Abstract
Nucleotides of transfer RNAs (tRNAs) are highly modified, particularly at the anticodon. Bacterial tRNAs that read A-ending codons are especially notable. The U34 nucleotide canonically present in these tRNAs is modified by a wide range of complex chemical constituents. An additional two A-ending codons are not read by U34-containing tRNAs but are accommodated by either inosine or lysidine at the wobble position (I34 or L34). The structural basis for many N34 modifications in both tRNA aminoacylation and ribosome decoding has been elucidated, and evolutionary conservation of modifying enzymes is also becoming clearer. Here we present a brief review of the structure, function, and conservation of wobble modifications in tRNAs that translate A-ending codons.
tRNA MODIFICATIONS
Base pairing rules established in the context of the DNA double helix generally hold true in RNA, although the greater structural and functional diversity of RNA is facilitated by a wide variety of chemical modifications. Transfer RNA (tRNA) serves a key function in genetic information transfer, accurately deciphering each trinucleotide codon of messenger RNA (mRNA) to produce the encoded polypeptide product. tRNA is the most chemically modified RNA species in any organism, with nucleotide modifications impacting tRNA structure, dynamics, and function. To date over 100 unique RNA modifications have been identified, more than 90 of which are found in tRNA (1–3). On average 17% of tRNA nucleotides are post-transcriptionally modified, more than 10 times the frequency observed in larger RNAs such as ribosomal RNA (rRNA), which is modified at 1–2% (1, 4). Post-transcriptional modifications have been shown to influence translational accuracy by directly regulating base pairing on the ribosome (3, 5), dictating aminoacyl-tRNA synthetase activity (6–9), facilitating peptidyl-tRNA translocation (10), and fine-tuning ribosomal kinetics (11, 12). Post-transcriptional modifications occur throughout tRNA; however, nucleotides in the anticodon loop are particularly highly decorated. Nucleotides N34, the wobble position, and N37, just outside the anticodon, display a larger diversity of modification chemistry than any other tRNA position (1, 4, 13). This review will consider modifications at the N34 wobble position in bacterial tRNAs decoding NNA codons.
NNA DECODING
tRNAs translating NNA codons are typically encoded with a uridine at position 34 (U34), as expected according to Watson-Crick rules, and have the intrinsic potential to decode both NNA and NNG codons by classical wobble pairing (14). Such expanded decoding can be advantageous for an organism, as a single tRNA can read both codons. In some cases, however, codon–anticodon wobble pairing could be detrimental to genetic code fidelity. In either situation organisms have evolved post-transcriptional modification schemes to enforce the correct decoding function of each tRNA (15, 16). The nature of these modifications varies among tRNA isoacceptors and across the domains of life, but the high frequency of U34 modification is conserved.
ESCHERICHIA COLI U34 MODIFICATIONS
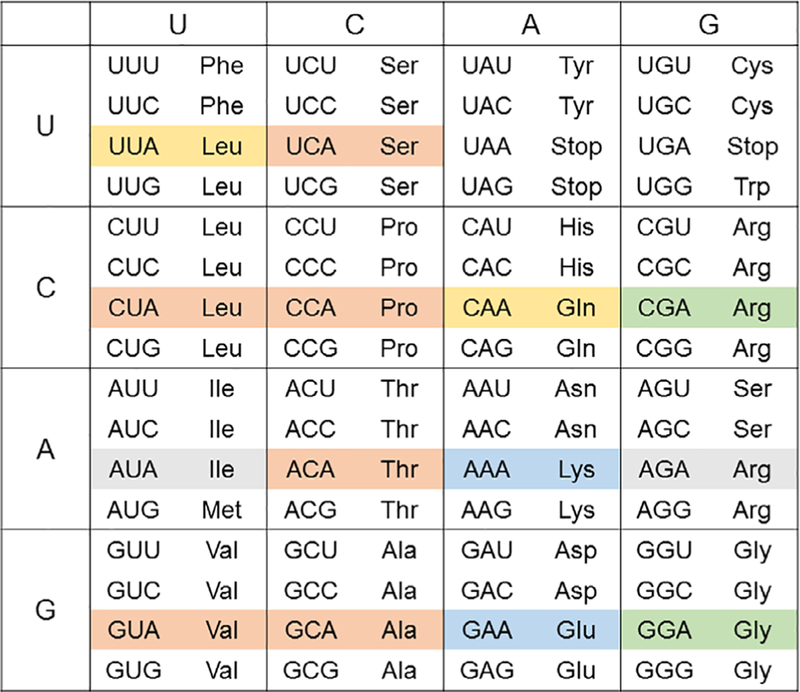
There are 16 A-ending trinucleotides in the Universal Genetic Code, of which two are the ochre (UAA) and opal (UGA) stop codons. Escherichia coli encodes 29 U34-containing tRNAs to translate 12 sense codons (highlighted in Fig. 1) (17), leaving two A-ending sense codons to be decoded through adaptation of other tRNAs. All U34-containing E. coli tRNAs are post-transcriptionally modified at the wobble position with a total of five unique species (15). The most frequent modification is uridine 5-oxyacetic acid (cmo5U), which is observed in tRNALeuUAG, tRNAValUAC, tRNASerUGA, tRNAThrUGU, tRNAAlaUGC, and tRNAProUGG (16, 18). Codons read by cmo5U-containing tRNAs are highlighted in orange on the Universal Genetic Code table (Fig. 1). Next, tRNAArgUCG and tRNAGlyUCC both contain a 5-methylaminomethyluridine (mnm5U) to translate codons CGA and GGA (highlighted in green in Fig. 1) (19, 20). tRNAGluUUC and tRNALysUUU also have mnm5U34 but are further differentiated through the addition of a 2-thio group, forming 5-methylaminomethyl-2-thiouridine (mnm5s2U) (reading codons highlighted in blue, Fig. 1) (21). tRNAGln is found with the 5-carboxymethylaminomethyl-2-thiouridine (cmnm5s2U) at position 34 while tRNALeuUAA presents a 5-carboxymethylaminomethyl-2´-O-methyluridine (cmnm5Um) (reading codons highlighted in yellow, Fig. 1) (22, 23). Each category of modification will be addressed below in the context of bacterial translation, with respect to structure of the chemical moiety, the enzymatic pathway, how decoding is facilitated, and evolutionary conservation, if known.
FIG 1.
The universal genetic code. Codons highlighted in orange are translated in bacteria by tRNAs containing cmo5U, those in green by tRNAs with mnm5U, those in blue by tRNAs with mnm5s2U, and those in yellow by tRNAs with cmnm5Um. Codons highlighted in gray are translated by tRNAs with L34 or I34.
CMO5U MODIFICATION
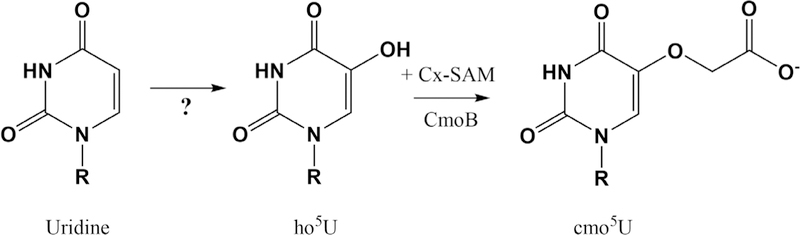
All E. coli tRNAs with a cmo5U modification also have a purine at nucleotide 35 and read codons from fourfold degenerate codon boxes (Fig. 1). The pathway for cmo5U formation is incomplete, as the enzyme responsible for conversion of uridine to 5-hydroxyuridine (ho5U) remains unknown (24). Installation of the carboxylmethyl group is achieved in E. coli by carboxy-S-adenosyl-L-methionine synthase (CmoA), which generates an unusual Cx-SAM moeity, and tRNA U34 carboxymethyltransferase (CmoB), which transfers Cx-SAM to ho5U (Fig. 2) (25–27). The presence of the cmo5U modification was originally proposed on theoretical grounds to expand the base pairing ability of the tRNA beyond Watson-Crick and wobble pairing so as to include the ability to form the cmo5U:U base pair (18). Subsequent in vivo work using knockouts of individual tRNA or modifying enzyme genes demonstrated that some but not all cmo5U34-containing tRNAs are able to efficiently decode even NNC codons (16, 27). For example, Salmonella enterica decodes its four CCN proline codons with three tRNAs, but all four codons can be read with the single tRNAProcmo5UUG species to maintain robust cell growth in the absence of G34- and C34-containing isoacceptors (27). Similarly, E. coli tRNAVal and tRNAAla species with cmo5U34 can partially rescue growth phenotypes arising from knockdown of the other cellular valine and alanine tRNAs (16). However, the expanded decoding efficiency is not used equally, even within a given species. A similar attempt to decode all S. enterica ACN threonine codons with a single tRNAThrcmo5UGU was not successful (16). Depletion of the S. enterica cmoB gene results in tRNAs devoid of cmo5U; inefficient decoding of G-ending proline, valine, and alanine codons demonstrated that cmo5U is important even for the predicted “normal” wobble pairing to occur (16).
FIG 2.
Biosynthesis of uridine 5-oxyacetic acid (cmo5U). R represents the 5′-phosphoribosyl group of the tRNA backbone. The enzyme responsible for hydroxylation remains unknown. Carboxy-S-adenosyl-Lmethionine (Cx-SAM) is synthesized by carboxy-S-adenosyl-L-methionine synthase (CmoA) and transferred to ho5U by tRNA U34 carboxymethyltransferase (CmoB).
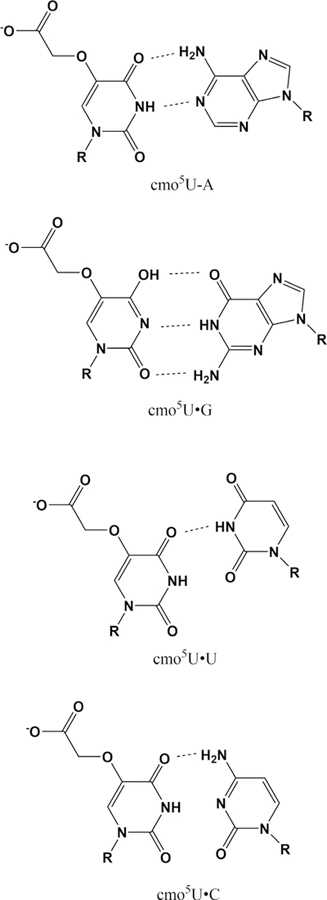
The structural basis for enhanced decoding by cmo5U incorporation was observed in crystal structures of the Thermus thermophilus 30S subunit in complex with GUN-containing mRNA oligonucleotides and a cmo5U tRNAVal anticodon stem-loop (ASLVal) (28). Surprisingly, both the cmo5U:A and cmo5U: G-containing complexes exhibit Watson-Crick geometry; this indicates that the modified uridine adopts the enol tautomer in the cmo5U:G pair. The cmo5U:U and cmo5U:C pairs exhibit a single hydrogen bond in each case (Fig. 3). The nonwobble geometry enables stacking of cmo5U with ASLVal A35 (position 35 is always a purine for cmo5U-containing tRNAs) (28). While earlier NMR studies on nucleotide monophosphates suggested the ribose of cmo5U would adopt a C2’-endo conformation (18), all four crystal structures exhibit a C3′-endo conformation for the modified uridine (28). A parallel solution study of ASLValUAC in the absence and presence of cmo5U revealed that the modification serves to preorder the anticodon loop for codon binding (29).
FIG 3.
Observed cmo5U pairs in decoding. R represents the 5′-phosphoribosyl group of the RNA backbone. The observed cmo5U G geometry suggests an enol tautomer for the modified base. Figure adapted from Weixlbaumer et al., Nat. Struct. Mol. Biol., 2007, 14, 498–502 (28).
XM5U MODIFICATIONS
This class of modifications in bacteria include mnm5U, cmnm5Um, and the thiolated derivatives mnm5s2U and cmnm5s2U, which are generated by a network of modifying enzymes in multiple steps (Fig. 4) (30–33). These modifications are parallel to the mcm5U and mcm5s2U nucleotides found in eukaryotic tRNAs (2, 32). The bacterial xm5U modifications (where x is a methylamino or carboxymethylamino moiety) are primarily found in tRNAs that decode two-fold degenerate codon boxes, those split between pyrimidine-ending and purine-ending trinucleotides (33). The exceptions are tRNAArgmnm5UCU and tRNAGlymnm5UCC, which read the purine-ending codons in fourfold degenerate boxes. The formation of xm5U modifications is catalyzed by the heterotetrameric MnmEG complex (previously annotated as TrmE and GidA) (33, 34). MnmEG can use either NH3 or glycine as its substrate resulting in the formation of nm5U or cmnm5U, respectively (33). The cmnm5U is then converted to mnm5U through a FAD-and SAM-dependent manner by MnmC1/2; nm5U conversion to mnm5U requires only the SAM-dependent MnmC2 step (35–37). mnm5U can then be further derivatized to mnm5s2U by the IscS-MnmA enzyme complex using cysteine as the sulfur donor (38). Isolation of tRNA harboring either the mnm5U or the s2U partially modified states indicates that the modifications are independent of each other (39, 40). The original cmnm5U can also be further derivatized by either the IscS-MnmA or TrmL to yield cmnm5s2U or cmnm5Um, respectively (38, 41, 42).
FIG 4.
Synthesis of xm5U modifications. R represents the 5′-phosphoribosyl group of the tRNA backbone. Installation of s2U is a multi-step sulfur trafficking pathway. Figure adapted from Armengod et al., Biochimie, 94, 1510–1520 (33).
MnmE and MnmG are nearly universal in bacteria, missing only in Mycoplasma suis, while TrmL is missing only in six species of mollicutes (43). Conservation of these enzymes suggests that xm5U34 modifications are important for translational fidelity.
Unlike cmo5U, the xm5U modifications do not expand the base pairing potential past the Watson-Crick and wobble pairing seen in unmodified U but they do enhance pairing to codon A and G nucleotides (30–32). An atomic-level perspective was obtained from crystal structures of E. coli tRNALysmnm5s2UUU bound to the T. thermophilus 70S subunit programmed with mRNA containing either AAA or AAG in the A-site (44). The hypermodified wobble base decodes its two cognate codons with distinct geometries (Fig. 5). While the mnm5s2U34-A pair has canonical Watson-Crick pairing, the mnm5s2U34 G did not adopt a typical wobble conformation, in which the U shifts toward the major groove of the codon–anticodon duplex. Instead, the modified uridine shifts toward the minor groove and apparently makes two hydrogen bonds to the N1 and N2 atoms of the codon guanine. The authors rationalize this geometry as arising from either a zwitterionic modified base or an unusual enol tautomer. One possible ionization state and one possible tautomer is shown (Fig. 5).
FIG 5.
Observed mnm5s2U pairs in decoding. R represents the 5′-phosphoribosyl group of the RNA backbone. The mnm5s2U-A pair exhibits Watson-Crick geometry (top), while the mnm5s2U G base edge distances suggest the modified uridine is either ionized (bottom left) or adopts an enol tautomer (bottom right). Figure adapted from Rozov et al., Nat. Commun., 7, 10457 (44).
ROLE OF S2U MODIFICATION
tRNAs containing U34 together with U35 (those decoding Gln, Glu, and Lys codons) are further modified by thioketone substitution at the O2 position of U34 (45–48). As demonstrated in both solution studies and crystal structures, the larger atomic radius of sulfur compared to oxygen forces a ribose C3′-endo conformation (13). This further enhances stacking interactions with U35, serving to preorder the anticodon for ribosomal decoding. The s2U is thought to restrict and stabilize the conformation of both U34 and U35 in these cases (49, 50). NMR experiments of tRNALys ASL in various states of modification showed that not only does the 2-thio modification promote base stacking between U34 and 35 but also with nucleotide 36, enhancing the interactions between nucleotides 35 and 36 of the anticodon and position one and two of the codon (51, 52).
Early studies using both chemical derivatization and sulfur starvation demonstrated the importance of the s2U modification for aminoacylation of tRNALys, tRNAGln, and tRNAGlu by their corresponding aminoacyl-tRNA synthetases (AARSs) (48, 53). The role of s2U34 in these tRNAs was revealed by comparing in vitro transcribed tRNAs devoid of modifications with partially or fully modified tRNAs. These experiments demonstrated that s2U34 contributed more to aminoacylation activity than did other modifications, including the xm5U34 substitutions (47). In particular the KM of the transcript tRNAGlu was 200-fold higher than that of the native tRNAGlu, suggesting that the wobble modification is a strong identity element for E. coli GluRS (54). The importance of the s2U34 modification in aminoacylation may also be derived from its influence on overall tRNA structure. Among the 20 aminoacyl-tRNA synthetases, only three require tRNA binding for the first step of aminoacylation, amino acid activation by condensation with ATP to form an enzyme-bound aminoacyl-adenylate. These three are the class I enzymes GluRS, GlnRS, and ArgRS (55). Modification-dependent structural variation was observed upon chemical probing of E. coli tRNAGlu “modivariants,” tRNAGluUUC species isolated from cells with modification heterogeneity due to over-production of the tRNA on a plasmid (56). Kinetic parameters for glutamyl-adenylate formation (in the presence of tRNAGlu) and aminoacylation were also impaired by about two orders of magnitude (6). This led to the suggestion that lack of the thioketone in particular may alter the structure of tRNAGlu in a manner that impacts both adenylate formation and amino acid transfer.
Biochemical observations regarding 2-thioketone importance were further validated when crystal structures for GlnRS: tRNAGln and LysRS:tRNALys complexes were solved, as both enzymes make direct contact with the modified wobble position (57, 58). One question that remained was how AARSs can recognize both modified and unmodified anticodon bases in a set of isoaccepting tRNAs. Crystal structures of E. coli GlnRS bound to tRNAGln isoacceptors reveal that the enzyme makes several hydrogen bonds with C34 of tRNAGlnCUG but U34 of the unmodified tRNAGlnUUG isoacceptor lacks any favorable hydrogen bonds (7). Furthermore, the s2U moiety of fully modified tRNAGlncmnm5s2UUG is nestled tightly into a pocket of GlnRS that contains both polar and hydrophobic groups. In the corresponding structure of unmodified tRNAGlnUUG the 2-oxo group is displaced 1.5 Å from the protein compared to the location of the 2-thio group, suggesting that the protein pocket is ideally suited for the large, polarizable sulfur moiety and that unmodified uridine is electrostatically excluded (7). Thus the enzyme is conformationally plastic with respect to its cognate anticodons while binding only weakly to the tRNA with an unmodified U34.
The importance of s2U34 in bacterial tRNAGln, tRNAGlu, and tRNALys isoacceptors is highlighted by the conservation of the thiouridylase enzyme MnmA, which is proposed to be in the minimal set of genes required for bacteria and is retained even in organisms with severely condensed genomes (43, 59). The network of sulfur relay enzymes required to enable tRNA thiolation by MnmA is not conserved, however. Instead of the IscS-MnmA complex described above, Bacillus subtilis and other gram-positive bacteria use an alternate path that includes the cysteine desulfurase YrvO and direct sulfur transfer to MnmA (60).
tRNAs THAT DECODE NNA CODONS WITHOUT U34
The CGA and the AUA codons in E. coli (highlighted in gray, Fig. 1) are translated by tRNAs lacking the expected U34. The CGA arginine codon is translated by a tRNA originally transcribed with an ACG anticodon, which is then modified by a tRNA-specific adenosine deaminase (TadA) enzyme to form the mature inosine base (61, 62). The AUA isoleucine codon is translated by a tRNA originally transcribed with a CAU anticodon, canonically dedicated to reading the lone AUG methionine codon. The specificity of this minor tRNAIle2 is switched from AUG to AUA by incorporation of a lysidine modification (L) at the wobble position (8, 63). These two modification pathways will be discussed separately.
INOSINE MODIFICATION
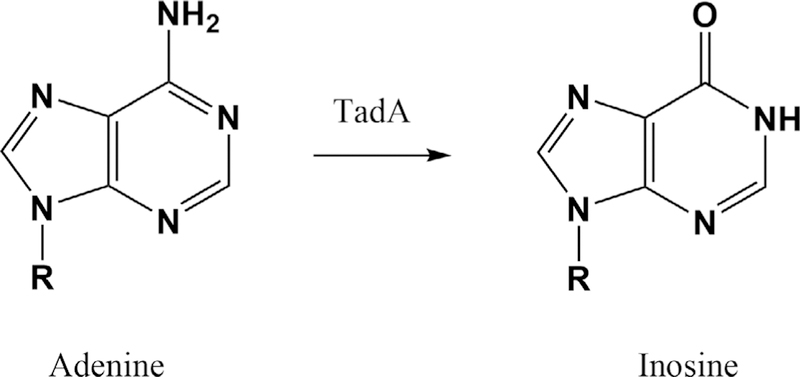
Inosine at position 34 expands the base pairing potential of a tRNA anticodon to include cytosine, adenosine, and uridine, as predicted in Crick’s original wobble hypothesis (14). Inosine was the first nonstandard nucleotide identified in a tRNA anticodon (64). Deamination of A34 to generate inosine is catalyzed in bacteria by TadA (Fig. 6), likely evolved from a cytosine deaminase (65). tRNAArgACG is the canonical target of TadA, which is essential in E. coli and other bacteria that use inosine to decode the arginine GCU/C/A codons (43, 59). Eukaryotes have a wider range of I34-containing tRNAs, produced by the action of adenosine deaminase acting on tRNA (ADAT) (66); archaea lack I34 tRNAs (67). A recent tRNAome analysis of A34 distribution suggested that some bacteria could expand their use of inosine-wobbling tRNAs. Indeed, Oenococcus oeni tRNALeuAAG was shown to contain I34, presumably generated by TadA (65). Although essential in E. coli, TadA is not widely distributed (4, 43, 68). Loss of TadA function correlates with the emergence of new tRNA genes to cover all arginine codons (69).
FIG 6.
Inosine formation. R represents the 5′-phosphoribosyl group of the tRNA backbone. A34 is deaminated by bacterial tRNA adenosine deaminase A (TadA) to produce I34-containing tRNAArg.
LYSIDINE MODIFICATION
The AUN codon box (Fig. 1) is the only position in the Universal Genetic Code where purine ending codons (NNR) are split between two amino acids, isoleucine and methionine. In all other cases either both NNR codons are assigned to the same amino acid or one is a stop codon. Use of a tRNAIleUAU to decode the AUA isoleucine codon would be detrimental to translational accuracy because of the potential to decode the AUG methionine by standard wobble. Thus the AUN codon box presents an example of cellular wobble avoidance. To circumvent isoleucine versus methionine ambiguity, each branch in the tree of life has evolved a distinct conserved strategy for AUA decoding. Bacteria use tRNA isoleucine lysidine synthetase (TilS) to convert immature tRNAIle2CAU to tRNAIle2LAU, which enables efficient AUA translation and AUG exclusion (Fig. 7) (63, 70, 71). Archaea adopt a parallel modification strategy in which tRNA isoleucine agmatidine synthetase (TiaS) generates agmatidine on the tRNAIle2CAU wobble position (5, 72, 73). Eukaryotes use the expanded decoding capacity of inosine to translate the three isoleucine codons (74).
FIG 7.
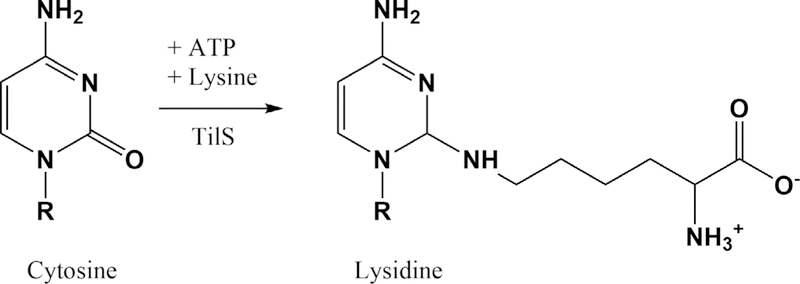
Lysidine synthesis. Lysidine (k2C or L) enables decoding of the minor AUA isoleucine codon while preventing wobble with the AUG methionine codon.
Lysidine (k2C or L) is formed by the attachment of a lysine moiety to the 2-carbon of C34; this reaction is ATP-dependent and proceeds through a cytosine adenylate intermediate (70, 75). Unlike the previously described modifications occurring in tRNAs decoding the NNA codon, lysidine eliminates the potential for base pairing with any codon other than AUA, so here modification restricts rather than expands decoding potential (8, 63).
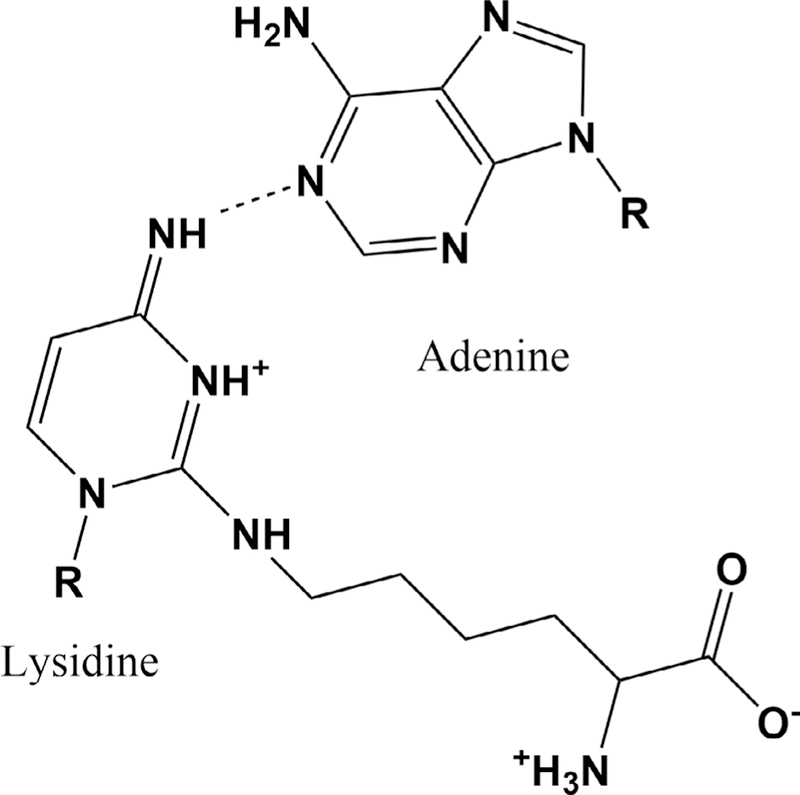
While there is no available crystal structure of tRNAIle2LAU decoding, the parallel agmatidine-containing tRNAIle2 from Haloarcula marismortui was solved in complex with T. thermophilus 70S ribosomes and an A-site AUA codon (76). The structure suggests a single H-bond between N1 of the codon adenine and the imine N4 of agm2C34. Additionally, the terminal amine of agmatidine makes a hydrogen bond with the down-stream mRNA backbone (76). Similar contacts can be made in the bacterial lysidine-adenine pair (Fig. 8). Lysidine in the wobble position of tRNAIle2 appears to exclude the AUG methionine codon because of steric clash with the guanine exocyclic amine.
FIG 8.
Proposed lysidine:adenine base pair. There are several tautomers of lysidine that could form one or two hydrogen bonds with adenine; this pair is suggested by the observed agmatidine:adenine structure in the context of ribosome decoding.
The ability of IleRS to aminoacylate both tRNAIle2LAU and tRNAIleGAU suggests that the synthetase does not use lysidine as a positive determinant for aminoacylation. In the crystal structure of Staphylococcus aureus IleRS in complex with tRNAIleGUA, the anticodon adopts an unusual conformation but there is no direct readout of the wobble base (77). As unmodified tRNAIle2CAU is unable to be aminoacylated by E. coli IleRS, it is more likely that the C34 nucleotide serves as an antideterminant for aminoacylation by IleRS than that L34 is a positive identity element (8). However, tRNAIle2CAU productively interacts with E. coli MetRS, which suggests a critical role for TilS in maintaining translational fidelity (8, 9). The extent to which this interaction occurs seems to be species dependent, as MetRS enzymes from varying organisms have a preference for tRNAMet over unmodified tRNAIle2 ranging from twofold to 2000-fold (78). This suggests that in some organisms, unmodified tRNAIle2 is a viable substrate for MetRS while in others it is not a likely substrate. Furthermore, the relative usage frequency of the isoleucine AUA codon varies across bacterial species. Both TilS efficiency and MetRS selectivity may vary with the cellular need for tRNAIle2LAU, and codon-biased gene expression may be a regulatory strategy used by some organisms.
While TilS is among the most highly conserved modifying enzymes in bacteria (present in 98% of bacteria studied in one analysis) (43, 59, 68), there are alternative routes to AUA decoding. A thermosensitive conditionally lethal mutant of tilS was generated in B. subtilis, and suppressor mutants were obtained. The two suppressor variants that occurred outside the tilS gene were located in genes for tRNAIleGAU, which had the wobble position nucleotide mutated to thymine, resulting in a tRNAIleUAU (79). While these suppressors were able to incorporate isoleucine at AUA codons in the absence of TilS, a low rate of mistranslation (AUA to methionine and AUG to isoleucine) was also observed (79). In contrast to the engineered depletion of TilS function in B. subtilis, Mycoplasma mobile lacks the tilS gene entirely (80). While a tRNAIle2UAU is used to translate the AUA codon, discrimination against AUG is maintained. Although the mechanism to ensure accurate AUA/AUG translation is not fully characterized, it seems the M mobile ribosome has adapted to reject the tRNAIle2UAU/AUG wobble (80).
WOBBLE MODIFICATIONS IN TRANSLATIONAL REGULATION
In more than 50 years since the Wobble Hypothesis was first proposed, much has been learned about the extent of chemical modifications present in tRNAs, the enzymes that catalyze their synthesis, and the structural basis for efficient wobble decoding (or its exclusion). This rich knowledge base now enables the study of dynamic changes in modification patterns during the cellular life cycle (81). Given the role of wobble modifications in recognition of particular codons, it is not surprising that modifications to the wobble uridine described here are among those utilized for codon-biased response to cellular stress (82). For example, hypoxia increases the level of cmo5U modification in Mycobacterium bovis tRNAThrUGU, leading to enhanced translation of codon-biased persistence genes (83). Rare codons that require wobble modifications for decoding, such as the isoleucine AUA, are a prime candidate for modification-regulated proteome adaptation.
ACKNOWLEDGEMENTS
This work was supported by NSF MCB-181831 (RWA) and by a pilot grant from the Wake Forest University Center for Molecular Signaling. EMN was supported by an NIH T32 fellowship (GM095440–06) to Wake Forest School of Medicine.
Abbreviations:
- AARS
aminoacyl-tRNA synthetase (individual AARS enzymes are named using the 3-letter abbreviations for their cognate amino acids)
- ASL
anticodon stem-loop
- I
inosine
- L
lysidine
- N
any standard ribonucleotide (A, C, G, U)
- NMR
nuclear magnetic resonance
Footnotes
The authors declare they have no conflict of interest.
REFERENCES
- [1].Jackman JE, and Alfonzo JD (2013) Transfer RNA modifications: nature’s combinatorial chemistry playground. Wiley Interdiscip Rev RNA 4, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rozenski J, Crain PF, and McCloskey JA (1999) The RNA modification database: 1999 update. Nucleic Acids Res. 27, 196–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agris PF, Narendran A, Sarachan K, Väre VYP, and Eruysal E (2017) The importance of being modified: the role of RNA modifications in translational fidelity. Enzyme 41, 1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Machnicka MA, Olchowik A, Grosjean H, and Bujnicki JM (2014) Distribution and frequencies of post-transcriptional modifications in tRNAs. RNA Biol. 11, 1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Numata T (2015) Mechanisms of the tRNA wobble cytidine modification essential for AUA codon decoding in prokaryotes. Biosci. Biotechnol. Biochem 79, 347–353. [DOI] [PubMed] [Google Scholar]
- [6].Madore E, Florentz C, Giegé R, Sekine S, Yokoyama S, et al. (1999) Effect of modified nucleotides on Escherichia coli tRNAGlu structure and on its aminoacylation by glutamyl-tRNA synthetase. Predominant and distinct roles of the mnm5 and s2 modifications of U34. Eur. J. Biochem 266, 1128–1135. [DOI] [PubMed] [Google Scholar]
- [7].Rodriguez-Hernandez A, Spears JL, Gaston KW, Limbach PA, Gamper H, et al. (2013) Structural and mechanistic basis for enhanced translational efficiency by 2-thiouridine at the tRNA anticodon wobble position. J. Mol. Biol 425, 3888–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, et al. (1988) Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature 336, 179–181. [DOI] [PubMed] [Google Scholar]
- [9].Soma A, Ikeuchi Y, Kanemasa S, Kobayashi K, Ogasawara N, et al. (2003) An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol. Cell 12, 689–698. [DOI] [PubMed] [Google Scholar]
- [10].Phelps SS, Malkiewicz A, Agris PF, and Joseph S (2004) Modified nucleotides in tRNA(Lys) and tRNA(Val) are important for translocation. J. Mol. Biol 338, 439–444. [DOI] [PubMed] [Google Scholar]
- [11].Ranjan N, and Rodnina MV (2017) Thio-modification of tRNA at the wobble position as regulator of the kinetics of decoding and translocation on the ribosome. J. Am. Chem. Soc 139, 5857–5864. [DOI] [PubMed] [Google Scholar]
- [12].Krüger MK, Pedersen S, Hagervall TG, and Sørensen MA (1998) The modification of the wobble base of tRNAGlu modulates the translation rate of glutamic acid codons in vivo. J. Mol. Biol 284, 621–631. [DOI] [PubMed] [Google Scholar]
- [13].Agris PF, Eruysal ER, Narendran A, Väre VYP, Vangaveti S, et al. (2018) Celebrating wobble decoding: half a century and still much is new. RNA Biol. 15, 537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crick FH (1966) Codon-anticodon pairing: the wobble hypothesis. J. Mol. Biol 19, 548–555. [DOI] [PubMed] [Google Scholar]
- [15].Schaffrath R, and Leidel SA (2017) Wobble uridine modifications-a reason to live, a reason to die?! RNA Biol. 14, 1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Näsvall SJ, Chen P, and Björk GR (2007) The wobble hypothesis revisited: uridine-5-oxyacetic acid is critical for reading of G-ending codons. RNA 13, 2151–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chan PP, and Lowe TM (2016) GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 44, D184–D189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yokoyama S, Watanabe T, Murao K, Ishikura H, Yamaizumi Z, et al. (1985) Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl. Acad. Sci. U. S. A 82, 4905–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sierzputowska-Gracz H, Sochacka E, Malkiewicz A, Kuo K, Gehrke CW, et al. (1987) Chemistry and structure of modified uridines in the anticodon, wobble position of transfer RNA are determined by thiolation. J. Am. Chem. Soc 109, 7171–7177. [Google Scholar]
- [20].Sakamoto K, Kawai G, Niimi T, Satoh T, Sekine M, et al. (1993) A modified uridine in the first position of the anticodon of a minor species of arginine tRNA, the argU gene product, from Escherichia coli. Eur. J. Biochem 216, 369–375. [DOI] [PubMed] [Google Scholar]
- [21].Carbon J, David H, and Studier MH (1968) Thiobases in Escherchia coli transfer RNA: 2-Thiocytosine and 5-Methylaminomethyl-2-thiouracil. Science 161, 1146–1147. [DOI] [PubMed] [Google Scholar]
- [22].Horie N, Yamaizumi Z, Kuchino Y, Takai K, Goldman E, et al. (1999) Modified nucleosides in the first positions of the anticodons of tRNA(Leu)4 and tRNA(Leu)5 from Escherichia coli . Biochemistry 38, 207–217. [DOI] [PubMed] [Google Scholar]
- [23].Yamada Y, Murao K, and Ishikura H (1981) 5-(carboxymethylaminomethyl)-2-thiouridine, a new modified nucleoside found at the first letter position of the anticodon. Nucleic Acids Res. 9, 1933–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].El Yacoubi B, Bailly M, and de Crécy-Lagard V (2012) Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet 46, 69–95. [DOI] [PubMed] [Google Scholar]
- [25].Byrne RT, Whelan F, Aller P, Bird LE, Dowle A, et al. (2013) S-Adenosyl-S-carboxymethyl-L-homocysteine: a novel cofactor found in the putative tRNA-modifying enzyme CmoA. Acta Crystallogr. D Biol. Crystallogr 69, 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim J, Xiao H, Bonanno JB, Kalyanaraman C, Brown S, et al. (2013) Structure-guided discovery of the metabolite carboxy-SAM that modulates tRNA function. Nature 498, 123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Näsvall SJ, Chen P, and Björk GR (2004) The modified wobble nucleoside uridine-5-oxyacetic acid in tRNAPro(cmo5UGG) promotes reading of all four proline codons in vivo. RNA 10, 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Weixlbaumer A, Murphy FV, Dziergowska A, Malkiewicz A, Vendeix FAP, et al. (2007) Mechanism for expanding the decoding capacity of transfer RNAs by modification of uridines. Nat. Struct. Mol. Biol 14, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vendeix FAP, Dziergowska A, Gustilo EM, Graham WD, Sproat B, et al. (2008) Anticodon domain modifications contribute order to tRNA for ribosome-mediated codon binding. Biochemistry 47, 6117–6129. [DOI] [PubMed] [Google Scholar]
- [30].Yarian C, Townsend H, Czestkowski W, Sochacka E, Malkiewicz AJ, et al. (2002) Accurate translation of the genetic code depends on tRNA modified nucleosides. J. Biol. Chem 277, 16391–16395. [DOI] [PubMed] [Google Scholar]
- [31].Kurata S, Weixlbaumer A, Ohtsuki T, Shimazaki T, Wada T, et al. (2008) Modified uridines with C5-methylene substituents at the first position of the tRNA anticodon stabilize U.G wobble pairing during decoding. J. Biol. Chem 283, 18801–18811. [DOI] [PubMed] [Google Scholar]
- [32].Takai K, and Yokoyama S (2003) Roles of 5-substituents of tRNA wobble uridines in the recognition of purine-ending codons. Nucleic Acids Res. 31, 6383–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Armengod M-E, Moukadiri I, Prado S, Ruiz-Partida R, Benítez-Páez A, et al. (2012) Enzymology of tRNA modification in the bacterial MnmEG pathway. Biochimie 94, 1510–1520. [DOI] [PubMed] [Google Scholar]
- [34].Yim L, Moukadiri I, Björk GR, and Armengod M-E (2006) Further insights into the tRNA modification process controlled by proteins MnmE and GidA of Escherichia coli. Nucleic Acids Res. 34, 5892–5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bujnicki JM, Oudjama Y, Roovers M, Owczarek S, Caillet J, et al. (2004) Identification of a bifunctional enzyme MnmC involved in the biosynthesis of a hypermodified uridine in the wobble position of tRNA. RNA 10, 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hagervall TG, Edmonds CG, McCloskey JA, and Björk GR (1987) Transfer RNA(5-methylaminomethyl-2-thiouridine)-methyltransferase from Escherichia coli K-12 has two enzymatic activities. J. Biol. Chem 262, 8488–8495. [PubMed] [Google Scholar]
- [37].Roovers M, Oudjama Y, Kaminska KH, Purta E, Caillet J, et al. (2008) Sequence-structure-function analysis of the bifunctional enzyme MnmC that catalyses the last two steps in the biosynthesis of hypermodified nucleoside mnm5s2U in tRNA. Proteins 71, 2076–2085. [DOI] [PubMed] [Google Scholar]
- [38].Kambampati R, and Lauhon CT (2003) MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry 42, 1109–1117. [DOI] [PubMed] [Google Scholar]
- [39].Elseviers D, Petrullo LA, and Gallagher PJ (1984) Novel E. coli mutants deficient in biosynthesis of 5-methylaminomethyl-2-thiouridine. Nucleic Acids Res. 12, 3521–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sullivan MA, Cannon JF, Webb FH, and Bock RM (1985) Anti-suppressor mutation in Escherichia coli defective in biosynthesis of 5-methylaminomethyl-2-thiouridine. J. Bacteriol 161, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ikeuchi Y, Shigi N, Kato J-I, Nishimura A, and Suzuki T (2006) Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thio-uridine biosynthesis at tRNA wobble positions. Mol. Cell 21, 97–108. [DOI] [PubMed] [Google Scholar]
- [42].Zheng C, Black KA, and Dos Santos PC (2017) Diverse mechanisms of Sulfur decoration in bacterial tRNA and their cellular functions. Biomolecules 7 pii: E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Grosjean H, Breton M, Sirand-Pugnet P, Tardy F, Thiaucourt F, et al. (2014) Predicting the minimal translation apparatus: lessons from the reduc-tive evolution of mollicutes. PLoS Genet. 10, e1004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rozov A, Demeshkina N, Khusainov I, Westhof E, Yusupov M, et al. (2016) Novel base-pairing interactions at the tRNA wobble position crucial for accurate reading of the genetic code. Nat. Commun 7, 10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Murphy FV, Ramakrishnan V, Malkiewicz A, and Agris PF (2004) The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat. Struct. Mol. Biol 11, 1186–1191. [DOI] [PubMed] [Google Scholar]
- [46].Väre VYP, Eruysal ER, Narendran A, Sarachan KL, and Agris PF (2017) Chemical and conformational diversity of modified nucleosides affects tRNA structure and function. Biomolecules 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rogers KC, Crescenzo AT, and Söll D (1995) Aminoacylation of transfer RNAs with 2-thiouridine derivatives in the wobble position of the anticodon. Biochimie 77, 66–74. [DOI] [PubMed] [Google Scholar]
- [48].Agris PF, Söll D, and Seno T (1973) Biological function of 2-thiouridine in Escherichia coli glutamic acid transfer ribonucleic acid. Biochemistry 12, 4331–4337. [DOI] [PubMed] [Google Scholar]
- [49].Kumar RK, and Davis DR (1997) Synthesis and studies on the effect of 2-thiouridine and 4-thiouridine on sugar conformation and RNA duplex stability. Nucleic Acids Res. 25, 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Smith WS, Sierzputowska-Gracz H, Sochacka E, Malkiewicz A, and Agris PF (1992) Chemistry and structure of modified uridine dinucleosides are determined by thiolation. J. Am. Chem. Soc 114, 7989–7997. [Google Scholar]
- [51].Sundaram M, Durant PC, and Davis DR (2000) Hypermodified nucleosides in the anticodon of tRNALys stabilize a canonical U-turn structure. Biochemistry 39, 12575–12584. [DOI] [PubMed] [Google Scholar]
- [52].Durant PC, Bajji AC, Sundaram M, Kumar RK, and Davis DR (2005) Structural effects of hypermodified nucleosides in the Escherichia coli and human tRNALys anticodon loop: the effect of nucleosides s2U, mcm5U, mcm5s2U, mnm5s2U, t6A, and ms2t6A. Biochemistry 44, 8078–8089. [DOI] [PubMed] [Google Scholar]
- [53].Seno T, Agris PF, and Söll D (1974) Involvement of the anticodon region of Escherichia coli tRNAGln and tRNAGlu in the specific interaction with cognate aminoacyl-tRNA synthetase. Alteration of the 2-thiouridine derivatives located in the anticodon of the tRNAs by BrCN or sulfur deprivation. Biochim. Biophys. Acta Theriol 349, 328–338. [DOI] [PubMed] [Google Scholar]
- [54].Sylvers LA, Rogers KC, Shimizu M, Ohtsuka E, and Söll D (1993) A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry 32, 3836–3841. [DOI] [PubMed] [Google Scholar]
- [55].Ibba M, and Söll D (2000) Aminoacyl-tRNA synthesis. Annu. Rev. Biochem 69, 617–650. [DOI] [PubMed] [Google Scholar]
- [56].Madore E, Lipman RS, Hou YM, and Lapointe J (2000) Evidence for unfolding of the single-stranded GCCA 3’-end of a tRNA on its aminoacyl-tRNA synthetase from a stacked helical to a foldback conformation. Biochemistry 39, 6791–6798. [DOI] [PubMed] [Google Scholar]
- [57].Rould MA, Perona JJ, Söll D, and Steitz TA (1989) Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 Å resolution. Science 246, 1135–1142. [DOI] [PubMed] [Google Scholar]
- [58].Cusack S, Yaremchuk A, and Tukalo M (1996) The crystal structures of T. thermophilus lysyl-tRNA synthetase complexed with E. coli tRNA(Lys) and a T. thermophilus tRNA(Lys) transcript: anticodon recognition and conformational changes upon binding of a lysyl-adenylate analogue. EMBO J. 15, 6321–6334. [PMC free article] [PubMed] [Google Scholar]
- [59].Gil R, Silva FJ, Peretó J, and Moya A (2004) Determination of the core of a minimal bacterial gene set. Microbiol. Mol. Biol. Rev 68, 518–537. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Black KA, and Dos Santos PC (2015) Abbreviated pathway for biosynthesis of 2-Thiouridine in Bacillus subtilis. J. Bacteriol 197, 1952–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wolf J, Gerber AP, and Keller W (2002) tadA, an essential tRNA-specific adenosine deaminase from Escherichia coli. EMBO J. 21, 3841–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kim J, Malashkevich V, Roday S, Lisbin M, Schramm VL, et al. (2006) Structural and kinetic characterization of Escherichia coli TadA, the wobble-specific tRNA deaminase. Biochemistry 45, 6407–6416. [DOI] [PubMed] [Google Scholar]
- [63].Muramatsu T, Yokoyama S, Horie N, Matsuda A, Ueda T, et al. (1988) A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J. Biol. Chem 263, 9261–9267. [DOI] [PubMed] [Google Scholar]
- [64].Holley RW, Apgar J, Everett GA, Madison JT, Marquisee M, et al. (1965) Structure of a ribonucleic acid. Science 147, 1462–1465. [DOI] [PubMed] [Google Scholar]
- [65].Rafels-Ybern À, Torres AG, Camacho N, Herencia-Ropero A, Roura Frigolé H, et al. (2019) The expansion of Inosine at the wobble position of tRNAs, and its role in the evolution of proteomes. Mol. Biol. Evol 36, 650–662. [DOI] [PubMed] [Google Scholar]
- [66].Gerber AP, and Keller W (1999) An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science 286, 1146–1149. [DOI] [PubMed] [Google Scholar]
- [67].Marck C, and Grosjean H (2002) tRNomics: analysis of tRNA genes from 50 genomes of Eukarya, Archaea, and bacteria reveals anticodon-sparing strategies and domain-specific features. RNA 8, 1189–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Diwan GD, and Agashe D (2018) Wobbling forth and drifting Back: the evolutionary history and impact of bacterial tRNA modifications. Mol. Biol. Evol 35, 2046–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yokobori S, Kitamura A, Grosjean H, and Bessho Y (2013) Life without tRNAArg-adenosine deaminase TadA: evolutionary consequences of decoding the four CGN codons as arginine in mycoplasmas and other Mollicutes. Nucleic Acids Res. 41, 6531–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Grosjean H, and Björk GR (2004) Enzymatic conversion of cytidine to lysidine in anticodon of bacterial isoleucyl-tRNA--an alternative way of RNA editing. Trends Biochem. Sci 29, 165–168. [DOI] [PubMed] [Google Scholar]
- [71].Suzuki T, and Miyauchi K (2010) Discovery and characterization of tRNAIle lysidine synthetase (TilS). FEBS Lett. 584, 272–277. [DOI] [PubMed] [Google Scholar]
- [72].Ikeuchi Y, Kimura S, Numata T, Nakamura D, Yokogawa T, et al. (2010) Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nat. Chem. Biol 6, 277–282. [DOI] [PubMed] [Google Scholar]
- [73].Terasaka N, Kimura S, Osawa T, Numata T, and Suzuki T (2011) Biogenesis of 2-agmatinylcytidine catalyzed by the dual protein and RNA kinase TiaS. Nat. Struct. Mol. Biol 18, 1268–1274. [DOI] [PubMed] [Google Scholar]
- [74].Paris Z, Fleming IMC, and Alfonzo JD (2012) Determinants of tRNA editing and modification: avoiding conundrums, affecting function. Semin. Cell Dev. Biol 23, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ikeuchi Y, Soma A, Ote T, Kato J, Sekine Y, et al. (2005) Molecular mechanism of lysidine synthesis that determines tRNA identity and codon recognition. Mol. Cell 19, 235–246. [DOI] [PubMed] [Google Scholar]
- [76].Voorhees RM, Mandal D, Neubauer C, Köhrer C, RajBhandary UL, et al. (2013) The structural basis for specific decoding of AUA by isoleucine tRNA on the ribosome. Nat. Struct. Mol. Biol 20, 641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Silvian LF, Wang J, and Steitz TA (1999) Insights into editing from an ile-tRNA synthetase structure with tRNAile and mupirocin. Science 285, 1074–1077. [PubMed] [Google Scholar]
- [78].Jones TE, Brown CL, Geslain R, Alexander RW, and Ribas de Pouplana L (2008) An operational RNA code for faithful assignment of AUG triplets to methionine. Mol. Cell 29, 401–407. [DOI] [PubMed] [Google Scholar]
- [79].Fabret C, Dervyn E, Dalmais B, Guillot A, Marck C, et al. (2011) Life without the essential bacterial tRNA Ile2-lysidine synthetase TilS: a case of tRNA gene recruitment in Bacillus subtilis. Mol. Microbiol 80, 1062–1074. [DOI] [PubMed] [Google Scholar]
- [80].Taniguchi T, Miyauchi K, Nakane D, Miyata M, Muto A, et al. (2013) Decoding system for the AUA codon by tRNAIle with the UAU anticodon in Mycoplasma mobile. Nucleic Acids Res. 41, 2621–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cai WM, Chionh YH, Hia F, Gu C, Kellner S, et al. (2015) A platform for discovery and quantification of modified Ribonucleosides in RNA: application to stress-induced reprogramming of tRNA modifications. Meth. Enzymol 560, 29–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chan C, Pham P, Dedon PC, and Begley TJ (2018) Lifestyle modifications: coordinating the tRNA epitranscriptome with codon bias to adapt translation during stress responses. Genome Biol. 19, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chionh YH, McBee M, Babu IR, Hia F, Lin W, et al. (2016) tRNA-mediated codon-biased translation in mycobacterial hypoxic persistence. Nat. Commun 7, 13302. [DOI] [PMC free article] [PubMed] [Google Scholar]