Abstract
Sirtuins are type III histone deacetylases (HDAC) that uses nicotinamide adenine dinucleotide as co-substrate. Dysfunction of sirtuins is implicated in wide varieties of human diseases. As such, there has been increased interest in development of small molecule to modulate sirtuin activities. Besides deacetylase activity, recent studies suggest SIRT1, 2, 3, and 6 efficiently remove fatty acyl groups on lysine. In vitro sirtuin enzymatic activity assays established so far are mainly based on the deacetylation activity. Here, we describe a fluorogenic assay for monitoring defatty-acylase activity of SIRT1,2,3 and 6 using peptide substrates. This assay can be utilized to evaluate sirtuin modulators in high-throughput manners.
Keywords: Fluorogenic assay, enzymatic assay, SIRT1, SIRT2, SIRT3, SIRT6, Defatty-acylase
1. Introduction
Sirtuins (SIRTs), which are evolutionarily conserved in all domains of life, are the type III histone deacetylases (HDACs). Seven sirtuins have been identified in mammals and can be classified into four classes. SIRT1, 2, 3 belong to class I. SIRT4 is in class II, SIRT5 is in class III, while SIRT6 and 7 are both in class IV [1,2]. Sirtuins are known to influence a wide range of physiological processes, such as aging, transcription, stress response, and inflammation [2,3]. Initially, sirtuins were known to act as nicotinamide adenine dinucleotide (NAD+)-dependent protein lysine deacetylases. In recent years, however, it was discovered that SIRT1, 2, 3, and 6 can remove long chain fatty acyl groups, like myristoyl and palmitoyl, protein lysine residues with high catalytic efficiency [4–7]. Of importance, by removing fatty acyl groups, SIRT2 positively affects the transforming ability of KRas4A [8], while SIRT6 functions as a tumor suppressor by disrupting the plasma membrane localization of RRas2 [9]. These findings highlight the emerging key roles of sirtuin-regulated lysine fatty acylation in regulating cell functions.
Because of sirtuins’ extensive regulatory activities and their pivotal role in human diseases, there has been increased interest in developing activators and inhibitors of sirtuins for various potential therapeutic applications [10,11]. Since deacetylation was the initial accepted activity of sirtuins, established high-throughput assays for screening sirtuin modulators are mainly based on their deacetylation activity. Very few methods have been developed to assess defatty-acylation activity. It is possible that the same compound may exhibit different abilities to inhibit the deacetylation and defatty-acylation activities of sirtuins. Thus, screening assays based on the defatty-acylation activities of sirtuin can be useful. Here, we describe a high-throughput assay protocol for screening small molecule modulators of the defatty-acylase activity of SIRT1, 2, 3 and 6 [12,13].
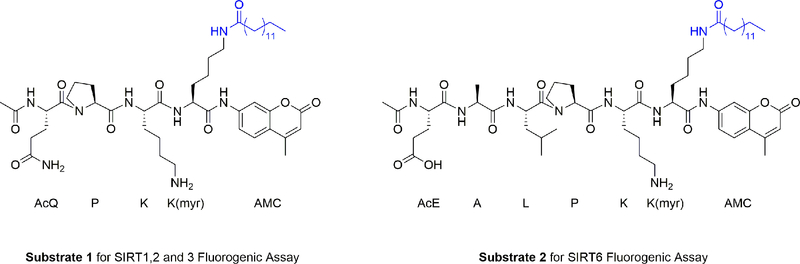
The fluorogenic substrates used in this assay are designed based on sirtuins’ natural substrate peptides, p53 for SIRT1, 2, and 3 and tumor necrosis factor-α (TNF-α) for SIRT6, with myristoylated lysine and 7-amino-4-methylcoumarin moiety (AMC) at the C-termini of the peptide chains. Our group has previously demonstrated that these substrates give stronger fluorescence read out than other substrates tried [12,13]. Substrate 1 (Fig. 1) is used for SIRT1, 2 and 3, while substrate 2 is used for SIRT6. Using NAD+ as the co-substrate, the sirtuins remove the myristoyl group on the lysine residues in the peptides, which allows trypsin to cleave off the AMC group. Once released, the AMC group is fluorescent (excitation/emission maximal wavelengths: 355/460 nm). The strength of the fluorescent signal portrays the sirtuin activity. In principle, a stronger signal reflects a higher activity of the sirtuin. This assay can be performed in 96-well plates, which allows testing multiple compounds at different concentrations at the same time. If a small molecule inhibits the sirtuin effectively, the well treated with the small molecule will exhibit a substantially lower fluorescent signal than vehicle-treated wells. Based on the results, the concentration of the inhibitor and the fluorescent signal can be plotted to calculate the IC50 or EC50 values (concentrations needed to reduce or activate the enzyme by 50 percent).
Fig 1.
Fluorogenic assay substrates.
2. Materials
2.1. Expression and Purification of Sirtuins
SIRT1 cDNA (full-length) in pET28a vector with N-terminal His6
SIRT2 cDNA (aa 38–256) in pET28a vector with N-terminal His6-SUMO
SIRT3 cDNA (aa 102–399) in pET28a vector with N-terminal His6
SIRT6 cDNA (full-length) in pET28a vector with an N-terminal His6 tag
Competent E. coli BL21 cells (use for SIRT1, SIRT2, and SIRT3)
Competent E. coli BL21 pRARE2 cells (use for SIRT6)
Culture Medium: Luria Broth, 20 μg/ml chloramphenicol, 50 μg/ml kanamycin
1 M isopropyl β-D-1-thiogalactopyranoside (IPTG) in water
TNGP buffer: 20 mM Tris-HCl (pH 8.0), 500 mM NaCl, 10% glycerol and 1 mM phenylmethane sulfonyl fluoride (PMSF)
TN buffer: 20 mM Tris-HCl (pH 8.0) and 500 mM NaCl
Imidazole Elution Buffers for step gradient: TN buffer with 20 mM, 50 mM, 100 mM, 200 mM, or 500 mM Imidazole
Nickel column
Dialysis tubing with 14,000 Molecular Weight Cutoff
Superdex 75 gel filtration column
50% glycerol
ULP1 (SUMO protease)
2.2. Fluorogenic Assay
2.3. Fluorogenic Assay for the Demyristoylase Activity of SIRT1, 2, and 3
Reaction buffer: 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2.7 mM KCl, 1 mM MgCl2. (see Note 3)
Enzyme: 20 μM of SIRT1, SIRT2, or SIRT3 in 20 mM Tris-HCl (pH 8.0), 500 mM NaCl, and 10% glycerol (see Note 4).
NAD+: stock solution of 25 mM in water (see Note 5).
Quench buffer: 10x Trypsin-EDTA (0.5%) without phenol red, 4 mM nicotinamide (see Note 6).
Substrate 1: 0.5 mM in DMSO (see Note 7).
Activator/Inhibitor: various concentrations dissolved in vehicle solvent (e.g. DMSO).
Vehicle control: vehicle solvent that the activator/inhibitor is dissolved in.
2.4. Fluorogenic Assay for the Demyristoylase Activity of SIRT6
Reaction Buffer: 20 mM Tris-HCl (pH 8.0), 1 mM dithiothreitol (DTT), 1 mg/mL of bovine serum albumin (BSA) (see Note 8)
Enzyme: 20 μM of SIRT6 in 20 mM Tris-HCl (pH 7.2), 500 mM NaCl, and 10% glycerol.
NAD+: solution of 25 mM in water (see Note 5).
Quench buffer: 10x Trypsin-EDTA (0.5%) with 4 mM nicotinamide without phenol red (see Note 6).
Substrate 2: 0.5 mM in DMSO (see Note 7).
Activator/Inhibitor: varied concentrations in vehicle solvent (e.g. DMSO).
Vehicle control: vehicle solvent that the activator/inhibitor is dissolved in.
3. Method
3.1. Purification of SIRT1, SIRT3, or SIRT6
Transform the SIRT1 or SIRT3 plasmid into E. coli BL21 cells; transform the SIRT6 plasmid into E. coli BL21 cells - pRARE2 strain.
Pick a colony from an agar plate, and grow in 25 mL Luria Broth with corresponding bacterial resistance antibiotic overnight at 37 °C.
Inoculate 2L culture medium with a bacterial colony from the SIRT1 or SIRT3 transformants.
Incubate the cells at 37 °C for 2 to 4 hours.
When the optical density at 600 nm reaches ~0.6, add 40 μL 1M IPTG to the culture (final concentration, 20 μM) to induce expression of SIRT1.
Incubate the cells for an additional 16 to 20 h at 15 °C and 200 rpm.
Collect the cells by centrifugation at 4 °C, 8000 rpm for 5 min using Beckman Coulter JA-10 rotor.
Suspend the cell pellet in 20 mL of TNGP buffer and lyse using a cell disrupter.
Remove cell debris by centrifuging at 4 °C, 20,000 rpm for 30 min using Beckman Coulter JA-20 rotor.
Apply the supernatant containing the SIRT protein to a nickel column equilibrated with TN buffer.
Use a step gradient to elute the SIRT protein from the nickel resin. Add 1.5 mL of elution buffers containing increasing concentrations (20 mM, 50 mM, 100 mM, 200 mM, 500 mM) of imidazole sequentially; collect as individual fractions.
Resolve aliquots of the elutions by SDS-PAGE and stain with Coomassie Blue to identify fractions containing SIRT.
Pool the SIRT fractions and dialyze at 4 °C for 16 to 20 h in TN buffer to remove imidazole.
If the protein still contains other impurities, further purify SIRT1 by gel filtration using a Superdex 75 gel filtration column equilibrated in TN buffer.
After the purification, concentrate the protein using a centrifugal concentrator.
Add 50% glycerol to the enzyme solution to a final concentration of 10%. The desired final enzyme concentration is 20 μM.
Store the purified protein at −80 °C in aliquots.
3.2. Purification of SIRT2.
Transform the SIRT2 plasmid into E. coli BL21 cells.
Follow steps 2 – 11 in Section 3.1.
Pool fractions with SIRT2 into dialysis tubing, adding 0.1 mL of ULP1, the SUMO protease that will cleave the His6-SUMO tag on SIRT2.
Dialyze the mixture at 4 °C for 16 to 20 h in TN buffer.
If desired, further purify SIRT2 by gel filtration on a Superdex 75 column.
Follow steps 14–16 in Section 3.1.
3.3. Fluorogenic Assay for the Demyristoylase Activity of SIRT1, 2, and 3 (see Note 9)
Thaw the purified SIRT1, 2, or 3 on ice.
To 48.4 μL of the reaction buffer, add 2.4 μL of 25 mM NAD+, and 6 μL of 20 μM sirtuin enzyme. Place the plate on ice (see Note 10).
Add 2 μL of activator/inhibitor or vehicle control to the reaction buffer, mix well by pipetting up and down, and incubate the plate at 37 °C for 15 minutes (see Note 11).
Add 1.2 μL of 0.5 mM fluorogenic substrate 1 to the well and mix. The total volume of the reaction system is 60 μL and the final concentration of the substrate is 10 μM (see Note 12).
Incubate the plate at 37 °C for 45 minutes.
Add 60 μL of the quench buffer to the well and mix by pipetting up and down a few times.
Incubate the plate at 37 °C for 30 minutes.
Measure the fluorescence at excitation of 355 nm and emission of 460 nm.
3.4. Fluorogenic Assay for the Demyristoylase Activity of SIRT6 (see Note 13)
Thaw the purified SIRT6 on ice.
To 51.4 μL of the reaction buffer, add 2.4 μL of 25 mM NAD+(see Note 10).
Add 3 μL of 20 μM SIRT6 enzyme to the well.
Add 2 μL of activator/inhibitor or vehicle control to the reaction buffer, mix, and incubate the plate at 37 °C using the dry block incubator for 15 minutes (see Note 11).
Add 1.2 μL of 0.5 mM fluorogenic substrate 2 to the well and mix (see Note 12).
Incubate the plate at 37 °C for 2 hours.
Add 60 μL of the quench buffer and mix.
Incubate the plate at 37 °C for 30 minutes.
Measure the fluorescence at excitation of 355 nm and emission of 460 nm.
4. Notes
We have been using a Costar Assay Plate, 96 well, Flat Bottom, Non-treated Non-sterile, Black Polystyrene plate from Corning (Cat#3916).
The plate reader should be equipped to measure the fluorescence which excites at 355 nm and emits at 460 nm.
Stock solutions for the reaction buffer: 1 M Tris-HCl (pH 8.0) (20X solution), 1.5 M NaCl (10X solution), 2.7 M KCl (1000X solution) and 1M MgCl2 (1000X solution).
Avoid repetitive freeze-thaw of the enzyme. Each time the enzyme is thawed, it may lose activity slightly. Aliquot the enzyme into smaller portions and store at −80 °C for future usage.
Prepare NAD+ stock solution fresh every time.
Nicotinamide, a universal sirtuin inhibitor, will stop the enzyme activity during the trypsin cleavage.
The fluorogenic substrates are prepared by solid-state peptide synthesis, and AMC is coupled to the C-terminal of the peptides using isobutyl chloroformate and N-methylmorpholine in dichloromethane. For more detailed synthesis procedures, please refer to Chiang et al. (Org. Biolmol. Chem., 2016, 14, 2186) for substrate 1 and Hu et al. (Org. Biomol. Chem., 2013, 11, 5213) for substrate 2.
Prepare a stock solution of the reaction buffer. To 41.8 μL of water, add 1.2 μL of 1 M Tris-HCl (pH 8.0), 2.4 μL of 25 mM DTT and 6 μL of 10 mg/mL BSA in H2O. This can be scaled up depending on how many reactions are needed (each reaction volume is 60 μL)
In the assay, SIRT1, 2, or 3 is incubated with fluorogenic substrate 1, NAD+, and a small molecule modulator or vehicle control. If the small molecule is a mechanism-based inhibitor, the enzyme is pre-incubated with NAD+ and the inhibitor before the addition of the fluorogenic substrate. After 15 minutes of pre-incubation, the fluorogenic substrate is added to the reaction system, and the reaction is stopped by adding the quench buffer, which contains trypsin and nicotinamide. Trypsin which will cleave off the AMC group from the demyristoylated peptide, producing a fluorescent signal. An excessive amount of nicotinamide, which inhibits the sirtuins, is added to quench the activity of the enzyme [14]. The stronger the fluorescent signal is, the stronger the sirtuin activity. Thus, if the activator is working efficiently, the signal will be significantly stronger than that of the control group.
A master mix of the reaction components multiplied by the number of the reaction tubes can be utilized. The components include everything except the inhibitor and the fluorogenic peptide substrate, which will be added separately. This way, one can test multiple compounds at multiple concentrations simultaneously.
This pre-incubation can be skipped, if the tested modulator is not mechanism-based. For the vehicle control sample, add the vehicle solution instead of the modulator.
Using a multi-channel pipettor throughout the assay can expedite the process.
The assay principle for SIRT6 is that same as that described in Note 9.
References
- 1.Imai S-i, Guarente L (2014) NAD+ and sirtuins in aging and disease. Trends in Cell Biology 24 (8):464–471. doi: 10.1016/j.tcb.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu J, Jing H, Lin H (2014) Sirtuin inhibitors as anticancer agents. Future Medicinal Chemistry 6 (8):945–966. doi: 10.4155/fmc.14.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarente L (2011) Sirtuins, Aging, and Medicine. New England Journal of Medicine 364 (23):2235–2244. doi: 10.1056/NEJMra1100831 [DOI] [PubMed] [Google Scholar]
- 4.Feldman JL, Baeza J, Denu JM (2013) Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem 288 (43):31350–31356. doi: 10.1074/jbc.C113.511261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He B, Hu J, Zhang X, Lin H (2014) Thiomyristoyl peptides as cell-permeable Sirt6 inhibitors. Org Biomol Chem 12 (38):7498–7502. doi: 10.1039/c4ob00860j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng Y-B, Jing H, Aramsangtienchai P, He B, Khan S, Hu J, Lin H, Hao Q (2015) Efficient Demyristoylase Activity of SIRT2 Revealed by Kinetic and Structural Studies. Scientific Reports 5:8529. doi: 10.1038/srep08529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Khan S, Jiang H, Antonyak MA, Chen X, Spiegelman NA, Shrimp JH, Cerione RA, Lin H (2016) Identifying the functional contribution of the defatty-acylase activity of SIRT6. Nat Chem Biol 12 (8):614–620. doi: 10.1038/nchembio.2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jing H, Zhang X, Wisner SA, Chen X, Spiegelman NA, Linder ME, Lin H (2017) SIRT2 and lysine fatty acylation regulate the transforming activity of K-Ras4a. eLife Sciences 6. doi: 10.7554/eLife.32436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Spiegelman NA, Nelson OD, Jing H, Lin H (2017) SIRT6 regulates Ras-related protein R-Ras2 by lysine defatty-acylation. eLife Sciences 6:e25158. doi: 10.7554/eLife.25158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet J-C, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science (New York, NY) 317 (5837):516–519. doi: 10.1126/science.1143780 [DOI] [PubMed] [Google Scholar]
- 11.Jing H, Hu J, He B, Negrón Abril YL, Stupinski J, Weiser K, Carbonaro M, Chiang Y-L, Southard T, Giannakakou P, Weiss RS, Lin H (2016) A SIRT2-Selective Inhibitor Promotes c-Myc Oncoprotein Degradation and Exhibits Broad Anticancer Activity. Cancer Cell 29 (3):297–310. doi: 10.1016/j.ccell.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang YL, Lin H (2016) An improved fluorogenic assay for SIRT1, SIRT2, and SIRT3. Org Biomol Chem 14 (7):2186–2190. doi: 10.1039/c5ob02609a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, He B, Bhargava S, Lin H (2013) A fluorogenic assay for screening Sirt6 modulators. Org Biomol Chem 11 (32):5213–5216. doi: 10.1039/c3ob41138a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avalos JL, Bever KM, Wolberger C (2005) Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell 17 (6):855–868. doi: 10.1016/j.molcel.2005.02.022 [DOI] [PubMed] [Google Scholar]