Abstract
Purpose.
To characterize folate receptor alpha (FRα) expression in archival and fresh biopsy tumor samples from relapsed ovarian cancer patients.
Methods.
Patients with ovarian tumors amenable to biopsy were eligible to enroll. Eligibility included a minimum requirement of FRα positivity in archival tumor samples (≥25% of cells with ≥2+ staining intensity). Patients received mirvetuximab soravtansine at 6 mg/kg once every 3 weeks. Core needle biopsies were collected before and after treatment and FRα levels assessed by immunohistochemistry. Descriptive statistics were used to summarize the association between receptor expression and response.
Results.
Twenty-seven heavily pre-treated patients were enrolled. Six individuals (22%) did not have evaluable pre-treatment biopsies due to insufficient tumor cells. The concordance of FRα expression in archival and biopsy tissues was 71%, and no major shifts in receptor expression were seen in matched pre- and post-treatment biopsy samples. Adverse events were generally mild (≤grade 2) with keratopathy (48%), fatigue (44%), diarrhea, and blurred vision (each 37%) being the most common treatment-related toxicities. The confirmed objective response rate (ORR) was 22%, including two complete responses and four partial responses. Superior efficacy measures were observed in the subset of patients with the highest FRα levels (ORR, 31%; progression-free survival, 5.4 months).
Conclusion.
Concordance of FRα expression in biopsy versus archival tumor samples suggests that archival tissue can reliably identify patients with receptor-positive tumors and is appropriate for patient selection in mirvetuximab soravtansine clinical trials. Regardless of the tissue source analyzed, higher FRα expression was associated with greater antitumor activity.
Keywords: Ovarian cancer, Antibody-drug conjugate, Clinical trial, Folate receptor alpha, Biopsy
1. Introduction
Folate is a B vitamin that plays essential roles in cellular metabolism as well as DNA synthesis, methylation, and repair [1]. Since folate is lipophilic, it requires active transport into cells. This primarily occurs through interaction with the reduced folate carrier (RFC), a ubiquitously-expressed protein that binds folate and promotes cellular uptake via a bidirectional anion-exchange mechanism [2]. Folate may also enter cells via the process of receptor-mediated endocytosis, facilitated by the folate receptor (FR) family of glycoproteins [3], the best characterized member of which is folate receptor alpha (FRα). In contrast to a highly restricted distribution pattern in normal tissues, aberrant over-expression of FRα is characteristic of a variety of epithelial tumors, including ovarian carcinoma [4]. Although absent from normal ovarian epithelium, approximately 80% of epithelial ovarian cancer (EOC) tumors constitutively express FRα [5]; moreover, elevated receptor expression may be a negative prognostic factor with respect to chemo-therapeutic response in this malignancy [6]. Thus, FRα has emerged as an attractive candidate for molecularly targeted approaches designed to exploit its differential distribution pattern as a novel avenue of therapeutic intervention in EOC [7].
A number of experimental FR-targeting agents have undergone clinical evaluation, including the humanized anti-FRα monoclonal antibody farletuzumab and small molecule folate-cytotoxic agent conjugate vintafolide [8–10]. While well tolerated in human studies, the development of these agents has been hampered due to a lack of meaningful single-agent activity in ovarian cancer patients and only modest efficacy in pivotal trials [11,12]. Notably, the ability of FRα to internalize large molecules makes this receptor well suited for antibody-drug conjugate (ADC)-based therapeutic approaches. ADCs are complex engineered molecules comprised of a monoclonal antibody, directed toward tumor-associated antigens, conjugated via a stable linker to a potent cytotoxic agent. In this manner, ADCs couple the targeting and pharmacokinetic features of the antibody moiety with the additional cancer-killing impact of the cytotoxic payload [13]. Mirvetuximab soravtansine (IMGN853) is an ADC consisting of a humanized anti-FRα antibody linked to the maytansinoid DM4 [14]. High affinity binding of the conjugate to FRα, followed by internalization, results in intracellular accumulation of DM4 - which subsequently acts as a potent antimitotic agent through its ability to suppress microtubule dynamics [15,16]. In addition, the cleavable linker incorporated into mirvetuximab soravtansine allows active DM4 metabolites to diffuse into proximal tumor cells and kill them, an effect termed bystander killing [17]. Confirming design expectations, mirvetuximab soravtansine exhibits robust antitumor activity against FRα-expressing tumors, including preclinical models of EOC [18].
Importantly, a maturing clinical profile has revealed manageable safety and encouraging signs of antitumor activity for mirvetuximab soravtansine in patients with advanced ovarian cancer [19,20]. Continued development of this promising investigational agent requires reliable methods for detecting and quantifying FRα expression in human tumors. Patient selection for studies of mirvetuximab soravtansine is based on FRα positivity of archival tumor samples assessed by immunohistochemistry (IHC). Here we report the findings of an expansion cohort study, opened as part of a Phase I trial of mirvetuximab soravtansine monotherapy (), designed to characterize receptor expression in archival and fresh biopsy samples obtained from a heterogeneous population of relapsed ovarian cancer patients with FRα-positive tumors.
2. Patients and methods
2.1. Patient selection and eligibility criteria
Adults with relapsed EOC, primary peritoneal, or fallopian tube cancer that was amenable to biopsy were eligible to enroll. Patients with primary refractory disease, clear cell or low grade ovarian cancer were excluded. Patients had to have met the minimum requirement of FRα positivity by immunohistochemistry (IHC; ≥25% of tumor staining at ≥2+ intensity), based on assessment of archival tumor specimens. There was no upper limit on the number of prior treatment regimens received. Patients had measurable or non-measureable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [21]. Patients were also required to be ≥18 years of age; have an Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; and have adequate hematologic, renal, and hepatic function. Key exclusion criteria included neuropathy greater than grade 1; any active or chronic corneal disorder; or a previous solid tumor malignancy with <3-year disease-free interval except for adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, or in situ breast cancer. All patients provided written informed consent in accordance with federal, local and institutional guidelines.
2.2. Treatment
Patients were administered mirvetuximab soravtansine intravenously once every 3 weeks at 6.0 mg/kg (adjusted ideal body weight), established as the recommended phase 2 dose (RP2D) during the dose-finding stage of the trial [19]. Patients continued on mirvetuximab soravtansine until intolerable toxicity or adverse events (AEs), disease progression, or investigator/patient decision. The study was conducted in accordance with the US Food and Drug Administration regulations, the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. The study was compliant with Institutional Review Board and Independent Ethics Committee requirements. This trial is registered at Clinical trials.gov ().
2.3. Biomarker analyses
Fresh core needle biopsies were collected prior to the first dose of mirvetuximab soravtansine (pre-treatment) and at Cycle 2, Day 8 (post-treatment). Immunohistochemical (IHC) staining of archival tissues and biopsy samples was performed using an anti-FRα antibody (FOLR1 2.1), generated at ImmunoGen, in an assay developed in collaboration with, and validated at, Ventana Medical Systems (Tucson, AZ). The assay was run on a Benchmark XT IHC staining platform. For scoring purposes, tumor samples were evaluated in a blinded fashion by an independent, board-certified pathologist trained in the scoring algorithm. Percent positivity of all viable tumor cells was reported based on membranous FRα staining intensity, which was scored 0–3 for none (0), weak (1), moderate (2), or strong staining (3), respectively. The presence of ≥100 viable tumor cells was required for scoring. Tumor tissues, both archival and fresh biopsy, were scored for FRα positivity as follows: low, 25–49% of tumor cells with ≥2+ staining intensity; medium, 50–74% of cells with ≥2+ intensity; and high, ≥75% of cells with ≥2+ intensity. Concordance between FRα positivity of archival and fresh biopsy samples was determined by estimating the percentage agreement of matched patient samples that met the enrollment criteria of ≥25% of tumor cells with ≥2+ staining intensity.
2.4. Evaluation of toxicity
Baseline assessments included medical history and physical examination, ECOG performance status, blood chemistry and hematology, ophthalmologic exam, and electrocardiogram. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03 and monitored continuously throughout the study from the time of the first study dose until 28 days after treatment cessation.
2.5. Assessment of clinical activity
During screening, radiological imaging of the chest, abdomen, and pelvis was performed. For patients with measureable disease, overall tumor response was defined by RECIST 1.1 and assessed using computerized tomography scans performed every 6 weeks.
2.6. Statistical considerations
Descriptive statistics were used to summarize demographic and baseline characteristics and additional analyses were performed using SAS statistical software (version 9.4). For the safety evaluations, baseline was defined as the last available assessment prior to Cycle 1, Day 1 and any adverse event with the same onset date as the start of study treatment or later was reported as treatment-emergent. For the efficacy assessments, all response-evaluable patients who had a post-baseline assessment were included in the objective response rate analyses, along with the corresponding exact 95% CIs based on Clopper-Pearson method. Progression-free survival was analyzed using Kaplan-Meier estimates. Subgroup analysis of objective response rate and progression-free survival by FRα expression level was conducted to evaluate the relationship between receptor expression and antitumor activity. No formal statistical hypothesis was tested for association between expression and response.
3. Results
3.1. Patient characteristics
Twenty-seven patients with relapsed ovarian cancer were enrolled in the biopsy expansion cohort and treated with mirvetuximab soravtansine at 6.0 mg/kg. Patient demographics and baseline characteristics are summarized in Table 1. The median age was 62 years (range, 38–76) and the majority of patients were diagnosed with either epithelial ovarian (81%) or fallopian tube cancer (11%). Most patients were white (93%) and had an ECOG performance status of 1 (63%). All individuals were heavily pretreated, with a median of 4 prior systemic therapies (range, 1–11), and all had received prior platinum and taxane exposure.
Table 1.
Patient demographics and baseline characteristics.
| Characteristic | N = 27 N (%) |
|---|---|
| Age, years | |
| Median (range) | 62 (38–76) |
| Race | |
| White | 25 (93) |
| Asian | 2 (7) |
| Primary diagnosis | |
| Epithelial ovarian cancer | 22 (81) |
| Fallopian tube cancer | 3 (11) |
| Carcinosarcoma | 1 (4) |
| Papillary carcinoma | 1 (4) |
| Performance status | |
| 0 | 10 (37) |
| 1 | 17 (63) |
| No. of prior systemic therapies | |
| Median (range) | 4 (1–11) |
| 1–3 | 10 (37) |
| 4–6 | 11 (41) |
| 7+ | 6 (22) |
| Platinum resistance | |
| Yes | 20 (74) |
| No | 7 (26) |
| Prior compound exposure | |
| Platinum | 27 (100) |
| Taxane | 27 (100) |
| Bevacizumab | 23 (85) |
| PARP inhibitor | 9 (33) |
3.2. Adverse events
All 27 patients were included in the safety analyses. A total of 26 (96%) had a treatment emergent adverse event (TEAE) deemed related to study drug. Treatment-related TEAEs occurring in >10% of patients are summarized in Table 2. The major adverse reactions seen across the cohort were keratopathy (48%), fatigue (44%), diarrhea, and blurred vision (each 37%). In all cases these events were either grade 1 or 2 and managed with appropriate supportive care; the corneal abnormalities and blurred vision were mild and reversible. Two patients (7%) reported a grade ≥3 related TEAE. One patient experienced grade 3 hypokalemia and another had grade 4 organizing pneumonia which led to discontinuation but resolved after withdrawal from study. No deaths related to study drug were seen during the study.
Table 2.
Treatment-related adverse events reported in >10% of patients.
| Grade 1 | Grade 2 | Grades 3–4 | All grades | |||||
|---|---|---|---|---|---|---|---|---|
| Adverse event | N | % | N | % | N | % | N | % |
| Keratopathya | 7 | 25.9 | 6 | 22.2 | 0 | 0 | 13 | 48.1 |
| Fatigue | 6 | 22.2 | 6 | 22.2 | 0 | 0 | 12 | 44.4 |
| Diarrhea | 9 | 33.3 | 1 | 3.7 | 0 | 0 | 10 | 37.0 |
| Vision blurred | 4 | 14.8 | 6 | 22.2 | 0 | 0 | 10 | 37.0 |
| Nausea | 6 | 22.2 | 1 | 3.7 | 0 | 0 | 7 | 25.9 |
| Peripheral neuropathyb | 5 | 18.5 | 2 | 7.4 | 0 | 0 | 7 | 25.9 |
| AST increased | 7 | 25.9 | 0 | 0 | 0 | 0 | 7 | 25.9 |
| ALT increased | 6 | 22.2 | 0 | 0 | 0 | 0 | 6 | 22.2 |
| Headache | 4 | 14.8 | 1 | 3.7 | 0 | 0 | 5 | 18.5 |
| Pneumonitis | 3 | 11.1 | 1 | 3.7 | 0 | 0 | 4 | 14.8 |
| Dry eye | 2 | 7.4 | 2 | 7.4 | 0 | 0 | 4 | 14.8 |
| Decreased appetite | 2 | 7.4 | 1 | 3.7 | 0 | 0 | 3 | 11.1 |
| Dyspnea | 1 | 3.7 | 2 | 7.4 | 0 | 0 | 3 | 11.1 |
| Platelet count decreased | 3 | 11.1 | 0 | 0 | 0 | 0 | 3 | 11.1 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Grouped term that includes corneal cyst, corneal deposits, corneal epithelial microcysts, keratitis, keratopathy, and punctate keratitis.
Grouped term that includes neuropathy peripheral, peripheral sensory neuropathy, and paresthesia.
3.3. Concordance of FRα expression in archival and biopsy samples
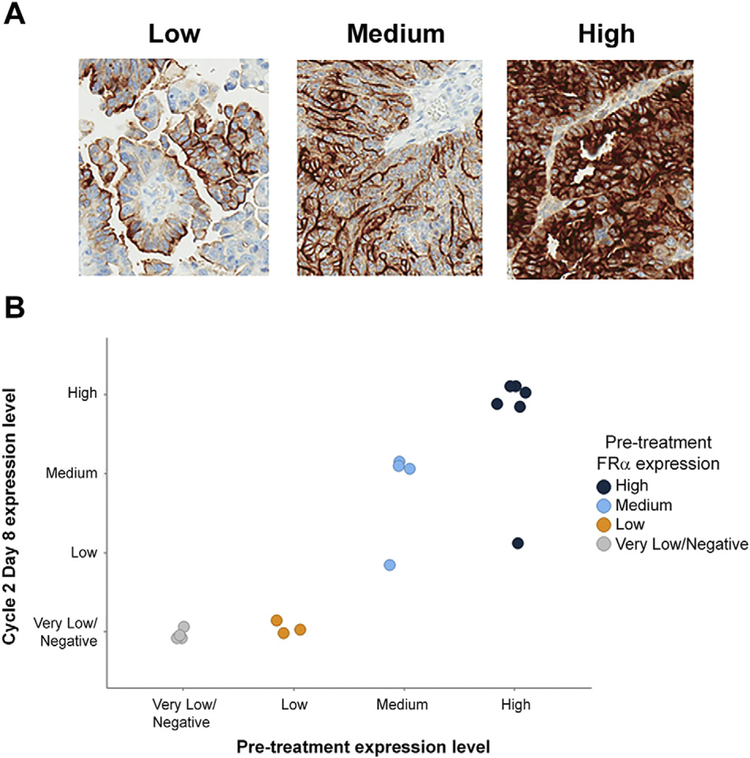
Representative staining images from archival samples are shown in Fig. 1A. Enrollment into the expansion cohort was determined from archival tissues for each patient; all met the enrollment criteria of ≥25% of tumor cells with ≥2+ staining intensity i.e. at least low FRα expression. Six patients (22%) did not have pre-treatment biopsies evaluable for FRα IHC due to a lack of sufficient tumor cells present. Of the 21 evaluable pre-treatment samples, 15 met the eligibility criteria, resulting in a 71% concordance rate with the archival tumor results. The remaining 6 were scored as very low/negative (i.e., <25% with ≥2+ staining). Of note, 2/5 patients deemed to be low expressers based on archival tissue (25–49% with ≥2+ intensity) had medium or high FRα expression in their pre-treatment biopsies (i.e. ≥50%).
Fig. 1.
FRα expression scoring and receptor levels while on treatment with mirvetuximab soravtansine. (A) Representative low, medium, and high staining patterns for FRα (25–49%, 50–74%, and ≥75% of cells with ≥2+ intensity, respectively) from archival tumor specimens. (B) Comparative FRα expression in matched pre- and post-treatment biopsy samples (N = 17). Color coding is based on receptor expression scored for pre-treatment specimens; individual samples are plotted according to Cycle 2, Day 8 groupings. Very low/Negative, <25% of cells with ≥2+ staining intensity.
3.4. Treatment effect on FRα expression in matched biopsy samples
In addition to pre-treatment sampling, biopsies were also taken at Cycle 2, Day 8 in order to evaluate any changes in FRα receptor expression while patients were undergoing mirvetuximab soravtansine therapy. Seventeen matched pre- and post-treatment biopsy pairs were available for analysis. In the majority of cases, FRα expression remained stable following two doses of mirvetuximab soravtansine, although reductions in post-treatment levels were seen in some patients (primarily low expressers; Fig. 1B).
3.5. Clinical activity based on FRα expression
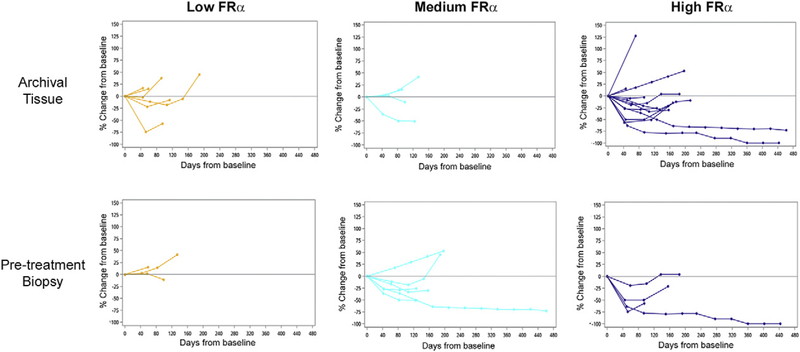
All 27 patients were evaluable for efficacy and included in the analyses. Fig. 2 plots changes in patient target lesion burden as a function of time, with individuals grouped according to FRα levels. Regardless of the tissue source analyzed (archival or pre-treatment biopsy), higher FRα expression was associated with greater antitumor activity. Confirmed tumor responses were observed in 6 patients, comprising 2 complete responses (CR) and 4 partial responses (PR), for an overall objective response rate (ORR) of 22% (Table 3). When patients were sorted based on archival FRα positivity, the ORR in the cohort of patients with high FRα expression was 31% (5/16), which included the two CRs. This was compared to 20% (1/5 patients) observed in the medium cohort, and lack of any objective responses in individuals with low FRα-expressing tumors. Further evidence of a trend toward improved outcomes in patients with higher receptor expression was provided by the progression-free survival (PFS) results (Table 3). The median PFS was 4.2 months (95% CI, 2.8 to 5.4 months) for the overall population, 5.4 months (2.8, −) for the high FRα cohort, 3.9 months (2.6, 12.7) for the medium subset, and 2.8 months (1.3, 5.4) for the low expressers.
Fig. 2.
Percent tumor change in target lesions by archival and pre-treatment biopsy FRα expression. Data are presented from 22 and 13 patients (archival and biopsy, respectively) as target lesion measurements and/or tumor samples evaluable for IHC were not available for some individuals.
Table 3.
Relationship of antitumor activity with archival FRα expression level.
| FRα expression | No. of patients | CR | PR | ORR N (%) | PFS (months) | PFS 95% CI |
|---|---|---|---|---|---|---|
| Low | 6 | 0 | 0 | 0 (0.0) | 2.8 | (1.3, 5.4) |
| Medium | 5 | 0 | 1 | 1 (20.0) | 3.9 | (2.6, 12.7) |
| High | 16 | 2 | 3 | 5 (31.3) | 5.4 | (2.8, −) |
| Overall | 27 | 2 | 4 | 6 (22.2) | 4.2 | (2.8, 5.4) |
CR, complete response; PR, partial response; ORR, objective response rate; PFS, progression-free survival; CI, confidence interval.
4. Discussion
Ovarian cancer is considered a malignancy particularly amenable to the application of FRα-targeting therapeutics [7]. The development of highly selective, directed therapies of this type has necessitated reliable quantification of tumor FRα expression, in order to use this measure as a response-predictive biomarker for patient selection [4]. The initial clinical evaluations of the first folate receptor-targeting agents (e.g. the humanized antibody farletuzumab and the folate-cytotoxic agent conjugate vintafolide) provided critical proof-of-concept evidence for FRα as a druggable target for cancer treatment [22–25]; unfortunately these agents exhibited only modest single-agent activity and neither demonstrated meaningful efficacy over chemotherapy alone when evaluated as part of combination regimens in advanced-stage, recurrent EOC in Phase III trials. Of note, it has been suggested that a contributing factor to these disappointing results, at least for farletuzumab, was a lack of a priori patient selection for FRα expression [8]. In contrast, mirvetuximab soravtansine as monotherapy has shown encouraging evidence of clinical activity in the setting of platinum-resistant disease in early human studies [20], sufficient to prompt the initiation of a pivotal Phase III monotherapy trial in this patient population (FORWARD I, ).
Accurate assessment of a patients’ receptor status has important prognostic and diagnostic implications for the development of FRα-selective investigational agents, particularly in light of the molecular and cellular heterogeneity of EOC. Different methods have been used to detect FRα expression in tumors, including recent efforts to develop non-invasive folate-targeting radioimaging agents [26]. In this regard, 99mTc-etarfolide, a technetium–labeled folate conjugate and companion imaging agent to vintafolide [27], has shown promise in the clinical setting as a means for identifying patients likely to respond to vintafolide treatment [25,28]. Moreover, imaging with this agent demonstrated that folate receptor-positive target lesions were found in over 80% of patients on trial [28], a number in agreement with other reports evaluating receptor expression in ovarian tumors using alternative detection methods [5].
Patient selection for human trials of mirvetuximab soravtansine is based on IHC assessment of archival tumor tissue. This detection method offers the ability to evaluate uniformity and intensity of FRα expression with sensitivity and specificity, as well as the distribution pattern in order to differentiate between membrane and cytoplasmic localization. One caveat of this approach is the possibility that the level of FRα expression observed in archival tissue may not be representative of FRα expression at the time of treatment. Importantly, Despierre and colleagues have reported that FRα expression is not significantly altered in response to chemotherapy, further supporting the rationale for targeting this receptor in the treatment of EOC, whether newly diagnosed or at the time of recurrence [29]. However expression levels may change during the intervening period before enrollment, due to other prior treatment modalities or tumorigenic evolution of the recurrent and/or metastatic lesions. Indeed, biopsy sampling typically occurs in lesions distinct from the original, primary tumors and the inherent degree of cellular heterogeneity exhibited by EOC may influence receptor expression. Therefore this expansion cohort was opened in order to compare FRα expression in archival tumor and fresh biopsy samples from a heterogeneous population of patients with relapsed ovarian cancer. A drawback of biopsy collection uncovered in the study was that a notable proportion (22%) of pre-treatment samples lacked sufficient tumor tissue for evaluation. This was likely related to the intrinsically small sample volume provided by a core biopsy compared to the amount of tissue procured as part of archival tumor collection. However, the high concordance of FRα expression in evaluable pre-treatment biopsies versus archival tumor samples suggests that archival tissue can be reliably used to identify patients with receptor-positive tumors, specifically with respect to pre-specified thresholds of FRα expression. Of interest, two of five patients considered low expressers based on their archival results were subsequently shown to express either medium or high FRα expression in their baseline biopsies. Accordingly, fresh biopsy may now be considered as an alternative option for enrollment purposes in mirvetuximab soravtansine trials if archival tissue is not available or archival FRα levels fail to meet eligibility criteria.
Another objective was to compare potential receptor expression changes in response to mirvetuximab soravtansine therapy using matched pre- and post-treatment biopsy samples. It was found that grouping based on FRα expression was unaffected for the majority of cases following two doses of mirvetuximab soravtansine. Some reductions were observed, particularly in patients whose pre-treatment biopsies showed low FRα levels. Contributing factors to this observation may include ADC-induced receptor internalization, heterogeneous intratumoral expression of FRα, and/or elimination of cells with higher relative receptor levels in response to treatment.
The dosing regimen used here, established as the RP2D during the escalation phase of the same trial [19], was well tolerated by patients, with the majority of toxicities being grade ≤2 and only one patient in this cohort discontinued due to an AE (organizing pneumonia). Ocular disorders, primarily corneal keratopathy and blurred vision (all grade 1 or 2), were among the most frequently observed treatment-related adverse events and were similar in nature to those reported for other DM4-conjugated antibodies [30]. Due to the absence of FRα expression in the eye, it is reasonable therefore to conclude that these effects were off-target and independent of target antigen expression. Indeed, ocular TEAEs have emerged as an important clinical consideration for a wide range of ADCs that are directed toward different antigens and utilize a variety of tubulin-interacting payloads [31]. The other principal events, fatigue and diarrhea, were readily managed with supportive care.
In this heavily pretreated ovarian cancer patient population, mirvetuximab soravtansine demonstrated encouraging signs of clinical activity, consistent with previously reported findings in relapsed EOC [19,20]. Although the small sample sizes precluded formal statistical analysis of the relationship between tumor FRα levels and efficacy, higher FRα expression (regardless of the tissue source analyzed) appeared to be associated with greater antitumor response, indicative of on-target activity. Based on our assay and pre-specified groupings, approximately 60% of patients expressed either medium or high levels of FRα, among which two-thirds were categorized as high expressers. Moreover, the improved ORR (including two CRs) and extended PFS observed in this high expressing subset further underscores the importance of incorporating patient stratification, based on receptor expression status, into the design of clinical trials.
In summary, the concordance of FRα levels in archival tissues and fresh biopsy samples seen in this heterogeneous cohort of relapsed ovarian cancer patients suggests that tumor receptor expression was preserved in the interval between original diagnosis and recurrence, even after multiple lines of prior therapy. Moreover, trends toward improved efficacy outcomes were observed in patients with higher FRα levels. Therefore immunohistochemical assessment of archival tissue is appropriate to guide treatment decisions in the recurrent setting, and more specifically for patient selection in clinical trials of mirvetuximab soravtansine, including the pivotal FORWARD I study in platinum-resistant EOC. Finally, mirvetuximab soravtansine continues to demonstrate manageable safety and promising clinical activity in a population of patients for which few effective therapeutic options are currently available.
HIGHLIGHTS.
Folate receptor alpha (FRα) is a rational therapeutic target in ovarian cancer.
FRα expression was compared in paired archival and fresh biopsy tumor tissues.
71% concordance seen between samples obtained from relapsed ovarian patients.
Archival tissue can reliably identify patients with receptor-positive tumors.
Higher FRα expression was associated with greater antitumor activity in this trial.
Acknowledgments
We wish to thank all the patients who participated and their families, as well as co-investigators, nurses, study coordinators, and operations staff at each of the clinical sites. We thank Marian Themeles for the technical support with the immunohistochemical analysis of the biopsy samples. We also thank Dr. Richard Bates, Sr. Manager of Publications at ImmunoGen, who provided drafts and editorial assistance during the production of this manuscript.
Disclosure statement
This study was supported by ImmunoGen, Inc. Ying Su, Anna Berkenblit, and Rodrigo Ruiz-Soto are current (Y.S., A.B.) or former (R.R.-S.) employees of ImmunoGen. There are no other conflicts of interest to declare.
References
- [1].Choi SW, Mason JB, Folate and carcinogenesis: an integrated scheme, J. Nutr 130 (2000) 129–132. [DOI] [PubMed] [Google Scholar]
- [2].Zhao R, Diop-Bove N, Visentin M, Goldman ID, Mechanisms of membrane transport of folates into cells and across epithelia, Annu. Rev. Nutr 31 (2011) 177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Elnakat H, Ratnam M, Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy, Adv. Drug Deliv. Rev 56 (2004) 1067–1084. [DOI] [PubMed] [Google Scholar]
- [4].Vergote IB, Marth C, Coleman RL, Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications, Cancer Metastasis Rev. 34 (2015) 41–52. [DOI] [PubMed] [Google Scholar]
- [5].Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, et al. , Folate receptor alpha as a tumor target in epithelial ovarian cancer, Gynecol. Oncol 108 (2008) 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Toffoli G, Russo A, Gallo A, Cernigoi C, Miotti S, Sorio R, et al. , Expression of folate binding protein as a prognostic factor for response to platinum-containing chemotherapy and survival in human ovarian cancer, Int. J. Cancer 79 (1998) 121–126. [DOI] [PubMed] [Google Scholar]
- [7].Marchetti C, Palaia I, Giorgini M, De Medici C, Iadarola R, Vertechy L, et al. , Targeted drug delivery via folate receptors in recurrent ovarian cancer: a review, Onco. Targets Ther 7 (2014) 1223–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ledermann JA, Canevari S, Thigpen T, Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments, Ann. Oncol 26 (2015) 2034–2043. [DOI] [PubMed] [Google Scholar]
- [9].Assaraf YG, Leamon CP, Reddy JA, The folate receptor as a rational therapeutic target for personalized cancer treatment, Drug Resist. Updat 17 (2014) 89–95. [DOI] [PubMed] [Google Scholar]
- [10].Lutz RJ, Targeting the folate receptor for the treatment of ovarian cancer, Trans. Cancer Res. 4 (2015) 118–126. [Google Scholar]
- [11].Vergote I, Armstrong D, Scambia G, Teneriello M, Sehouli J, Schweizer C, et al. , A randomized, double-blind, placebo-controlled, phase III study to assess efficacy and safety of weekly farletuzumab in combination with carboplatin and taxane in patients with ovarian cancer in first platinum-sensitive relapse, J. Clin. Oncol 34 (2016) 2034–2043. [DOI] [PubMed] [Google Scholar]
- [12].Endocyte, Inc., Merck and Endocyte Announce Independent DSMB Recommends Vintafolide PROCEED Phase 3 Trial Be Stopped for Futility Following Interim Analysis, Avaialble at http//investor.endocyte.com/releasedetail.cfm?releaseid=844838 2014.
- [13].Chari RV, Miller ML, Widdison WC, Antibody-drug conjugates: an emerging concept in cancer therapy, Angew. Chem. Int. Ed. Engl 53 (2014) 3796–3827. [DOI] [PubMed] [Google Scholar]
- [14].Gunderson CC, Moore KN, Mirvetuximab soravtansine. FRa-targeting ADC; treatment of epithelial ovarian cancer, Drugs Future 41 (2016) 539–545. [Google Scholar]
- [15].Oroudje E, Lopus M, Wilson L, Audette C, Provenzano C, Erickson H, et al. , Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability, Mol. Cancer Ther. 9 (2010) 2700–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Goldmacher VS, Audette CA, Guan Y, Sidhom EH, Shah JV, Whiteman KR, et al. , High-affinity accumulation of a maytansinoid in cells via weak tubulin interaction, PLoS One 10 (2015), e0117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, et al. , Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen, Cancer Res. 66 (2006) 3214–3221. [DOI] [PubMed] [Google Scholar]
- [18].Ab O, Whiteman KR, Bartle LM, Sun X, Singh R, Tavares D, et al. , IMGN853, a folate receptor-alpha (FRalpha)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRalpha-expressing tumors, Mol. Cancer Ther. 14 (2015) 1605–1613. [DOI] [PubMed] [Google Scholar]
- [19].Moore KN, Borghaei H, O’Malley DM, Jeong W, Seward SM, Bauer TM, et al. , Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor a-targeting antibody-drug conjugate, in patients with solid tumors, Cancer (2017) 10.1002/cncr.30736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moore KN, Martin LP, O’Malley DM, Matulonis UA, Konner JA, Perez RP, et al. , Safety and activity of Mirvetuximab Soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study, J. Clin. Oncol 35 (2017) 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1), Eur. J. Cancer 45 (2009) 228–247. [DOI] [PubMed] [Google Scholar]
- [22].Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, et al. , Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study, Clin. Cancer Res. 16 (2010) 5288–5295. [DOI] [PubMed] [Google Scholar]
- [23].Sasaki Y, Miwa K, Yamashita K, Sunakawa Y, Shimada K, Ishida H, et al. , A phase I study of farletuzumab, a humanized anti-folate receptor alpha monoclonal antibody, in patients with solid tumors, Investig. New Drugs 33 (2015) 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lorusso PM, Edelman MJ, Bever SL, Forman KM, Pilat M, Quinn MF, et al. , Phase I study of folate conjugate EC145 (Vintafolide) in patients with refractory solid tumors, J. Clin. Oncol 30 (2012) 4011–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA SA, et al. , PRECEDENT: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer, J. Clin. Oncol 31 (2013) 4400–4406. [DOI] [PubMed] [Google Scholar]
- [26].Sega EI, Low PS, Tumor detection using folate receptor-targeted imaging agents, Cancer Metastasis Rev. 27 (2008) 655–664. [DOI] [PubMed] [Google Scholar]
- [27].Maurer AH, Elsinga P, Fanti S, Nguyen B, Oyen WJ, Weber WA, Imaging the folate receptor on cancer cells with 99mTc-etarfolatide: properties, clinical use, and future potential of folate receptor imaging, J. Nucl. Med 55 (2014) 701–704. [DOI] [PubMed] [Google Scholar]
- [28].Morris RT, Joyrich RN, Naumann RW, Shah NP, Maurer AH, Strauss HW, et al. , Phase II study of treatment of advanced ovarian cancer with folate-receptor-targeted therapeutic (vintafolide) and companion SPECT-based imaging agent (99mTc-etarfolatide), Ann. Oncol 25 (2014) 852–858. [DOI] [PubMed] [Google Scholar]
- [29].Despierre E, Lambrechts S, Leunen K, Berteloot P, Neven P, Amant F, et al. , Folate receptor alpha (FRA) expression remains unchanged in epithelial ovarian and endometrial cancer after chemotherapy, Gynecol. Oncol 130 (2013) 192–199. [DOI] [PubMed] [Google Scholar]
- [30].Parslow AC, Parakh S, Lee F, Gan HK, Scott AM, Antibody-drug conjugates for cancer therapy, Biomedicine 4 (2016) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eaton JS, Miller PE, Mannis MJ, Murphy CJ, Ocular adverse events associated with antibody-drug conjugates in human clinical trials, J. Ocul. Pharmacol. Ther 31 (2015) 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]