Abstract
Images projected onto the retina of an animal eye are rarely still. Instead, they usually contain motion signals originating either from moving objects or from retinal slip caused by self-motion. Accordingly, motion signals tell the animal in which direction a predator, prey, or the animal itself is moving. At the neural level, visual motion detection has been proposed to extract directional information by a delay-and-compare mechanism, representing a classic example of neural computation. Neurons responding selectively to motion in one but not in the other direction have been identified in many systems, most prominently in the mammalian retina and the fly optic lobe. Technological advances have now allowed researchers to characterize these neurons’ upstream circuits in exquisite detail. Focusing on these upstream circuits, we review and compare recent progress in understanding the mechanisms that generate direction selectivity in the early visual system of mammals and flies.
Keywords: direction selectivity, motion detection, visual system, retina, optic lobe
INTRODUCTION
Neurons that signal the direction of visual motion are pervasive in animals that see and have been the focus of neuroscience research for over 100 years (Exner 1894). Such neurons were first recorded in primary visual cortex of the awake cat by David Hubel (1959) and are considered a basic computational unit of cortex of all mammalian species, including primate (Livingstone & Hubel 1988, Priebe & Ferster 2008). Direction-selective cells can also be found earlier in visual processing 2–3 synapses downstream of photoreceptors. Their contribution to cortical processing is yet unclear (Cruz-Martin et al. 2014). However, due to their experimental accessibility such early direction-selective neurons hold great promise to reach a comprehensive understanding of the fundamental cellular mechanisms underlying visual motion detection.
Direction-selective ganglion cells (DSGCs) in the mammalian retina were first observed more than 50 years ago in rabbit, and encode the direction of motion in their spiking patterns (Figure 1a) (Barlow & Hill 1963). DSGCs have been extensively studied in mouse and in rabbit, where they comprise a large percentage of retinal ganglion cells. In flies, tangential cells of the lobula plate of the optic lobe respond to patterns moving in one direction by a graded depolarization, whereas they hyperpolarize during opposite pattern motion (Figure 1b) (Dvorak et al. 1975). Regardless of the animal, neurons with different directional preferences receive signals from the same array of photoreceptors. Therefore, directional information must be computed in the interjacent circuits.
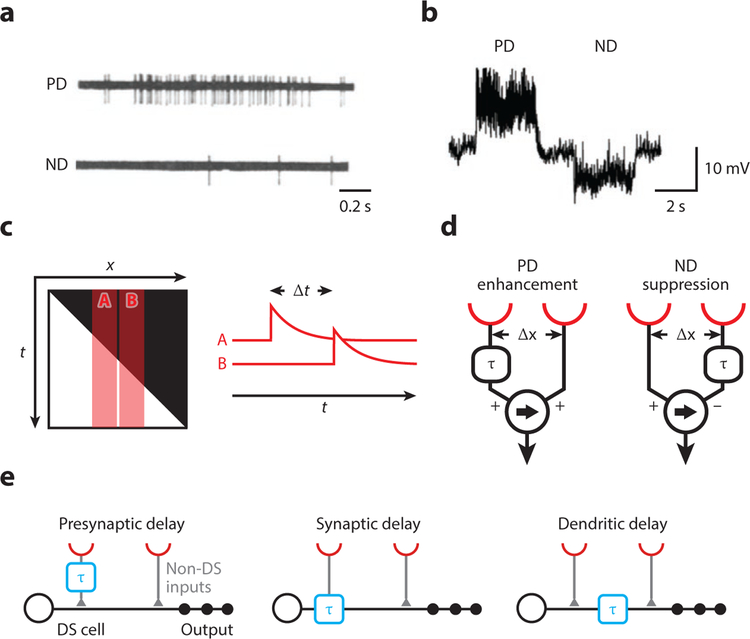
Figure 1. Cells and models for visual motion detection.
(a) Spiking response of a direction-selective unit in the rabbit retina to a moving light spot. Panel adapted with permission from Barlow and Levick (1965).
(b) Response of a lobula plate tangential cell in Drosophila to a grating moving in opposite directions. Panel adapted with permission from Joesch et al. (2008).
(c) The brightness profile of a light edge moving to the right shown in space-time (x-t). A and B represent the receptive fields of two adjacent photoreceptors, which are activated sequentially with a delay Δt. The traces A and B depict a high-pass-filtered version of a signal, highlighting illumination changes.
(d) Two alternative motion detector models. Both generate direction-selective responses by a delay-and-compare mechanism but differ in their nonlinear integration. In the Hassenstein-Reichardt model on the left, a delayed signal (denoted by τ) enhances a direct signal, for instance by a multiplication. In the Barlow-Levick model on the right, a delayed signal suppresses a direct signal, for instance by a division. In both models, the arrow indicates the preferred direction.
(e) Potential cellular implementations of local motion detection (not mutually exclusive). Spatially offset signals are conveyed through different cell types or different synaptic receptors with different dynamics (left and middle, respectively). Temporal delays might also arise by dendritic filtering (right). Note that “presynaptic delay” could be generated by an arbitrary mechanism in the upstream circuit. Abbreviations: DS, direction-selective; ND, null-direction; PD, preferred-direction.
Because visual motion involves the displacement of brightness profiles across a retina over time, both spatial and temporal information must be encoded (Figure 1c). To detect the sequence of photoreceptor activation, signals need to be compared across space and time. Two simple models have been conceived for how neurons generate direction-selective output, both of which are based on a delay-and-compare mechanism (Figure 1d). First, delays between neighboring inputs, when activated in sequence during motion, are reduced by an asymmetric filtering operation (τ). Next, coincidence is detected through a nonlinear processing step: In one model, one signal is enhanced by the other (Hassenstein & Reichardt 1956), and in the other model, it is suppressed by the other (Barlow & Levick 1965). The site of the relative temporal delay (in visual space) and the nature of the nonlinearity determine the directional preference of the detector. In order to understand visual motion detection at the cellular level, three issues need to be addressed: 1) Which is the underlying algorithm endowing neurons with direction selectivity (Figure 1d)? 2) Where and how do the asymmetric filtering operations take place (Figure 1e)? 3) How are asymmetrically filtered signals integrated in a non-linear way?
Both the mammalian retina and the optic lobe of dipteran flies have been studied extensively in this context. Although the two neuropils appear quite different at first sight, they share certain design principles, such as a retinotopic representation of visual space intersected by synaptic laminae and horizontal connections (Sanes & Zipursky 2010). Signals from each point in visual space are processed in parallel channels—for instance, for increments and decrements of light separately (Borst & Helmstaedter 2015)—forming the basis for visual feature extraction.
Historically, the underlying mechanism for direction selectivity in DSGCs in mammals was suggested to involve null-direction suppression at the level of DSGCs, with spatially offset inhibitory suppression of excitation establishing the null-direction (Barlow & Levick 1965). By contrast, tangential cells in flies were suggested to receive input from arrays of local motion detectors that compute directional information by preferred-direction enhancement (Haag et al. 2004, Single & Borst 1998). Despite decades of research, the neuronal and synaptic underpinnings of such models are still not fully understood. Here, focusing on DSGCs, tangential cells, and their respective presynaptic elements, we review recent surprising findings about the circuits and mechanisms leading to direction selectivity in the mammalian and fly visual systems.
MAMMALIAN CIRCUITS FOR DIRECTION SELECTIVITY
Integration of Excitation and Inhibition in Direction-Selective Ganglion Cells
DSGCs fall into two main classes, ON and ON-OFF, which respond to light increments or both increments and decrements, respectively. DSGCs receive excitatory inputs from glutamatergic bipolar cells, which themselves receive their primary inputs from cones. DSGCs also receive inhibitory and excitatory input from a class of interneurons called starburst amacrine cells (SACs) (Figure 2a). The SAC is radially symmetric, GABAergic, and cholinergic, with dendrites stratifying in either the OFF or ON layer of the inner plexiform layer (Figure 2b). Recent research has led to several hypotheses of how directional information is computed in the retina. As originally conceived by Barlow and Levick (1965), direction selectivity may arise in the ganglion cell itself, though not necessarily by their postulated mechanism. Furthermore, it has become clear that direction selectivity also emerges at the level of SACs which in turn convey direction-selective inhibition to DSGCs. Evidence now suggests that several of these mechanisms may function in parallel, under different stimulus conditions, or both. Here, we review evidence for these different mechanisms at the level of the DSGC and then examine closely the presynaptic mechanisms in SACs and bipolar cells.
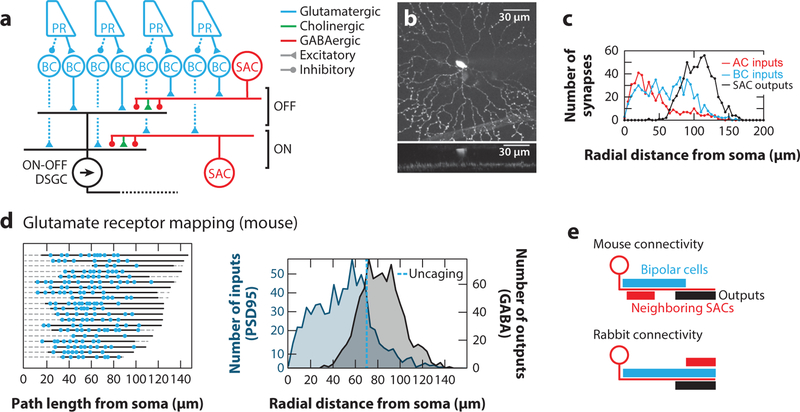
Figure 2. Visual motion detection in the mammalian retina.
(a) Schematic of the mammalian retinal circuit elements for direction selectivity. Photoreceptor signals are split into an ON and an OFF pathway at the level of bipolar cells via different glutamate receptors. ON and OFF bipolar cells synapse individually onto direction-selective ON and OFF SACs, respectively, and jointly onto ON-OFF DSGCs. DSGC receive additional asymmetric GABAergic and symmetric cholinergic input from SACs. Note that only selected aspects of the connectivity are captured in this simplified schematic.
(b) A z-projection of an ON SAC filled with a fluorescent dye showing the radial dendrites and stratification.
(c) Distribution of inputs and outputs determined by serial electron microscopy reconstructions of mouse ON SACs as well as their pre- and postsynaptic partners. Panel adapted with permission from Ding et al. (2016).
(d) (left) Distributions of glutamatergic inputs on SAC dendrites determined using glutamate uncaging. Each row is a different SAC dendrite, the solid line is the mapped region of the dendrite, and blue dots are glutamatergic synapses as revealed by glutamate uncaging. (right) Comparison of glutamatergic inputs determined by labeling SACs with the postsynaptic marker for glutamatergic synapses, PSD95-YFP (blue) and outputs determined using serial electron microscopy reconstructions. The average location of the most distal synapse measured with uncaging is represented by the dotted line. Panel adapted with permission from Vlasits et al. (2016).
(e) Schematic of dendritic locations of inputs (blue, bipolar cells; red, neighboring SACs) and outputs (black) in mouse and rabbit SACs. Abbreviations: AC, amacrine cell; BC, bipolar cell; DSGC, direction-selective ganglion cell; EM, electron microscopy; PR, photoreceptor; SAC, starburst amacrine cell.
In the main model of how DSGCs integrate their inputs to produce direction-selective firing, During null side stimulation, the inhibition from SACs provides a strong shunt, of excitation from bipolar cells and thus prevents spiking. This shunting process during null stimulation relies on the relative timing of excitatory and inhibitory inputs (as in the Barlow-Levick model) (Figure 1d, right) as well as the strength or direction selectivity of the inhibitory inputs. Active properties of the DSGC dendrites are thought to further enhance direction selectivity.
The relative importance of the input timing versus input strength mechanism likely depends on features of the stimulus, such as spatial frequency (Lipin et al. 2015). Input timing appears crucial to direction-selective tuning of DSGCs at low contrast (when cholinergic signaling dominates), as GABAergic currents lag cholinergic currents significantly in the preferred-direction (Sethuramanujam et al. 2016). Two recent studies altered the relative timing of excitation and inhibition onto DSGCs, which resulted in DSGCs being tuned to motion in what was originally their null-direction (Rivlin-Etzion et al. 2012, Vlasits et al. 2014). Whether these circuit dynamics are used endogenously remains to be determined but could underlie adaptation to motion stimuli such as described by Masland (1969).
DSGCs respond in a direction-selective manner across many orders of magnitude of light intensity. Thus, maintaining a balance between the strength of excitation and inhibition for a given motion direction across this stimulus space is important for the direction selectivity. This was recently shown in studies that explored DSGC spiking as a function of stimulus contrast. Poleg-Polsky & Diamond (2016a) showed that the excitatory inputs to SACs have higher contrast sensitivity than the excitatory inputs to DSGCs, leading to apparently matched contrast sensitivity after the SAC dendrite’s nonlinearity. Rabbit and guinea pig use another strategy: SACs have a low contrast threshold for activation and saturate at high contrast (Lipin et al. 2015). These properties lead to E-I imbalances at high contrast and therefore a preference for medium contrast—an example of a species difference that may relate to differences in the ratio of nocturnal to diurnal behavior.
In addition to the role of synaptic input timing and strength, several active properties of DSGC dendrites enhance the difference between preferred- and null-direction firing. In rabbit, recordings of dendritic spikes from rabbit DSGCs revealed that DSGCs have active, voltage-gated sodium conductances necessary for sharp directional tuning (Oesch et al. 2005). Current clamp recordings directly from the dendrites of rabbit DSGCs confirmed the presence of dendritic sodium spikes and demonstrated that excitation and inhibition compete locally within the dendrites, with GABAergic inputs capable of silencing dendritic spikes (Sivyer & Williams 2013). Thus, at the level of the dendrite, the absence of spiking during null-direction stimulation likely results from suppression of dendritic spikes by GABA receptors opening in DSGC dendrites.
N-methyl-d-aspartate (NMDA) receptors (NMDARs) are another active component of DSGC dendrites. In rabbit, NMDA is the primary glutamatergic conductance onto DSGCs and important for producing direction-selective tuning (Tjepkes & Amthor 2000). In mouse, one surprising finding is that NMDARs may be primed to open due to depolarization of the DSGC by acetylcholine receptor (AChR) activation in some stimulus conditions (Sethuramanujam et al. 2016). Poleg-Polsky & Diamond (2016b) described a role for NMDARs in enhancing direction-selective signals: Local interactions between GABA receptors and voltage-dependent NMDARs lead to a balance between excitation and inhibition that aids DSGCs in maintaining directional tuning in noisy stimulus conditions.
Beyond active channels enhancing direction selectivity, one type of DSGC, the Hb9+ type, relies in part on asymmetric anatomy of its dendrites for selectivity (Trenholm et al. 2014). In addition to the usual SAC-mediated direction-selective tuning, the asymmetric dendrites confer direction-selective tuning at slow velocities by integrating excitatory inputs preferentially during motion stimulation in the preferred-direction—a putative Hassenstein-Reichardt motif. In addition, Hb9+ DSGCs are gap-junction coupled to one another. This syncytium of DSGCs leads to correlated firing in the Hb9+ DSGC population, a property that may enhance coding in ambiguous stimulus conditions.
The Emergence of Direction Selectivity in Starburst Amacrine Cells
The SAC’s central role in the direction-selective circuit has been demonstrated unequivocally. Several studies have now shown that SACs provide the asymmetric inhibition that DSGCs use to produce direction-selective spiking, both through manipulations that perturb SAC development and morphology (Kostadinov & Sanes 2015, Sun et al. 2013) and through manipulations that remove the SAC’s participation in the circuit, either reversibly using pharmacogenetics (Vlasits et al. 2014) or irreversibly by killing SACs (Amthor et al. 2002, Yoshida et al. 2001). Thus, numerous recent studies have focused on the wiring and computational properties of SACs.
SACs have both inputs and outputs on their dendrites. They provide direction-selective inhibition to DSGCs through a combination of two properties. First, SAC release sites prefer stimuli moving outward from the soma toward the end of the dendrites (centrifugally) to motion inward toward the soma (centripetally), as first shown by Euler et al. (2002) through two-photon calcium imaging. Thus, quadrants of SAC dendrites behave like local motion detectors. Second, Briggman et al. (2011) used calcium imaging and electron microscopy (EM) to reveal that DSGCs receive inhibition predominantly from SAC dendrites pointing in their null-direction. For example, a nasally preferring DSGC receives more inputs from SAC dendrites pointing in the temporal direction. Thus, DSGCs receive inputs from SAC dendrites that have the largest influx of calcium during stimulation in a DSGC’s null-direction, presumably providing the largest amount of GABA release to the null stimulus.
To understand how SAC dendrites accomplish direction detection, researchers have elucidated the specific connectivity of the mouse SACs’ presynaptic partners. Dense EM reconstructions revealed the specific bipolar cell types that contact SAC dendrites (Ding et al. 2016, Kim et al. 2014). These EM reconstructions also showed that inputs from bipolar cells are excluded from the distal dendrites of mouse SACs (Figure 2c), also observed as a shift in the distribution of a fluorescently labeled PSD95, a scaffolding protein that marks the location of excitatory synapses (Figure 2d) (Vlasits et al. 2016). Functional mapping of excitatory inputs based on whole-cell recordings paired with either glutamate uncaging or light stimulation confirmed that the skewed distribution of receptors observed anatomically corresponds to a skewed distribution of functional receptors (Vlasits et al. 2016). EM reconstructions also described the inhibitory inputs to SACs, revealing that contacts from neighboring SACs occur in the proximal third of the dendritic arbor (Figure 2c) (Ding et al. 2016). The inhibition from SACs onto the proximal dendrites of neighboring SACs was functionally confirmed in a study that determined the intersoma distances of pairs of SACs (Kostadinov & Sanes 2015).
Using this detailed connectivity pattern to create computer simulations of SAC dendrites, one can now describe how the distribution of synaptic inputs influences the direction selectivity of SAC dendrites. One simulation based entirely on excitatory inputs (Vlasits et al. 2016) suggests that direction selectivity across the release site region of the SAC dendrite is conferred by the high input-impedance of the narrow-diameter (approximately 200–300 nm) dendrites and the fact that the presynaptic release sites are located near the end of the dendrites, where the sealed end of the dendrite creates a high-resistance compartment. When excitatory inputs in this simulation are stimulated sequentially, summation is augmented for outward motion and the depolarization is consistent across the output region because of the spatial offset of the excitatory and inhibitory input regions.
However, this model does not appear to apply to rabbit SACs, which have excitatory inputs across the entire length of their dendrites (Figure 2e) (Famiglietti 1991, Koizumi et al. 2011, Lee & Zhou 2006). This alternative was addressed by a second computer simulation that included inhibition onto SACs (Ding et al. 2016), which is confined to the proximal part of the SACs in mice and distributed more distally in rabbits. This second simulation suggests that both the mouse and the rabbit arrangements produce direction-selective responses and that the different arrangements might serve to optimize each animal’s motion detection to different velocity ranges experienced on the retina as a direct consequence of these species’ different eye diameters. Thus, throughout mammalian evolution, different solutions to the computation of direction in the SAC dendrites may have evolved.
Two papers have hypothesized an alternative mechanism for SAC direction selectivity based on bipolar cell release kinetics (Greene et al. 2016, Kim et al. 2014). As they radiate outward, SAC dendrites traverse different depths of the inner plexiform layer. EM studies indicate that the bipolar cells they contact reflect this change in depth: OFF SACs receive more contacts from bipolar cell types 1 and 2 near the soma and types 3a and 3b further from the soma; ON SACs receive more contacts from bipolar cell type 7 near the soma and from the several type 5s further from the soma. Because bipolar cells differ in the kinetics of their calcium transients and glutamate release (Baden et al. 2013, Borghuis et al. 2013), researchers have proposed they represent spatially offset and asymmetrically filtered inputs to SAC dendrites—a potential basis for preferred-direction enhancement (Figure 1e, left). However, an additional EM reconstruction using conventional staining methods, allowing the identification of synapses, questioned the clear proximal versus distal delineation of the bipolar cell types (Ding et al. 2016). Furthermore, two papers have failed to find differences in excitatory input kinetics fv (Stincic et al. 2016, Vlasits et al. 2016). These data also argue against the possibility that delays are generated by synaptic receptors with different temporal properties in SACs (Figure 1e, middle). Bipolar cell–SAC paired recordings will be needed to determine whether bipolar cell release or synaptic receptor kinetics play a role in the computation.
In addition to the excitatory inputs, researchers have debated the role of inhibition in shaping the direction-selective computation in SAC dendrites. First, we will review evidence for a role for inhibition in general, followed by the evidence of a role for mutual inhibition between SACs (SAC-SAC inhibition) specifically. Pharmacological results in rabbit showed that SACs lose direction selectivity in the absence of inhibition, suggesting that inhibition is necessary for producing direction selectivity in their dendrites (Lee & Zhou 2006). However, two studies in rabbit observed direction-selective responses in the absence of inhibition, suggesting that inhibition was not necessary for the computation at all (Euler et al. 2002, Hausselt et al. 2007). One possible explanation for the discrepancies in these experiments was suggested by Ding et al. (2016). They demonstrated that blocking inhibition in mouse reduces the direction-selective response to a high-contrast stimulus but has a more modest effect at low contrast. Thus, the importance of SAC-SAC inhibition in the direction-selective computation may depend heavily on stimulus conditions.
More recent experiments in mouse suggest that GABAergic inhibition plays a role in enhancing direction-selective responses. Blocking GABA pharmacologically greatly reduces the centrifugal preference of calcium responses in ON SAC dendrites, but some residual direction selectivity remains (Ding et al. 2016, Vlasits et al. 2016). Another study showed that knocking out the GABA a2 receptor from SACs resulted in reduced direction selectivity in OFF SACs, but not ON SACs (Chen et al. 2016), suggesting a difference between the computation in the ON and OFF channels. However, that study also showed that ON responses in DSGCs are impaired in noisy conditions, again suggesting that the importance of inhibition in the ON SAC computation depends on stimulus conditions.
One classic theory was that mutual inhibition between SACs was the origin of the direction selectivity in the circuit. SACs cofasiculate extensively and synapse onto one another. A modeling study showed that direction selectivity could originate from SAC-SAC inhibition (Münch & Werblin 2006), and many studies that used pharmacology to block GABA receptors in the retina globally assumed that this perturbation primarily influenced SAC-SAC inhibition. However, a recent study in mouse that knocked out GABA release from SACs demonstrated that SAC release sites remained strongly direction-selective (Chen et al. 2016), suggesting that SAC-SAC inhibition plays no role in the computation. Thus, other inhibitory inputs may be contributing to this computation, which remains to be investigated.
Overall, these results suggest that SACs produce a direction-selective response independent of inhibition that is then augmented by inhibition. Thus, the mouse SAC dendrite appears to have features of a Hassenstein-Reichardt detector (preferential summing of excitatory inputs for outward motion) (Figure 1d, left) but also relies on spatially offset, direction-selective inhibition from neighboring SACs to produce strong direction-selective signals.
Having described the connectivity between SACs and their presynaptic partners, we can now address how dendritic properties of SACs transform these inputs into the direction-selective GABAergic output. Beyond the role of the dendrites’ passive membrane properties in supporting a direction-selective computation (Ding et al. 2016, Tukker et al. 2004, Vlasits et al. 2016), several mechanisms for producing nonlinearities in the dendrite have been investigated, including voltage-gated channels (Hausselt et al. 2007) and gradients of ion pumps (Dmitriev et al. 2012, Gavrikov et al. 2006). Most simulations agree that some nonlinearity, at the very least the voltage-gated calcium channel that triggers GABA release, will be necessary to produce the strong centrifugal selectivity observed experimentally (Hausselt et al. 2007, Tukker et al. 2004).
Excitatory Inputs to Direction-Selective Ganglion Cells
Although SACs’ role in producing direction-selective inhibition has been well described, it remains difficult to determine whether DSGCs receive direction-selective excitation. Voltage clamp recordings from DSGCs during motion stimulation often produce tuned excitation (Borg-Graham 2001, Taylor & Vaney 2002, Taylor et al. 2000), but this tuning may be due to voltage clamp errors produced by a strong and varying inhibitory shunt (Poleg-Polsky & Diamond 2011). In addition, the relative contributions of cholinergic and glutamatergic input to DSGCs’ computations has remained a puzzle. Recent work has begun to address these issues.
One classic hypothesis was that SACs would synapse onto bipolar cells, providing direction-selective inhibition to their axons and thereby decreasing glutamate release in the null-direction (Taylor & Vaney 2002). This would lead to both asymmetric inhibition (more in the null-direction) and asymmetric excitation (more in the preferred-direction) onto DSGCs. However, EM reconstructions do not reveal such connections (Ding et al. 2016). In addition, several papers have failed to detect either direction-selective glutamate release using a glutamate sensor expressed on DSGCs or direction-selective calcium signals from bipolar cell terminals (Chen et al. 2014, Park et al. 2014, Yonehara et al. 2013). However, a recent study using a mouse lacking the vesicular GABA transporter in SACs suggests that DSGCs remain direction selective in the absence of direction-selective inhibition. Pei et al. (2015) demonstrated that, with GABAergic inputs from SACs diminished and symmetric, some DSGCs are still direction selective owing to direction-selective cholinergic signaling from SACs onto DSGCs. This suggests that excitation alone can provide direction information to DSGCs.
One puzzling aspect of ACh transmission is that SACs’ specific wiring and centrifugal motion preference would lead to more ACh release in the null compared to the preferred-direction (Figure 2a). Work in the rabbit retina showed that SACs release both ACh and GABA onto DSGCs but that kinetics and mechanisms of release differ (Lee et al. 2010), suggesting that ACh and GABA are released from separate synapses. A recent study in mouse provided further clues to answer this cholinergic puzzle. Sethuramanujam et al. (2016) demonstrated that cholinergic signaling is required for excitatory drive to DSGCs during visual stimulation with natural scenes, in which the motion edges were primarily low contrast. They further showed that stimulating SACs with Channelrhodopsin was sufficient to produce direction-selective firing in DSGCs without participation of bipolar cells. Using conductance analysis, they showed that in low-contrast conditions, roughly equal amounts of ACh are released during stimulation in either direction. Others have also observed that ACh transmission onto DSGCs is symmetric to motion (Park et al. 2014). This suggests either that SACs’ cholinergic synapses with DSGCs are symmetric or that paracrine ACh release dominates cholinergic signaling. How these results relate to the finding that ACh can provide asymmetric excitation in the absence of inhibition from SACs (Pei et al. 2015) remains to be determined.
Recent findings in mouse have revealed that the simple circuit of bipolar cells, SACs, and DSGCs is not complete (Ding et al. 2016). Elucidating the precise identity of these additional synaptic partners, their roles in circuit function, and their modulation are exciting areas for future research.
FLY CIRCUITS FOR DIRECTION SELECTIVITY
The Source of Direction-Selective Excitation in Lobula Plate Tangential Cells
Lobula plate tangential cells have been described in great detail in various fly species (Dvorak et al. 1975, Hausen 1984, Hengstenberg et al. 1982, Joesch et al. 2008, Pierantoni 1976, Schnell et al. 2010, Scott et al. 2002). They are characterized by their large dendrites on which they receive retinotopically arranged direction-selective inputs across large receptive fields (Hopp et al. 2014, Krapp & Hengstenberg 1996). Indirect evidence based on anatomy and cell-unspecific 2-deoxyglucose activity labeling suggested that arrays of small-field T4/T5 neurons supply these local direction-selective signals to tangential cells (Buchner et al. 1984, Fischbach & Dittrich 1989). Drosophila genetics has made it possible to target and manipulate T4/T5 neurons to test this hypothesis directly. Two-photon imaging of T4/T5 terminals expressing a genetically encoded calcium indicator has shown that they are indeed direction selective and segregated according to their directional preference into four lobula plate layers (Fisher et al. 2015b, Maisak et al. 2013). Predominantly monostratified tangential cell dendrites overlap with T4/T5 terminals of matching directional preference. Furthermore, silencing T4/T5 neurons genetically by expressing the K+ channel Kir2.1 (Baines et al. 2001) or a temperature-sensitive form of dynamin, shibire-ts (Kitamoto 2001) abolished all motion responses in tangential cells (Schnell et al. 2012). In addition, optogenetic activation of T4/T5 cells expressing Channelrhodopsin-2 (Pulver et al. 2009) lead to short-latency (2–3 ms) excitatory synaptic potentials in tangential cells (Mauss et al. 2014). Those synaptic potentials were sensitive to nicotinic antagonists, in line with the T4/T5 cells’ cholinergic phenotype (Mauss et al. 2014, Shinomiya et al. 2014).
T4 and T5 cells can be discriminated anatomically by the location of their dendrites in the medulla and lobula, respectively. Based on this anatomical difference, T4 and T5 cells have been suggested to lie downstream of two visual pathways segregating one synapse below the photoreceptors at the level of lamina cells L1 and L2 (Bausenwein et al. 1992) (Figure 1a). L1 and L2, with contribution from L3, feed into separate motion processing streams for increments and decrements of light, respectively (Joesch et al. 2010; Silies et al. 2013; reviewed extensively in Borst & Helmstaedter 2015). This ON/OFF split has been observed at the level of direction-selective T4 and T5 cells as well, as they respond selectively to moving ON and OFF edges, respectively (Fisher et al. 2015b, Maisak et al. 2013). Moreover, selective blockade has revealed that T4 and T5 cells are necessary for ON and OFF responses, respectively, at the level of postsynaptic tangential cells (Maisak et al. 2013). Extending the silencing approach to behavioral assays has confirmed T4/T5 cells’ role in processing ON and OFF motion signals. In fact, all behavioral responses to visual motion stimuli tested so far (such as moving edges, gratings and bars, as well as looming stimuli) depend on T4/T5 neurons; thus, they arguably represent an essential stage in visual motion processing (Bahl et al. 2013, Leonhardt et al. 2016, Maisak et al. 2013, Schilling & Borst 2015).
The Source of Direction-Selective Inhibition in Lobula Plate Tangential Cells
In addition to direction-selective excitation, tangential cells also receive inhibition in response to opposite directions of motion (Figure 1b), which requires T4/T5 cell activity (Schnell et al. 2012). Since T4/T5 neurons are excitatory, this suggested a feed-forward inhibitory element in the circuit. Because the lobula plate is organized such that adjacent layers represent opposite directions of motion (rather than directions shifted by 90°), the existence of a bistratified inhibitory relay neuron was postulated (Mauss et al. 2014). Indeed, such a neuron has been found, termed the lobula plate intrinsic (LPi) cell (Mauss et al. 2015). LPi cells receive T4/T5 input in one layer and convey an inhibitory glutamatergic signal to tangential cells expressing a glutamate-gated Cl− channel in the neighboring motion-opponent layer. Tangential cells thus integrate two sources of local, direction-selective information: direct excitation from ON- and OFF-selective T4 and T5 cells in joint lobula plate layers and indirect inhibition from bistratified LPi cells activated by neighboring T4/T5 terminals (Figures 3a).
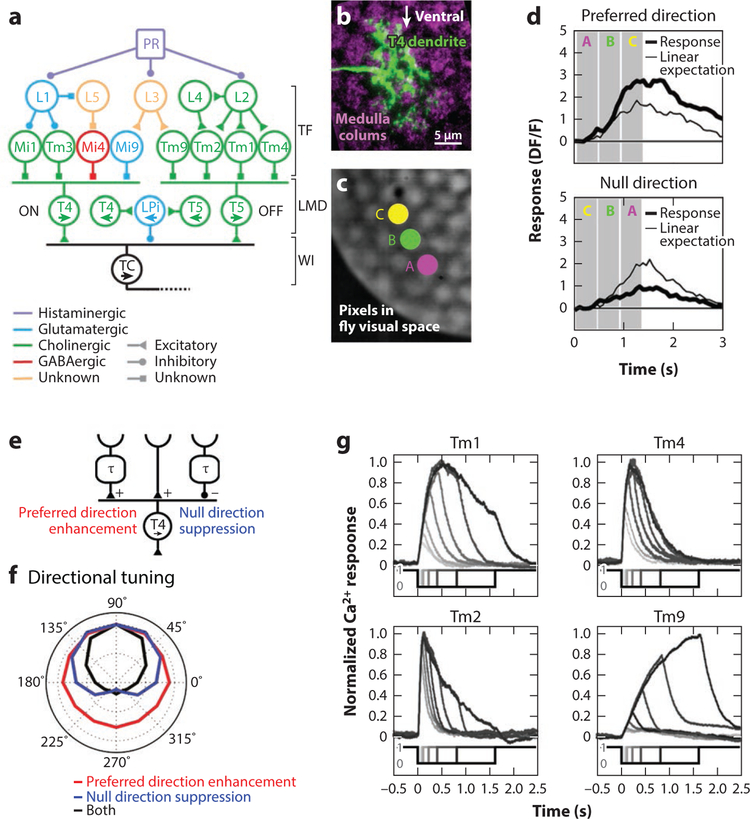
Figure 3. Visual motion detection in the Drosophila optic lobe.
(a) Schematic representation of the motion detection circuit. Photoreceptor signals are split into an ON (left) and an OFF (right) pathway processing motion information independently. Lamina (L) and medulla (Mi and Tm) cells constitute different temporal filters whose spatially offset outputs are integrated in the T4 and T5 dendrites. There, direction-selective signals are generated. T4/T5 cells thus constitute local motion detectors. Note that the spatial arrangement of T4/T5 inputs as shown here is meaningless and that inputs to leftward-tuned T4/T5 cells are omitted for clarity. Tangential cells of the lobula plate integrate direct excitatory T4/T5 signals and indirect sign-inverted signals via LPi neurons with opposite tuning (illustrated by opposing arrows) across large receptive fields.
(b) Dendrite of a T4 cell (green) tuned to upward motion. Medulla columns representing individual points in visual space are labeled with a synaptic marker in magenta. Note that the T4 dendrite spreads across multiple columns. Image courtesy of J. Pujol-Marti.
(c) Image of compound eye illuminated from the inside. This can be implemented to precisely stimulate single points in fly visual space, individually or sequentially.
(d) Calcium signals of a T4 cell, obtained by two-photon imaging. Sequential stimulation (apparent motion) of three adjacent pixels in an upward direction (A to C) produces a combined response, which is larger than the sum of the three individual responses (superlinear). Conversely, apparent downward motion (C to A) produces a combined response, which is smaller than the sum of the three individual responses (sublinear).
(e) T4 cells generate direction-selective signals by two complementary mechanisms: preferred-direction excitation and null-direction suppression.
(f) In simulations, null-direction suppression or preferred-direction enhancement alone produce broadly tuned direction-selective responses. A detector combining both elements yields markedly increased direction selectivity (matching experimentally obtained data), highlighting a potential benefit for combining two mechanisms. Panels c–f adapted with permission from Haag et al. (2016). (g) Responses to brightness decrements of varying durations of the four nondirection-selective inputs to direction-selective T5 cells, measured by two-photon calcium imaging. Tm cells can be characterized as fast and transient (Tm2 and Tm4), intermediate (Tm1), and slow (Tm9). Onsets and offsets of light stimuli are indicated below; 0 represents light off, 1 represents light on. Panel adapted with permission from Serbe et al. (2016). Abbreviations: DF/F, delta fluorescence/fluorescence (relative change of calcium indicator fluorescence); L, lamina cell; LMD, local motion detector; LPi, lobula plate-intrinsic cell; Mi, medulla-intrinsic cell; PR, photoreceptor; TC, tangential cell; TF, temporal filter; Tm, transmedullary cell; WI, wide-field integrator.
Wide-field neurons functionally reminiscent of fly tangential cells have been identified in a variety of animals and brain structures, including the accessory optic system of pigeons and the dorsal medial superior temporal area of the extrastriate visual cortex of macaque monkeys (Duffy & Wurtz 1991, Wylie et al. 1998). Owing to their large receptive fields, such neurons are commonly thought to detect panoramic flow fields arising by self-motion through visual reafference. Depending on the maneuver, flow fields on the eyes may contain mostly unidirectional motion (e.g., as experienced during body rotation about the left-right body axis), or they may be dominated by expansion (e.g., as occurs during forward translation). Often, as in flies, wide-field motion-sensitive neurons respond not only with depolarization to their preferred-direction but also with inhibition to the opposite direction. The identification and genetic control over LPi neurons allowed researchers to address the significance of this property. Recordings from tangential cells have shown that they are selectively depolarized by unidirectional flow fields and much less so by expanding flow. Genetically silencing the LPi cells (via expression of tetanus light chain) (Sweeney et al. 1995) impaired this selectivity: Tangential cells now responded unselectively to a variety of moving patterns containing opposite directions of motion (Mauss et al. 2015). Thus, the tangential cells’ reduced responses to such stimuli under normal conditions can be explained by response cancellation of opponent inputs impinging on different parts of the receptive field. Motion-opponent subtraction seems therefore essential for flow-field selectivity in wide-field motion-sensitive neurons.
The Emergence of Direction Selectivity in T4-ON Cells
T4 and T5 cells represent the outputs of two motion-detection pathways that process ON and OFF signals independently (Eichner et al. 2011, Fisher et al. 2015b, Joesch et al. 2013, Leonhardt et al. 2016, Maisak et al. 2013). The focus has shifted recently to their dendrites, where the underlying local computations take place. T4 dendrites are located in the medulla, where they arborize across various columns (approximately 7–9) (Figure 3b) (Haag et al. 2016), indicating that they receive input from several points in visual space. Dense reconstruction of their presynaptic partners based on chemical synapses revealed that >80% of identified T4 inputs (with 50% of inputs unidentified) are from medulla intrinsic neuron 1 (Mi1) and and transmedullary neuron 3 (Tm3), both of which in turn are downstream of lamina cell L1 (Takemura et al. 2013) (Figure 3a). To examine whether Mi1 and Tm3 provide spatially offset input to T4, as an indication for a potential role in motion detection, their connections onto T4 dendrites were mapped in retinotopic coordinates. Whereas both Mi1 and Tm3 inputs were distributed across the multicolumnar dendrite, the center of masses differed: Mi1 inputs were skewed toward the preferred-direction side and Tm3 synapses toward the null-direction side of the T4 dendrite. This spatial offset corresponded to 1° in visual space and is therefore significantly smaller than the spatial resolution of the Drosophila eye (~ 4.6°) (Götz 1964). Nonetheless, Mi1 and Tm3 were suggested to constitute the two arms of a candidate motion detector, predicting differential signal delays in the two cell types (Takemura et al. 2013).
A subsequent study based on whole-cell recordings from Mi1 and Tm3 cells revealed that neither cell type is direction selective and that both respond to brightness increments with depolarization (Behnia et al., 2014), in line with the model. In addition, the average Mi1 impulse response peaks later than the one of Tm3 (at 71 versus 53 ms). Taken together, these data indicate that Mi1 inputs onto T4 cells are delayed and spatially shifted to the preferred-direction side relative to Tm3 (Behnia et al. 2014, Takemura et al. 2013). Such a configuration is consistent with a Barlow-Levick–type motion detector (Figure 1d, right), with Mi1 providing an inhibitory shunt of Tm3-mediated excitation during null-direction motion.
However, this model needs to be reconciled with the following observations: First, robust direction selectivity, in particular under noisy conditions, seems difficult to achieve with the above-reported small spatial and temporal offsets. In simulations, even much more pronounced offsets yield poorly tuned local motion detectors, in stark contrast to experimental data (Haag et al. 2016, Maisak et al. 2013). Second, impulse responses of Mi1 and Tm3 measured subsequently with a fast voltage indicator did not exhibit any differences in the time-to-peak (Yang et al. 2016). Third, both Mi1 and Tm3 express cholinergic markers (Hasegawa et al. 2011, Pankova et al. 2017) and are therefore likely excitatory (rather than inhibitory). And fourth, although tangential cell responses to moving ON edges were affected strongly by genetic silencing of Mi1 (expression of the K+ channel Kir2.1), this was not generally the case for Tm3 block, leaving responses to slowly moving ON edges unaffected. This suggests that at least one other cell type is required (Ammer et al. 2015).
What could the role of such a cell be? Using a telescopic stimulation device, Haag et al. (2016) managed to place apparent motion stimuli precisely onto the columnar raster of the fly’s optic lobe (Figure 3c). Calcium signals in T4 neurons revealed responses to sequential stimulation of columns in preferred and null-directions that were markedly different from the expected linear sum of individual columnar stimulation (Figure 3d). Similar to conventional apparent motion stimulation from a screen in front of the eye (Fisher et al. 2015b), preferred-direction responses were larger, in agreement with an underlying enhancement of spatially offset and asymmetrically filtered signals (Figure 1d, left). However, Haag et al. (2016) also observed that sequential responses to null-direction stimulation on average fell not only below the sum of both individual stimulations but even below the flicker response to an individual column. This strongly indicates that slowed inhibitory signals impinge on the dendrite and suppress spatially offset local flicker inputs, therefore contributing to direction selectivity (Figure 1d, right). Interestingly, the two different mechanisms appear to be offset in their receptive fields: preferred-direction enhancement is observed predominantly for sequential stimulation of inputs on the null side and null-direction suppression for inputs on the preferred side (Haag et al. 2016). Given the strict retinotopic organization of medulla cell terminals, this suggests a corresponding mechanistic segregation on the T4 dendrite spreading across approximately 3–4 columns in each dimension (Figure 3b). In conclusion, after being discussed in the literature as alternative motion detection models for almost 60 years, both preferred-direction enhancement and null-direction suppression turn out to be combined at the level of single T4 neurons to generate direction selectivity (Figure 3e). Because either algorithm alone produces direction selectivity, the question arises why a combination thereof is advantageous. Comparisons between simulations implementing preferred-direction enhancement and null-direction suppression individually or in combination revealed a sharp directional tuning only when both algorithms are combined (Figure 3f), matching the experimentally obtained T4/T5 data (Haag et al. 2016, Maisak et al. 2013). Importantly, the sharp tuning of T4/T5 cells was not affected by silencing their own synaptic output (Haag et al. 2016). Thus, in contrast to mammalian SACs, feedback inhibition between oppositely tuned T4/T5 cells—for instance, via inhibitory bistratified LPi neurons in the lobula plate (Mauss et al. 2015)—does not seem to be required for robust direction selectivity.
Regarding potential input lines to T4 dendrites, apart from known Mi1 and Tm3 cells, recent connectomic data suggest Mi4 and Mi9 as two additional promising candidates (https://web.archive.org/web/20150218101857/http://emanalysis.janelia.org/flyem_tables.php). For a biophysical understanding of local motion detection in the ON pathway, it will be necessary to examine Mi4 and Mi9’s spatiotemporal receptive fields, requirement for motion detection, and functional connectivity with T4 cells.
The Emergence of Direction Selectivity in T5-OFF Cells
T5 cells represent the outputs of the OFF motion detection pathway. Their dendrites are located in the lobula, where they arborize across various columns, similar to T4 dendrites in the medulla. Dense reconstructions from EM data revealed the T5 cells’ chemical synapses from identified medulla (Tm) neurons (Shinomiya et al. 2014). Four candidate presynaptic partners, namely Tm1, Tm2, Tm4, and Tm9 cells, constitute more than 80% of all inputs (Figure 3a). As for Mi1 and Tm3 inputs to T4 dendrites in the ON pathway, the OFF medulla cell contacts on the T5 dendritic arbors (of unknown directional preference) were not homogenously distributed, hinting toward potential spatial offsets that could be implemented in local motion detection.
The response properties of OFF pathway Tm cells have been examined using various approaches, including somatic whole-cell patch-clamp recordings (Behnia et al. 2014) as well as functional two-photon imaging using genetically encoded voltage (Yang et al. 2016) and calcium indicators (Fisher et al. 2015b, Meier et al. 2014, Serbe et al. 2016). In line with their presumed role in OFF pathway motion detection, all four Tm cell types responded with strong activation to light decrements. None of the Tm cells responded in a direction-selective way, demonstrating that this property indeed arises in T5 dendrites. Regarding their temporal properties, the time-to-peak voltage responses have been extracted for Tm1 and Tm2 (Behnia et al. 2014, Yang et al. 2016). Both studies found a small yet consistent average difference. Much more pronounced temporal differences became apparent in a comparison of all four Tm cells’ calcium signals in response to brightness steps (fast/ transient Tm2 and Tm4, intermediate Tm1, and tonic/ slow Tm9) (Serbe et al. 2016) (Figure 3g). Assuming a suitable spatial offset, all pairwise combinations except Tm2 and Tm4 can produce biologically plausible direction-selective responses when combined nonlinearly, as explored in simulations.
Serbe and colleagues (2016) have additionally tested the requirement of candidate Tm cells for motion detection at the level of downstream tangential cell activity and behavior by silencing them using genetic shibire-ts expression. Interestingly, silencing of each cell type reduced direction-selective responses markedly, together with their different dynamics strongly suggesting that at least some of the cells represent differentially filtered inputs to a motion detector (Figure 1e, left). However, neither single-block nor any pairwise combination turned out to completely abolish direction selectivity in T5 cells. Assuming a complete synaptic block, this outcome suggests an implementation of motion detection clearly involving more than two input lines and some level of redundancy. Regarding the OFF pathway Tm cells’ spatial filtering properties, Serbe et al. (2016) have measured small receptive fields for all elements with an average half-width of 4–5°, corresponding roughly to one point in fly visual space. Neurons with such a property represent suitable local inputs for a local motion detector (Figure 1c,d). However, Fisher et al. (2015a) report wide-field receptive fields for Tm9 larger than 60°, indicating the possibility that Tm9 provides a gating mechanism for direction-selective T5 neurons, rather than a local signal for correlation.
In conclusion, T5 cells receive at least four nondirection-selective and differentially filtered input types from which they compute direction-selective signals. Regarding the underlying algorithm, spatiotemporal receptive field measurements of T5 cells are compatible with the implementation of both preferred-direction enhancement and null-direction suppression (Leong et al. 2016), as shown for T4 cells (Haag et al. 2016). However, the source of null-direction suppression is enigmatic, since all identified T5 inputs are reported to be cholinergic (Raghu et al. 2011, Shinomiya et al. 2014, Takemura et al. 2011) and inhibitory actions of acetylcholine are yet unknown in flies. Thus, the biophysical basis for generating direction selectivity in T5 dendrites remains to be established.
Behavioral Tuning of Motion Detectors
The output of a correlation-type motion detector is not proportional to arbitrary motion velocities. Rather, it has a velocity optimum, which results from the spatiotemporal filtering steps in the upstream circuit. Recent evidence suggests that wide-field tangential cells become more sensitive to higher velocity ranges during walking and flight. These effects are mediated through the neuromodulatory action of the biogenic amine octopamine released in the optic lobe during behavioral activity (Chiappe et al. 2010, Jung et al. 2011, Longden & Krapp 2010, Suver et al. 2012). Thus, the delay-and-compare mechanisms underlying the detection of visual motion are not fixed but can be flexibly tuned. Behavioral modulation of the motion detection circuit can be interpreted such that the dynamic velocity range of motion detectors matches the expected stimulus statistics and, in turn, resources are conserved during quiescence. It will be very interesting to examine octopaminergic behavioral modulation in the circuit upstream of tangential cells. One prediction is a concomitant tuning shift in local motion detectors, the T4/T5 cells. Regarding nondirection-selective T4/T5 inputs, the Mi and Tm cells, a meaningful change in their temporal dynamics might lend further credence to the notion that those cell types indeed correspond to the asymmetrically filtered input lines to a local motion detector.
CONCLUSIONS
Since the first identification of direction-selective neurons in rabbits and flies, research in the past decades—afforded by technological advances such as two-photon microscopy, genetic manipulations, and dense EM reconstructions—has made tremendous progress in identifying the upstream circuit elements and their properties. Nonetheless, the conceptually modest problem of local motion detection has been remarkably resilient to an understanding at the biophysical level.
Theoretical models posit an asymmetry in the organization of inputs to local motion detectors, such that signals from different points in space are differently temporally filtered. In retinotopically organized neuropils, spatial integration is already given for neurons that receive inputs in multiple adjacent locations, as is the case for direction-selective SAC branches and T4/T5 neurons. Asymmetric filtering could originate in different presynaptic neuron types, differentially connected to direction-selective cells depending on retinotopic location (Figure 1e, left). In flies, there is good evidence for this possibility, as some of the T4/T5 input cells exhibit markedly different dynamics. Moreover, silencing these cell types generally reduces direction selectivity, demonstrating their necessity. In mammals, the evidence for space-time wiring of bipolar cells to different parts of SAC dendrites seems less likely based on updated EM reconstructions and recordings from SACs. Instead, a delay seems to be implemented in the SAC dendrite itself by electrotonic filtering (Figure 1e, right). An asymmetry is given by distally located release sites, where centrifugally moving signals summate optimally. Direction selectivity is further enhanced by the precise wiring of excitatory inputs to the proximal portion of the dendritic tree, at least in mouse, as well as by reciprocal inhibition among neighboring SACs. As a third possibility, differential delays could also be implemented in direction-selective cells by asymmetrically distributed synapses with receptors of different dynamics, though as yet there is no direct evidence for this (Figure 1e, middle).
Models of motion detection further require nonlinear integration in direction-selective cells, which can be of an enhancing or suppressing type. SACs appear to integrate nondirection-selective inputs in a purely enhancing fashion but also rely on suppression in some stimulus conditions. In the T4/T5 dendrites, both preferred-direction enhancement and null-direction suppression take place. Although the physiological bases for these nonlinear interactions remain to be identified, a plausible implementation for a superlinear preferred-direction enhancement could be an amplification of coinciding signals through voltage-gated Ca+ or Na+ channels. Null-direction suppression could in turn be achieved by a shunting inhibition through opening of K+ or Cl− conductances.
In addition to the first local computation of motion direction performed by SACs and T4/T5 cells, signals are further processed in their downstream circuits via additional circuit motifs. There is evidence for spatially offset excitatory enhancement and inhibitory suppression to DSGCs and additional evidence of nonlinearities in DSGC dendrites that enhance signals in the preferred-direction. These mechanisms support robust detection of motion direction that persists in varying stimulus conditions and behavioral settings. T4/T5 cells already exhibit sharply tuned directional preference. However, their output is spatially integrated in downstream tangential cells across large receptive fields. This feature underlies the detection of panoramic optic flow as experienced during self-motion. The comparable mechanisms in distantly related species can be understood as convergent solutions to general problems underlying feature extraction. The differences, by contrast, are likely products of divergent anatomical, physiological, and behavioral constraints throughout evolutionary history.
ACKNOWLEDGMENTS
We are grateful to Jesus Pujol-Marti for providing the single T4 cell image shown in Figure 3. We would also like to thank Alexander Arenz for critically reading the manuscript and helpful discussions.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Ammer G, Leonhardt A, Bahl A, Dickson BJ, Borst A. 2015. Functional specialization of neural input elements to the Drosophila ON motion detector. Curr. Biol 25(17):2247–53 [DOI] [PubMed] [Google Scholar]
- Amthor FR, Keyser KT, Dmitrieva NA. 2002. Effects of the destruction of starburst-cholinergic amacrine cells by the toxin AF64A on rabbit retinal directional selectivity. Vis. Neurosci 19(4):495–509 [DOI] [PubMed] [Google Scholar]
- Baden T, Berens P, Bethge M, Euler T. 2013. Spikes in mammalian bipolar cells support temporal layering of the inner retina. Curr. Biol 23(1):48–52 [DOI] [PubMed] [Google Scholar]
- Bahl A, Ammer G, Schilling T, Borst A. 2013. Object tracking in motion-blind flies. Nat. Neurosci 16(6):730–38 [DOI] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. 2001. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci 21(5):1523–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Hill RM. 1963. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science 139(3553):412–14 [DOI] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. 1965. The mechanism of directionally selective units in rabbit’s retina. J. Physiol 178(3):477–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausenwein B, Dittrich AP, Fischbach KF. 1992. The optic lobe of Drosophila melanogaster. II. Sorting of retinotopic pathways in the medulla. Cell Tissue Res. 267(1):17–28 [DOI] [PubMed] [Google Scholar]
- Behnia R, Clark DA, Carter AG, Clandinin TR, Desplan C. 2014. Processing properties of ON and OFF pathways for Drosophila motion detection. Nature 512(7515):427–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Graham LJ. 2001. The computation of directional selectivity in the retina occurs presynaptic to the ganglion cell. Nat. Neurosci 4(2):176–83 [DOI] [PubMed] [Google Scholar]
- Borghuis BG, Marvin JS, Looger LL, Demb JB. 2013. Two-photon imaging of nonlinear glutamate release dynamics at bipolar cell synapses in the mouse retina. J. Neurosci 33(27):10972–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A, Helmstaedter M. 2015. Common circuit design in fly and mammalian motion vision. Nat. Neurosci 18(8):1067–76 [DOI] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. 2011. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471(7337):183–88 [DOI] [PubMed] [Google Scholar]
- Buchner E, Buchner S, Bülthoff I. 1984. Deoxyglucose mapping of nervous activity induced in Drosophila brain by visual movement. J. Comp. Physiol. A 155(4):471–83 [Google Scholar]
- Chen M, Lee S, Park SJH, Looger LL, Zhou ZJ. 2014. Receptive field properties of bipolar cell axon terminals in direction-selective sublaminas of the mouse retina. J. Neurophysiol 112(8):1950–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Pei Z, Koren D, Wei W. 2016. Stimulus-dependent recruitment of lateral inhibition underlies retinal direction selectivity. Elife. 5:e21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappe ME, Seelig JD, Reiser MB, Jayaraman V. 2010. Walking modulates speed sensitivity in Drosophila motion vision. Curr. Biol 20(16):1470–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, et al. 2014. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature. 507(7492):358–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Smith RG, Poleg-Polsky A, Diamond JS, Briggman KL. 2016. Species-specific wiring for direction selectivity in the mammalian retina. Nature 535(7610):105–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV, Gavrikov KE, Mangel SC. 2012. GABA-mediated spatial and temporal asymmetries that contribute to the directionally selective light responses of starburst amacrine cells in retina. J. Physiol 590(7):1699–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. 1991. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large-field stimuli. J. Neurophysiol 65(6):1329–45 [DOI] [PubMed] [Google Scholar]
- Dvorak DR, Bishop LG, Eckert HE. 1975. On the identification of movement detectors in the fly optic lobe. J. Comp. Physiol. A 100(1):5–23 [Google Scholar]
- Eichner H, Joesch M, Schnell B, Reiff DF, Borst A. 2011. Internal structure of the fly elementary motion detector. Neuron 70(6):1155–64 [DOI] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. 2002. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418(6900):845–52 [DOI] [PubMed] [Google Scholar]
- Exner S. 1894. Entwurf zu einer physiologischen Erklärung der psychischen Erscheinungen. I. Theil. Deuticke, Leipzig & Wien, pp. 179–202. [Google Scholar]
- Famiglietti EV. 1991. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J. Comp. Neurol 309(1):40–70 [DOI] [PubMed] [Google Scholar]
- Fischbach KF, Dittrich APM. 1989. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 258(3):441–75 [Google Scholar]
- Fisher YE, Leong JCS, Sporar K, Ketkar MD, Gohl DM, et al. 2015a. A class of visual neurons with wide-field properties is required for local motion detection. Curr. Biol 25(24):3178–89 [DOI] [PubMed] [Google Scholar]
- Fisher YE, Silies M, Clandinin TR. 2015b. Orientation selectivity sharpens motion detection in Drosophila. Neuron. 88(2):390–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrikov KE, Nilson JE, Dmitriev AV, Zucker CL, Mangel SC. 2006. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. PNAS 103(49):18793–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz KG. 1964. Optomotorische Untersuchung des visuellen Systems einiger Augenmutanten der Fruchtfliege Drosophila. Kybernetik 2(2):77–92 [DOI] [PubMed] [Google Scholar]
- Greene MJ, Kim JS, Seung HS, EyeWirers. 2016. Analogous convergence of sustained and transient inputs in parallel On and Off pathways for retinal motion computation. Cell Rep. 14(8):1892–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag J, Arenz A, Serbe E, Gabbiani F, Borst A. 2016. Complementary mechanisms create direction selectivity in the fly. eLife 5:e17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag J, Denk W, Borst A. 2004. Fly motion vision is based on Reichardt detectors regardless of the signal-to-noise ratio. PNAS 101(46):16333–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa E, Kitada Y, Kaido M, Takayama R, Awasaki T, et al. 2011. Concentric zones, cell migration and neuronal circuits in the Drosophila visual center. Development 138(5):983–93 [DOI] [PubMed] [Google Scholar]
- Hassenstein B, Reichardt W. 1956. Systemtheoretische Analyse der Zeit-, Reihenfolgen- und Vorzeichenauswertung bei der Bewegungsperzeption des Rüsselkäfers Chlorophanus. Z. Naturforsch. B 11(9–10):513–24 [Google Scholar]
- Hausen K 1984. The lobula-complex of the fly: structure, function and significance in visual behaviour In Photoreception and Vision in Invertebrates, ed. Ali MA, pp. 523–59. New York/London: Plenum [Google Scholar]
- Hausselt SE, Euler T, Detwiler PB, Denk W. 2007. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLOS Biol. 5(7):e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg R, Hausen K, Hengstenberg B. 1982. The number and structure of giant vertical cells (VS) in the lobula plate of the blowfly Calliphora erythrocephala. J. Comp. Physiol. A 149(2):163–77 [Google Scholar]
- Hopp E, Borst A, Haag J. 2014. Subcellular mapping of dendritic activity in optic flow processing neurons. J. Comp. Physiol. A 200(5):359–70 [DOI] [PubMed] [Google Scholar]
- Hubel DH. 1959. Single unit activity in striate cortex of unrestrained cats. J. Physiol 147(2):226–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joesch M, Plett J, Borst A, Reiff DF. 2008. Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr. Biol 18(5):368–74 [DOI] [PubMed] [Google Scholar]
- Joesch M, Schnell B, Raghu SV, Reiff DF, Borst A. 2010. ON and OFF pathways in Drosophila motion vision. Nature 468(7321):300–4 [DOI] [PubMed] [Google Scholar]
- Joesch M, Weber F, Eichner H, Borst A. 2013. Functional specialization of parallel motion detection circuits in the fly. J. Neurosci 33(3):902–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SN, Borst A, Haag J. 2011. Flight activity alters velocity tuning of fly motion-sensitive neurons. J. Neurosci 31(25):9231–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Greene MJ, Zlateski A, Lee K, Richardson M, et al. 2014. Space-time wiring specificity supports direction selectivity in the retina. Nature 509(7500):331–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T 2001. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol 47(2):81–92 [DOI] [PubMed] [Google Scholar]
- Koizumi A, Jakobs TC, Masland RH. 2011. Regular mosaic of synaptic contacts among three retinal neurons. J. Comp. Neurol 519(2):341–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostadinov D, Sanes JR. 2015. Protocadherin-dependent dendritic self-avoidance regulates neural connectivity and circuit function. eLife 4:e08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp HG, Hengstenberg R. 1996. Estimation of self-motion by optic flow processing in single visual interneurons. Nature 384(6608):463–66 [DOI] [PubMed] [Google Scholar]
- Lee S, Kim K, Zhou ZJ. 2010. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron 68(6):1159–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhou ZJ. 2006. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron 51(6):787–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong JCS, Esch JJ, Poole B, Ganguli S, Clandinin TR. 2016. Direction selectivity in Drosophila emerges from preferred-direction enhancement and null-direction suppression. J. Neurosci 36(31):8078–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt A, Ammer G, Meier M, Serbe E, Bahl A, Borst A. 2016. Asymmetry of Drosophila ON and OFF motion detectors enhances real-world velocity estimation. Nat. Neurosci 19(5):706–15 [DOI] [PubMed] [Google Scholar]
- Lipin MY, Taylor WR, Smith RG. 2015. Inhibitory input to the direction-selective ganglion cell is saturated at low contrast. J. Neurophysiol 114(2):927–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. 1988. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 240(4853):740–49 [DOI] [PubMed] [Google Scholar]
- Longden KD, Krapp HG. 2010. Octopaminergic modulation of temporal frequency coding in an identified optic flow-processing interneuron. Front. Syst. Neurosci 4:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisak MS, Haag J, Ammer G, Serbe E, Meier M, et al. 2013. A directional tuning map of Drosophila elementary motion detectors. Nature 500(7461):212–16 [DOI] [PubMed] [Google Scholar]
- Masland RH. 1969. Visual motion perception: experimental modification. Science 165(3895):819–21 [DOI] [PubMed] [Google Scholar]
- Mauss AS, Meier M, Serbe E, Borst A. 2014. Optogenetic and pharmacologic dissection of feedforward inhibition in Drosophila motion vision. J. Neurosci 34(6):2254–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss AS, Pankova K, Arenz A, Nern A, Rubin GM, Borst A. 2015. Neural circuit to integrate opposing motions in the visual field. Cell 162(2):351–62 [DOI] [PubMed] [Google Scholar]
- Meier M, Serbe E, Maisak MS, Haag J, Dickson BJ, Borst A. 2014. Neural circuit components of the Drosophila OFF motion vision pathway. Curr. Biol 24(4):385–92 [DOI] [PubMed] [Google Scholar]
- Münch TA, Werblin FS. 2006. Symmetric interactions within a homogeneous starburst cell network can lead to robust asymmetries in dendrites of starburst amacrine cells. J. Neurophysiol 96(1):471–77 [DOI] [PubMed] [Google Scholar]
- Oesch N, Euler T, Taylor WR. 2005. Direction-selective dendritic action potentials in rabbit retina. Neuron 47(5):739–50 [DOI] [PubMed] [Google Scholar]
- Pankova K, Borst A. 2017. Transgenic line for the identification of cholinergic release sites in Drosophila melanogaster. J. Exp. Biology (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJH, Kim I-J, Looger LL, Demb JB, Borghuis BG. 2014. Excitatory synaptic inputs to mouse on-off direction-selective retinal ganglion cells lack direction tuning. J. Neurosci 34(11):3976–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z, Chen Q, Koren D, Giammarinaro B, Acaron LH, Wei W. 2015. Conditional knock-out of vesicular GABA transporter gene from starburst amacrine cells reveals the contributions of multiple synaptic mechanisms underlying direction selectivity in the retina. J. Neurosci 35(38):13219–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantoni R 1976. A look into the cock-pit of the fly: the architecture of the lobular plate. Cell Tissue Res. 171(1):101–22 [DOI] [PubMed] [Google Scholar]
- Poleg-Polsky A, Diamond JS. 2011. Imperfect space clamp permits electrotonic interactions between inhibitory and excitatory synaptic conductances, distorting voltage clamp recordings. PLoS ONE. 6(4):e19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleg-Polsky A, Diamond JS. 2016a. Retinal circuitry balances contrast tuning of excitation and inhibition to enable reliable computation of direction selectivity. J. Neurosci 36(21):5861–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleg-Polsky A, Diamond JS. 2016b. NMDA receptors multiplicatively scale visual signals and enhance directional motion discrimination in retinal ganglion cells. Neuron 89(6):1277–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. 2008. Inhibition, Spike Threshold, and Stimulus Selectivity in Primary Visual Cortex. Neuron. 57(4):482–97 [DOI] [PubMed] [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. 2009. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J. Neurophysiol 101(6):3075–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varija Raghu S, Reiff DF, Borst A. 2011. Neurons with cholinergic phenotype in the visual system of Drosophila. J. Comp. Neurol 519(1):162–76 [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Wei W, Feller MB. 2012. Visual stimulation reverses the directional preference of direction-selective retinal ganglion cells. Neuron 76(3):518–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. 2010. Design principles of insect and vertebrate visual systems. Neuron 66(1):15–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T, Borst A. 2015. Local motion detectors are required for the computation of expansion flow-fields. Biol. Open 4(9):1105–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell B, Joesch M, Forstner F, Raghu SV, Otsuna H, et al. 2010. Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J. Neurophysiol 103(3):1646–57 [DOI] [PubMed] [Google Scholar]
- Schnell B, Raghu SV, Nern A, Borst A. 2012. Columnar cells necessary for motion responses of wide-field visual interneurons in Drosophila. J. Comp. Physiol. A 198(5):389–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EK, Raabe T, Luo L. 2002. Structure of the vertical and horizontal system neurons of the lobula plate in Drosophila. J. Comp. Neurol 454(4):470–81 [DOI] [PubMed] [Google Scholar]
- Serbe E, Meier M, Leonhardt A, Borst A. 2016. Comprehensive characterization of the major presynaptic elements to the Drosophila OFF motion detector. Neuron 89(4):829–41 [DOI] [PubMed] [Google Scholar]
- Sethuramanujam S, McLaughlin AJ, deRosenroll G, Hoggarth A, Schwab DJ, Awatramani GB. 2016. A central role for mixed acetylcholine/GABA transmission in direction coding in the retina. Neuron 90(6):1243–56 [DOI] [PubMed] [Google Scholar]
- Shinomiya K, Karuppudurai T, Lin T-Y, Lu Z, Lee C-H, Meinertzhagen IA. 2014. Candidate neural substrates for off-edge motion detection in Drosophila. Curr. Biol 24(10):1062–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silies M, Gohl DM, Fisher YE, Freifeld L, Clark DA, Clandinin TR. 2013. Modular use of peripheral input channels tunes motion-detecting circuitry. Neuron. 79(1):111–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Single S, Borst A. 1998. Dendritic integration and its role in computing image velocity. Science 281(5384):1848–50 [DOI] [PubMed] [Google Scholar]
- Sivyer B, Williams SR. 2013. Direction selectivity is computed by active dendritic integration in retinal ganglion cells. Nat. Neurosci 16(12):1848–56 [DOI] [PubMed] [Google Scholar]
- Stincic T, Smith RG, Taylor WR. 2016. Time course of EPSCs in ON-type starburst amacrine cells is independent of dendritic location. J. Physiol 594(19):5685–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LO, Jiang Z, Rivlin-Etzion M, Hand R, Brady CM, et al. 2013. On and Off retinal circuit assembly by divergent molecular mechanisms. Science 342(6158):1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suver MP, Mamiya A, Dickinson MH. 2012. Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila. Curr. Biol 22(24):2294–302 [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. 1995. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14(2):341–51 [DOI] [PubMed] [Google Scholar]
- Takemura S-Y, Karuppudurai T, Ting C-Y, Lu Z, Lee C-H, Meinertzhagen IA. 2011. Cholinergic circuits integrate neighboring visual signals in a Drosophila motion detection pathway. Curr. Biol 21(24):2077–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S-Y, Bharioke A, Lu Z, Nern A, Vitaladevuni S, et al. 2013. A visual motion detection circuit suggested by Drosophila connectomics. Nature 500(7461):175–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, He S, Levick WR, Vaney DI. 2000. Dendritic computation of direction selectivity by retinal ganglion cells. Science 289(5488):2347–50 [DOI] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. 2002. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J. Neurosci 22(17):7712–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjepkes DS, Amthor FR. 2000. The role of NMDA channels in rabbit retinal directional selectivity. Vis. Neurosci 17(2):291–302 [DOI] [PubMed] [Google Scholar]
- Trenholm S, McLaughlin AJ, Schwab DJ, Turner MH, Smith RG, et al. 2014. Nonlinear dendritic integration of electrical and chemical synaptic inputs drives fine-scale correlations. Nat. Neurosci 17(12):1759–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukker JJ, Taylor WR, Smith RG. 2004. Direction selectivity in a model of the starburst amacrine cell. Vis. Neurosci 21(4):611–25 [DOI] [PubMed] [Google Scholar]
- Vlasits AL, Bos R, Morrie RD, Fortuny C, Flannery JG, et al. 2014. Visual stimulation switches the polarity of excitatory input to starburst amacrine cells. Neuron 83(5):1172–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasits AL, Morrie RD, Tran-Van-Minh A, Bleckert A, Gainer CF, et al. 2016. A role for synaptic input distribution in a dendritic computation of motion direction in the retina. Neuron 89(6):1317–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie DR, Bischof WF, Frost BJ. 1998. Common reference frame for neural coding of translational and rotational optic flow. Nature 392(6673):278–82 [DOI] [PubMed] [Google Scholar]
- Yang HH, St-Pierre F, Sun X, Ding X, Lin MZ, Clandinin TR. 2016. Subcellular imaging of voltage and calcium signals reveals neural processing in vivo. Cell 166(1):245–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Farrow K, Ghanem A, Hillier D, Balint K, et al. 2013. The first stage of cardinal direction selectivity is localized to the dendrites of retinal ganglion cells. Neuron 79(6):1078–85 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. 2001. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron 30(3):771–80 [DOI] [PubMed] [Google Scholar]