Abstract
Paroxysmal Nocturnal Hemoglobinuria (PNH) is a rare blood disorder characterized by chronic intravascular hemolysis, thromboses in unusual sites and cytopenias related to bone marrow failure. The diagnosis is based on the Flow Cytometric (FCM) detection of peripheral blood cell clones lacking the surface molecules linked to the GPI anchor, which is altered by mutations.
Consensus studies have developed standardized and robust multicolor FCM assays to disclose PNH clones among red cells, neutrophils and monocytes at a high level of sensitivity and accuracy. High-resolution procedures have been also established to detect small PNH clones at a sensitivity level of around 0.01% in red cells and neutrophils. Cell clone type and size have been put into correlation with the clinical presentations of the associated diseases, and recommendations for the clinical follow-up have been established. The recent advent of the therapeutic monoclonal antibody Eculizumab has dramatically improved both the quality of life and the life expectancy of the affected patients, further increasing the importance of an accurate FCM detection and monitoring of the clones. The technical features of the FCM diagnostic workup and the many critical aspects of the analytical process are discussed here.
Key words: paroxysmal nocturnal hemoglobinuria, PNH, flow cytometry, FLAER, monoclonal antibodies, ICCS/ESCCA guidelines
INTRODUCTION
Paroxysmal Nocturnal Hemoglobinuria (PNH) is a rare, acquired clonal blood disorder of the hematopoietic stem cell characterized by a triad of signs and symptoms: chronic intravascular hemolysis with recurrent crises, thromboses in unusual sites and cytopenias related to bone marrow failure (BMF).
Its name was coined early in the 20th century, but after the extensive research made during the last few decades, nowadays the three key terms characterizing the disease may sound somewhat misleading.
While some 90 percent of patients experience hemoglobinuria at some point of their clinical course (1), hemoglobinuria is seen in about one quarter of cases only at diagnosis (2). Chronic hemolysis with hemoglobinuria occurs not only during nighttime, but 24 hours per day, it is most often asymptomatic and may present as ‘paroxysmal’ only occasionally (2).
PNH is also known as ‘the most vicious acquired thrombophilic state’ (3, 4), probably through the free hemoglobin itself (5) and the reduced intravascular nitric oxide, although its multi-factorial biological basis still remains partly unknown (6).
The strong association between PNH and BMF syndromes, including myelodysplasia (MDS) and aplastic anemia (AA), is well known and recommendations have been published to include PNH screening in the clinical workup of the BMF syndromes, MDS and AA (7-9).
A number of other apparently incoherent clinical signs and symptoms are often experienced by the affected patients, namely fatigue, dyspnea, abdominal pain, pulmonary hypertension, renal insufficiency, dysphagia, erectile dysfunction, thus making the clinical assessment long and difficult, with frequent misdiagnosis and mismanagement (1, 10).
Except for Direct Antiglobulin Test (DAT)-negative hemolysis tests and cytopenias, which may focus medical attention, ordinary laboratory assessments usually do not provide specific clues for the diagnosis of PNH (11).
The uncontrolled complement activation, leading to life-threatening hemolysis and thromboses forms the basis of symptomatic PNH. The pathogenic mechanism of PNH resides in the faulty synthesis of the cell membrane protein anchor Glycosyl-Phosphatidyl Inositol (GPI) due to mutations occurring in the PIG-A gene located on chromosome X (12). GPI is the anchor for many functional cell surface glycoproteins (13) (Figure 1): the reduced or absent expression of the GPI-linked complement-regulator molecules on red blood cells (RBC), the Decay Accelerating Factor (DAF, also known as CD55) and the Membrane Inhibitor of Reactive Lysis (MIRL, also known as CD59) are the cause of hemolysis in PNH (2-4, 14). In PNH all blood cell lines deriving from a mutated stem cell carry the same genetic and phenotypic defect, and can coexist as ‘PNH clones’ along with normal, unmutated counterparts. The flow cytometric (FCM) demonstration of the absence of GPI-linked molecules in a sizable fraction of peripheral blood red cells, neutrophils and monocytes is the cornerstone of the diagnostic process of PNH (15-17). Three types of clinical presentation of PNH are schematically considered: the classical form, associated to overt hemolysis and large PNH cell clones; the PNH associated to BMF/AA with hemolysis and usually small clones, and the subclinical PNH, with no or minimal hemolysis and variable signs of BMF. A more extensive discussion on the clinical spectrum of PNH presentations and the associated laboratory features can be found in the recent literature (7, 18-20).
Figure 1.
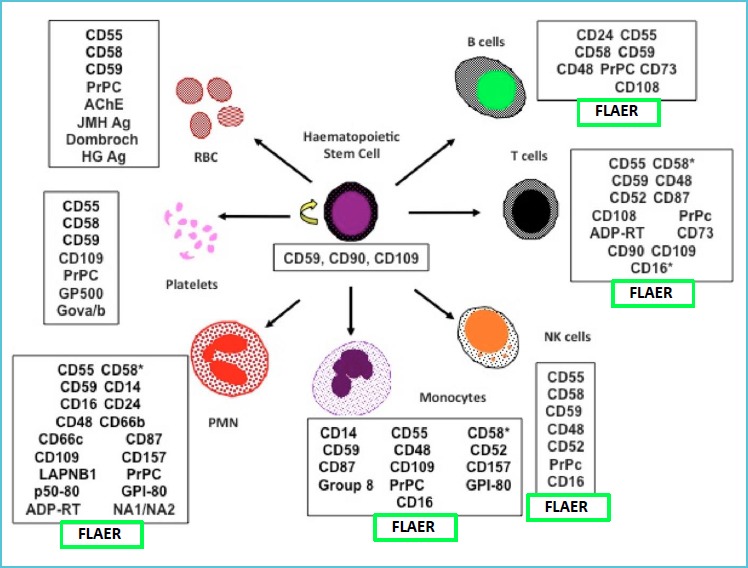
The major GPI-linked cell surface molecules that can be studied by flow cytometry in the diagnostic process of PNH
Blood transfusions, anticoagulants and steroids have been the only therapeutic options for PNH for decades (19, 21, 22). Patients with PNH occurring in the context of BMF take advantage from the specific treatment of the underlying bone marrow disorder (7, 19, 23). In recent years, the advent of the complement inhibitory monoclonal antibody Eculizumab has dramatically improved the prognosis and the quality of life of patients with symptomatic PNH (24-28), although the only cure may reside in allogeneic stem cell transplantation in selected cases (25).
FLOW CYTOMETRIC DIAGNOSIS OF PNH
The reasons for testing
The classical list of signs and symptoms which may prompt a testing order for PNH includes haemoglobinuria or haemosiderinuria, unexplained DAT-negative haemolysis, aplastic or hypoplastic anaemia, MDS, thrombosis in unusual sites, dystonic symptoms (dysphagia, abdominal pain, erectile dysfunction) (29). However, not all such signs and symptoms have the same diagnostic efficiency in helping the definition of a clinical PNH, especially when occurring separately. In general, when the above listed signs and symptoms combine, the clinical yield increases according to Morado et al. (30) (Table 1). For instance, testing for an underlying PNH in case of thrombosis alone may account for a clinical yield as low as 0.4 to 2.7%, (28, 30), whereas if thrombosis is associated with anemia and/or other cytopenias, the diagnostic efficiency may raise up to about 14% (30).
Table 1.
Frequency of cases showing GPI-deficient cells in blood at diagnosis in 3,938 PNH+ patients according to the reasons for testing in a multicenter study
| Medical indications for PNH screening | Frequency of PNH+ cases |
|---|---|
| Individuals with clinical and biological signs/symptoms of PNH in the absence of a previous hematological disorder (n = 3,032) | 8.7% |
| Hemoglobinuria (n = 73) | 47.9% |
| Hemolytic anemia (n = 382) | 18.6% |
| Subtotal hemolysis (n = 455) | 23.3% |
| Unexplained cytopenias including anemia (n = 393) | 22.4% |
| Unexplained cytopenia without anemia (n = 772) | 5.1% |
| Anemia, not otherwise specified (n = 468) | 3.6% |
| Subtotal cytopenia (n = 1,633) | 8.8% |
| Thrombosis with nonhemolytic anemia and/or other cytopenias (n = 73) | 13.7% |
| Thrombosis without anemia and/or other cytopenia (n = 800) | 0.4% |
| Subtotal thrombosis (n = 873) | 1.5% |
| Iron deficiency (n = 57) | 0% |
| Other (n= 14) | 0% |
| Subtotal others (n = 71) | 0% |
| Patients with hematological disorders (n= 906) | 29.8% |
| Aplastic/hypoplastic anemia (n= 541) | 44.9% |
| Myelodysplastic syndrome (n = 261) | 9.8% |
| Subtotal BM failure (n = 802) | 33.3% |
| Chronic myeloproliferative neoplasm (n = 21) | 4.8% |
| Other hematological and/or immunological disorders (n= 78) | 0% |
| Subtotal other non-BM failure disorders (n = 99) | 1.0% |
| Total (n = 3,938) | 14.3% |
Reproduced by permission from (30).
Preanalytical Issues
The available guidelines on FCM analysis of PNH have not addressed the specific preanalytical requirements of PNH analysis (1) or have provided only very concise indications (15, 31). In the year 2000 guidelines, the maximum blood sample age for analysis was reported as 7 days for RBC and 48 hours for white blood cells (WBC), with the recommendation to refrigerate samples at 4°C if storage time exceeds 24 hours (15). The more recent guidelines (17) just recommend less than 48 hour storage time for RBC with no further details.
It is important to consider carefully the peculiar analytical context of FCM analysis of PNH, where the absence rather than the presence of cell surface markers has to be demonstrated. The cell viability in anticoagulated blood samples progressively decreases with storage time, and may introduce unpredictable changes in the cell staining features, both increasing or decreasing artifactually the binding properties of conjugated antibodies or of other reagents.
Pre-analytical procedures, especially ammonium chloride lysis, may kill cells which are already damaged in aged samples. Dead and apoptotic cells can strongly influence PNH clone detection, while also causing non-specific antibody binding, increased autofluorescence and cell aggregation.
To avoid under- or overestimation of clone size a viability dye, i.e. DRAQ7 (32), can be used to exclude dead cells. DRAQ7 is a fluorescent impermeant dye (excited at 630 nm, emitting at >670 nm) that does not enter intact cells. When the cell membrane integrity is compromised DRAQ7 enters the cells and readily binds to nuclei to report cell death, thus improving the assay specificity and reducing artifacts (Figure 2).
Figure 2.
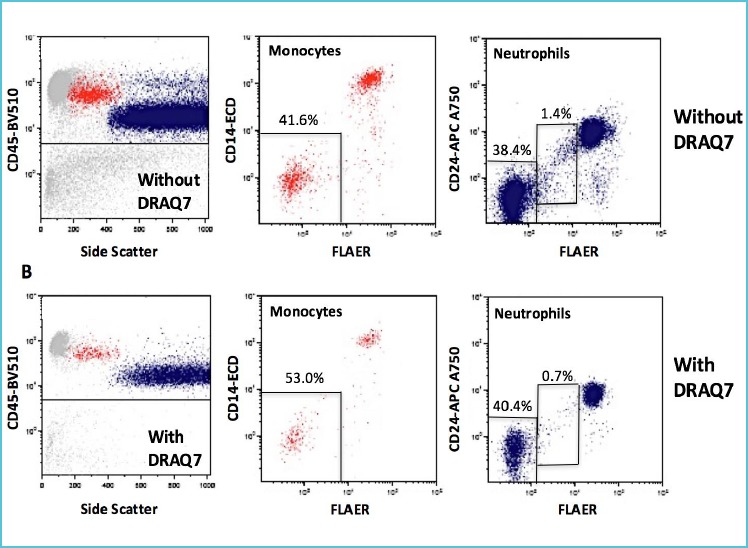
Artifacts induced by dead cells on WBC PNH clones in a suboptimal aged sample with reduced cell viability*
The usage of erythrocyte bulk lysis procedures by ammonium chloride buffer before staining has been found useful in reducing the background signal and to increase the resolution between positive and negative cell populations (33, 34).
The FLAER reagent
The Aeromonas hydrophila toxin Aerolysin was demonstrated to be a highly specific ligand for the GPI molecule on white blood cells, thus making it a suitable indicator of GPI-deficient leucocytes in PNH (35, 36).
An inactivated non-toxic, fluorochrome-conjugated molecular variant was then developed under the acronym FLAER (FLuorescent AERolysin) and extensively applied as a highly effective non-antibody reagent to be used in FCM studies of PNH leucocytes, along with other conjugated monoclonal antibodies (MoAb) (17, 37-39).
The binding properties of FLAER on blood cells have been accurately studied, and the higher signal resolution obtained by the pre-staining bulk lysis of the sample has been demonstrated (33, 34).
Cell staining protocols
The 2018 ICCS/ESCCA guidelines (17, 31) have provided a fully comprehensive series of technical recommendations on sample preparation, reagent titration, MoAb clone and fluorochrome selection.
Bulk erythrocyte lysis of 1-2 ml of anticoagulated blood with ammonium chloride-containing buffer is useful to enrich cytopenic samples and to concentrate the cell pellet for high-resolution analyses requiring the collection of large numbers of events (33, 34).
For every cell type to be analyzed, gating antibodies are first used to capture the target populations, then GPI-deficient cells are defined in each subset using the appropriate MoAb combination and FLAER, where applicable (Table 2). Several possible staining alternatives have been studied and optimized (17).
Table 2.
Examples of validated antibody mixtures for 6-color PNH analysis using recommended fluorochromes and clones, for Becton Dickinson and Beckman-Coulter instrument users*
| Becton Dickinson FACSCanto (2-Laser) | FLAER-Alexa 488 | CD24-PE | CD15-PerCP-Cy5.5 | CD64-PE-Cy7 | CD14-APC | CD45-APCH7 |
| (Clones SN3, ML5) | (Clone MEM-158) | (Clones 10.1, 22) | (Clone MφpP9) | (Clone 2D1) | ||
| Beckman Coulter Navios (3-Laser) | FLAER-Alexa 488 | CD24-PE | CD15-PC5 | CD64-PC7 | CD14-APC700 | CD45-KO |
| (Clones SN3, ALB9) | (Clone 80H5) | (Clones 10.1, 22) | (Clone RM052) | (Clone J33) |
* From (17), where several alternative reagent mixtures and clones are described.
FLAER: FLuorescent AERolysin; Alexa: Alexa-Fluor 488nm fluorochrome; PE: Phycoerythrin; PerCP: Peridinin-Chlorophyll Protein; Cy: Prefix of Cyanine-series fluorochromes; APC: Allophycocyanine and its tandem conjugates; PC5: PE-Cyanine 5 tandem; PC7: PE-Cyanine 7 tandem; KO: Krome Orange.
It is important to restrict the analysis to mature WBC populations using MoAb reactive with GPI molecules fully expressed by peripheral blood mature cells and not by their progenitors, since these cells usually express GPI-linked markers at a lower density (8).
To determine GPI-deficient RBC clones, of course, a lysis procedure should not be used. RBC are first diluted 1:100 in phosphate buffer (PBS), stained with anti-glycophorin-A (CD235a) for gating and CD59 for GPI-deficiency, then two washes using PBS supplemented with bovine serum albumin (BSA) are mandatory before reading. The FSC-W vs FSC-A pulse analysis of RBC physical parameters can be also of help in defining the RBC gate (Figure 3).
Figure 3.
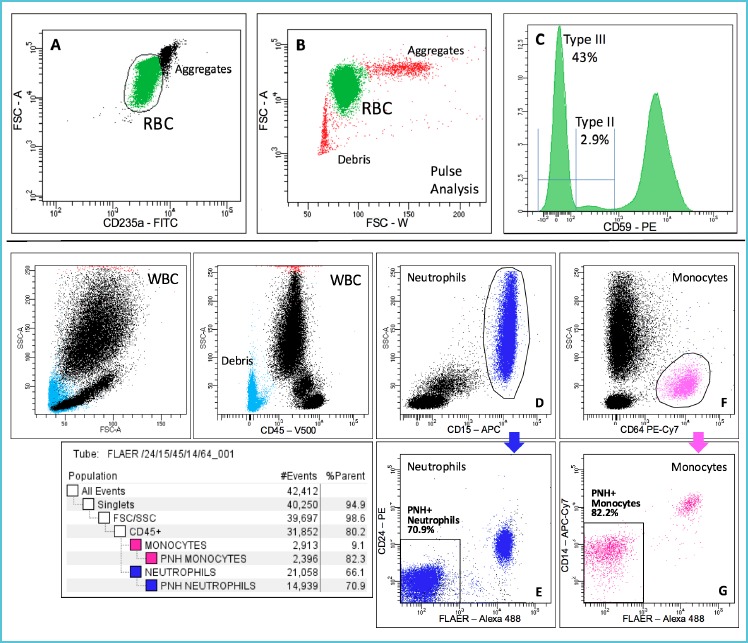
An example of PNH analysis using the recommended guidelines in a highly symptomatic patient, off therapy
Thiazole orange pre-staining of reticulocytes within the RBC cluster can be also used to measure the proportion between normal and GPI-deficient erythropoiesis, which is very useful in evaluating the red cell compartment response to therapy (40).
The state-of-the-art FCM technique to study PNH clones among WBC is a five- to six-color combination that is adjusted slightly according to instrument brand, optical filter sets and laser types.
For WBC analysis whole blood is preferably bulklysed and washed twice with PBS+BSA before staining. CD45 and side scatter are used along with CD15 and CD64 to gate neutrophils (with exclusion of eosinophils that express CD15 in lower amount) and monocytes, respectively. FLAER and CD24 is the optimal reagent combination to identify GPI-deficient neutrophils, whereas FLAER and CD14 are used to study PNH monocyte clones (Figure 3).
Lymphocytes are not useful in PNH diagnosis, due to their very long lifespan and to an excess variability in the expression of surface GPI-linked molecules (15). The role of lymphocytes in PNH analysis is currently limited to internal staining controls (17).
Platelets in PNH have a normal lifespan despite expressing the same GPI-molecule deficiency of RBC, and are not included in routine diagnostic procedures (6).
The reported limited availability or the high cost of FLAER in certain countries (17) have prompted the development of non-FLAER based staining protocols, introducing CD157 as a replacement for CD24 and CD14 to determine PNH clones in neutrophils and monocytes, respectively (41, 42). However, the reduced CD157 expression by eosinophil granulocytes may cause artifacts in case of imprecise gating of CD15+ neutrophils (41), and genetic or ethnic variants of CD157 have been described which may account for the detection of false PNH clones in some individuals (43, 44).
Recently, additional multicolor FCM protocols have been described, coupling FLAER to CD157 (7-colors) (45) or adding CD5 and CD19 as a dump channel to reduce background fluorescence (8-colors) (46). The advantages provided by this increase in fluorochrome number and complexity are still to be fully evaluated.
Instrument setup, optimization and gating syntax
Modern digital flow cytometers are equipped with very efficient setup and calibration bead suspensions that should be used to optimize automatically the photomultiplier voltages, the thresholds, the intra- and inter-laser fluorescence spillover compensation, ensuring instrumental consistency with time and comparable results among laboratories using the same type of equipment (47-50). Therefore, if manufacturer’s directions are strictly applied, the FCM setup is nowadays easy to standardize, thus making the instrument-related variables almost negligible.
The creation of an appropriate gating syntax, however, is still a manual process that requires skilled operators and sample-to-sample adjustments in order to correct for the unavoidable inter-patient variability in cell cluster positioning (17, 45).
The limits of detection – coping with background events
As mentioned, in PNH analysis the lack rather than the presence of cell surface molecules is the indicator of the disease-related abnormalities. The presence of some GPI-negative elements in the various blood cell subsets of healthy subjects is a well-known phenomenon and represents the assay background (33, 51, 52).
Furthermore, the presence of blasts or other immature cells, as it occurs in MDS, may strongly influence the amount of background events. This requires a strict check of the analytical process using a cohort of healthy donors to control and minimize the background event frequency, which in turn vary in the different cell populations and in the different clinical settings. Moreover, it is mandatory to pay attention to the numerous biological and staining artifacts that may generate negatively stained cells and false ‘PNH events’ in the background, such as the presence of autofluorescent events that come as diagonally shaped or ‘angle of dangle’ parasite events in fluorescence plots (53).
Before the advent of FLAER the high background level in healthy controls and other technical limitations made it difficult to enumerate accurately PNH events below 1% (54). Following the more recent FLAER-based guidelines on high-resolution PNH analysis (16, 17, 41, 45), a clearer picture of the lower limits of detection (LLOD) and of quantitation (LLOQ) of PNH events can be obtained. These issues have been thoroughly discussed and validated in the recent study by Payne (53).
It is important to mention here how the cytometry researchers have tried over the years to translate the concepts of lower limit of blank (LLOB), LLOD and LLOQ, typical of chromatographic or spectrophotometric procedures (55, 56), into a technology based on event accumulation (57). This transition was made - rather simplistically - by analogy with clinical chemistry, but surely without applying the same methodological care, and it is still matter of debate.
With the use of a negative or normal sample, the events falling into the final acquisition window may be taken as the LLOB, and ratioed to their cellular counterpart (the ‘clean cell denominator’) to obtain the ‘blank’ level to be subtracted from the analysis of the relevant cell population.
The Poisson’s statistics governing rare cellular event analysis mandates the acquisition of at least 100 relevant events to generate measures with the acceptable coefficient of variation of 10%. In the delicate field of high-resolution FCM, however, the collection of 100 events can be sometimes out of reach, so a compromise has been developed, proposing a lower limit of 20 (31), 25 (53) or 30 (58) relevant events for the calculation of LLOD in high-sensitivity PNH and leukemia minimal residual disease analyses, keeping a universally accepted limit of 50 events for the LLOQ. Little attention has been paid however to the inherent variance of such a new approach, which dramatically increases with the lowering of the relevant event number. Despite increasing the acquisition to millions of clean cells, the lower the established minimum level of the detectable relevant events, the wider the confidence interval and the variability of the cytometric measure, according to Poisson’s statistics.
In the daily practice, assuming 20 events (31) or 30 events (58) as the minimum size of a relevant cell event cluster for detection, and 50 events as the minimum for quantitation (31, 58), the calculation of LLOD% is: 2,000 (or 3,000) / total number of clean events; and the calculation of LLOQ% is: 5,000 / total number of clean events.
When the same criteria for minimal residual disease studies with high resolution FCM analysis of hematological malignancies are applied (i.e. from 500,000 to >1,000,000 clean cell events as the denominator (58), the median background ‘PNH’ Type III RBC events in normals are from 3 to 5 per million, with a range from 0 to 17 per million or 0.002-0.007% and a LLOQ around 0.005% (31, 52). Type II RBC events are often difficult to dissect precisely from Type I and Type III populations, moreover they can be generated by artifacts. As a consequence, Type II detection levels are usually not addressed in high-resolution PNH studies (53). For CD15+ neutrophils the average background in normals ranges from 2 to 10 per million or 0 to 0.001% (8, 31, 52). Similar figures in normal subjects have been consistently reported by other groups (33, 51, 53). For CD64+ monocytes, the low number of events that can be acquired in clinical samples greatly reduces the achievable sensitivity level, with background levels ranging around 0.1% (34, 52).
PNH clone patterns – diagnostic criteria
The total absence of GPI-linked molecules defines a Type III PNH clone, whereas a partial deficiency defines a Type II PNH clone. It is customary to quantify and report the presence of Type II clones in the RBC population only, while WBC Type II (if detectable) and Type III clones are usually summed up in the reporting, with no further details (31).
RBC PNH clone size may vary remarkably according to the occurrence of hemolytic crises, to the administration of blood transfusions or Eculizumab, so RBC PNH clones may not reflect accurately the disease extent, are often smaller than the respective WBC clones and are not an ideal target for PNH quantitation at diagnosis (38, 59). The established guidelines state that the absence of two GPI-linked molecules must be demonstrated in WBC lineages to corroborate the diagnosis (15-17). Neutrophil and monocyte PNH clones usually tend to be of similar magnitude. In a small percentage of patients, however, marked discrepancies may occur, more often with PNH monocyte clones outnumbering neutrophil clones (20, 31). PNH clone size and type can also change with time, and can both increase or decrease in an unpredictable fashion, thus indicating the need for a follow up with time (20). The correlations between PNH cell clones and the associated clinical pictures - also in pediatric patients - have been extensively reviewed, as well as the recommended criteria for the longitudinal follow-up of small clones (10, 20, 59, 60).
Patients under Eculizumab treatment usually display expanded RBC clones, due to the increased lifespan of GPI-deficient erythrocytes (3, 4, 24, 25, 27). In such patients the abrupt discontinuation of Eculizumab may increase the intensity and the severity of hemolytic crises (25).
The high-resolution PNH analysis and assay precision
Until a few years ago a technical distinction between ‘routine diagnostic’ and ‘high-resolution’ PNH analysis was commonplace in the literature and considered in external quality assessment exercises. With the advent of the highly standardized recent protocols such a difference has lost its significance (17), since using the same sensitive laboratory technique both routine diagnostic and high-resolution analysis of PNH clones can be accomplished accurately (62). It is well known that PNH+ patients can have a neutrophil PNH clone of <1% in some 42% of cases (31) (Figure 4), thus stressing the need for the systematic usage of a very sensitive FCM technique. Small PNH clones are more common in patients with BMF syndromes, may change their size with time and seem positively correlated to the response to immunosuppressive treatments (63). The term ‘High resolution FCM’ refers mainly to the collection of an adequate number of cell events (i.e. from 500,000 to 1-2 million), which enables analyses at a sensitivity levels around 10-4 with LLOQ equal or less than 0.005%. This feature can be easily applied to RBC and neutrophil analyses, but such a level of resolution is almost impossible to achieve in the routine with monocytes, due to their low level in peripheral blood.
Figure 4.
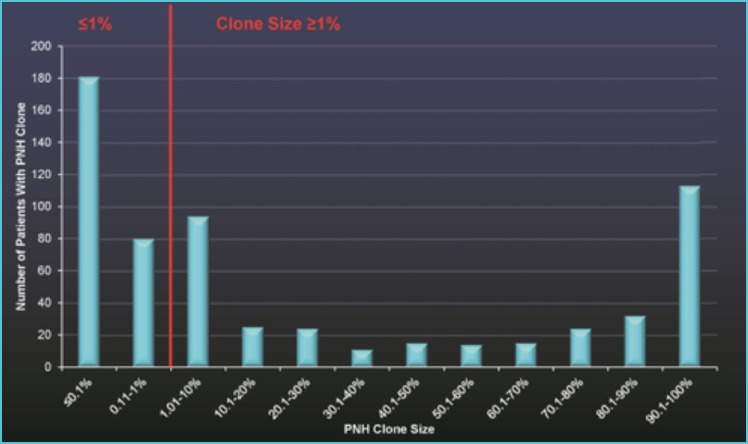
PNH clone size distribution in 633 PNH+ patients out of 10,236 screened subjects (clinical yield 6.18%) using neutrophils as clone indicators. 42% of PNH+ patients had a PNH clone of <1%.*
As expected in any FCM rare event analyses, the lower the PNH clone percentages the worse the assay precision, ranging from 14 to 25% at the lowest limit of sensitivity (31, 52). Moreover, the precision in detecting a very small but well resolved Type III clone is usually better that the one achieved with larger but less clearly separated Type II clones (53, 61). The acceptable limit of precision for clinical purposes is not yet well established in the literature. Very small PNH clones, however, do not make a diagnosis of clinical PNH and usually do not indicate the need of therapeutic measures, although a strict monitoring also of small clones over the time is mandatory in any instance.
Assay results – reporting PNH analysis
In the reporting of PNH analyses it is important to use a standardized terminology, in order to avoid a common shortcoming, namely the over-diagnosis of clinical PNH when only small GPI-deficient clones are detected. The recommended reporting terminology to be used is: ‘Presence of a PNH Clone [specify the cell type(s)] ‘ in case of cell clones >1%; ‘Presence of minor [specify the cell type] PNH population(s)’ from 0.1% to 1%; ‘Presence of rare GPI-deficient cells [specify the cell type]’ when <0.1% (31, 64). The achieved sensitivity level should be specified in the reporting for every cell subset, since it may vary according to the patient’s status and the overall cell collection. As mentioned, the presence of Type II clones should be reported for RBC only (31).
Screening tests versus diagnostic confirmation
PNH is a very rare disease, with a diagnostic yield ranging from 6% to 14% under the best reason for testing conditions. This means that testing for PNH will give negative results in the vast majority of the checked cases. In the literature, a primary screening test before a diagnostic confirmation assay in selected patients has been repeatedly proposed to rule out the more numerous negative cases (16, 20, 34, 38, 65). The current 6- or 7-color technical guidelines can be costly, complex and demanding for small peripheral laboratories, thus preventing a diffuse first-level disease surveillance, which should be practiced in every blood transfusion center and hematology laboratory. Due to their favorable features, neutrophils and monocytes may serve better than RBC as the preliminary index population for the screening of new PNH cases (38) in patients with the appropriate reasons for testing (Figure 4). In some countries samples with an initially positive first-level screening test for PNH are then referred to an accredited central facility for diagnostic confirmation, using a state-of-the-art multicolor technique (34). A simplified and inexpensive PNH screening test, which can be performed upon the initiative of the laboratory itself and affordably repeatable over the time, is a cost-effective option especially in resource-restricted countries. This approach proved valuable in disclosing new PNH cases in local studies (20, 34), and can be a practical solution to overcome the disappointing level of disease surveillance reported in some settings (66).
External quality assessment for PNH
The blood stabilization technology implemented by United Kingdom National External Quality Assurance Schemes (UK NEQAS) has been applied in developing stable PNH samples to be used as process control and in external quality assessment studies (67). This advancement has greatly facilitated the diffusion of the FCM analysis of PNH and stimulated the participating centers in selecting the optimal procedures, antibody clones and fluorochromes (62, 68). The periodic surveys made by UK NEQAS, however, still highlight a certain degree of variability in the interpretation and usage of the available guidelines by some participants, which may account for an excess of non-specific assay results in some instances (i.e. PNH clone erroneously detected in healthy donor samples). Two separate PNH schemes - ordinary and high-sensitivity - have been developed initially, and were subsequently merged for the reasons discussed above.
Pifalls and artifacts
Provided the reasons for testing are clinically appropriate and the recommended techniques are applied, the FCM operators involved in PNH analysis may come through a series of technical artifacts and pitfalls with real-life samples, that require an in-depth expertise for the full control of the technology.
Despite CD55 is traditionally included in the list of GPI-linked RBC molecules, Anti-CD55 MoAbs have been progressively dismissed from FCM panels due to the weak antigen expression (17). When RBC are stained with anti-CD59, at least two washing steps are then required to eliminate excess fluorochrome and non-specific MoAb binding, which greatly interfere with positive/negative discrimination (15).
A typical problem with WBC analysis is represented by the appearance of putative small Type II clones, especially in the monocyte gating, often due to errors in mixing the sample with the reagents, to an imprecise gating or to cell adhesion phenomena. Basophils and NK cells that can be erroneously captured in the neutrophil or in the monocyte gate are characteristically stained dimly by FLAER, and can simulate Type II PNH clones. Myeloid blasts that often circulate in patients with BMF syndromes behave the same way, and can be captured in the monocyte gate, especially if they express CD64. Large platelet aggregates can also be dragged into the monocyte gate, and may account for FLAER-negative events (34). This artifact can be effectively prevented by bulk lysis and extensive washings.
Another common problem is represented by the acquisition of an insufficient number of cell events and by the calculation of improper PNH clone percentages, in the erroneous belief to have achieved a sufficient level of assay sensitivity (62). The latter problem is particularly evident with monocytes, that in some instances cannot be collected in sufficient number for a true high-sensitivity analysis.
CONCLUDING REMARKS
Flow Cytometry is today the laboratory technique of choice to provide an accurate, sensitive, standardized and widely applicable diagnosis of PNH. The diagnostic efficiency largely resides in the appropriateness of the reasons for testing, which may be a major conditioning factor in the clinical workup of this rare and elusive disease.
The state-of-the art multicolor FCM technology is mature, robust, covered by international ISO17043-certified external quality assessment schemes, and enables the detection also of very small PNH clones with good accuracy and precision. The implementation of a diffuse screening network in partnership with central, second-level reference facilities seems today a rational and affordable model to favor the disclosure of new PNH cases.
REFERENCES
- 1.Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, Rosse W, Socié G. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 2005; 106: 3699-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rother RP, Mojcik CF, Brodsky RA, Bell R. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nature Biotechnology 2007; 25: 1256-1264. [DOI] [PubMed] [Google Scholar]
- 3.Luzzatto L, Risitano AM, Notaro R. Paroxysmal nocturnal hemoglobinuria and eculizumab. Haematologica 2010; 95: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luzzatto L. Recent advances in the pathogenesis and treatment of paroxysmal nocturnal hemoglobinuria. F1000Research 2016; 5(F1000 Faculty Rev): 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Bijnen STA, Van Heerde WL, Muus P. Mechanisms and clinical implications of thrombosis in paroxysmal nocturnal hemoglobinuria. J Thromb Haemost 2012; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 6.Peacock-Young B, Macrae FL, Newton DJ, Hill A, Ariëns RAS. The prothrombotic state in paroxysmal nocturnal hemoglobinuria: a multifaceted source. Haematologica 2018; 103: 9-17. [DOI] [PubMed] [Google Scholar]
- 7.Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti G, Snowden JA, Samarasinghe S, Wood A, Marsh JCW. Guidelines for the diagnosis and management of adult aplastic anaemia. Brit J Haematol 2016; 172: 187-207. [DOI] [PubMed] [Google Scholar]
- 8.Wang SA, Pozdnyakova O, Jorgensen JL, Medeiros LJ, Stachurski D, Anderson M, Raza A, Woda BA. Detection of paroxysmal nocturnal hemoglobinuria clones in patients with myelodysplastic syndromes and related bone marrow diseases, with emphasis on diagnostic pitfalls and caveats. Haematologica 2009; 94: 29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young NS. Paroxysmal nocturnal hemoglobinuria and myelodysplastic sydromes: clonal expansion of PIG-A-mutant hematopoietic cells in bone marrow failure. Haematologica 2009; 94: 3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrezenmeier H, Muus P, Socié G, Szer J, Urbano-Ispi-zua A, Maciejewski JP, Brodsky RA, Bessler M, Kanakura Y, Rosse W, Khursigara G, Bedrosian C, Hillmen P. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica 2014; 99: 922-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauss JS. Laboratory diagnosis of paroxysmal nocturnal hemoglobinuria. Ann Clin Lab Sci 2003; 33: 401–406. [PubMed] [Google Scholar]
- 12.Brodsky RA. Advances in the diagnosis and therapy of Paroxysmal Nocturnal Hemoglobinuria. Blood Rev 2008; 22: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotoli B, Nafa K. Paroxysmal nocturnal hemoglobinuria. In: Principles of Molecular Medicine. Jameson JL. (ed). Springer Science & Business Media, New York: 1998, pp. 227-232. [Google Scholar]
- 14.Parker CJ. The pathophysiology of paroxysmal nocturnal hemoglobinuria. Experimental Hematology 2007; 35: 523–533. [DOI] [PubMed] [Google Scholar]
- 15.Borowitz MJ, Craig FE, Di Giuseppe JA, Illingworth AJ, Rosse W, Sutherland DR, Wittwer CT, Richards SJ. Guidelines for the Diagnosis and Monitoring of Paroxysmal Nocturnal Hemoglobinuria and Related Disorders by Flow Cytometry. Cytometry Part B (Clinical Cytometry) 2010; 78B: 211-230. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland DR, Keeney M, Illingworth A. Practical guidelines for the high-sensitivity detection and monitoring of paroxysmal nocturnal hemoglobinuria clones by flow cytometry. Cytometry Part B (Clinical Cytometry) 2012; 82B: 195–208. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland DR, Illingworth A, Marinov I, Ortiz F, An-dreasen J, Payne D, Wallace PK, Keeney M. ICCS/ESCCA Consensus guidelines to detect GPI-deficient cells in Paroxysmal Nocturnal Hemoglobinuria (PNH) and related disorders. Part 2 - Reagent selection and assay optimization for high-sensitivity testing. Cytometry Part B (Clinical Cytometry) 2018; 94B: 23–48. [DOI] [PubMed] [Google Scholar]
- 18.Pu JJ, Mukhina G, Wang H, Savage WJ, Brodsky RA. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol 2011; 87: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devalet B, Mullier F, Chatelain B, Dogné JM, Chatelain C. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015; 95: 190-198. [DOI] [PubMed] [Google Scholar]
- 20.Cannizzo E, Raia M, De Propris MS, Triolo A, Scarpati B, Marfia A, Stacchini A, et al. Features, Reason for Testing and Changes with Time of 583 Paroxysmal Nocturnal Hemoglobinuria Clones from 529 Patients: a Multicenter Italian Study. Annals of Hematology 2019; 98: 1083-1093. [DOI] [PubMed] [Google Scholar]
- 21.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med 1995; 333: 1253-1258. [DOI] [PubMed] [Google Scholar]
- 22.Peffault De Latour R, Mary JY, Salanoubat C, Terriou L, Etienne G, Mohty M, Roth S, de Guibert S, Maury S, Cahn JY, Socié G. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood 2008; 112: 3099-3106. [DOI] [PubMed] [Google Scholar]
- 23.Rho H, Wells RA. A Game of Clones: The complex interplay of Aplastic Anaemia, Myelodysplastic Syndrome, and Paroxysmal Nocturnal Haemoglobinuria. European Medical Journal 2018; 3: 108-115. [Google Scholar]
- 24.Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, Röth A, Szer J, Elebute MO, Nakamura R, Browne P, Risitano Hill A, Schrezenmeier H, Fu CL, Maciejewski J, Rollins SA, Mojcik CF, Rother RP, Luzzatto L. The complement inhibitor Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N Engl J Med 2006; 355: 1233-1243. [DOI] [PubMed] [Google Scholar]
- 25.Hill A, Richards SJ, Hillmen P. Recent developments in the understanding and management of paroxysmal nocturnal haemoglobinuria. Brit J Haematol 2007; 137: 181-192. [DOI] [PubMed] [Google Scholar]
- 26.Hill A, Rother RP, Arnold L, Kelly R, Cullen MJ, Richards SJ, Hillmen P. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica 2010; 95: 567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loschi M, Porcher R, Barraco F, Terriou L, Mohty M, de Guibert S, Mahe B, Lemal R, Dumas PY, Etienne G, Jardin F, Royer B, Bordessoule D, Rohrlich PS, Fornecker LM, Salanoubat C, Maury S, Cahn JY, Vincent L, Sene T, Rigaudeau S, Nguyen S, Lepretre AC, Mary JY, Corront B, Socié G, Peffault de Latour R. Impact of eculizumab treatment on paroxysmal nocturnal hemoglobinuria: a treatment versus no-treatment study. Am J Hematol 2016; 91: 366–370. [DOI] [PubMed] [Google Scholar]
- 28.Griffin M, Kulasekararaj A, Gandhi S, Munir T, Richards S, Arnold L, Benson-Quarm N, Copeland N, Duggins I, Riley K, Hillmen P, Marsh J, Hill A. Concurrent treatment of aplastic anemia/paroxysmal nocturnal hemoglobinuria syndrome with immunosuppressive therapy and eculizumab: a UK experience. Haematologica 2018; 103: e345-e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weitz IC. Paroxysmal nocturnal haemoglobinuria - BMJ Best Practice. January 2016. www.bestpractice.bmj.com.
- 30.Morado M, Freire Sandes A, Colado E, Subirà D, Isusi P, Noya MS, Vidriales MB, Sempere A, Diaz JA, Minguela A, Alvarez B, Serrano C, Caballero T, Rey M, Pérez Corral A, Fernàndez Jiménez MC, Magro E, Lemes A, Benavente C, Bañas H, Merino J, Castejon C, Gutierrez O, Rabasa P, Vescosi Gonçalves M, Perez-Andres M, Orfao A. Diagnostic screening of paroxysmal nocturnal hemoglobinuria: Prospective multicentric evaluation of the current medical indications. Cytometry Part B (Clinical Cytometry) 2017; 92B: 361–370. [DOI] [PubMed] [Google Scholar]
- 31.Illingworth A, Marinov I, Sutherland DR, Wagner-Ballon O, Del Vecchio L. ICCS/ESCCA Consensus Guidelines to detect GPI-deficient cells in Paroxysmal Nocturnal Hemoglobinuria (PNH) and related Disorders Part 3 – Data Analysis, Reporting and Case Studies. Cytometry Part B (Clinical Cytometry) 2018; 94B: 49–66. [DOI] [PubMed] [Google Scholar]
- 32.Wlodkowic D, Akagi J, Dobrucki J, Errington R, Smith PJ, Takeda K, Darzynkiewicz Z. Kinetic viability assays using DRAQ7 probe. Curr Protoc Cytom 2013. July; Chapter 9: Unit 9.41 doi:10.1002/0471142956.cy0941s65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahmani A, Roudot H, Cymbalista F, Letestu R. Evaluation of fluorescently labeled aerolysin as a new kind of reagent for flow cytometry tests. Optimization of use of FLAER, hints and limits. Am J Clin Pathol 2016; 145: 407-417. [DOI] [PubMed] [Google Scholar]
- 34.Gatti A, Del Vecchio L, Geuna M, Della Porta MG, Brando B. Multicenter validation of a simplified method for paroxysmal nocturnal hemoglobinuria screening. Eur J Haematol. 2017; 99: 27–35. [DOI] [PubMed] [Google Scholar]
- 35.Brodsky RA, Mukhina GL, Nelson KL, Lawrence TS, Jones RJ, Buckley T. Resistance of Paroxysmal Nocturnal Hemoglobinuria cells to the glycosylphosphatidylinositol-binding toxin aerolysin. Blood 1999; 93: 1749-1756. [PubMed] [Google Scholar]
- 36.Brodsky RA, Mukhina GL, Li S, MD, Nelson KL, Chiurazzi PL, Buckley JT, Borowitz MJ. Improved detection and characterization of Paroxysmal Nocturnal Hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol 2000; 114: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland DR, Kuek N, Davidson J, Barth D, Chang H, Yeo E, Bamford S, Chin-Yee I, Keeney M. Diagnosing PNH with FLAER and Multiparameter Flow Cytometry. Cytometry Part B (Clinical Cytometry) 2007; 72B: 167–177. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland DR, Kuek N, Azcona-Olivera J, Anderson T, Acton E, Barth D, Keeney M. Use of a FLAER-Based WBC Assay in the Primary Screening of PNH Clones. Am J Clin Pathol 2009; 132: 564-572. [DOI] [PubMed] [Google Scholar]
- 39.Sachdeva MUS, Varma N, Chandra D, Bose P, Malhotra P, Varma S. Multiparameter FLAER-based flow cytometry for screening of paroxysmal nocturnal hemoglobinuria enhances detection rates in patients with aplastic anemia. Ann Hematol 2015; 94: 721–728. [DOI] [PubMed] [Google Scholar]
- 40.Höchsmann B, Rojewski M, Schrezenmeier H. Paroxysmal nocturnal hemoglobinuria (PNH): higher sensitivity and validity in diagnosis and serial monitoring by flow cytometric analysis of reticulocytes. Ann Hematol 2011; 90: 887-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland DR, Acton E, Keeney M, Davis BH, Illingworth A. Use of CD157 in FLAER-based assay for high sensitivity PNH granulocyte and PNH monocyte detection. Cytometry Part B (Clinical Cytometry) 2014; 86B: 44–55. [DOI] [PubMed] [Google Scholar]
- 42.Marinov I, Illingworth AJ, Benko M, Sutherland DR. Performance Characteristics of a non-fluorescent aerolysin-based paroxysmal nocturnal hemoglobinuria (PNH) assay for simultaneous evaluation of PNH neutrophils and PNH monocytes by flow cytometry, following published PNH guidelines. Cytometry Part B (Clinical Cytometry) 2018; 94B: 257–263. [DOI] [PubMed] [Google Scholar]
- 43.Blaha J, Schwarz K, Fischer C, Schauwecker P, Höchsmann B, Schrezenmeier H, Anliker M. The Monoclonal Anti-CD157 Antibody Clone SY11B5, Used for High Sensitivity Detection of PNH Clones on WBCs, Fails to Detect a Common Polymorphic Variant Encoded by BST-1. Cytometry Part B (Clinical Cytometry) 2018; 94B: 652-659. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland DR, Musani R. Re: Blaha J et al.: The Monoclonal Anti-CD157 Antibody Clone SY11B5, Used for High Sensitivity Detection of PNH Clones on WBCs, Fails to Detect a Common Polymorphic Variant Encoded by BST-1 (Letter). Cytometry Part B (Clinical Cytometry) 2019; 96B: 16-18. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland DR, Ortiz F, Quest G, Illingworth A, Benko M, Nayyar R, Marinov I. High-sensitivity 5-, 6-, and 7-Color PNH WBC Assays for Both Canto II and Navios Platforms. Cytometry Part B (Clinical Cytometry) 2018; 94B: 637-651. [DOI] [PubMed] [Google Scholar]
- 46.Chan RCF, Leung RH, Posadas A, Lorey TS, Shaw AJ. High sensitivity 8-color flow cytometry assay for paroxysmal nocturnal hemoglobinuria granulocyte and monocyte detections. Biomedical Reports 2018; 8: 224-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalina T, Flores-Montero J, van der Velden VHJ, Martin-Ayuso M, Böttcher S, Ritgen M. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012; 26: 1986-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cossarizza A, Chang HD, Radbruch A, Akdis M, Andrä I, Annunziato F, Bacher P, Barnaba V, Battistini L, Bauer WM, Baumgart S, Becher B, Beisker W, Berek C, Blanco A, Borsellino G, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol 2017; 47: 1584-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nováková M, Glier H, Brdičková N, Vlková M, Santos AH, Lima M, Roussel M, Flores-Montero J, Szczepanski T, Böttcher S, van der Velden VHJ, Fernandez P, Mejstříková E, Burgos L, Paiva B, van Dongen JJM, Orfao A, Kalina T. How to make usage of the standardized EuroFlow 8-color protocols possible for instruments of different manufacturers. J Immunol Methods 2017; pii: S0022-1759(17)30139-4. [DOI] [PubMed] [Google Scholar]
- 50.Solly F, Rigollet L, Baseggio L, Guy J, Borgeot J, Guérin E, Debliquis A, Drenou B, Campos L, Lacombe F, Béné MC. Comparable flow cytometry data can be obtained with two types of instruments, Canto II, and Navios. A GEIL study. Cytometry Part A 2013; 83A: 1066-1072. [DOI] [PubMed] [Google Scholar]
- 51.Damianaki A, Stagakis E, Mavroudi I, Spanoudakis M, Koutala H, Papadogiannis F, Kanellou P, Pontikoglou C, Papadaki HA. Minor populations of paroxysmal nocturnal hemoglobinuria-type cells in patients with chronic idiopathic neutropenia. European Journal of Haematology 2016; 97: 538-546. [DOI] [PubMed] [Google Scholar]
- 52.Liew M, Farley M, Andreasen J, Parker CJ, Wittwer CT. Rare event counting of CD59- red cells in human blood: A 47-month experience using PNH consensus guidelines for WBC and RBC testing in a reference lab. Cytometry Part B (Clinical Cytometry) 2015; 88B: 261-269. [DOI] [PubMed] [Google Scholar]
- 53.Payne D, Johansson U, Bloxham D, Couzens S, Carter A, Holtom P, Baker B, Hughes M, Knill T, Milne T, Morilla A, Morilla R, O’Brien D, Thomas L. Inter-laboratory validation of a harmonized PNH flow cytometry assay. Cytometry Part B (Clinical Cytometry) 2018; 94B: 736-743. [DOI] [PubMed] [Google Scholar]
- 54.Battiwalla M, Hepgur M, Pan D, McCarthy PL, Ahluwalia MS, Camacho SH, Starostik P, Wallace PK. Multiparameter Flow Cytometry for the Diagnosis and Monitoring of Small GPI-Deficient Cellular Populations. Cytometry Part B (Clinical Cytometry) 2010; 78B: 348-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008; 29 Suppl i: S49-S52. [PMC free article] [PubMed] [Google Scholar]
- 56.Clinical and Laboratory Standards Institute. Protocols for determination of limits of detection and limits of quantitation, Approved Guideline. CLSI document EP17-A2, Vol. 32 n.8, 2012. Wayne, PA USA: CLSI; 2012. [Google Scholar]
- 57.Wood B, Jevremovic D, Béné MC, Yan M, Jacobs P, Litwin V. Validation of cell-based fluorescence assays: Practice guidelines from the ICSH and ICCS - Part V - Assay performance criteria. Cytometry Part B (Clinical Cytometry) 2013; 84B: 315–323. [DOI] [PubMed] [Google Scholar]
- 58.Arroz M, Came N, Lin P, Chen W, Yuan C, Lagoo A, Monreal M, de Tute R, Vergilio JA, Rawstron AC, Paiva B. Consensus guidelines on plasma cell myeloma minimal residual disease analysis and reporting. Cytometry Part B (Clinical Cytometry) 2016; 90B: 31-39. [DOI] [PubMed] [Google Scholar]
- 59.Dezern AE, Borowitz MJ. ICCS/ESCCA consensus guidelines to detect GPI-deficient cells in Paroxysmal Nocturnal Hemoglobinuria (PNH) and related Disorders. Part 1 – Clinical Utility. Cytometry Part B (Clinical Cytometry) 2018; 94B: 16-22. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Chuhjo T, Yasue S, Omine M, Nakao S. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood 2002; 100: 3897–3902. [DOI] [PubMed] [Google Scholar]
- 61.Sipol AA, Babenko EV, Borisov VI, Naumova EV, Boyakova EV, Yakunin DI, Glazanova TV, Chubukina ZV, Pronkina NV, Popov AM, Saveliev LI, Lugovskaya SA, Lisukov IA, Kulagin AD, Illingworth AJ. An inter-laboratory comparison of PNH clone detection by high-sensitivity flow cytometry in a Russian cohort. Hematology (Amsterdam) 2015; 20(1): 31-38. [DOI] [PubMed] [Google Scholar]
- 62.Fletcher M, Whitby L, Whitby A, Barnett D. Current international flow cytometric practices for the detection and monitoring of paroxysmal nocturnal haemoglobinuria clones: A UK NEQAS survey. Cytometry Part B (Clinical Cytometry) 2017; 92B: 266-274. [DOI] [PubMed] [Google Scholar]
- 63.Kulagin A, Lisukov I, Ivanova M, Golubovskaya I, Kruch-kova I, Bondarenko S, Vavilov V, Stancheva N, Babenko E, Sipol A, Pronkina N, Kozlov V, Afanasyev B. Prognostic value of paroxysmal nocturnal haemoglobinuria clone presence in aplastic anaemia patients treated with combined immunosuppression: results of two-centre prospective study. Br J Haematol 2014; 164: 546-554. [DOI] [PubMed] [Google Scholar]
- 64.Davis BH, Keeney M, Brown R, Illingworth AJ, King MJ, Kumpel B, Meier ER, Sandler SG, Shaz BH, Sutherland DR. CLSI H52-A2 Red blood cell diagnostic testing using flow cytometry; Approved guideline, 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2014. ISBN: 1–56238–957–2. [Google Scholar]
- 65.Peghini PE, Fehr J. Clinical evaluation of an aerolysinbased screening test for paroxysmal nocturnal haemoglobinuria. Cytometry Part B (Clinical Cytometry) 2005; 67: 13-18. [DOI] [PubMed] [Google Scholar]
- 66.Whitby L, White J, Fletcher M, Whitby A, Milkins C, Barnett D. Paroxysmal nocturnal haemoglobinuria testing in blood transfusion laboratories: do they go with the flow? Transfus Med 2018; 28(6): 451-456. [DOI] [PubMed] [Google Scholar]
- 67.Richards SJ, Whitby L, Cullen MJ, Dickinson AJ, Granger V, Reilly JT, Hillmen P, Barnett D. Development and evaluation of a stabilized whole-blood preparation as a process control material for screening of paroxysmal nocturnal hemoglobinuria by flow cytometry. Cytometry Part B (Clinical Cytometry) 2009; 76B: 47-55. [DOI] [PubMed] [Google Scholar]
- 68.Fletcher M, Sutherland DR, Whitby L, Whitby A, Richards SJ, Acton E, Keeney M, Borowitz M, Illingworth A, Reilly JT, David Barnett D. Standardizing leucocyte PNH clone detection: An international study. Cytometry Part B (Clinical Cytometry) 2014; 86B: 311-318. [DOI] [PubMed] [Google Scholar]


