crystals are available, but application of crystallography to these integral membrane proteins has proven to be challenging. X-ray crystal structures have been achieved only until very recently for a limited number of TRP channels. In 2013, Cheng and Julius and colleagues published their groundbreaking papers reporting near-atomic resolution structures of TRPV1 determined by single-particle cryo-EM without the need of protein crystals [38,39], which have transformed the field of structural biology of membrane proteins. With the advent of ‘Resolution Revolution’ in cryo-EM [40], near-atomic structures of TRP channels from all subfamilies, including TRPV, TRPA, TRPC, TRPM, TRPML and TRPP, have rapidly emerged. Additionally, distinct conformations representing multiple functional states during channel gating have been determined for a number of TRP channels. The wealth of structural information has enormously advanced our understanding of TRP channel structure and function. This review briefly summarizes exciting progress in structural biology of thermosensitive TRPV1–4 channels and its mechanistic insights into ion permeation and gating mechanisms, and remaining important questions.
2. Architecture of TRPV channels
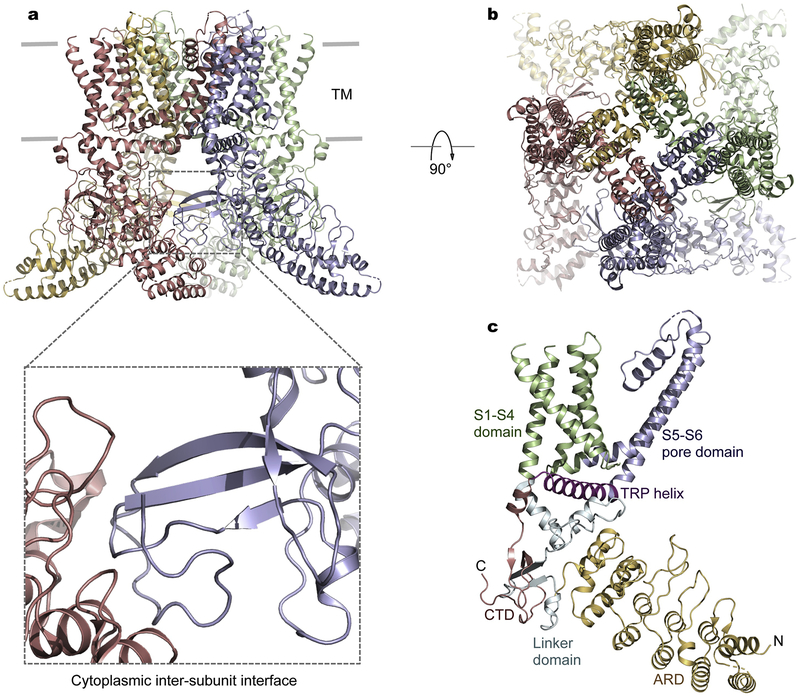
TRPV channels share a similar architecture includi6ng a characteristic cytoplasmic ankyrin repeat domain (ARD) consisting of six ankyrin repeats and a transmembrane domain (TMD) resembling that of VGICs such as Kv channels [41] (Fig. 1). In the transmembrane region, the peripheral S1–S4 domains, akin to the voltage-sensor domains in VGICs, are attached to the central ion-conduction pore domains in a domain-swapped configuration as seen in many VGICs and other TRP channels (Fig. 1). The cytoplasmic assembly consists of both N- and C-terminal segments. Following the N-terminal ARD, a linker domain preceding the S1–S4 domain, together with the intracellular C-terminal domain following the amphipathic TRP helix, mediates an extensive cytoplasmic subunit-subunit assembly interface that was hypothesized to be critical for channel gating (Fig. 1a) [38,42,43]. This notion was elegantly illuminated by recent structural and functional analyses of TRPV3 [44,45]. Disruption of a salt-bridge interaction at this cytoplasmic interface leads to considerable structural rearrangements involving a loop-to-helix switch in the distal C-terminal domain, which dictates tight coupling between the cytoplasmic and transmembrane domains, a prerequisite for TRPV3 activation [44]. Importantly, the distal C-terminal portion undergoing secondary structure transition is conserved in amino acid sequence among thermoTRPVs. Further examination of the electron density maps at this region in other thermoTRPV structures suggests that the loop-to-helix transition may serve as a common conformational switch in activation of thermoTRPVs [44].
Fig. 1.
Architecture of TRPVs. a, Representative structure of a TRPV3 channel (PDB: 6MHO) with the most complete atomic model. Each subunit of the tetrameric channel is in a distinct color. The cytoplasmic inter-subunit interface critical for channel gating is highlighted. The linker domain preceding the transmembrane domain and the C-terminal domain following the TRP helix are both involved in inter-subunit packing with the ARD of an adjacent subunit. b, View from the extracellular side. c, Domain structure of a single channel subunit.
Following the pore-lining helix S6, the membrane-proximal C-terminal amphipathic TRP helix, a conserved sequence motif among TRP channels critical for regulation of channel activity [46], is strategically located between the cytoplasmic and transmembrane domains and poised to mediate allosteric interactions between these modular domains. The TRP helix is in close proximity with the S4–S5 linker connecting the S1–S4 and pore domains and the cytoplasmic linker domain bridging the ARD and S1 (Fig. 1c). As observed in TRPV structures, the invariant tryptophan in the middle of the TRP helix is engaged in strong hydrogen bonding between its indole nitrogen and the main-chain carbonyl oxygen in the S4–S5 linker. Mutations of this tryptophan residue result in gain-of-function phenotypes in TRPV3 (W692G) and TRPV4 (W733R) [47,48], further supporting the critical involvement of the TRP helix in channel activation.
3. Ion selectivity
Thermosensitive TRPV1–4 channels are non-selective cation channels displaying slightly higher permeability for physiological divalent ions such as Ca2+ than monovalent ions such as Na+ and K+ [7,8,11,49], whereas TRPV5–6 are highly selective for Ca2+ with a permeability ratio of PCa/PNa over 100 [50,51]. To reveal the molecular basis of ion selectivity in these channels, unambiguous determination of ion binding sites along the permeation pathway is necessary. Toward this end, recent X-ray crystallographic studies of TRPV2, TRPV4, and TRPV6 channels have greatly contributed to our understanding of ion selectivity among TRPV channels [43,52,53].
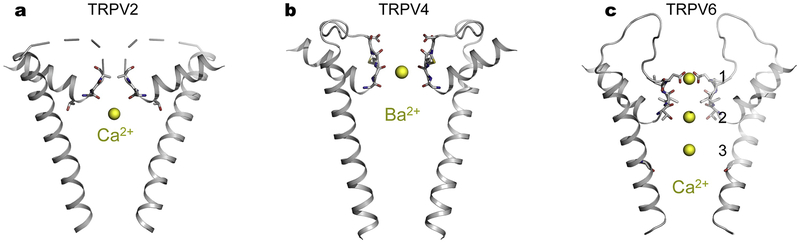
The crystal structure of TRPV2-Ca2+ shows that one Ca2+ ion is bound at the selectivity filter (Fig. 2a) [52]. Anomalous X-ray diffraction of TRPV4 crystals in the presence of monovalent, divalent and trivalent ions (Cs+, Ba2+ and Gd3+) identifies a single cation-binding site at the selectivity filter (Fig. 2b), which has opening wide enough to accommodate a hydrated ion [43]. Regardless of the charges of the bound ion carries, a single binding site exists at virtually the same location in the selectivity filter of TRPV4 [43]. One ion-binding site seen in TRPV2 and TRPV4, together with the absence of specific side chains coordinating the bound ion, explains non-selectivity in that multi-ion occupancy is necessary for achieving selectivity, as demonstrated in the NaK channel and mutants with altered selectivity by varying the number of ion-binding sites [54–56]. With a single ion-binding site, an entering ion could spontaneously exit to either side of the selectivity filter, and thus it permeates. In contrast, when multiple ion binding sites align in single file in the selectivity filter, an entering ion with lower affinity would be rejected by a previously occupied ion with higher affinity, and hence the entering ion would be forced back to the same side it enters.
Fig. 2.
Ion binding sites at the selectivity filter region of TRPVs revealed by X-ray crystallography. a, TRPV2 (PDB: 6BWM). b, TRPV4 (PDB: 6C8G). c, TRPV6 (PDB: 5IWP). Bound divalent ions are shown as yellow spheres.
Three Ca2+ ions have been identified along the ion-conduction pathway in the crystal structure of the Ca2+-selective channel TRPV6 (Fig. 2c) [53]. The first high-affinity Ca2+-binding site near the extracellular entrance is constructed by the aspartate side chains from four subunits, which are arranged to coordinate a dehydrated Ca2+ ion [53]. Site 2 is formed by backbone carbonyls and threonine side chains and site 3 appears to involve water-mediated weak hydrogen bonding interactions [53]. Three consecutive Ca2+-binding sites along the ion permeation path render TRPV6 highly selective for Ca2+.
4. Gating mechanism
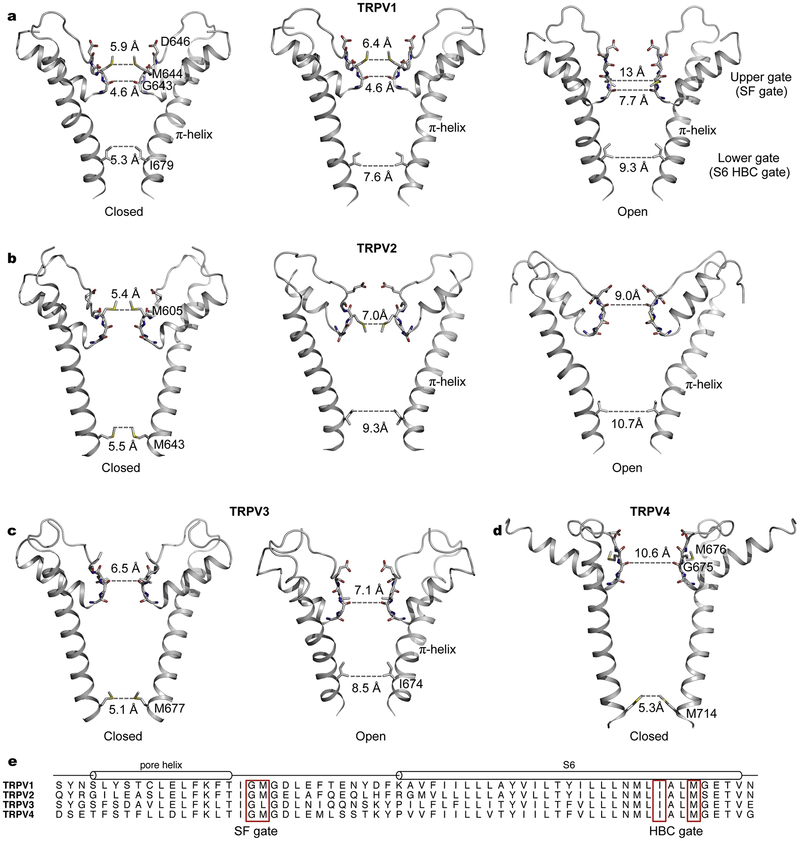
Evident from cryo-EM structures of TRPV1 in distinct functional states, two prominent physical constrictions along the ion permeation pathway, the upper selectivity-filter (SF) gate and the lower helix bundle-crossing (HBC) gate, undergo considerable structural rearrangements during channel opening (Fig. 3a) [38,39]. In the apo state, both the SF and HBC gates are closed. At the SF gate, the side chains of M644 point to the central pore axis and the carbonyl oxygens of G643 form the narrowest constriction (4.6 Å in diameter), whereas at the HBC gate, the side chains of I679 create a hydrophobic seal at the intracellular end of S6 (5.3 Å in diameter) [39]. With the vanilloid agonist capsaicin bound to a pocket in the vicinity of the S4–S5 linker, the lower HBC gate is expanded to 7.6 Å in diameter while the upper SF gate in the outer pore region remains unchanged [39]. With the application of a more potent agonist resiniferatoxin (RTX) and a peptide toxin DkTx, which specifically interacts with the outer pore region, the channel enters a fully open state with substantial opening at both gates [39]. At the SF gate, the pore helix moves downward and the pore diameter at G643 is increased to 7.7 Å. At the HBC gate, conformational changes of the lower half of S6 accompanied by a rotation of the I679 side chains away from the central pore axis result in a further widening to 9.3 Å in diameter [39]. TRPV1 structures suggest a dual-gating mechanism involving pronounced structural rearrangements at both the SF and HBC gates [39]. These structural findings elegantly explain previous observations that the outer pore region of TRPV1 actively contributes to gating elicited by multiple physiological stimuli, including spider toxins, protons, and heat [20,57–61]. Thus the gating transition of TRPV1 appears to be fundamentally different from that of the architecturally related VGICs such as Kv channels [62], in which only the lower S6 HBC gate is engaged in channel activation and the selectivity filter is relatively rigid under normal physiological settings. Additionally, in VGICs, the voltage-sensor S1–S4 domains move in response to membrane depolarization to promote gate opening [62], but the analogous S1–S4 domains in TRPV1 remain static during gating [39].
Fig. 3.
Pore profiles of thermoTRPV channels representing distinct functional states. a, The TRPV1 ion-conduction pore in the apo, closed (PDB: 3J5P), capsaicin-bound (PDB: 3J5R), and RTX/DkTx-bound, open states (PDB: 3J5Q). b, The TRPV2 ion permeation pathway in distinct conformational states (from left to right, PDB ID: 5AN8, 6BO5, and 6BO4). c, Closed (PDB: 6MHO) and open (PDB: 6DVZ) ion-conduction pore of TRPV3. d, Ion-conduction pore of TRPV4 (PDB: 6BBJ). e, Sequence alignment of human TRPV1–4 in the pore region. Residues forming the gates are highlighted in red boxes.
The cryo-EM structure of rabbit TRPV2 in the apo state also reveals both the SF and HBC gates (Fig. 3b) [42]. The SF gate is defined by the methionine side chains and backbone carbonyls analogous to those in TRPV1 (Fig. 3b). In contrast, the S6 HBC gate is instead constructed by the side chains of M643 [42], which is one helical turn closer to the cytosol in S6 compared to the gating isoleucine (I679) in TRPV1. The position shift of the HBC gate in TRPV2 is a consequence of different secondary structures in S6, evident by comparison of the non-conducting pores of TRPV1 and TRPV2 [42]. In the closed TRPV1 structure S6 contains a π-helical turn in the middle (Fig. 3a), whereas in TRPV2 S6 is entirely α-helical (Fig. 3b) [42]. This observation has led to the proposal that the observed TRPV2 structure represents a desensitized state and that an α-to-π secondary structure transition in S6 is involved in channel gating [42], which was first observed in opening of TRPV6 [63].
Surprisingly, cryo-EM structures of rat TRPV2 with and without an intact pore turret in the apo state show distinct pore profiles from that of rabbit TRPV2 in the apo state [42,64]. With the complete pore turret, the rat TRPV2 structure is consistent with a fully open conformation with both gates exhibiting substantial expansion (Fig. 3b) [64]. Comparison with the non-conducting rabbit TRPV2 structure confirms that the α-to-π transition in S6 occurs upon pore opening, and in the open conformation the HBC pore region is lined by the equivalent isoleucine as in TRPV1 [64]. The rat TRPV2 variant with a shortened turret generated a partially open conformation, where the S6 HBC gate is wide open but the SF gate remains closed with the methionine side chains protruding to the central ion-conduction pore [64]. Remarkably, all structures of TRPV2 representing distinct conformations were obtained in the absence of any ligand [42,64], therefore it remains unknown what dictates these conformational states. Whether these differentiating conformations originate from species differences or biochemical preparations needs further clarification. Nevertheless, the collective conformations of TRPV1 and TRPV2 support the notion that dual gating might be a common feature distinguishing TRP channels from VGICs. Intriguingly, the observed closed (or desensitized) states of TRPV1 and TRPV2 display different secondary structures in the pore-lining helix S6 (Figs. 3a and 3b). S6 in TRPV1 contains a π-helical turn and the isoleucine side chains constitute the HBC gate [38], whereas S6 in TRPV2 is completely α-helical and the methionine side chains form the HBC gate [42]. A hypothetical gating model, involving rotation of a conserved asparagine in S6 to hydrate the pore for ion conduction, suggests the possibility of an α-to-π transition in TRPV1 gating [65], and a closed TRPV1 conformation with α-helical S6 has yet to be experimentally verified.
Like in TRPV2, S6 in the apo closed conformations of human and mouse TRPV3 is entirely α-helical and the equivalent methionine side chains line the HBC gate (Fig. 3c) [66,67]. In marked contrast to TRPV2, the selectivity filter of TRPV3 in the closed conformations appears to be wide enough for ion conduction [66,67]. In the agonist 2-APB bound open conformation of TRPV3, the HBC gate is widened following an α-to-π transition in S6 [66], akin to opening of the HBC gate in TRPV2. However, the selectivity filter has a similar dimension sufficiently wide for ion conduction in both the closed and open states, suggesting the absence of any SF gate in TRPV3 [66,67].
In the apo nonconductive conformation of TRPV4, S6 is fully α-helical as in the apo closed TRPV2 and TRPV3 structures and the equivalent methionine side chains (M714) constitute the HBC gate (Fig. 3d) [43]. In stark contrast, the selectivity filter is exceptionally wide in TRPV4 compared with other thermoTRPV channels though the selectivity-filter sequences TIGM(L)GD(E) are well conserved (Fig. 3e) [43]. The narrowest point at the selectivity filter is 10.6 Å in diameter lined by carbonyls of G675 [43], considerably wider than that in the open state of TRPV1 (7.7 Å) [39]. The hydrophobic side chains of M676 in the pore loop point away from the central pore axis and thus do not interfere with ion conduction [43]. Like TRPV3, TRPV4 may only contain the S6 HBC gate but lack the SF gate [43]. Consistently, in contrast to direct involvement of the selectivity filter region of TRPV1 in channel activation by spider toxins, protons and heat [20,57–61], no known physiological stimuli specifically interact with the selectivity filter region of TRPV4 to open the channel.
Structures of thermoTRPV channels raise an important question: how conserved is the dual gating mechanism among these channels? The observed pore profiles of TRPV1 and TRPV2 certainly support the existence of two physical gates, the SF and HBC gates. Additionally, partially open conformations of TRPV1 and TRPV2 suggest the possibility of step-wise opening of the two gates, which are likely allosterically coupled during gating induced by distinct stimuli. In contrast, for TRPV3 and TRPV4, the selectivity filters in the apo non-conducting states are wide enough for ion passage, and therefore appear to be not involved in gating. Alternatively, it is also possible that additional closed conformations with a closed SF gate have so far escaped our studies and remain to be captured. In all TRPV3 structures, including the closed, sensitized, and open conformations, the selectivity filter is sufficiently wide for partially dehydrated ions [66,67]. However, several point mutations (N643S, I644S, N647Y, L657I and Y661C) specifically abolishing temperature activation [68], cluster at the outer pore region, and cysteine-scanning experiments identified two nearby residues I652 and L655 undergoing temperature-dependent conformational changes [69]. These results suggest that the selectivity filter and its immediate surrounding regions are directly involved in thermosensation. Therefore TRPV3 may also engage a dual gating mechanism and further structural and biochemical studies are necessary to address this possibility.
Structural biology of thermoTRPV channels has provided remarkable insights into ligand but not temperature activation mechanisms. The physicochemical underpinnings of exquisite temperature sensitivity exhibited by thermoTRP channels have been a tremendous endeavor in the field approached by numerous functional studies but remain largely unknown even in light of recent structures [70,71]. A large number of point mutations throughout the channel sequences, including the intracellular N- and C- termini and the pore domains, have been identified to affect temperature activation, and thus the ‘temperature sensor’ accounting for the extraordinary temperature sensitivity is unclear [70,71]. Large changes in heat capacity associated with gating rearrangements and allosteric coupling between sensing domains likely contribute to temperature sensing [70–72]. Undoubtedly, future studies combining structural, functional, biophysical and computational approaches are needed to elucidate molecular mechanisms underlying thermosensation.
5. Symmetry reduction
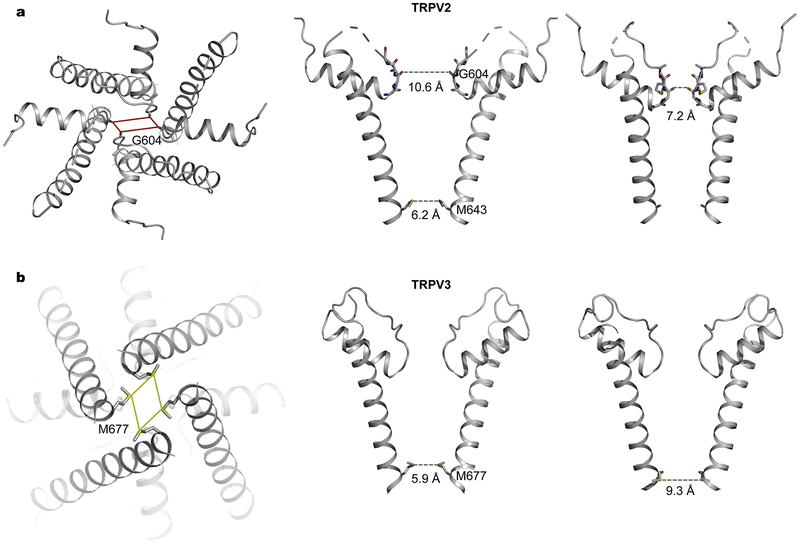
Homotetrameric TRP channels are expected to maintain four-fold symmetric arrangements during gating transitions. Surprisingly, crystal structures of rabbit TRPV2 captured conformations with two-fold symmetry instead [52]. The crystal structure of an engineered rabbit TRPV2 construct in complex with the vanilloid ligand RTX shows more pronounced deviation from four-fold symmetry [52]. RTX binds to all four subunits but the modes of interaction differ in the two adjacent subunits, giving rise to distinct hinge orientations of the pore around the S4–S5 linker and subsequently different subunit arrangements [52]. The selectivity filter exhibits marked two-fold symmetric opening compatible for permeation of large organic cations [52], therefore providing structural evidence explaining the ‘pore dilation’ phenomenon (Fig. 4a) [73]. Subsequently, cryo-EM structures of TRPV2-RTX in amphipols and in nanodiscs demonstrated that two-fold symmetry is an intrinsic property of the channel, which was not induced by crystal packing interactions [74]. Interestingly, RTX-induced two-fold symmetric opening of the SF gate is more prominent for channels embedded in lipid nanodiscs, small patches of lipid bilayer encircled by amphipathic scaffolding proteins, suggesting that two-fold symmetric conformations may occur in biological membranes during physiological activation [74]. Together, X-ray and cryo-EM structures have provided a glimpse into the intricate conformational landscape of TRPV2 [42,52,64,74,75], revealing an intriguingly plastic selectivity filter with varying degrees of opening, which is in marked contrast to the relatively rigid selectivity filter of K+ channels under normal physiological conditions [76].
Fig. 4.
Two-fold symmetry in tetrameric TRPV channels. a, Crystal structure of TRPV2 in complex with RTX (PDB: 6BWJ) showing pronounced symmetry reduction at the selectivity filter. The selectivity filter dimension is shown for each pair of two diagonal subunits. Also shown are the side chains of M643 at the S6 bundle-crossing gate. b, Cryo-EM structure of human TRPV3 in the presence of 2-APB (PDB: 6MHW) showing two-fold symmetry at the S6 bundle-crossing gate. The side chains of M677 are shown for each pair of two diagonal subunits.
In the crystal structure of RTX-bound TRPV2, the SF gate assumes two-fold symmetric opening but S6 remains α-helical and the HBC gate stays closed [52]. RTX binds to the same vanilloid pocket as in TRPV1, which is composed of the intracellular half of S3, S4, S6 and the S4–S5 linker, and therefore it is well positioned to influence channel gating by directly interacting with these critical structural components surrounding the intracellular S6 HBC gate [39,52]. Remarkably, ligand binding stabilizes opening of distinct gates in these two channels, the lower S6 HBC gate in TRPV1 but the upper SF gate in TRPV2 [39,52].
Ligand-induced symmetry reduction from four- to two- fold was also observed in TRPV3 [67]. The apo closed structures of TRPV3 are four-fold symmetric [66,67], whereas in the presence of the agonist 2-APB multiple conformations of TRPV3 distinct from both the apo closed and open structures exist, including two-fold symmetric conformations (Fig. 4b), in which S6 is α-helical and the methionine residues constitute the lower HBC gate as in the apo closed state [67]. However, the distances between pairs of diagonal methionine residues range from 5.2 to 9.3 Å [67], representing dimensions of closed and open gates, respectively. The ligand 2-APB was not identified in these two-fold symmetric structures, and thus ligand-induced conformational changes leading to reduction of symmetry remain unknown [67]. The open conformations of mouse TRPV3 and human TRPV3 K169A return to the canonical four-fold symmetric arrangement with a π-helical turn in S6 and the isoleucine residues forming the HBC gate [44,66].
Collectively, these two-fold symmetric structures, especially structures of TRPV2 embedded in membrane-like nanodiscs [74], have provided evidence that these channels may enter two-fold symmetric conformations induced by stimuli in physiological settings. Interestingly, the two-fold symmetric arrangement is substantial at the SF gate in TRPV2 but at the S6 HBC gate in TRPV3 [52,67]. Ensemble structures with varying degrees of opening at the upper SF and lower HBC gates indicate the plasticity of both gates, which may be linked to the well-appreciated rich gating behavior of TRP channels. So far, the observed fully open conformations of TRPV channels remain four-fold symmetric, and therefore two-fold symmetric arrangements may represent intermediate states en route to opening. It is tempting to speculate that multiple distinct open conformations, such as with two-fold symmetry or with completely α-helical S6, may exist for TRPV channels under certain activation pathways. These alternative open conformations would possess distinctive pore properties stemming from different sets of pore-lining residues and/or structural rearrangements of these residues, further increasing the plasticity and dynamics of the permeation pathway. It remains unknown whether other TRPV channels adopt two-fold symmetry during gating.
6. Channel-lipid interactions
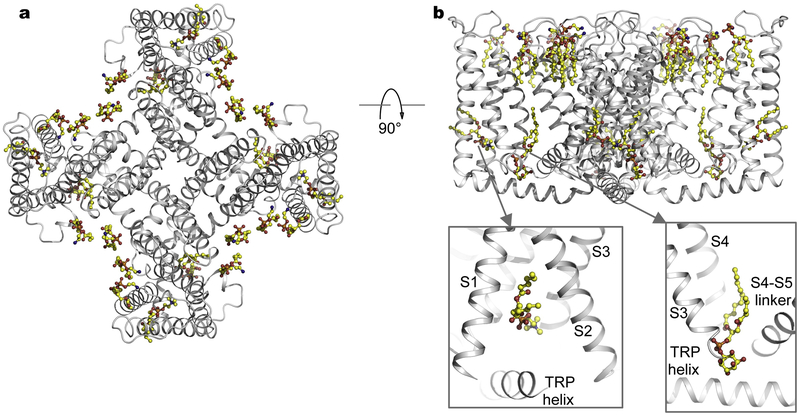
It has been well documented that membrane lipids actively regulate the function of many ion channels and other membrane proteins including TRP channels [77–81], thereby playing a central role in physiology. Single-particle cryo-EM of membrane proteins reconstituted into lipid nanodiscs mimicking biological membranes, has enabled detailed characterization of protein-lipid interactions [82]. Structures of TRPV1 embedded in nanodiscs revealed well-resolved lipid-like densities surrounding the channel (Fig. 5) [83]. Annular lipids in the outer leaflet fill the crevices between channel subunits surrounding the pore domain [83]. The peptide toxin DkTx specifically binds to the outer pore region, stabilizing the open conformation [39]. In the membrane-like environment, tripartite channel-toxin-lipid interface and channel/lipid movement upon toxin binding indicate the involvement of lipids in mediating channel-toxin interactions [83]. A putative phosphatidylinositol lipid molecule resides precisely in the vanilloid-binding pocket of the un-liganded channel, and comparison of the apo and RTX-bound structures suggests that vanilloid ligands competitively displace the resident phosphatidylinositol lipid to activate the channel (Fig. 5b) [83]. Another lipid density tentatively assigned as phosphatidylcholine is located in the hydrophobic cleft within the intracellular half of the S1–S4 domain facing the TRP helix (Fig. 5b) [83]. Interestingly, this site is a common lipid- or ligand- binding pocket recognized in several other TRP channels [42,63,66,84–86]. Structures of TRPV2-RTX in amphipols and nanodiscs showed marked differences [74], further emphasizing the intimate relationship between channel and lipids and potential caveats of structural analyses of membrane proteins in non-lipid environments. Therefore, structures of channels embedded in lipid membranes are crucial for physiological interpretations.
Fig. 5.
Channel-lipid interactions. a, Cryo-EM structure of TRPV1 in lipid nanodiscs (PDB: 5IRZ). Lipid molecules are shown in ball-and-stick representation. b, Orthogonal view as in (a). Also shown are closed-up views of lipid binding in the pocket formed by the TRP helix and the intracellular half of the S1–S4 domain (bottom left) and in the vanilloid pocket (bottom right).
7. Conclusions and future perspectives
Recent breakthroughs in single-particle cryo-EM have facilitated exponential growth of the number of near-atomic structures of TRP channels, which have provided unparalleled insights into ion permeation, ligand recognition, lipid regulation, and gating mechanisms. The intriguingly complex conformational landscapes of TRP channels such as reduced symmetry and varying degrees of gate opening have also emerged, as exemplified by TRPV2 and TRPV3 channels. However, fundamental mechanisms such as temperature activation remain to be elucidated. Structural biology will certainly continue to make strides in advancing our understanding of these fascinating molecules central for physiology.
Highlights.
Cryo-EM and X-ray structures of TRPV channels
Structural basis of ion selectivity and gating
Conformational landscapes of TRPV channels
Channel-lipid interactions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Clapham DE, TRP channels as cellular sensors., Nature. 426 (2003) 517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- [2].Ramsey IS, Delling M, Clapham DE, An introduction to TRP channels, Annu. Rev. Physiol 68 (2006) 619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- [3].Venkatachalam K, Montell C, TRP channels., Annu. Rev. Biochem 76 (2007) 387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Julius D, TRP channels and pain, Annu. Rev. Cell Dev. Biol 29 (2013) 355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- [5].Nilius B, Owsianik G, The transient receptor potential family of ion channels, Genome Biol. 12 (2011) 218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nilius B, Szallasi A, Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine, Pharmacol. Rev 66 (2014) 676–814. doi: 10.1124/pr.113.008268 [doi]. [DOI] [PubMed] [Google Scholar]
- [7].Caterina MJ, a Schumacher M, Tominaga M, Rosen T. a, Levine JD, Julius D, The capsaicin receptor: a heat-activated ion channel in the pain pathway., Nature. 389 (1997) 816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- [8].Caterina MJ, Rosen T. a, Tominaga M, Brake a J., Julius D, A capsaicin-receptor homologue with a high threshold for noxious heat., Nature. 398 (1999) 436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- [9].Güler AD, Lee H, Iida T, Shimizy I, Tominaga M, Caterina M, Heat-evoked activation of the ion channel, TRPV4, J. Neurosci 22 (2002) 6408–6414. doi:20026679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin J-P, Ooi L, Egerton J, Charles KJ, Smart D, Randall a D., Anand P, Davis JB, TRPV3 is a temperature-sensitive vanilloid receptor-like protein., Nature. 418 (2002) 186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- [11].Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE, TRPV3 is a calcium-permeable temperature-sensitive cation channel, Nature. 418 (2002) 181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- [12].Patapoutian A, TRP Channels and Thermosensation, Chem. Senses 30 (2005) i193–i194. doi: 10.1093/chemse/bjh180. [DOI] [PubMed] [Google Scholar]
- [13].Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D, Impaired nociception and pain sensation in mice lacking the capsaicin receptor, Science. 288 (2000) 306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- [14].Bang S, Yoo S, Yang TJ, Cho H, Hwang SW, Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3, J. Biol. Chem 285 (2010) 19362–19371. doi: 10.1074/jbc.M109.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD, Hypotonicity induces TRPV4-mediated nociception in rat, Neuron. 39 (2003) 497–511. doi: 10.1016/S0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- [16].Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD, Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat, J. Neurosci 24 (2004) 4444–4452. doi: 10.1523/jneurosci.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD, OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity., Nat. Cell Biol. 2 (2000) 695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- [18].Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S, Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor., Cell. 103 (2000) 525–535. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liedtke W, Friedman JM, Abnormal osmotic regulation in trpv4−/− mice., Proc. Natl. Acad. Sci. U. S. A 100 (2003) 13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jordt SE, Tominaga M, Julius D, Acid potentiation of the capsaicin receptor determined by a key extracellular site., Proc. Natl. Acad. Sci. U. S. A 97 (2000) 8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McNamara FN, Randall A, Gunthorpe MJ, Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1), Br. J. Pharmacol 144 (2005) 781–790. doi: 10.1038/sj.bjp.0706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lou Gauthier M, Beaudry F, Vachon P, Intrathecal [6]-gingerol administration alleviates peripherally induced neuropathic pain in male sprague-dawley rats, Phyther. Res 27 (2013) 1251–1254. doi: 10.1002/ptr.4837. [DOI] [PubMed] [Google Scholar]
- [23].Nilius B, Appendino G, Spices: The savory and beneficial science of pungency, Rev. Physiol. Biochem. Pharmacol 164 (2013) 1–76. doi: 10.1007/112_2013_11. [DOI] [PubMed] [Google Scholar]
- [24].Siemens J, Zhou S, Piskorowski R, Nikai T, a Lumpkin E, Basbaum AI, King D, Julius D, Spider toxins activate the capsaicin receptor to produce inflammatory pain., Nature. 444 (2006) 208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- [25].Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX, 2-Aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3, J. Biol. Chem 279 (2004) 35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- [26].Hu H, Grandl J, Bandell M, Petrus M, Patapoutian A, Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4., Proc. Natl. Acad. Sci. U. S. A 106 (2009) 1626–1631. doi: 10.1073/pnas.0812209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bisogno T, Hanuš L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V, Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide, Br. J. Pharmacol 134 (2001) 845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Qin N, Neeper MP, Liu Y, Hutchinson TL, Lou Lubin M, Flores CM, TRPV2 Is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons, J. Neurosci 28 (2008) 6231–6238. doi: 10.1523/jneurosci.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, Di Marzo V, Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8, J. Pharmacol. Exp. Ther 325 (2008) 1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- [30].de Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, di Marzo V, Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation, Acta Physiol. 204 (2012) 255–266. doi: 10.1111/j.1748-1716.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- [31].Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE, Bisandrographolide from Andrographis paniculata activates TRPV4 channels, J. Biol. Chem 281 (2006) 29897–29904. doi: 10.1074/jbc.M605394200. [DOI] [PubMed] [Google Scholar]
- [32].Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ESR, Gordon E, Evans L, Misajet BA, DeMarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD, N-((1S)-1- −3-hydroxypropanoyl)-1-piperazinyl]carbonyl}−3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a Novel and Potent Transient Receptor Potential Vanilloid 4 Channel Agonist Induces Urinary Bladder Contraction and Hyperactivity: Part I, J. Pharmacol. Exp. Ther 326 (2008) 432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- [33].Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B, Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives, J. Biol. Chem 277 (2002) 13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- [34].Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B, Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels., Nature. 424 (2003) 434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- [35].Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y, TRPV2 Is a Component of Osmotically Sensitive Cation Channels in Murine Aortic Myocytes, Circ. Res 93 (2003) 829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- [36].Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M, A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel, J. Cell Biol. 161 (2003) 957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wissenbach U, Bödding M, Freichel M, Flockerzi V, Trp12, a novel Trp related protein from kidney, FEBS Lett. 485 (2000) 127–134. doi: 10.1016/S0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- [38].Liao M, Cao E, Julius D, Cheng Y, Structure of the TRPV1 ion channel determined by electron cryo-microscopy., Nature. 504 (2013) 107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cao E, Liao M, Cheng Y, Julius D, TRPV1 structures in distinct conformations reveal activation mechanisms., Nature. 504 (2013) 113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kühlbrandt W, The Resolution Revolution, Science. 343 (2014) 1443–1444. doi: 10.1126/science.1251652. [DOI] [PubMed] [Google Scholar]
- [41].Long SB, Campbell EB, MacKinnon R, Crystal structure of a mammalian voltage-dependent shaker family K+ channel, Science. 309 (2005) 897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- [42].Zubcevic L, Herzik MA, Chung BC, Liu Z, Lander GC, Lee S-Y, Cryo-electron microscopy structure of the TRPV2 ion channel, Nat. Struct. Mol. Biol 23 (2016) 180–186. doi: 10.1038/nsmb.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Deng Z, Paknejad N, Maksaev G, Sala-Rabanal M, Nichols CG, Hite RK, Yuan P, Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms, Nat. Struct. Mol. Biol 25 (2018) 252–260. doi: 10.1038/s41594-018-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zubcevic L, Borschel WF, Hsu AL, Borgnia MJ, Lee S-Y, Regulatory switch at the cytoplasmic interface controls TRPV channel gating, Elife 8 (2019). doi: 10.7554/eLife.47746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Macikova L, Vyklicka L, Barvik I, Sobolevsky AI, Vlachova V, Cytoplasmic Inter-Subunit Interface Controls Use-Dependence of Thermal Activation of TRPV3 Channel, Int. J. Mol. Sci 20 (2019) 3990. doi: 10.3390/ijms20163990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gregorio-Teruel L, Valente P, Liu B, Fernández-Ballester G, Qin F, Ferrer-Montiel A, The Integrity of the TRP Domain Is Pivotal for Correct TRPV1 Channel Gating, Biophys. J 109 (2015) 529–541. doi: 10.1016/j.bpj.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, Chen L, Hu X, Wang H, Wang X, Zhang P, Liu X, Guan L, Tang Y, Yang H, Tu P, Bu D, Zhu X, Wang K, Li R, Yang Y, Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome, Am. J. Hum. Genet 90 (2012) 558–564. doi: 10.1016/j.ajhg.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Teng J, Loukin SH, Anishkin A, Kung C, L596–W733 bond between the start of the S4–S5 linker and the TRP box stabilizes the closed state of TRPV4 channel, Proc. Natl. Acad. Sci (2015) 201502366. doi: 10.1073/pnas.1502366112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bödding M, Droogmans G, Nilius B, Molecular determinants of permeation through the cation channel TRPV4, J. Biol. Chem 277 (2002) 33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- [50].Vennekens R, Hoenderop JGJ, Prenen J, Stuiver M, Willems PHGM, Droogmans G, Nilius B, Bindels RJM, Permeation and gating properties of the novel epithelial Ca2+ channel, J. Biol. Chem 275 (2000) 3963–3969. doi: 10.1074/jbc.275.6.3963. [DOI] [PubMed] [Google Scholar]
- [51].Nilius B, Vennekens R, Prenen J, Hoenderop JGJ, Bindels RJM, Droogmans G, Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC, J. Physiol 527 (2000) 239–248. doi: 10.1111/j.1469-7793.2000.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zubcevic L, Le S, Yang H, Lee SY, Conformational plasticity in the selectivity filter of the TRPV2 ion channel, Nat. Struct. Mol. Biol 25 (2018) 405–415. doi: 10.1038/s41594-018-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Saotome K, Singh AK, Yelshanskaya MV, Sobolevsky AI, Crystal structure of the epithelial calcium channel TRPV6, Nature. 534 (2016) 506–511. doi: 10.1038/nature17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shi N, Ye S, Alam A, Chen L, Jiang Y, Atomic structure of a Na+- and K+- conducting channel, Nature. 440 (2006) 570–574. doi: 10.1038/nature04508. [DOI] [PubMed] [Google Scholar]
- [55].Derebe MG, Sauer DB, Zeng W, Alam A, Shi N, Jiang Y, Miller C, Tuning the ion selectivity of tetrameric cation channels by changing the number of ion binding sites., Proc. Natl. Acad. Sci. U. S. A 108 (2011) 598–602. doi: 10.1073/pnas.1013636108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sauer DB, Zeng W, Canty J, Lam Y, Jiang Y, Sodium and potassium competition in potassium-selective and non-selective channels, Nat. Commun 4 (2013) 2721. doi: 10.1038/ncomms3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Grandl J, Kim SE, Uzzell V, Bursulaya B, Petrus M, Bandell M, Patapoutian A, Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain., Nat. Neurosci 13 (2010) 708–14. doi: 10.1038/nn.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cui Y, Yang F, Cao X, Yarov-Yarovoy V, Wang K, Zheng J, Selective disruption of high sensitivity heat activation but not capsaicin activation of TRPV1 channels by pore turret mutations, J. Gen. Physiol 139 (2012) 273–283. doi: 10.1085/jgp.201110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D, A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain, Cell. 141 (2010) 834–845. doi: 10.1016/j.cell.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Myers BR, Bohlen CJ, Julius D, A Yeast Genetic Screen Reveals a Critical Role for the Pore Helix Domain in TRP Channel Gating, Neuron. 58 (2008) 362–373. doi: 10.1016/j.neuron.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yang F, Cui Y, Wang K, Zheng J, Thermosensitive TRP channel pore turret is part of the temperature activation pathway, Proc. Natl. Acad. Sci 107 (2010) 7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Long SB, Campbell EB, Mackinnon R, Voltage sensor of Kv1.2 : structural basis of electromechanical coupling, Science. 309 (2005) 903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- [63].McGoldrick LL, Singh AK, Saotome K, Yelshanskaya MV, Twomey EC, Grassucci RA, Sobolevsky AI, Opening of the human epithelial calcium channel TRPV6, Nature. 553 (2018) 233–237. doi: 10.1038/nature25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Dosey TL, Wang Z, Fan G, Zhang Z, Serysheva II, Chiu W, Wensel TG, Structures of TRPV2 in distinct conformations provide insight into role of the pore turret, Nat. Struct. Mol. Biol 26 (2019) 40–49. doi: 10.1038/s41594-018-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kasimova MA, Yazici AT, Yudin Y, Granata D, Klein ML, Rohacs T, Carnevale V, A hypothetical molecular mechanism for TRPV1 activation that invokes rotation of an S6 asparagine, J. Gen. Physiol 150 (2018) 1554–1566. doi: 10.1085/jgp.201812124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Singh AK, McGoldrick LL, Sobolevsky AI, Structure and gating mechanism of the transient receptor potential channel TRPV3, Nat. Struct. Mol. Biol 25 (2018) 805–813. doi: 10.1038/s41594-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zubcevic L, Herzik MA, Wu M, Borschel WF, Hirschi M, Song AS, Lander GC, Lee SY, Conformational ensemble of the human TRPV3 ion channel, Nat. Commun 9 (2018) 4773. doi: 10.1038/s41467-018-07117-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Grandl J, Hu H, Bandell M, Bursulaya B, Schmidt M, Petrus M, Patapoutian A, Pore region of TRPV3 ion channel is specifically required for heat activation., Nat. Neurosci 11 (2008) 1007–1013. doi: 10.1038/nn.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kim SE, Patapoutian A, Grandl J, Single Residues in the Outer Pore of TRPV1 and TRPV3 Have Temperature-Dependent Conformations, PLoS One. 8 (2013) e59593. doi: 10.1371/journal.pone.0059593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Feng Q, Temperature Sensing by Thermal TRP Channels. Thermodynamic Basis and Molecular Insights., in: Curr. Top. Membr, Academic Press Inc., 2014: pp. 19–50. doi: 10.1016/B978-0-12-800181-3.00002-6. [DOI] [PubMed] [Google Scholar]
- [71].Castillo K, Diaz-Franulic I, Canan J, Gonzalez-Nilo F, Latorre R, Thermally activated TRP channels: Molecular sensors for temperature detection, Phys. Biol 15 (2018) 021001. doi: 10.1088/1478-3975/aa9a6f. [DOI] [PubMed] [Google Scholar]
- [72].Clapham DE, Miller C, A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 19492–19497. doi: 10.1073/pnas.1117485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chung MK, Güler AD, Caterina MJ, TRPV1 shows dynamic ionic selectivity during agonist stimulation, Nat. Neurosci 11 (2008) 555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- [74].Zubcevic L, Hsu AL, Borgnia MJ, Lee SY, Symmetry transitions during gating of the TRPV2 ion channel in lipid membranes, Elife 8 (2019). doi: 10.7554/eLife.45779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Huynh KW, Cohen MR, Jiang J, Samanta A, Lodowski DT, Zhou ZH, Moiseenkova-Bell VY, Structure of the full-length TRPV2 channel by cryo-EM., Nat. Commun 7 (2016) 11130. doi: 10.1038/ncomms11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R, The open pore conformation of potassium channels., Nature. 417 (2002) 523–546. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- [77].Hilgemann DW, Ball R, Regulation of cardiac Na+, Ca2+- exchange and KATP potassium channels by PIP2, Science. 273 (1996) 956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- [78].Hilgemann DW, Feng S, Nasuhoglu C, The complex and intriguing lives of PIP2 with ion channels and transporters., Sci. STKE 2001 (2001) re19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- [79].Hille B, Dickson EJ, Kruse M, Vivas O, Suh BC, Phosphoinositides regulate ion channels, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1851 (2015) 844–856. doi: 10.1016/j.bbalip.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hansen SB, Lipid agonism: The PIP2 paradigm of ligand-gated ion channels, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1851 (2015) 620–628. doi: 10.1016/j.bbalip.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rohacs T, Phosphoinositide regulation of TRPV1 revisited, Pflugers Arch. Eur. J. Physiol 467 (2015) 1851–1869. doi: 10.1007/s00424-015-1695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Denisov IG, Sligar SG, Nanodiscs for structural and functional studies of membrane proteins, Nat. Struct. Mol. Biol 23 (2016) 481–486. doi: 10.1038/nsmb.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gao Y, Cao E, Julius D, Cheng Y, TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action, Nature. 534 (2016) 347–351. doi: 10.1038/nature17964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hughes TET, Lodowski DT, Huynh KW, Yazici A, Del Rosario J, Kapoor A, Basak S, Samanta A, Han X, Chakrapani S, Zhou ZH, Filizola M, Rohacs T, Han S, Moiseenkova-Bell VY, Structural basis of TRPV5 channel inhibition by econazole revealed by cryo-EM, Nat. Struct. Mol. Biol 25 (2018) 53–60. doi: 10.1038/s41594-017-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Singh AK, Saotome K, McGoldrick LL, Sobolevsky AI, Structural bases of TRP channel TRPV6 allosteric modulation by 2-APB, Nat. Commun 9 (2018) 2465. doi: 10.1038/s41467-018-04828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yin Y, Le SC, Hsu AL, Borgnia MJ, Yang H, Lee SY, Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel, Science. 363 (2019) eaav9334. doi: 10.1126/science.aav9334. [DOI] [PMC free article] [PubMed] [Google Scholar]